Abstract
It has been proposed that the elkhorn coral, Acropora palmata, is genetically separated into two distinct provinces in the Caribbean, an Eastern and a Western population admixing in western Puerto Rico and around the Mona Passage. In this study, the genetic structure of A. palmata sampled at 11 Puerto Rican localities and localities from Curaçao, the Bahamas and Guadeloupe were examined. Analyses using five microsatellite markers showed that 75% of sampled colonies had unique genotypes, the rest being clone mates. Genetic diversity among genets was high (HE = 0.761) and consistent across localities (0.685 to 0.844). FST ranged from −0.011 to 0.047 supporting low but significant genetic differentiation between localities within the previously reported Eastern and Western genetic provinces. Plots of genetic per geographic distances and significant Mantel tests supported isolation-by-distance (IBD) within Puerto Rico. Analysis with the software Structure favored a scenario with weak differentiation between two populations, assigning eastern Puerto Rican locations (Fajardo and Culebra), Guadeloupe and Curaçao to the Caribbean Eastern population and western Puerto Rican locations (west of Vega Baja and Ponce), Mona and the Bahamas to the Caribbean Western population. Vieques and San Juan area harbored admixed profiles. Standardized FSTs per 1,000 km unit further supported higher differentiation between localities belonging to different Structure populations, with IBD being stronger within Puerto Rico than on larger regional scales. This stronger genetic transition seems to separate localities between putative Eastern and Western provinces in the eastern Puerto Rican region, not around the Mona Passage.
PROBLEM
Genetic diversity and structure in scleractinian corals vary significantly, reflecting the evolutionary differences between species, but also the type of genetic markers employed, microsatellite markers being more successful at detecting weak genetic structure than mitochondrial markers, ITS or allozymes (Palumbi 2003; Vollmer & Palumbi 2004; Van Oppen & Gates 2006). Interestingly, even related species with similar life-histories and dispersal potentials might exhibit different population structure (Severance & Karl 2006; Hemond & Vollmer 2010). With a few exceptions (Benzie et al. 1995; Ayre & Hughes 2000), panmixia is generally observed within small distances (10s of Km; Ng & Morton 2003; Magalon et al. 2005), where connectivity is assured over one-generation-spawning events (Palumbi 2003). In contrast, varying patterns of genetic structuring are generally the rule over larger geographic distances and are characterized by a combination of discrete populations with Isolation-By-Distance (IBD, MacKenzie et al. 2004; Maier et al. 2005). Studies in the Caribbean are less numerous than in the Indo-Pacific but have typically shown significant genetic structuring, perhaps as a result of limited gene flow (Vollmer & Palumbi 2007).
With over 100 species, Acropora is one of the most broadly distributed coral genera (Wallace 1999; Veron & Stafford-Smith 2000). Acropora species harbor diverse patterns of genetic structuring (e.g. Benzie et al. 1995; Ayre & Hughes 2000; MacKenzie et al. 2004; Baums et al. 2005b). Despite the extreme diversity of acroporids in the Indo-Pacific Ocean, there are only two species in the Caribbean, A. palmata and A. cervicornis (Van Oppen et al. 2000; Vollmer & Palumbi 2002). While molecular data of A. cervicornis across the Caribbean has supported significant genetic divergence between regions separated by several hundreds of kilometers or more (e.g. Florida vs. the Bahamas vs. Curaçao), genetic differentiation between reefs separated by a few kilometers is generally not significant, except when introgression of A. palmata alleles is observed (Vollmer & Palumbi 2007; Garcia Reyes & Schizas 2010; Hemond & Vollmer 2010). On the other hand, such short-scale structure was recently evidenced in A. cervicornis using spatial autocorrelation of nuclear and mtDNA data (Palumbi et al. 2012).
Microsatellites analysis of A. palmata sampled throughout the Caribbean in Structure, a software that has been widely used to find the number of biological populations in a given dataset (Evanno et al. 2005), suggested that the species comprised an Eastern and a Western population (Baums et al. 2005b, 2006a, 2006b). The population break in the southern Caribbean seemed to occur at the Guajira Peninsula, Colombia, while in the northern Caribbean, the break was located around Puerto Rico. Depending on the model used, western Puerto Rican localities either clustered with the western population or presented admixed genotypes reminiscent of a hybrid zone between populations (Baums et al. 2005b, 2006a), suggesting that the Mona channel might act as a natural filter in A. palmata, as was reported for several other marine species (Colin 2003; Dennis et al. 2005; Galindo et al. 2006; Taylor & Hellberg 2006; Andras et al. 2013).
Further genetic characterization of elkhorn corals around Puerto Rico is necessary because it is unclear where the two proposed populations stop or merge. Indeed, it is arguable to assume two well differentiated biological populations based on Structure results, since the existence of discrete populations is an implicit assumption of Structure, which makes its use inadequate to describe continuously distributed genetic differentiation such as in the case of isolation-by-distance (Pritchard et al. 2000). Furthermore, only a few Puerto Rican reefs have been studied and they only represented the west and south coasts of the island (Baums et al. 2005b, 2006b). Additionally, a detailed description of the genetic diversity and structure of A. palmata in Puerto Rican reefs might improve local management of the species, following the example of the Tres Palmas Marine Reserve, implemented in 2004 to protect elkhorn coral stands (Valdés-Pizzini et al. 2009). In order to obtain this much needed and improved understanding of genetic structure on the Puerto Rico Shelf, we sampled A. palmata around Puerto Rico by alternating small geographic distances between reefs (few kilometers) and moderate distances (tens of km) between neighboring reefs as recommended by Guillot et al. (2009). Samples from the Bahamas, Curaçao and Guadeloupe were also included to represent distant reefs (hundreds to thousands of km), representing both inferred populations of the Eastern and Western regions (Baums et al. 2005b, 2006b). We assessed clonality and genetic diversity within these reefs, and explored patterns of IBD versus patterns of population structuring resulting from the existence of discrete populations in the dataset.
MATERIAL AND METHODS
Sampling
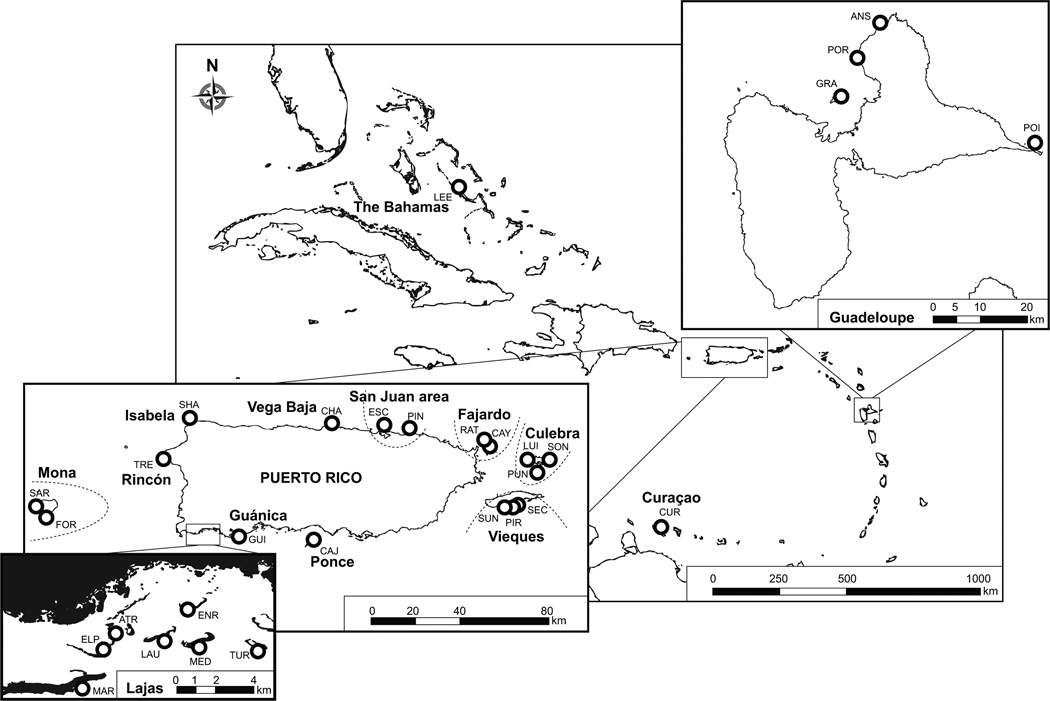
Twenty-four reefs were located in Puerto Rico (including Mona, Culebra and Vieques) (Figure 1, Table 1). Special effort was dedicated to (1) alternate small geographic distances (few kilometers) with moderate distances (tens of km) between reefs and (2) select reefs from areas all over the Puerto Rican archipelago in a comprehensive design. The other six reefs represented samples from the Eastern (Curaçao, Guadeloupe) and Western populations (the Bahamas). All samples were taken between 2006 and 2009 and were collected opportunistically (non-randomized pattern). Particular efforts were made to sample both potential clone colonies (various ramets of a same genet) and potentially different genotypes. Hence, for each reef we sampled tissue from colonies within a 5 meter radius (likely to be clones) as well as colonies separated by tens to hundreds of meters (unlikely to be clones). Whenever possible, 20–50 colonies per reef were collected, preferentially by snapping the tip of branches. Samples from 412 colonies were obtained, including 86 from Garcia Reyes & Schizas (2010).
