Abstract
Background
Cardiovascular disease including left ventricular hypertrophy, diastolic dysfunction and ectopic valvular calcification are common in patients with chronic kidney disease (CKD). Both S100A12 and fibroblast growth factor 23 (FGF23) have been identified as biomarkers of cardiovascular morbidity and mortality in patients with CKD. We tested the hypothesis that human S100/calgranulin would accelerate cardiovascular disease in mice subjected to CKD.
Methods
This review paper focuses on S100 proteins and their receptor for advanced glycation end products (RAGE) and summarizes recent findings obtained in novel developed transgenic hBAC-S100 mice that express S100A12 and S100A8/9 proteins. A bacterial artificial chromosome of the human S100/calgranulin gene cluster containing the genes and regulatory elements for S100A8, S100A9 and S100A12 was expressed in C57BL/6J mice (hBAC-S100). CKD was induced by ureteral ligation, and hBAC-S100 mice and WT mice were studied after 10 weeks of chronic uremia.
Results
hBAC-S100 mice with CKD showed increased FGF23 in the heart, left ventricular hypertrophy (LVH), diastolic dysfunction, focal cartilaginous metaplasia and calcification of the mitral and aortic valve annulus together with aortic valve sclerosis. This phenotype was not observed in WT mice with CKD or in hBAC-S100 mice lacking RAGE with CKD, suggesting that the inflammatory milieu mediated by S100/RAGE promotes pathological cardiac hypertrophy in CKD. In vitro, inflammatory stimuli including IL-6, TNFα, LPS, or serum from hBAC-S100 mice up regulated FGF23 mRNA and protein in primary murine neonatal and adult cardiac fibroblasts.
Conclusions
Taken together, our study shows that myeloid-derived human S100/calgranulin is associated with the development of cardiac hypertrophy and ectopic cardiac calcification in a RAGE dependent manner in a mouse model of CKD. We speculate that FGF23 produced by cardiac fibroblasts in response to cytokines may act in a paracrine manner to accelerate LVH and diastolic dysfunction in hBAC-S100 mice with CKD. We suggest that S100/RAGE-mediated chronic sustained systemic inflammation is linked to pathological cardiac remodeling via direct up regulation of FGF23 in cardiac fibroblasts, thereby providing a new mechanistic understanding for the common association between CKD, diabetes, metabolic syndrome, or hypertension with left ventricular hypertrophy with diastolic dysfunction.
Keywords: S100 proteins, Receptor for advanced glycation end products (RAGE), Fibroblast growth factor 23 (FGF23), Chronic kidney disease, Ectopic cardiac calcification, Left ventricular hypertrophy
1. S100/calgranulins are ligands to RAGE
There has been recent interest in S100/calgranulins since several clinical studies established their value as biomarker of acute and chronic sustained inflammation. S100A8 (Calgranulin A, or myeloid related proteins-8), S100A9 (Calgranulin B, or myeloid related protein-14), and S100A12 (Calgranulin C, or extracellular newly identified RAGE-binding protein, EN-RAGE) have structural and functional homologies and are known as“S100/calgranulins”. S100/calgranulins are members of the EF-hand calcium binding proteins family. They are abundantly expressed in myeloid cells including neutrophils, monocytes, and dendritic cells. For example, S100A8/9 and S100A12 comprise 40% and 5% of cytosolic protein in neutrophils, respectively [1–2]. In addition, under pathological conditions including responses to injury such as cytokines or mechanical stretch, S100/calgranulins are induced in various cell types such as vascular smooth muscle cells, endothelial cells, epithelial cells, and fibroblasts [3–6]. S100/calgranulins are implicated in regulating a variety of cellular functions including calcium homeostasis, cell maturation, inflammation and immune responses [7].
Besides their intracellular activities, S100/calgranulins exert extracellular cytokine-like effects through the activation of cell surface receptors, including RAGE (receptor for advanced glycation end products) [3, 8] and toll-like receptor 4 [9, 10]. S100/calgranulins are therefore belong to the class of Damage-Associated Molecular Pattern (DAMP) proteins, a heterogenous group of endogenous pro-inflammatory molecules activating innate immunity pathways. A remarkable characteristic of RAGE is that it binds multiple ligands: AGEs, S100 proteins (S100A8/9, S100A12, S100A4, S100B, and S100P), amyloid beta peptide, beta sheet fibrils, and high mobility group box 1 protein. There is growing evidence that sustained activation of RAGE is an important mechanism leading to endothelial dysfunction, neointima formation upon local injury, and vascular inflammation associated with atherosclerosis [11–13]. While these discoveries were primarily derived from animal models of human disease, they are supported by many clinical translational studies that establish a strong positive association between serum concentrations of the RAGE-ligand S100/calgranulins, and a negative association of esRAGE with cardiovascular disease activity as further outlined below.
