Abstract
Rationale
Acute cocaine administration produces an initial rewarding state followed by a dysphoric/anxiogenic “crash”.
Objective
To determine whether individual differences in the relative value of cocaine’s positive and negative effects would account for variations in subsequent drug self-administration.
Methods
The dual actions of cocaine were assessed using a conditioned place test (where animals formed preferences for environments paired with the immediate rewarding effects of 1.0 mg/kg i.v. cocaine or aversions of environments associated with the anxiogenic effects present 15 min post-injection) and a runway test (where animals developed approach-avoidance “retreat” behaviors about entering a goal-box associated with cocaine delivery). Ranked scores from these two tests were then correlated with each other and with the escalation in the operant responding of the same subjects observed over 10 days of 1- or 6-h/day access to i.v. (0.4 mg/inj) cocaine self-administration.
Results
a) larger place preferences were associated with faster runway start latencies (rs=−0.64), but not with retreat frequency or run times; b) larger place aversions predicted slower runway start times (rs=0.62) and increased run times (rs=0.65) and retreats (rs=0.62); c) response escalation was observed in both the 1-h and 6-h self-administration groups and was associated with increased CPPs (rs=0.58) but not CPAs, as well as with faster run times (rs=−0.60).
Conclusions
Together, these data suggest that animals exhibiting a greater positive than negative response to acute (single daily injections of) cocaine are at the greatest risk for subsequent escalated cocaine self-administration, a presumed indicator of cocaine addiction.
Keywords: cocaine, drug self-administration, extended access, cocaine addiction, conditioned place test, operant runway, opponent processes, drug abuse, drug reward, drug aversion
INTRODUCTION
Acute cocaine administration has been described by users as producing two diametrically opposing affective states: an initial euphoric/rewarding “high” that is followed in time by a dysphoric/anxiogenic “crash” (Gawin 1991; Gawin and Kleber 1986; Koob and LeMoal 2006; Lambert et al. 2006; Port and 1983; van Dyke and Byck, 1982; Williamson et al. 1997). These positive and negative actions of cocaine have also been reported in animal studies. For example, cocaine can induce conditioned preferences for distinctive places paired with its administration (e.g., Bardo, et al. 1995; Mucha et al. 1982; Mueller and Stewart 2000; Tzschentke 1998), it reduces thresholds for rewarding brain stimulation (Ahmed et al. 2002; Markou and Koob 1991, 1992), and is readily self-administered (e.g., Ettenberg et al. 1982; Foltin and Fischman 1994; Goeders 1988; Roberts et al. 1977; Wolverton 1992). In contrast to its rewarding actions, cocaine also induces aversions for places associated with its delayed anxiogenic effects (Ettenberg et al. 1999; Jhou et al. 2013; Knackstedt et al. 2002), enhances measures of an animal’s anxiety in an open field or elevated-plus maze (Rogerio and Takahashi, 1992; Simon et al. 1994; Yang et al. 1992), and sensitizes subjects’ startle responses to conditioned fear stimuli (Borowski et al. 1994; Willick and Kokkinidis 1995). These behavioral effects are paralleled by the drug’s neurochemical actions in that cocaine enhances neurotransmission within putative dopaminergic reward pathways (Dackis and O’Brien 2001; Koob et al. 1994; Roberts et al. 1977; Wise et al. 1995) while also inducing the release of corticosteroids associated with anxiogenic states (Goeders 1997; McReynolds et al. 2014; Moldow and Fischman 1987; Palamarchouk et al. 2009). Additionally, its administration has been associated with the emission of ultrasonic vocalizations that are reportedly associated with both positive and negative subjective states (Barker et al. 2014; Browing et al. 2011; Covington and Miczek 2003; Ma et al. 2010).
Our laboratory has extensively studied these putative dual actions of cocaine using two behavioral test procedures: a modified conditioned place test and a runway model of drug self-administration (see review by Ettenberg 2004). In the former, we have shown that while animals exhibit conditioned place preferences (CPPs) for a distinct environment paired with the immediate effects of i.v. cocaine, the same doses of cocaine produce conditioned place aversions (CPAs) for the same environment when paired with the effects present 15-min post-injection (Ettenberg et al. 1999; Knackstedt et al. 2002; see also Jhou et al. 2013). In the runway test, undrugged rats are permitted to traverse a straight-arm alley once daily for a single i.v. infusion of cocaine delivered upon goal-box entry (Ettenberg 2009). In this procedure, animals leave the start box faster and faster each day (an indication of their motivation to seek the drug) but develop over trials a unique approach-avoidance conflict about goal box entry. They run quickly to the goal box threshold, but then stop and retreat toward the start box; a behavior that the subjects repeat with increasing frequency over trials before eventually entering the goal box and receiving the cocaine reinforcer (Ettenberg and Geist 1991, 1993). Both the place test and runway test are therefore uniquely sensitive to both the positive and negative actions of the same dose of cocaine.
The demonstration that acute single daily injections of cocaine have both positive and negative consequences implies that the decision or motivation to seek cocaine is likely related to the relative valence of these two opposing actions during initial experiences with the drug. Presumably, individuals for whom the acute negative consequences outweigh the positive would be less likely to re-engage in drug-seeking behavior compared to those for whom the positive outweighs the negative effects of the drug. To examine this possibility in the animal laboratory, we sequentially employed the conditioned place test and runway test to assess the positive and negative attributes of the cocaine experience in the same animals. These subjects were then permitted to self-administer cocaine for either 1-h or 6-h per day. Previous work has shown that rats provided extended 6-h/day of access to cocaine produce escalations in their rate of cocaine self-administration over trials – a response pattern that some have interpreted as evidence for and a model of cocaine “addiction” (e.g., Ahmed and Koob 1998, 1999; Ben Shahar et al. 2004; Calipari et al. 2014; Ferrario et al. 2005). In the present study, we correlated the performance of the same animals in the place conditioning, runway and drug self-administration tests as a means of assessing whether or not the acute affective response to cocaine has predictive value for the development of cocaine “addiction” as measured by the magnitude of response escalation observed in the self-administration test.
MATERIALS AND METHODS
Subjects
Thirty male albino Sprague-Dawley rats (Charles River Laboratories, Hollister, CA) weighing 275 – 325 g at the start of the experiment served as the subjects. Animals were housed in pairs in plastic ventilated cages located within a temperature-controlled (22° C), 12/12 h light/dark cycle (lights on at 20:00) vivarium. Free access to food (Purina Rat Chow) and water were provided throughout the study. All animal handling and experimental procedures adhered to the NIH Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the University of California at Santa Barbara’s Institutional Animal Care and Use Committee (IACUC).
Surgery
Rats were acclimated to human handling for 1 wk prior to i.v. catheterization, which was completed during deep anesthesia induced by an i.m. injection of a mixed solution consisting of 56 mg/kg ketamine and 7.5 mg/kg xylazine. The catheter consisted of PE-20 tubing (Dow Corning Corp, Midland, MI, USA) inserted into the right jugular vein with the open end affixed to a stainless steel guide cannula (Item 313G; Plastics One, Roanoke, VA, USA) that was passed subcutaneously and protruded through a 2mm hole on the midline of the animal’s back. The guide cannula was cemented to a 2-cm square of Mersilene surgical mesh (Bard; Warwick, RI, USA) that was laid flat on the animal’s back and secured by suture to the subdermal tissue. Following surgery, rats received the antibiotic ticarcillin disodium/clavulanate potassium (Timetin; 50 mg/kg, i.v.) and 0.1 ml of heparin (6.0 IU/0.1 ml prepared in 0.9% physiological saline, i.v.) as a prophylactic measure against microbial infection and to promote catheter patency. One week was provided for recovery during which the catheters were flushed daily with 0.1 ml of the antibiotic ticarcillin disodium/clavulanate potassium (Timetin® 20 mg/kg, i.v.) and 0.1 ml of heparinized 0.9% physiological saline to maintain catheter patency. Catheter patency was confirmed weekly by observing the impact of an i.v. injection of the fast-acting barbiturate, methohexital sodium (Brevital; 2.0 mg/kg/0.1 ml). Animals that did not lose their righting reflex were removed from the study and their data not included in the statistical analysis. For more complete details both the surgical and post-surgical treatment protocols see Wenzel et al. (2014).
