Abstract
CD8+ T-cell immune response to liver antigens is often functionally diminished or absent. This may occur via deletion of these autoaggressive T cells, through the acquisition of an anergic phenotype, or via active suppression mediated by other cell populations. We generated a double transgenic model in which mice express CD8+ T cells specific for the lymphocytic choriomeningitis virus nucleoprotein (LCMV-NP) and LCMV-NP as a hepatic neo-autoantigen, to study the immunological response of potentially liver antigen autoagressive CD8+ T cells. Autoreactive transgenic CD8+ T cells were analyzed for functionality and cytotoxic effector status. Despite severe peripheral deletion of liver-specific CD8+ T cells, a fraction of autoreactive NP-specific CD8+ T cells accumulate in liver, resulting in hepatocyte injury and production of autoantibodies in both male and female mice. NP-specific intrahepatic T cells showed capacity to proliferate, produce cytokines and up-regulate activation markers. These data provide in vivo evidence that autoreactive CD8+ T cells are activated in the liver and developed an inflammatory process, but require additional factors to cause severe autoimmune destruction of hepatocytes. Our new model will provide a valuable tool for further exploration of the immunological response involved in inflammatory liver diseases, including autoimmune hepatitis.
Keywords: autoimmune hepatitis, transgenic mouse model, autoreactive CD8+ T cells, peripheral tolerance mechanisms
1. Introduction
In addition to metabolic functions, the liver has immunological properties playing an essential role in maintaining a delicate balance between tolerance and immunity [1, 2]. The liver is exposed to endotoxins and other microbial degradation products that reach the liver via portal blood; many unique molecular mechanisms, including immune and non-immune cells, contribute to promote the development of immune tolerance. Similar mechanisms avoid hepatocytes damage from specific autoimmune process [3]. Therefore, the liver is particularly successful in the development of peripheral tolerance. However in specific circumstances, tolerance to hepatic self-proteins is broken, usually in genetically susceptible hosts, resulting in development of autoimmune hepatitis (AIH) [4].
AIH is the consequence of selective and progressive destruction of hepatic parenchyma by an inflammatory process [4]. Many cell types are involved in liver lesions characteristic of AIH, including antigen-presenting cells (APC), B lymphocytes and both CD4+ and CD8+ T lymphocytes [5]. The presence of autoreactive T cells (CD4+ and CD8+) in the liver and auto-antibodies (auto-Ab) production directed against various hepatic antigens, reflects the autoimmune character of the disease [6, 7]. However, neither their specific contribution to the disease pathogenesis nor how they are recruited and primed in the liver has been resolved.
There are convincing arguments showing that CD8+ T cells contribute to the pathogenic autoimmune process in organ specific autoimmune diseases (AID) [8]. First, adoptive transfer of autoimmune disorders by CD8+ T cells in syngenic immunodeficient mice proves that these cells are essential for the initiation of autoimmunity [9]. In addition, some CD8+ T cell clones have been shown to kill specific targets without the help of CD4+ T cells [10]. Autoreactive CD8+ T cells can be detected in the peripheral blood of human patients with AID, including patients with AIH. Thus, CD8+ T cell clones specific for ASGPR or CYP2D6 from patients with AIH have the ability to produce IFN-γ cytokine and to exert cytotoxicity after recognition of CYP2D6 peptide-pulsed targets [11]. Hepatic self antigens (HAgs) are readily accessible to class I-restricted CD8+ T-lymphocytes and many studies in mice suggest that autoreactive CD8+ T lymphocytes play a key role in the pathogenesis of AIH [12, 13]. Transgenic (Tg) or knockout animal models have been excellent tools for dissecting pathogenesis and have provided crucial information on mechanisms responsible for the fragile and complex balance between tolerance and autoimmunity in the liver [14, 15]. Moreover, knowledge of the mechanisms responsible for autoreactive T-lymphocytes activation, proliferation, clonal deletion, anergy, or ignorance in the periphery, have been enhanced by the use of TCR Tg mouse strains [16]. Thus, several murine models have highlighted the role of HAgs-specific CD8+ T cells. However, most previous works were based on transfer of CD8+ T cells against liver specific antigens expressed as neoself in Tg mice [17, 18]. Unfortunately, transfer of autoreactive CD8+ T cells was not sufficient to induce chronic inflammation [19]. Thus, these approaches showed severe limitations in the induction of immune-mediated hepatitis as observed in humans. Heterogeneity of T cell clones activation in vitro, impact of number of transferred cells and the fact that the liver plays a robust role in peripheral tolerance could explain the disappointing results reported. To bypass this obstacle, Tg murine models have been produced to develop a spontaneous chronic progressive AIH using double Tg mice expressing a TCR carried by CD8+ T cells with specificity for a neoself-autoAg expressed by hepatocytes [20–22]. However, these models show a central clonal deletion or a persistence of high avidity autoreactive Tg CD8+ T cells in the periphery in the absence of chronic liver inflammation. For example, double Tg mice on C57BL/6 (B6) background expressing the neoself gag protein (FMuLVgag) from Friend virus in the thymus and liver under Albumin promoter (Alb-Gag) showed no signs of autoimmunity. Gag-specific Tg CD8+ T cells escaping central tolerance were maintained in a tolerant state in the periphery and lost the ability to proliferate and produce IL-2 in response to antigen stimulation [20]. In contrast, double Tg mice expressing influenza virus-hemagglutinin (HA) as neoself-Ag only in the liver under hepatocyte-specific albumin promoter developed a moderate and transient form of hepatitis only in males. However, most liver-infiltrating HA-specific CD8+ T cells had an anergic status [22]. In this model, the development of a chronic T-cell mediated liver autoimmunity on mixed genetic background (DBA2, B6 and Balb/c) could be impaired by the interaction of autoimmunity-related susceptibility loci and/or the background of these strains.
To further analyze the liver specific autoimmune response and the role of the liver in peripheral tolerance, the double Tg model presented in this study was generated directly on B6 background to avoid the impact of genetic heterogeneity on T cell development and tolerance breakdown. This model shows specific signs of autoimmunity (cellular and humoral immune responses) and develops mild liver inflammation in both male and female mice. As such the model reported here should be of unique value for dissecting the molecular basis for the liver’s role in the balance between tolerance and immune mediated autoimmune liver injury.
2. Methods
2.1. Cells
The T CD8+ NP18 clone, which has previously been shown to have specific reactivity to amino acids 396–404 of the nucleoprotein (NP) from lymphocytic choriomeningitidis virus (LCMV), is restricted by the MHC class I H-2Db molecule [23]. Mice thymocytes, splenocytes, PBMC and lymph node cells were prepared according to standard protocols with minor modifications and cultured in RPMI+Glutamax medium (Invitrogen), 10% SBF, 5×10−5 M 2-ME, 100 U/ml penicillin G and 100 μg/ml streptomycin.
