Abstract
As an obvious candidate for a p-type dopant in ZnO, nitrogen remains elusive in this role. Nitrogen containing precursors are a potential means to incorporate nitrogen during MOCVD growth. One class of nitrogen-containing precursors are zinc acetate amines, yet, they have received little attention. The synthesis and single crystal X-ray structure of [Zn(acetate)2(en)], and the synthesis of [Zn(acetate)2(en)2], [Zn(acetate)2(benzylamine)2], [Zn(acetate)2(butylamine)2], [Zn(acetate)2(NH3)2], and [Zn(acetate)2(tris)2], where en = ethylenediamine and tris = (tris[hydroxymethyl]aminomethane) are reported. The compounds were characterized by thermogravimetric analysis and pyrolyzed in air and inert gas to yield ZnO. These compounds are useful single source precursors to ZnO bulk powders by alkali precipitation and ZnO thin films by spray pyrolysis. The amine bound to the zinc influences the ZnO crystal size and shape and acts as a nitrogen donor for preparing nitrogen-doped ZnO during alkali precipitation. Thin films of ZnO prepared by spray pyrolysis using the precursors had a (100) preferred orientation and measured n-type to intrinsic conductivity.
Keywords: ZnO, single source precursors, crystal structure
Introduction
Zinc oxide has received much interest in recent years because of its attractive electronic properties, ease of synthesis, low cost and for being more environmentally friendly than some alternative materials. These properties make it the material of choice for use in applications such as, but not limited to photovoltaics [1], photoacoustic wave devices [2], gas sensors [3], devices requiring a transparent conductive oxide [4], uses in biomedical applications [5-7], photocatalysis [8, 9], and as a UV absorber in many sunscreens. Military interest in ZnO arises from its potential use in UV transceivers [10], and for space electronics applications due to its superior “radiation hardness” over other semiconductors [11]. The prediction that manganese doped ZnO would exhibit room-temperature carrier-mediated ferromagnetism spurred more interest in the material [12]. The development of dilute magnetically doped semiconductors, with magnetic spins that can be manipulated at or above room temperature, is essential for the development of spin-based electronics (spintronics), and transparent semiconductors, such as ZnO, offer great potential for the development of these magnetic-optical-electronic devices [13].
For many of the electronic applications, p-type ZnO would be critical to their implementation. As typically grown, ZnO is n-type because of donors from impurities and defects [14]. Doping ZnO with elements containing fewer valence electrons than oxygen, such as nitrogen or phosphorous, introduces acceptors that compensate the intrinsic donors. Many groups have prepared nitrogen doped ZnO by numerous routes, including sputtering [15-17], laser ablation [18], molecular beam epitaxy [19], chemical vapor deposition [20], sol-gel synthesis [21], hydrothermal synthesis [22], spray pyrolysis [23], ball milling and combustions techniques [24, 25]. Additionally, many of the applications require processing control of particle size and morphology, as size and shape of the crystals can alter electronic, optical and chemical properties of the material [26]. Crystals of ZnO have been prepared in a vast array of sizes and shapes, including nanotubes [27], nanowires [28], nanorods [28], nanosheets [29], plates [30], and exotic snowflake- and flower-like structures [31, 32]. For solution-based synthesis, ZnO crystal size and shape have been influenced by not only synthesis method (hydrothermal, sol-gel, precipitation, etc.) but also solvent [30], time of precipitation [32], ions and molecules present during precipitation and growth [33-35], and annealing temperature and time [36, 37].
This research explores the use of [Zn(acetate)2(amine)x] (x = 1 or 2) compounds as single source precursors to ZnO films and bulk powders, and the use of the different amines to modify morphology, crystal size, and ZnO formation temperature. The ability of the amine to act as a nitrogen donor to yield nitrogen doped ZnO was also explored. The precursors prepared were [Zn(acetate)2(en)], [Zn(acetate)2(en)2], [Zn(acetate)2(benzylamine)2], [Zn(acetate)2(butylamine)2], [Zn(acetate)2(NH3)2], and [Zn(acetate)2(tris)2], where en = ethylenediamine and tris = (tris[hydroxymethyl]aminomethane). The synthesis of [Zn(acetate)2(NH3)2] has been previously reported [38], but this is the first report of the synthesis of the others. This paper is also the first reported use of [Zn(acetate)2(amine)x] compounds to prepare ZnO. The synthesis and characterization of these compounds are straightforward, making them convenient compounds to prepare and use as chemical precursors to ZnO. Crystal morphology of bulk ZnO powders can be tuned from needles to plates by using different amines.
Experimental Procedure
Techniques and Materials
Zinc acetate dihydrate (Fisher Chemicals) was used without further purification. Amines were either used as received or distilled under nitrogen prior to use. All synthesis was done at room temperature under ambient conditions unless otherwise stated. Solvents were either ACS or HPLC grade and used as received. Ultrapure water for reactions was obtained from a Millipore Milli-Q system. Melting points were measured in a nitrogen filled glovebox. All NMR spectra were measured on a Varian Mercury 300 MHz Spectrometer.
Synthesis of [Zn(acetate)2(ethylenediamine)]•1.6 H2O (1)
Zinc acetate dihydrate (4.5068g, 20.5 mmol) and ethylenediamine (1.38 ml, 20.6 mmol) were dissolved in water (25 mL). The solution was stirred for three days and then evacuated to dryness. The product was washed with dichloromethane (15 ml) then filtered and washed with additional dichloromethane (2×5 ml) to give a fine, white powder. Yield: 3.9554g (95.4%). Soluble in methanol and ethanol. Mp: 181-187°C. Elem. Anal. for ZnC6H17.2N2O5.6: C = 26.46; H = 6.36; N = 10.22%. Found: C = 26.67; H = 6.73; N = 10.23%. 1H NMR (CD3OD): δ 1.954 (s,6H, OOCCH3); 2.802 (s,4H,H2NCH2CH2NH2). Crystals suitable for X-ray diffraction were grown by slow evaporation of an ethanol solution of the product.