Although S100A12 and S100A8/9 are often regulated in a similar manner, differences exist between S100A12 and S100A8/9 in their ability to bind to RAGE. Using surface plasmon resonance, our laboratory demonstrated that increasing concentrations of heparin sulfate only minimally impaired binding of S100A12 to human (or murine) RAGE while heparin sulfate competed with binding of S100A9 or S100A8/9 to RAGE [14]. Moreover, Srikrishna et al showed that glycan-enrichment of the receptor RAGE led to a 30-fold higher binding capacity of S100A12/mole RAGE (Bmax 44.4) compared to non-glycated RAGE (Bmax 1.2). Increased affinity of RAGE for S100A12 due to glycan-dependent enrichment was specific to S100A12 and attenuated for S100A8/9 or S100A11 [15], suggesting that S100A12 may have the strongest potential to activate RAGE in conditions of posttranslational modification of RAGE associated with diabetes or uremia. Collectively, these data may suggest that S100A12 is a major ligand for RAGE.
Another important dissimilarity between S100A12 and S100A8/9 relates to their post-translational modifications. Both of S100A8 and S100A9 have a single highly conserved cysteine (Cys) residue. The Cys residue can be readily oxidized by peroxide, hypochlorite and nitric oxide (NO) [16, 17]. Notably, the oxidative modifications of S100A8/9 are irreversible, which may profoundly affect their function. In contrast, S100A12 is resistant to covalent modification by oxidants because it lacks Cys and methionine (Met) residues. This indicates that the pro-inflammatory properties of S100A12 are likely stable in oxidative environments typical of inflammatory sites. S100A8/9 can also be readily S-nitrosylated (the covalent coupling of NO to Cys residues) by NO donors [18] while S-nitrosylation is not observed for S100A12.S100A8/9 oxidation and S100A8/9S-nitrosylation may exert important protective roles in inflammation with pleiotropic effects.
2. Pleiotropic effects of S100/calgranulins
As discussed above, DAMP molecules S100/calgranulins can result in the activation of Pattern Recognition Receptors such as RAGEandTLR4, indicating a pro-inflammatory role for extracellular S100/calgranulins. Elevated serum concentrations of S100/calgranulins are present in many chronic or acute inflammatory diseases, including coronary artery disease, Kawasaki arteritis, asthma, diabetes, obesity, rheumatoid arthritis, chronic inflammatory bowel disease, and others. While the extracellular pro-inflammatory functions of S100/calgranulins have been extensively studied, there is also increasing evidence of anti-inflammatory/protective properties of S100A8/9. Oxidant scavenging functions of S100A8/9 have been described, which might be mediated by their irreversible oxidative modifications [19]. The high sensitivity of S100A8/9 to oxidation may protect the host against severe tissue damage from reactive oxygen species (ROS), particularly because S100A8/9 are so abundantly expressed in activated neutrophils and macrophages, both of which produce high levels of myeloperoxidase and peroxide that generate ROS. In addition, S-nitrosylation ofS100A8 stabilizes nitric oxide and transports it to hemoglobin, suggesting a function in NO transport and vascular homoeostasis. Additionally, S-nitrosylated S100A8 reduces leukocyte transmigration in the vasculature and inhibits mast cell activation [20]. S-glutathionylation of S100A9 reduces neutrophil adhesion to the extracellular matrix as well as their binding affinity to endothelial cells, which may regulate the magnitude of neutrophil migration in the extra vasculature [21]. Interestingly, a recent study showed that S100A9-deficient mice are extremely sensitive to infection with Streptococcus pneumoniae and proposed that key chemokines, granulocyte colony-stimulating factor and interleukin (IL)-6, which contribute to neutrophil recruitment in this infection, are regulated by S100A8/9 [22]. Moreover, S100A8/9 expression is enhanced by glucocorticoids and IL-10, and despite their activation of the TLR/IL-1 pathway, are considered as pleiotropic molecules in regulating acute and chronic inflammation [23].