2.2. Transgenic mice generation
To determine TCR-α and -β gene usage, synthesized cDNA from total RNA of T CD8+ NP18 clone was amplified with a panel of Vα and Vβ specific primers as described [24] and PCR amplification products were subcloned and sequenced. TCR transgenes were constructed by subcloning PCR amplified regions encoding rearranged Vα8.5Jα5.1 and Vβ12D2Jβ2.3 domains into pT-Vα and pT-Vβ TCR transgenic vectors, respectively [25]. The TCR chain expression of both constructs was confirmed by transient transfection into 58 α−/β− T cell hybridomas and RT-PCR. Their ability to respond to NP396-404 peptide was confirmed using an IL-2 production assay. Transgenic constructs coding for the functional VJα and VDJβ rearrangements of NP18 clone were co-injected in fertilized eggs from C57BL/6 (H-2b) mice.
Genomic DNA from founder mice and their offspring was screened for the presence of TCRα and TCRβ chains DNA by PCR amplification, using specific primers. TCR chains expression was proved by RT-PCR amplification of total RNA from Tg splenocytes. Two transgenic (Tg) TCR mouse lines (TNP4 and TNP5) were kept and only TNP5 was used in further experiments in this study. The double transgenic TNP5/TTR-NP mice line was obtained after breeding of TCR transgenic mice (TNP5/B6) with the TTR-NP/B6 mice [26]. All transgenic mice used for this study were bred in the animal facility and were kept under specific pathogen-free conditions. All animal experiments were performed according to national and institutional guidelines.
2.3. Flow cytometry
Thymocytes, splenocytes, PBMC or lymph node cells (1×06) were labeled with fluorescence-conjugated antibodies specific for TCR β chain, CD4, CD8, CD25 or CD69. All antibodies were purchased from eBioscience, except the Extravidin-PE (Sigma). The H2-Db-NP396-404 tetramer (NP-tet) was produced as previously described [27] and was used to stain NP-specific effector cells. Four-color flow cytometry was performed on a FACScalibur (BD Biosciences), and data were analyzed using Cell Quest Pro software (BD Biosciences).
2.4. Transgenic CTLs functionality
Proliferation assays were performed on splenocytes from Tg mice. Briefly, cells were stained with 5 μM CFSE (eBioscience), then 1×106 cells per well were seeded in a 24-wells plate. Cells were stimulated with 10 μg/ml of the cognate NP396-404 peptide (FQPQNGQFI) or CMVpp65 (NLVPMVATV) irrelevant peptide as specificity control. Intrahepatic lymphocytes were isolated as described [28] and combined with syngeneic splenocytes loaded with specific peptides. Unstimulated and PMA stimulated controls were included in all experiments. Cells were harvested after 48–72 hours incubation at 37°C and proliferation of CFSE-labeled CD8+ T cells was analyzed by FACS using an APC-conjugated anti-CD8 antibody (eBioscience).
For degranulation assays, 2×105 transgenic mice splenocytes were seeded in 96-well plates and incubated for 4 hours with 10 μg/ml of NP396-404 peptide and a mAb specific for CD107a (Alexa Fluor 647-conjugated anti-mouse CD107a, eBioscience) [29]. An anti-CD8 antibody (FITC-conjugated anti-mouse CD8, eBioscience) was added for the last hour of incubation. Cells were harvested and washed twice with PBS and then processed in four-color flow cytometry.
For cytokines measurements, single Tg TCR mice or double Tg mice splenocytes (1×106 cells/wells) were incubated, with or without NP396-404 peptide. Supernatants were collected after 48 or 72 hours incubation and stored at −20°C. They were used for IL-2 and IFN-γ measurement with respective READY-SET-GO ELISA kits (eBioscience).
2.5. Monitoring liver-specific autoimmunity
Liver samples from groups of 3–12 wk-old TNP5 and TNP5/TTR-NP Tg mice were dehydrated, embedded in paraffin, sectioned and stained with H&E for histopathological and morphological analysis. The TUNEL assay was performed using The Apoptag® plus peroxidase in situ Apoptosis Detection Kit (Millipore, CA) according to the manufacturer’s protocol.
Serum ALT levels were measured in a Beckman-Synchron CX9 apparatus, from blood taken from mice of 3, 6, 10 and 12 weeks old. The presence of anti-NP autoantibodies in serum was revealed by ELISA as described [26].
2.6. Statistical analysis
Student t-test was used to determine the statistical significance of differences between groups, which was set at the 95% confidence level.
3. Results
3.1. Phenotypic analysis of transgenic T cells expressing a TCR specific for LCMV-NP
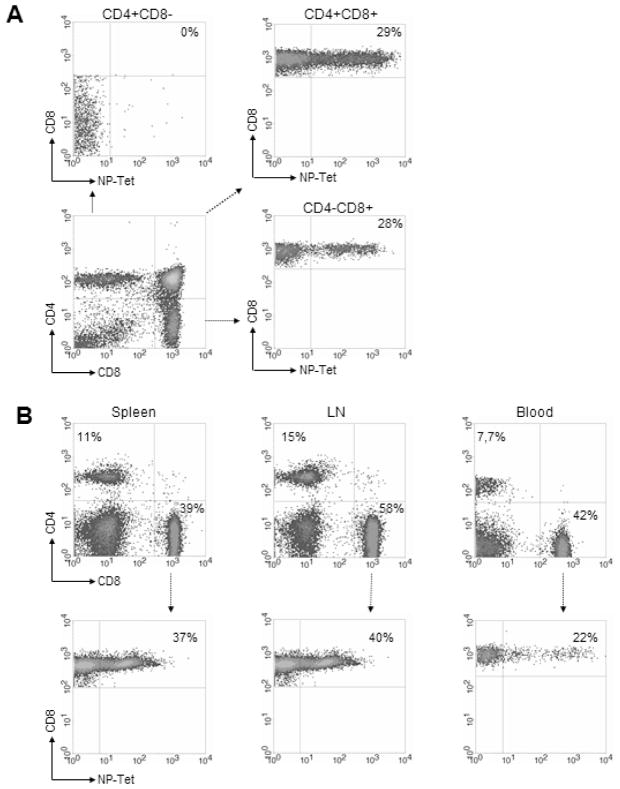
We amplified and cloned the genomic rearranged TCR genes from NP396-404 epitope (FQPQNGQFI) of LCMV-NP-specific CD8+ T clone (NP18) to generate TCR Tg mice. The αand β TCR chains coding for Vβ12-D2-Jβ2.3 and Vα8.5-Jα5.1 receptors were identified as specific chains recognizing NP396-404 epitope. The generated mouse strain (TNP5) expressing the Tg TCR on CD8+ T cells in the thymus and the periphery, showed an increased proportion of CD8+ T-cells and reduced CD4 T-cells compared with wild type B6 mice (data not shown). As expected in CD8+ Tg models, the ratio of CD4/CD8 thymocytes showed skewing toward the CD8+ T cell compartment. Assessment of Tg TCR expression on CD8 T cells by flow cytometry using tetramer–Db-NP396-406 (NP-tet) confirmed that Tg TCR are present only on CD8+ single-positive (SP) or CD4+ CD8+ double positive (DP) thymocytes (fig 1A).