Drugs
Cocaine hydrochloride was provided by the National Institute of Drug Abuse. The drug was dissolved in a sterile vehicle solution of 0.9% physiological saline and delivered in a volume of 0.1 ml over a period of 4.3 s via a 10 ml syringe seated in a motorized syringe pump (Razel Scientific Instruments, St Albans, VT, USA). Control injections consisted of a comparable volume of saline alone. The dose of cocaine used in the place conditioning and runway tests (1.0 mg/kg i.v.) was selected on the basis of optimal results observed in prior studies (Ettenberg and Bernardi 2007; Ettenberg et al. 1999; Raven et al. 2000). The cocaine dose in the self-administration task (0.4 mg/injection i.v.) was selected for equivalency (based on the subjects’ weights) to the dose (0.25mg/inj; corresponding to a dose of 0.75/mg/kg) previously used by us and others in studies of response escalation among animals given 6-h daily access to the drug (e.g., Ahmed and Koob 1998, 1999; Ben-Shahar et al. 2004, 2005; Paterson and Markou 2003).
Behavioral Apparatus
a) Place Conditioning Apparatus
The apparatus consisted of two identical wooden rectangular enclosures (156-L × 34-W × 30-H cm) each of which could be subdivided into separate compartments using removable walls. Two larger compartments (61-L × 34-W × 30-H cm) were at opposite ends separated by a smaller middle chamber (34-L × 24-W × 30-H cm). The larger compartments differed in their visual (black or white), tactile (floor Plexiglas or soft bedding) and olfactory (acetic acid scent or no scent) properties. The middle section of the apparatus was painted gray and had a wooden floor. Situated above each apparatus was a digital camera connected by interface to a desktop PC running Any-Maze software (Stoelting Co, Wood Dale, IL, USA) that recorded the precise location of the animal in real time.
b) Runways
The apparatus consisted of two identical wooden straight-arm runways (160-L × 12-W × 44-H cm) each with a start and goal box (each 23 × 20 × 44 cm) attached at opposite ends. Automated sliding doors separated the goal and start boxes from the long alley portion of the apparatus. Thirteen pairs of infrared photocell emitter-detectors were evenly distributed 2.5 cm above the floor along the length of each runway with the first pair located within the start box and the final pair within the goal box. The infrared sensors were connected to a custom interface (Hamilton-Kinder Co, San Diego, CA., USA) controlled by a desktop computer running Any-Maze software that recorded the precise location of the animal in the runway in real time. The software also controlled the start box and goal box doors, recorded start latencies, run times, and retreat frequencies (see Procedures below), and delivered the cocaine reinforcer via activation of a syringe pump (Med Associates, St. Albans, VT, USA) upon the animal’s entry into the goal box. For a more complete description of the runway apparatus see Geist and Ettenberg (1990).
c) Operant Self-Administration Chambers
Ten standard Med Associates operant conditioning boxes (25-L × 29-W × 30-H cm) each located within its own sound-attenuating chamber were used for the self-administration test. Each chamber contained a food trough, a food-pellet dispenser, a 2.8-W cue light and a retractable lever. Reinforcer delivery (food or drug), the control of the cue light and lever, and data collection were all accomplished by a desktop PC running Med Associates software (MED-PC for Windows, Version 1.17; St. Albans, VT, USA). An Instech Laboratory liquid swivel was centered on the roof of each operant conditioning box to provide the animal with freedom of movement. PE-20 tubing connected a drug-filled 10 ml syringe (seated in a Med Assoc. syringe pump) to the swivel and the swivel to the connector on each animal’s back.
Procedures
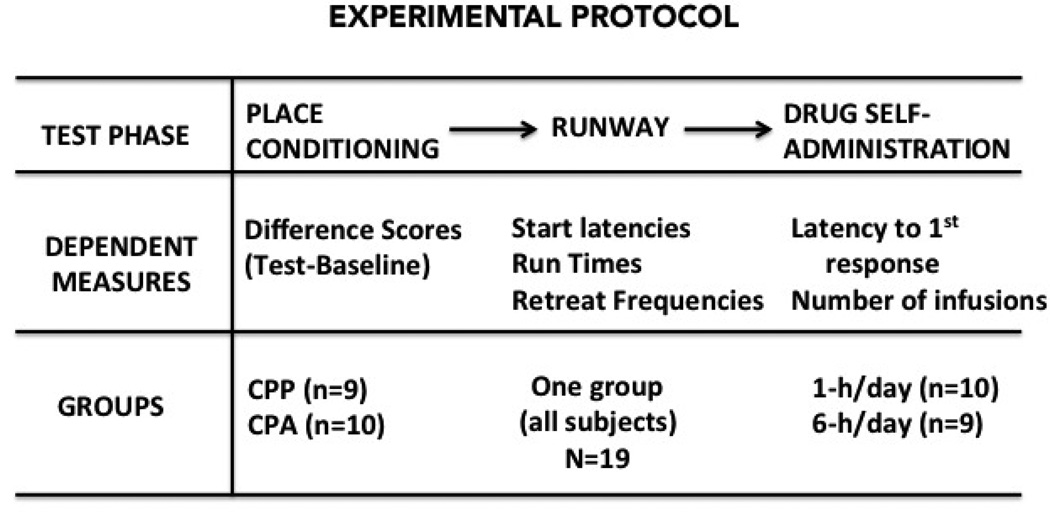
Subjects were run first in the Place Conditioning Test, then the runway, and then in the self-administration test. Since the goal of this research was to assess the relationship between the performance of the same subjects in three different behavioral tests, only those animals that completed the entire test sequence (n=19) were included in the data analyses. In each case, the test procedures employed here were precisely as described in several of our previous reports (e.g., see Ben Shahar et al. 2004, 2005; Ettenberg 2004, 2009; Ettenberg et al. 1999). A schematic summary of the experimental protocol is provided in Figure 1.
Figure 1.
Schematic representation of the experimental protocol depicting the order of events, dependent measures, and group designations in each of three tests conducted sequentially: the place conditioning, runway, then self-administration test.
a) Place Conditioning
Place conditioning was conducted in three stages: a 15-min preconditioning baseline during which the time spent in each of the three compartments was measured, 16 place-conditioning trials, and a final post-conditioning preference test conducted identically to baseline. The 16 place-conditioning trials each consisted of an injection of either cocaine (1.0 mg/kg i.v.) or saline followed by confinement to either the white or black compartment for 5 min. On alternating days the subjects were provided the alternate treatment such that at the end of conditioning each rat had received 8 cocaine pairings with one side of the apparatus and 8 saline pairings with the alternate side. The use of 16 conditioning trials was to ensure robust results that minimize the within-group variability common to place conditioning procedures particularly given the short 5-min duration of the conditioning trials employed here. Approximately half of the animals were placed into the conditioning apparatus immediately after cocaine injection (a conditioned place preference [CPP] group; n=9) while the remaining animals were placed into the apparatus 15-min post-injection (a conditioned place aversion [CPA] group; n=10). The drug-paired environments and the order of treatments were counterbalanced within each group. Note that the CPAs produced with this method are thought to be a consequence of the aversive affective response to cocaine and not to some aspect of drug withdrawal since cocaine levels in both brain and plasma remain high 15-min post-IV injection (e.g., Barbieri et al. 1992; Booze et al. 1997). Following conditioning, a single test trial was conducted as described for baseline. Difference Scores (Time in the drug-paired side on Test Day minus the time spent there on baseline) identified whether each rat exhibited a shift toward (CPP) or away (CPA) from the cocaine-paired environment as a consequence of conditioning.
b) Cocaine-seeking in the Runway
Forty-eight hours after completion of the place-conditioning test, each animal was allowed to freely explore the apparatus (with the goal door closed) during a single 10-min habituation trial. The next day, the subjects, now combined into one group (n=19), began a 14-day test protocol consisting of single daily trials as previously described (e.g., see Ettenberg et al. 2009). Three dependent measures were recorded for each animal on every trial: Start Latency (time to leave the start box once the start door was opened), Run Time (time required to enter the goal box once a subject had left the start box), and the number of approach-avoidance Retreat behaviors (defined as forward movement for a distance of at least 3 photo-beams followed by a stop and a retreat back for a distance of at least 2 photo-beams). Upon the subject’s entry into the goal-box, the goal door was automatically closed and a single injection of cocaine (1.0 mg/kg i.v.) administered. Each rat remained in the goal box for 5-min after which it was returned to its home cage.
c) Lever-Press Self-Administration
Forty-eight hours after completion of the final runway trial, the rats were transitioned to the lever-press self-administration phase of the study. We employed here the same procedures as previously described (e.g., Ben Shahar et al. 2004, 2005; Su et al. 2013). To facilitate the learning of the operant response, the rats were placed on a restricted diet of 15g of Purina® rat chow per day, and then shaped to lever-press for 45 mg food pellets (P.J. Noyes Company, Lancaster, NH, USA) delivered on a FR1 schedule during daily 1-h sessions conducted over two consecutive days. All rats successfully learned the operant behavior by the 2nd day of training after which they were again provided ad libitium access to food in their homes cages.
