Abstract
Objectives
1) To determine the prevalence of fibroids in asymptomatic young black and white women (ages 18–30yo); 2) To determine other differences in uterine and adnexal anatomy; 3) To obtain preliminary data for sample size calculations.
Design
A pilot cross-sectional study.
Setting
An academic medical center
Patients
101 non-parous black and white women, ages 18–30 years old, with no known diagnosis of fibroids or clinically suggestive symptoms.
Interventions
A transvaginal ultrasound was performed in the follicular phase in all subjects.
Main Outcome Measure(s)
1) Presence of fibroids; 2) endometrial thickness; 3) ovarian findings.
Results
Of the 101 participants (mean age 24.5 ± 3.5), 43% self-identified as black and 57% as white. The prevalence of ultrasound-diagnosed fibroids was 15% overall (26% in black women and 7% in white women). The mean fibroid size was 2.3 ± 2.1 cm. There was a significant difference in endometrial thickness between races, even after adjusting for contraception use and fibroid presence.
Conclusions
Racial differences in fibroid prevalence exist even before women become symptomatic. Findings of thicker endometrium in black women could have clinical implications and warrants further investigation.
Keywords: endometrial thickness, fibroids, health disparities, race, ultrasound
Introduction
Uterine leiomyomas, or fibroids as they are commonly called, occur in up to 65% of women by the age of 50 and are the single most common indication for hysterectomies, with race being an important epidemiological risk factor (1–3). While not all fibroids are symptomatic, they can be associated with significant morbidity including abnormal bleeding, and reproductive dysfunction, consuming a significant amount of health care resources and costing the United States an estimated $34 billion annually (4–6).
Rates of uterine fibroids are known to increase with age throughout the reproductive years, and it is well established that there is a higher prevalence among black women, who tend to have larger and more symptomatic fibroids than white women (1, 3, 7–10). A study of women undergoing hysterectomy for noncancerous conditions reported that 59% of the white women and 89% of black women had fibroids, with average age of diagnosis of 37.5 years in black women vs. 41.6 in white women (3). In the Nurses Health Study cohort of over 95,000 premenopausal females, the rate of new diagnoses of fibroids per year was reported as 12.8 per 1000 women-years, with the age-specific rate among black women peaking earlier than rates in other groups (8). However, only 5% of the study population was black (8). The Black Women’s Health Study reported ultrasound and hysterectomy confirmed incidence rates as high as 29.7 per 1000 women-years, which translates to approximately 3% of premenopausal black women being diagnosed with fibroids yearly (10).
Existing estimates of incidence and prevalence of fibroids as described above and in other descriptive studies are largely based on self-reported symptoms or findings at time of hysterectomy, which potentially excludes asymptomatic women and therefore only represents a fraction of the overall prevalence of the disease. The true prevalence of fibroids, independent of clinical symptoms, is best assessed with applied ultrasound imaging to a randomly selected population.
To date, such data is limited and focuses on women in later reproductive years. The prevalence of fibroids in asymptomatic younger women (ages < 30) is not well established. Laughlin et al reported a prevalence of 6% in African American women and 4% in white women under the age of 25 (11), but this study was done only in pregnant women, and since parity may be protective against fibroids it likely underestimated the true incidence. In a Swedish study of randomly selected asymptomatic women aged 25–40 years old, the prevalence of ultrasound diagnosed fibroids was 5.4%, however this study was conducted in a homogenous population of Caucasian women (12). Baird et al.(1) reported ultrasound evidence of fibroids in over 50% of women aged 35–49 who had no previous diagnosis, with a cumulative incidence by age 50 of greater than 80% in black women compared to 70% in white women. While such studies suggest that black women are disproportionately affected by uterine fibroids, it is unknown if this disparity exists prior to the age of thirty. Improved understanding of when fibroids begin to develop may allow for targets for intervention that could reduce fibroid associated morbidity. Given the paucity of information on the prevalence of fibroids and racial differences in young women before they become clinically significant, this pilot study sought to compare the prevalence of ultrasound-diagnosed fibroids in asymptomatic black and white women ages 18–30 years old, as well as differences in uterine and adnexal anatomy among this cohort.
