Abstract
Optical coherence tomography (OCT) is a three-dimensional optical imaging technique that has been successfully implemented in ophthalmology for imaging the human retina, and in studying animal models of disease. OCT can nondestructively visualize structural features in tissue at cellular-level resolution, and can exploit contrast agents to achieve molecular contrast. Photothermal OCT relies on the heat-producing capabilities of antibody-conjugated gold nanoparticles to achieve molecular contrast. A pump laser at the nanoparticle resonance wavelength is used to heat the nanoparticles in the sample, and the resulting changes in the index of refraction around the nanoparticles are detected by phase-sensitive OCT. Lock-in detection of the pump beam amplitude-modulated frequency and the detector frequency allow for high-sensitivity images of molecular targets. This approach is attractive for nondestructive three-dimensional molecular imaging deep (approximately 2 mm) within biological samples. The protocols described here achieve a sensitivity of 14 parts per million (weight/weight) nanoparticles in the sample, which is sufficient to differentiate EGFR (epidermal growth factor receptor)-overexpressing cells from minimally expressing cells in three-dimensional cell constructs.
Keywords: Nanospheres, Microscopy, Optical coherence tomography, Gold, Contrast media, Epidermal growth factor receptor, Fourier analysis, Equipment design, MDA-MB-435, MDA-MB-468
1 Introduction
Molecular imaging is a powerful tool for investigating biological signaling, disease processes, and potential therapies in both in vivo and in vitro systems. Microscopy, including confocal and multiphoton microscopy, has been the standard for high-resolution molecular imaging in live cells and tissues. However, these microscopy techniques suffer from relatively shallow imaging depths. MRI and PET have been the standard for functional imaging deep within the body, with the caveat of relatively poor resolution. Optical coherence tomography (OCT) fills a niche between high-resolution microscopy techniques and whole-body imaging techniques with relatively good resolution (~1–10 μm) and penetration depths (~1–2 mm) in tissue. Molecular imaging in this regime would be a powerful tool for scientists and clinicians. Two examples with potentially high impact are imaging the effects of antiangiogenic treatment in age-related macular degeneration, and whole-tumor imaging of molecular microenvironments.
OCT is intrinsically insensitive to incoherent scattering processes such as fluorescence and spontaneous Raman scattering, which are central to optical molecular imaging, because OCT depends on coherent detection of scattered light. Gold nanoparticles are attractive contrast agents for OCT because they are biocompatible, do not exhibit photobleaching or cytotoxicity, and are tunable through a broad range of wavelengths including the visible and near-infrared regions. Gold nanoparticles currently under development for molecular contrast OCT include highly scattering gold nanoshells [1], gold nanocages [2], and gold nanorods [3]. Gold nanoshells are also under development as photothermal contrast agents [4].
Photothermal imaging [5–7] provides one potential method for increasing the molecular contrast of OCT over a highly scattering background. In photothermal imaging, strong optical absorption of a small metal particle at its plasmon resonance results in a change in temperature around the particle (photothermal effect). This temperature change leads to a variation in the local index of refraction that can be optically detected with an amplitude-modulated heating beam that spatially overlaps with the focus position of the sample arm of an interferometer. Previous work has shown that photothermal interference contrast images of gold nanoparticles from a modified DIC microscope are insensitive to a highly scattering background [5]. We have applied this concept to OCT with the added benefits of depth resolution and increased imaging depth. Our photothermal OCT system has a measured sensitivity of 14 parts per million (ppm, weight/weight), and we have used this system to measure epidermal growth factor receptor (EGFR) expression from live monolayers of cells and in three-dimensional tissue constructs [8]. Protocols for nanoparticle conjugation, labeling of cell monolayers and three-dimensional cell constructs, dark-field microspectral imaging, and photothermal OCT imaging follow.
2 Materials
2.1 Cell Culture
Cells that overexpress EGFR (MDA-MB-468) [9] and cells that express low levels of EGFR (MDA-MB-435) [10] were obtained from the American Type Culture Collection (ATCC).
Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco/BRL, Bethesda, MD) supplemented with 10 % fetal bovine serum (FBS, HyClone, Ogden, UT).
Chambered coverglasses (Laboratory-Tek).
Low-gelling point agarose (Sigma-Aldrich Co.).
24-well inserts (6.5 mm diameter, Transwell, Fisher Scientific).
10 % dimethyl sulfoxide (DMSO).
2.2 Gold Nanosphere Antibody Conjugation
Anti-epidermal growth factor receptor (anti-EGFR) mAb (E2156, Sigma, ~1.5 mg/mL).
HEPES buffer.
Gold colloids of 60 nm diameter (Ted Pella, Inc.).
