Abstract
Numerous epidemiological studies link vascular disorders, such as hypertension, diabetes, and stroke, with Alzheimer’s disease. Hypertension, specifically, is an important modifiable risk factor for late onset Alzheimer’s disease. To examine the link between midlife hypertension and the onset of Alzheimer’s disease later in life, we chemically induced chronic hypertension in the TgSwDI mouse model of Alzheimer’s disease in early adulthood. Hypertension accelerated cognitive deficits in the Barnes maze test (p<0.05 after 3 months of treatment; p<0.001 after 6 months), microvascular deposition of beta-amyloid (p<0.001 after 3 months of treatment; p<0.05 after 6 months), vascular inflammation (p<0.05 in the dentate gyrus and p<0.001 in the dorsal subiculum after 6 months of treatment), blood brain barrier leakage (p<0.05 after 3 and 6 months of treatment), and pericyte loss (p<0.05 in the dentate gyrus and p<0.01 in the dorsal subiculum after 6 months of treatment) in these mice. Additionally, hypertension induced hippocampal neurodegeneration at an early age in this mouse line (43% reduction in the dorsal subiculum, p<0.05), establishing this as a useful research model of Alzheimer’s disease with mixed vascular and amyloid pathologies.
Keywords: Alzheimer’s disease, hypertension, neurodegeneration, cerebral amyloid angiopathy, blood brain barrier
Alzheimer’s disease (AD) is the most common form of dementia, and there is no universal cause or treatment to delay or stop its progression. Late onset AD (LOAD) often occurs without the contribution of known genetic risk factors and results in memory loss, irritability, and eventually death1, 2. Cerebral amyloid angiopathy (CAA), the deposition of beta-amyloid (Aβ) along vessel walls in the central nervous system (CNS), is observed in approximately 94% of AD patients3.
In addition to CAA, researchers have observed numerous ultrastructural and functional changes within the AD microvasculature. Alterations in every cellular component of the neurovascular unit (NVU), the tightly regulated network of cells that couples neuronal energy demands to modulation of blood flow, have been observed in AD patients. Furthermore, both endothelial cells and pericytes degenerate in brain capillaries in AD4, 5. Loss of these cell types has detrimental effects on blood brain barrier (BBB) integrity as well as neuronal perfusion and function6–8. Astrocyte endfeet, which ensheath brain capillaries, help regulate capillary blood flow, and maintain the extracellular milieu, are swollen in the presence of CAA6, 7. The direct cause of these cellular abnormalities has not been determined, though Aβ is toxic to neurons and other cell types in vitro9. Additionally, the presence of Aβ deposits amongst the cells of the NVU could interfere with signaling between cell types, alter cellular health and function, and result in reduced flow-mediated dilation, an indicator of vessel reactivity, also observed in AD brains10. Thus, CAA may contribute to the reduced neurovascular coupling reported in multiple AD mouse lines10–13.
More than 30% of AD cases exhibit cerebrovascular pathology in addition to CAA14 and the frequent co-incidence of stroke and AD suggests that the cerebrovascular changes that occur during AD progression compromise vascular integrity and function15. Clinical evidence suggests that cardiovascular risk factors, such as hypertension, are linked to AD onset14. In non-AD individuals, hypertension induces pathological changes in the brain including impaired cerebral autoregulation, vascular remodeling, cerebral microbleeds, and cerebral atrophy16–18. Given the vasoactive properties of Aβ19, it is unclear whether midlife hypertension is an early symptom of the vascular pathology present in AD or if it contributes to the onset of the disease. It is possible that elevated blood pressure (BP) during midlife compromises vascular integrity and leads to cellular, basement membrane, and/or BBB damage. Given the prevalence of cardiovascular risk factors in middle-aged individuals and the relevance of these risk factors to AD susceptibility, we induced chronic hypertension in an AD mouse model. We examined behavioral, cellular, and ultrastructural changes to determine the impact of chronic hypertension prior to AD onset on disease pathogenesis.
Methods
An extended methods section is available in the online-only supplement (http://hyper.ahajournals.org).
Animals
TgSwDI+/− (AD) and −/− (WT) littermates were used to conduct the experiments described. Both males and females were used in approximately equal ratios for all experiments. Animal procedures were approved by the Institutional Animal Care and Use Committee at The Rockefeller University.
L-NAME-Induced Hypertension
Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME, Sigma-Aldrich) was dissolved in drinking water so that each mouse consumed approximately 100 mg/kg body weight/day. Control mice consumed only water. Treatment was initiated at 3–4 months-of-age and continued for 3 or 6 months. BP was measured one week prior to and every week throughout treatment by tail cuff plethysmography (Kent Scientific). An average of three BP readings was obtained for each animal during measurement.
