Abstract
Rationale
Several studies have demonstrated a correlation between extracellular glutamate concentration in the mesolimbic reward pathway and alcohol craving. Extracellular glutamate concentration is regulated by several glutamate transporters. Glial glutamate transporter 1 (GLT1) is one of them that regulates the majority of extracellular glutamate concentration. In addition cystine/glutamate antiporter (xCT) is another transporter that regulates extracellular glutamate.
Objectives
We focus in this study to determine the effects of ceftriaxone, β-lactam antibiotic, on glial proteins such as GLT1 isoforms, xCT, GLAST and several associated signaling pathways as well as ethanol intake in P rats. Additionally, to examine the onset of signaling pathways associated with GLT1 upregulation following ceftriaxone treatment, we tested two-day versus five-day daily dosing of ceftriaxone.
Results
Ceftriaxone treatment (100 mg/kg), two-day and five day, resulted in about five-fold reduction in ethanol intake by P rats. The reduction in ethanol intake was associated with significantly enhanced expression of GLT1, GLT1a, GLT1b, and xCT in the NAc and PFC of five-day ceftriaxone treated P rats. Two-day treated P rats showed marked changes in expression of these glutamate transporters in the PFC but not in the NAc. Importantly, ceftriaxone treated P rats (two-day and five-day) demonstrated enhanced phosphorylation of Akt and nuclear translocation of NFκB in the NAc and PFC compared to control animals.
Conclusions
These findings demonstrate that ceftriaxone treatment induced upregulation of GLT1, GLT1 isoforms, and xCT in association with activation of Akt-NFκB signaling pathway.
Keywords: Glutamate, GLT1, EAAT2, P rats, ceftriaxone, nucleus accumbens, prefrontal cortex
INTRODUCTION
Ceftriaxone, a β-lactam antibiotic, is a known modulator of the major glutamate transporters, glutamate transporter 1 (GLT1) and cystine/glutamate antiporter, xCT (system xc−) (Knackstedt et al. 2010; Lewerenz et al. 2009; Rao and Sari 2014a; Rothstein et al. 2005). Ceftriaxone treatment has proven to be effective in several drug abuse animal models (Abulseoud et al. 2012; Alajaji et al. 2013; Rasmussen et al. 2011; Sari et al. 2013a; Sari et al. 2011; Sondheimer and Knackstedt 2011). Furthermore, ceftriaxone treatment has shown promising results in drug reinstatement studies as well (Knackstedt et al. 2010; Qrunfleh et al. 2013).
The association between increased extracellular glutamate cocnentrations in the mesolimbic reward pathway and alcohol craving has been studied extensively (Bauer et al. 2013; Griffin et al. 2013; Kapasova and Szumlinski 2008). This increase in extracellular glutamate concentration was suggested to be a result of decreased glutamate clearance (Melendez et al. 2005). Furthermore, chronic alcohol intake in P rats resulted in downregulation of GLT1 expression in the nucleus accumbens (NAc), both the core and the shell, as compared to ethanol naïve animals (Alhaddad et al. 2014b; Sari and Sreemantula 2012; Sari et al. 2013b). We have recently demonstrated that ceftriaxone-induced upregulation of GLT1 expression in the prefrontal cortex (PFC) and NAc has been effective in reducing ethanol consumption in P rats (Sari et al. 2013a; Sari and Sreemantula 2012; Sari et al. 2013b). Additionally, we have revealed that chronic ethanol intake induced downregulation of xCT, a subunit of system xc, mesocorticolimbic reward pathways in P rats (Alhaddad et al. 2014a).
The major glutamate transporter in the brain, GLT1, is known to be encoded in at least three isoforms: GLT1a, GLT1b, and GLT1c (Chen et al. 2002; Pines et al. 1992; Rauen et al. 2004; Reye et al. 2002; Schmitt et al. 2002). These isoforms are known to assemble as homo-oligomers and hetero-oligomers (Gendreau et al. 2004; Gonzalez-Gonzalez et al. 2009). GLT1a isoform is mainly expressed in glial cells but is also found on pre-synaptic neurons (Chen et al. 2004; Melone et al. 2009). Similarly, GLT1b isoform was found predominantly in astrocytes (Berger et al. 2005; Holmseth et al. 2009; Maragakis et al. 2004; Schmitt et al. 2002). Thus, we have investigated changes in the expressions of these major GLT1 isoforms, GLT1a and GLT1b with ceftriaxone treatment.
While GLT1 is predominantly involved in the uptake of glutamate and control of extracellular glutamate concentrations, xCT is responsible for the extrasynaptic release of glutamate (For review, see Bridges et al. 2012). Importantly, xCT can regulate the synaptic glutamate concentration via activation of extrasynaptic mGluR2/3 metabotropic receptors, which negatively modulate synaptic glutamate release. Considering the significant impact of xCT on glutamate homeostasis, studies have shown significant changes in the expression and activity of this protein in drug-dependence and reinstatement drug-seeking models (Baker et al. 2003a; Baker et al. 2003c; Knackstedt et al. 2009; Madayag et al. 2007). Therefore, the effect of ceftriaxone treatment on the expression of xCT was also determined in this study.
Furthermore, we have also determined changes in several signaling pathways associated with GLT1 upregulation in P rats following ceftriaxone administration. Since in vitro studies have confirmed that GLT1 upregulation may be mediated, in part, by Akt phosphorylation and nuclear translocation of the transcription factor nuclear factor kappaB (NFκB) (Lee et al. 2008), we investigated the occurrence of these changes in ceftriaxone-treated P rats. Expression of phospho-Akt/total-Akt, nuclear translocation of NFκB, and cytoplasmic levels of IkBa following ceftriaxone treatment were determined. Furthermore, xCT and GLAST protein levels were determined in the PFC and NAc of saline- and ceftriaxone-treated P rats. Studies have shown that five-day treatment with ceftriaxone upregulated GLT1 expression in the mesocorticolimbic pathway (Miller et al. 2008; Rothstein et al. 2005; Sari et al. 2011; Sari et al. 2009), and hence this treatment period was chosen for this study. Importantly, to observe the onset of effects of ceftriaxone treatment on GLT1 expression, and to establish the timeline for associated pathway changes, two-day as well as five-day treatment regimens were included in this study.