Figure 1. Sampling locations of Acropora palmata.
30 reefs were sampled (24 reefs in Puerto Rico, including Mona, Vieques and Culebra, 4 reefs in Guadeloupe, 1 reef in Curaçao, and 1 reef in the Bahamas). Reefs are represented by codes of three-upper case letters, referring to the reef names in Table 1. The corresponding 14 localities grouping those reefs in the same table were also marked as full lower cape names on the map. Maps modified from maps generated on reefbase.org and Garcia Reyes & Schizas (2010).
Table 1.
Samples origin, number of sampled colonies and number of unique genotypes (genets) in this study.
| Island | Region | Locality | Reef | Abb. | Latitude (N) | Long. (W) | Genets | Colonies |
|---|---|---|---|---|---|---|---|---|
| Bahamas | Bahamas | Bahamas | Lee Stocking Island | LEE | 23°45'41" | 76°05'15" | 11 | 16 |
| Curaçao | Curaçao | Curaçao | Curaçao | CUR | 12°11'10" | 69°00'05" | 10 | 10 |
| Puerto Rico | North PR | San Juan area | Piñones | PIN | 18°27'44" | 65°59'47" | 6 | 6 |
| Escambrón | ESC | 18°28'05" | 66°05'29" | 11 | 25 | |||
| Vega Baja | Chalets | CHA | 18°29'27" | 66°24'52" | 14 | 37 | ||
| Culebra | Luis Peña | LUI | 18°19'05" | 65°19'31" | 1 | 1 | ||
| Soni beach | SON | 18°19'09" | 65°15'07" | 1 | 1 | |||
| Punta Soldado | PUN | 18°16'58" | 65°17'24" | 17 | 19 | |||
| Fajardo | Cayo Lobos | CAY | 18°22'32" | 65°34'03" | 7 | 11 | ||
| Ratón | RAT | 18°22'53" | 65°35'03" | 3 | 4 | |||
| NorthEast PR | Vieques | Pirates cove | PIR | 18°06'32" | 65°23'55" | 6 | 7 | |
| Secret beach | SEC | 18°06'31" | 65°23'59" | 9 | 9 | |||
| Sun beach | SUN | 18°05'20" | 65°27'55" | 15 | 15 | |||
| NorthWest PR | Isabela | Shack | SHA | 18°30'58" | 67°05'58" | 5 | 5 | |
| Rincón | Tres Palmas | TRE | 18°20'47" | 67°15'48" | 38 | 52 | ||
| SouthWest PR | Lajas | Atravesao | ATR | 17°56'38" | 67°05'12" | 3 | 3 | |
| Enrique | ENR | 17°57'11" | 67°02'48" | 1 | 2 | |||
| Laurel | LAU | 17°56'24" | 67°03'44" | 1 | 1 | |||
| Margarita | MAR | 17°55'04" | 67°06'24" | 8 | 13 | |||
| Media Luna | MED | 17°56'20" | 67°02'36" | 15 | 24 | |||
| Tumurote | TUR | 17°56'10" | 67°01'09" | 1 | 1 | |||
| El Palo | ELP | 17°55'53" | 67°05'38" | 1 | 1 | |||
| South PR | Guánica | Guilligan | GUI | 17°56'26" | 66°52'07" | 3 | 9 | |
| Ponce | Caja de Muerto | CAJ | 17°54'05" | 66°30'35" | 25 | 30 | ||
| Guadeloupe | Guadeloupe | Guadeloupe | Anse-Bertrand | ANS | 16°29'16" | 61°29'42" | 15 | 15 |
| Grand-Cul-de-Sac-Marin | GRA | 16°21'36" | 61°35'37" | 20 | 20 | |||
| Pointe-des-Châteaux | POI | 16°15'03" | 61°10'53" | 9 | 11 | |||
| Port-Louis | POR | 16°25'35" | 61°32'04" | 3 | 3 | |||
| Mona | Mona | Mona | Sardinera | SAR | 18°05'29" | 67°56'23" | 41 | 50 |
| Fortuna Reefer | FOR | 18°03'24" | 67°52'05" | 9 | 11 | |||
| TOTAL | 309 | 412 |
Abb.: three letters reef abbreviation; Long.: longitude.
Molecular techniques
From each sample, 5–10 polyps were cut-off and total genomic DNA was extracted with the DNeasy Blood & Tissue Kit (Qiagen) following the manufacturer’s animal tissue protocol. Each sample was then screened for 5 polymorphic microsatellite markers, following a modified protocol from Baums et al. (2005a). The selected markers, namely #166, #181, #182, #192 and #207, were the same as those used in Baums et al. (2005a; 2006a; 2006b). PCR amplifications were done in 10 µl reactions, containing 1 µl genomic DNA (5–15 ng ul−1), 0.8 mM dNTPs, 0.1 µM of forward primer with M13 tail, 0.1 µM of M13 fluorescently labeled with FAM (markers #166 and #182) or HEX (markers #181, #192 and #207), 0.2 µM of reverse primer, MgCl2 (2 mM), 0.3 µl of 1U µl−1 Taq DNA polymerase (Fermentas), and 1X of the PCR buffer. Temperature cycling was performed by denaturing 1 min at 94°C, followed by 20 cycles of 20 s at 94°C, 35 s at 56°C and 30 s at 72°C, 15 cycles of 20 s at 94°C, 35 s at 50°C and 30 s at 72°C and a 10 min extension step at 72°C. Amplicons were diluted up to 50X to approach 10–20 ng µl−1, pooled whenever possible (#166 with #207 and #182 with #192) and were run on an ABI3130xl Genetic Analyzer with ROX labeled size standards. Microsatellite alleles were scored using the software GeneMapper® 4.0.
Genetic diversity and structure
The Probability of Identity (PI) is the probability that two genetically different samples have identical multilocus genotypes given a set of genetic markers. Computation of PI was performed in Genalex 6.1 (Peakall & Smouse 2006). Identical multilocus genotypes were then considered ramets of the same genet (clones of a same genotype) with a confidence probability PI. All subsequent analyses were performed by reducing the dataset to the number of unique genets. Because ramets were represented by a single genet, the final dataset had no further information on genotype frequency. Genetic indices of diversity, tests of linkage disequilibrium (50,000 permutations), pair-wise FST between localities (50,000 permutations) and Hardy-Weinberg disequilibrium (HWE, 10,000 burn-in, 1,000,000 permutations) were estimated in Arlequin 3.5 (Excoffier & Lischer 2010) and p-values were adjusted to control for False Discovery Rate (FDR; Benjamini & Hochberg 1995) with the stats package in R (R Development Core Team 2010). FST was preferred to RST because it is a more suitable measure of genetic distance between populations when the number of markers <20 (Gaggiotti et al. 1999). The number of K populations was estimated in Structure 2.3.3 (Pritchard et al. 2000; Hubisz et al. 2009) and repeated ten times for each value of K, ranging from K=1 to K=5. Using the FullSearch algorithm in CLUMPP 1.1.2 we permuted the independent replicate runs (Jakobsson & Rosenberg 2007). The mean of the permuted matrices across replicates was visualized in Distruct 1.1 (Rosenberg 2004). To find the number of discrete populations in the dataset, we used Structure under three different models. The model ADM used only allelic information to find population structure. In contrast, the model POPINFO added a priori population assignments for some individuals to assign the remaining individuals to the a priori populations. Finally, the LOCPRIOR model enabled the incorporation of the sampling locations as a priori information. While these analyses were performed assuming 14 sampling localities (see Table 1), analyses were also repeated using the alternate “reef”, “region” and “island” grouping for the LOCPRIOR model. Structure analyses, including the different methods to select the number of populations K that best describe the dataset, are further detailed in the supporting information (Text S1 and accompanying Figure S1 and S2). A matrix of genetic distances FST/(1-FST) was generated and tested against the geographic distance matrix to explore IBD patterns. Reflecting the short pelagic life of their larvae, A. palmata populations seem to be largely self-recruiting, while connectivity across large distances seems to approximate a rather one-dimensional path following shallow water habitats (Baums et al. 2006b), in particular along the Lesser Antilles. Hence we generated a geographic distance matrix based on the shortest shallow nautical (SSN) distances in Google Earth 6.2.1.6014 at the 1,000,000:1 scale. Alternatively, another matrix based on the shortest-nautical (SN) distances was constructed to explore IBD patterns involving direct connectivity, in particular for distant reefs such as the Bahamas, Guadeloupe and Curaçao. Matrices were appended in Table S1. Significance of IBD patterns was tested with Mantel tests (30,000 permutations) while Reduced Major Axis (RMA) regressions were used to estimate the strength of genetic differentiation (RMA slope, jackknife 95% CI). Because the data is only known with error, RMA was favored over Ordinary Least Square regression (Hellberg 1994, Jensen et al. 2005). Mantel tests and RMA calculations were performed in IBDWS (Jensen et al. 2005) by comparing the matrix of pair-wise Slatkin’s FST (FST/1-FST) with the geographic distance matrices after setting genetic distances <0 to 0. A log10 transformation was applied to the SN matrix to account for bi-dimensionality (Slatkin 1993, Rousset 1997). On the other hand, connectivity between distant reefs in the SSN matrix was rather one-dimensional since it followed shallow water habitats (from one island to the next), thus no logarithmic transformation was applied to the SSN matrix (Rousset 1997). Comparisons were first made with all studied localities, but because some populations had fewer individuals (n<15), part of the observed patterns was not highly supported (Cornuet et al. 1999). As a balance between improved statistical confidence and data loss, the results were compared anew by including only localities with a certain minimum of different genotypes (n=15, n=20 and n=30). When the data could not be shaped into a usable matrix for IBDWS, RMA for Java (Bohonak et al. 2004) was used instead (one-delete jackknife with 30,000 bootstraps for 95% CI), as was the case for RMA calculations excluding within-Puerto Rico pair-wise comparisons or to obtain the regression slopes between a specific location (Mona or Culebra) and mainland Puerto Rico.