Figure 1.
Representative T lymphocyte populations in TNP5 TCR-transgenic mice. (A) Thymocytes from 6–12 weeks-old TNP5 TCR-Tg mice were stained with fluorescence-labeled antibodies specific for CD4, CD8, and MHC class I tetramer Db-NP396-404 (NP-Tet). Expression of CD4 and CD8 on total thymocytes and of Vα8-Vβ12 Tg TCR on T cells were gated on double positive and single positive thymocytes. Numbers indicate the percentage of live cells or the percentage of NP-Tet positive cells in CD8 subset. (B) Peripheral lymphocyte profiles from spleen, lymph nodes and blood were determined by flow cytometry using anti-CD4 and anti-CD8 (top). Expression of TCR-α /β transgenes specific for NP396-406 peptide was analyzed by tetramer. The percentage of positive cells gated on CD8+ T cells was determined (bottom).
CD4 and CD8 profiles in spleen, lymph nodes or blood showed that CD8+ population was markedly enhanced compared to B6 littermates (data not shown). These results confirmed marked skewing towards the CD8 single-positive phenotype in Tg TCR mice (fig1B). The staining by anti-CD8+ antibody and NP-tet showed that Tg NP-specific CD8+ T cells represent about 37% of CD8 population in the spleen, 40% in LN, and 22% in blood (fig1B). No specific phenotype develops in TCR single Tg mice (TCR-STg).
3.2. Hepatic Ag-specific CD8+ T cells population in double Tg mouse model
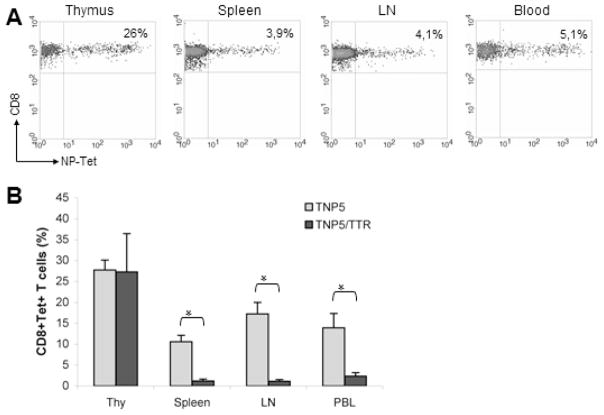
Double Tg mice (DTg) were obtained by crossing TTR-NP single Tg mice (STg), which express the neo self-antigen LCMV-NP in the hepatocytes [26], with TNP5 STg mice producing NP-specific CD8+ T lymphocytes. The Tg TCR T cells in TNP5/TTR-NP DTg mice were not deleted in the thymus and the percentage of NP-specific CD8+ thymocytes gated in CD4−CD8+ SP thymocytes was similar to those in TNP5 STg mice compare (Fig 1A vs Fig 2A). This result is consistent with a positive selection in the absence of neo-self LCMV-NP expression in the thymus as described in TTR-NP Tg mice [26] and confirmed in DTg mice by RT-PCR (data not shown). Unexpectedly, NP-tet specific CD8+ T cells frequencies from the total of peripheral CD8+ T cells in DTg mice compared to STg mice showed a marked decrease from 37% to 3.9%, from 40% to 4% and from 22% to 5.1% in the spleen, lymph nodes and peripheral blood respectively (fig 1B and fig 2A). Thus, these potentially autoreative CD8+ T cells in DTg mice undergone partial but significant peripheral deletion compared to STg mice.
Figure 2.
Central and peripheral tolerance in TNP5\TTR-NP double Tg mice. (A) Typical NP-Tet positive thymocytes and peripheral lymphocytes populations from double Tg mice, gated on CD8+ T cells. (B) The histogram represents the NP-Tet positive CD8+ TCR Tg cells from total T cells in single (TNP5) and double (TNP5/TTR) Tg mice (mean ± SD of 5 independent experiments, n= 4–7 mice/group) *p<0.05
3.3. Functional capacity of potential autoaggressive NP-specific CD8 T cells
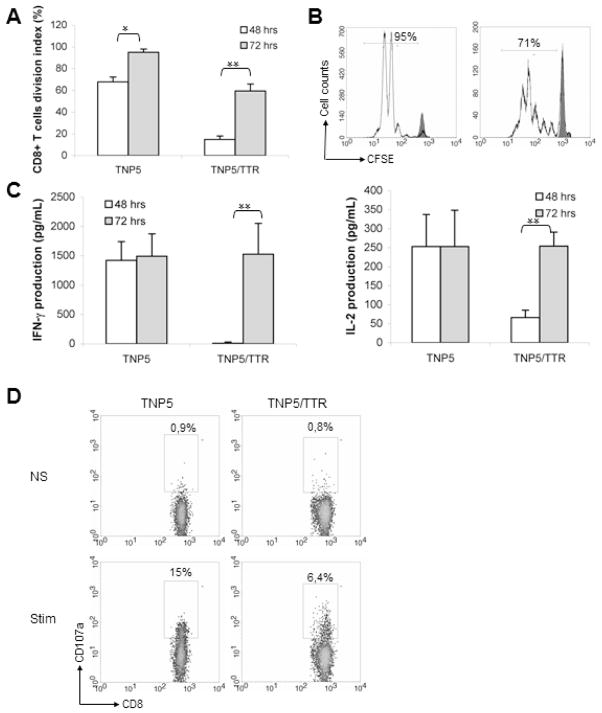
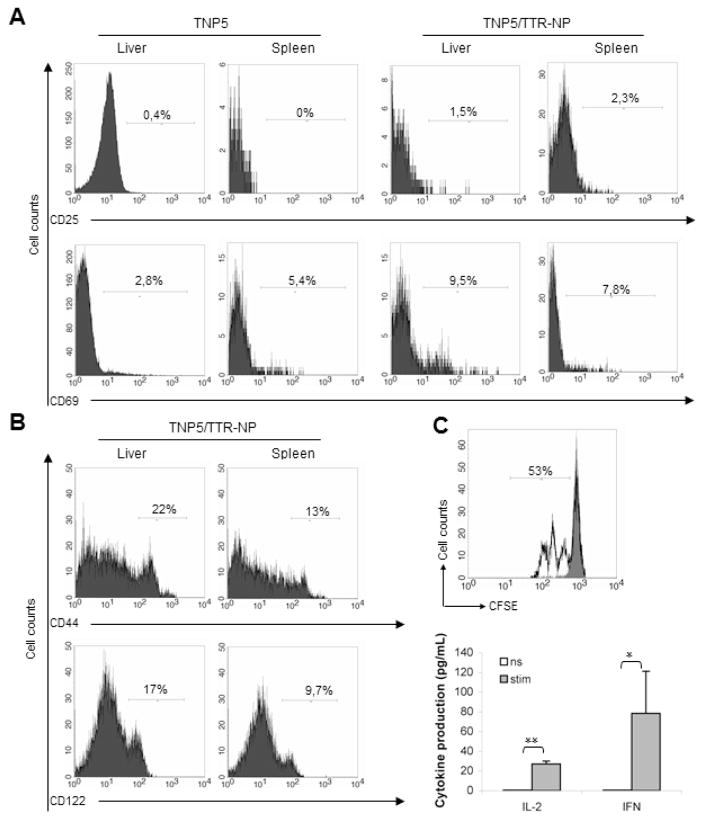