In contrast, S100A12 tends to exert predominantly pro-inflammatory role due to activating mast cells and resistance to covalent modification by oxidants or NO. That it does not requireIL-10 is also consistent with a pro-inflammatory effect. However, low S100A12 concentrations (50nmol/L) suppressed pro-inflammatory cytokines induced by Serum Amyloid A (SAA) in activated monocytes and macrophages, whereas S100A8 or S100A9 were ineffective [24]. This study suggests that localized S100A12 is likely to modulate sterile inflammation via attenuating pro-inflammatory properties of SAA. Additionally, in mice engineered to express human S100A12 driven by the smooth muscle 22-α promoter, our laboratory demonstrated attenuated lung inflammation in a mouse model of allergic inflammation [25]. These findings were unexpected, and it was our hypothesis that S100A12 would exacerbate the allergic inflammation since S100A12 activates RAGE and both proteins are abundantly expressed in the airways of human asthma. However, our unexpected results of lunted allergic lung inflammation in S100A12 transgenic mice underscore pleiotropic effects of S100A12 in modulating inflammation.
3. Association of S100/calgranulins with cardiovascular diseases
Recent reviews of S100A8/9 [7] and of S100A12 [26] summarize the pathological role of S100/calgranulins for cardiovascular diseases. Many epidemiological studies have demonstrated an association of S100A12 with atherosclerotic disease. For example, serum S100A12 serves as an independent predictor for cardiovascular mortality in patients with CKD, even after adjustment for other confounding pro-inflammatory markers including white blood cell count, IL-6, and C-reactive protein [27, 28]. Recent studies by Hara et al. established a strong positive correlation between serum S100A12 and S100A12 mRNA in leukocytes, implicating that peripheral blood leukocytes are the major source of serum S100A12 [29]. Several other studies showed an association of S100A12 with atherosclerotic disease, including carotid intimal–media thickness, peripheral artery disease, coronary artery disease and myocardial infarction [30–38]. To further study whether S100/calgranulins directly mediates cardiovascular disease, we generated two different models of transgenic mice, i.e S100A12 expression in vascular smooth muscle, or S100/calgranulin expression targeted to myeloid cells.
We exploited the fact that S100A12 is absent in mice and generated transgenic mice expressing human S100A12 in vascular smooth muscle driven by the smooth muscle (SM) 22α promoter. Although S100A12 is not expressed in human arteries under physiological conditions, it is induced in smooth muscle cells under pathological conditions such as acute myocardial infarction with coronary artery plaque rupture [39] or in thoracic aortic aneurysms and dissections [40]. In this SM22α-S100A12 mouse model, we found a phenotype switch of the smooth muscle cells to a more synthetic state with increased production of IL-6, transforming growth factor-β signaling, matrix metalloproteases-2, and increased reactive oxidative species. This was accompanied by a loss of contractile fibers in the smooth muscle and the development of thoracic aortic aneurysms in this mouse model [4]. Importantly, while aged SM22α-S100A12 mice maintained on normal rodent chow showed only scant spontaneous vascular medial calcification, young SM22α-S100A12 mice challenged with hyperlipidemia (SM22α-S100A12/ApoE null mice on chow diet [41]) or surgical induced chronic renal insufficiency [42] developed a significant amount of vascular calcification. In contrast, hyperlipidemia or chronic kidney disease alone did not result in vascular calcification in wild type littermate mice, but the presence of SM22α targeted human S100A12 greatly induced intimal or medical calcification in those two models. The enhanced intimal and medical calcification induced by SM22α targeted expression of S100A12 was initiated, at least in part, by induction of an osteogenic gene regulatory program in vascular smooth muscle, and was partially reversed in cultured murine aortic smooth muscle cells by treatment with inhibitors to NADPH-oxidase diphenylene iodonium (DPI) and apocynin, and by treatment with soluble RAGE, indicating a role for oxidative stress and RAGE in S100A12- mediated smooth muscle calcification. In addition, SM22α-S100A12 mice with an atherosclerosis-prone ApoE null background exhibited accelerated atherosclerosis [41], and we recently reported reduced atherosclerosis and a stabilization of atherosclerotic plaques in SM22α-S100A12/ApoE null mice by treatment with quinoline-3-carboxamide ABR-215757, a small molecule that has immune-modulatory effects by selectively binding S100A12 and S100A8/9 and thereby preventing access to RAGE and TLR4[14, 43]. ABR-215757 treated SM22α-S100A12/ApoE null mice showed normalization of a heighten T-cell infiltration in atherosclerotic lesions. These findings suggest a causative involvement of S100A12 in aortic aneurysm, atherosclerosis and vascular calcification.