Results
L-NAME-induced chronic hypertension accelerates cognitive decline in TgSwDI mice
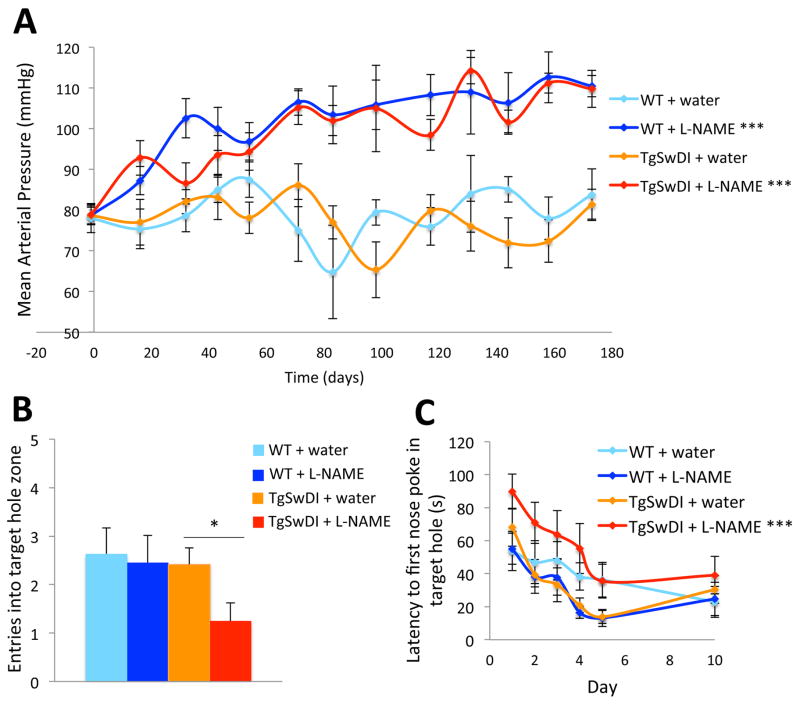
L-NAME was used to chemically induce hypertension in TgSwDI and WT mice. L-NAME treatment alone was used to induce hypertension to reduce the likelihood of hemorrhage and increase survivorship during long-term treatment20, 21. Our protocol was designed to mimic the pattern of hypertension that predisposes humans to LOAD, which occurs in individuals with midlife hypertension prior to indications of cognitive decline18, 22, 23. Since TgSwDI mice are predisposed to AD, we induced hypertension at 3–4 months-of-age, which corresponds to early adulthood in humans, and before we observed any dramatic AD-related pathological changes in this mouse line (not shown). Mice were treated for 3 or 6 months and were 6–7 or 9–10 months-of-age during testing/analysis, respectively. L-NAME-treated mice exhibited a significant increase in BP compared to water-treated animals (Figure 1A). There were no differences in levels of CD31 or α-smooth muscle actin between normotensive and hypertensive brains, suggesting that hypertension did not affect vessel number in the brains of these animals (not shown). The Barnes maze24 was used to compare the cognitive abilities of normotensive and hypertensive WT and TgSwDI mice. After 3 months of L-NAME treatment, TgSwDI mice performed significantly worse during the 5-day probe trial compared to water-treated TgSwDI mice and all WT mice (Figure 1B), indicating that chronic hypertension impacts cognition after only 3 months, well before significant cognitive impairment is evident from the transgene alone. TgSwDI mice treated with L-NAME for 6 months exhibited a similar trend (not shown). Following 6 months of treatment, hypertensive TgSwDI mice performed worse during Barnes maze training, taking significantly longer to find the escape hole compared to all other groups (Figure 1C), an effect that was not observed in TgSwDI mice after 3 months of treatment. There were no differences in baseline locomotor activity between any of the groups (not shown). Interestingly, this cohort of water-treated TgSwDI mice (9–10 months-of-age) did not have cognitive deficits relative to WT mice in this test, though chronic hypertension was able to induce significant cognitive dysfunction.
Figure 1. Chronic L-NAME treatment induces hypertension in WT and TgSwDI mice and exacerbates AD-related cognitive decline.
(A) WT and TgSwDI mice administered L-NAME in their drinking water exhibited significantly elevated BP compared to water-treated groups (***p<0.001 for effect of treatment for each genotype by 2-way ANOVA). Mice treated with water maintained constant BP. Treatments started on day 0, after baseline BP was measured. (B) TgSwDI mice treated with L-NAME for 3 months exhibited cognitive deficits in the Barnes maze relative to untreated TgSwDI and WT groups. During the probe trial carried out 5 days after training, hypertensive TgSwDI mice approached the target hole less frequently than normotensive TgSwDI (*p<0.05) and WT (p<0.05) mice. There was no effect of hypertension on cognitive function in WT mice. (C) Long-term hypertension, induced by L-NAME treatment for 6 months, affected learning in TgSwDI mice, which exhibited extended latencies to find the target hole during training compared to normotensive TgSwDI mice (***p<0.001), an effect not observed after 3 months of L-NAME treatment (not shown). [n=8–12/group (A), 10–12/group (B,C)].
Chronic hypertension induces vascular amyloid deposition in TgSwDI mice
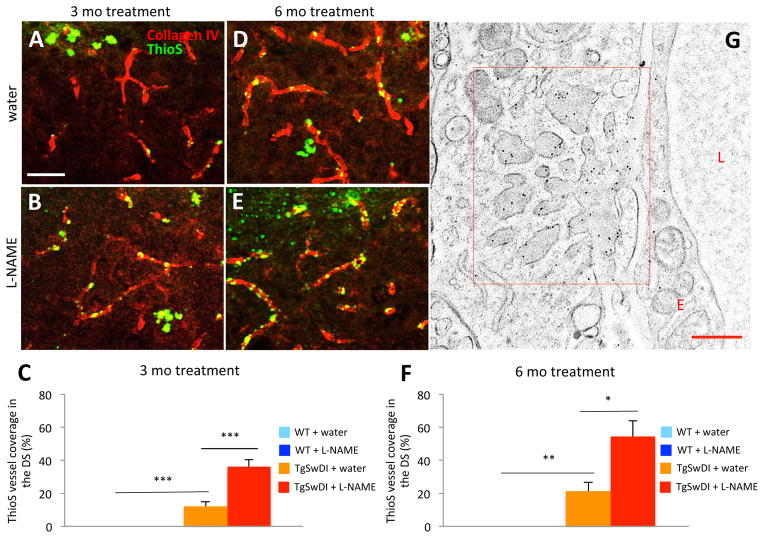
To determine if increased levels of Aβ in the hippocampus were responsible for the cognitive deficits observed in our hypertensive TgSwDI mice, we used Thioflavin-S (ThioS) to detect fibrillar Aβ. We co-stained sections with an anti-collagen IV antibody, which recognizes the basement membrane of capillaries. After quantifying the total length of collagen IV-positive capillaries in the dorsal subiculum (DS), we quantified the length of vessels co-stained by ThioS to calculate the percent of capillary CAA. We found that Aβ in the DS of normotensive TgSwDI mice (Figure 2A,C) was both microvascular and parenchymal. Hypertensive TgSwDI mice (Figure 2B,E), however, had fewer parenchymal deposits and exhibited significantly more microvascular CAA (Figure 2C,F). Though localization of Aβ deposition in the brains of normotensive and hypertensive TgSwDI mice was different, ThioS intensity overall was not (not shown), suggesting that the amount of Aβ in these groups was similar. Immunostaining with anti-Aβ40- and 42-specific antibodies revealed no significant difference in levels of either peptide between normotensive and hypertensive TgSwDI mice, though both were slightly elevated in the hypertensive group (not shown), suggesting that both peptides contribute to CAA-load in these mice. Immunoelectron microscopy confirmed that Aβ deposition appeared as finger-like clusters around microvessels (Figure 2G).