MATERIALS AND METHODS
Animals
Male P rats were obtained from the Indiana School of Medicine (Indianapolis, IN) breeding colonies. Animals were single-housed in wood chip-bedded plastic cages in a temperature (21°C) and humidity (50%) controlled environment on a 12/12-hour light/dark cycle. Animal protocol employed for this study was approved by the Institutional Animal Care and Use Committee of The University of Toledo, Health Science Campus, Toledo, OH. Protocols were based on the guidelines set forth by the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals. Animals had ad lib access to food and water throughout the duration of the study. At the age of three months, P rats were divided into four groups: 1) Two-day saline vehicle-treated group (n=7); 2) Two-day ceftriaxone (100 mg/kg, i.p.) treated group (n=7); 3) Five-day saline vehicle-treated group (n=8); and 4) Five-day ceftriaxone (100 mg/kg, i.p.) treated group (n=8). Ceftriaxone was administered as a solution made in physiological saline.
Ethanol consumption
For the duration of study, P rats had free access to two concentrations of ethanol, 15% and 30%, in distilled water. Animals were provided free choice to ethanol for five consecutive weeks before the start of treatment. This model of ethanol drinking consisting of multiple choices of ethanol concentrations (15% and 30%) is known to increase ethanol intake in P rats (Rodd-Henricks et al. 2001; Sari et al. 2006). During the last two weeks before treatment (Week 4 and Week 5), ethanol intake, water consumption and body weight of all animals were measured three times per week (Monday, Wednesday and Friday). Data measurements during these two weeks served as baseline values. As reported in other studies from our lab, ethanol measurements were taken to the nearest tenth of a gram by subtraction of the weight of the bottle from its previous weight. Importantly, animals with a baseline ethanol intake of less than 4 g/day were not included in this study, and they were subsequently euthanized. After five weeks of exposure to ethanol, P rats were treated i.p., once daily, with ceftriaxone (100 mg/kg) or saline for either two days or five days, depending on their assigned groups. Following the start of treatment, P rats were monitored once daily for consumption of ethanol and water. The time of daily measurement was kept constant during the treatment period to closely assess the drinking behavior over a 24-hour period.
Brain tissue extraction
At the end of the two-day or five-day treatment, P rats were euthanized by exposure to CO2 inhalation and then decapitated. Brains were immediately removed and stored at −70 °C. Brain regions (NAc and PFC) were dissected using a cryostat apparatus maintained at −20°C. The dissections of the PFC and NAc were performed by following the stereotaxic coordinates in accordance with the Paxinos and Watson Atlas of the rat brain (Paxinos and Watson 2007). Surgical blades were used to extract these brain regions. The extracted brain regions were then kept frozen at −70°C until Western blot analysis.
Western blot analysis
Western blot analysis was performed on the brain tissue samples to determine the level of protein expression, employing a previously described procedure (Sari et al. 2010; Sari et al. 2011; Sari et al. 2009). To separate the nuclear and cytoplasmic fractions, brain samples were homogenized in buffer A (10 mM HEPES-KOH, pH 7.9; 1.5mM MgCl2; 10 mM KCl; 1 mM Dithiothreitol (DDT); 1 mM phenylmethylsulfonyl fluoride (PMSF); 10 uL of protease inhibitor cocktail/ml of buffer) and incubated on ice for 10 min before adding Nonidet P-40 (0.1%). Following the addition of Nonidet P-40, samples were incubated on ice for 2 min and centrifuged at 13,200 rpm at 4°C for 15 min. The supernatant, representing the cytosolic fraction, was collected and supplemented with NaF (50 mM), Na vanadate (10 mM), and Na pyrophosphate (0.1 mM). The pellet was re-suspended in buffer B (20 mM HEPES-KOH, pH 7.9; 25% glycerol; 420 mM NACl; 1.5 mM MgCl2; 1 mM DDT; 1 mM PMSF; 0.2 mM EDTA; 50 mM NaF; 10 mM Na vanadate; 0.1 mM Na pyrophosphate; 10 uL of protease inhibitor cocktail/ml of buffer), incubated on ice for 30 min, and centrifuged at 13,200 rpm for 15 min at 4°C to obtain the nuclear fraction.
Extracted proteins were loaded on 10-20% tris-glycine gel (Invitrogen) and then transferred electrophoretically from the gel onto a PVDF membrane. The following antibodies were used in this study: guinea pig anti-GLT1 (millipore;1:5000), rabbit anti-GLT1a (gift from Dr. Jeffery Rothstein, Johns Hopkins University;1:5000), rabbit anti-GLT1b (gift from Dr. Paul Rosenberg, Harvard Medical School; 1:5000), rabbit anti-GLAST (Abcam;1:1000), rabbit anti-xCT antibody (Novus;1:1000), rabbit anti p-Akt (Santa Cruz;1:1000), mouse anti t-Akt (Santa Cruz;1:1000), rabbit anti-NFκB (Santa Cruz;1:1000), rabbit anti-IκBa (Santa Cruz;1:500), and mouse anti-GAPDH (Millipore;1:3000). Kodak BioMax MR film was used to capture the chemiluminescent signal from the HRP, and films were further developed using an SRX-101A machine. Immunoblots were digitized using the MCID system, and the data were reported as the ratio of protein of interest/ GAPDH (loading control protein). For the nuclear fraction, however, Lamin was used as a loading marker to normalize NFκB expression. An equal number of samples from saline- and ceftriaxone-treated groups was compared for the protein of interest, and Western blot results were analyzed as percentage of saline response (saline group was treated as 100%). For comparing treatment-induced changes across various brain regions, the saline -normalized data for the protein of interest from all brain regions were assembled as a bar graph, along with a representative Western blot image.