Synthesis of [Zn(acetate)2(ethylenediamine)2] (2)
Zinc acetate dihydrate (0.9986g, 4.55 mmol) and ethylenediamine (0.61 ml, 8.96 mmol) were dissolved in anhydrous ethanol (20 mL). The solution was stirred overnight and then evacuated to dryness. The product was dried at 100°C for 30 minutes in a nitrogen flow containing a partial pressure of the ethylenediamine, which was generated by bubbling the nitrogen through the amine. Yield: 1.0705g (77%) of cream colored powder. Soluble in water and alcohols. Mp: 155-160°C. Elem. Anal. for ZnC8H22N4O4: C = 31.64; H = 7.30; N = 18.45%. Found: C = 31.52; H = 7.40; N = 18.49%. 1H NMR (CD3OD): δ 1.921 (s,6H, OOCCH3); 2.737 (s,8H,H2NCH2CH2NH2).
Synthesis of [Zn(acetate)2(tris)2] (3)
Zinc acetate dihydrate (1.0313 g, 47 mmol) and tris[hydroxymethyl]aminomethane (1.1376 g) were dissolved in anhydrous ethanol (20 mL) in a nitrogen-filled glove box. The solution was stirred for two hours and then was evaporated to dryness under vacuum. Yield: 1.4551g (72.8%). Soluble in dimethylsulfoxide and water. Mp: 118-124°C. Elem. Anal. for ZnC12H28N2O10: C = 33.85; H = 6.63; N = 6.58%. Found: C =33.89; H = 6.80; N = 6.67%. 1H NMR (CD3OD): δ 1.727 (s,6H, OOCCH3); 3.489 (s,12H,C(CH2)3).
Synthesis of [Zn(acetate)2(benzylamine)2] (4)
Zinc acetate dihydrate (0.9966g, 4.54 mmol), benzylamine (0.98 mL, 9.0 mmol), and anhydrous ethanol (20 mL), were added to a round bottom flask. The solution was stirred overnight and then evaporated to dryness. Yield: 1.7055g (94%) of white powder. Soluble in dimethyl sulfoxide and methanol. Mp: 136-143°C. Elem. Anal. for ZnC18H24N2O4: C = 54.35; H = 6.08; N = 7.04%. Found: C = 53.98; H = 6.04; N = 6.81%. 1H NMR (CDCI3): δ 1.952 (s,6H,COCH3); 3.584 (s,4H,NH2); 3.844 (s,4H,ph-CH2NH2) 7.260-7.312 (10H, Benzene ring).
Synthesis of [Zn(acetate)2(butylamine)2] (5)
Zinc acetate dihydrate (1.0287g, 4.69 mmol), butylamine (0.94 mL, 9.5 mmol), and anhydrous ethanol (20 mL), were added to a round bottom flask. The solution was stirred over night and was then evaporated to dryness. The product was dried at 80°C for 30 minutes in a nitrogen flow containing a partial pressure of the butylamine, which was generated by bubbling the nitrogen through the amine. Yield: 1.1093g (66.9%). Soluble in chloroform and methanol. Mp: 91-97°C. Elem. Anal. for ZnC12H28N2O4: C = 43.71; H = 8.56; N = 8.50%. Found: C = 43.65; H = 8.57; N = 8.32%. 1H NMR (CDCI3): δ 0.903 (t,6H,CH3); 1.286-1.362 (sextet,4H,CH3CH2CH2); 1.451-1.548 (pentet,4H, CH2CH2CH2) 1.990 (s,6H, COCH3); 2.768 (t,4H,CH2CH2NH2); 2.969 (s,4H, NH2).
Synthesis of [Zn(acetate)2(NH3)2] (6)
Zinc acetate dihydrate (10.10g, 46 mmol), aqueous ammonia (10 mL, 28%), and methanol (25 mL), were added to a round bottom flask. The solution was stirred for six days and then evaporated to dryness in vacuum. The product was dried at 100°C for 10 minutes in a flow of NH3. Yield: 10.03 g (100%) as a white powder. Soluble in dimethylsulfoxide and methanol. Mp: 140-148°C. Elem. Anal. for ZnC4H12N2O4: C = 22.09; H = 5.56; N = 12.88%. Found: C = 22.23; H = 5.55; N = 12.35%. 1H NMR (DMSO): δ 1.74 (s,6H, OOCCH3); 2.88 (s,6H,NH3).
Thermal Analysis
The thermal properties of the precursors and the alkali precipitated ZnO were measured using a Mettler Toledo TGA/DSC1 thermal analyzer connected to a Pfeiffer ThermoStar mass spectrometer. Thermal characterization was performed in air, nitrogen, argon and vacuum (0.1 atm), with ramp rates of 10-50°C/min from 50°C to 1000°C.
Bulk pyrolysis of precursors
All six precursors were thermally pyrolyzed in air and under a flow of nitrogen for two hours at temperature. Precursors 1 and 2 were pyrolyzed at 750°C, and 3-6 were pyrolyzed at 650-660°C.
Alkali precipitation of ZnO powders
Each precursor was added to distilled water maintained at a constant temperature ranging from 65-95°C. With stirring, 0.6M NaOH was added to bring the pH to 10.00 and cause the ZnO to precipitate from solution. The ZnO was then filtered, washed with deionized water, air dried, and then annealed under nitrogen for two hours. Annealing temperatures ranged from 150-300°C.
X-Ray Powder Diffraction
The materials were characterized by X-ray diffraction (XRD) at room temperature using a Philips X'Pert PRO diffractometer with a Cu Kα source (λ = 1.5418 Å) in Bragg–Brentano geometry. Phase composition was determined by comparison to the Joint Committee of Powder Diffraction Standards (JCPDS) patterns. Crystallite size was calculated using the Scherrer equation, with a Scherrer constant value of 0.94 [39]. Average sizes were determined for each material using data from the (100), (002) and (101) reflections.
Single crystal X-Ray Diffraction
Crystals of 1 suitable for X-ray diffraction were obtained as described above. Data for 1 was collected at 100(±2)°K on a Bruker APEX CCD diffractometer with Mo K radiation (λ = 0.71073 Å) and a detector-to-crystal distance of 4.94 cm. A full sphere of data was collected utilizing four sets of frames, 600 frames per set, with 0.5° rotation about ω between frames and an exposure time of 10 s per frame. Data integration, correction for Lorentz and polarization effects, and final cell refinement were performed using SAINTPLUS and corrected for absorption using SADABS. The structure of 1 was solved using direct methods followed by successive least-squares refinement on F2 using the SHELXTL 5.12 software package [40]. All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were placed on the appropriate carbon atoms using the standard riding model. Relevant crystallographic data and data collection parameters are summarized in Table 1.