To overcome the limitations of forced transgenic expression of S100A12 in smooth muscle cells, we have now generated a novel “humanized” mouse model with transgenic expression of the human S100/calgranulins in cells and tissues similar to the expression pattern in humans by utilizing a bacterial artificial chromosome containing 60 kb of human DNA of the S100/calgranulins gene cluster containing the genes and endogenous regulatory elements for S100A8, S100A12, and S100A9 (hBAC-S100 mice). hS100/calgranulins are expressed in this model in myeloid blood cells, bone marrow, and splenic cells. Serum S100A12 is elevated in hBAC-S100 mice with levels comparable to S100A12 concentrations measured in human serum samples, indicating that this novel transgenic hBAC-S100 mouse model is compatible with human physiology in regard to S100A12 expression [44]. Importantly, many genes associated with inflammation or neutrophil activation are up regulated in peripheral blood mononuclear cells in hBAC-S100 mice, and this is associated with increased serum levels of IL-6 and IL-22. This enhanced systemic inflammation present in hBAC-S100 mice was abolished in hBAC-S100 mice lacking RAGE, the receptor for S100/calgranulins. Together, these data demonstrate that myeloid-derived S100/calgranulins are sufficient to induce a sustained systemic inflammatory milieu in a RAGE-dependent manner, and therefore should be suitable to study the effects of elevated serum S100A12 on the development of cardiovascular diseases.
4. S100/RAGE-mediated sustained inflammation promotes pathological cardiac remodeling and is associated with FGF23 expression in cardiac fibroblasts
In our most recent study entitled“S100/Calgranulin- Mediated Inflammation Accelerates Left Ventricular Hypertrophy and Aortic Valve Sclerosis in Chronic Kidney Disease in a Receptor for Advanced Glycation End Products-Dependent Manner” [44], we used the novel hBAC-S100 mouse model together with a model of surgically induced CKD to test the hypothesis that elevated serum levels of S100A12 in mice with CKD would accelerate pathological cardiac remodeling. We found left ventricular hypertrophy (LVH) and diastolic dysfunction together with focal cartilaginous metaplasia and calcification in the fibroblast-rich annulus of the mitral and aortic valves, and thickening of the aortic valve leaflets in hBAC-S100 mice with CKD, but not in WT mice with CKD. We identified S100/calgranulins-mediated inflammation, at least in part, as an underlying mechanism of this pathological cardiac remodeling, because hBAC-S100 mice lacking RAGE had attenuated systemic inflammation and did not develop pathological cardiac remodeling when exposed to the same degree of CKD. Our data implicate S100/calgranulins/RAGE for the first time as an accelerating factor for the development of cardiac hypertrophy and diastolic dysfunction in CKD.
Similar to S100A12, fibroblast growth factor 23 (FGF23) has recently emerged in several epidemiological studies as another powerful biomarker that predicts progression of CKD as well as morbidity and mortality of cardiovascular disease in patients with CKD [45–47]. FGF23 is a hormone produced by osteoblasts/osteocytes in bone that regulates vitamin D metabolism and phosphate homeostasis. Principally acting in the kidney, FGF23 inhibits 1, 25-dihydroxyvitamin D synthesis and promotes urinary phosphate excretion. In addition, FGF23 acts in the parathyroid glands to suppress parathyroid hormone (PTH) synthesis and secretion. Meanwhile, FGF23 is regulated by 1, 25-dihydroxyvitamin D and PTH, which creates a feedback loop between FGF23 and 1, 25-dihydroxyvitamin D or between FGF23 and PTH [47]. Elevated serumFGF23 levels have been independently related to LVH, and the heart has been identified as an “off-target”. Important work by Faul and colleagues demonstrated that recombinant FGF23 can directly cause hypertrophy of cardiac myocytes in vitro and in vivo [48].
Our study reveals a potential link between S100A12 and FGF23 in the development of LVH in CKD. Although serum FGF23 was equally elevated in hBAC-S100 and WT mice with CKD, cardiac expression of FGF23 mRNA and protein was only increased in the hearts of hBAC-S100 mice. Immunohistochemistry of serial cardiac sections demonstrated that FGF23 was expressed in valvular interstitial cells of hBAC-S100/CKD and only minimally in cardiac fibrous tissue from WT/CKD. The mechanism whereby FGF23 gene transcription and protein secretion are augmented in the hearts of hBAC-S100 mice with CKD likely represents a response to inflammation because several cytokines (IL-6, tumor necrosis factor-α, LPS, and serum from hBAC-S100 mice) were capable of inducing FGF23 in cultured cardiac fibroblasts or aortic smooth muscle cells. However, recombinant S100A12 (≤2 μg/mL), alone or together with ossification-inducing high-phosphate medium, failed to induce FGF23 in cardiac fibroblasts, suggesting that inflammation per se, but not an “osteoblastic switch” of cardiac fibroblasts is responsible for FGF23 induction in cardiac fibroblasts. Our hypothesis that FGF23 can be induced in response to inflammatory signals is supported by a recent study by Pöss et al [49] demonstrating a 100-fold increase in serum FGF23 in patients with cardiogenic shock. Although cytokines were not reported in this study, we speculate that the cytokine storm commonly associated with cardiogenic shock may contribute to the severe increase in serum FGF23.