Statistical analyses
General linear model (GLM) repeated measures were used for statistical analysis of data related to ethanol consumption, water intake, and P rat body weight. One-way ANOVA was used to determine the day by effect of treatment. Changes in protein expression were analyzed statistically using independent t-test by comparing results between saline vehicle- and ceftriaxone-treated groups. Spearman rank correlational analysis between changes in ethanol consumption on Day 2/Day 5 and corresponding GLT1 expression in ceftriaxone treated P rats was performed. All statistical tests were based on p<0.05 level of significance. The analytical SPSS software and Graph Pad prism were used for this study.
RESULTS
Ceftriaxone treatment: effects on ethanol consumption, water intake, and body weight
The effect of ceftriaxone treatment on ethanol consumption was monitored for both the two-day (Table 1) and five-day paradigms (Table 2). The baseline value represents the average volume of ethanol consumed by the P rats over the two-week period preceding the first injection. GLM repeated measures analysis comparing ethanol consumption between ceftriaxone- and saline-treated animals revealed a significant main effect of Day [F(1,2)=7.846, p<0.05)] and a significant Day X treatment interaction effect [F(1,2)=28.206, p<0.05)] for the two-day paradigm as well as a significant main effect of Day [F(1,5)=21.576, p<0.05)] and a significant Day X treatment interaction effect [F(1,5)=18.782, p<0.05)] for the five-day paradigm. One-way NOVA revealed that the ceftriaxone treatment-induced reduction of ethanol intake was statistically significant (p<0.05) on all days of ceftriaxone treatment for both paradigms.
Table 1.
Effect of two-day ceftriaxone treatment on ethanol drinking, water consumption, and body weight of P rats. Data are expressed as Mean±SEM.
| Ethanol drinking (g/kg/day) |
Water drinking (g/kg/day) |
Body weight (g) |
||||
|---|---|---|---|---|---|---|
| SALINE | CEF | SALINE | CEF | SALINE | CEF | |
| Baseline | 4.9±0.2 | 5.2±0.2 | 26.7±1.8 | 27.8±2.8 | 456.9±9.6 | 434.8±19.1 |
| Day 1 | 4.3±0.6 | 2.8±0.2* | 28.4±4.2 | 36.9±6.9 | 468.5±9.9 | 449.9±20.5 |
| Day 2 | 6.5±1.0 | 1.3±0.3* | 26.3±2.9 | 40.2±7.3 | 470.7±10.3 | 446.2±20.6 |
significant difference between treatment groups (p<0.05)
Table 2.
Effect of five-day ceftriaxone treatment on ethanol drinking, water consumption, and body weight of P rats. Data are expressed as Mean±SEM.
| Ethanol drinking (g/kg/day) |
Water drinking (g/kg/day) |
Body weight (g) |
||||
|---|---|---|---|---|---|---|
| SALINE | CEF | SALINE | CEF | SALINE | CEF | |
| Baseline | 5.3±0.2 | 5.5±0.3 | 21.1±2.5 | 23.6±1.1 | 461.5±7.7 | 460.7±13.8 |
| Day 1 | 4.5±0.5 | 2.9±0.2* | 20.9±2.8 | 30.2±4.5 | 479.7±7.9 | 477.3±14.6 |
| Day 2 | 6.0±0.5 | 1.2±0.1* | 20.7±3.3 | 48.9±4.2* | 479.6±8.2 | 474.3±13.8 |
| Day 3 | 4.3±0.5 | 1.6±0.2* | 22.0±3.9 | 51.7±2.9* | 484.0±8.0 | 481.8±13.4 |
| Day 4 | 4.6±0.4 | 1.3±0.2* | 15.6±2.3 | 36.1±4.2* | 486.0±8.7 | 485.3±13.4 |
| Day 5 | 5.3±0.5 | 2.4±0.4* | 16.5±1.7 | 38.5±4.0* | 486.8±8.5 | 481.8±12.5 |
significant difference between treatment groups (p<0.05)
GLM repeated measures analysis comparing water intake between ceftriaxone- and saline-treated animals revealed no significant effect of treatment for the two-day paradigm (Table 1). However, GLM repeated measures analysis comparing water intake between five-day ceftriaxone- and saline-treated animals revealed a significant main effect of Day [F(1,5)=7.77, p<0.05)] and a significant Day X treatment interaction effect [F(1,5)=6.82, p<0.05)]. One-way ANOVA revealed that the ceftriaxone treatment-induced increase of water consumption was statistically significant (p<0.05) from Day 2 through Day 5 (Table 2). GLM repeated measures analysis comparing body weights of animals in the ceftriaxone- and saline-treated groups revealed no significant change following two-day and five-day treatment regimens (Tables 1 and 2).