Despite the fact that the majority of NP-specific CD8+ T cells encountering LCMV-NP as a self-Ag in the periphery, mainly in the liver, of DTg mice are deleted and their number decreased by around 10 fold in secondary lymphoid tissues, they showed vigorous activation in vitro. To compare rates of proliferation of NP-specific CD8+ T cells, splenocytes were only stimulated with NP396-404 peptide and no exogenous APC were added to the culture. Measurement of the level of proliferation of NP-specific CD8+ T cells showed that these specific T cells from STg mice proliferated with higher rate (4 fold) than those from DTg mice (fig 3A) after 2 days of culture (70% vs 17%). However, after 3 days of culture, extensive proliferation of NP-specific T cells from DTg was observed with several rounds of division (5 to 6 divisions) (71% vs 95%) (fig 3B). Moreover, this response was highly Ag specific, because splenic cells failed to proliferate in absence of peptide or when exposed to a control peptide. Splenocytes from both Tg mice were tested for production of IL-2 and IFN-γ after stimulation with NP396-404 peptide during 48h or 72h. Supernatants from proliferation culture assayed by quantitative cytokine ELISA analysis showed that CD8+ T cells from STg mice produced significantly more IFN-γ and IL-2 than NP-specific CD8+ T cells from the DTg mice after 48h. However, specific NP- CD8+ T cells of both Tg mice produced equivalent amounts of IFN-γ and IL-2 after 72 of culture (fig 3C). This result indicates that autoreactive T cells from STg and DTg were able to acquire a full cytotoxic effector capacity.
Figure 3.
Functional capacity of Tg TCR CD8+ T lymphocytes. (A) The cell division index (CDI) was assessed for the proliferative capacity of NP-specific Tg CD8+ T cells after 2 or 3 days of culture in both STg and DTg mice. The CDI was calculated by substracting the percentage of CFSElow in the CD8+ subset (%CFSElow /CD8+) found in unstimulated cultures from %CFSElow /CD8+ found in stimulated cultures (mean ± SD of 5 independent experiments, n=5–7 mice per group). *p<0.05; **p<0.001. (B) Representative FACS analysis of NP396-404-stimulated splenocytes and unactivated (gray filled) after 3 days of culture. Equivalent numbers of naive spleen cells from either TNP5 or TNP5/TTR-NP mice were assessed for proliferation in response to NP396-404 peptide. Numbers in the diagrams display the percentages of proliferating NP-specific CD8+ T-cells gated on CD8+ T cells subset. The background proliferation (M1) for both mice was 2.5–4 % (data not shown). (C) Supernatants of NP396-404 peptide-stimulated splenocytes were analyzed for IFN-γ and IL-2 production in both STg and DTg mice. Non-Tg mice were used as negative controls (data not shown). (Mean ± SD of 3 independent experiments, n=5–6 mice per group). **p<0.001. (D) To assess the cytotoxic function, Tg splenocytes stimulated with NP396-404 peptide or without (NS) after 4h of culture were used in exocytosis assays in which CD107a (LAMP-1) detection reflects the release of granzymes by CTL to kill specific targets. The representative flow cytometry showed the proportion of CD107a+ cells within the CD8+ T lymphocyte population from TNP5 and TNP5/TTR-NP mice.
To compare the functional cytotoxic status of these cells, we evaluated the degranulation of NP-specific CD8+ T cells from STg and DTg mice. Tg CD8+ T cells were evaluated directly ex vivo for surface expression of CD107a (LAMP-1), a surrogate marker for recent cytolytic activity. As around 4% of splenic NP-specific CD8+ T cells were found in DTg mice and similar number of splenocytes express CD107a suggest that all or most of them showed a cytotoxic activity. However, one third of those from STg mice were positive for CD107a marker (15% of total CD8+ T cells) (fig. 3D). These data indicate that CTLs isolated from DTg mice undergone full activation and suggest that NP-specific CD8+ T cells in DTg mice had been exposed to their antigen and were not naïve.