Figure 2. Hypertensive TgSwDI mice exhibit increased vascular Aβ deposition.
ThioS (green) and an anti-collagen IV antibody (red) were used to determine levels of CAA in normotensive (A,D) and hypertensive (B,E) TgSwDI mice. CAA levels were significantly increased after 3 (B,C) and 6 (E,F) months of L-NAME treatment (***p<0.001 or **p<0.01 normotensive TgSwDI vs. normotensive WT; ***p<0.001 or *p<0.05 normotensive vs. hypertensive TgSwDI; scale bar=50 μm; n=7–8/group). (G) Microvascular Aβ deposition, observed by immunoelectron microscopy, appeared as dark finger-like projections adhering to the basement membrane of capillaries in hypertensive TgSwDI brains (L, lumen; E, endothelial cell; scale bar=500 nm).
Abundant microglia surround microvessels of hypertensive TgSwDI mice
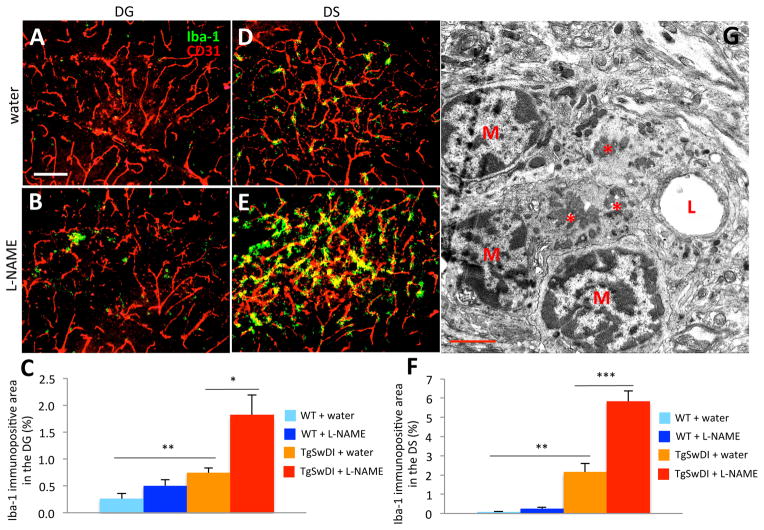
Since activated astrocytes and microglia are often abundant in Aβ-laden tissue25, we examined glial markers in normotensive and hypertensive TgSwDI and WT brains. Though levels of astrocyte markers were similar in both TgSwDI groups (not shown), the microglial marker Iba-1 was upregulated in hippocampal subregions of hypertensive TgSwDI mice compared to those of normotensive mice (Figure 3A,D vs B,E). Iba-1 expression was significantly elevated in water-treated TgSwDI mice compared to water-treated WT mice, and was even higher in L-NAME-treated TgSwDI mice (Figure 3C,F). The pattern of Iba-1 staining in the DG appears patchy (Figure 3B), likely due to the presence of larger parenchymal plaques in this region, compared to the DS, where the staining appears vascular (Figure 3E). We examined Aβ-laden microvessels by electron microscopy (EM) and confirmed that CAA is often surrounded by infiltrating microglia (M in Figure 3G).
Figure 3. Hypertensive TgSwDI mice display increased vascular microgliosis.
Microgliosis was examined in tissues using an anti-Iba-1 antibody (green) and vessels were stained with an endothelial cell-specific anti-CD31 antibody (red). Compared to water-treated TgSwDI (A,D) and WT (not shown) groups, the DG (B) and DS (E) of hypertensive TgSwDI brains exhibited significantly more Iba-1 staining after 6 months of treatment (C,F; **p<0.01 normotensive TgSwDI vs. normotensive WT; *p<0.05 or ***p<0.001 normotensive vs. hypertensive TgSwDI; scale bar=100 μm; n=6–9/group). (G) The infiltration of microglia was observed by EM of brain capillaries in hypertensive TgSwDI samples. Capillaries laden with Aβ (*) were surrounded by infiltrating microglial nuclei (M, microglia; L, lumen; scale bar=2 μm).
Hypertension disrupts tight junctions and decreases BBB integrity in TgswDI mice
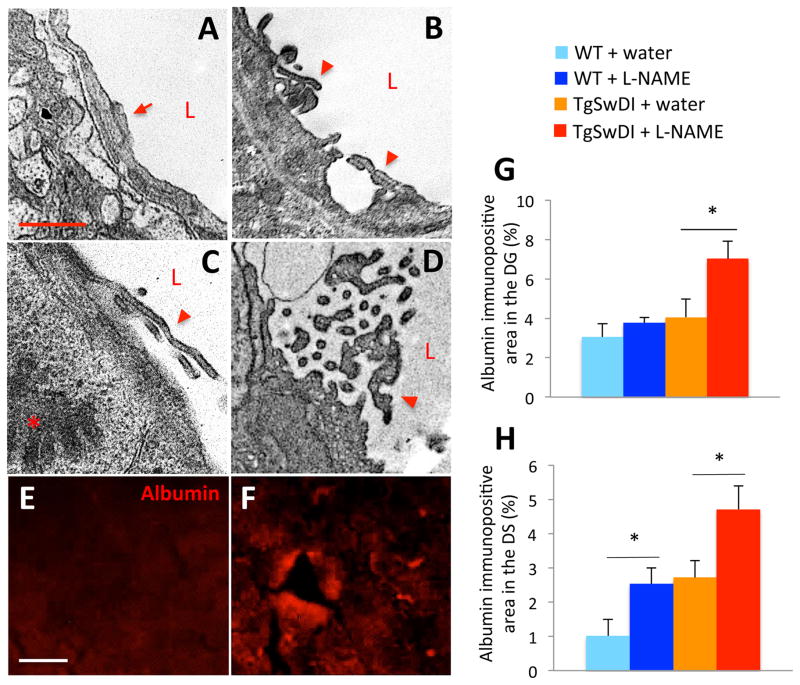
Since the BBB is disrupted in AD patients and mouse models26–28, we examined BBB integrity to determine if hypertension affects this feature of AD pathology. Under normal conditions, tight junctions, which form the basis of the BBB29, appear continuous and lay flat, preventing diffusion of blood components into the brain30 (Figure 4A). However, the tight junctions in samples from hypertensive TgSwDI mice appeared to be breaking off into the capillary lumen and lifting slightly from the endothelial cell layer (arrowheads in Figure 4B–D), providing an opportunity for BBB leakage. Given these structural alterations, we examined tissue for the presence of blood components that could enter the brain if BBB integrity were compromised. Albumin was elevated in the DS and dentate gyrus (DG) of hypertensive TgSwDI brains when compared to normotensive TgSwDI brains after only 3 months of treatment, indicating that BBB integrity was compromised due to hypertension (Figure 4E–H).