Table 1.
Crystal data and Structure refinement for [Zn(acetate)2en] 1.
| Empirical formula | C6H14N2O4Zn |
| Formula weight | 243.56 |
| T (K) | 100(2) |
| l (Å) | 0.71073 |
| Crystal system | Orthorhombic |
| Space group | Pbcn |
| a (Å) | 12.2486(13) |
| b (Å) | 7.7063(8) |
| c (Å) | 10.1889(11) |
| α (deg) | 90 |
| β (deg) | 90 |
| γ (deg) | 90 |
| V (Å3) | 961.74(18) |
| Z | 4 |
| Dcalc (Mg/m3) | 1.682 |
| abs coeff (mm-1) | 2.540 |
| cryst size (mm3) | 0.28×0.16×0.04 |
| θ range for data collection (deg) | 3.12–32.27 |
| Index ranges | -18≤ h ≤18, |
| -11≤ k ≤11, | |
| -14≤ l ≤ 15 | |
| reflns collected | 10708 |
| indep reflns | 1697 |
| [Rint = 0.0364] | |
| abs correction | SADABS |
| Data / restraints / parameters | 1697/0/88 |
| GOFF2 | 1.068 |
| Final R indices | R1 = 0.0293, |
| [I > 2σ(I)] | R2 = 0.0705 |
| R indices | R1 = 0.0432, |
| (all data) | R2 = 0.0773 |
| CCDC no. | 972792 |
X-ray photoelectron spectroscopy
All ZnO samples were analyzed by X-ray photoelectron spectroscopy using a Physical Electronics Versaprobe system equipped with a 100 W monochromated Al Kα X-ray source. Samples were pressed into indium foil and secured under a molybdenum mask. Sample charging was minimized using low energy electrons and Ar ions, and spectra were shifted and charge referenced to the C 1s peak at 284.8 eV. Samples were sputter cleaned using a 4 kV, 5 μA Ar ion beam rastered over a 2 × 2 mm area for 1 minute at an Ar pressure of ∼5 × 10-6 Pa.
Scanning Electron Microscope
All ZnO products were characterized by field emission scanning electron microscopy (FESEM) (Hitachi, S-4500) to determine size and morphology. Quartz PCI Version 8 image processing software was used to acquire SEM micrographs. Accelerating voltage was varied from 7 kV to 20 kV depending on the sample requirements. Magnification ranged from 1,000X to 200,000X. Particulate size of each powder dictated the magnification necessary to distinguish morphology. Outgassing was avoided in the FESEM vacuum chamber via a specific sample preparation. A diamond scribe was used to mark through the oxide layer on the sample stage. To secure the sample onto the sample stage and to avoid sample charging, a colloidal solution of Pelco silver paint was applied to the sample stage and allowed to air dry. A second coat of silver was applied to the sample stage, and the ZnO powder was deposited onto the wet silver paint. The paint was again allowed to dry. Immediately before inserting the mounted sample into the chamber, the sample was sprayed with compressed air to remove any loose particulates.
Conductivity type measurements
Conductivity type measurements were performed on the ZnO thin films by the rectifying three-point probe method [41, 42], using an Agilent 4156C Precision Semiconductor Parameter Analyzer using three of the four available source measurement units (2 μV and 1 fA resolution), an Agilent E5250A Low Leakage Solid State Switch Matrix, and three DCM-210-M micromanipulators with tungsten probe tips connecting the devices enclosed in a Faraday cage to minimize electromagnetic noise. In the rectifying three-point probe technique, three tungsten probes were placed in parallel and approximately equidistant from each other (several millimeters apart) from left to right onto the ZnO film forming a metal-semiconductor contact (figure 1a). A bias, Vb, was applied to probe 1 while probe 2 was kept at ground, and the output voltage was measured at probe 3 relative to probe 2 (figure 1a & b). In theory, by biasing the metal-semiconductor contact over both negative and positive voltages, the rectification behavior of metal-semiconductor contacts will reveal the conductivity type. If V32 value is close to a positive Vb when Vb is swept positively and V32 is zero volts (ground) when Vb is swept negatively, the semiconductor is n-type (figure 1c). Conversely, if V32 is proximate to a negative Vb, when Vb is biased in the negative direction and V32 is measured to be zero volts when Vb is biased positively, then the semiconductor is p-type (figure 1c). Finally, if V32 is near the value of positive Vb when Vb is biased positively, and V32 is near the value of negative Vb when Vb is biased negatively suggests the semiconductor is intrinsic-like. The rectifying three-point probe method was carried out on both n-type and p-type Si wafers to verify the technique, and the outcome is shown in figure 1c. For the ZnO thin films, Vb was swept from -4V to 4V while V32 was measured. Since the three-point probe technique assumes rectifying (i.e., Schottky) contacts, energy band diagram simulations of the metal-semiconductor contacts were performed to examine the validity of the results.
Figure 1.
The rectifying three-point probe technique. a. Three tungsten probes in which Vb is applied at probe 1 and current is measured at probe 2 while V32 is measured at probe 3. b. The circuit representation of the configuration shown in a. c. The applied bias, Vb, versus measured bias at probe 3 relative to probe 2, V32, showing the expected behavior for n-type and p-type semiconductors and data for n-type Si and p-type Si. Adapted from references [41, 42].