3.4. Self-specific CD8+ and CD4+ T cells accumulate in the liver
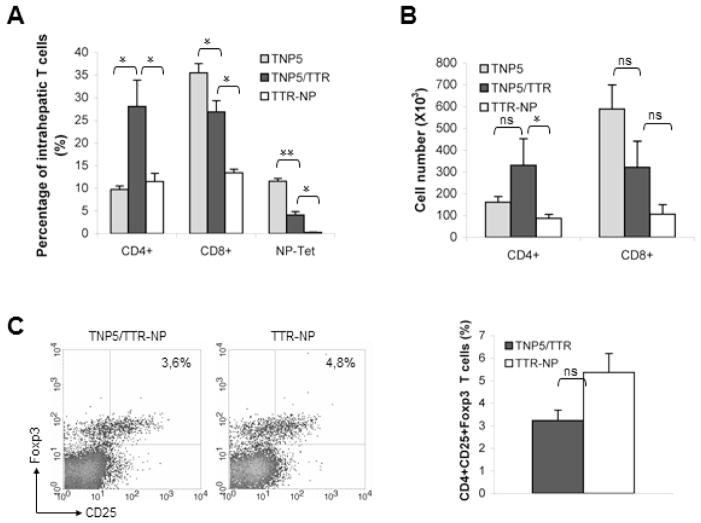
The percentages of CD8 T cells in liver (average four mice for all groups) are 26.7 ± 2.5 % in DTg mice and 32.1 ± 1% in TCR STg mice compared with 12.7 ± 2.1% in TTTR-NP (fig 4A). Furthermore, the ratio of total number of CD8/CD4 single positive cells was 1:1 in DTg mice or TTR-NP mice compared to 4:1 in TCR STg mice. Moreover, the frequency of NP-specific CD8+T-cells in liver of naïve TCR STg mice was similar to those found in spleen (32% vs 37%, fig 4A). In contrast, NP-specific CD8+T-cells (gated on CD8+ population) in liver of DTg mice was significantly higher than in lymphoid peripheral organs and blood and resulted in an increase of approximate four- to five-fold (15% vs 3%). Thus, these data indicate that the accumulation of autoaggressive CD8+ T-cells in the liver of DTg mice is specific to the hepatic neo-Ag expression. The observation that the proportion and the number of CD4+ T cells in double Tg mice were higher than in STg TCR or TTR-NP mice led to the hypothesis that these cells may have developed toward a regulatory phenotype. However, the proportion of intrahepatic Foxp3+ CD25+CD4+ Tregs from DTg TCR/TTR-NP was similar to those from TTR-NP control mice (fig 4C). These data suggest that Tregs are not modulated in the liver of DTg mice and the accumulation of self-reactive CD8+ T cells in liver appears to be related to conventional CD4+ T cells that could provide help to promote this specific CD8+ T cells activation.
Figure 4.
Autoreactive CD8+ T lymphocytes escape from clonal deletion and accumulate in the liver of TNP5/TTR-NP mice. (A) Intrahepatic lymphocytes from TNP5, TNP5/TTR-NP and control TTR-NP mice were isolated, and the percentage of CD4+ T cells, CD8+ T cells, NP specific CD8+ tetramer-bound T cells was determined by flow cytometry (mean ± SD of 2 independent experiments, n=3–4 mice per group) *p<0.05 and **p<0.001. (B) Total number of intrahepatic T cells in indicated mice was determined by cell counting. The total cell numbers per liver are presented as means ± SD from 3–4 livers for each group. *p<0.05. (C) Gated intrahepatic CD4+ T cells from DTg TNP5/TTR-NP and control mice (TTR-NP) were analyzed for CD25 and intracellular Foxp3 expression (left). The frequency of CD4+ T cells that are FoxP3+ and CD25+ in liver of DTg TNP5/TTR-NP mice was not significantly different compared with that of STg TTR-NP (right, p=.330) (mean ± SD representative of 2 independent experiments, n=3–4 mice per group).
3.5. Liver-specific CD8+ T cells bear the phenotypic signature of activated/memory T cells
Increased percentage of intra-liver autoreactive T cells in DTg mice compared to peripheral organ suggested possible priming in vivo by resident APC, including hepatocytes. NP-specific CD8+ T cells do not become anergic, because they display numerous phenotypic markers and functional traits that are found in Ag-driven activated CD8+ T cells. CD69 and CD25 expression, considered to be a marker of T cell activation, was up-regulated in CD8+ T cells from both spleen and liver of DTg mice upon in vitro stimulation (data not shown). In contrast, on unstimulated intrahepatic CD8+ T cells, only CD69 was increased when compared to those from STg mice (9,5% vs. 2,8%). Increased expression of CD69 seemed to result from stimulation in the liver itself because its expression in spleen was identical from both Tg mice (7.8% in DTg vs. 5.4% in STg) (fig 5A). Moreover, these unstimulated intrahepatic CD8+ T cells from Dtg mice exhibit strong up-regulation of effector/memory CD44 or CD122 markers compared to splenocytes (fig 5B). Furthermore, NP-specific CD8+ T cells from liver proliferate vigorously to NP396-404 peptide and produce IFN-γ and IL-2 upon antigenic stimulation in vitro (fig 5C).
Figure 5.
Proliferation, activation and acquisition of effector function by liver infiltrating NP-specific CD8+ T cells. (A) Representative results of unstimulated CD8+ T cells from liver or spleen of both Tg mice, analyzed for CD69 and CD25 as early activation markers. (B) Expression of CD8+ T cell effector memory-related surface markers is shown in DTg mice gated on CD8+ T lymphocyte population from indicated organs. Numbers on histograms indicate percentage of CD8+ T cells upregulating a given marker. (C) Intraliver lymphocytes isolated from DTg mice were cocultured with syngeneic splenocytes from wild type mice and NP396-404 peptide for 3 days. Proliferation capacity was analyzed for NP-specific CD8+ T-cells gated on CD8+ T cells. Cells without stimulation or stimulated with 20 ng/mL PMA or 1 mg/mL conA served as a negative and a positive control, respectively (top). Supernatants from these proliferation experiments were analyzed by ELISA to assess secretion of IFN-γ and IL-2 cytokines as effector markers. (bottom) (mean ± SD of 2 independent experiments, n=4–5 mice per group). *p<0.05 and **p<0.001.
3.6. Liver inflammation and autoimmunity signs
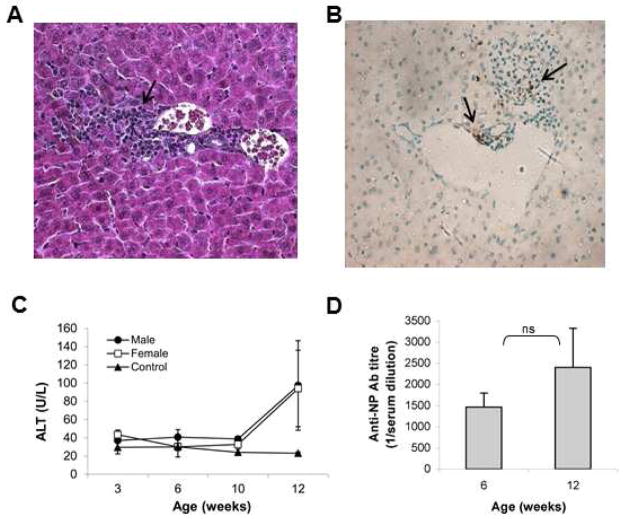
Autoagressive T cells activation in vivo could result in the development of an inflammatory process that would lead to the destruction of hepatocyte expressing LCMV-NP. Such specific injury could be mediated by the potential pathogenicity of these effector NP-specific CD8+ T cells as shown by histological analysis (fig 6A and B) and increased serum transaminase (ALT) levels, in both males and females (fig 6C). DTg mice developed liver inflammation and the early phase of liver infiltration was first found in 3 weeks old mice (fig 6C). Spontaneous inflammation in the DTg mice was detected in each generation (F2-F10) and was specific because we did not observe any sign of liver inflammation (ALT ≈ 20-30 U/L) in single Tg mice (TCR or TTR-NP). Moreover, the histologic analysis in DTg mice revealed an intralobular inflammation persistent at least until 12 weeks old mice of both sexes. Moreover, anti-NP auto-Abs were found in the serum of both male and female DTg mice (fig 6D). These results suggest that a T-cell mediated liver inflammation occurred together with a humoral response that would be secondary to hepatocytes damage.