Polyethylene glycol (PEG) compound (Sigma P2263).
2.3 Imaging Instrumentation
-
Dark-field microspectroscopy system [11, 12].
Inverted microscope (Axiovert 200, Carl Zeiss, Inc.).
Color camera (CoolSnap cf, Photometrics).
Line-imaging spectrometer (SpectraPro 2150i, Action Research).
-
Photothermal optical coherence tomography (OCT) system (see Note 1) [8].
Diode pumped solid-state frequency doubled Nd:YAG laser as the heating laser (Coherent, Verdi; see Note 2).
Super luminescent diode light source (Superlum) centered at 840 nm with a full width at half-maximum bandwidth of 52 nm (resulting in 6 μm axial resolution).
30 mm focal length focusing lens (20 μm imaging spot size on the sample; see Note 3).
Two-dimensional scanning mirrors (galvos, Cambridge Technology).
Custom spectrometer with 1,024 pixel line-scan CCD camera (ATMEL, Aviiva).
Software to control lateral scanning, perform data acquisition, rescaling from wavelength to wavenumber, Fourier Transform, two-dimensional B-scan display, and data archiving in real time (Bioptigen Inc.).
Optical chopper to amplitude-modulate the heating laser (Thorlabs).
3 Methods
3.1 Gold Nanosphere Antibody Conjugation [11–14]
Dilute 1 mL of the 60 nm diameter gold colloid with 125 mL 20 μM HEPES buffer.
Dilute 30 μL anti-EGFR mAb in 20 mM HEPES buffer to prepare a 3 % (volume/volume) anti-EGFR solution.
Adjust the pH of the colloid and antibody preparations to 7.0 ± 0.2 by the addition of 100 nM K2CO3.
Mix the pH-adjusted colloid and antibody preparations and allow to conjugate at room temperature for 20 min on an oscillator operating at 190 cycles/min.
After verifying antibody attachment (see Note 4), add 200 μL of 1 % PEG compound to the remainder of the conjugated nanoparticle suspension and allow the solution to interact at room temperature for 10 min.
At the end of the interaction period, centrifuge the solution at 2245 × g until a pellet is formed (~10 min) (see Note 5).
Withdraw the supernatant and resuspend the nanoparticle pellet in 1 mL of phosphate buffered saline (see Note 6).
3.2 Cell Monolayer Experiments
Suspend 80,000 cells in 1 mL of media, plate onto 2.0 mL chambered cover glasses, and incubate for 12–16 h to allow for cell adhesion.
Exchange media with a mixture of 0.5 mL antibody-conjugated nanosphere suspension (2.35 × 1010 nanospheres/mL; see Note 7) and 0.5 mL fresh media.
Incubate at 37°°C for 20 min, remove the media, rinse cells twice with fresh media, and add fresh media once more for imaging immediately afterward (see Note 8).
Image labeled monolayers using dark-field microspectroscopy and photothermal OCT (see Note 9 and Fig. 1).
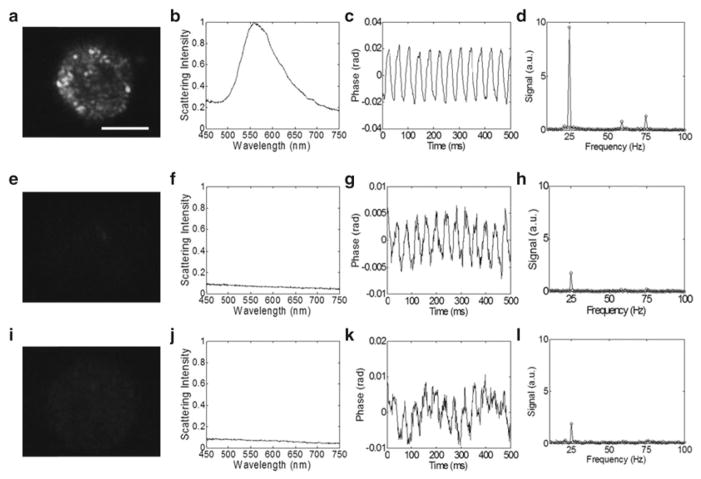
Fig. 1.
EGFR expression and nanoparticle labeling was con fi rmed in EGFR+/nanosphere+ cells (a–d), with two control groups that include EGFR-/nanosphere+ (e–h) and EGFR+/nanosphere- (i–l) using dark-field microscopy (panels a, e, i) and microspectroscopy (panels b, f, j). The phase as a function of time in the photothermal system is plotted for the experimental (panel c) and control groups (panels g, k), and the Fourier transform of the phase confirms oscillations at 25 Hz (the pump laser modulation frequency) (panels d, h, l). In three repeated experiments, the photothermal signal from overexpressing cell monolayers was at least 9 dB higher than the highest signal from the cells that express low levels of EGFR. The repeated experiments were performed at pump powers of 7.5–8.5 kW/cm2. Scale bar is 20 μm. Reproduced with permission from ref. [8]
3.3 Dark-Field Microspectroscopy
Acquire color images of cells to confirm spatial distribution nanoparticle labeling.