Results and Discussion
Several [Zn(acetate)2(amine)x] (x = 1 or 2) compounds were prepared, and their stoichiometries and compositions were confirmed by NMR and elemental analysis. Compound 1, [Zn(acetate)2(en)], was obtained from a stoichiometric reaction of zinc acetate dihydrate with ethylenediamine in ultrapure water, followed by crystallization from ethanol. When the reaction was performed with excess ethylenediamine in anhydrous ethanol and dried at 100°C in a flow of nitrogen, saturated with ethylenediamine, a compound with the stoichiometry of [Zn(acetate)2(en)2] (2) was obtained, as determined by 1H NMR and elemental analysis. Although attempts were not made to obtain a single crystal X-ray structure of the second compound, NMR results are consistent with an octahedral coordination for the zinc and the acetate ligands bound trans to each other. The crystalline structure of [Zn(acetate)2(en)] (1) was determined by single crystal X-ray diffraction. The unit cell of 1 contains four molecules, with the molecules stacking in layers normal to the (101) plane. The [Zn(acetate)2(en)] molecule (figure 2) shows the tetrahedral geometry expected for the d10 zinc-containing precursor. The Zn1-N bond distance is 2.0739(13)Å, and the N1-Zn1-N1A angle is 85.57(8)°. This angle is less than the ideal 109.2° angle of tetrahedral geometry due to the geometry of the ethylenediamine molecule, which does not allow the nitrogen atoms to separate to ideal tetrahedral configuration. The restricted N1-Zn1-N1A angle allows the O1-Zn1-O1A bonds to spread to an angle of 126.09(7)°. The Zn-O bonds to the acetates are 1.9714(12) Å. All bond lengths and angles correlate well to values reported for similar compounds (Table 2) [43-46].
Figure 2.
Thermal ellipsoid diagram of [Zn(acetate)2(ethylenediamine)] (1). Atoms are represented by ellipsoids at 50% probability.
Table 2.
Selected bond lengths (Å) and bond angles (degrees) for 1.
| Bond | Bond Length, Å | Bond | Bond Angle, ° |
|---|---|---|---|
| Zn-O(1) | 1.9714(12) | O(1)-Zn-O(1A) | 126.09(7) |
| Zn-N(1) | 2.0739(13) | N(1)-Zn-N(1A) | 85.57(8) |
| O(1)-Zn-N(1) | 107.45(5) | ||
| O(1)-Zn-N(1A) | 111.43(5) |
The thermal properties of compounds 1-6 were probed using thermogravimetric analysis/differential scanning calorimetry - mass spectroscopy (TGA/DSC-MS) in both air and argon atmospheres. When heated in air, the precursors thermally decompose in multiple steps to yield ZnO (Figure 3). The temperature of ZnO formation varies with the amine bound to the zinc acetate (Table 3), with benzylamine and ammonia yielding the lowest formation temperatures and tris producing the highest. The high ZnO formation temperature of [Zn(acetate)2(tris)2] is attributed to tris being the least volatile of the monodentate amines with a boiling point of 220°C, and the tris molecule thermally cracking rather than vaporizing intact, as determined by TGA-MS (Figure SI-1). The temperature for ZnO formation using [Zn(acetate)2(benzylamine)2] is slightly lower than that observed for the oxidation Zn(acetate)2· 2H2O to ZnO, which occurs at 350°C. Therefore, by choosing different amines, the ZnO formation temperature may be tuned from 335 – 590°C. When heated in an argon atmosphere, the compounds containing benzylamine, ammonia and butylamine have ZnO formation temperature similar to that observed in air, but the final weight loss step for the tris- and ethylenediamine-containing precursors extends over several hundred degrees (Figure 4).
Figure 3.
Thermogravimetric analysis (TGA) curves for the heating of the six chemical precursor to 1000°C in dry air at a rate of 20°C/min. The figure illustrates how the amine bound to the zinc affects the ZnO formation temperature.
Table 3.
Thermal data for [Zn(acetate)2(amine)x] compounds pyrolyzed in dry air.
| Precursor compound | ZnO Formation Temp. | Residue (%) |
|---|---|---|
| Zn(acetate)2(en) | 530°C | 34.10 |
| Zn(acetate)2(en)2 | 530°C | 26.03 |
| Zn(acetate)2(tris)2 | 590°C | 19.14 |
| Zn(acetate)2(benzylamine)2 | 335°C | 17.58 |
| Zn(acetate)2(butylamine)2 | 350°C | 24.94 |
| Zn(acetate)2(NH3)2 | 355°C | 35.01 |
Figure 4.
Thermogravimetric analysis (TGA) curves for the heating of the six chemical precursor to 1000°C in argon at a rate of 20°C/min.
Using the TGA data as a guide, larger quantities of each of the precursors were pyrolyzed in nitrogen and air, and the products were characterized by powder X-ray diffraction (XRD) and scanning electron microscopy. All the precursors pyrolyzed in air were white in color, whereas precursors pyrolyzed under an inert atmosphere were all dark gray to black due to carbon incorporation. All the pyrolyzed samples yielded wurtzite ZnO, as determined by powder X-ray diffraction (JCPDS 00-036-1451) (figures 5 & 6), and were generally very crystalline. The exception was [Zn(acetate)2(tris)2], which yielded a material with lower crystallinity or smaller crystallite size when pyrolyzed under inert conditions. This result is consistent with the weight loss observed in the TGA for this compound, which extends well above the 650°C pyrolysis temperature. Average crystallite sizes for the pyrolyzed materials were calculated using the Sherrer equation [39]. Average crystallite sizes were determined to be 40 nm, 48 nm, 43 nm, 40 nm, 38 nm and 40 nm for material from [Zn(acetate)2(tris)2], [Zn(acetate)2(en)], [Zn(acetate)2(en)2], [Zn(acetate)2(butylamine)2], [Zn(acetate)2(NH3)2], and [Zn(acetate)2(benzylamine)2], respectively when pyrolyzed in air and 7 nm, 38 nm, 43 nm, 31 nm, 36 nm and 38 nm, respectively when pyrolyzed in nitrogen.
Figure 5.
Powder X-ray diffraction data for the products obtained from the pyrolysis of compounds 1-6 in air.
Figure 6.
Powder X-ray diffraction data for the products obtained from the pyrolysis of compounds 1-6 in an inert atmosphere.
