Abstract
Introduction
Despite decades of study, a clear understanding of autonomic nervous system activity in space remains elusive. Differential interpretation of fundamental data have driven divergent theories of sympathetic activation and vasorelaxation.
Methods
This paper will review the available in-flight autonomic and hemodynamic data in an effort to resolve these discrepancies. The NASA NEUROLAB mission, the most comprehensive assessment of autonomic function in microgravity to date, will be highlighted. The mechanisms responsible for altered autonomic activity during spaceflight, which include the effects of hypovolemia, cardiovascular deconditioning, and altered central processing, will be presented.
Results
The NEUROLAB experiments demonstrated increased sympathetic activity and impairment of vagal baroreflex function during short-duration spaceflight. Subsequent non-invasive studies of autonomic function during spaceflight have largely reinforced these findings, and provide strong evidence that sympathetic activity is increased in space relative to the supine position on Earth. Others have suggested that microgravity induces a state of relative vasorelaxation and increased vagal activity when compared to upright posture on Earth. These ostensibly disparate theories are not mutually exclusive, but rather directly reflect different pre-flight postural controls.
Conclusion
When these results are taken together, they demonstrate that the effectual autonomic challenge of spaceflight is small, and represents an orthostatic stress less than that of upright posture on Earth. In-flight countermeasures, including aerobic and resistance exercise, as well as short-arm centrifugation have been successfully deployed to counteract these mechanisms. Despite subtle changes in autonomic activity during spaceflight, underlying neurohumoral mechanisms of the autonomic nervous system remain intact and cardiovascular function remains stable during long-duration flight.
Overt symptoms of autonomic dysfunction related to spaceflight were observed in the earliest stages of our manned space program. Orthostatic hypotension during 70 degree head-up tilt was seen in post-flight testing of a Mercury astronaut after a mission of only 30 hours duration (30). Further efforts to evaluate and characterize the clinical observation of post-flight orthostatic intolerance have served as stimuli for experimental work on basic cardiovascular and autonomic function in microgravity. Despite a breadth of study spanning nearly five decades, fundamental questions, including the impact of spaceflight on basic physiologic properties like blood pressure and heart rate, remain unclear.
Post-flight orthostatic intolerance in returning astronauts continued to be a common observation through the early stages of the shuttle program (4). As the clinical features of post-flight orthostatic intolerance resembled patients suffering from various disorders of autonomic dysfunction, autonomic function related to spaceflight became a focus of investigation. Early work indicated impaired vagal baroreflex responses after microgravity exposure (21, 22). Astronauts who experienced severe orthostatic intolerance were found to have decreased baseline supine and standing peripheral vascular resistance, and demonstrated blunted augmentation of post-flight norepinephrine levels and peripheral vascular resistance with standing (24). These findings led to the initial hypothesis that subnormal baseline vascular resistance, as well as both vagal and sympathetic baroreflex impairment during flight, led to inadequate tachycardic and vasocontrictor responses to upright positioning upon return to Earth and contributed to post-flight orthostasis. Flaws in experimental design and execution led to questions regarding the accuracy of these early studies. There was also significant variability in experimental testing of other indicators of autonomic activity, including in-flight catecholamine levels, which were found to be decreased (40), unchanged (39) or increased (38, 53).
The NEUROLAB Experiments
It was in this background that the NEUROLAB mission (STS-90) was developed and launched, with the goal of providing a more comprehensive understanding of the neurophysiological consequences of microgravity. At its core were a set of experiments which evaluated key components of basic autonomic function and their perturbation induced by spaceflight. These included vagal and sympathetic responses to Valsalva's maneuver, baroreflex responses to orthostatic stress, as well as sympathetic responses to peripheral afferent stimulation. The NEUROLAB mission was the first in-flight utilization of muscle sympathetic nerve activity (MSNA) for direct and real-time measurement of sympathetic activity (Figure 1).
Figure 1.