Materials and Methods
Subjects
This study was reviewed and approved by the Northwestern University Institutional Review Board. Written informed consent was obtained from every subject prior to participation. English-speaking, healthy women ages 18–30 years old were recruited from the general Chicago area. A screening questionnaire written by the authors was verbally administered to determine if women met inclusion criteria. Inclusion criteria were as follows: 18–30 years old, black or white race, have had vaginal intercourse, not currently pregnant, no history of delivering a baby, periods last no longer than seven days, do not soak more than one pad per hour during menses, have periods every 25–35 days, no history of cancer, no diagnosis of fibroids. Given the need for a trans-vaginal ultrasound (TVUS) and concern for tolerance, only women who were vaginally sexually active were recruited. Women were excluded if pregnant, parous, or had a known diagnosis of uterine fibroids or had a history of cancer. Subjects who met inclusion criteria as determined during a telephone or in-person interview were scheduled for an ultrasound during the follicular phase (cycle D4–11) of their menstrual cycle. Demographic and relevant medical information was obtained. Participants were compensated for their time with a gift card and parking / transportation costs.
On the study day, weight and height were measured. A trans-vaginal ultrasound was then performed by one of two sonographers using a General Electric Medical (Milwaukee, WI) machines equipped with high frequency, high resolution 5–9 MHz vaginal transducers able to detect myomas as small as 5mm. A standardized imaging protocol was followed, with assessment of the dimensions and contour of the uterus; number, location and size of fibroids and evaluation of the endometrium and bilateral adnexa. The endometrial thickness was measured as the widest distance from the reflective interface between the endometrium and myometrium of one side to the opposing side on a sagittal view of the uterus. Both sonographers were American Registry of Diagnostic Medical Sonographers (ARDMS) certified with over 7 years of obstetric/gynecologic imaging experience individually.
Images were reviewed by a single experienced Obstetrician/Gynecologist with extensive experience in pelvic ultrasounds (LC), who was blinded to the race, medical history, and background information of the subjects. Analyzed images and survey responses were coded and entered into a database.
Statistical Analysis
Chi-square test, Student t-test, and ANOVA were used to analyze the data. All comparisons were two-tailed, and a p-value of less than .05 was considered to be statistically significant. All statistical analyses were performed in SPSS version 18 software.
Results
Demographics
In total, 202 subjects were screened with 134 meeting inclusion criteria. Of these, 101 women participated and had a TVUS performed. The remaining 33 women did not present on the scheduled day or could not be scheduled due to conflicts. Demographic data are summarized in Table 1. Of the 101 participants (mean age 24.5 ± 3.5), 43% self-identified as black and 57% as white. 62.4% reported active contraception use, and of those using contraception, 65.1% used oral contraceptive pills, 12.7% used Nuvaring, 12.7% used an IUD, 3.2% used Depo-Provera, and 6.3% reported “other.” Maternal history of fibroids was more likely to be reported by black women (32.6% vs. 10.3%, P= .02), who were also less likely to report active contraception use (41.9% vs. 77.6%, P <.001). The mean cycle day at time of imaging did not differ between groups (8.3±2.1 vs. 7.8±2.2; P=.327).
Table 1.