Daily 1-h cocaine self-administration sessions were initiated 24-h after the completion of operant training. Rats were individually placed into an operant conditioning box, followed 30–90 s later by the extension of the lever. Lever-presses were then reinforced on a FR1 schedule by i.v. infusion of cocaine (0.4 mg/inj). To prevent accidental overdose, no cocaine was delivered over the next 20-s during which time the cue light remained illuminated. The termination of cue light signaled the availability of the next reinforcer. After seven days of self-administration training, rats were randomly assigned to one of two groups both of which were given the opportunity to self-administer cocaine for 10 additional daily sessions. A “brief-access” group (n=10) continued with 1-h sessions and an “extended-access” (n=9) group self-administered cocaine for 6-h/day.
RESULTS
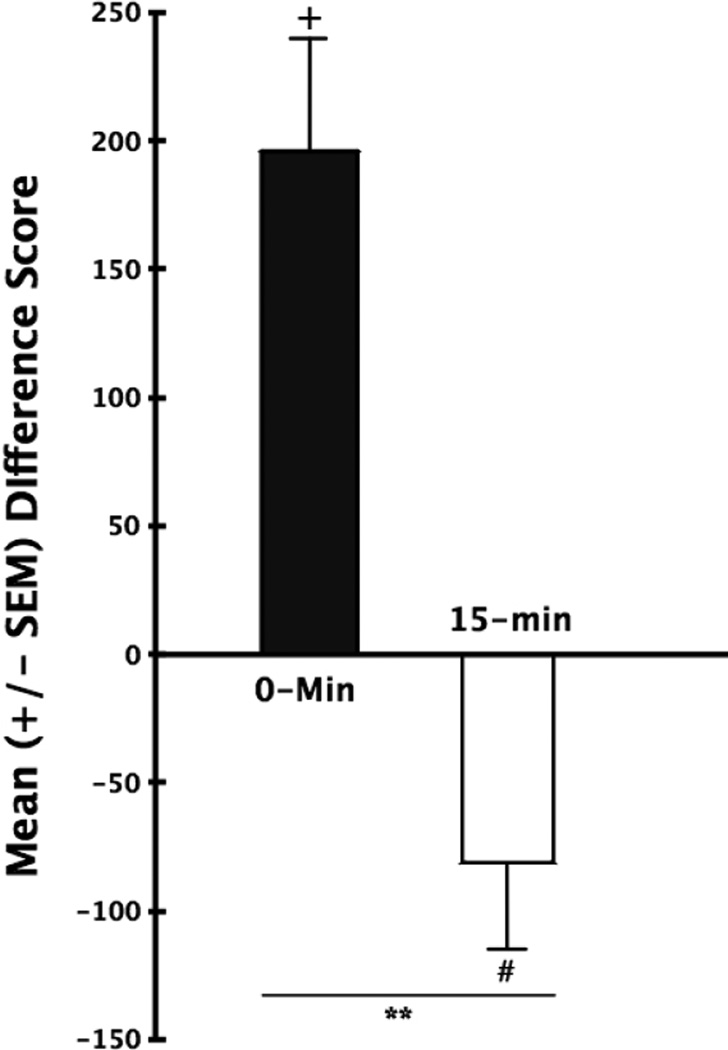
Place Conditioning
Figure 2 depicts the mean (+SEM) difference scores of animals conditioned to the immediate positive effects of cocaine (the 0-min group in the figure) and those conditioned to the delayed negative effects of the drug (the 15-min group). While the initial times spent on the white or black sides of the apparatus were not different from one another for either group on baseline (repeated measures two-tailed t-tests, p>.05; data not shown) as Figure 2 illustrates, animals exhibited a post-conditioning shift in preference toward the cocaine-paired side of the apparatus in the 0-min group and an avoidance of the side paired with the delayed effects of cocaine in the 15-min group. The statistical reliability of these CPPs and CPAs were confirmed by one-group t-tests comparing the value of each group’s difference score from “0” (for the CPP group: t(8) = 4.93, p<.002; and for the CPA group t(9)=2.39, p<.05). An independent group two-tailed t-test computed on these data additionally confirmed a statistically significant difference in the behavior of the two groups: t(17) = 3.650, p < 0.002.
Figure 2.
Mean (±SEM) Difference Scores (time spent in the cocaine-paired environment on test day minus the time spent there prior to conditioning). The score above the X-axis reflects a shift toward the side associated with cocaine (a conditioned place preference; CPP) while the score below the line represents avoidance of the cocaine-paired environment (conditioned place aversions; CPA). Each group’s mean difference score was significantly different from “0” (+ p<.002; # p<.05). The two groups also performed differentially from one another (**p<.0.01).
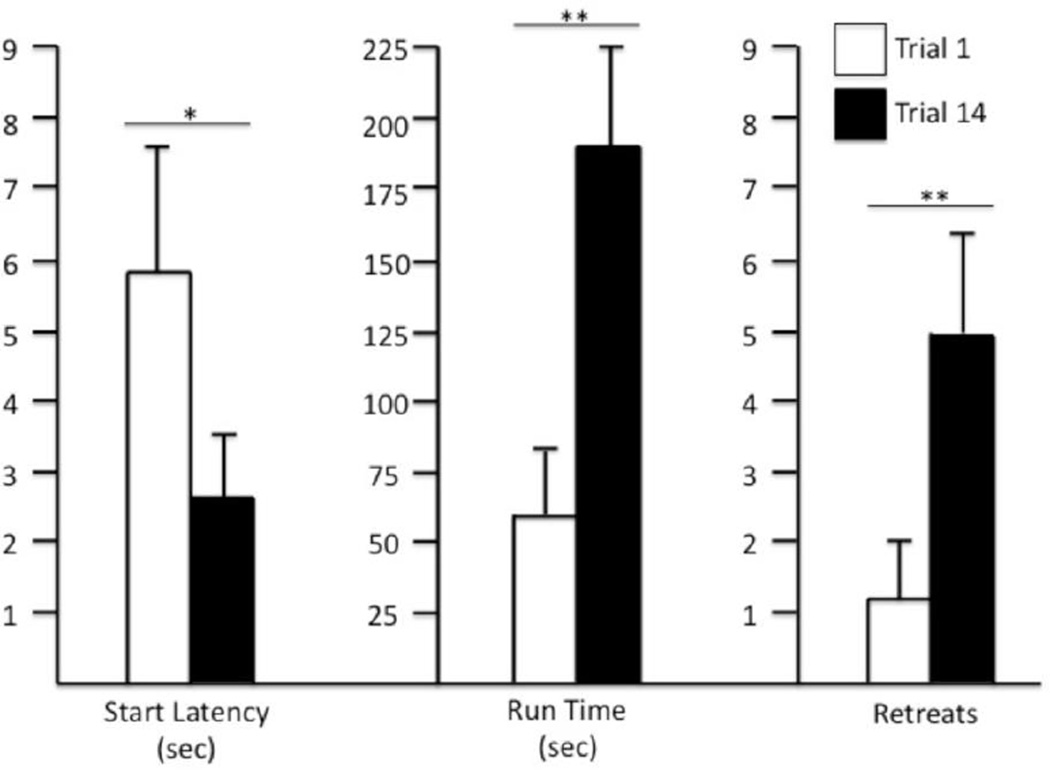
Runway
Individual One-Way repeated measures ANOVAs were computed on the Start Latencies, Run Times and Retreat Behaviors of the subjects during the 14 trials/days of runway testing. The animals’ latency to initiate responding decreased over trials (Start Latency, F(13,234) = 4.48, p<.006) while the number of approach-avoidance retreat behaviors, and as a consequence the time to enter the goal box, both increased over trials (Retreat frequency, F(13,234) = 3.368, p<.02; Run Time, F(13,234) = 2.31, p<.045). These results are reflected in Figure 3, which shows the group’s mean performance on each of the dependent measures during the first versus the final day of testing. Separate repeated-measures two-tailed t-tests computed on the data depicted in Figure 3 confirmed that while subjects initiated responding faster by the end of testing (start latencies, Trial 1 vs Trial 14, t(18)=2.15, p<.05), run times and approach-avoidance retreat behaviors increased from the first to last trial (Retreats, t(18)=2.99, p<.008; Run Times, t(18)=2.80, p<.02).