RESULTS
Clonality and genetic diversity
Based on our dataset, the probability for two genetically different samples to have identical multilocus genotypes by chance (PI) using the five microsatellite markers was ~1.48 × 10−7, which closely matched the ~1.5 × 10−7 estimate in Baums et al. (2005b, 2006a). Therefore, it was a reasonable assumption that identical genotypes represented biological clones. We identified 309 unique microsatellite multilocus genotypes (genets; 75%) and an additional 103 clones (25.0%) from a total of 412 colonies (Table 1). In order to avoid artificial, misleading signals resulting from the presence of those clones in our dataset, the remaining analyses, including estimates of genetic diversity and population structure, were based on the 309 unique genets identified in this study.
Mean genetic diversity across localities and loci was high (HE = 0.761) and consistent across localities (0.685 to 0.844). Mean allelic diversity per locality across loci was 9.7 (Table 2). Allelic diversity ranged from 8 to 23, from the least (#181) to the most polymorphic locus (#166). Mean allelic range per location across loci was 11.3, ranging from 13 to 24 depending on the marker (#181 and #166, respectively). After correcting for multiple comparisons with the FDR, no test of pair-wise linkage disequilibrium was significant (140 pair-wise tests comparing all loci at each of the 14 localities). Therefore, the five loci were assumed to be unlinked. FDR corrected p-values for tests of HWE at each locus were not significant (70 tests, 5 loci for 14 localities), indicating that the sampled populations were at equilibrium.
Table 2.
Summary of genetic diversity indices among the 14 localities.
| Locality | Ng | Ng/N | A | HO | HE | R |
|---|---|---|---|---|---|---|
| Bahamas | 11 | 0.69 | 9.2 | 0.873 | 0.844 | 11.6 |
| Curaçao | 10 | 1.00 | 7.4 | 0.695 | 0.741 | 8.8 |
| San Juan area | 17 | 0.55 | 9.2 | 0.694 | 0.746 | 11.2 |
| Vega Baja | 14 | 0.38 | 9.6 | 0.771 | 0.795 | 12.0 |
| Culebra | 19 | 0.90 | 9.4 | 0.768 | 0.740 | 10.6 |
| Fajardo | 10 | 0.67 | 7.2 | 0.700 | 0.685 | 10.8 |
| Vieques | 30 | 0.97 | 12.2 | 0.740 | 0.754 | 13.2 |
| Isabela | 5 | 1.00 | 5.8 | 0.790 | 0.777 | 7.6 |
| Rincón | 38 | 0.73 | 12.2 | 0.754 | 0.766 | 13.2 |
| Lajas | 30 | 0.67 | 11.8 | 0.780 | 0.764 | 13.0 |
| Guánica | 3 | 0.33 | 4.2 | 0.667 | 0.760 | 5.4 |
| Ponce | 25 | 0.83 | 12.0 | 0.704 | 0.764 | 13.0 |
| Guadeloupe | 47 | 0.96 | 12.2 | 0.706 | 0.744 | 12.2 |
| Mona | 50 | 0.82 | 13.8 | 0.756 | 0.780 | 16.0 |
| TOTAL | 309 | |||||
| MEAN | 0.75 | 9.7 | 0.743 | 0.761 | 11.3 |
Ng: number of genets. Ng/N: ratio of the number of genets by the number of colonies. Allelic diversity (A), observed heterozygosity (HO), expected heterozygosity (HE) and allelic range (R) were averaged across loci.
Number of populations with Structure
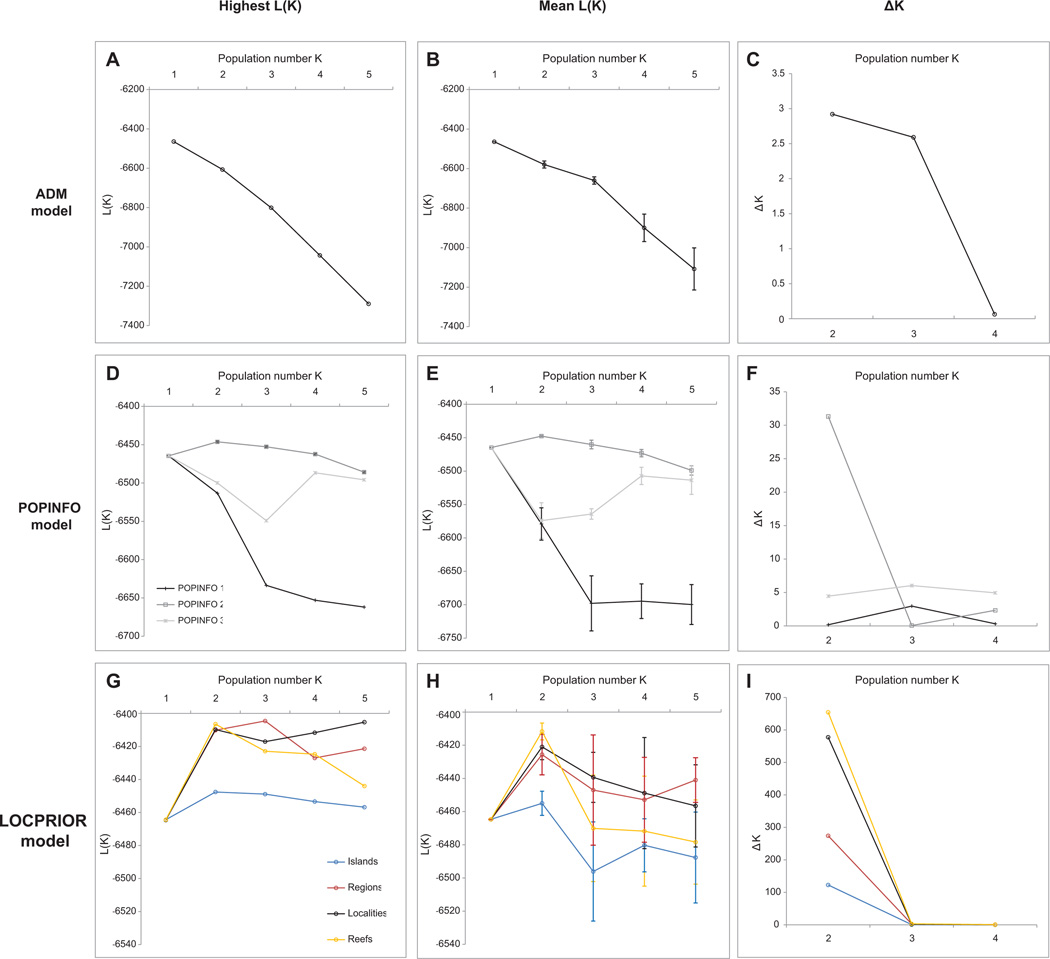
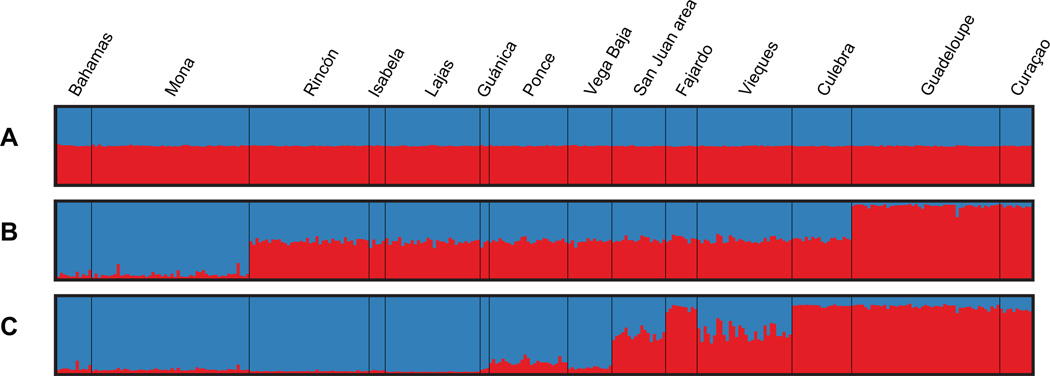
Simulations performed under the ADM model (without a priori information on sampling locations) had a higher probability L(K) for K = 1 (Figure 2A, B). Furthermore, the summary statistics α and F did not stabilize, indicating that Structure did not detect population structure with this algorithm (Pritchard et al. 2000) as visually confirmed in Figure 3A. This contrasted with the findings of Baums et al. (2005b) who found an optimal number of population K = 2 using the same algorithm. In the POPINFO model, which assigns some genotypes to user-specified a priori populations, the results were similar (Figure 3B and S2D, E). Again, the summary statistics did not stabilize, indicating that Structure could not find a likely assignment for the given genotypes. Under the LOCPRIOR model which uses sampling locations as a priori information, the optimum burn-in period was found to be relatively long, with r (the contribution of the predefined sampling locations to the end probabilities assigned to the individuals), α and F generally stabilizing after 5 to 10 million iterations. These control steps were necessary to have confidence in the resulting probability for K (Hubisz et al. 2009). Also, the variance of L(K) within and between runs was relatively high when K > 2, requiring a high number of iterations and the use of replicate runs. Based on the arithmetic mean of L(K) across replicate runs, the optimal number of K found by Structure in all models was K = 2 (Figure 2H). This was also true for other recommended methods, such as using only the best L(K) among replicate runs (Figure 2G) or the ΔK parameter described in Evanno et al. (2005) (Figure 2I). When performing alternate LOCPRIOR analyses, i.e. by assigning individuals to a priori locations corresponding to “reefs”, “regions” or “islands” (Figure 2G, H) the original “localities” model was generally found to reach superior probabilities across the range of tested K (1 to 5), although at K = 2, the “reef” model had a slightly higher L(K) than the “localities” model (mean L(K)reefs = −6,412 and mean L(K)localities = −6,421, respectively). Following the population model at K = 2, the Bahamas, Mona, Rincón, Lajas, Guánica, Ponce, Isabela and Vega Baja were clustered into one population. Curaçao, Guadeloupe, Culebra, Fajardo clustered into another population. Finally, Vieques and the San Juan area had admixed origin of populations (Figure 3C).