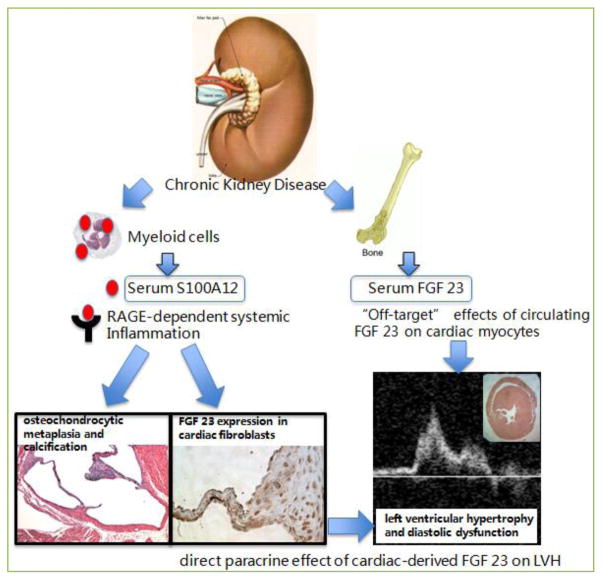
Our study is the first to show expression of FGF23 in cardiac fibroblasts, and we believe that cardiac-derived FGF23 mediates LVH. This view is supported by the findings that hBAC-S100 mice lacking RAGE had no increased expression of FGF23 in the heart (presumably attributable to the lack of systemic inflammation) and were protected from LVH despite equally elevated serum FGF23 and CKD. We speculate that FGF23 produced by cardiac fibroblasts in response to cytokines might be a potential link between S100/calgranulins/RAGE- mediated systemic inflammation and development of cardiac hypertrophy and diastolic dysfunction in vivo (Figure 1).
Figure 1. Proposed model of LVH in CKD.
Increased serum concentration of S100/calgranulins in mice with CKD promotes systemic inflammation in a RAGE-dependent manner, and this is associated with cartilagenous metaplasia and calcification of the valve annulus. Importantly, systemic inflammation in vivo (and in cultured cardiac fibroblasts upon treatment with cytokines), up regulates endogenous FGF23 in cardiac fibroblasts, which may act in a paracrine manner to promote LVH and diastolic dysfunction. Elevated serum FGF23 has been previously associated with LVH and recombinant FGF23 was shown to directly cause hypertrophy of cardiac myocytes in vitro and in vivo [48]. Reprinted with permission [44].
Another critical finding of our study utilizing hBAC-S100 mice exposed to CKD was the development of sclerotic aortic valve disease with leaflet thickening and chondrocytic hypertrophy of the mitral and aortic valve annulus and spotty cardiac calcification. Calcific aortic valve disease (CAVD) is commonly associated in clinical epidemiological studies with aging, chronic kidney disease, bicuspid aortic valves, diabetes, hypertension, and metabolic syndrome. Although inflammatory cells are commonly seen within the calcified aortic valves, the role of inflammation for the development of CAVD is not clear. Our animal model shows that enhanced systemic inflammation in hBAC-S100 mice is sufficient to induce pathological remodeling of the aortic valve in a manner similar to human CAVD. However, it remains unknown whether S100/RAGE-mediated systemic inflammation with increased levels of cytokines directly affect valvular cells, or whether altered hemodynamic force due to left ventricular hypertrophy with increased biomechanical stress on valvular endothelial and interstitial cells initiates cell activation leading to aortic sclerosis in hBAC-S100 mice.
5. Future directions
Our study provides mechanistic insights into how S100A12 and FGF23, both biomarkers of increased cardiovascular mortality in CKD, could synergize to produce an environment where S100/calgranulins/ RAGE-mediated inflammation promotes up regulation of cardiac-derived FGF23, LVH, and diastolic dysfunction. However, several important questions remain.
We first implicate systemic inflammation initiated by S100/calgranulins and RAGE as mechanism to upregulate FGF23 in cardiac fibroblasts. Future experiments should examine if this is specific to S100/RAGE or if other models of inflammation also increase FGF23 expression in the hearts. Moreover, do other models of cardiac hypertrophy show increased expression of FGF23 in cardiac fibroblasts? Although FGF23 is commonly measured in serum and correlates positively with LVH in patients with CKD, future studies are needed to explore cardiac expression of FGF23 and whether this mediates LVH in a paracrine manner. It would be interesting to test whether the blockade of cardiac FGF23 in hBAC-S100 RAGE+/+mice with CKD is sufficient to prevent LVH, as selective blockade of cardiac FGF23 in vivo has not been previously reported. Genetic ablation of FGF23 in mice led to a markedly short lifespan and a number of abnormal morphological and biochemical features [50]. Pharmacological blockade by treatment with the pan-FGF-Receptor blocker PD 173074 reduces LVH as previously demonstrated by Faul et al[48], but this is not a cardiac selective approach. Future experiments using cardiac-specific FGF23 knockout mice are needed to better understand the relationship ofFGF23 and cardiac hypertrophy. Moreover, future studies are needed to probe whether cardiacmyocytes lacking RAGE have a similar hypertrophic response to FGF23 as was recently shown for WT cardiac myocytes [48].