Figure 3.
Mean (+SEM) Start Latency, Run Time and Retreat Frequency on the first (white bars) and final (dark bars) trial for rats running a straight alley for single daily injections of i.v. cocaine. Separate repeated measures t-tests confirmed changes in performance over the course of testing. *p<.05, **p<.01.
Self-Administration
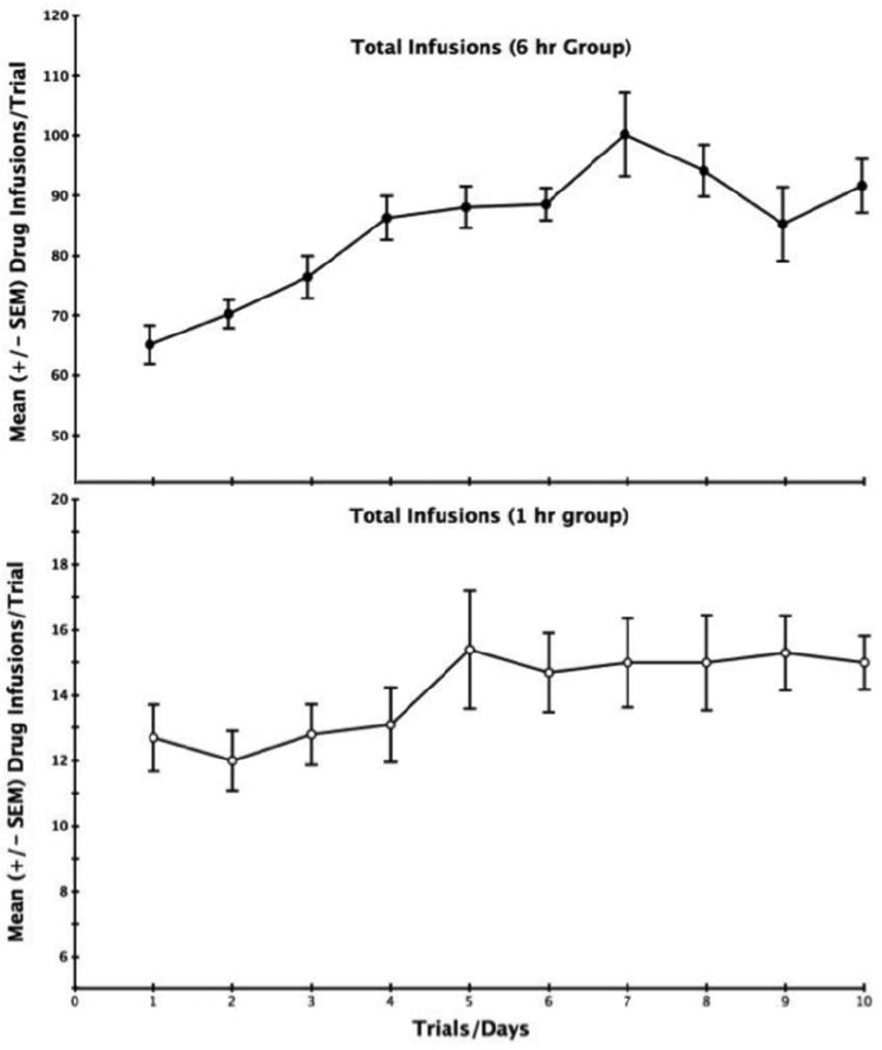
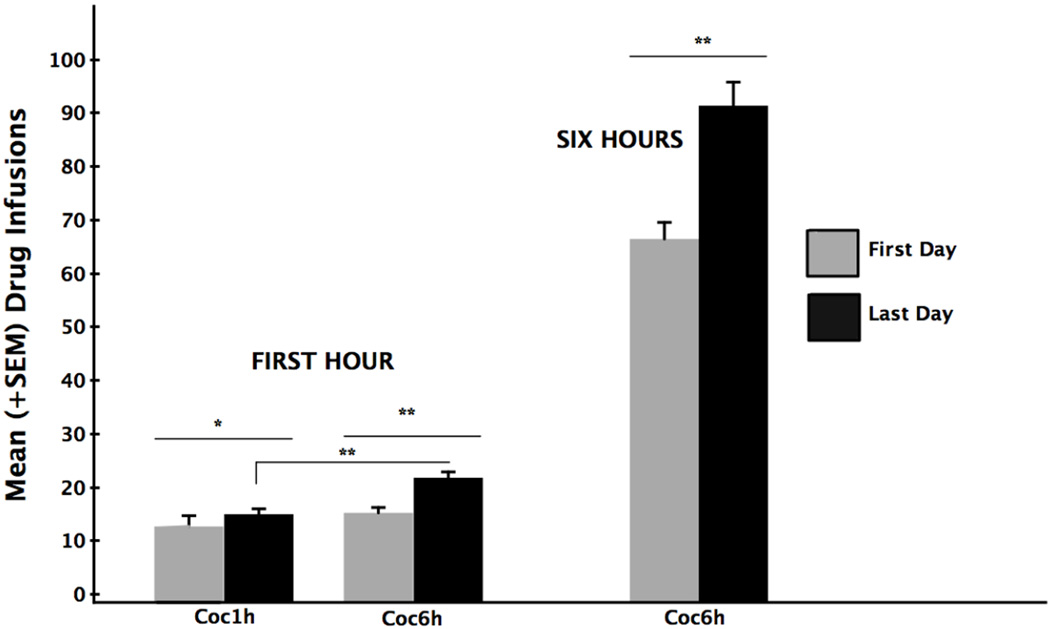
In the self-administration test, both the extended access (6-h) and brief-access (1-h) groups were comparably and highly motivated to initiate responding. The mean latency (± SEM) to emit the first lever-press once the lever was introduced into the operant conditioning box was 0.22 s (±0.12) in the 6-h group and 0.35 s (±0.14) in the 1-h (independent group t-test p>.05). Thus, once placed into the operant chamber, the animals hovered in the vicinity of the lever and began responding quickly as soon as the lever was fully extended. Once the responding was initiated, group differences in performance emerged. The mean (±SEM) number of cocaine-reinforced responses earned by the 6-h and 1-h groups over the 10-days of testing is depicted in Figure 4. Separate repeated measures one-way ANOVAs computed on the data for each of the two groups confirmed that both the brief-access and extended-access animals significantly increased their earned drug intake over trials. Brief access (1-h) animals increased their number of self infusions from an average of 12.7 on the first trial to 15.0 infusions on Day 10 [F(9,81) = 2.69, p < 0.01], while extended access (6-h) animals increased reinforced responding from an average of 65.4 infusions on the first day to 91.2 infusions on the last day [F(9,72) = 3.29, p < 0.003]. While both groups increased their drug intake over trials, the rate at which they did so (i.e., the slopes of the curves depicted in the top versus bottom portions of Figure 4) was significantly greater in the 6-h group than in the 1-hour group (independent group single-factor ANOVA [F(1,17) = 32.07, p <0.001] reflecting stronger escalation over trials in the 6-h group.