Subject Demographics
| Total (N=101) n (%) |
Black (N=43) n (%) |
White (N=58) n (%) |
P value | |
|---|---|---|---|---|
| Age, years* | 24.5±3.5 | 23.5±3.8 | 25.2±3.1 | .019 |
| 18–20 | 18(17.8) | 13(30.2) | 5(8.6) | |
| 21–24 | 30 (29.7) | 11(25.6) | 19(32.8) | |
| 25–27 | 27 (26.7) | 9(20.9) | 18(31.0) | |
| 28–30 | 26(25.7) | 10(23.3) | 16(27.6) | |
| All | 101(100) | 43 (100) | 58 (100) | |
| Education | ||||
| <College | 24(23.8) | 17 (39.5) | 7 (12.1) | .001 |
| College or greater | 77(76.2) | 26 (60.5) | 51 (87.9) | |
| Employed | ||||
| Yes | 61(60.4) | 26 (60.5) | 35 (60.3) | .990 |
| No | 40 (39.6) | 17 (39.5) | 23 (39.7) | |
| Household Income | ||||
| ≤$40,000 | 58(57.4) | 24 (55.8) | 34 (58.6) | .778 |
| >$40,000 | 43(42.6) | 19 (44.2) | 24 (41.4) | |
| Smoking | ||||
| Never/Past | 99 (98.0) | 42 (97.7) | 57(98.3) | .830 |
| Current | 2 (2.0) | 1 (2.3) | 1 (1.7) | |
| Married/Living as married | ||||
| Yes | 28 (27.7) | 9 (21.0) | 19 (32.8) | .424 |
| No | 73 (72.3) | 34 (79.1) | 39 (67.2) | |
| Body Mass Index (Kg/m2)* | 24.6±4.6 | 26.4±5.1 | 23.3±3.8 | .001 |
| < 25 | 63(62.4) | 21 (48.8) | 42(72.4) | |
| ≥ 25 | 38(37.6) | 22 (51.2) | 16(27.6) | |
| Contraception use | ||||
| Yes | 63(62.4) | 18(41.9) | 45 (77.6) | <.001 |
| No | 38(37.6) | 25 (58.1) | 13 (22.4) | |
| Age at Menarche, years | ||||
| <10 | 1 (1.0) | 1 (2.3) | 0 (0) | .031 |
| 10–12 | 48 (47.5) | 26 (60.5) | 22 (37.9) | |
| >12 | 52 (51.5) | 16(37.2) | 36 (62.1) | |
| Maternal history of fibroids | ||||
| Yes | 20 (19.8) | 14 (32.6) | 6 (10.3) | .006 |
| No | 81 (80.2) | 29 (67.4) | 52 (89.7) |
N= number of subject
mean±standard deviation
Ultrasound Findings
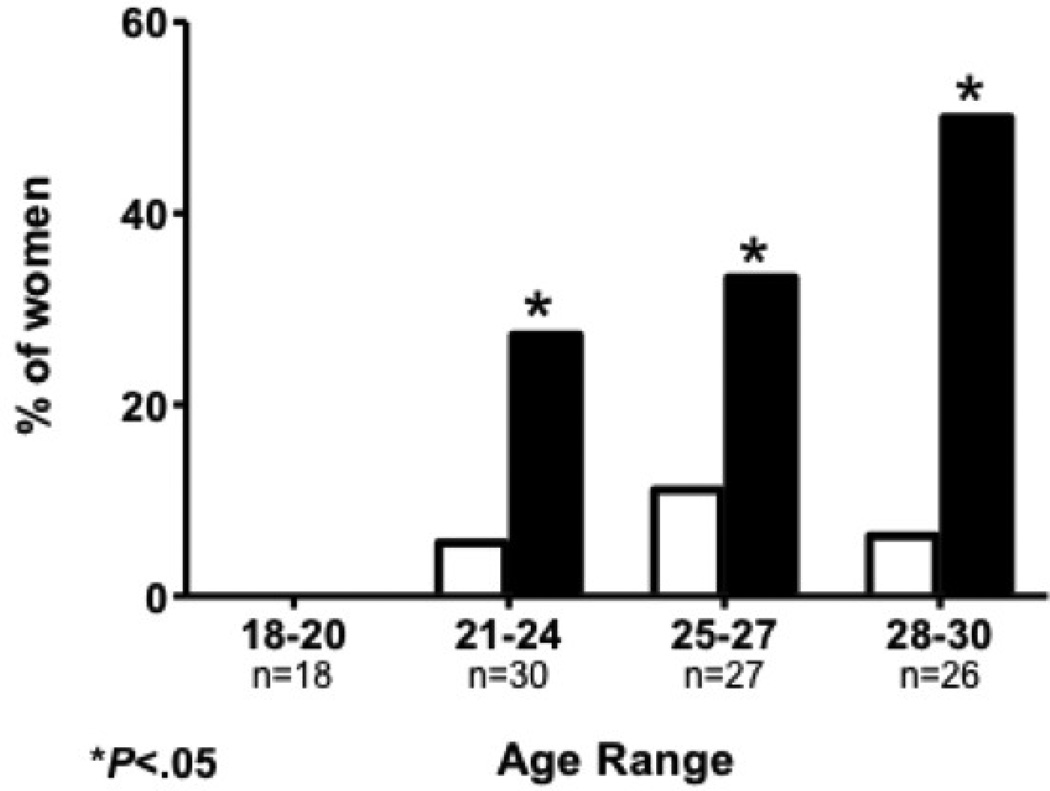
In 15 women, at least one uterine fibroid was found on ultrasound giving an overall prevalence of 14.9%. Fibroids were more common in black women compared to white women (25.6% vs. 6.9%; P= .009). This finding was consistent across age groups (Figure 1). However there was no significant difference in the mean age of black versus white women diagnosed with fibroids (26.4±2.6 vs. 26.0±2.9 years old, P=.821). Women with fibroids were older on average (26.3±2.6 vs. 24.2±3.5years old; P= .030). The presence of fibroids did not correlate with contraception, age at menarche or Body Mass Index (BMI). 67% of identified fibroids were subserosal in location. No submucosal fibroids were identified. The mean fibroid size was 2.6±2.3cm in black women and 1.7±1.4cm in white women but this did not approach statistical significance (P = .474) (Table 2). Reported maternal history of fibroids was associated with the presence of fibroids on TVUS (P = .005).