Figure 2. Results of Structure for different parameter sets.
From top to bottom, the first row contains the results of the ADM model (A, B, C), the second row contains the results of the three POPINFO models (D, E, F), the third row (G, H, I) contains the results of the LOCPRIOR model for the four grouping variants proposed to explore the dataset at different scales. A, D and G plot the highest L(K) out of ten runs for each K assumed a priori. B, E and H plot the mean L(K) and standard deviation (vertical whiskers) over the ten runs for each K. C, F, and I plot ΔK, following Evanno et al.(2005).
Figure 3. Structure results for K = 2 with the three parameter sets defined in this study.
All parameter sets used the admixture algorithm and performed 5,000,000 burn-in followed by 15,000,000 iterations per run. Each figure plots the mean assignments of each individual genotype to K a priori populations (here K=2) among 10 replicate runs. A. ADM parameter set: admixture without a priori information. B. POPINFO 2 parameter set: admixture model with the Bahamas and Mona a priori assigned in a first population (Western population, blue), and Guadeloupe and Curaçao a priori assigned in a second population (Eastern population, red). C. LOCPRIOR parameter set run with a priori information for the 14 localities.
Genetic structure and isolation-by-distance
The genetic distances between localities were small (FST ranging from −0.011 to 0.047) but often significant, even after correcting for multiple comparisons (Table 3). Conversely, they were not significant for those locations with the lowest numbers of genets, such as Guánica or Isabela (n=3 and n=5, respectively). In general, genetic distances were higher between the most distant localities, in conformity with a model of IBD. In the West, pair-wise FSTs indicated that despite a relatively low number of genotypes (n=11) the Bahamas showed significant divergence with all localities, except Mona, Rincón and Vega Baja. Interestingly, Mona showed no significant difference with the closest localities in eastern Puerto Rico (e.g. Rincón, Lajas). In the East, Culebra (n=19), Guadeloupe (n=47) and Curaçao (n=10) had non-significant FSTs among them. On the other hand, these locations exhibited significant pair-wise differences against most other localities.
Table 3.
Pair-wise genetic distances and pair-wise genetic distances per 1,000 km unit between localities.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bahamas | - | 0.016 | 0.016* | 0.001 | 0.017* | 0.005 | 0.026* | 0.015 | 0.029* | 0.023* | 0.036* | 0.029* | 0.024* | 0.011 |
| 2 | Mona | 0.017 | - | 0.070 | −0.208 | 0.049 | −0.084 | 0.117* | 0.006 | 0.064 | 0.062* | 0.054 | 0.071* | 0.025* | 0.011* |
| 3 | Rincón | 0.018* | 0.005 | - | −0.899 | −0.071 | −0.368 | 0.042 | −0.050 | 0.067 | 0.035 | 0.032 | 0.120* | 0.018* | 0.013* |
| 4 | Isabela | 0.001 | −0.020 | −0.025 | - | −0.201 | −0.238 | −0.061 | −0.401 | −0.179 | 0.040 | 0.056 | 0.090 | 0.000 | 0.015 |
| 5 | Lajas | 0.021* | 0.004 | −0.004 | −0.017 | - | −1.914 | 0.055 | −0.013 | 0.037 | 0.018 | 0.034 | 0.151* | 0.024* | 0.015* |
| 6 | Guánica | 0.006 | −0.009 | −0.030 | −0.026 | −0.031 | - | −1.018 | −0.187 | −0.107 | −0.066 | −0.056 | 0.097 | 0.007 | 0.005 |
| 7 | Ponce | 0.034* | 0.017* | 0.005 | −0.009 | 0.003 | −0.039 | - | −0.038 | 0.000 | 0.062 | 0.082 | 0.145* | 0.021* | 0.013 |
| 8 | V. Baja | 0.019 | 0.001 | −0.005 | −0.029 | −0.002 | −0.034 | −0.008 | - | −0.205 | 0.038 | 0.102 | 0.154 | 0.014 | 0.009 |
| 9 | S. J. area | 0.037* | 0.013 | 0.009 | −0.019 | 0.007 | −0.022 | 0 | −0.007 | - | 0.093 | 0.023 | 0.206 | 0.011 | 0.004 |
| 10 | Vieques | 0.031* | 0.016* | 0.008 | 0.008 | 0.003 | −0.010 | 0.007 | 0.005 | 0.008 | - | −0.255 | 0.224 | 0.020 | 0.005 |
| 11 | Fajardo | 0.047* | 0.014 | 0.006 | 0.009 | 0.006 | −0.009 | 0.01 | 0.009 | 0.001 | −0.011 | - | −0.303 | −0.012 | −0.004 |
| 12 | Culebra | 0.039* | 0.020* | 0.026* | 0.017 | 0.029* | 0.017 | 0.020* | 0.018 | 0.015 | 0.007 | −0.008 | - | 0.022 | −0.003 |
| 13 | Guadeloupe | 0.043* | 0.018* | 0.012* | 0.000 | 0.015* | 0.004 | 0.012* | 0.008 | 0.006 | 0.009 | −0.006 | 0.01 | - | 0.004 |
| 14 | Curaçao | 0.034 | 0.022* | 0.025* | 0.029 | 0.028* | 0.010 | 0.024 | 0.016 | 0.008 | 0.008 | −0.007 | −0.006 | 0.005 | - |
Below diagonal: FST. Above diagonal: standardized FST per 1,000 km (SSN distances). FST per 1,000 km > 0.05 and are printed in bold to indicate areas of higher genetic differentiation.
False Discovery Rate corrected p-value < 0.05 for multiple comparisons.