Acquire dark-field scattering spectra from the labeled cells to confirm plasmon resonance peak of the functionalized nanoparticles.
3.4 Photothermal OCT Imaging
Acquire multiple OCT depth scans (A-scans) at each position in the sample.
-
Calculate the photothermal signal at each point in the cross-sectional image.
Take the Fourier transform of the phase vs. time at each point in the image.
The photothermal signal is the peak at the pump laser frequency, minus the background (Fig. 2).
Fig. 2.

Phase of the tissue-like phantom (polystyrene spheres with μs = 100 cm−1) with 84 ppm nanospheres and 25 Hz pump frequency (a). The definition of the photothermal signal (b) in the Fourier-transformed phase is the peak at 25 Hz (arrow 1, the pump beam modulation frequency in these experiments) minus the background (arrow 2, 27–50 Hz). Reproduced with permission from ref. [8]
3.5 Three-Dimensional Tissue Culture
Mix 3 % low-gelling point agarose with a cell suspension in media (100×106 cells/mL) to obtain a homogeneous cell distribution.
Pour agarose/cell/media mixture into 24-well inserts at 2–3 mm thickness for gelation at room temperature (see Note 10).
After gelation, topically label cell constructs with antibody-conjugated nanospheres in the same manner as the monolayer experiment (see Subheading 3.1), except add 10 % DMSO to the antibody-conjugated nanosphere suspension to allow the solution to penetrate the three-dimensional construct, and incubate for 30 min [10].
Wash construct two times with saline.
Place a coverslip on the construct for photothermal OCT imaging.
Photothermal OCT imaging parameters included 1,000 sequential A-scans (1 ms integration time for each A-scan) at each of 110 lateral positions across the top of the construct (1 mm scan length in the lateral dimension). See Fig. 3.
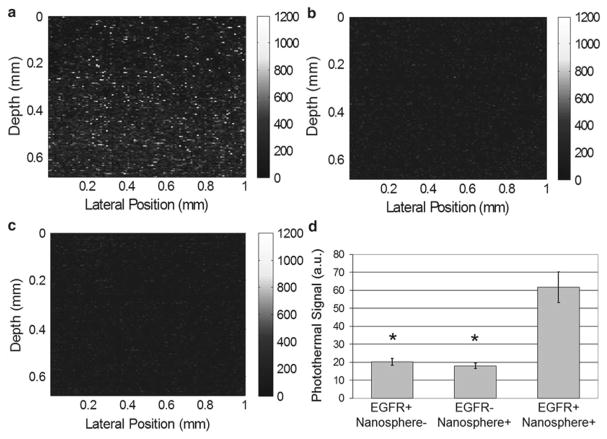
Fig. 3.
Images of EGFR expression in three-dimensional cell constructs containing EGFR+ cells (MDA-MB-468) with and without antibody-conjugated nanospheres (a and c, respectively) and EGFR- cells (MDA-MB-435) with antibody-conjugated nanospheres (b). There was a significant increase in the photothermal signal from EGFR-overexpressing cell constructs labeled with antibody-conjugated nanospheres (d) compared to the two controls (EGFR+/Nanosphere- and EGFR-/Nanosphere+). N = 17 images for each group, (*, p < 0.0001). Pump power 8.5 kW/cm2. Reproduced with permission from ref. [8]
Footnotes
Commercial spectral domain OCT systems can be purchased from a variety of sources (Thorlabs, Bioptigen, Inc., etc.), and may be used with the heating laser and chopper to perform photothermal OCT measurements.
The pump laser wavelength must overlap with the resonance peak of the nanoparticle of interest, and must provide sufficient power to produce a measurable photothermal signal. In these experiments, 20 mW of 532 nm light was incident on the sample.
The focused spot of the pump beam overlaps with the focused spot of the imaging beam (20 μm pump beam spot size on the sample).
Verify antibody attachment by removing 200 μL of the resulting conjugated colloid and mix with 10 μL of 10 % NaCl. It is well known that the addition of NaCl will cause nanoparticle aggregation [15], resulting in a color change to the solution, unless the nanoparticle surface has been well coated.
Centrifugation step can be repeated if necessary to form a pellet.
Resuspend in 0.5 mL of phosphate buffered saline twice to ensure complete removal of the excess PEG.