Effect of ceftriaxone treatment on expression of GLT1, GLT1a, GLT1b in NAc and PFC
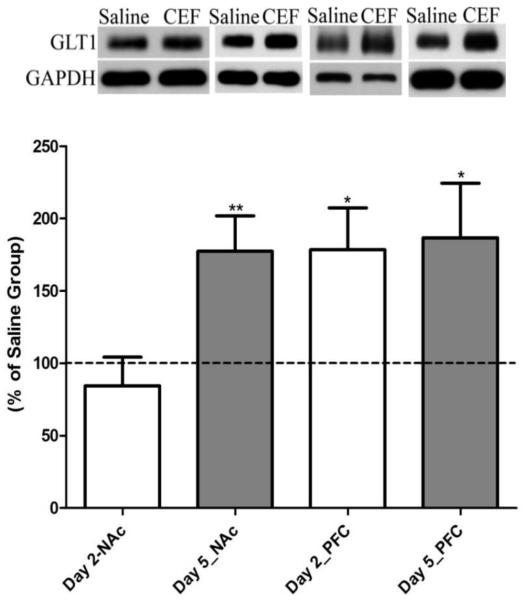
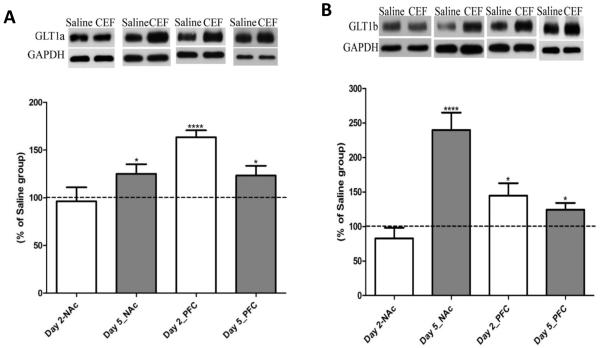
Ceftriaxone-mediated changes in levels of GLT1 (Fig. 1) and its isoforms (Fig. 2), GLT1a and GLT1b, were determined. In the NAc, ceftriaxone treatment for five days (five-day paradigm) significantly increased the levels of GLT1 (p<0.01; n=8), GLT1a (p<0.05; n=8), and GLT1b (p<0.0001; n=8) as compared to saline-treated groups. Importantly, in the PFC, ceftriaxone treatment induced significant upregulation of the levels of GLT1 (p<0.05; n=8), GLT1a (p<0.05; n=8), and GLT1b (p<0.05; n=7), as compared to the saline-treated group, for the five-day treatment paradigm and significantly upregulated the levels of GLT1 (p<0.05; n=7), GLT1a (p<0.0001; n=7), and GLT1b (p<0.05; n=7) for the two-day treatment paradigm. Moreover, as summarized in Table 3, the ceftriaxone induced enhanced expression of GLT1 in NAc and PFC demonstrated a significant correlation with reduced ethanol intake on last day of ceftriaxone treatment in P rats.
Figure 1.
Effect of ceftriaxone treatment on the expression of GLT1 in the nucleus accumbens (NAc) and prefrontal cortex (PFC) compared to the respective saline-treated control groups. Representative immunoblot for GLT1 and GAPDH (loading control) expression along with quantitative analysis comparing ratios of GLT1/GAPDH expression between saline- (served as 100%) and ceftriaxone-treated P rats for the corresponding brain regions. Data are expressed as mean ± SEM. (*p<0.05; **p<0.01).
Figure 2.
A) Effect of ceftriaxone treatment on the expression of GLT1a in the nucleus accumbens (NAc) and prefrontal cortex (PFC) compared to the respective saline-treated control groups presented in one figure. Representative immunoblot for GLT1a and GAPDH (loading control) expression along with quantitative analysis comparing ratios of GLT1a/GAPDH expression between saline- (served as 100%) and ceftriaxone treated P rats for the corresponding brain regions. Data are expressed as mean ± SEM. (*p<0.05; ****p<0.0001). B) Effect of ceftriaxone treatment on the expression of GLT1b in the nucleus accumbens (NAc) and prefrontal cortex (PFC) compared to the respective saline-treated control groups. Representative immunoblot for GLT1b and GAPDH (loading control) expression along with quantitative analysis comparing ratios of GLT1b/GAPDH expression between saline- (served as 100%) and ceftriaxone treated P rats for the corresponding brain regions. Data are expressed as mean ± SEM. (*p<0.05; ****p<0.0001).
Table 3.
Spearman rank correlation analysis between changes in ethanol consumption and GLT1 expression in two-day and five-day ceftriaxone treated P rats.
|
Two-day treatment |
Drinking | GLT1_NAc | GLT1_PFC | |
| Correlation coefficient | 1.0 | −0.018 | −0.537 | |
| Significance | 0.952 | 0.047 | ||
| N | 14 | 14 | 14 | |
|
Five-day
treatment |
Correlation coefficient | 1.0 | −0.685 | −0.575 |
| Significance | 0.003 | 0.02 | ||
| N | 16 | 16 | 16 |
Effect of ceftriaxone treatment on expression of xCT and GLAST in NAc and PFC
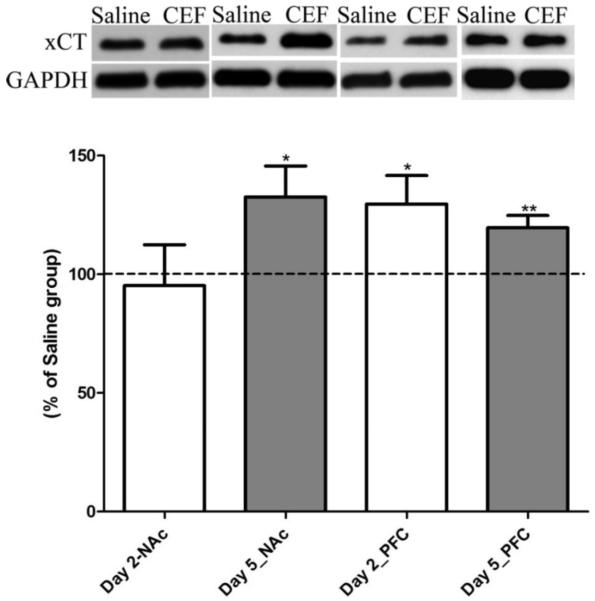
Expression of xCT, a subunit of system xc−, was analyzed following two-day and five-day treatment paradigms in P rats (Fig. 3). Ceftriaxone treatment for five days induced significant upregulation in the level of xCT (p<0.05; n=8) in the NAc. Furthermore, ceftriaxone induced significant upregulation of the levels of xCT in the PFC after two-day (p<0.05; n=7) and five-day (p<0.01; n=8) treatments. Ceftriaxone treatment paradigms, both two-day and five-day, did not change GLAST levels in the NAc or PFC compared to the saline-treated groups.