Figure 1.
Fibroid prevalence by age and race. * indicates a statistically significant difference (P < .01)
 White women
White women  Black women
Black women
Table 2.
Ultrasound findings by race (all subjects)
| Total (N*=101) |
Black (N=43) |
White (N=58) |
P value | |
|---|---|---|---|---|
| Mean cycle day | 8.1±2.2 | 8.3±2.1 | 7.8±2.2 | .327 |
| Presence of fibroid | 15 (14.9) | 11 (25.6) | 4 (6.9) | .009 |
| Submucosal | 0 | 0 | 0 | |
| Intramural | 4 (26.7) | 2 (18.2) | 2 (50.0) | |
| Subserosal | 10 (66.7) | 8 (72.7) | 2 (50.0) | |
| Pedunculated | 1 (6.7) | 1 (9.1) | 0 | |
| Largest diameter of fibroid (cm) | 2.3±2.1 | 2.6±2.3 | 1.7 ±1.4 | .474 |
| Uterine volume (cc) | 50.7±43 | 64.4±62.3 | 41.1±14.6 | .007 |
| Endometrial thickness (mm) | 5.0±2.7 | 6.0±2.8 | 4.3±2.4 | .002 |
| Volume of right ovary (cc) | 7.6±8.7 | 10.4±12.2 | 5.5±3.5 | .005 |
| Volume of left ovary (cc) | 5.2±3.3 | 6.2±3.9 | 4.5±2.6 | .012 |
N = number of subjects;
Data reported as N (%) or mean ± standard deviation
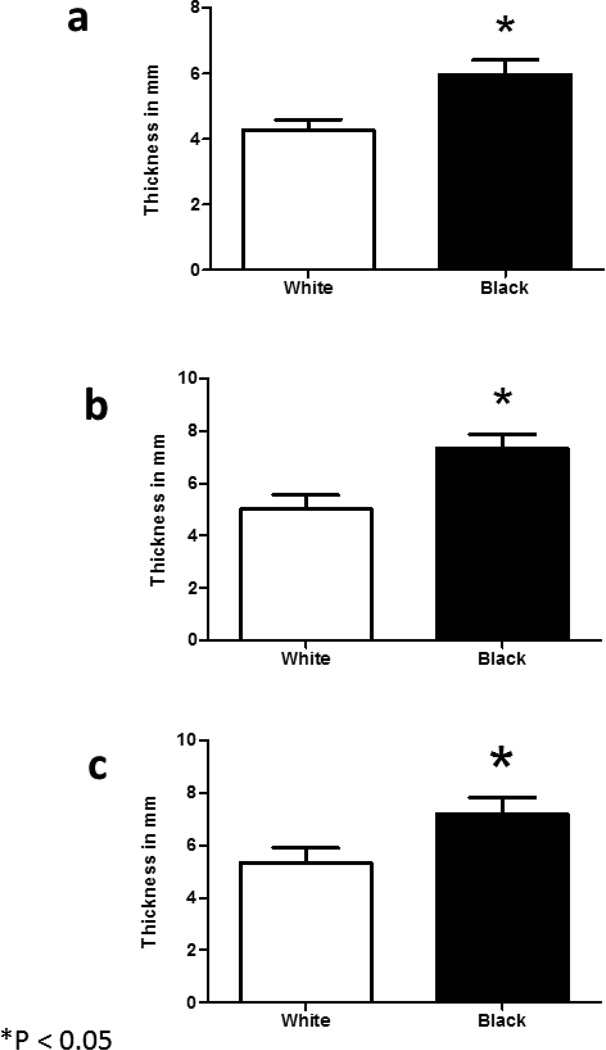
Overall, women using contraception had a significantly thinner endometrium (4.1±2.3mm vs. 6.6±2.6mm; P <0.001) and smaller uterine volume (42.1±18.4 vs. 65.2±64.0cc;P=.009) than women not on contraception. In comparing black and white women, there was a significant difference in mean endometrial thickness (6.0±2.8 vs. 4.3±2.4mm; P =.002), uterine volume (64.4±62.3 vs. 41.1±14.6 cc; P =.007), volume of right ovary (10.4±12.2 vs. 5.5±3.5cc; P=.005) and volume of left ovary (6.2±3.9 vs. 4.5±2.6cc; P=.012) (Table 2). The difference in endometrial thickness between races persisted even when controlling for contraception use and presence of fibroids (Figure 2).