Figure 4. Hypertensive TgSwDI mice have increased BBB disruption.
Compared to capillary structure in water-treated mouse vessels (A), capillaries in brains of hypertensive TgSwDI mice exhibited tight junction alterations (arrowheads in B–D). Compared to the structure of normal tight junctions (arrow in A), those of hypertensive TgSwDI mice were often lifting or fragmented (arrowheads in B–D). The plasma protein albumin, which was present at low levels in the DS of normotensive TgSwDI brains (E), was significantly enriched in the DS (F–H; *p<0.05) and DG (not shown) of hypertensive TgSwDI mice after 3 months of L-NAME treatment. Compared to normotensive WT mice, hypertensive WT mice also exhibited significant leakage of albumin from the vasculature into the DS after 3 months of L-NAME treatment (H, *p<0.05; scale bar in A=1 μm; E=20 μm; n=4–6/group).
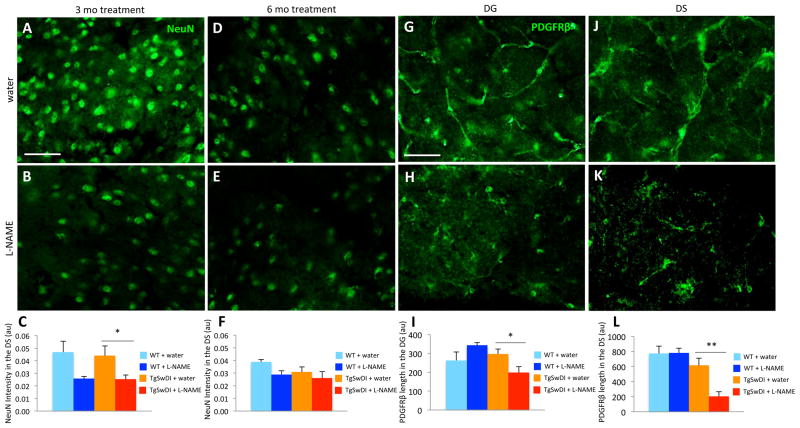
Chronic hypertension results in the loss of neurons and pericytes in TgSwDI brains
Under normal conditions, many AD mouse models, including TgSwDI mice, do not exhibit neurodegeneration31, 32, a hallmark of AD pathology. Due to the accelerated time course of other pathologies in L-NAME-treated TgSwDI mice, we examined hippocampal subregions for neuronal death. Neurodegeneration was not evident in normotensive TgSwDI mice (Figure 5A,C), but significant neuronal loss was observed after short-term L-NAME treatment (3 months) and at only 6–7 months-of-age (Figure 5B,C). Normotensive TgSwDI mice at 9–10 months-of-age began to exhibit subtle signs of neuronal loss, making hypertension-induced cell death at this age less dramatic (Figure 5D–F).
Figure 5. Hypertensive TgSwDI mice exhibit early neuron and pericyte loss.
Anti-NeuN antibody was used to identify neurons in the DS of normotensive (A,D) and hypertensive (B,E) TgSwDI mice. (C) Reduced NeuN intensity was observed in hypertensive TgSwDI mice after 3 months of L-NAME treatment (*p<0.05, n=3–9/group). (F) Since NeuN intensity was slightly decreased in normotensive TgSwDI animals after 6 months of treatment due to advanced age (9–10 months-of-age), there was no longer a significant difference between groups. Although NeuN intensity appeared reduced in the DS of hypertensive WT mice compared to normotensive WT mice after 3 and 6 months of treatment, neither change was significant (C,F). Similarly, anti-PDGFRβ antibody was used to examine pericyte coverage of vessels in normotensive (G,J) and hypertensive (H,K) TgSwDI mice. (I,L) PDGFRβ levels were significantly reduced in hypertensive TgSwDI mice in the DG and DS compared to control groups after 6 months of treatment (*p<0.05, **p<0.01; scale bars=50 μm; n=5–9/group).
Perictye loss occurs in severely affected AD patients33 and in older TgSwDI mice12, so we examined levels of PDGFRβ to determine if the expression of this pericyte marker is altered in our hypertensive groups. After 6 months of L-NAME treatment, hypertensive TgSwDI mice exhibited a significant decrease in PDGFRβ staining in both the DG and DS (Figure 5G,J vs H,K; I,L). Taken together, these results suggest that neuronal and pericytic loss can occur at a much earlier age when there is concomitant hypertension.
Discussion
Under normal conditions, hypertension induces pathological changes within the cerebral vasculature, resulting in impaired autoregulation, microbleeds, and lacunar infarcts, as well global downstream changes, such as white matter lesions and atrophy16, 34–39. In fact, these changes within the cerebral vasculature of hypertensive individuals also occur in AD patients in the absence of hypertension40–42. Additionally, midlife hypertension is a significant risk factor for the development of AD later in life14. Hypertension may initiate vascular damage prior to the onset of AD, allowing symptoms to be more pronounced and progress more quickly. Additionally, compromised vessels may be more vulnerable to the deleterious effects of Aβ. For example, since hypertension results in reduced BBB integrity (Figure 4), blood components may enter the brain before large-scale accumulation of Aβ occurs. These blood components may serve as a seed for Aβ deposition and increase vascular inflammation (Figure 3), resulting in cellular damage and the release of toxic molecules.