Figure 3.
Effect of ceftriaxone treatment on the expression of xCT in the nucleus accumbens (NAc) and prefrontal cortex (PFC) compared to the respective saline-treated control groups. Representative immunoblot for xCT and GAPDH (loading control) expression along with quantitative analysis comparing ratios of xCT/GAPDH expression between saline- (served as 100%) and ceftriaxone-treated P rats for the corresponding brain regions. Data are expressed as mean ± SEM. (*p<0.05; **p<0.01).
Effect of ceftriaxone treatment on levels of nuclear NFκB and cytoplasmic IkBa in NAc and PFC
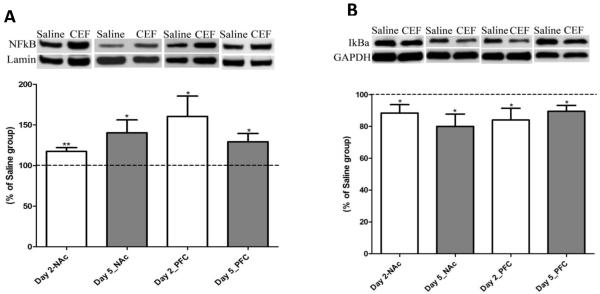
In this study, we also determined the levels of NFκB (nuclear) and IkBa (cytoplasmic) in the NAc and PFC from two-day and five-day treatment groups (Fig. 4). Ceftriaxone treatment resulted in an increased nuclear translocation of NFκB in the NAc, compared to the saline-treated group, for both the two-day treatment (p<0.01; n=7) and five-day treatment (p<0.05; n=8) groups. Similarly, ceftriaxone treatment increased the level of nuclear NFκB in the PFC for twoday (p<0.05; n=7) and five-day (p<0.05; n=7) treatment paradigms. Accordingly, ceftriaxone treatment resulted in a statistically significant reduction in the level of cytoplasmic IkBa in the NAc for both two-day (p<0.05; n=7) and five-day (p<0.05; n=7) treatment paradigms as compared to respective saline-treated groups. Similarly, ceftriaxone treatment significantly decreased the levels of cytoplasmic IkBa in the PFC for two-day (p<0.05; n=7) and five-day (p<0.05; n=7) treatment paradigms.
Figure 4.
A) Effect of ceftriaxone treatment on the expression of NFκB in the nuclear fraction of nucleus accumbens (NAc) and prefrontal cortex (PFC) compared to the respective saline-treated control groups. Representative immunoblot for NFκB and Lamin (nuclear protein loading control) expression along with quantitative analysis comparing ratios of NFκB/Lamin expression between saline- (served as 100%) and ceftriaxone treated P rats for the corresponding brain regions. Data are expressed as mean ± SEM. (*p<0.05; **p<0.01). B) Effect of ceftriaxone treatment on the expression of IkBa in the nucleus accumbens (NAc) and prefrontal cortex (PFC) compared to the respective saline-treated control groups. Representative immunoblot for IkBa and GAPDH (loading control) expression along with quantitative analysis comparing ratios of IkBa/GAPDH expression between saline- (served as 100%) and ceftriaxone treated P rats for the corresponding brain regions. Data are expressed as mean ± SEM. (*p<0.05).
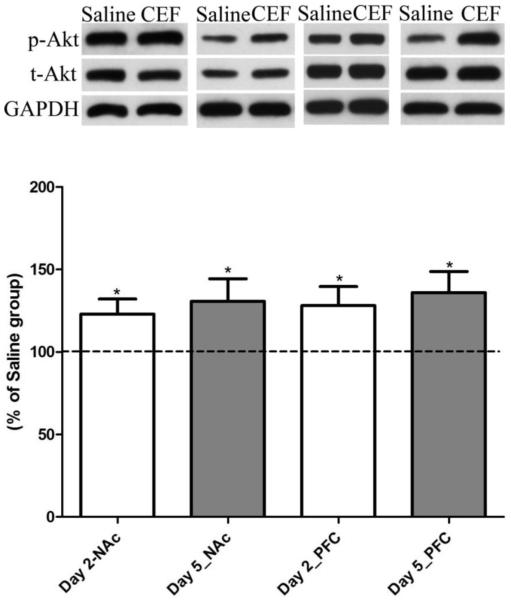
Effect of ceftriaxone treatment on expression of p-Akt/Akt in NAc and PFC
Phosphorylation of signaling molecule Akt in association with ceftriaxone treatment was also determined. We found that ceftriaxone treatment induced a significant increase in the phosphorylation of protein Akt in the NAc following the two-day (p<0.05; n=7) and five-day (p<0.05; n=8) treatment paradigms and in the PFC for the two-day (p<0.05; n=6) and five-day (p<0.05; n=8) treatment paradigms, as compared to saline-treated groups (Fig. 5).
Figure 5.
Effect of ceftriaxone treatment on the expression of p-Akt/Akt in the nucleus accumbens (NAc) and prefrontal cortex (PFC) compared to the respective saline treated control groups. Representative immunoblot for phospho-Akt, total-Akt, and GAPDH (loading control) expression along with quantitative analysis comparing ratios of p-Akt/Akt (normalized to GAPDH) expression between saline- (served as 100%) and ceftriaxone treated P rats for the corresponding brain regions. Data are expressed as mean ± SEM. (*p<0.05).