Figure 2.
Difference in Endometrial thickness by race. * indicates a statistically significant difference (P < .01)
 White women
White women  Black women
Black women
2a) Endometrial thickness (all subjects)
2b) Endometrial thickness (no contraception)
2c) Endometrial thickness (no contraception or fibroids)
Discussion
Our pilot study provides data on the prevalence of ultrasound-diagnosed fibroids in a population of nulliparous, asymptomatic women ages 18–30 years old. While fibroids are relatively more common and often become clinically significant in late reproductive years, the onset of fibroid development is unknown. Isolated cases of fibroids in adolescents, although rare, have been reported (13), but limited data exists on the prevalence of fibroids in women under ago 30. The prevalence rate of 15% found in this study is higher than the 3.3% rate reported by Borgfeldt et al. (12) in a Swedish cohort study of asymptomatic women ages 25–32. While fibroids were similarly diagnosed by ultrasound in that study, parous women were included which may explain the reported low prevalence given the suggested protective effect of parity on development of fibroids in certain populations (14). Additionally, the aforementioned study population was a homogenous European population compared to our study population comprised of black and white women. Nevertheless, the fibroid prevalence rate of 6.9% in the cohort of white women in our study is also higher than that reported in the Borgfeldt study. The statistically significant higher prevalence of fibroids in young black women compared to white women as reported in our study, suggests that racial differences exist in the early reproductive years even before women become symptomatic.
The effect of hormonal contraception on the risk of fibroids remains controversial with an inverse association reported in some studies and no protective effect in others (7, 10, 15). While white women in our study were more likely to use contraception than black women (78% vs. 42%), contraception use did not significantly correlate with the absence of fibroids. However, due to small numbers we were unable to differentiate hormonal versus non-hormonal contraception and correlation with the presence of fibroids. In addition, information on duration and age of onset of contraceptive use was not obtained which is a limitation in the interpretation of this finding, as it is plausible that protective effects may be more apparent with prolonged contraception use. Still, one may theorize that any protective effects of contraception on the development of fibroids may be negated by the inherent non-parous state associated with contraception use. On the contrary, the Nurses Health Study (8) reported a significantly increased risk of uterine fibroids (RR=1.9) for women who had first used oral contraceptives between 13 and 16 years of age. Multiple confounding factors may contribute to this finding and Payson et al (16) suggest that perhaps early contraceptive use may be a marker for other risk factors for fibroids rather than a cause itself. No submucosal fibroids were identified in our study population, which is not unexpected as these are more likely to be clinically relevant with patients reporting menorrhagia symptoms, which would have excluded them from participating in the study.