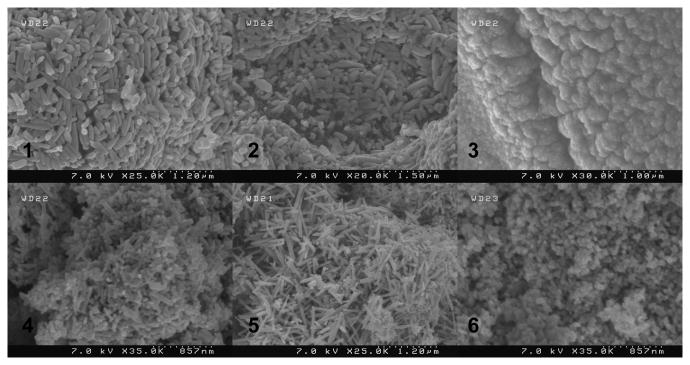
The pyrolyzed materials were characterized by field emission scanning electron microscopy to explore the effect of the different amines on crystal morphology and size (Figures 7 and 8). All of the materials heated in air have a sintered/melted appearance. With the exception of the benzyl- and butyl containing precursors, the products have poorly defined morphology and vary in size from 75 – 200 nm for [Zn(acetate)2(tris)2], from 100 – 300 nm for [Zn(acetate)2(NH3)2] and from 120 – 360 nm for [Zn(acetate)2(en)2]. The material obtained from the pyrolysis of [Zn(acetate)2(en)] form aggregates with sizes ranging up to 1.2 μm and with grain sizes as small as 100 nm. In contrast, the benzyl- and butyl containing precursors yield products with rod-shaped morphology. The rods produced from [Zn(acetate)2(benzylamine)2] have a 90 nm cross section and range in length from 300 – 500 nm, whereas the rod produced using [Zn(acetate)2(butylamine)2] have a similar cross section but with lengths ranging up to 600 nm.
Figure 7.
Field emission scanning electron microscopy images obtained from the pyrolysis of compounds 1-6 in dry air. The number on the image corresponds to the compound used to produce the material.
Figure 8.
Field emission scanning electron microscopy images obtained from the pyrolysis of compounds 1-6 in an inert atmosphere.
When pyrolyzed under inert conditions all of the precursors, except [Zn(acetate)2(tris)2] and [Zn(acetate)2(NH3)2], yielded well defined rod-shaped crystals. The benzyl- and butyl-containing precursors produced the smallest diameter rods of 60 nm and lengths ranging from 160-450 nm for the benzyl precursor and 350-650 nm for butyl-containing precursor. The two ethylenediamine-containing precursors produced rods with approximately 100 nm diameters and lengths ranging from 240-480 nm for 1 and 180-750 nm for 2. The [Zn(acetate)2(NH3)2] precursor yielded poorly defined crystals with diameters of approximately 80 nm. The [Zn(acetate)2(tris)2] precursor produced aggregated crystals with 50 nm grain size. Therefore, the different amines bound to the zinc influence both the size and morphology of the ZnO produced by pyrolysis of compounds 1–6.
Precursors 1 and 3-6 were also used to prepare bulk powders of ZnO by alkali precipitation. The ZnO was precipitated from aqueous solutions at a pH = 10 by the addition of NaOH. The precipitation was carried out at 65°C and 75°C and the time for precipitation titration was varied from 5 minutes to 30 minutes. All precipitations yielded wurtzite ZnO, as determined by powder X-ray diffraction (figure 9). Average crystallite size for the materials characterized in figure 9 were calculated using the Sherrer equation to be 33 nm, 43 nm, 35 nm, 40 nm, and 35 nm for material from [Zn(acetate)2(tris)2], [Zn(acetate)2(en)], [Zn(acetate)2(butylamine)2], [Zn(acetate)2(NH3)2], and [Zn(acetate)2(benzylamine)2], respectively. Temperature, rate of precipitation and the amine bound to the zinc were all found to have a pronounced effect on the morphology and particle size of the material produced. Consistent with the Ostwald ripening mechanism, higher temperatures and slower precipitation rates yielded larger particles for a given morphology [47], as has been observed using other ZnO precursors [33]. The amine bound to the zinc was found to direct the morphology of the particles, with ethylenediamine yielding needles, tris producing plates, benzylamine and butylamines yielding short needles, and NH3 producing hexagonal rods. The benzylamine and butylamine products often resembled grains of rice. The materials produced using tris were either well defined hexagonal plates or as shown in figure 10, plates with rounded edges. Scanning electron micrographs of the materials obtained at 75°C and precipitated over 30 minutes are provided in figure 10. Garcia has shown that the denticity of carboxylate ligands in solution during ZnO crystal growth has a pronounced impact on the crystal aspect ratio, with monodentate ligands yielding rods, tridentate ligands producing plates, and bidentate ligands yielding intermediate short rods [35]. The use of monodentate and bidentate amines to control crystal aspect ratio in this work, suggests that the effects of denticity on crystal aspect ratio is more complex than previously understood.
Figure 9.
Powder X-ray diffraction data for ZnO powders obtained by alkali precipitation of the different precursors at 75°C. Sodium hydroxide was added over the course of 25-30 minutes to precipitate the products. All products were annealed at 200°C for two hours in a nitrogen atmosphere.
Figure 10.
Field emission scanning electron microscopy images of ZnO obtained from the alkali precipitation over the course of 30 minutes at 75°C for compounds 1 and 3-6. All samples were annealed at 200°C for two hours under nitrogen.
A primary goal of this work was to explore the use of these compounds to prepare bulk quantities of nitrogen-doped ZnO powders. Zinc oxide powders obtained by precipitation were characterized by XPS. All of the materials contained minimal nitrogen, with percent nitrogen concentrations ranging from 0.3-0.5% for ZnO prepared from the precursors containing NH3, butylamine, and ethylenediamine to 1.1-1.6% for benzylamine and tris. X-ray photoelectron spectroscopy analysis is provided in the supporting information (Figures SI-2 and SI-3 and Table SI-1). The observed nitrogen content is consistent with the t-butyl group of the tris molecule and the benzyl group being more stable leaving groups, yielding ZnO with higher nitrogen doping. Although the amine bound to the precursor does affect nitrogen concentrations in the resulting ZnO, these nitrogen concentration levels are not expected to produce p-type ZnO [48, 49].