DISCUSSION
Findings from this study reveal that ceftriaxone treatment for two days significantly upregulated the levels of GLT1, including its major isoforms, and xCT only in the PFC and not in the NAc. However, ceftriaxone treatment for five days upregulated these glutamate transporters in both PFC and NAc. Importantly, for the first time, significant changes in signaling pathways previously implicated in ceftriaxone-mediated upregulation of GLT1 have been observed in the present study. Ceftriaxone treatment was associated with significantly increased levels of phosphorylated Akt, increased nuclear translocation of NFκB, and reduced cytoplasmic levels of IkBa in both the NAc and the PFC for two- and five-day treatment paradigms as compared to the saline-treated group. Although there was no change in the expression of GLT1, GLT1a and GLT1b isoforms, and xCT in the NAc following two-day ceftriaxone treatment, the signaling pathways previously reported for ceftriaxone-mediated upregulation of GLT1, including Akt and NFκB, were found to be activated. These data suggest a possible delay in ceftriaxone-induced upregulation of the identified glutamate transporters in the NAc as compared to the PFC. Alternatively, we demonstrated that ceftriaxone upregulated the identified proteins and reduced ethanol intake, but there is less known about whether this drug interacts with ethanol itself. Studies are warranted to determine any drug interaction mechanism between ceftriaxone and ethanol.
We have recently demonstrated the effectiveness of ceftriaxone treatment in reducing ethanol-drinking and reinstatement-like ethanol drinking in P rats (Rao and Sari 2014b; Sari et al. 2013a; Sari et al. 2011; Sari et al. 2013b). These effects were associated with upregulation of GLT1 expression in the PFC and NAc after five days of ceftriaxone treatment. Importantly, a significant correlation between attenuation of ethanol consumption on the last day of treatment (Day 2 or Day 5) and increased GLT1 expression has been observed (Table 3) (p<0.05). Except, we did not see any correlation between ethanol consumption and GLT1 expression in NAc from two-day ceftriaxone treated group. In addition, we revealed for the first time that ceftriaxone induced upregulation of GLT1 and its isoforms, along with xCT expressions, in the PFC after only two daily doses. These results might be due to the fact that the PFC receives and sends glutamatergic projections to and from other brain reward regions involved in addictive behavior; however, the NAc only receives the glutamatergic projections from several brain regions, including key reward regions. As we have postulated previously, neuroadaptations may occur as a consequence of differences in the neuroanatomical distribution of GLT1, its isoforms, and xCT as well as the levels of expression of these transporters in the PFC and NAc, and possibly in other brain reward regions [For review, see ref. (Danbolt 2001)].
The xCT is the major source of non-synaptic glutamate and is a crucial element in regulating the extracellular glutamate levels in the NAc and PFC (Baker et al. 2002; McBean 2002; Moran et al. 2003). Changes in the expression of xCT have been implicated in drug-seeking behavior, reinstatement to drug abuse, and the development of drug dependence (Baker et al. 2003b; Kau et al. 2008; Knackstedt et al. 2009). Importantly, we have recently reported that chronic ethanol consumption was associated with significant downregulation of xCT levels in the NAc and PFC in P rats (Alhaddad et al. 2014a). Ceftriaxone is known to upregulate xCT expressions and was found effective in abolishing drug-seeking behavior (Knackstedt et al. 2010; Lewerenz et al. 2009; Rao and Sari 2014a; Trantham-Davidson et al. 2012). Given the possible role of xCT in the development of addiction, we have revealed in this study the ability of ceftriaxone to upregulate xCT expressions in the NAc and PFC of ethanol-dependent P rats compared to saline-treated animals.
In regard to the target signaling pathways involved in the upregulation of the identified glutamate transporters, protein kinase Akt is known to modulate the nuclear translocation of transcription factor NFκB (Dan et al. 2008; Madrid et al. 2001; Ozes et al. 1999). Based on previous in in vitro studies, Akt has been identified as a key regulator of GLT1 expression (Ji et al. 2011; Li et al. 2006). In addition, it has been demonstrated that NFκB is responsible for the transcription of GLT1 (Ghosh et al. 2011; Yang et al. 2009). The present work has revealed for the first time that the activation of previously established cellular pathways, Akt phosphorylation and nuclear translocation of NFκB, occur in association with ceftriaxone-induced upregulation of GLT1 expressions in ethanol-dependent P rats. Interestingly, the levels of phospho-Akt/total-Akt and nuclear NFκB were significantly higher than corresponding saline-treated group, while levels of GLT1 were not upregulated in the NAc at two-day ceftriaxone treatment paradigm. We hypothesized that this results from an increase in the activity of the cellular machinery responsible for the upregulation of GLT1. Since the five-day treatment paradigm is followed by a distinct upregulation in GLT1 expression in the NAc, we rationalized the changes in GLT1 expression occurring between the two-day and five-day paradigms. Furthermore, the increase in nuclear translocation of the transcription factor was associated with a significant reduction in the level of IkBa in the cytoplasm, indicating the possible proteasomal degradation of IkBa following nuclear translocation of p65. The reduction in cytoplasmic IkBa level was observed in both the NAc and PFC following two-day and five-day treatments with ceftriaxone.
The dysfunction of the PFC as a key reward brain region has been well-studied in the development of drug addiction (for review (Goldstein and Volkow 2011)). The inputs received by the ventral tegmental area (VTA) from the PFC play a critical role in the development of addiction, and disruption of these inputs arising from the PFC interfere with the development of addiction (Chen et al. 2011; Dong et al. 2005; Kalivas 1993; Li et al. 1999; Tong et al. 1995). Moreover, results from several studies highlight the critical role played by the PFC in the development of ethanol dependence (Barker et al. 2012; Costin et al. 2013; Echeverry-Alzate et al. 2012; Kerns et al. 2005; Seo et al. 2013; Vetreno et al. 2013). Interestingly, results of the present work indicate that ceftriaxone treatment-induced changes in the expression of GLT1 and xCT are evident in the PFC as early as 24h after two daily doses, compared to the saline-treated group, in contrast to its effects in the NAc. These findings reveal the possibility of the PFC playing a major role in reducing ethanol intake following ceftriaxone administration during the initial stages via modulation of the glutamatergic inputs to the NAc and other brain regions. Following five days of ceftriaxone treatment, GLT1 and xCT expressions are significantly higher in both the NAc and PFC, indicating an overall establishment of glutamate homeostasis in the mesocorticolimbic reward pathway.