Endometrial thickness is known to increase in the follicular phase in women with normal ovulatory cycle as confirmed in this study (17). The clinical significance of the thicker endometrium seen in black women even after adjusting for contraception use is unclear, as current literature on endometrial thickness has focused on the postmenopausal age group. While studies have shown that a 5mm or less postmenopausal endometrial thickness is associated with benign histology in white women, this was not confirmed in an Asian population (18–21). A subsequent study of Afro-Caribbean Jamaican women with postmenopausal bleeding found that an endometrial thickness of <5mm did not exclude endometrial cancer (22). In a retrospective cohort study evaluating the predictive value of cycle characteristics for clinical pregnancy in Caucasian women after fresh embryo transfer, Traub et al (23) reported that having a thicker endometrial stripe was a positive predictor of clinical pregnancy. Although there was no difference in the cycle day of endometrial stripe measurement between Caucasian and African-American women, there is data by Marsh et al that suggests that the hormonal milieu between the two groups could be different with African-American women having higher estradiol levels than Caucasian women (24). These differences, however, were seen in the mid-cycle and luteal phase estradiol levels and not in the follicular phase when these scans were performed. Further understanding of any physiologic and normative differences in endometrial thickness between races would be helpful in monitoring during assisted reproduction cycles or interpretation of endometrial stripe measurements in women with post-menopausal bleeding. Larger studies with serum collection are needed to assess the potential clinical significance of this difference.
No serum was collected to assess sex steroid levels in this pilot study, so correlation between serum hormone levels and endometrial thickness cannot be determined. We can, however, speculate as to what accounts for the differences in endometrial thickness seen between black and white women. One explanation for the increased endometrial thickness seen in black women is differences in serum hormonal mileau, as mentioned above (24). Another possibility is that racial differences exist in the biology of the endometrium, which could include differences in local aromatase (25). While the study by Ishikawa et al did not explicitly look at endometrium, it is certainly possible that the racial differences seen in myometrium and leiomyoma extend to endometrial tissue. Another difference may be in responsiveness to estrogen at the endometrial level, such as differences in estrogen receptor responsiveness (26). While this has not been specifically shown in endometrium, different estrogen receptor polymorphisms have been shown in leiomyoma in black versus white women (26).
Similar to other fields of medicine, racial and ethnic differences in the prevalence and presentation of benign gynecologic conditions such as fibroids have been attributed to a complex set of genetic, physiologic, cultural and economic factors that are not well understood (27). In the study by Baird et al in which 35–49 year old women were screened for fibroids with a pelvic ultrasound, fibroids were diagnosed at a younger age in black women and they were nearly 3 times as likely to have fibroids compared with white women. Multiple other studies as previously mentioned have reported similar disparities establishing race as a risk factor (3, 8, 28). Within our cohort of young women, while the prevalence differed significantly between races, the mean age of women with fibroids did not differ between groups, contrary to findings in other studies where black women with fibroids were relatively younger. With ongoing research in the medical community to elucidate gene polymorphisms, lifestyle and clinical factors that contribute to the development of fibroids, understanding the age of onset and how the presentation differs among various races could allow for targeted early interventions that may reduce associated morbidity.
As this was a pilot study done in part to determine size needed for a cohort study, a limitation of this study is the small sample size. Despite the size, however, statistically significant differences were noted. We hypothesize that with a larger sample size, the racial differences in fibroid prevalence would be even more apparent. The sample size of this pilot study was not large enough to allow correlative analysis of specific type of contraception used (i.e. hormonal versus non-hormonal) and presence of fibroids or endometrial thickness, but this is the goal of future studies. In addition, this sample size did not allow for multivariate analysis. Due to the anticipated small sample size of this pilot study, participation was limited to nulliparous women. It is known that parity is associated with reduced fibroid risk (10, 15, 29), therefore if parous women are included in a study numbers must be large enough to control for parity. Future larger studies would not need to be limited to nulliparous women.
In our pilot study BMI was higher in black women than in white women, however we did not have a large enough sample size to do regression analysis on BMI. BMI is important to consider, as previous studies have shown that higher BMI is associated with an increased prevalence of fibroids (30). A preliminary chi square analysis of this data did not show a statistically different association between BMI and fibroid prevalence. A future study would allow for further analysis of the association between BMI, fibroid prevalence, ovarian volume, and endometrial thickness.
Strengths of this study lie in the use of ultrasound to define true fibroid status given its high sensitivity (99%) and specificity (91%) (31). Also, all imaging was done with a transvaginal probe, which is of superior quality than the trans-abdominal approach. The uniform imaging in the follicular phase by one of two ultrasonographers allowed for comparisons within and between groups while minimizing confounding factors. Potential bias in reviewing of ultrasound images to identify fibroids was eliminated by blinding the lone reading physician, who has over 20 years of ultrasound experience, to the racial background of the subjects.