To further investigate the usefulness of these precursors, they were used to deposit thin films of ZnO in air by spray pyrolysis. Methanol solutions of each precursor were sprayed onto pyrex substrates, with the substrate temperature held at 550°C. Both air and argon were used as the carrier gas, but argon was found to yield higher quality films. Precursors [Zn(acetate)2(en)], [Zn(acetate)2(butylamine)2], and [Zn(acetate)2(NH3)2] yielded high quality, well adhered, optically transparent films (Figure SI-4). The precursor [Zn(acetate)2(tris)2] yielded powdery, optically opaque films. This result is consistent with the TGA data, which gives the thermal decomposition of [Zn(acetate)2(tris)2] at or above the substrate temperature. The compound [Zn(acetate)2(benzylamine)2] yielded very thin films contaminated with partially decomposed precursor, as they contained high concentrations of both carbon and nitrogen, as determined by XPS. All of the precursors yielded wurtzite ZnO films, with a (100) preferred growth orientation (figure 11). The amorphous broad low intensity peak in Figure 11 is attributed to the glass substrate, as the film had an average thickness of 1.1 μm. Sherrer equation calculations give an average crystallite size of 24 nm for the film used for Figure 11. The films were characterized by XPS, but again the high quality films revealed minimal nitrogen incorporation, likely from the formation of volatile nitrogen oxide compounds during precursor decomposition. The optical energy band gap, as measured by optical transmission, varied from 3.29 eV to 3.32 eV, similar to the reported value of 3.37 eV [50]. Lower measured optical band gaps are possibly due to increased scattering from the high surface roughness of the films.
Figure 11.
X-ray powder diffraction of a ZnO thin film grown using [Zn(acetate)2(NH3)2].
Conductivity type measurements using the rectifying three-point probe method (Figure 1) revealed that n-type to somewhat intrinsic type conductivity was prevalent in all samples, consistent with intrinsic ZnO, with the films having varying degrees of conductivity. Representative data revealing the conductivity type behavior of the ZnO thin films are shown in Figure 12. Theoretically, if V32 follows the value of Vb in a one-to-one ratio as Vb is swept positively and V32 is at ground (i.e., 0 V) as Vb is swept negatively, then the semiconductor is n-type. Alternatively, if V32 follows the value of Vb in a one-to-one ratio as Vb is swept negatively, and V32 is at ground as Vb is swept positively, then the semiconductor is p-type. However, it is apparent that the results are not so well defined. The conductivity results of the ZnO thin films seem to fall into three regimes of V32. Hence, the criteria of V32 values in ratios of Vb were developed and applied as outlined in Table 4. Based on these criteria, the conductivity type results of the ZnO thin films were organized into 3 categories: n-type behavior, moderate n-type behavior, and intrinsic-like behavior (Table 4). As seen in Table 4, most of the samples were observed to exhibit moderately n-type, with [Zn(acetate)2(butylamine)2] and [Zn(acetate)2(en)] yielding n-type to moderately n-type films and [Zn(acetate)2(NH3)2] producing films of moderately n-type to intrinsic behavior. Films prepared from [Zn(acetate)2] solutions, with no added amine also revealed moderately n-type behavior. The criteria may appear somewhat subjective, but what is clear from the results is that p-type behavior in the ZnO thin films is not observed.
Figure 12.
Representative conductivity type measurement data from several ZnO thin films – two films exhibit n-type behavior, two exhibit moderate n-type while one shows intrinsic-like behavior. Theoretical lines for n-type and p-type semiconductor are included to help guide the reader.
Table 4.
Precursor, conductivity type, and criteria to determine conductivity type of ZnO thin film samples.
| Precursor | Conductivity Type (n-, p-, intrinsic) |
Criteria: V32 at +Vb | Criteria: V32 at -Vb |
|---|---|---|---|
| [Zn(acetate)2(butylamine)] | n-type | ∼+1Vb to ∼+1/2Vb | ∼0V to ∼-1/4V |
| [Zn(acetate)2(en)] | |||
|
| |||
| [Zn(acetate)2(butylamine)] | ∼+1/2Vb to ∼+1/4Vb | ∼0V | |
| [Zn(acetate)2(en)] | Moderately n-type | or | or |
| [Zn(acetate)2(NH3)2] | ∼+1Vb to ∼+3/4Vb | ∼-1/2V to ∼-1/4V | |
| [Zn(acetate)2] | |||
|
| |||
| [Zn(acetate)2(NH3)2] | Intrinsic behavior | ∼+1/2Vb to ∼+1/4Vb | ∼-1/2V to ∼-1/4V |
Energy band diagram simulations were performed using the energy band diagram simulation tool established at Boise State University [51, 52]. The simulations require an intrinsic material between the semiconductor and the metal. It is reasonable to assume this scenario since intrinsic behavior of semiconductor surfaces can be induced by the presence of surface states or native oxides. Thus, very thin (0.5nm) intrinsic native oxides were incorporated in simulating both the W-ZnO and the W-Si contacts. Assuming the intrinsic thin native oxides used in the simulations are to first order charge-free, their presence do not affect the outcome because the work function differences (i.e., built-in voltage) are dictated by the work function (i.e., Fermi energy) differences between only the metal and the doped semiconductor. The materials parameters used for the simulations are listed in Table 5. A tungsten thickness of 5 nm was chosen to maximize energy band diagram visualization and has no bearing on its work function, φw. The doping concentration of all semiconductors, 2×1018 cm-3, was kept constant to minimize ambiguities for comparisons between tungsten contacts to ZnO and silicon. The high doping concentration was chosen because ZnO ni (1×1016 cm-3) [53] is so large considering the energy band gap (Eg) for ZnO is a relatively large ∼3.44eV. Typically, ni scales inversely to the semiconductor energy band gap (e.g. ni: GaAs ∼106 cm-3, Si ∼1010 cm-3, Ge∼1013 cm-3; Eg: GaAs ∼1.42eV, Si ∼1.12eV, Ge ∼0.66 eV) [51, 52]. The large ni,Zno is owed to its high defect density [53]. For simulation purposes due to the limitations of the energy band diagram program, the doping concentration needs to be ∼200 times greater than ni. The high doping concentration is consistent with recently reported p-type nitrogen-doped ZnO with carrier densities ranging from 1019 to 1023cm-3[54].
Table 5.