The antibody-conjugated nanosphere suspension of 2.35 × 1010 nanospheres/mL corresponds to 1.3 optical density in a 1 cm path-length cuvette.
For photothermal OCT imaging of cell monolayers, replace the media (which contains phenol red that could interfere with the photothermal experiments) with saline.
Confirm viability of cells after photothermal imaging using try-pan blue exclusion. Our previous experiments show no loss of cell viability due to photothermal imaging.
The viability of the cells in the three-dimensional construct can be verified by applying a live-dead fluorescence stain(Invitrogen) without nanosphere labeling. Confocal microscopy can be used to quantify the ratio of live (green fluorescence) to dead (red fluorescence) cells.
References
- 1.Agrawal A, Huang S, Wei Haw Lin A, et al. Quantitative evaluation of optical coherence tomography signal enhancement with gold nanoshells. J Biomed Opt. 2006;11:041121. doi: 10.1117/1.2339071. [DOI] [PubMed] [Google Scholar]
- 2.Cang H, Sun T, Li ZY, et al. Gold nanocages as contrast agents for spectroscopic optical coherence tomography. Opt Lett. 2005;30:3048–3050. doi: 10.1364/ol.30.003048. [DOI] [PubMed] [Google Scholar]
- 3.Oldenburg AL, Hansen MN, Zweifel DA, Wei A, Boppart SA. Plasmon-resonant gold nanorods as low backscattering albedo contrast agents for optical coherence tomography. Opt Express. 2006;14(15):6724–6738. doi: 10.1364/oe.14.006724. [DOI] [PubMed] [Google Scholar]
- 4.Adler DC, Huang S-W, Huber R, Fujimoto JG. Photothermal detection of gold nanoparticles using phase-sensitive optical coherence tomography. Opt Express. 2008;16:4376–4393. doi: 10.1364/oe.16.004376. [DOI] [PubMed] [Google Scholar]
- 5.Boyer D, Tamarat P, Maali A, Lounis B, Orrit M. Photothermal imaging of nanometer-sized metal particles among scatterers. Science. 2002;297:1160–1163. doi: 10.1126/science.1073765. [DOI] [PubMed] [Google Scholar]
- 6.Telenkov SA, Dave DP, Sethuraman S, Akkin T, Milner TE. Differential phase optical coherence probe for depth-resolved detection of photothermal response in tissue. Phys Med Biol. 2004;49:111–119. doi: 10.1088/0031-9155/49/1/008. [DOI] [PubMed] [Google Scholar]
- 7.Zharov VP, Galanzha EI, Tuchin VV. Integrated photothermal flow cytometry in vivo. J Biomed Opt. 2005;10:051502. doi: 10.1117/1.2070167. [DOI] [PubMed] [Google Scholar]
- 8.Skala MC, Crow MJ, Wax A, Izatt JA. Photothermal optical coherence tomography of epidermal growth factor receptor in live cells using immunotargeted gold nanospheres. Nano Lett. 2008;8:3461–3467. doi: 10.1021/nl802351p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey KE, Costantini DL, Cai Z, et al. Epidermal growth factor receptor inhibition modulates the nuclear localization and cytotoxicity of the Auger electron emitting radiopharmaceutical 111 In-DTPA human epidermal growth factor. J Nucl Med. 2007;48:1562–1570. doi: 10.2967/jnumed.107.044073. [DOI] [PubMed] [Google Scholar]
- 10.Aaron J, Nitin N, Travis K, et al. Plasmon resonance coupling of metal nanoparticles for molecular imaging of carcinogenesis in vivo. J Biomed Opt. 2007;12:034007. doi: 10.1117/1.2737351. [DOI] [PubMed] [Google Scholar]
- 11.Curry A, Hwang WL, Wax A. Epi-illumination through the microscope objective applied to darkfield imaging and microspectroscopy of nanoparticle interaction with cells in culture. Opt Express. 2006;14:6535–6542. doi: 10.1364/oe.14.006535. [DOI] [PubMed] [Google Scholar]
- 12.Curry AC, Crow M, Wax A. Molecular imaging of epidermal growth factor receptor in live cells with refractive index sensitivity using dark-field microspectroscopy and immunotargeted nanoparticles. J Biomed Opt. 2008;13:014022. doi: 10.1117/1.2837450. [DOI] [PubMed] [Google Scholar]
- 13.El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 14.Sokolov K, Follen M, Aaron J, et al. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63:1999–2004. [PubMed] [Google Scholar]
- 15.Sato K, Hosokawa K, Maeda M. Rapid aggregation of gold nanoparticles induced by non-cross-linking DNA hybridization. J Am Chem Soc. 2003;125:8102–8103. doi: 10.1021/ja034876s. [DOI] [PubMed] [Google Scholar]