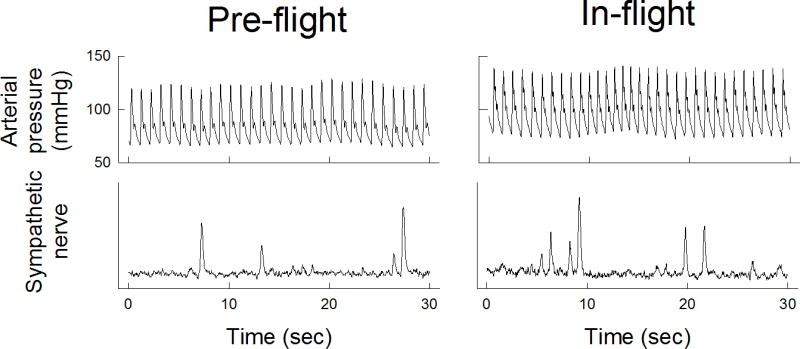
Continuous arterial blood pressure (top panels) and muscle sympathetic nerve activity (MSNA, bottom panels) tracings during pre-flight and in-flight during NEUROLAB mission. Both arterial blood pressure and MSNA were increased in-flight. (figure from Ertl et al 2002)
This mission was also the first time that norepinephrine spillover and norepinephrine clearance have been studied in-flight. Norepinephrine spillover and clearance provide a detailed understanding of norepinephrine kinetics. Norepinephrine spillover is better correlated with MSNA than the serum norepinephrine concentration and provides a more accurate representation of overall sympathetic nervous system activity (18).
The NEUROLAB studies revealed increased sympathetic activity during short-duration spaceflight, as compared to supine pre-flight controls. This was reflected in increases in MSNA (Figure 1) as well as norepinephrine spillover (Figure 2) (17). Furthermore, sympathetic reflex mechanisms, both during and post-flight, remained intact (26, 41). Vagal baroreflex sensitivity, however, was reduced during flight (Figure 3) (13).
Figure 2.
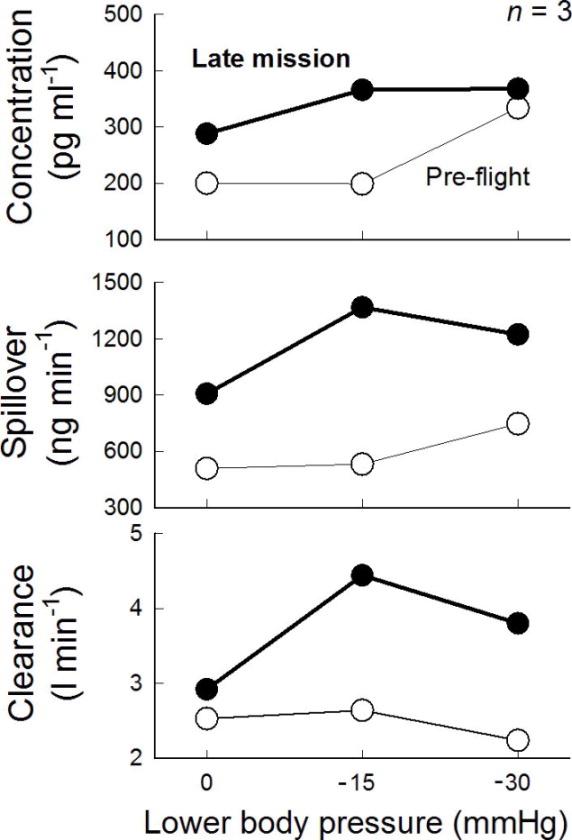
In-flight norepinephrine concentration (top), spillover (middle), and clearance (bottom) during NEUROLAB mission. All three were increased during flight. (figure from Ertl et al 2002)
Figure 3.