It would be also interesting to test whether binding of S100/calgranulins to small molecule ABR-215757 attenuates LVH in hBAC-S100 mice with CKD. It is our hypothesis that ABR-215757 attenuates activation of RAGE by binding S100/calgranulins and thereby reduces inflammation and LVH, similar to our previous studies demonstrating attenuated atherosclerosis in ABR-215757 treated SM22α-S100A12 ApoE null mice [14].
Taken together, S100/RAGE is an accelerating factor not only for atherosclerosis, but also for the development of cardiac hypertrophy and diastolic dysfunction, possibly via enhanced FGF23 secretion in cardiac fibroblasts. We speculate that the enhanced systemic inflammation present in hBAC-S100 mice leads to the observed increased expression of FGF23 in the cardiac tissue, since cytokines (TNFα, IL-6, LPS) directly induce FGF23 in cultured primary cardiac fibroblasts. We hypothesize that the inflammation-mediated expression of FGF23 in cardiac fibroblasts may act in a paracrine manner to induce LVH, thereby providing a possible link between inflammation and LVH with diastolic dysfunction. Reducing systemic inflammation by, for instance, the promotion of healthy life style changes, weight loss and increased cardiovascular fitness, or possibly pharmacological interventions aimed at reducing cardiovascular inflammation, should have beneficial effects on LVH and diastolic dysfunction.
Acknowledgments
This work was supported by the National Health, Lung, and Blood Institute (1R01HL4821 to M.A. Hofmann Bowman)
Footnotes
Conflict of interests
The authors declare that there is no conflict of interests.
References
- 1.Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8, 14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266:7706–7713. [PubMed] [Google Scholar]
- 2.Vogl T, Pröpper C, Hartmann M, Strey A, Strupat K, van den Bos C, et al. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem. 1999;274:25291–25296. doi: 10.1074/jbc.274.36.25291. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann Bowman M, Wilk J, Heydemann A, Kim G, Rehman J, Lodato JA, et al. S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ Res. 2010;106:145–154. doi: 10.1161/CIRCRESAHA.109.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbas A, Aukrust P, Dahl TB, Bjerkeli V, Sagen EB, Michelsen A, et al. High Levels of S100A12 Are Associated With Recent Plaque Symptomatology in Patients With Carotid Atherosclerosis. Stroke. 2012;43:1347–1353. doi: 10.1161/STROKEAHA.111.642256. [DOI] [PubMed] [Google Scholar]
- 6.Christaki E, Lazaridis N, Opal SM. Receptor for advanced glycation end products in bacterial infection: is there a role for immune modulation of receptor for advanced glycation end products in the treatment of sepsis? Curr Opin Infect Dis. 2012;25:304–311. doi: 10.1097/QCO.0b013e3283519b82. [DOI] [PubMed] [Google Scholar]
- 7.Averill MM, Kerkhoff C, Bornfeldt KE. S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol. 2012;32:223–229. doi: 10.1161/ATVBAHA.111.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- 9.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, et al. Mrp8 and Mrp14 are endogenous activators of toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 10.Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, et al. The Toll-like receptor 4 ligands Mrp8 andMrp14 are crucial in the development of auto reactive CD8+ T cells. Nat Med. 2010;16:713–717. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 11.Ramasamy R, Yan SF, Herold K, Clynes R, Schmidt AM. Receptor for advanced glycation end products: fundamental roles in the inflammatory response: winding the way to the pathogenesis of endothelial dysfunction and atherosclerosis. Ann N Y Acad Sci. 2008;1126:7–13. doi: 10.1196/annals.1433.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bu DX, Rai V, Shen X, Rosario R, Lu Y, D’Agati V, et al. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ Res. 2010;106:1040–1051. doi: 10.1161/CIRCRESAHA.109.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan L, Bjork P, Butuc R, Gawdzik J, Earley J, Kim G, et al. Beneficial effects of quinoline-3-carboxamide (ABR-215757) on atherosclerotic plaque morphology in S100A12 transgenic ApoE null mice. Atherosclerosis. 2013;228:69–79. doi: 10.1016/j.atherosclerosis.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srikrishna G, Nayak J, Weigle B, Temme A, Foell D, Hazelwood L, et al. Carboxylated N-glycans on RAGE promote S100A12 binding and signaling. J Cell Biochem. 2010;110:645–659. doi: 10.1002/jcb.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison CA, Raftery MJ, Walsh J, Alewood P, Iismaa SE, Thliveris S, et al. Oxidation regulates the inflammatory properties of the murine S100 protein S100A8. J Biol Chem. 1999;274:8561–8569. doi: 10.1074/jbc.274.13.8561. [DOI] [PubMed] [Google Scholar]