To our knowledge, this is the first published study establishing an ultrasound diagnosed fibroid prevalence rate in women under 25. We show here a 15% prevalence rate in asymptomatic young woman ages 18–30 with a significantly increased prevalence in black women. The thicker endometrium in black women and the associated clinical implications warrant further investigation. By recognizing that fibroids develop in early reproductive years, perhaps early interventions may reduce fibroid-associated morbidity and disparities. These options for earlier interventions could include opportunity for myomectomy or minimally invasive radiologic techniques while uterine size is still amenable to these techniques. Furthermore there is growing data that dietary supplementation, such as with Vitamin D, may be of benefit (32–35). The significant differences seen in this pilot study of prevalence of uterine fibroids in asymptomatic young women emphasize the need for a larger and more in depth study.
Acknowledgements
The authors would like to acknowledge Keoshia Parker, ARDMS and Rossella Passerella, ARDMS of the Northwestern University’s Division of Diagnostic Ultrasound who performed all the transvaginal ultrasounds for this study. The authors would also like to acknowledge Hannah Recht and Marissa Ghant for their help with statistical analysis and manuscript review.
Financial Support: NIH K12HD050121 Women’s Reproductive Health Research Scholar Program at Northwestern (EEM), NIH P01HD57877 (EEM), Feinberg School of Medicine – Northwestern University, and Northwestern Memorial Hospital, Robert Wood Johnson Foundation (EEM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. Cohen is a consultant for General Electric Ultrasound, Philips Ultrasound and Samsung Medison. He teaches and lectures on subjects related to 3-D ultrasound.
None of the other authors have a conflict of interest.
Presentation Information: Oral Presentation at American Society of Reproductive Medicine Annual Meeting, San Diego, CA, October 21–24, 2012
References
- 1.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229–234. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- 3.Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med. 1996;41:483–490. [PubMed] [Google Scholar]
- 4.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211 e1–211 e9. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn M, Jamison M, Datta S, Myers E. Health care resource use for uterine fibroid tumors in the United States. Am J Obstet Gynecol. 2006;195:955–964. doi: 10.1016/j.ajog.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 7.Faerstein E, Szklo M, Rosenshein N. Risk factors for uterine leiomyoma: a practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. Am J Epidemiol. 2001;153:1–10. doi: 10.1093/aje/153.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 9.Moore AB, Flake GP, Swartz CD, Heartwell G, Cousins D, Haseman JK, et al. Association of race, age and body mass index with gross pathology of uterine fibroids. J Reprod Med. 2008;53:90–96. [PubMed] [Google Scholar]
- 10.Wise LA, Palmer JR, Harlow BL, Spiegelman D, Stewart EA, Adams-Campbell LL, et al. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol. 2004;159:113–123. doi: 10.1093/aje/kwh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound-screening study. Obstet Gynecol. 2009;113:630–635. doi: 10.1097/AOG.0b013e318197bbaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borgfeldt C, Andolf E. Transvaginal ultrasonographic findings in the uterus and the endometrium: low prevalence of leiomyoma in a random sample of women age 25–40 years. Acta obstetricia et gynecologica Scandinavica. 2000;79:202–207. [PubMed] [Google Scholar]
- 13.Fields KR, Neinstein LS. Uterine myomas in adolescents: case reports and a review of the literature. Journal of pediatric and adolescent gynecology. 1996;9:195–198. doi: 10.1016/s1083-3188(96)70030-x. [DOI] [PubMed] [Google Scholar]
- 14.Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology. 2003;14:247–250. doi: 10.1097/01.EDE.0000054360.61254.27. [DOI] [PubMed] [Google Scholar]
- 15.Marshall LM, Spiegelman D, Goldman MB, Manson JE, Colditz GA, Barbieri RL, et al. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril. 1998;70:432–439. doi: 10.1016/s0015-0282(98)00208-8. [DOI] [PubMed] [Google Scholar]