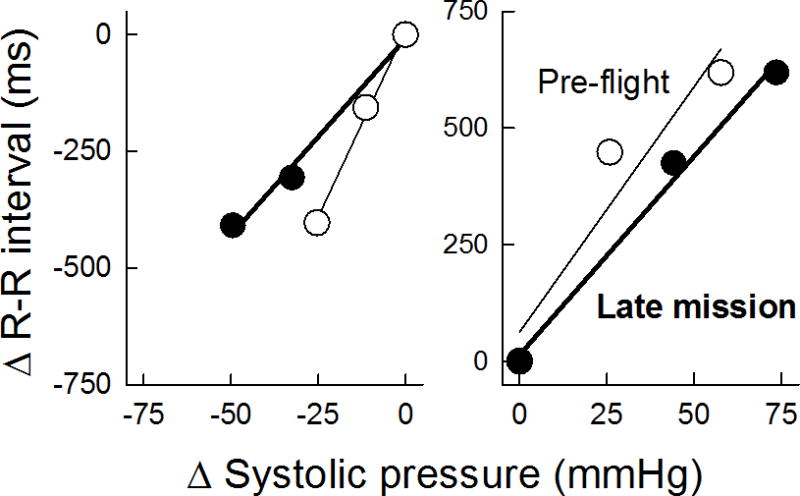
Relationship between changes in R–R interval and systolic pressure pre-flight and in-flight during phase IV of Valsalva maneuver, reflective of vagal baroreceptor function during NEURO- LAB. The slope, i.e., vagal baroreceptor sensitivity, was diminished in-flight (figure from Cox et al 2002)
In-flight increases in HR and BP were also observed. These findings were consistent across subjects, and consistent between multiple investigative modalities, including norepinephrine spillover and MSNA.
Noninvasive Measurement of Sympathetic Activity during Spaceflight
In-flight sympathetic activity has been indirectly evaluated during subsequent missions using several non-invasive techniques, including power spectral analysis of heart rate variability and post-flight platelet norepinephrine concentrations. Heart rate variability is largely affected by neural mechanisms and the interplay between sympathetic and vagal modulation. Decreased standard deviation of heart rate variability is found with increased sympathetic activity and/or decreased parasympathetic activity (37, 50). Power spectrum analysis of heart rate variability yields further insight into clinical autonomic function (43). Under known conditions of sympathetic activation (e.g. during orthostatic stress) low-frequency (LF) power, as well as the ratio of LF and high-frequency (HF) power, can reflect relative sympathetic activation (54).
This method has been used to indirectly assess in-flight sympathetic activity during several recent short- and long-duration flights. The results of these studies are summarized in Table 1 (2, 3, 31, 45, 59).
Table 1.
Results of power spectral analysis of pre-flight and in-flight heart rate variability during short- and long-duration spaceflight.
| n | Data Acquisition Period | RRI LF (ms2) | RRI HF (ms2) | RRI LF/HF | Respirations | 1-G Position | |
|---|---|---|---|---|---|---|---|
| Di Rienzo (2008) | 4 | Pre-flight | 607 ± 144† | 161 ± 35† | 0.82 ± 0.11 | Unknown | Sitting |
| <30h | 899 ± 250† | 485 ± 195† | 0.32 ± 0.15 | Unknown | |||
| Days 6-14 | 447 ± 115† | 136 ± 30† | 0.52 ± 0.10 | Unknown | |||
| Beckers (2009) | 5 | Pre-flight | 1317 ± 575 | 462 ± 167 | 3 ± 1 | Spontaneous | Supine/Sitting/Stand |
| Day 8 | 1492 ± 423 | 435 ± 166 | 7 ± 4 | Spontaneous | |||
| Hughson (2012)† | 6 | Pre-flight | 503 ± 45 | 340 ± 56 | 1.48 ± 2.7 | Spontaneous | Supine/Sitting |
| Days 14-21 | 507 ± 72 | 209 ± 38 | 2.44 ± 2.11 | Spontaneous | |||
| Months 2-6 | 614 ± 68 | 230 ± 34 | 2.64 ± 1.77 | Spontaneous | |||
| Migeotte (2003) | 4 | Pre-flight | — | — | 1.67 ± 0.40 | Paced | Supine |
| Days 5-6 | — | — | 1.77 ± 0.76 | Paced | |||
| Days 11-16 | — | — | 1.68 ± 0.77 | Paced | |||
| Baevsky (2007) | 8 | Pre-Flight | 876 ± 387 | 400 ± 167 | 2.31 ± 0.48 | Spontaneous | Semi-Supine |
| Month 1 | 1215 ± 544 | 369 ± 94 | 4.44 ± 1.82 | Spontaneous | |||
| Month 6 | 1948 ± 815 | 345 ± 107 | 6.43 ± 2.18 | Spontaneous | |||
| Pre-Flight | 263 ± 44 | 452 ± 178 | 1.82 ± 0.80 | Paced | |||
| Month 1 | 452 ±104 | 715 ± 211 | 1.17 ± 0.43 | Paced | |||
| Month 6 | 401 ± 139 | 779 ± 374 | 0.90 ± 0.34 | Paced |
Converted from logarithmic plots. Standard error calculated from percentage of mean.