Figure 4.
Mean (±SEM) earned cocaine infusions/trial for the 6-hr (top) and 1-hr (bottom) groups over the course of a 10-day test self-administration period. While both groups exhibited statistically reliable increases in responding over trials (1-h group, p<.01; 6-h group, p<.0003) the average rate of rise in responding (i.e., the average slope of the response curves) was greater in the 6-h than 1-h group (p<.001).
To further facilitate direct comparisons of the two groups, the mean (±SEM) number of infusions during the first hour of Trial 1 and Trial 10 are provided for each group on the left side of Figure 5. The average number of infusions during the entire session for the extended access (6-h) group is also included on the right side of the figure. The data from the first hour of testing (the four bars on the left side of the figure) were subjected to a Group × Trial repeated measures ANOVA which identified: a significant main effect for Trial [F(1,17) = 36.58, p < 0.001] reflecting the overall increase in reinforced responding observed in both groups from Day 1 to day 10; a main effect of Group [F(1,17) = 15.54, p < 0.001] confirming that the 6-h group emitted more reinforced responses than did the 1-h group when averaged across the first and last trial; and a Group × Trial interaction [F(1,17) = 9.559, p < 0.008] indicating that while both groups exhibited an increase in cocaine self-administration over trials, the magnitude of that escalation was greater in the 6-h group (a 37.2% increase) than the 1-h group (21.6%). Post-hoc analyses confirmed that while the two groups responded comparably during the first hour of the first Day (p>.05), and while both the short-access and extended-access groups responded more for cocaine on Day 10 than on Day 1 (p<.02 and p<.002, respectively), the 6-h group earned more infusions during the first hour of Day 10 than did the 1-h group (p<.05). A significant increase in drug infusions from Day 1 to Day 10 was also observed in the 6-h group across the entire 6-h test session (right side of Figure 4; repeated measures two-tailed t-test; t(8) = 6.49, p<.001). Finally, note that the increases in self-administration responding developed over the course of the final 10 days of testing; there were no differences between the responding of the two groups during the 7-day self-administration training period nor, as described above, during the first hour of the first day of testing (p>.05).
Figure 5.
Mean (±SEM) number of earned cocaine infusions during the first and last trial/day for the 1-h and 6-h self-administration groups. The four bars to the left reflect reinforced responding during the first hour of testing while the two bars to the right show the change in responding over the entire 6-h session for the extended-access group alone. *p<.05, **p<.01
Correlations
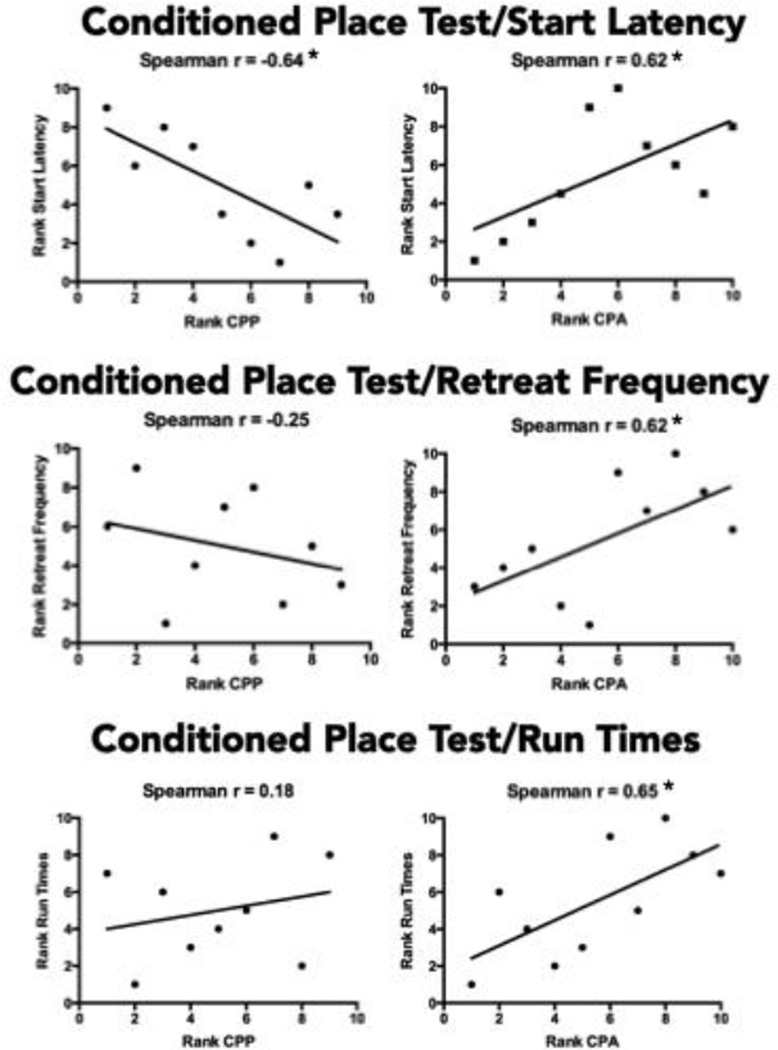
Spearman Rank-Order correlations (rs) were computed to assess the relationship between the subjects’ performance across the three behavioral tasks. Figure 6 depicts the ranking of each subject’s performance in the conditioned place test relative to that observed in the runway test. CPP scores correlated negatively with start latencies in the runway (rs = −0.64) in that animals exhibiting the largest preferences for the cocaine-paired environment (a presumed measure of cocaine reward) later exhibited the fastest start latencies in the runway task. Differences in the ranks of CPP scores accounted for 41% of the variance in the rankings of the same subjects’ start latencies. Note that CPP magnitude did not correlate with run time or retreat frequency, both measures that are highly sensitive to the negative/anxiogenic properties of cocaine. In contrast, the rank-order magnitude of conditioned place aversions correlated positively with start latency (rs = 0.62), run time (rs = 0.65), and retreat frequency (rs = 0.62). Thus, rats exhibiting the strongest adverse reactions to cocaine took the longest to initiate running and to reach the goal box, and exhibited the most approach-avoidance conflict behavior in the runway. Differences in the rank order of the subjects’ CPAs accounted for between 38–42% of the variance in the measures of the runway test.
Figure 6.