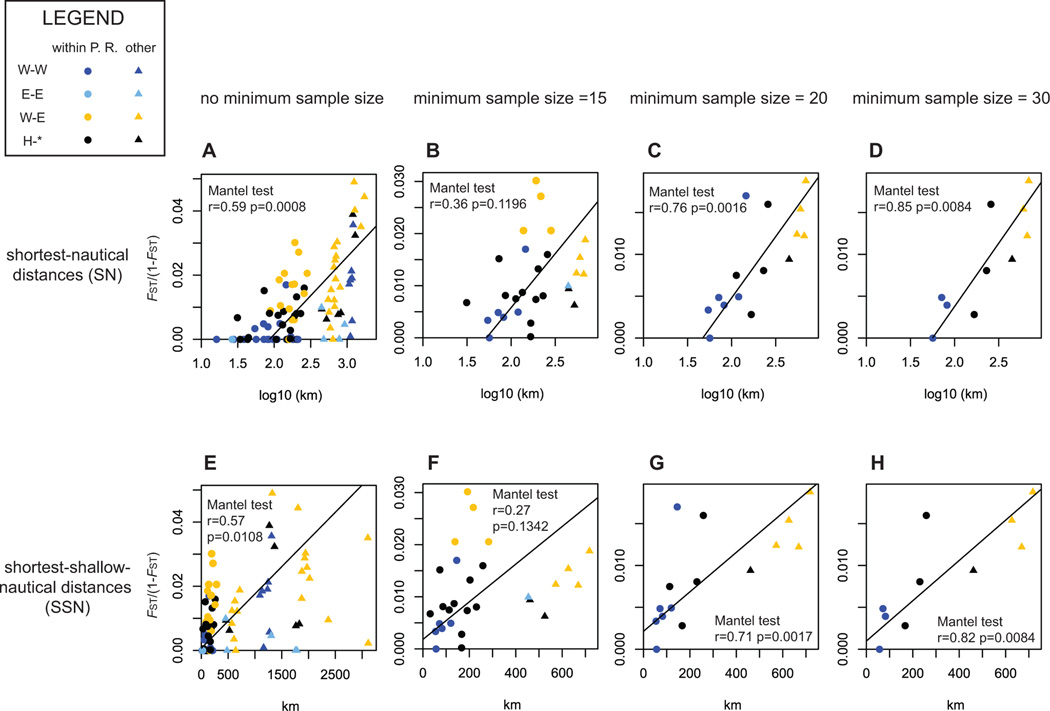
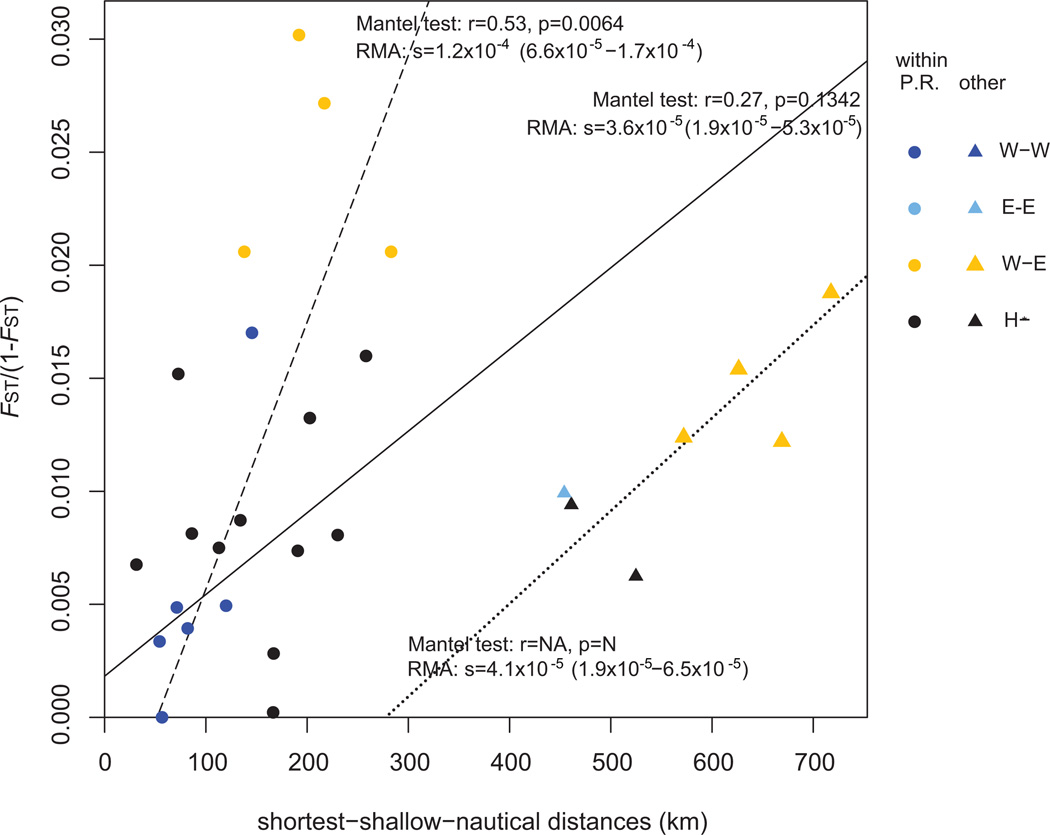
IBD plots showed consistent patterns of genetic differentiation via IBD, regardless of the type of geographic distance matrix or of the minimum sample size for each population (Figure 4). Mantel tests were high and significant (p<0.05) except when minimum sample size per population was set to 15 different genotypes. In such cases, removing the pair-wise comparisons involving localities outside Puerto Rico effectively resulted in significant Mantel test results within Puerto Rico (Figure 5, dash line r=0.53, p=0.0064), suggesting different patterns and strengths of differentiation within and outside Puerto Rico. This was supported by marginally non-overlapping confidence intervals ranges (95% CI) between the slopes of their respective RMA regression, respectively 6.6×10−5 – 1.7×10−4 for within Puerto Rico pair-wise comparisons and 1.9×10−5 – 6.5×10−5 for the remaining pair-wise comparisons (Figure 5). However, there was no clear, separate clustering pattern characteristic of discrete populations (Figure 4 and 5). Ideally, population structure in discrete populations would show separate clustering of within-population genetic distances from clustering of between-population genetic distances. However, an intermediate clustering pattern can be observed: for near-identical geographic distances, pair-wise comparisons between a priori Western and Eastern populations in Figures 4 and 5 (orange symbols) were characterized by higher genetic distances than pair-wise comparisons within each of these groups (light blue and blue symbols, respectively). Although stronger statistical support could be achieved by increasing the low number of genets in the Bahamas and Curaçao (respectively n=11 and n=10), pair-wise FSTs per 1,000 km unit were higher within Puerto Rico than when locations thousands of kilometers apart were compared, supporting the presence of a zone of further differentiation in Puerto Rico (Table 3). In this regard, Culebra was characterized by the highest FST per 1,000 km. In fact, the RMA regression slope of pair-wise comparisons between Culebra and mainland Puerto Rico (s=1.2×10−4, SE=2.5×10−5) was superior to the slope for Mona-mainland Puerto Rico (s=7.0×10−5, SE=2.7×10−5), although their respective 95% CI overlapped (8.3×10−5 – 1.5×10−4 and −4.6×10−5 – 1.9×10−4, respectively).
Figure 4. Pair-wise genetic distances in function of pair-wise geographical distances.
The first, second, third and fourth columns (left- right) show the plots of genetic versus geographic distances when the analysis include all localities (n=14), only localities with >=15 genotypes (n=8), >=20 genotypes (n=6) and >=30 genotypes (n=5). The top (A to D) and bottom rows (E to H) respectively show the plots for shortest-nautical (SN) and shortest-shallow-nautical (SSN) geographic distance. SN values were log10 transformed due to the bi-dimensionality of the model assumed. In all cases, FST(1-FST) between pair-wise served as a genetic distances. For each plot, RMA regressions were drawn with black lines. Results of the Mantel tests realized in each case were directly appended on the graphs. W-W: comparisons between two localities from the Western region. E-E: comparisons between two localities from the Eastern region. W-E: comparisons between one Western locality and one Eastern locality. H-*: comparisons between one hybrid locality and any other locality. The region of origin of each locality was determined using Structure results (Figure 3).
Figure 5. Pair-wise genetic distances in function of pair-wise shortest-shallow-nautical distances.
The following population assignments were based on Structure results using the LOCPRIOR option and K = 2. E-E: Pair-wise comparisons within the Eastern population. W-W: Pair-wise comparisons within the Western population. W-E: Pair-wise comparisons between Western and Eastern populations. H-*: Pair-wise comparisons involving admixed localities (San Juan area and/or Vieques). RMA regression for all points is represented by the full line. Two additional regressions were also performed to test the difference in IBD strength within Puerto Rico (circles, dash line) or with alternate locations (involving Curaçao, Bahamas and/or Guadeloupe; triangles, dot line). For each group, Mantel test and RMA regression results between FST/(1-FST) and geographic distances are appended next to the corresponding line whenever possible.
DISCUSSION
Sampling strategy and clone proportions
Clone distribution in the three-dimensional reef space depends on a variety of factors (Coffroth & Lasker 1998). For example, the genetic disposition of individual genets is likely to be important for the successful settlement of new ramets. Environmental factors such as hurricane disturbance, reef orientation and inclination, current dynamics and competition for space with other reef organisms will be responsible for part of the observed clone distribution, frequency and density (Highsmith et al. 1980). Because we wished to avoid overrepresentation of clones for the benefit of genetic structure analyses, we favored a non-random, opportunistic sampling strategy. Hence, population dynamics implications based on the frequency and density of ramets in this study should be interpreted with caution. We found that unique genets represented 75% of the samples (Ng/N = 0.75, mean Ng/N per locality = 0.75). In contrast, Baums et al. (2006a) used randomized circle plots and opportunistic sampling and found that both sampling strategies yielded similar results (mean Ng/N per reef = 0.52 and 0.51 respectively). Since genotypes were scored with the same markers in both studies, differences at the molecular level are unlikely to explain the difference in the proportion of clones. Because clonality varies grandly among reefs, the choice of sampling localities might explain some amount of discordance. The rest of the differences can probably be explained by a sampler effect (personal preferences for certain coral colonies during sampling) and/or other uncontrolled factors, e.g. the depth of sampling. A lower value of unique haplotypes (42%) was estimated from reefs of west and southwest Puerto Rico, where 46 unique mitochondrial haplotypes were detected from 110 distinct colonies (Garcia Reyes & Schizas 2010). The difference with the current results is not surprising, because microsatellites are usually more variable than mitochondrial markers, detecting more unique genets (Baums et al. 2005a).