Neither short- nor long-duration studies have demonstrated a statistically significant change in the in-flight LF/HF ratio, though most demonstrate trends toward an increase in the LF/HF ratio, suggesting in-flight sympathetic activation. Only two studies have evaluated power spectral analysis of heart rate variability during long-duration flight aboard the ISS, demonstrating similar non-specific increases in the LF/HF ratio during flight (2, 31).
There are several important factors complicating the interpretation of in-flight power spectral analysis. The first is the inconsistent use of pre-flight controls using supine or sitting or standing positions, which makes both interpretation of these results, as well as inter-study comparisons, extremely difficult. The second are changes in respiratory frequency during space flight. Respiratory dynamics have an intrinsic effect on the amplitude and frequency of heart rate variability spectral power peaks (12). Findings from several studies suggest that observed increases in the LF/HF ratio during spaceflight might be secondary to respiratory interference. Baevsky et al found that the non-statistically significant increases in the LF/HF ratio observed during spontaneous breathing are absent when a paced breathing protocol is used. Other studies using a paced breathing protocol have failed to demonstrate any change in the in-flight LF/HF ratio during short-duration flight (45). The current results of in-flight power spectal analysis of heart rate variability studies should be interpreted cautiously.
In-flight sympathetic activity has also been indirectly measured using post-flight platelet norepinephrine and epinephrine concentrations. During their circulatory lifespan, platelets take up both norepinephrine and epinephrine in plasma through active and passive processes. Measurement of platelet catecholamine concentrations can be used as a reflection of average circulating levels in the plasma. Importantly, platelet catecholamine concentrations are most elevated following periods of sustained sympathetic activation, as compared to acute periods of stress (6). Hence, relative post-flight platelet catecholamine concentrations, drawn shortly after landing, should reflect changes in overall sympathetic activity, which occur during flight. In a study of 5 cosmonauts, post-flight platelet norepinephrine and epinephrine concentrations were significantly increased compared to pre-flight values, consistent with in-flight sympathetic activation (7). These data were only obtained following short-duration flight, and similar study following long-duration missions has not been performed.
Indirect assessment of vascular tone has also been studied in microgravity. Pulse wave transit time (PWTT) is decreased during long-duration spaceflight, as compared to a 1-G semi-supine control (2). PWTT represents the elapsed time for arterial pulse wave propagation from initial aortic ejection to the distal arteries. It is a marker of vascular tone and vessel stiffness. PWTT is also strongly related to sympathetic activity. Shortening of PWTT is directly correlated with increased sympathetic activity (60). Decreased PWTT during long-duration space flight suggests increased vascular tone, a finding also supported by evidence of increased calf vascular resistance during flight relative to a 1-G supine control (65).