- 17.Lim SY, Raftery MJ, Goyette J, Hsu K, Geczy CL. Oxidative modifications of S100 proteins: functional regulation by redox. J Leukoc Biol. 2009;86:577–587. doi: 10.1189/jlb.1008608. [DOI] [PubMed] [Google Scholar]
- 18.Lim SY, Raftery M, Cai H, Hsu K, Yan WX, Hseih HL, et al. S-nitrosylated S100A8: novel anti-inflammatory properties. J Immunol. 2008;181:5627–5636. doi: 10.4049/jimmunol.181.8.5627. [DOI] [PubMed] [Google Scholar]
- 19.Goyette J, Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids. 2011;41:821–842. doi: 10.1007/s00726-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 20.Geczy CL, Chung YM, Hiroshima Y. Calgranulins may contribute vascular protection in atherogenesis. Circ J. 2014;78:271–280. doi: 10.1253/circj.cj-13-1505. [DOI] [PubMed] [Google Scholar]
- 21.Lim SY, Raftery MJ, Goyette J, Geczy CL. S-glutathionylation regulates inflammatory activities of S100A9. J Biol Chem. 2010;285:14377–14388. doi: 10.1074/jbc.M109.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Filippo K, Neill DR, Mathies M, Bangert M, McNeill E, Kadioglu A, et al. A new protective role for S100A9 in regulation of neutrophil recruitment during invasive pneumococcal pneumonia. FASEB J. 2014;28:3600–3608. doi: 10.1096/fj.13-247460. [DOI] [PubMed] [Google Scholar]
- 23.Endoh Y, Chung YM, Clark IA, Geczy CL, Hsu K. IL-10-dependent S100A8 gene induction in monocytes/macrophages by double-stranded RNA. J Immunol. 2009;182:2258–2268. doi: 10.4049/jimmunol.0802683. [DOI] [PubMed] [Google Scholar]
- 24.Chung YM, Goyette J, Tedla N, Hsu K, Geczy CL. S100A12 suppresses pro-inflammatory, but not pro-thrombotic functions of serum amyloid A. PLoS One. 2013;8:e62372. doi: 10.1371/journal.pone.0062372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofmann Bowman MA, Heydemann A, Gawdzik J, Shilling RA, Camoretti-Mercado B. Transgenic expression of human S100A12 induces structural airway abnormalities and limited lung inflammation in a mouse model of allergic inflammation. Clin Exp Allergy. 2011;41:878–889. doi: 10.1111/j.1365-2222.2011.03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofmann Bowman MA, Schmidt AM. S100/calgranulins EN-RAGE ing the blood vessels: implications for inflammatory responses and atherosclerosis. Am J Cardiovasc Disease. 2011;1:92–100. [PMC free article] [PubMed] [Google Scholar]
- 27.Nakashima A, Carrero JJ, Qureshi AR, Miyamoto T, Anderstam B, Bárány P, et al. Effect of circulating soluble receptor for advanced glycation end products (sRAGE) and the proinflammatory RAGE ligand (EN-RAGE, S100A12) on mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:2213–2219. doi: 10.2215/CJN.03360410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiotsu Y, Mori Y, Nishimura M, Sakoda C, Tokoro T, Hatta T, et al. Plasma S100A12 level is associated with cardiovascular disease in hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:718–723. doi: 10.2215/CJN.08310910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara M, Ando M, Morito T, Nokiba H, Iwasa Y, Tsuchiya K, et al. S100A12 gene expression is increased in peripheral leukocytes in chronic kidney disease stage 4–5 patients with cardiovascular disease. Nephron Clin Pract. 2013;123:202–208. doi: 10.1159/000353808. [DOI] [PubMed] [Google Scholar]
- 30.Mori Y, Kosaki A, Kishimoto N, Kimura T, Iida K, Fukui M, et al. Increased plasma S100A12 (EN-RAGE) levels in hemodialysis patients with atherosclerosis. Am J Nephrol. 2009;29:18–24. doi: 10.1159/000148646. [DOI] [PubMed] [Google Scholar]
- 31.Kim JK, Park S, Lee MJ, Song YR, Han SH, Kim SG, et al. Plasma levels of soluble receptor for advanced glycation end products (sRAGE) and proinflammatory ligand for RAGE (EN-RAGE) are associated with carotid atherosclerosis in patients with peritoneal dialysis. Atherosclerosis. 2012;220:208–214. doi: 10.1016/j.atherosclerosis.2011.07.115. [DOI] [PubMed] [Google Scholar]