The frequency of clones in each locality was unrelated to a purely geographic division between Western and Eastern provinces. Rather, difference in clonal structure is more likely explained by differences in environmental conditions such the size and depth of the shelf area (Baums et al. 2006a). The areas with the most clones were found in shallow, extensive shelves, such as Vega Baja and Escambrón. The back reefs of these locations varied in depth between <1 to two meters, providing asexual recruits with space to settle and shelter from waves. Tres Palmas was also well represented by clonal genets, although its shallow shelf is short and rapidly reaches unsuitable depths for dense A. palmata stands. On the contrary, reefs with no or few clones were often representative of areas with less suitable habitat. For example, the Anse-Bertrand reef is positioned against a small cliff, and elkhorn coral colonies were found at depth where they were growing in an encrusting fashion, limiting their ability to produce asexual recruits via fragmentation. Reefs at Piñones and Sun beach had simply no space for settlement of asexual recruits. Reefs at Shack, Pointe-des-Châteaux and Grand-Cul-de-Sac-Marin were positioned in shallow, extensive shelves, but numerous dead stands of A. palmata separated the sampled colonies, most likely the ghosts of disease or bleaching past. In the event of disease outbreaks, sensitive genets will disappear first (Reusch et al. 2005). Hence, because of their low genetic diversity, highly clonal reefs are more likely to lose extensive coral cover than heterogeneous reefs.
High genetic diversity
An important step in the genetic diversity and structure analyses is that the frequency of each genotype was removed from the dataset prior to analyses. This precaution avoided unreasonable assumptions for the estimation of gene flow and genetic structure (Baums et al. 2005b). In A. palmata, asexual reproduction happens by fragmentation, when broken branches rise into new, identical clones near the original colony (Bruckner 2002; Reusch et al. 2005). Thus, the genetic fingerprint of clones within a reef will mostly be the result of environmental hazards, not of sexual reproduction and gene flow. On the other hand, the probability of inbreeding and gene flow depends on the effective population size, and genets having many ramets will contribute more to sexual reproduction (Coffroth & Lasker 1998). Hence, some amount of information is inevitably lost in the analysis. The genetic diversity found in this study (HE = 0.761) was higher than the genetic diversity in Acropora nasuta (MacKenzie et al. 2004) or A. cytherea (Concepcion et al. 2009) but surprisingly lower than for A. muricata and A. digitifera using the same microsatellite markers used in this study (Tang et al. 2010). On the other hand, our results were consistent with those of Baums et al. (2005b) for A. palmata. Interestingly, genetic diversity was also consistent across all localities in our study and was higher than the genetic diversity found in a study of 14 rare and common species of Indo-Pacific Acropora using nine neutral microsatellite markers (including the five markers used in the present study; Richards et al. 2012) With respect to conservation efforts, these results suggest that there is high genetic diversity in A. palmata despite the dramatic losses during the last decades, in particular to the White Band disease (Bruckner 2002). The high genetic diversity estimated with microsatellites, however, is not depicted in the mitochondrial nucleotide diversity (π = 0.00075) of A. palmata in Puerto Rico, a value amongst the lowest reported values for scleractinian corals (Garcia Reyes & Schizas 2010). Part of the discrepancy can be explained by the low levels of genetic variability observed in mitochondrial DNA of corals compared to nuclear genes (Hellberg 2006).
Clustering of Western and Eastern populations in Puerto Rico
In the study of Baums et al. (2005b), two populations, roughly divided east and west of Puerto Rico, were detected using Structure. Sampling around the Mona Passage, the proposed region of population admixture, was limited to 36 unique genets from Mona and 90 unique genets along the west and southwest coast of Puerto Rico (Rincón, Lajas, Bajo Gallardo). In order to improve our understanding of population differentiation in the region, further sampling efforts in the south of Puerto Rico and in the Dominican Republic were conducted in Baums et al. (2006b). In their study, western and southwestern Puerto Rican reefs were pre-assigned to the Eastern population via the POPINFO option in Structure, in disagreement with the initial Structure results of Baums et al. (2005b), where the same Puerto Rican reefs clustered with the Western population when no a priori assignments were made. The added samples from the Dominican Republic clustered with the Western population (Baums et al. 2006b), but the a priori assignment of western and southwestern Puerto Rican samples to the Eastern cluster did little to improve our understanding of population differentiation around Puerto Rico. By including new samples and locations around the proposed region of population admixture (Baums et al 2005b), in particular where sampling was missing (northern and eastern Puerto Rico), the present study further details the population structure of elkhorn coral in this region of particular interest.
In the present study, in accord with high genetic diversity and low estimates of population structure (FSTs, AMOVA analyses presented in supporting information in Text S2 and accompanying Table S2), genetic differentiation with Structure was detected but weak: the basic admixture model (ADM) in Structure was not able to detect population structure in the data, while a high number of iterations was needed to reach statistical stability and find population structure when using prior information on locations via the LOCPRIOR model, recommended in case of weak population structure (Hubisz et al. 2009). In contrast, Baums et al. (2005b) were able to find population structure separating a Western from an Eastern population using the same markers and admixture model without a priori information, thus proposing that two populations of A. palmata meet at Puerto Rico. Differences between the two studies might be due to the more extensive geographic coverage and larger number of unique genotypes in Baums et al. (2005b) than in this study (n = 709 and n = 309, respectively) or user-related differences in the assessment of marker states.
Baums et al. (2005b) performed additional runs in Structure, using the built-in POPINFO option to define a priori assignments of non-Puerto Rican localities to their respective Eastern or Western cluster, thus allowing the software to estimate population admixture of the unassigned Puerto Rican genotypes. The resulting assignments of evenly admixed Puerto Rican colonies were then interpreted as hybrid genotypes between the Eastern and the Western populations. Similarly, we also used the POPINFO option to define a priori assignments of selected locations. Various configurations combining Western and Eastern a priori assignments invariably resulted in (1) the lack of stabilization of control parameters during the runs and (2) similar patterns of totally admixed genotypes, in equal proportions between K populations, for all individuals that were not assigned to a priori cluster. This pattern suggests that those admixed assignments resulted from a lack of discriminative power of the algorithm, and represented undecided assignments rather than perfectly admixed genotypes (with 1:1 proportions of each population of origin in the case of K=2) (Pritchard et al. 2000).
Using the LOCPRIOR option in Structure, which was not available when Baums et al. (2005b, 2006b) were published, we further detailed the population structure of A. palmata in Puerto Rico. Our results agree with Baums et al. (2005b), with K = 2 having the highest probability to explain the data, even when reefs outside Puerto Rico were not included. In Puerto Rico, the Western population would include Mona Island and the west coast of Puerto Rico, as was described in Baums et al. (2005b). The Western population most likely extends (1) northwest and north of Puerto Rico reaching past Vega Baja; (2) southwest and south past Lajas and Guánica, reaching past Ponce. In contrast, the Eastern population probably includes Culebra and reaches past Fajardo. A transitional area occurs somewhere between Vega Baja and Fajardo in the North, explaining the admixed profiles of the San Juan area. In the south/southeast of the island, the transitional area is located between Ponce, Fajardo and Vieques. Keeping in mind that the low number of genets for some of these localities might affect our results (Cornuet et al. 1999), we also find significant FST and AMOVA results supporting the genetic structure evidenced by our Structure results.
Patterns of IBD vs. discrete populations
Structure explicitly allocates individuals into an a priori number of groups that are discrete and whose members minimally violate the assumptions of HWE (Pritchard et al. 2000). In instances where genetic differentiation is correlated with geographic distance, individuals separated by sufficiently large geographic distances may violate the assumptions of a population at HWE when placed into one group, and therefore two or more groups may better explain the clustering of these individuals. This, however, does not mean that these groups are discrete, and that individuals with intermediate genotypes represent admixed individuals between two discrete populations, but rather indicates that the program is forcing continuous variation into discrete and discontinuous clusters. Because Bayesian clustering programs such as Structure can overestimate the number of clusters in datasets characterized by IBD, IBD should also be tested and interpretations based on the results of all analyses (Frantz et al. 2009).