Spearman rank-order correlations between measures in the Conditioned Place Test and the Runway Test. CPP = conditioned place preference; CPA = conditioned place aversion. Animals with the numerically lowest CPP/CPA ranks (e.g., ranks 1 or 2) exhibited the smallest preferences or aversions; higher ranked animals (e.g. ranks of 9 or 10) exhibited larger preferences or aversions. Animals with numerically lower ranks for start latency had shorter (faster) mean response initiation times over the course of runway testing, while those with higher ranks exhibited longer (slower) start times. Subjects with numerically lower ranked retreat frequencies exhibited fewer retreats than those with higher ranks. For Run Times, subjects with lower ranks entered the goal box sooner (shorter run times) than animals with higher ranks (longer run times). The correlations show that larger CPPs were associated with faster start times but not with retreat frequency or run time, while larger CPAs were associated with slower start times, longer run times, and greater retreat frequency. *p<.05
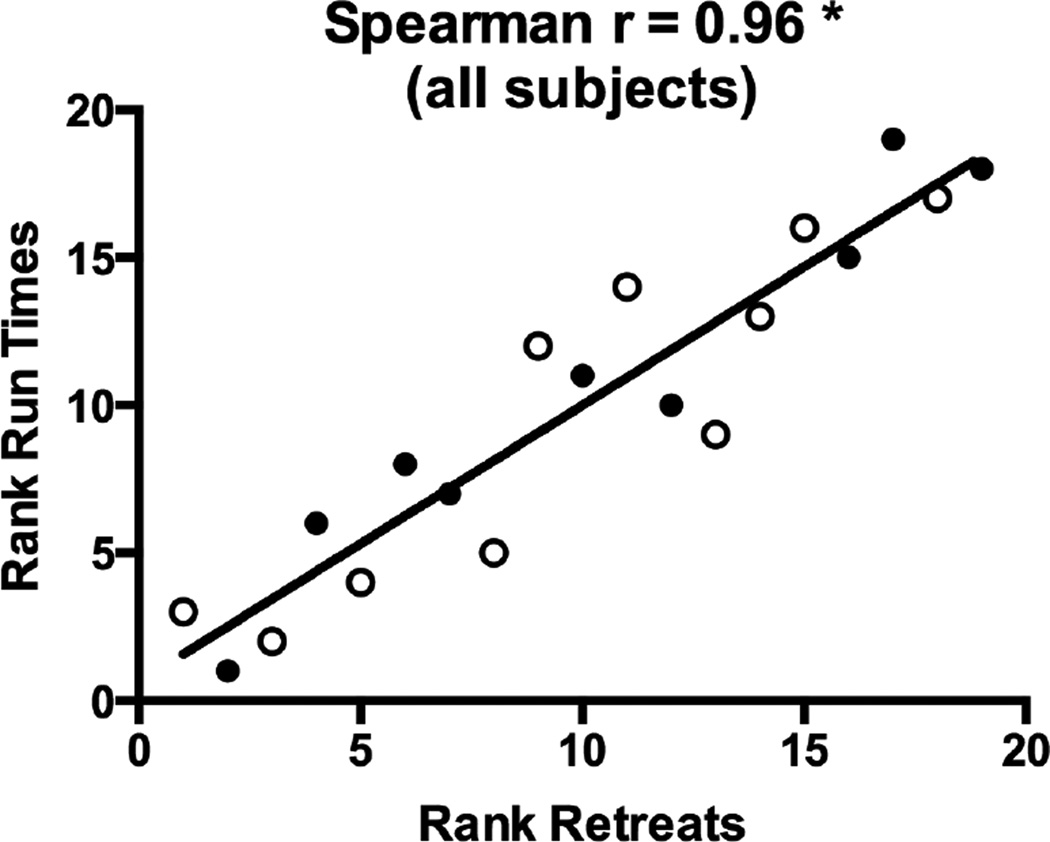
Other statistically significant correlations included the relationship between mean run time and mean retreat frequency (rs = 0.96, see Figure 7) reflecting the fact that animals emitting the most retreats not surprisingly took the longest to enter the goal box, and vice versa (ranked scores for retreat frequency accounted for 92% of the variation in ranked run time scores). Another strong positive correlation was obtained between ranked mean start latencies in the runway and ranked mean latency to emit the first lever-press each day in the operant self-administration chamber (rs = 0.81, data not shown). Thus, faster response initiation in one task (assumed to be an index of the subject’s motivation to seek the drug) was predictive of faster response initiation in the other task. Indeed, ranked start latencies in the runway test accounted for 78% of the variation in response latencies if the self-administration test.
Figure 7.
Spearman rank-order correlations between Run Times and Retreat Frequency in the runway. Animals that emitted the most retreats before entering the goal box tended to record longer Run Times (i.e., took longer to enter the goal box). Open circles identify subjects in the 6-hr self-administration group; closed circles identify subjects in the 1-hr group. *p<.05
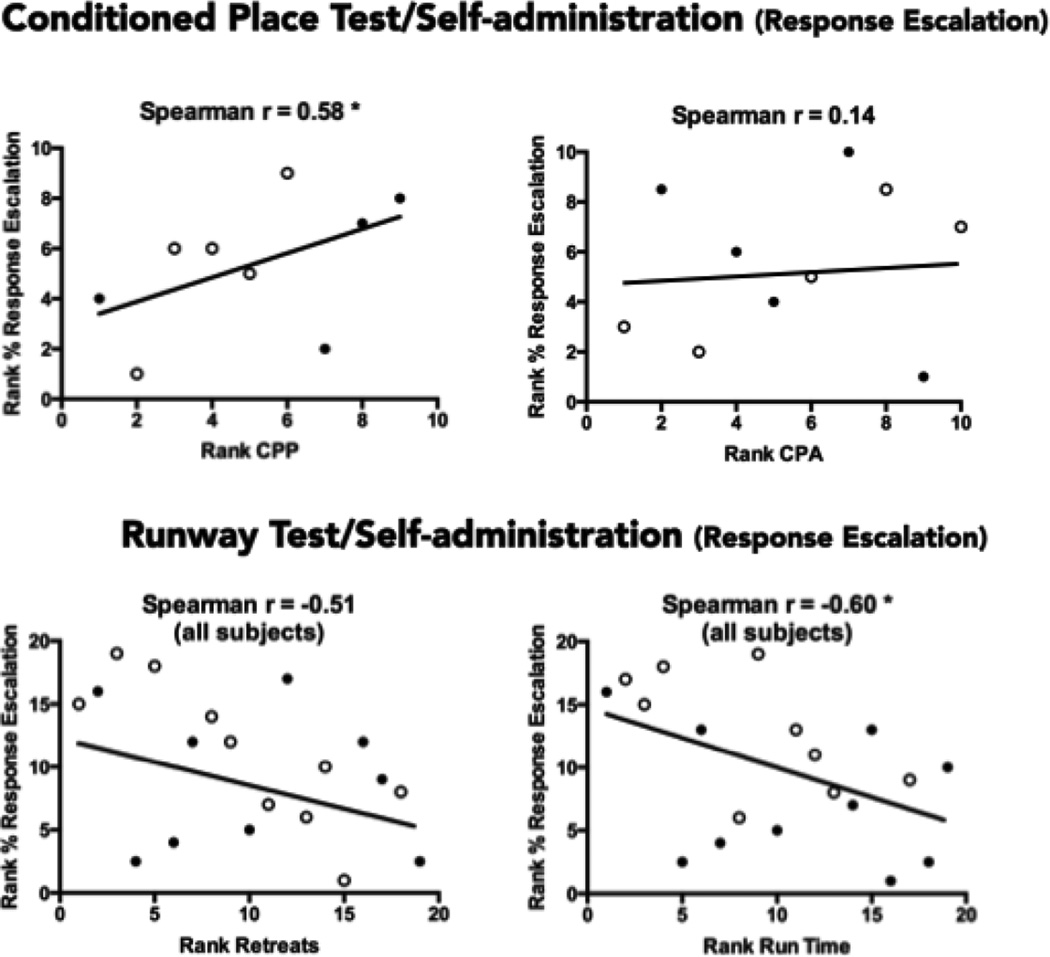
Of primary interest in the current study was the relationship between indices of the positive and negative effects of cocaine (in the place conditioning and runway tests) and the magnitude of response escalation over trials in the self-administration test. Those relationships are depicted in Figure 8. Animals that exhibited the greatest escalation in responding from Trial 1 to trial 10 tended to be those subjects that had the strongest positive response to cocaine. More specifically, the magnitude of CPPs was significantly and positively correlated with the ranked magnitude of response escalation (rs = 0.58) accounting for 34% of the variance in ranked escalation scores; in contrast CPA magnitude was unrelated to self-administration responding (rs = 0.14, p>.05). Similarly, animals exhibiting the fastest (shortest) run times exhibited the greatest response escalation in the self-administration test (rs = −0.60, p<.05) accounting for 36% of the variation in escalation magnitude. Despite the high correlation between retreats and run times, retreat frequency was only marginally associated with escalation (rs = −0.51, p=0.051) accounting for 25% of the variation in response escalation. Run times also negatively correlated with the mean number of self-administered drug infusions per trial (rs = −0.67) and the mean number of infusions during the first hour (rs = −0.67) when averaged across both the 1-h and 6-h groups (data not shown). Variations in run times therefore accounted for 45% of the variation in each of the two measures of cocaine infusions. Thus, animals that entry into the runway goal box most quickly tended to exhibit the greatest response escalation over the course of self-administration testing.
Figure 8.