Materials and materials parameters at 300K used for the energy band diagram simulations.
| Material\Parameters | Work Function, φ (eV) | Electron Affinity, χ (eV) | Band Gap Energy, Eg (eV) | Intrinsic Carrier Concentration, ni (carriers/cm-3) | Relative Dielectric Constant | Reference |
|---|---|---|---|---|---|---|
| Tungsten, W | 4.5 | N/A | N/A | N/A | N/A | [49,50] |
| Silicon, Si | * | 4.05 | 1.125 | 1.41×1010 | 11.7 | [49,50] |
| Zinc Oxide, ZnO | * | 4.6 | 3.44 | 1×1016 | 8.75 | [49,50] |
Depends on whether n-type or p-type
The primary difference between W-Si and W-ZnO contacts is that tungsten contacts to p-Si, n-Si and p-ZnO are Schottky, but the tungsten contact on n-ZnO is ohmic. The reason is two-fold. The primary reason is attributed to the sufficiently large electron affinity of ZnO (χzno ∼4.6eV) where φw is less than χzno. Not as significant as the first reason but nonetheless impactful is the large value of ni,zno – it is so large for a rather large energy band gap semiconductor as compared to ni,si (Table 5) that the Fermi energies of both p- and n-type ZnO (i.e., Ef,p and Ef,n) are near the mid-energy band gap of ZnO (Ei,zno) and hence Ef,p and Ef,n will not differ significantly from one another. That is, Ef,p, Ef,n, and Ei,zno are relatively similar. Hence, the tungsten-ZnO contact for n-type is ohmic, whereas the p-type W-ZnO contact is Schottky (i.e., rectifying, blocking).
Will the W-nZnO ohmic contact significntly affect the electrical measurements? Since eVBI,nZno is small (table SI-3), the ohmic contact does not significantly affect biasing conditions. That is, the amount of voltage (Vb) required to bend the n-ZnO energy bands to the Schottky condition (reverse bias condition) is less than 2V. By examining the band bending in the W-nZnO contact over the range of biasing conditions for Vb (i.e., -4V to 4V) as shown in Figure SI-5, the band bending in n-ZnO is very similar to that of n-Si except at equilibrium conditions (i.e., Vb = 0V).
Overall, the simulations reveal interesting aspects, without which, are not immediately apparent. It can be seen that to obtain p-type conductivity in ZnO with such a high intrinsic carrier concentration, the concentration of nitrogen donors must be at least 1018 cm-3 in order to observe p-type behavior. However, at these concentrations, one needs to be concerned with the solubility limit of nitrogen in ZnO.
Closing Remarks
The [Zn(acetate)2(amine)x] (x = 1 or 2) compounds prepared for this study may be used as single source precursors to ZnO bulk powders by alkali precipitation and to ZnO thin films by spray pyrolysis. The amine bound to the zinc during precipitation influences ZnO crystal size and shape and acts as a nitrogen donor to give nitrogen doped ZnO. However, nitrogen doping was minimal, with the best amine (tris) only yielding 1.6% nitrogen incorporation. The six compounds investigated in this study were used because the solid form could be stored for extended periods and readily redissolved. Many other amines were examined and the products characterized by solution NMR, but the solid [Zn(acetate)2(amine)2] compounds would become insoluble in common solvents after a few days in air and some within a week when stored in a nitrogen filled glovebox. However, many of the [Zn(acetate)2(amine)2] compounds that were not storable in solid form were prepared in situ, and the solutions were used to generate ZnO bulk powders. All of the amines used were found to influence crystal size and shape. Our study of these materials will be reported elsewhere. Additionally, the authors note that amine selection does influence morphology by more complex pathways than previously reported [35]. Three of the precursors were useful for preparing high quality, well adhering, optically transparent films of ZnO by spray pyrolysis. The films had n-type to somewhat intrinsic type conductivity. Films grown using [Zn(acetate)2(tris)] were powdery and opaque, and films prepared using [Zn(acetate)2(benzylamine)2] contained high concentrations of carbon and nitrogen. Films were also deposited using solutions of [Zn(acetate)2(amine)2] compounds generated in situ, as discussed above, and those results will also be reported elsewhere.
Supplementary Material
Acknowledgments
We gratefully acknowledge the financial support from the National Science Foundation through grants DMR 0722699, DMR 0840265, and CHE 0226402, support by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant #P20GM103408, NASA Idaho Space Grant Consortium, M.J. Murdock Charitable Trust, and Northwest Nazarene University's Science, Math Associates.
Footnotes
Supporting Information Available: A complete listing of anisotropic displacement parameters, interatomic distances, angles, hydrogen coordinates and displacement parameters for [1] are available upon request from the authors. CCDC 972792 also contains the supplementary crystallographic data for [1], and can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Thermogravimetric analysis – mass spectrometry data for [Zn(acetate)2(tris)2] heated in argon, XPS analysis data for ZnO powders, a photo of representative thin films grown on pyrex substrates, tables of polarity and bias conditions for the rectifying three-point probe conductivity type method, a table of contact types, built-in voltages, and built-in voltage differences, as well as figures of energy band diagrams of W/iZnO/ZnO and W/iSiO2/Si from the conductivity simulations are available in the supplemental material.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ennaoui A, Weber M, Scheer R, Lewerenz HJ. Sol Energy Mater. Sol Cells. 1998;54:277–286. [Google Scholar]