Structure analyses indicate two clusters with genetically intermediate individuals and localities occurring in western Puerto Rico (Figure 3). This result is broadly comparable to that of Baums et al. (2005b, 2006a). However, our interpretation, based mainly on the addition of localities and samples in the proposed area of admixture, differs slightly. Analyses of pair-wise FSTs and of correlations between genetic and geographic distances suggested a pattern of IBD, which normally characterizes populations with limited connectivity, such as in a stepping stone model (Hellberg 2007). This is consistent with what is now known of limited larvae dispersal in corals and other marine species (Palumbi 2003; Cowen et al. 2006; Galindo et al. 2006; Hellberg 2007; Andras et al. 2013). Furthermore, the choice of a different geographic distance matrix or limiting analyses to localities with a certain minimum sample size (no minimum, 15, 20 or 30 genotypes) did not affect the interpretation of genetic differentiation by IBD. Our data suggests that the strength of IBD measured as the slope of the correlation between genetic and geographic distances differs along the studied seascape inhabited by A. palmata. The slope was steeper in comparisons involving the small geographic distances around Puerto Rico than Caribbean wide comparisons, suggesting that IBD is much stronger within the region of Puerto Rico than outside this region. This could reflect the stronger genetic changes associating with the hybridization of the Western and Eastern populations in the Puerto Rican region (Baums et al. 2005b). While Structure consistently placed the Bahamas within the putative Western population along several Puerto Rican localities, the Bahamas were found to be significantly differentiated from almost all other locations in this study, a result reminiscent of the genetic separation of the Bahamas from other Caribbean locations in A. cervicornis (Galindo et al. 2006; Vollmer & Palumbi 2007; Garcia Reyes & Schizas 2010; Hemond & Vollmer 2010) or Orbicella (previously Montastraea) annularis (Foster et al. 2012). East of Puerto Rico (Culebra, Guadeloupe and Curaçao), there was little genetic divergence between localities, suggesting near-panmixia within the eastern region (Table 3), a result largely consistent with the Eastern cluster described by Baums et al. (2005b) but also with the strong separation of Eastern locations from the West in both experimental data and dispersal models in O. annularis (Foster et al. 2012).
Although our findings within Puerto Rico were well supported, further studies should be conducted to confirm our observations regarding non-Puerto Rican localities because of the low number of sites (n=3) and genets per site (n=11 in the Bahamas and n=10 in Curaçao) analyzed outside of Puerto Rico in this study.
Re-assessing population structure in the elkhorn coral
Genetic structure in this study was characterized by a non-uniform IBD across the seascape inhabited by A. palmata, suggesting that connectivity, at least among some A. palmata reefs, is limited. IBD seemed to be stronger among localities from Puerto Rico, in particular in eastern Puerto Rico. IBD was weaker to insignificant outside of the Puerto Rico Shelf, as suggested by mostly non-significant genetic structuring within the Eastern population. When the distribution of the genetic diversity is interpreted in the framework of a discrete population structure as implemented in Structure, one then observes a zone of transition in eastern Puerto Rico between apparently two discrete genetic groups. This zone of transition did not seem to associate directly with the Mona Passage, west of Puerto Rico, which was suggested to be a significant barrier to gene flow in A. palmata (Baums et al. 2006b). Foster et al. (2012) also found an East-West break in the genetic structure of O. annularis, but noted that its location was ambiguous since their results pointed to a separation between the British Virgin Islands and Dominica. Understanding why the genetic transition could be more pronounced in the east of Puerto Rico, as suggested by the slopes of RMA regressions between mainland Puerto Rico and Mona vs. mainland Puerto Rico and Culebra, is an intriguing challenge. The filtering effect of the Mona Passage, coupled with asymmetric net gene flow in the easterly direction, as is generally the case for surface currents during the spawning season on the north coast of Puerto Rico, could provide an explanation. Admittedly, the genetic structure in the south of the island would then be expected to respond to gene flow following opposite currents in the westerly direction, which does not seem to be the case, unless gene flow in the southeast is hindered by another, unidentified barrier. Residual genetic structure inherited from historical shifts in the geographical ranges of Caribbean species during the late Quaternary (Lighty et al. 1982; Toscano & Macintyre 2003) could explain some of the present genetic patterns, as did the last glaciation event on Indo-Pacific population dynamics of marine species (Benzie et al. 1999). Less than 15 genotypes were recovered from two of the three non-Puerto Rican localities in this study (Curaçao and the Bahamas). Because Structure and other analyses of population structuring are context-dependent, adding molecular markers, new locations in the larger Caribbean and increasing sample size per population seems essential for further, improved analyses (e.g. when assigning individuals to populations of origin, see Cornuet et al. 1999). These additions will likely provide us with a better understanding of possible IBD and other genetic structuring of A. palmata populations outside of Puerto Rico. New sampling efforts concentrated in the northeast, southeast and east of Puerto Rico are also needed to identify with more accuracy the location(s) of the strongest genetic shift(s) between Eastern and Western populations. Meanwhile, building on the knowledge that most of the extant genetic diversity of A. palmata is present on the Puerto Rico Shelf, we exert local decision-makers to implement new conservation measures for the elkhorn coral, as effective protection at a local scale could be beneficial for preserving the global genetic diversity of A. palmata.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jenny Acevedo, Silvia Planas, Ximena Velez Zuazo, Juan Marrero and Elsie Rivera for their help, Claude Bouchon and Olivier Gros for their logistic support in Guadeloupe, and Edwin Hernandez for providing samples from Mona. The manuscript was also greatly improved from the comments of various reviewers. Funding was provided by a Caribbean Coral Reef Institute Grant, a CMRC Program Development Grant (05-PRKS-01-05A) awarded to NVS and via NIH-SCORE grant S06GM08102 and NSF-HRD grants 0734826 and 0931659 to TH. Collection permits were issued by PR–D.N.E.R. and D.R.A.M. of Guadeloupe.
REFERENCES
- Andras JP, Rypien KL, Harvell CD. Range-wide population genetic structure of the Caribbean sea fan coral. Gorgonia ventalina. Molecular Ecology. 2013;22:56–73. doi: 10.1111/mec.12104. [DOI] [PubMed] [Google Scholar]
- Ayre DJ, Hughes TP. Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution. 2000;54:1590–1605. doi: 10.1111/j.0014-3820.2000.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Baums IB, Hugues CR, Hellberg RE. Mendelian microsatellite loci for the Caribbean coral Acropora palmata. Marine Ecology Progress Series. 2005a;288:115–127. [Google Scholar]
- Baums IB, Miller MW, Hellberg RE. Regionally isolated populations of an imperiled Caribbean coral, Acropora palmata. Molecular Ecology. 2005b;14:1377–1390. doi: 10.1111/j.1365-294X.2005.02489.x. [DOI] [PubMed] [Google Scholar]
- Baums IB, Miller MW, Hellberg RE. Geographic variation in clonal structure in a reefbuilding Caribbean coral, Acropora palamata. Ecological Monographs. 2006a;76:503–519. [Google Scholar]
- Baums IB, Paris CB, Chérubin LM. A bio-oceanographic filter to larval dispersal in a reef-building coral. Limnology and Oceanography. 2006b:1969–1981. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Benzie J, Haskell A, Lehman H. Variation in the genetic composition of coral (Pocillopora damicornis and Acropora palifera) populations from different reef habitats. Marine Biology. 1995;121:731–739. [Google Scholar]
- Benzie JAH. Genetic structure of coral reef organisms: ghosts of dispersal past. American Zoologist. 1999;39:131–145. [Google Scholar]
- Bohonak A, Van Der Linde K. [Accessed February 2012];RMA: software for reduced major axis regression, Java version 3. 21. 2004 http://ibdws.sdsu.edu.