Spearman rank-order correlations computed on the response escalation of subjects in the Self-administration test (based on their ranked percentage increase in reinforced responding from Trial 1 of Trial 10) and their ranked scores in the Conditioned Place Preference (CPP) test (top left), the Conditioned Place Aversion (CPA) test (top right), the frequency of retreats exhibited in the Runway Test (bottom left), and their average run-times in the Runway test (bottom right). Animals with the numerically lower CPP/CPA ranks exhibited the smallest preferences or aversions; higher ranked animals exhibited larger preferences or aversions. Animals with numerically lower ranks for retreat frequency exhibited fewer retreats than those with higher ranks, while for Run Times, subjects with lower ranks entered the goal box sooner (shorter run times) than animals with higher ranks (longer run times). Larger CPPs, but not CPAs, were associated with a greater escalation in self-administration responding. In the runway test, escalations in self-administration responding were associated with shorter run-times and marginally fewer retreats (p=0.051). Open circles identify subjects in the 6-hr self-administration group; closed circles identify subjects in the 1-hr group. *p<.05
DISCUSSION
The rationale for this study was to determine whether or not individual differences in the positive and negative affective responses to single daily acute injections of cocaine, would have predictive value for the escalation in self-administration responding observed over trials in animals provided extended 6-h daily access to the drug (a proposed model of cocaine “addiction”; e.g. Ahmed and Koob 1998; Ben Shahar et al. 2009). Two behavioral tests were employed to measure the affective consequences of cocaine administration: in the conditioned place test rats exhibited preferences for a distinct environment paired with the immediate (rewarding) effects of cocaine and aversions of places associated with the anxiogenic effects of cocaine present 15-min post-injection (as we have previously reported e.g., Ettenberg et al. 1999; Knackstedt et al. 2002; Su et al. 2013); in the runway test subjects developed over trials an ambivalence (i.e., approach-avoidance “retreat” behaviors) about entering the goal box – an effect that has been shown to stem from concurrent associations of the goal with the rewarding and anxiogenic consequences of cocaine administration (see review by Ettenberg 2004). The novelty of the current findings lies in the results of the correlational analyses between these two tests. Despite the inherent procedural differences between the place conditioning test, which employs a CS-US (place-drug) classical conditioning paradigm, and the runway test, which employs a hybrid operant and classical conditioning paradigm in which animals emit an operant response (running) to enter a place associated with drug delivery, the results from the two procedures appear to be sensitive to the same underlying processes. As depicted in figure 6, larger preferences for the cocaine-paired environment in the place test were associated with shorter start latencies in the runway. Given that drug-induced conditioned place preferences are generally interpreted as a measure of drug “reward” (e.g., Bardo and Bevins 2000; Carr et al. 1989; Tzschentke 1998, 2007; White and Carr 1985), these results suggest that greater cocaine “reward” was predictive of greater motivation to subsequently initiate cocaine-seeking in the runway. However, CPPs were not predictive of run times or retreats in the runway indicating that these latter two dependent measures were associated with some other aspect of cocaine action. Indeed, as animals developed ambivalence about goal-box entry in the runway test (i.e. as approach-avoidance retreat behavior strengthened over trials) run times also grew, undoubtedly a consequence of the subjects spending more time in the alley-way before entering the goal. This is of course confirmed by the strong positive (rs=0.96) correlation between run times and retreat frequency. Previous work in our laboratory has long suggested that the development of retreat behavior in animals running for cocaine stems from the drug’s dual rewarding and anxiogenic effects, both of which come to be associated with the goal box (Ettenberg 2004, 2009). This position is further supported by the current observation that while CPPs were unrelated to retreat frequency or run times, CPAs were positively and significantly correlated with both measures. Thus, the more negative the drug experience is for subjects (as measured by CPA magnitude) the longer those same subjects subsequently took to leave the start box (rs=0.62), the more retreats they made in the runway (rs=0.62), and the longer they took to finally enter the goal box (rs=0.65). Finally we note that although animals were all tested first in the place condition test and then in the runway, there was no evidence to show that the modest prior drug exposure from the CPP/CPA tests had in any way altered subsequent runway behavior – i.e., the runway test produced response patterns and magnitudes that were highly comparable to those that we had previously reported in animals that did not have any prior cocaine exposure (e.g., see recent studies by Ettenberg et al. 2011; Su et al. 2012; Wenzel et al. 2014).
Of particular interest in the current study was whether or not the magnitude of these dual affective responses to acute (once daily injections of) cocaine, predicted the behavior of animals permitted to freely self-administer the drug. In that regard, and as might be expected, runway start latency (the time it took the subject to leave the start box) was strongly and positively correlated with the latency to initiate self-administration responding each day (rs=0.81) – indicating that high motivation to seek cocaine in one test, predicted high motivation to do so in the other test. Run Time (the time required to enter the goal box once the subject had left the start box) was significantly and negatively correlated with several measures in the self-administration test including the average number of reinforcers that animals earned during each trial (across both the 1-h and 6-h groups; rs= −0.67), the mean number of reinforcers earned during the first hour of testing (rs= −0.67), and, perhaps most importantly, the degree of response escalation observed over the course of testing (i.e., the difference score between the first and last day of cocaine self-administration; rs= −0.60). Retreat behaviors were also negatively associated with response escalation (rs= −0.51) although this correlation narrowly missed statistical significance (p=0.051). Ahmed and Koob (1998) have suggested that the occurrence of escalated responding in animals provided 6-h of daily access to cocaine reflects the development of cocaine addiction and results from underlying neuroadaptations in the brain’s reward system that renders the drug less rewarding and hence motivates compensatory enhanced responding to overcome this form of drug tolerance (Ahmed and Koob 1998; Ahmed et al. 2002; Koob et al. 2004). If that hypothesis is correct, then the current results suggest that the less aversive the effects of acute cocaine (i.e., the faster the animals made it into the goal box of the runway) the greater the likelihood that they would exhibit escalated self-administration responding (i.e., the greater their predisposition to self-administer cocaine at rates that would induce the development of an addictive state). This suggests that individual differences among organisms in the development of cocaine addiction are likely related to the relative valence of the drug’s positive and negative consequences during initial acute exposure. Indeed, others have similarly pointed out the importance of assessing a drug’s positive and negative actions for understanding drug abuse (e.g., Cunningham et al. 2009; Gaiardi et al. 1991; Riley 2011; Verendeev and Riley 2012). The current results, for example, are very much line with those of Cunningham et al. (2009), who used conditioned taste aversions to assess the negative properties of drugs of abuse and reported that animals found to be more sensitive to the aversive effects of a drug later self-administer less drug than those who found the drug to be less aversive (see also: Cannon et al. 1994; Riley, 2011; Risinger and Cunningham, 1998). The current data showing that CPPs but not CPAs were positively and significantly correlated with the escalation in self-administration responding is clearly consistent with these prior results.
An unexpected but important finding in the current study was the observation that response escalation (from Trial 1 to Trial 10) in cocaine self-administration was observed in both the 1-h and 6-h groups. It had previously been presumed that such escalation in drug-reinforced responding over trials only occurred with 6-h but not with 1-h of daily access to cocaine and reflected a transition from “recreational” to “addicted” drug use (e.g., Ahmed and Koob, 1998). Here we show that response escalation occurred even in the 1-h group, a result that likely stems from the fact that the 1-h group in the current study had experienced prior exposure to cocaine in both the place conditioning (8 injections) and runway (14 injections) tests. While it is true that the degree of escalation was significantly greater in the 6-h versus the 1-h group, the fact that response escalation (and hence presumably the development of underlying compensatory neuronal adaptations) was present in the 1-h group suggests that the key factor in the escalation of drug consumption (and hence quite possibly the development of cocaine addiction) may not be the duration of daily access to the drug but rather the cumulative amount of drug exposure over time. Thus even “recreational” drug users who expose themselves to a relatively limited amount of drug in a given day or week, are likely to be at risk for the development of addiction if that exposure persists over time.
Acknowledgments
Funding for this research was provided by National Institute of Drug Abuse grants DA05041 and 033370 awarded to AE. The data described herein were collected as part of the undergraduate honors theses of VF and KK. We thank Dr. Osnat Ben Shahar for her invaluable assistance and advice.
Footnotes
The authors have no conflicts of interest to report.