The hippocampus coordinates memory consolidation and spatial navigation and is one of the first regions in the brain to suffer damage in AD43. The DG receives all hippocampal inputs and passes them through the hippocampus proper (CA1-4) to the subiculum, which projects to the entorhinal cortex44. Thus, proper functioning of the DG and subiculum is essential to memory consolidation and spatial navigation. The subiculum may also be involved in the spread of Aβ, as lesions in this region inhibit plaque formation in other brain areas45.
We found that the location, but not abundance, of Aβ deposits is altered in hypertensive TgSwDI mice, which also exhibit early and dramatic cognitive decline. Our findings suggest that CAA contributes more to cognitive decline than parenchymal plaques. We also observed that microvascular Aβ adheres to capillary basement membranes, which normally bind cells of the NVU together. Therefore, CAA may act as a physical barrier to cell signaling at the NVU. Moreover, CAA appears to obstruct binding of astrocytic endfeet and contribute to pericyte loss (Figure 5). Both astrocytes and pericytes are involved in the recruitment of blood flow during neuronal activity, and damage to these cell types in hypertensive TgSwDI mice may impair neurovascular coupling.
Aβ is thought to be deposited as CAA due to failed clearance across the BBB and along perivascular spaces46. Since L-NAME treatment results in increased CAA in TgSwDI mice without an overall increase in Aβ levels, hypertension may have a detrimental effect on either or both of these clearance pathways. L-NAME inhibits endothelial nitric oxide synthase (eNOS) and thereby prevents the production of the vasodilator nitric oxide (NO). Alternatively, it could be that NO-mediated vasodilation in normotensive mice is required for effective clearance of Aβ across the BBB or along perivascular drainage pathways.
The neuronal loss we observed in hypertensive TgSwDI mice could result from reductions in blood flow as well as impaired function of NVU support cells that either degenerate (pericytes; Figure 5) or are chronically activated (microglia; Figure 3). Since brain atrophy is associated with hypertension in humans16 and hypertensive WT mice exhibit a trend towards reduced neuron number, it is possible that vascular changes that occur during hypertension in WTs, such as BBB leakage (Figure 4), affect neuronal health. However, the changes specific to hypertensive TgSwDI mice, such as increased microgliosis (Figure 3) and severe CAA (Figure 2), are likely to contribute in some way. In addition to the cytotoxic effects of Aβ itself47–49, activated microglia are known to produce cytotoxic molecules50, which may damage neurons.
We used L-NAME to induce hypertension due to its long-term tolerability and ease of administration to large cohorts of mice. L-NAME treatment resulted in elevated BP similar to other treatment types but circumvented drawbacks of other surgical and chemical techniques. Surgical wounds are susceptible to infection or injury, often requiring animals to be singly-housed and excluded from behavioral experiments. Techniques requiring infusion pumps are shorter in duration, hindering the implementation of chronic hypertension, and more severe treatment regimes, such as the pairing of L-NAME with other molecules, resulted in seizures and intracerebral hemorrhage in our mice (not shown). Therefore, long-term L-NAME treatment was best suited to mimic chronic hypertension in humans, which can last decades.
Though models of comorbid hypertension and AD are limited, our results are consistent with what has been observed in other models. Similar to our findings, surgically-induced hypertension in WT mice results in cognitive deficits and subtle amyloid pathology51, while angiotensin-II infusion in APPPS1 mice results in a more rapid onset of AD pathology52. However, it is possible that longer-term L-NAME-induced hypertension exacerbates previously observed pathologies, such as cognitive decline (Figure 1) and microvascular Aβ deposition (Figure 2), and reveals new neuropathologies not previously observed, such as microglia activation (Figure 3), BBB leakage (Figure 4), and pericytic and neuronal loss (Figure 5).
Studies linking hypertension with AD define midlife as the time between the ages of 40 and 64 years. Midlife hypertension is associated with an increased risk of developing AD, but late-life hypertension (typically defined as age ≥65) does not have this association. Importantly, studies linking hypertension with AD examine BP prior to onset of AD symptoms. Given that untreated TgSwDI mice develop some AD pathology around 6 months-of-age (Figures 2,3) and cognitive decline around 10–12 months(not shown), we initiated hypertension at an age corresponding to early adulthood developmentally, to maximize the duration of hypertension before the onset of AD symptoms. Though midlife hypertension is a significant risk factor for AD and treatment alleviates this risk18, 19, dramatic reduction in BP often occurs at later stages of AD, after which point antihypertensives are deleterious to cognitive function18, 53. Hypertension may compromise vascular integrity during midlife and lead to cellular, basement membrane, and/or BBB damage. However, after the onset of AD symptoms, low BP may aggravate the brain hypoperfusion already present in AD due to other types of vascular damage.
We found that hypertension dramatically accelerated various features of AD pathology in our model. Microvascular Aβ deposition was increased in hypertensive TgSwDI mice without an increase in overall Aβ deposition (Figure 2), suggesting that Aβ is recruited to capillaries in hypertensive individuals. Since we showed that L-NAME-treated TgSwDI mice have deficits in cognitive function (Figure 1B,C), our results suggest that capillary CAA is detrimental to cognitive function relative to other forms of deposited Aβ, an idea that has been proposed by others in the field54, 55. It is possible that the deposition of microvascular Aβ compromises the survival of NVU cells such as pericytes and endothelial cells. Although others have shown pericyte loss in TgSwDI mice at 18 months-of-age12, we found that chronic hypertension induced pericyte loss significantly earlier (9–10 months-of-age; Figure 5G–L). Furthermore, neuronal loss has never been reported in this transgenic model, yet hypertensive TgSwDI mice demonstrated significant neurodegeneration in a subregion of the hippocampus (Figure 5A–F). Moreover, not only did hypertension result in quantifiable neuronal loss, but it did so quite early (6–7 months-of-age) relative to the few other mouse lines that exhibit this feature of AD56. At 6–7 months-of-age, the first indications of AD (Aβ deposition, neuroinflammation, cognitive decline) are often just becoming apparent in other AD mouse models56–58. Our finding is notable, since neuronal loss is widely accepted as a hallmark of AD, yet does not occur in many commonly used AD models59. If hypertension indeed accelerates AD pathogenesis, then a reasonable hypothesis is that neuronal loss is a downstream effect of the abundant microvascular amyloid pathology observed in TgSwDI mice. Pairing hypertension with genetic predisposition to AD may represent a more complete model of AD than the traditional AD mouse model.