- Bruckner AW. NOAA Technical Memorandum NMSF-OPR-24. Silver Spring, MD: 2002. Proceedings of the Caribbean Acropora workshop--potential application of the US Endangered Species Act as a conservation strategy. [Google Scholar]
- Coffroth MA, Lasker HR. Population structure of a clonal gorgonian coral: the interplay between clonal reproduction and disturbance. Evolution. 1998;52:379–393. doi: 10.1111/j.1558-5646.1998.tb01639.x. [DOI] [PubMed] [Google Scholar]
- Colin PL. Larvae retention: genes or oceanography? Science. 2003;300:1657–1659. doi: 10.1126/science.300.5626.1657c. [DOI] [PubMed] [Google Scholar]
- Concepcion GT, Polato NR, Baums IB, Toonen RJ. Development of microsatellite markers from four Hawaiian corals: Acropora cytherea, Fungia scutaria, Montipora capitata and Porites lobata. Conservation Genetics Resources. 2010;2:11–15. [Google Scholar]
- Cornuet J-M, Piry S, Luikart G, Estoup A, Solignac M. New methods employing multilocus genotypes to select or exclude populations as origins of individuals. Genetics. 1999;153:1989–2000. doi: 10.1093/genetics/153.4.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen RK, Paris CB, Srinivasan A. Scaling of connectivity in marine populations. Science. 2006;311:522–527. doi: 10.1126/science.1122039. [DOI] [PubMed] [Google Scholar]
- Dennis GD, Smith-Vaniz WF, Colin PL, Hensley DA, McGehee MA. Shore fishes from the islands of the Mona Passage, Greater Antilles with comments on their zoogeography. Caribbean Journal of Science. 2005;41:716–743. [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Fong P, Lirman D. Hurricanes cause population expansion of the branching coral Acropora palmata (Scleractinia): wound healing and growth patterns of asexual recruits. Marine Ecology. 1995;16:317–335. [Google Scholar]
- Foster NL, Paris CB, Kool JT, Baums IB, Stevens JR, et al. Connectivity of Caribbean coral populations: complementary insights from empirical and modelled gene flow. Molecular Ecology. 2012;21:1143–1157. doi: 10.1111/j.1365-294X.2012.05455.x. [DOI] [PubMed] [Google Scholar]
- Frantz AC, Cellina S, Krier A, Schley L, Burke T. Using spatial Bayesian methods to determine the genetic structure of a continuously distributed population: clusters or isolation by distance? Journal of Applied Ecology. 2009;46:493–505. [Google Scholar]
- Gaggiotti O, Lange O, Rassmann K, Gliddon C. A comparison of two indirect methods for estimating average levels of gene flow using microsatellite data. Molecular Ecology. 1999;8:1513–1520. doi: 10.1046/j.1365-294x.1999.00730.x. [DOI] [PubMed] [Google Scholar]
- Galindo HM, Olson DB, Palumbi SR. Seascape genetics: a coupled oceanographic-genetic model predicts population structure of Caribbean corals. Current Biology. 2006;16:1622–1626. doi: 10.1016/j.cub.2006.06.052. [DOI] [PubMed] [Google Scholar]
- Garcia Reyes J, Schizas NV. No two reefs are created equal: fine-scale population structure in the threatened coral species Acropora palmata and A. cervicornis. Aquatic Biology. 2010;10:69–83. [Google Scholar]
- Guillot G, Leblois R, Coulon A, Frantz AC. Statistical methods in spatial genetics. Molecular Ecology. 2009;18:4734–4756. doi: 10.1111/j.1365-294X.2009.04410.x. [DOI] [PubMed] [Google Scholar]
- Hellberg ME. Relationships between inferred levels of gene flow and geographic distance in a philopatric coral, Balanophyllia elegans. Evolution. 1994;48:1829–1854. doi: 10.1111/j.1558-5646.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Hellberg ME. No variation and low synonymous substitution rates in coral mtDNA despite high nuclear variation. BMC Evolutionary Biology. 2006;6:24. doi: 10.1186/1471-2148-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg ME. Footprints on water: the genetic wake of dispersal among reefs. Coral Reefs. 2007;26:463–473. [Google Scholar]
- Hemond EM, Vollmer SV. Genetic diversity and connectivity in the threatened staghorn coral (Acropora cervicornis) in Florida. PLoS one. 2010;5:e8652. doi: 10.1371/journal.pone.0008652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith RC, Riggs AC, D'Antonio CM. Survival of hurricane-generated coral fragments and a disturbance model of reef calcification/growth rates. Oecologia. 1980;46:322–329. doi: 10.1007/BF00346259. [DOI] [PubMed] [Google Scholar]
- Hubisz MJ, Falush D, Stephens M, Pritchard JK. Inferring weak population structure with the assistance of sample group information. Molecular Ecology Resources. 2009;9:1322–1332. doi: 10.1111/j.1755-0998.2009.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson M, Rosenberg NA. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics. 2007;23:1801–1806. doi: 10.1093/bioinformatics/btm233. [DOI] [PubMed] [Google Scholar]
- Jensen J, Bohonak A, Kelley S. Isolation by distance, web service. BMC Genetics. 2005;6:13. doi: 10.1186/1471-2156-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighty RG, Macintyre IG, Stuckenrath R. Acropora palmata reef framework: a reliable indicator of sea level in the western Atlantic for the past 10,000 years. Coral Reefs. 1982;1:125–130. [Google Scholar]
- MacKenzie JB, Munday PL, Willis BL, Miller DJ, Van Oppen MJH. Unexpected patterns of genetic structuring among locations but not colour morphs in Acropora nasuta (Cnidaria; Scleractinia) Molecular Ecology. 2004;13:9–20. doi: 10.1046/j.1365-294x.2003.02019.x. [DOI] [PubMed] [Google Scholar]
- Magalon H, Adjeroud M, Veuille M. Patterns of genetic variation do not correlate with geographical distance in the reef-building coral Pocillopora meandrina in the South Pacific. Molecular Ecology. 2005;14:1861–1868. doi: 10.1111/j.1365-294X.2005.02430.x. [DOI] [PubMed] [Google Scholar]
- Maier E, Tollrian R, Rinkevich B, Nürnberger B. Isolation by distance in the scleractinian coral Seriatopora hystrix from the Red Sea. Marine Biology. 2005;147:1109–1120. [Google Scholar]
- Ng W, Morton B. Genetic structure of the scleractinian coral Platygyra sinensis in Hong Kong. Marine Biology. 2003;143:963–968. [Google Scholar]
- Palumbi SR. Population genetics, demographic connectivity, and the design of marine reserves. Ecological Applications. 2003;13:146–158. [Google Scholar]
- Palumbi SR, Vollmer S, Romano S, Oliver, J Ladner T. The role of genes in understanding the evolutionary ecology of reef building corals. Evolutionary Ecology. 2012;26:317–335. [Google Scholar]
- Peakall R, Smouse PE. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes. 2006;6:288–295. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- Reusch TBH, Ehlers A, Hämmerli A, Worm B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proceedings of the National Academy of Sciences, USA. 2005;102:2826. doi: 10.1073/pnas.0500008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards ZT, van Oppen MJH. Rarity and genetic diversity in Indo–Pacific Acropora corals. Ecology and Evolution. 2012;2:1867–1888. doi: 10.1002/ece3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg NA. DISTRUCT: a program for the graphical display of population structure. Molecular Ecology Notes. 2004;4:137–138. [Google Scholar]
- Rousset F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics. 1997;145:1219–1228. doi: 10.1093/genetics/145.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Karl SA. Contrasting population genetic structures of sympatric, mass-spawning Caribbean corals. Marine Biology. 2006;150:57–68. [Google Scholar]
- Slatkin M. Isolation by distance in equilibrium and non-equilibrium populations. Evolution. 1993;47:264–279. doi: 10.1111/j.1558-5646.1993.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Tang PC, Wei NV, Chen CW, Wallace CC, Chen CA. Comparative study of genetic variability of AAT and CT/GT microsatellites in staghorn coral, Acropora (Scleractinia: Acroporidae) Zoological Studies. 2010;49:657–668. [Google Scholar]
- Taylor MS, Hellberg ME. Comparative phylogeography in a genus of coral reef fishes: biogeographic and genetic concordance in the Caribbean. Molecular Ecology. 2006;15:695–707. doi: 10.1111/j.1365-294X.2006.02820.x. [DOI] [PubMed] [Google Scholar]
- Toscano MA, Macintyre IG. Corrected western Atlantic sea-level curve for the last 11,000 years based on calibrated 14C dates from Acropora palmata framework and intertidal mangrove peat. Coral Reefs. 2003;22:257–270. [Google Scholar]
- Valdés-Pizzini M, Schärer-Umpierre M, Carrelo-Morales CJ, Ferández-Arribas M, Muñoz-Hincapié M. Plan de Manejo de la Reserva Marina de Tres Palmas Rincón. Equipo de facilitación del Centro Interdisciplinario de Estudios del Litoral (CIEL) Puerto Rico: Universidad de Puerto Rico en Mayagüez; 2009. [Google Scholar]
- Van Oppen MJ, Willis HWJA, Van Vugt BL, Miller DJ. Examination of species boundaries in the Acropora cervicornis group (Scleractinia, Cnidaria) using nuclear DNA sequence analyses. Molecular Ecology. 2000;9:1363–1373. doi: 10.1046/j.1365-294x.2000.01010.x. [DOI] [PubMed] [Google Scholar]
- Van Oppen MJH, Gates RD. Conservation genetics and the resilience of reef-building corals. Molecular Ecology. 2006;15:3863–3883. doi: 10.1111/j.1365-294X.2006.03026.x. [DOI] [PubMed] [Google Scholar]
- Veron J, S-S M. Corals of the world. Townsville, Australia: Australian Institute of Marine Science; 2000. [Google Scholar]
- Vollmer SV, Palumbi SR. Hybridization and the evolution of reef coral diversity. Science. 2002;296:2023–2025. doi: 10.1126/science.1069524. [DOI] [PubMed] [Google Scholar]
- Vollmer SV, Palumbi SR. Testing the utility of internally transcribed spacer sequences in coral phylogenetics. Molecular Ecology. 2004;13:2763–2772. doi: 10.1111/j.1365-294X.2004.02265.x. [DOI] [PubMed] [Google Scholar]
- Vollmer SV, Palumbi SR. Restricted gene flow in the Caribbean staghorn coral Acropora cervicornis: implications for the recovery of endangered reefs. Journal of Heredity. 2007;98:40–50. doi: 10.1093/jhered/esl057. [DOI] [PubMed] [Google Scholar]
- Wallace CC. Staghorn corals of the world: a revision of the coral genus Acropora (Scleractinia; Astrocoeniina; Acroporidae) worldwide, with emphasis on morphology, phylogeny and biogeography. Collingwood, Victoria, Australia: CSIRO Publishing; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.