- 2.Lee JB, Lee HJ, Seo SH, Park JS. Thin Solid Films. 2001;398-399:641–646. [Google Scholar]
- 3.Paraguay DF, Miki-Yoshida M, Morales J, Solis J, Estrada LW. Thin Solid Films. 2000;373:137–140. [Google Scholar]
- 4.Das R, Ray S. J Phys D Appl Phys. 2003;36:152–155. [Google Scholar]
- 5.Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A. Applied Physics Letters. 2007;90:213902. doi: 10.1063/1.2742324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansari SA, Husain Q, Qayyum S, Azam A. Food Chem Toxicol. 2011;49:2107–2115. doi: 10.1016/j.fct.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Thurber A, Wingett DG, Rasmussen JW, Layne J, Johnson L, Tenne DA, Zhang J, Hanna CB, Punnoose A. Nanotoxicology. 2012;6:440–452. doi: 10.3109/17435390.2011.587031. [DOI] [PubMed] [Google Scholar]
- 8.Velmurugan R, Swaminathan M. Sol Energy Mater Sol Cells. 2011;95:942–950. [Google Scholar]
- 9.Saucedo-Lucero JO, Arriaga S. Chem Eng J. 2013;218:358–367. [Google Scholar]
- 10.Look DC, Reynolds DC, Litton CW, Jones RL, Eason DB, Cantwell G. Applied Physics Letters. 2002;81:1830–1832. [Google Scholar]
- 11.Look DC, Reynolds DC, Hemsky JW, Jones RL, Sizelove JR. Applied Physics Letters. 1999;75:811–813. [Google Scholar]
- 12.Dietl T, Ohno H, Matsukura F, Cibert J, Ferrand D. Science. 2000;287:1019–1022. doi: 10.1126/science.287.5455.1019. [DOI] [PubMed] [Google Scholar]
- 13.Ohno H. Science. 1998;281:951–956. doi: 10.1126/science.281.5379.951. [DOI] [PubMed] [Google Scholar]
- 14.Claflin B, Look DC, Park SJ, Cantwell G. J Cryst Growth. 2006;287:16–22. [Google Scholar]
- 15.Xu HY, Liu YC, Xu CS, Liu YX, Shao CL, Mu R. Applied Physics Letters. 2006;24:242502-242502–242503. [Google Scholar]
- 16.Zhang JP, Zhang LD, Zhu LQ, Zhang Y, Liu M, Wang XJ, He G. J Appl Phys. 2007;102:114903. [Google Scholar]
- 17.Tabet N, Faiza M, Al-Oteibi A, Electron J. Spectrosc Relat Phenom. 2008;163:15–18. [Google Scholar]
- 18.Hirai M, Kumar A. J Vac Sci Technol A. 2007;25:1534–1538. [Google Scholar]
- 19.Iwata K, Tampo H, Yamada A, Fons P, Matsubara K, Sakurai K, Ishizuka S, Niki S. Appl Surf Sci. 2005;244:504–510. [Google Scholar]
- 20.Perkins CL, Lee SH, Li X, Asher SE, Courts TJ. J Appl Phys. 2005;97:034907. [Google Scholar]
- 21.Cao Y, Miao L, Tanemura S, Tanemura M, Kuno Y, Hayashi Y. Applied Physics Letters. 2006;88:251116-251116–251113. [Google Scholar]
- 22.Mahmood K, Park SB. J Cryst Growth. 2012;347:104–112. [Google Scholar]
- 23.Swapna R, Santhosh Kumar MC. Mater Sci Eng, B. 2013;178:1032–1039. [Google Scholar]
- 24.Zheng M, Wu J. Appl Surf Sci. 2009;255:5656–5661. [Google Scholar]
- 25.Shifu C, Wei Z, Sujuan Z, Wei L. Chem Eng J. 2009;148:263–269. [Google Scholar]
- 26.Burda C, Chen X, Narayanan R, El-Sayed MA. Chem Rev. 2005;105:1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Zeng HC. Nano Res. 2009;2:201–209. [Google Scholar]
- 28.Zhou H, Li Z. Mater Chem Phys. 2005:326–331. [Google Scholar]
- 29.Mina SK, Manea RS, Joob OS, Ganesha T, Choc BW, Han SH. Curr Appl Phys. 2009;9:492–495. [Google Scholar]
- 30.Li Y, Liu CS, translators. Nonferrous Met Soc China. 2009;19:399–403. [Google Scholar]
- 31.Xu ZZ, Ben Y, Chen ZL, Qi F. Mater Res Bull. 2013;48:1725–1727. [Google Scholar]
- 32.Sigoli FA, Davolos MR, M J., Jr J Alloys Compd. 1997;262-263:292–295. [Google Scholar]
- 33.Wang L, Muhammed M. J Mater Chem. 1999;9:2871–2878. [Google Scholar]
- 34.Liu Y, Liu Z, Wang G. J Cryst Growth. 2003;252:213–218. [Google Scholar]
- 35.Meagley KL, Garcia SP. Cryst Growth Des. 2012;12:707–713. [Google Scholar]
- 36.Andres-Verges M, Martinez-Gallego M. J Mater Sci. 1992;27:3756–3762. [Google Scholar]
- 37.Noack V, Eychmüller A. Chemistry of Materials. 2002;14:1411–1417. [Google Scholar]
- 38.Petrusenko SR, Kokozei VN. Zh Neorg Khim. 1996;41:1238–1240. [Google Scholar]
- 39.Langford JI, Wilson AJC, Appl J. Crystallogr. 1978;11:102–113. [Google Scholar]
- 40.XRD Single-Crystal Software. Bruker Analytical X-ray Systems; Madison, WI: 1999. [Google Scholar]
- 41.Schroder DK. Semiconductor Material and Device Characterization. Wiley-Interscience. 2006:39–40. [Google Scholar]
- 42.Henaux S, Mondon F, Gusella F, Kling I, Reimbold G. J Electrochem Soc. 1999;146:2737–2743. [Google Scholar]
- 43.Guilera G, Steed JW. Chem Commun. 1999:1563–1564. [Google Scholar]
- 44.Mimoun H, Laumer JYdS, Giannini L, Scopelliti R, Floriani C. J Am Chem Soc. 1999;121:6158–6166. [Google Scholar]
- 45.Zhao QH, Li HF, Ma YP, Fang RB. Polish J Chem. 2002;76:497–502. [Google Scholar]
- 46.Cheng L, Sun YY, Zhang YW, Xu GQ. Acta Cryst, Sect E. 2008;E64:m1246-m1246. doi: 10.1107/S1600536808027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostwald W. Zeitschrift für physikalische Chemie. 1897;22:289–330. [Google Scholar]
- 48.Wang D, Zhang J, Peng Y, Bi Z, Bian X, Zhang X, Hou X. J Alloys Compd. 2009;478:325–329. [Google Scholar]
- 49.Lyons JL, Janotti A, Van de Walle CG. Applied Physics Letters. 2009;95:252105. [Google Scholar]
- 50.Özgür Ü, Alivov YI, Liu C, Teke A, Reshchikov MA, Dog-brevean S, Avrutin V, Cho SJ, Morko H. J Appl Phys. 2005;98:041301. [Google Scholar]
- 51.Southwick RG, III, Knowlton WB. IEEE Transactions on Device and Materials Reliability. 2006;6:136–145. [Google Scholar]
- 52.Southwick RG, III, Sup A, Jain A, Knowlton WB. IEEE Transactions on Device and Materials Reliability. 2011;11:236–243. [Google Scholar]
- 53.Janotti A, Van de Walle CG. Rep Prog Phys. 2009;72:126501, 126529. [Google Scholar]
- 54.Herring NP, Panchakarla LS, El-Shall MS. Langmuir. 2014;30:2230–2240. doi: 10.1021/la404593w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.