Figure 6.
Capacity of Tg TCR CD8+ T cells to provoke liver-specific inflammatory process. (A) Histological examination of the liver from double Tg mice (TNP5/TTR-NP). Liver tissue section from single Tg TNP5 mice did not show any inflammatory cells (data not shown). In livers of double Tg mice, portal tract and intralobular lymphocytic infiltration were observed in both sexes. Magnification of hematoxylin–eosin microphotographs 400×. (B) Hepatocytes apoptosis (brown coloured nuclei) was only observed in double Tg mice (TNP5/TTR-NP) groups. The TUNEL-positive cells are shown by arrows. Magnification of TUNEL microphotographs 400×. (C) Serum ALT levels were determined at indicated age. Data represent mean values ±SD (n=5–6 mice per age group). Dotted line represents normal values in single Tg TNP5 mice. (D) ELISA for anti-NP autoantibodies. Serum anti-NP Abs were detected at 6 and 12 weeks old and similar levels were found in both sex. Values are expressed as mean ± SD (n=7–8 mice per group).
4. Discussion
Central T-cell tolerance contributes significantly to the prevention of autoimmunity, whereas peripheral tolerance controls self-reactive T cells that escape thymic negative selection to limit their activation and the induction of immune pathology [30]. Thus, these potentially autoreactive T cells that escape negative peripheral tolerance and form the peripheral self-reactive repertoire could be resistant to anergy, ignorance and T cells suppressive mechanisms and provoke autoimmunity against some organs vulnerable to autoimmune attacks, including pancreas and central nervous system [31, 32]. In contrast, liver appears to be better protected from allogenic immune response or autoimmunity, because transplanted livers are less rejected than other solid organs and autoimmune liver diseases are rare [33]. However, specific autoimmunity against hepatocytes can occur in the form of an autoimmune hepatitis. Despite some advances in the understanding of this disease both its etiology as well as most of its pathogenic mechanisms are unknown.
Generation of Tg mouse models has provided crucial information for better understanding mechanisms involved in peripheral tolerance breakdown by employing neo-self antigen- and TCR-double transgenic mice [34]. However, the development of tolerance to antigens expressed only in the liver has been poorly understood. In this study, we developed a novel line of Tg mice expressing dual transgenes for both LCMV-NP neoself-antigen expressed only in the liver, and the NP-specific CD8+ T cells. This model allowed us to investigate the functional outcomes of potentially autoreactive CD8+ T cells in mice. CD8+ T cells are more abundant in liver of patients with inflammatory liver diseases [35] and are effector cells responsible for the pathogenesis of numerous animal models of autoimmune diseases [8].
In our double Tg model (called TNP5/TTR-NP), the NP346-404-specific CD8+ T cells mediate a limited intralobular liver inflammation. Despite the fact that peripheral tolerance eliminates 90% of these highly pathogenic T cells from the repertoire, about 10% of potential autoreactive CD8+ T cells escape peripheral clonal deletion. Low proportion of neoself-Ag-specific CD8+ T cells (4–5% of total CD8+ T cells) express a Tg TCR composed of Vα8 and Vβ12 specfic to LCMV-NP and showed effector function in spleen and LNs of double Tg mice. The proportion of potential autoreactive CD8+ T cells in liver increase to 3- to 4-fold and keep the capacity to proliferate vigorously to NP396-404 peptide in vitro and to secrete IL-2 and IFN-γ cytokines. These results indicate that the NP-specific CD8+ T cells escaping from deletion gained effector functions mainly in the liver, after encountering the target self-Ag. Furthermore, the accumulation of functional autoagressive NP-specific CD8+ T cells in the liver raised the possibility that peripheral clonal deletion observed might be mediated by mechanisms relegated to the target organ in addition to a potential role of peripheral lymphoid tissues.
The proportion of auto-reactive CD8+ T cells in our model could be sufficient to initiate the autoimmune cascade. Thus, there is no evidence for thymic deletion of autoreactive CD8+ T cells in DTg mice, even when the proportion of total SP CD8+ cells was reduced to 7% that is higher than TTR-NP (1.5%) or normal age-matched littermates B6 mice (data not shown). Furthermore, the frequency of Tg CD8+ T cells observed in liver of DTg is physiologically relevant in contrast to other models expressing more than 90% of the Tg CD8+ population in thymus and 40–60% in periphery or liver [17, 18]. In addition, potentially autoreactive CD8+ T cells were specifically retained in the liver of DTg mice, as reflected by the higher proportion of NP-specific CD8+ T cells in the total CD8+ T cells in the liver (15%) compared with the spleen, LN or peripheral blood (3–5%) of DTg mice. In contrast, the proportion of these NP-specific CD8+T cells in the total CD8+ T cell population was similar in peripheral lymphoid organs, thymus and liver (around 30%) of STg mice. Thus, the presence of very few NP-specific CD8+ T cells in the blood of DTg mice, indicates that reactive CD8+ T cells were retained in the liver and do not recirculate between the liver and lymphoid tissues. These observations are similar to those reported from Tg models using adoptive transfer of HAg-specific self-reactive CD8+ T cells [5].
Mild liver inflammation in the liver lobule that we observed caused hepatocyte injury, as assessed by measuring serum ALT levels. The development of spontaneous inflammation in both sexes excludes any sex effect on the CD8+ TCR Tg cells in DTg mice. In addition, the priming of pathogenic Tg CD8+ T-cells could be driven by professional APCs within the liver and/or by direct recognition of Ag endogenously processed and presented by hepatocytes. Intrahepatic NP-Tg CD8+ T cells displayed elevated expression of CD69 marker related to activated phenotype in vivo and have the capacity to acquire effector function in vitro. Activation in the liver was Ag specific, CD69 up-regulation was detected in NP-specific CD8+ T cells (17% of CD8+ T cells),but not in naïve CD8+ T cells from the spleen or the liver of STg mice. Increased CD44 and CD122 expression on CD8+ T cells was more prevalent in liver than spleen from DTg, which is an indication that these Tg CD8+ T cells have previously encountered the Ag, and have been activated through their TCR. Thus, these NP-specific CD8+ T cells isolated from liver of DTg mice expressed noticeably increased levels of CD69 and CD44, which is consistent with an effector memory/ activated phenotype [36]. Thus, hepatocytes are capable of activating naïve CD8+ T cells, but imprint a distinct program of differentiation leading to unsustained expression of CD25 as described for adoptively transferred self reactive CD8+ T cells [37]. Altogether, our data strongly suggest that the liver is a site of primary activation for NP-specific CD8+ T cells in DTg mice, and that such mice did not show ignorance of the target Ags or anergic phenotype [20, 21, 38]. Therefore, retention and activation of NP-specific CD8+ T cells in the liver provide evidence that hepatocytes are able to activate CD8+ T cells in vivo.