Our results suggest that hypertension has a significant effect on the onset and progression of AD pathology. Treating hypertension in midlife may be an effective strategy for reducing the likelihood of AD onset later in life, as suggested by numerous epidemiological studies17, 53. Vascular damage due to untreated hypertension results in loss of vascular tone and hypotension in older individuals. Treatment with antihypertensives after the onset of cognitive symptoms in AD patients exacerbates cognitive decline60, possibly due to further reduction in neurovascular coupling. If blood flow were reduced to areas of the brain requiring energy substrates, then low BP would only exacerbate this effect and damage already-compromised neurons and NVU components61.
Perspectives
We show that hypertension accelerates cognitive decline and other AD pathologies – CAA, neuroinflammation, BBB leakage, pericyte loss, and neurodegeneration – in transgenic mice predisposed to AD. Since many individuals with LOAD experience some form of midlife cardiovascular-related disease, such as hypertension62, our mouse model of mixed vascular and amyloid pathologies may be more relevant for studying human AD pathophysiology than a mouse model of AD alone.
Supplementary Material
Novelty and Significance.
What is new?
We used L-NAME to chemically induce chronic hypertension in the TgSwDI mouse model of AD. Long-term L-NAME treatment was well-tolerated by both WT and TgSwDI mice and consistently elevated BP for many consecutive months. Not only did chronic L-NAME treatment accelerate known pathological changes in this model, but it induced neuronal loss, which has not been observed previously in TgSwDI mice.
What is relevant?
Hypertension in early adulthood hastens AD pathogenesis in TgSwDI mice. Pathologies known to occur in this mouse model, such as cognitive decline, microgliosis, BBB damage, and pericyte loss, occurred significantly earlier when mice had chronic hypertension. We also observed neuronal loss in hypertensive TgSwDI mice, a feature of AD pathology that has not yet been reported in this mouse line.
Summary
Since hypertension accelerates AD pathogenesis, our results suggest that neuronal loss could be part of the disease trajectory in this and possibly other mouse lines if mouse models were longer-lived. Given that 1) hypertensive TgSwDI mice exhibited neuronal loss and many other AD hallmarks and 2) many individuals with LOAD experience some form of midlife cardiovascular-related disease, our mouse model of mixed vascular and amyloid pathologies may be more relevant for studying human AD pathophysiology than a mouse model of AD alone.
Acknowledgments
We thank Strickland lab members, especially Odella Jno-Charles and Allison Richards for technical assistance; Drs. Daria Zamolodchikov, Zu-Lin Chen, and Yao Yao for experimental advice; and Dr. Karin Hultman for assistance with early experiments. We also thank Dr. Kunihiro Uryu for technical and experimental EM support.
Sources of Funding
This work was supported by the American Heart Association (14SDG18300001), NIH (NS0050537), Mellam Family Foundation, May and Samuel Rudin Family Foundation, Litwin Foundation, and by John A. Hermann, Jr.
Footnotes
Conflicts of Interest/Disclosures
None.
References
- 1.Isik AT. Late onset alzheimer’s disease in older people. Clin Interv Aging. 2010;5:307–311. doi: 10.2147/CIA.S11718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balin BJ, Hudson AP. Etiology and pathogenesis of late-onset alzheimer’s disease. Curr Allergy Asthma Rep. 2014;14:417. doi: 10.1007/s11882-013-0417-1. [DOI] [PubMed] [Google Scholar]
- 3.Jellinger KA, Attems J. Prevalence and pathogenic role of cerebrovascular lesions in alzheimer disease. J Neurol Sci. 2005;229–230:37–41. doi: 10.1016/j.jns.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Kalaria RN, Hedera P. Differential degeneration of the cerebral microvasculature in alzheimer’s disease. Neuroreport. 1995;6:477–480. doi: 10.1097/00001756-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Yamashita K, Miyakawa T, Katsuragi S. Vascular changes in the brains with alzheimer’s disease. Jpn J Psychiatry Neurol. 1991;45:79–84. doi: 10.1111/j.1440-1819.1991.tb00509.x. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi Y, Miyakawa T, Shimoji A, Katsuragi S. Ultrastructural changes of blood vessels in the cerebral cortex in alzheimer’s disease. Jpn J Psychiatry Neurol. 1987;41:283–290. doi: 10.1111/j.1440-1819.1987.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 8.Lyros E, Bakogiannis C, Liu Y, Fassbender K. Molecular links between endothelial dysfunction and neurodegeneration in alzheimer’s disease. Curr Alzheimer Res. 2014;11:18–26. doi: 10.2174/1567205010666131119235254. [DOI] [PubMed] [Google Scholar]
- 9.Carrillo-Mora P, Luna R, Colin-Barenque L. Amyloid beta: Multiple mechanisms of toxicity and only some protective effects? Oxid Med Cell Longev. 2014;2014:795375. doi: 10.1155/2014/795375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iadecola C. Neurovascular regulation in the normal brain and in alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 11.Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002;283:H315–323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- 12.Park L, Koizumi K, El Jamal S, Zhou P, Previti ML, Van Nostrand WE, Carlson G, Iadecola C. Age-dependent neurovascular dysfunction and damage in a mouse model of cerebral amyloid angiopathy. Stroke. 2014;45:1815–1821. doi: 10.1161/STROKEAHA.114.005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol Dis. 2002;9:61–68. doi: 10.1006/nbdi.2001.0460. [DOI] [PubMed] [Google Scholar]