In conclusion, we report for the first time that ceftriaxone-induced attenuation of ethanol consumption begins as early as 24h after two daily doses in ethanol-dependent P rats. This reduction in ethanol intake for the two-day ceftriaxone-treated group was associated with upregulation of expressions of xCT and GLT1 and its isoforms in the PFC. Following five daily doses of ceftriaxone, the expressions of these glutamate transporters were found significantly higher in both the NAc and PFC compared to saline-treated P rats. Importantly, both two-day and five-day ceftriaxone treatment paradigms were associated with increased Akt phosphorylation and nuclear translocation of transcription factor NFκB in P rats. These findings suggest the possible involvement of previously established signaling pathways in the ceftriaxone-induced upregulation of GLT1 expression in the mesocorticolimbic reward pathway.
Acknowledgments
This work was supported by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism. The authors thank Dr. Jeffery Rothstein from Johns Hopkins University and Dr. Paul Rosenberg from Harvard Medical School for proving our laboratory with GLT1a and GLT1b, respectively. The authors would like to thank Charisse Montgomery for editing this manuscript.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
REFERENCES
- Abulseoud OA, Miller JD, Wu J, Choi DS, Holschneider DP. Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain research. 2012;1456:14–21. doi: 10.1016/j.brainres.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alajaji M, Bowers MS, Knackstedt L, Damaj MI. Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology. 2013;228:419–26. doi: 10.1007/s00213-013-3047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 2014a doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SH, Wei Y, Sari Y. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Frontiers in behavioral neuroscience. 2014b;8:366. doi: 10.3389/fnbeh.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature neuroscience. 2003a;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature neuroscience. 2003b;6:743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW. N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann Ny Acad Sci. 2003c;1003:349–351. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Taylor JR. Low prefrontal PSA-NCAM confers risk for alcoholism-related behavior. Nature neuroscience. 2012;15:1356–8. doi: 10.1038/nn.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, Heindel W, Arolt V, Ohrmann P. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1401–8. doi: 10.1038/npp.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger UV, DeSilva TM, Chen W, Rosenberg PA. Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. The Journal of comparative neurology. 2005;492:78–89. doi: 10.1002/cne.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R, Lutgen V, Lobner D, Baker DA. Thinking Outside the Cleft to Understand Synaptic Activity: Contribution of the Cystine-Glutamate Antiporter (System x(c)(-)) to Normal and Pathological Glutamatergic Signaling. Pharmacol Rev. 2012;64:780–802. doi: 10.1124/pr.110.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Zhang D, Dragomir A, Kobayashi K, Akay Y, Akay M. Investigating the influence of PFC transection and nicotine on dynamics of AMPA and NMDA receptors of VTA dopaminergic neurons. Journal of neuroengineering and rehabilitation. 2011;8:58. doi: 10.1186/1743-0003-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Aoki C, Mahadomrongkul V, Gruber CE, Wang GJ, Blitzblau R, Irwin N, Rosenberg PA. Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2142–52. doi: 10.1523/JNEUROSCI.22-06-02142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Mahadomrongkul V, Berger UV, Bassan M, DeSilva T, Tanaka K, Irwin N, Aoki C, Rosenberg PA. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:1136–48. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costin BN, Wolen AR, Fitting S, Shelton KL, Miles MF. Role of adrenal glucocorticoid signaling in prefrontal cortex gene expression and acute behavioral responses to ethanol. Alcoholism, clinical and experimental research. 2013;37:57–66. doi: 10.1111/j.1530-0277.2012.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes & development. 2008;22:1490–500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu XT, Malenka RC, White FJ. Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:936–40. doi: 10.1523/JNEUROSCI.4715-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry-Alzate V, Tuda-Arizcun M, Buhler KM, Santos A, Gine E, Olmos P, Gorriti MA, Huertas E, Rodriguez de Fonseca F, Lopez-Moreno JA. Cocaine reverses the naltrexone-induced reduction in operant ethanol self-administration: the effects on immediate-early gene expression in the rat prefrontal cortex. Neuropharmacology. 2012;63:927–35. doi: 10.1016/j.neuropharm.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Gendreau S, Voswinkel S, Torres-Salazar D, Lang N, Heidtmann H, Detro-Dassen S, Schmalzing G, Hidalgo P, Fahlke C. A trimeric quaternary structure is conserved in bacterial and human glutamate transporters. The Journal of biological chemistry. 2004;279:39505–12. doi: 10.1074/jbc.M408038200. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Yang Y, Rothstein JD, Robinson MB. Nuclear factor-kappaB contributes to neuron-dependent induction of glutamate transporter-1 expression in astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9159–69. doi: 10.1523/JNEUROSCI.0302-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews Neuroscience. 2011;12:652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez IM, Garcia-Tardon N, Gimenez C, Zafra F. Splice variants of the glutamate transporter GLT1 form hetero-oligomers that interact with PSD-95 and NMDA receptors. Journal of neurochemistry. 2009;110:264–74. doi: 10.1111/j.1471-4159.2009.06125.x. [DOI] [PubMed] [Google Scholar]
- Griffin WC, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased Extracellular Glutamate In the Nucleus Accumbens Promotes Excessive Ethanol Drinking in Ethanol Dependent Mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, Danbolt NC. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 2009;162:1055–71. doi: 10.1016/j.neuroscience.2009.03.048. [DOI] [PubMed] [Google Scholar]