- 16.Payson M, Leppert P, Segars J. Epidemiology of myomas. Obstetrics and gynecology clinics of North America. 2006;33:1–11. doi: 10.1016/j.ogc.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Booske BC, Robert SA, Rohan AM. Awareness of racial and socioeconomic health disparities in the United States: the national opinion survey on health and health disparities, 2008–2009. Preventing chronic disease. 2011;8:A73. [PMC free article] [PubMed] [Google Scholar]
- 18.Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16–20. [PubMed] [Google Scholar]
- 19.Nasri MN, Coast GJ. Correlation of ultrasound findings and endometrial histopathology in postmenopausal women. British journal of obstetrics and gynaecology. 1989;96:1333–1338. doi: 10.1111/j.1471-0528.1989.tb03233.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsuda H, Kawabata M, Kawabata K, Yamamoto K, Hidaka A, Umesaki N, et al. Differences between Occidental and Oriental postmenopausal women in cutoff level of endometrial thickness for endometrial cancer screening by vaginal scan. Am J Obstet Gynecol. 1995;172:1494–1495. doi: 10.1016/0002-9378(95)90484-0. [DOI] [PubMed] [Google Scholar]
- 21.Varner RE, Sparks JM, Cameron CD, Roberts LL, Soong SJ. Transvaginal sonography of the endometrium in postmenopausal women. Obstet Gynecol. 1991;78:195–199. [PubMed] [Google Scholar]
- 22.Phillip H, Dacosta V, Fletcher H, Kulkarni S, Reid M. Correlation between transvaginal ultrasound measured endometrial thickness and histopathological findings in Afro-Caribbean Jamaican women with postmenopausal bleeding. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2004;24:568–572. doi: 10.1080/01443610410001722671. [DOI] [PubMed] [Google Scholar]
- 23.Traub ML, Van Arsdale A, Pal L, Jindal S, Santoro N. Endometrial thickness, Caucasian ethnicity, and age predict clinical pregnancy following fresh blastocyst embryo transfer: a retrospective cohort. Reprod Biol Endocrinol. 2009;7:33. doi: 10.1186/1477-7827-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh EE, Shaw ND, Klingman KM, Tiamfook-Morgan TO, Yialamas MA, Sluss PM, et al. Estrogen levels are higher across the menstrual cycle in African-American women compared with Caucasian women. J Clin Endocrinol Metab. 2011;96:3199–3206. doi: 10.1210/jc.2011-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa H, Reierstad S, Demura M, Rademaker AW, Kasai T, Inoue M, et al. High aromatase expression in uterine leiomyoma tissues of African-American women. J Clin Endocrinol Metab. 2009;94:1752–1756. doi: 10.1210/jc.2008-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-alpha polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertil Steril. 2006;86:686–693. doi: 10.1016/j.fertnstert.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 27.Jacoby VL, Fujimoto VY, Giudice LC, Kuppermann M, Washington AE. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. Am J Obstet Gynecol. 2010;202:514–521. doi: 10.1016/j.ajog.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huyck KL, Panhuysen CI, Cuenco KT, Zhang J, Goldhammer H, Jones ES, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168 e1–168 e9. doi: 10.1016/j.ajog.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best practice & research Clinical obstetrics & gynaecology. 2008;22:571–588. doi: 10.1016/j.bpobgyn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Wise LA, Palmer JR, Spiegelman D, Harlow BL, Stewart EA, Adams-Campbell LL, et al. Influence of body size and body fat distribution on risk of uterine leiomyomata in U.S. black women. Epidemiology. 2005;16:346–354. doi: 10.1097/01.ede.0000158742.11877.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. Am J Obstet Gynecol. 2002;186:409–415. doi: 10.1067/mob.2002.121725. [DOI] [PubMed] [Google Scholar]
- 32.Thota C, Farmer T, Garfield RE, Menon R, Al-Hendy A. Vitamin D Elicits Anti-Inflammatory Response, Inhibits Contractile-Associated Proteins, and Modulates Toll-Like Receptors in Human Myometrial Cells. Reprod Sci. 2012 doi: 10.1177/1933719112459225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halder SK, Sharan C, Al-Hendy A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod. 2012;86:116. doi: 10.1095/biolreprod.111.098145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al-Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril. 2011;95:247–253. doi: 10.1016/j.fertnstert.2010.07.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2011;96:E754–E762. doi: 10.1210/jc.2010-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]