- 14.Kalaria RN, Ballard C. Overlap between pathology of alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13 (Suppl 3):S115–123. doi: 10.1097/00002093-199912003-00017. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Yu JT, Wang HF, Meng XF, Tan CC, Wang J, Wang C, Tan L. Association between stroke and alzheimer’s disease: Systematic review and meta-analysis. J Alzheimers Dis. 2015;43:479–489. doi: 10.3233/JAD-140666. [DOI] [PubMed] [Google Scholar]
- 16.Beauchet O, Celle S, Roche F, Bartha R, Montero-Odasso M, Allali G, Annweiler C. Blood pressure levels and brain volume reduction: A systematic review and meta-analysis. J Hypertens. 2013;31:1502–1516. doi: 10.1097/HJH.0b013e32836184b5. [DOI] [PubMed] [Google Scholar]
- 17.Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH, Babarskiene MR, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R. Prevention of dementia in randomised double-blind placebo-controlled systolic hypertension in europe (syst-eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 18.Joas E, Backman K, Gustafson D, Ostling S, Waern M, Guo X, Skoog I. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59:796–801. doi: 10.1161/HYPERTENSIONAHA.111.182204. [DOI] [PubMed] [Google Scholar]
- 19.Townsend KP, Obregon D, Quadros A, Patel N, Volmar C, Paris D, Mullan M. Proinflammatory and vasoactive effects of abeta in the cerebrovasculature. Ann N Y Acad Sci. 2002;977:65–76. doi: 10.1111/j.1749-6632.2002.tb04799.x. [DOI] [PubMed] [Google Scholar]
- 20.Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab. 2010;30:56–69. doi: 10.1038/jcbfm.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francois H, Makhanova N, Ruiz P, Ellison J, Mao L, Rockman HA, Coffman TM. A role for the thromboxane receptor in l-name hypertension. Am J Physiol Renal Physiol. 2008;295:F1096–1102. doi: 10.1152/ajprenal.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldstein CA. Association between chronic blood pressure changes and development of alzheimer’s disease. J Alzheimers Dis. 2012;32:753–763. doi: 10.3233/JAD-2012-120613. [DOI] [PubMed] [Google Scholar]
- 23.Shah NS, Vidal JS, Masaki K, Petrovitch H, Ross GW, Tilley C, DeMattos RB, Tracy RP, White LR, Launer LJ. Midlife blood pressure, plasma beta-amyloid, and the risk for alzheimer disease: The honolulu asia aging study. Hypertension. 2012;59:780–786. doi: 10.1161/HYPERTENSIONAHA.111.178962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes CA, Eppich C, Rao G. Selective improvement of aged rat short-term spatial memory by 3,4-diaminopyridine. Neurobiol Aging. 1989;10:337–341. doi: 10.1016/0197-4580(89)90045-6. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999;93:182–193. doi: 10.1016/s0165-5728(98)00226-4. [DOI] [PubMed] [Google Scholar]
- 26.Claudio L. Ultrastructural features of the blood-brain barrier in biopsy tissue from alzheimer’s disease patients. Acta Neuropathol. 1996;91:6–14. doi: 10.1007/s004010050386. [DOI] [PubMed] [Google Scholar]
- 27.Leonardi A, Gandolfo C, Caponnetto C, Arata L, Vecchia R. The integrity of the blood-brain barrier in alzheimer’s type and multi-infarct dementia evaluated by the study of albumin and igg in serum and cerebrospinal fluid. J Neurol Sci. 1985;67:253–261. doi: 10.1016/0022-510x(85)90121-2. [DOI] [PubMed] [Google Scholar]
- 28.Wisniewski HM, Kozlowski PB. Evidence for blood-brain barrier changes in senile dementia of the alzheimer type (sdat) Ann N Y Acad Sci. 1982;396:119–129. doi: 10.1111/j.1749-6632.1982.tb26848.x. [DOI] [PubMed] [Google Scholar]
- 29.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dermietzel R, Leibstein AG. The microvascular pattern and perivascular linings of the area postrema. A combined freeze-etching and ultrathin section study. Cell Tissue Res. 1978;186:97–110. doi: 10.1007/BF00219657. [DOI] [PubMed] [Google Scholar]
- 31.German DC, Eisch AJ. Mouse models of alzheimer’s disease: Insight into treatment. Rev Neurosci. 2004;15:353–369. doi: 10.1515/revneuro.2004.15.5.353. [DOI] [PubMed] [Google Scholar]
- 32.Timmer NM, Metaxas A, van der Stelt I, Kluijtmans LA, van Berckel BN, Verbeek MM. Cerebral level of vglut1 is increased and level of glycine is decreased in tgswdi mice. J Alzheimers Dis. 2014;39:89–101. doi: 10.3233/JAD-130437. [DOI] [PubMed] [Google Scholar]
- 33.Szpak GM, Lewandowska E, Wierzba-Bobrowicz T, Bertrand E, Pasennik E, Mendel T, Stepien T, Leszczynska A, Rafalowska J. Small cerebral vessel disease in familial amyloid and non-amyloid angiopathies: Fad-ps-1 (p117l) mutation and cadasil. Immunohistochemical and ultrastructural studies. Folia Neuropathol. 2007;45:192–204. [PubMed] [Google Scholar]
- 34.Calcinaghi N, Wyss MT, Jolivet R, Singh A, Keller AL, Winnik S, Fritschy JM, Buck A, Matter CM, Weber B. Multimodal imaging in rats reveals impaired neurovascular coupling in sustained hypertension. Stroke. 2013;44:1957–1964. doi: 10.1161/STROKEAHA.111.000185. [DOI] [PubMed] [Google Scholar]
- 35.Heistad DD, Baumbach GL. Cerebral vascular changes during chronic hypertension: Good guys and bad guys. J Hypertens Suppl. 1992;10:S71–75. [PubMed] [Google Scholar]
- 36.Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, Kase CS, Wolf PA, Seshadri S. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the framingham heart study. Stroke. 2014;45:1492–1494. doi: 10.1161/STROKEAHA.114.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Swieten JC, Geyskes GG, Derix MM, Peeck BM, Ramos LM, van Latum JC, van Gijn J. Hypertension in the elderly is associated with white matter lesions and cognitive decline. Ann Neurol. 1991;30:825–830. doi: 10.1002/ana.410300612. [DOI] [PubMed] [Google Scholar]
- 38.Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, Knottnerus IL, Staals J. Higher ambulatory blood pressure relates to new cerebral microbleeds: 2-year follow-up study in lacunar stroke patients. Stroke. 2013;44:978–983. doi: 10.1161/STROKEAHA.111.676619. [DOI] [PubMed] [Google Scholar]