Biochemical (increased serum ALT levels) and histological (lobular inflammatory infiltrates) parameters observed in both sexes were likely caused by direct cytolytic effects against hepatocytes of specific autoreactive CD8+ T cells. Hepatocytes death leads to release of LCMV-NP autoantigen, and consequently to an induction of B cell autoantibody production. Altogether, these findings indicate that NP-specifc Tg CD8+ T cells are essential contributors to the earliest phases of liver inflammation leading to hepatocytes destruction.
Previous evidence suggests that autoantibodies in AIH are not pathogenic, and their titers may not correlate with the disease process after its initiation. Autoantibody production is likely secondary to pathogenic T cell responses [39, 40]. These observations of others are now supported experimentally by our results showing that the presence of NP-specific autoantibodies was secondary to hepatocytes injury by cytotoxic CD8+ T cells and cytokines production.
Given the importance of the helper function of CD4+ T cells in autoantibody production and CD8+ T cells cytotoxicity, the intrahepatic CD4+ T cells might play an integral role in the inflammatory process observed in this model. The only obvious difference observed regarding cell composition in DTg mice and STg control animals (TCR Tg or NP Tg mice) was elevated CD4+ T cell proportion in liver from DTg mice. Interestingly, these results indicate that the CD4+ T cell attraction was specific to activation status of auto-reactive CD8+ T cells in the liver and not due to differences in the frequencies of NP-specific CD8+ T cells or total CD8+ T cells.
In contrast to the results obtained for CD8+ T cell-mediated liver inflammation showing that more of 40% of intrahepatic CD4+ T cells are Tregs [22], the increased population of intra-liver CD4+ T cells in our model is not due to Foxp3+ CD25+ CD4+ Tregs, suggesting that intra-liver conventional CD4+ T cells in TNP5/TTR-NP could play a crucial role for NP-specific CD8+ T-cell responses and autoantibody production. Thus, these results do not support other findings, suggesting that accumulation of activated CD8+ T cells in the liver leads to a transient increase of intra-hepatic Tregs for the control of liver injury [22].
Several facts may account for the discrepancy in results between our DTg model and those used by others: A) Tg constructs used to generate hepatic neo-self Ag differ in promoter and nature of expressed Ags; B) the expression level of self Ag including the amount of the MHC class I-associated peptides presented by hepatocytes; C) the avidity and/or affinity of TCR; D) the number of potentially autoreactive Tg CD8+ T cells, that may affect the fate of T cell responses. Indeed, in a DTg mouse model expressing the main epitope of the LCMV-Glycoprotein (GP33) under control of the albumin promoter in the liver, but also in the thymus, and the GP-specific CD8+ TCR (P14), the thymic clonal deletion of more than 90% of GP33-specific CD8+ T cells and the transient form of hepatitis observed after transfer of GP33-specific CTL and LCMV infection indicate that the mechanisms leading to the abrogation of tolerance are diverse amongst the various mouse models [21].
Consequently, more fundamental questions, such as which population of T cells plays a role in triggering liver inflammation, remain unresolved. Our model described in this study will provide a valuable tool trying to clarify such unsolved pathophysiological mechanisms of T cell–mediated liver diseases. In view of our results, we propose that the presence of self-reactive CD8+ T cells is not sufficient to cause severe or fulminate liver disease because some additional cellular or molecular mechanisms are crucial to autoimmune disease progression. Thus, an inflammatory insult is required and CD8+ T cells play a critical role in initiation of liver injury but not in the establishment of severe disease. This suggestion is in line with our observation that lymphoid infiltrates are detectable in the liver of 3 weeks old DTg mice; in adult mice the inflammation reaches the steady state and does not progress to severe inflammation.
5. Conclusions
The accumulation of potential auto-reactive CD8+ T cells and conventional CD4+ T cells in the liver are not sufficient to explain the development of a severe autoimmune hepatitis and there are probably multiple mechanisms that can contribute to the onset of disease [41]. Indeed, the difference in data derived from different DTg models may reflect this complexity and the identification of key mechanism in the development of a chronic liver inflammatory process is likely to lead to new therapeutic interventions.
We generated a new double transgenic model of autoimmune hepatitis
Transgenic mice show an incomplete deletion of autoreactive CD8+ T cells
Remaining autoreactive CD8+ T cells accumulate in the liver
Autoreactive CD8+ T cells acquire an effector phenotype
Mice sera show elevated transaminases (ALT) level, auto-antibodies and inflammation
Acknowledgments
This work was supported by Grants from Research Center of CHU Ste-Justine (to I.DS), National Institutes of Health AI 009484 (to MB.O) and Ste-Justine Foundation scholarship (to S.C).