- Ji YF, Xu SM, Zhu J, Wang XX, Shen Y. Insulin increases glutamate transporter GLT1 in cultured astrocytes. Biochemical and biophysical research communications. 2011;405:691–6. doi: 10.1016/j.bbrc.2011.01.105. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain research Brain research reviews. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcoholism, clinical and experimental research. 2008;32:617–31. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Kau KS, Madayag A, Mantsch JR, Grier MD, Abdulhameed O, Baker DA. Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience. 2008;155:530–7. doi: 10.1016/j.neuroscience.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns RT, Ravindranathan A, Hassan S, Cage MP, York T, Sikela JM, Williams RW, Miles MF. Ethanol-responsive brain region expression networks: implications for behavioral responses to acute ethanol in DBA/2J versus C57BL/6J mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:2255–66. doi: 10.1523/JNEUROSCI.4372-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biological psychiatry. 2009;65:841–5. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 2010;67:81–4. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. The Journal of biological chemistry. 2008;283:13116–23. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Kornblum HI, Wiedau-Pazos M, Schubert D, Maher P, Methner A. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. Journal of neurochemistry. 2009;111:332–43. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- Li LB, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, Robinson MB. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. Journal of neurochemistry. 2006;97:759–71. doi: 10.1111/j.1471-4159.2006.03743.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu XT, Berney TG, Vartanian AJ, Stine CD, Wolf ME, White FJ. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse. 1999;34:169–80. doi: 10.1002/(SICI)1098-2396(19991201)34:3<169::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. Journal of Neuroscience. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr. Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. The Journal of biological chemistry. 2001;276:18934–40. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Dykes-Hoberg M, Rothstein JD. Altered expression of the glutamate transporter EAAT2b in neurological disease. Annals of neurology. 2004;55:469–77. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends in pharmacological sciences. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism, clinical and experimental research. 2005;29:326–33. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Melone M, Bellesi M, Conti F. Synaptic localization of GLT-1a in the rat somatic sensory cortex. Glia. 2009;57:108–17. doi: 10.1002/glia.20744. [DOI] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–37. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, Melendez R, Baker D, Kalivas PW, Seamans JK. Cystine/glutamate antiporter regulation of vesicular glutamate release. Annals of the New York Academy of Sciences. 2003;1003:445–7. doi: 10.1196/annals.1300.048. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sixth Edition Academic Press; New York: 2007. [Google Scholar]
- Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–7. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Qrunfleh AM, Alazizi A, Sari Y. Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. J Psychopharmacol. 2013;27:541–9. doi: 10.1177/0269881113482529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Sari Y. Effects of Ceftriaxone on Chronic Ethanol Consumption: a Potential Role for xCT and GLT1 Modulation of Glutamate Levels in Male P Rats. Journal of molecular neuroscience : MN. 2014a doi: 10.1007/s12031-014-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PSS, Sari Y. Effectiveness of Ceftriaxone Treatment in Preventing Relapse-like Drinking Behavior Following Long-term Ethanol Dependence in P Rats. J Addict Res Ther. 2014b;5:183. doi: 10.4172/2155-6105.1000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B, Unterwald EM, Rawls SM. Glutamate transporter subtype 1 (GLT-1) activator ceftriaxone attenuates amphetamine-induced hyperactivity and behavioral sensitization in rats. Drug and alcohol dependence. 2011;118:484–8. doi: 10.1016/j.drugalcdep.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauen T, Wiessner M, Sullivan R, Lee A, Pow DV. A new GLT1 splice variant: cloning and immunolocalization of GLT1c in the mammalian retina and brain. Neurochemistry international. 2004;45:1095–106. doi: 10.1016/j.neuint.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Reye P, Sullivan R, Scott H, Pow DV. Distribution of two splice variants of the glutamate transporter GLT-1 in rat brain and pituitary. Glia. 2002;38:246–55. doi: 10.1002/glia.10059. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res. 2001;25:1140–50. [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sari Y, Bell RL, Zhou FC. Effects of chronic alcohol and repeated deprivations on dopamine D1 and D2 receptor levels in the extended amygdala of inbred alcohol-preferring rats. Alcohol Clin Exp Res. 2006;30:46–56. doi: 10.1111/j.1530-0277.2006.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Franklin KM, Alazizi A, Rao PS, Bell RL. Effects of ceftriaxone on the acquisition and maintenance of ethanol drinking in peri-adolescent and adult female alcohol-preferring (P) rats. Neuroscience. 2013a;241:229–38. doi: 10.1016/j.neuroscience.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Prieto AL, Barton SJ, Miller BR, Rebec GV. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington’s disease. J Biomed Sci. 2010;17:62. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–46. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:9239–43. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012;227:327–35. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN, Lee MR, Choi DS. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. Journal of molecular neuroscience : MN. 2013b;51:779–87. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Asan E, Lesch KP, Kugler P. A splice variant of glutamate transporter GLT1/EAAT2 expressed in neurons: cloning and localization in rat nervous system. Neuroscience. 2002;109:45–61. doi: 10.1016/s0306-4522(01)00451-1. [DOI] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA psychiatry. 2013;70:727–39. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer I, Knackstedt LA. Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue- and cocaine-primed reinstatement of cocaine-seeking. Behavioural brain research. 2011;225:252–8. doi: 10.1016/j.bbr.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Chronic administration of (+)-amphetamine alters the reactivity of midbrain dopaminergic neurons to prefrontal cortex stimulation in the rat. Brain research. 1995;674:63–74. doi: 10.1016/0006-8993(94)01439-o. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:12406–10. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Qin L, Crews FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiology of disease. 2013;59:52–62. doi: 10.1016/j.nbd.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, Vidensky S, Brennan J, Poulsen D, Won Park J, Li Jeon N, Robinson MB, Rothstein JD. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–94. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]