References
- Ahmed SH, Koob GS. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Barbieri EJ, Ferko AP, DiGregorio GJ, Ruch EK. The presence of cocaine and benzoylecgonine in rat cerebrospinal fluid after intravenous administration of cocaine. Life Sci. 1992;51:1739–1746. doi: 10.1016/0024-3205(92)90303-7. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: A meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Simmons SJ, Servilio LC, Bercovicz D, Ma S, Root DH, Pawlak AP, West MO. Ultrasonic vocalizations: evidence for an affective opponent process during cocaine self-administration. Psychopharmacology (Berl) 2014;231:909–918. doi: 10.1007/s00213-013-3309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A. Prolonged daily exposure to IV cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav. 2005;82:411–416. doi: 10.1016/j.pbb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booze RM, Lehner AF, Wallace DR, Welch MA, Mactutus CF. Dose-response cocaine pharmacokinetics and metabolite profile following intravenous administration and arterial sampling in unanesthetized freely moving male rats. Neurotoxicol Teratol. 1997;19:7–15. doi: 10.1016/s0892-0362(96)00180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JR, Browning DA, Maxwell AO, Dong Y, Jansen HT, Panksepp J, Sorg BA. Positive affective vocalizations during cocaine and sucrose self-administration: a model for spontaneous drug desire in rats. Neuropharmacology. 2011;61:268–275. doi: 10.1016/j.neuropharm.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Jones SR. Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem. 2014;128:224–232. doi: 10.1111/jnc.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DS, Leeka JK, Block AK. Ethanol self-administration patterns and taste aversion learning across inbred rat strains. Pharmacol Biochem Behav. 1994;47:795–802. doi: 10.1016/0091-3057(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. In: Liebman JM, Cooper SJ, editors. The neuropharmacological basis of reward. New York: Clarendon Press/Oxford University Press; 1989. pp. 264–319. [Google Scholar]
- Covington HE, 3rd, Miczek KA. Vocalizations during withdrawal from opiates and cocaine: possible expressions of affective distress. Eur J Pharmacol. 2003;467:1–13. doi: 10.1016/s0014-2999(03)01558-9. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Genetic influences on conditioned taste aversion. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. New York: Oxford University Place; 2009. pp. 387–421. [Google Scholar]
- Dackis CA, O'Brien CP. Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treat. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology. 1991;103:455–461. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacol Biochem Behav. 1993;44:191–198. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–512. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27:721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi RE. Effects of buspirone on the immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. Pharmacol Biochem Behav. 2007;87:171–178. doi: 10.1016/j.pbb.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Ofer OA, Mueller CL, Waldroup S, Cohen A, Ben-Shahar O. Inactivation of the dorsal raphé nucleus reduces the anxiogenic response of rats running an alley for intravenous cocaine. Pharmacol Biochem Behav. 2011 Feb;97(4):632–639. doi: 10.1016/j.pbb.2010.11.008. 2011, Epub 2010 Nov 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Cocaine self-administration research: treatment implications. NIDA Res Monogr. 1994;145:139–162. [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A. A simple method for studying intravenous drug reinforcement in the runway. Pharmacol Biochem Behav. 1990;36:703–706. doi: 10.1016/0091-3057(90)90278-p. [DOI] [PubMed] [Google Scholar]
- Gaiardi M, Bartoletti M, Bacchi A, Gubellini C, Costa M, Babbini M. Role of repeated exposure to morphine in determining its affective properties: place and taste conditioning studies in rats. Psychopharmacology (Berl.) 1991;103:183–186. doi: 10.1007/BF02244201. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Intracranial cocaine self-administration. NIDA Res Monogr. 1988;88:199–216. 1988. [PubMed] [Google Scholar]
- Goeders NE. A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology. 1997;22:237–259. doi: 10.1016/s0306-4530(97)00027-9. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Samimi MM, Ettenberg A. Evidence for opponent-process actions of intravenous cocaine and cocaethylene. Pharmacol Biochem Behav. 2002;72:931–936. doi: 10.1016/s0091-3057(02)00764-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine B, Markou A, Pulvirenti L, Weiss F. Role for the mesocortical dopamine system in the motivating effects of cocaine. NIDA Res Monogr. 1994;145:1–18. [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Neurobiology of Addiction. London: Academic Press/Elsevier; 2006. [Google Scholar]
- Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101:713–725. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010;212:109–114. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Koob GF. Bromocriptine reverses the elevation in intracranial self-stimulation thresholds observed in a rat model of cocaine withdrawal. Neuropsychopharmacology. 1992;7:213–224. [PubMed] [Google Scholar]
- McReynolds JR, Peña DF, Blacktop JM, Mantsch JR. Neurobiological mechanisms underlying relapse to cocaine use: contributions of CRF and noradrenergic systems and regulation by glucocorticoids. Stress. 2014;17:22–38. doi: 10.3109/10253890.2013.872617. [DOI] [PubMed] [Google Scholar]
- Moldow RL, Fischman AJ. Cocaine induced secretion of ACTH, beta-endorphin, and corticosterone. Peptides. 1987;8:819–822. doi: 10.1016/0196-9781(87)90065-9. [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Palamarchouk V, Smagin G, Goeders NE. Self-administered and passive cocaine infusions produce different effects on corticosterone concentrations in the medial prefrontal cortex (MPC) of rats. Pharmacol Biochem Behav. 2009;94:163–168. doi: 10.1016/j.pbb.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administration cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Port RM, Contel NR. Human and animal studies of cocaine: implications for the development of behavioral pathology. In: Creese I, editor. Stimulants: Neurochemical, Behavioral and Clinical Perspectives. New York: Raven Press; 1983. pp. 169–203. [Google Scholar]
- Raven MA, Necessary BD, Danluck DA, Ettenberg A. Comparison of the reinforcing and anxiogenic effects of intravenous cocaine and cocaethylene. Exp Clin Psychopharmacol. 2000;8(1):117–124. doi: 10.1037/1064-1297.8.1.117. [DOI] [PubMed] [Google Scholar]
- Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Ethanol-induced conditioned taste aversion in BXD recombinant inbred mice. Alcohol Clin Exp Res. 1998;22:1234–1244. [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol Biochem Behav. 1992;43:631–633. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Su ZI, Kichaev G, Wenzel J, Ben-Shahar O, Ettenberg A. Weakening of negative relative to positive associations with cocaine-paired cues contributes to cue-induced responding after drug removal. Pharmacol Biochem Behav. 2012;100:458–463. doi: 10.1016/j.pbb.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z-I, Wenzel JM, Ettenberg A, Ben-Shahar O. Prior extended daily access to cocaine elevates the reward threshold in a Conditioned Place Preference test. Addict Biol. 2013;18:222–229. doi: 10.1111/adb.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog in Neurobiol. 1998;56:61–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Van Dyke C, Byck R. Cocaine. Scientific American. 1982;246:128–141. doi: 10.1038/scientificamerican0382-128. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. Conditioned taste aversion and drugs of abuse: history and interpretation. Neurosci Biobehav Rev. 2012;36:2193–2205. doi: 10.1016/j.neubiorev.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Cotten SW, Dominguez HM, Lane JE, Shelton K, Su ZI, Ettenberg A. Noradrenergic β-receptor antagonism within the central nucleus of the amygdala or bed nucleus of the stria terminalis attenuates the negative/anxiogenic effects of cocaine. J Neurosci. 2014;34:3467–3474. doi: 10.1523/JNEUROSCI.3861-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend. 1997;44:87–94. doi: 10.1016/s0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Willick ML, Kokkinidis L. Cocaine enhances the expression of fear-potentiated startle: evaluation of state-dependent extinction and the shock-sensitization of acoustic startle. Behav Neurosci. 1995;109:929–938. doi: 10.1037//0735-7044.109.5.929. [DOI] [PubMed] [Google Scholar]
- White NM, Carr GD. The conditioned place preference is affected by two independent reinforcement processes. Pharmacol Biochem Beha. 1985;23:37–42. doi: 10.1016/0091-3057(85)90127-3. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. Determinants of cocaine self-administration by laboratory animals. Ciba Found Symp. 1992;166:149–161. doi: 10.1002/9780470514245.ch9. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–650. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]