Footnotes
The authors declare to have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–63. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 2.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–66. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 4.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 5.Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114:701–12. doi: 10.1172/JCI21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teufel A, Galle PR, Kanzler S. Update on autoimmune hepatitis. World J Gastroenterol. 2009;15:1035–41. doi: 10.3748/wjg.15.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergani D, Mieli-Vergani G. Aetiopathogenesis of autoimmune hepatitis. World J Gastroenterol. 2008;14:3306–12. doi: 10.3748/wjg.14.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter U, Santamaria P. CD8+ T cells in autoimmunity. Curr Opin Immunol. 2005;17:624–31. doi: 10.1016/j.coi.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Bendelac A, Carnaud C, Boitard C, Bach JF. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987;166:823–32. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiLorenzo TP, Serreze DV. The good turned ugly: immunopathogenic basis for diabetogenic CD8+ T cells in NOD mice. Immunol Rev. 2005;204:250–63. doi: 10.1111/j.0105-2896.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 11.Longhi MS, Hussain MJ, Bogdanos DP, Quaglia A, Mieli-Vergani G, Ma Y, et al. Cytochrome P450IID6-specific CD8 T cell immune responses mirror disease activity in autoimmune hepatitis type 2. Hepatology. 2007;46:472–84. doi: 10.1002/hep.21658. [DOI] [PubMed] [Google Scholar]
- 12.Russell JQ, Morrissette GJ, Weidner M, Vyas C, Aleman-Hoey D, Budd RC. Liver damage preferentially results from CD8(+) T cells triggered by high affinity peptide antigens. J Exp Med. 1998;188:1147–57. doi: 10.1084/jem.188.6.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka A, Iwabuchi S, Takatori M, Ohno A, Yamada H, Hashimoto N, et al. Clonotypic analysis of T cells in patients with autoimmune and viral hepatitis. Hepatology. 1997;25:1070–6. doi: 10.1002/hep.510250504. [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–36. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 15.Lohr J, Knoechel B, Abbas AK. Regulatory T cells in the periphery. Immunol Rev. 2006;212:149–62. doi: 10.1111/j.0105-2896.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 16.Hardtke-Wolenski M, Jaeckel E. Mouse models for experimental autoimmune hepatitis: limits and chances. Dig Dis. 2010;28:70–9. doi: 10.1159/000282067. [DOI] [PubMed] [Google Scholar]
- 17.Bertolino P, Schrage A, Bowen DG, Klugewitz K, Ghani S, Eulenburg K, et al. Early intrahepatic antigen-specific retention of naive CD8+ T cells is predominantly ICAM-1/LFA-1 dependent in mice. Hepatology. 2005;42:1063–71. doi: 10.1002/hep.20885. [DOI] [PubMed] [Google Scholar]
- 18.Buxbaum J, Qian P, Allen PM, Peters MG. Hepatitis resulting from liver-specific expression and recognition of self-antigen. J Autoimmun. 2008;31:208–15. doi: 10.1016/j.jaut.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertolino P, Trescol-Biemont MC, Rabourdin-Combe C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur J Immunol. 1998;28:221–36. doi: 10.1002/(SICI)1521-4141(199801)28:01<221::AID-IMMU221>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 20.Ohlen C, Kalos M, Cheng LE, Shur AC, Hong DJ, Carson BD, et al. CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J Exp Med. 2002;195:1407–18. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voehringer D, Blaser C, Grawitz AB, Chisari FV, Buerki K, Pircher H. Break of T cell ignorance to a viral antigen in the liver induces hepatitis. J Immunol. 2000;165:2415–22. doi: 10.4049/jimmunol.165.5.2415. [DOI] [PubMed] [Google Scholar]
- 22.Zierden M, Kuhnen E, Odenthal M, Dienes HP. Effects and regulation of autoreactive CD8+ T cells in a transgenic mouse model of autoimmune hepatitis. Gastroenterology. 2010;139:975–86. 86 e1–3. doi: 10.1053/j.gastro.2010.05.075. [DOI] [PubMed] [Google Scholar]
- 23.Lewicki H, Tishon A, Borrow P, Evans CF, Gairin JE, Hahn KM, et al. CTL escape viral variants. I. Generation and molecular characterization. Virology. 1995;210:29–40. doi: 10.1006/viro.1995.1314. [DOI] [PubMed] [Google Scholar]
- 24.Baker FJ, Lee M, Chien YH, Davis MM. Restricted islet-cell reactive T cell repertoire of early pancreatic islet infiltrates in NOD mice. Proc Natl Acad Sci U S A. 2002;99:9374–9. doi: 10.1073/pnas.142284899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouskoff V, Signorelli K, Benoist C, Mathis D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. J Immunol Methods. 1995;180:273–80. doi: 10.1016/0022-1759(95)00002-r. [DOI] [PubMed] [Google Scholar]
- 26.Djilali-Saiah I, Lapierre P, Vittozi S, Alvarez F. DNA vaccination breaks tolerance for a neo-self antigen in liver: a transgenic murine model of autoimmune hepatitis. J Immunol. 2002;169:4889–96. doi: 10.4049/jimmunol.169.9.4889. [DOI] [PubMed] [Google Scholar]
- 27.Lacasse P, Denis J, Lapointe R, Leclerc D, Lamarre A. Novel plant virus-based vaccine induces protective cytotoxic T-lymphocyte-mediated antiviral immunity through dendritic cell maturation. J Virol. 2008;82:785–94. doi: 10.1128/JVI.01811-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapierre P, Beland K, Djilali-Saiah I, Alvarez F. Type 2 autoimmune hepatitis murine model: the influence of genetic background in disease development. J Autoimmun. 2006;26:82–9. doi: 10.1016/j.jaut.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 30.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–9. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 31.Han B, Serra P, Yamanouchi J, Amrani A, Elliott JF, Dickie P, et al. Developmental control of CD8 T cell-avidity maturation in autoimmune diabetes. J Clin Invest. 2005;115:1879–87. doi: 10.1172/JCI24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perchellet A, Stromnes I, Pang JM, Goverman J. CD8+ T cells maintain tolerance to myelin basic protein by ‘epitope theft’. Nat Immunol. 2004;5:606–14. doi: 10.1038/ni1073. [DOI] [PubMed] [Google Scholar]
- 33.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 34.Miyagawa F, Gutermuth J, Zhang H, Katz SI. The use of mouse models to better understand mechanisms of autoimmunity and tolerance. J Autoimmun. 2010;35:192–8. doi: 10.1016/j.jaut.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oo YH, Weston CJ, Lalor PF, Curbishley SM, Withers DR, Reynolds GM, et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol. 2010;184:2886–98. doi: 10.4049/jimmunol.0901216. [DOI] [PubMed] [Google Scholar]
- 36.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–48. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holz LE, Benseler V, Bowen DG, Bouillet P, Strasser A, O’Reilly L, et al. Intrahepatic murine CD8 T-cell activation associates with a distinct phenotype leading to Bim-dependent death. Gastroenterology. 2008;135:989–97. doi: 10.1053/j.gastro.2008.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benseler V, Warren A, Vo M, Holz LE, Tay SS, Le Couteur DG, et al. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc Natl Acad Sci U S A. 2011;108:16735–40. doi: 10.1073/pnas.1112251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vergani D, Alvarez F, Bianchi FB, Cancado EL, Mackay IR, Manns MP, et al. Liver autoimmune serology: a consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J Hepatol. 2004;41:677–83. doi: 10.1016/j.jhep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Vitozzi S, Lapierre P, Djilali-Saiah I, Alvarez F. Autoantibody detection in type 2 autoimmune hepatitis using a chimera recombinant protein. J Immunol Methods. 2002;262:103–10. doi: 10.1016/s0022-1759(02)00016-9. [DOI] [PubMed] [Google Scholar]
- 41.Bertolino P, McCaughan GW, Bowen DG. Role of primary intrahepatic T-cell activation in the ‘liver tolerance effect’. Immunol Cell Biol. 2002;80:84–92. doi: 10.1046/j.0818-9641.2001.01048.x. [DOI] [PubMed] [Google Scholar]