- 32.Shiotsu Y, Mori Y, Hatta T, Maki N, Iida K, Matsuoka E, et al. Plasma S100A12 levels and peripheral arterial disease in end-stage renal disease. Nephron Extra. 2011;1:242–250. doi: 10.1159/000335198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao P, Wu M, Yu H, Huang Y, Wang Y, Wang W, et al. Serum S100A12 levels are correlated with the presence and severity of coronary artery disease in patients with type 2 diabetes mellitus. J Investig Med. 2013;61:861–866. doi: 10.2310/JIM.0b013e318292fb1e. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg S, Elashoff MR, Beineke P, Daniels SE, Wingrove JA, Tingley WG, et al. Multicenter validation of the diagnostic accuracy of a blood-based gene expression test for assessing obstructive coronary artery disease in nondiabetic patients. Ann Intern Med. 2010;153:425–434. doi: 10.7326/0003-4819-153-7-201010050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahajan N, Malik N, Bahl A, Dhawan V. Receptor for advanced Glucation end products (RAGE) and its inflammatory ligand EN-RAGE in non-diabetic subjects with pre-mature coronary artery disease. Atherosclerosis. 2009;207:597–602. doi: 10.1016/j.atherosclerosis.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Tydén H, Lood C, Gullstrand B, Jönsen A, Nived O, Sturfelt G, et al. Increased serum levels of S100A8/A9 and S100A12 are associated with cardiovascular disease in patients with inactive systemic lupus erythematosus. Rheumatology (Oxford) 2013;52:2048–2055. doi: 10.1093/rheumatology/ket263. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Ren YG, Zhang LH, Tong YW, Kang L. Serum S100A12 concentrations are correlated with angiographic coronary lesions complexity in patients with coronary artery disease. Scand J Clin Lab Invest. 2014;74:149–154. doi: 10.3109/00365513.2013.864786. [DOI] [PubMed] [Google Scholar]
- 38.Goyette J, Yan WX, Yamen E, Chung YM, Lim SY, Hsu K, et al. Pleitropic roles of S100A12 in coronary atherosclerotic plaque formation and rupture. J Immunol. 2009;183:593–603. doi: 10.4049/jimmunol.0900373. [DOI] [PubMed] [Google Scholar]
- 39.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: A postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 40.Das D, Gawdzik J, Dellefave-Castillo L, McNally EM, Husain A, Raman J, et al. S100A12 expression in thoracic aortic aneurysm is associated with increased risk of dissection and perioperative complications. J Am Coll Cardiol. 2012;60:775–785. doi: 10.1016/j.jacc.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmann Bowman MA, Gawdzik J, Bukhari U, Husain AN, Toth PT, Kim G, et al. S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein E-null mice by activating an osteogenic gene regulatory program. Arterioscler Thromb Vasc Biol. 2011;31:337–344. doi: 10.1161/ATVBAHA.110.217745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gawdzik J, Mathew L, Kim G, Puri TS, Hofmann Bowman MA. Vascular remodeling and arterial calcification are directly mediated by S100A12 (EN-RAGE) in chronic kidney disease. Am J Nephrol. 2011;33:250–259. doi: 10.1159/000324693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Björk P, Björk A, Vogl T, Stenström M, Liberg D, Olsson A, et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7:e97. doi: 10.1371/journal.pbio.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan L, Mathew L, Chellan B, Gardner B, Earley J, Puri TS, et al. S100/Calgranulin-mediated inflammation accelerates left ventricular hypertrophy and aortic valve sclerosis in chronic kidney disease in a receptor for advanced glycation end products-dependent manner. Arterioscler Thromb Vasc Biol. 2014;34:1399–1411. doi: 10.1161/ATVBAHA.114.303508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80:806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 47.Quarles LD. Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res. 2012;318:1040–1048. doi: 10.1016/j.yexcr.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pöss J, Mahfoud F, Seiler S, Heine GH, Fliser D, Böhm M, et al. FGF-23 is associated with increased disease severity and early mortality in cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2013;2:211–218. doi: 10.1177/2048872613494025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]