- 39.Kaiser D, Weise G, Moller K, Scheibe J, Posel C, Baasch S, Gawlitza M, Lobsien D, Diederich K, Minnerup J, Kranz A, Boltze J, Wagner DC. Spontaneous white matter damage, cognitive decline and neuroinflammation in middle-aged hypertensive rats: An animal model of early-stage cerebral small vessel disease. Acta Neuropathol Commun. 2014;2:169. doi: 10.1186/s40478-014-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benedictus MR, Goos JD, Binnewijzend MA, Muller M, Barkhof F, Scheltens P, Prins ND, van der Flier WM. Specific risk factors for microbleeds and white matter hyperintensities in alzheimer’s disease. Neurobiol Aging. 2013;34:2488–2494. doi: 10.1016/j.neurobiolaging.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 41.Arai H, Kobayashi K, Ikeda K, Nagao Y, Ogihara R, Kosaka K. A computed tomography study of alzheimer’s disease. J Neurol. 1983;229:69–77. doi: 10.1007/BF00313444. [DOI] [PubMed] [Google Scholar]
- 42.Claassen JA, Diaz-Arrastia R, Martin-Cook K, Levine BD, Zhang R. Altered cerebral hemodynamics in early alzheimer disease: A pilot study using transcranial doppler. J Alzheimers Dis. 2009;17:621–629. doi: 10.3233/JAD-2009-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laczo J, Vlcek K, Vyhnalek M, Vajnerova O, Ort M, Holmerova I, Tolar M, Andel R, Bojar M, Hort J. Spatial navigation testing discriminates two types of amnestic mild cognitive impairment. Behav Brain Res. 2009;202:252–259. doi: 10.1016/j.bbr.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 44.Gorchetchnikov A, Grossberg S. Space, time and learning in the hippocampus: How fine spatial and temporal scales are expanded into population codes for behavioral control. Neural Netw. 2007;20:182–193. doi: 10.1016/j.neunet.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 45.George S, Ronnback A, Gouras GK, Petit GH, Grueninger F, Winblad B, Graff C, Brundin P. Lesion of the subiculum reduces the spread of amyloid beta pathology to interconnected brain regions in a mouse model of alzheimer’s disease. Acta Neuropathol Commun. 2014;2:17. doi: 10.1186/2051-5960-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: Sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2011;37:75–93. doi: 10.1111/j.1365-2990.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Chen S, Ahmed SH, Chen H, Ku G, Goldberg MP, Hsu CY. Amyloid-beta peptides are cytotoxic to oligodendrocytes. J Neurosci. 2001;21:RC118. doi: 10.1523/JNEUROSCI.21-01-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folin M, Baiguera S, Tommasini M, Guidolin D, Conconi MT, De Carlo E, Nussdorfer GG, Parnigotto PP. Effects of beta-amyloid on rat neuromicrovascular endothelial cells cultured in vitro. Int J Mol Med. 2005;15:929–935. [PubMed] [Google Scholar]
- 49.Liu ML, Hong ST. Early phase of amyloid beta42-induced cytotoxicity in neuronal cells is associated with vacuole formation and enhancement of exocytosis. Exp Mol Med. 2005;37:559–566. doi: 10.1038/emm.2005.69. [DOI] [PubMed] [Google Scholar]
- 50.Nakajima K, Kohsaka S. Microglia: Activation and their significance in the central nervous system. J Biochem. 2001;130:169–175. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- 51.Carnevale D, Mascio G, D’Andrea I, Fardella V, Bell RD, Branchi I, Pallante F, Zlokovic B, Yan SS, Lembo G. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60:188–197. doi: 10.1161/HYPERTENSIONAHA.112.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cifuentes D, Poittevin M, Dere E, Broqueres-You D, Bonnin P, Benessiano J, Pocard M, Mariani J, Kubis N, Merkulova-Rainon T, Levy BI. Hypertension accelerates the progression of alzheimer-like pathology in a mouse model of the disease. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.04139. [DOI] [PubMed] [Google Scholar]
- 53.Liu J, Liu S, Tanabe C, Maeda T, Zou K, Komano H. Differential effects of angiotensin ii receptor blockers on abeta generation. Neurosci Lett. 2014;567:51–56. doi: 10.1016/j.neulet.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 54.Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in alzheimer disease: Correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol. 2003;62:1287–1301. doi: 10.1093/jnen/62.12.1287. [DOI] [PubMed] [Google Scholar]
- 55.Soffer D. Cerebral amyloid angiopathy--a disease or age-related condition. Isr Med Assoc J. 2006;8:803–806. [PubMed] [Google Scholar]
- 56.Eriksen JL, Janus CG. Plaques, tangles, and memory loss in mouse models of neurodegeneration. Behav Genet. 2007;37:79–100. doi: 10.1007/s10519-006-9118-z. [DOI] [PubMed] [Google Scholar]
- 57.Janus C. Search strategies used by app transgenic mice during navigation in the morris water maze. Learn Mem. 2004;11:337–346. doi: 10.1101/lm.70104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lovasic L, Bauschke H, Janus C. Working memory impairment in a transgenic amyloid precursor protein tgcrnd8 mouse model of alzheimer’s disease. Genes Brain Behav. 2005;4:197–208. doi: 10.1111/j.1601-183X.2004.00104.x. [DOI] [PubMed] [Google Scholar]
- 59.Zahs KR, Ashe KH. ‘Too much good news’ - are alzheimer mouse models trying to tell us how to prevent, not cure, alzheimer’s disease? Trends Neurosci. 2010;33:381–389. doi: 10.1016/j.tins.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Kennelly S, Collins O. Walking the cognitive “minefield” between high and low blood pressure. J Alzheimers Dis. 2012;32:609–621. doi: 10.3233/JAD-2012-120748. [DOI] [PubMed] [Google Scholar]
- 61.de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012;2012:367516. doi: 10.1155/2012/367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yano Y, Stamler J, Garside DB, Daviglus ML, Franklin SS, Carnethon MR, Liu K, Greenland P, Lloyd-Jones DM. Isolated systolic hypertension in young and middle-aged adults and 31-year risk for cardiovascular mortality: The chicago heart association detection project in industry study. J Am Coll Cardiol. 2015;65:327–335. doi: 10.1016/j.jacc.2014.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.