Sympathovagal Balance During Spaceflight
The investigation of autonomic function in microgravity has spanned the better part of five decades, from the earliest manned space programs to long-duration flights aboard the ISS. Salient findings from these studies, including invasive testing during NEUROLAB and more recent non-invasive autonomic evaluation, have been presented above. Fundamentally, this work seeks to answer the question: does vagal or sympathetic tone dominate during spaceflight?
To appropriately answer this question, it is essential to recognize that sympathetic or vagal activation during flight must be interpreted relative to their respective pre-flight activity. The autonomic nervous system is responsible for maintaining physiologic homeostasis despite a number of perturbing intrinsic and extrinsic factors. In a healthy individual, under controlled circumstances, autonomic nervous system activity is largely influenced by position relative to gravity. The cardiovascular stress of gravity causes a reduction in cardiac preload and cardiac output, with resultant sympathetic activation directing increases in HR and peripheral vascular resistance to maintain a physiologic blood pressure. Postural changes relative to gravity (e.g. supine, sitting, or standing) produce incremental increases in orthostatic stress, resulting in proportional increases in sympathetic activity. To correctly understand changes in autonomic nervous system activity during spaceflight, relative sympathetic activity must be interpreted in the context of its pre-flight postural control.
When compared to the 1-G supine posture, sympathetic activity is increased during short-duration spaceflight. This is most comprehensively demonstrated by findings of the NEUROLAB experiments. Relative sympathetic activation was supported by both MSNA and norepinephrine kinetic data, and was repeatedly demonstrated in the context of the Valsalva maneuver, lower-body negative pressure, as well as peripheral chemo- and mechanoreceptor stressors. These findings have also been supported by contemporary non-invasive autonomic study during flight. Post-flight platelet norepinephrine concentrations are increased, reflecting sustained increases in sympathetic activity during flight. Studies of in-flight power spectral analysis of heart rate variability have also suggested sympathetic predominance during flight, though it should be noted that these results have not been statistically significant, and may be confounded by alterations in respiratory frequency. Decreased PWTT and evidence of increased calf vascular resistance are also consistent with increased sympathetic activity relative to the supine position on earth.
Seemingly in direct opposition, several groups have proposed a state of “vasorelaxation” during spaceflight (51, 52, 63, 64). Norsk et al showed that systemic vascular resistance (SVR) was reduced during short-duration flight. SVR was calculated from measured pre- and in-flight cardiac output (CO) and mean arterial pressure (MAP). The reduction in SVR suggests systemic vasodilatation, and led to the conclusion that exposure to microgravity results in vasorelaxation. Essential to the interpretation of these findings is that in-flight changes in CO and MAP in this study are relative to upright (seated) pre-flight controls. When Norsk et al compared in-flight measurements to supine pre-flight controls, SVR was increased during flight, consistent with in-flight sympathetic activation.
The assertion of vasorelaxation during spaceflight is not contradictory to the body of evidence suggesting sympathetic activation. In fact, the ostensibly divergent findings of Norsk et al provide important insight into the relative magnitude of the effective orthostatic stress of spaceflight. When compared to 1-G supine controls, the complex physiologic changes that occur in microgravity result in relative sympathetic activation. The effectual autonomic stress of spaceflight is small, such that when in-flight sympathetic activity is compared to upright pre-flight controls, relative sympathetic activity and peripheral vascular resistance is reduced. Microgravity, through a variety of neurophysiologic mechanisms, produces an orthostatic stress of magnitude somewhere between supine and upright posture on Earth.
This understanding of relative autonomic function during spaceflight is primarily a result of studies during short-duration flight, including NEUROLAB and data from Norsk et al. Whether mild sympathetic activation relative to 1-G supine posture persists during long-duration spaceflight is unclear. Few experimental attempts have addressed this question. Only two studies have studied power spectral analysis of heart rate variability during long duration flight (2, 31). Both show non-significant increases in LF/HF ratio during flight, and are plagued by ill-defined pre-flight postural controls and confounded by the use of spontaneous breathing protocols.
Baroreflex Function in Space
Baroreflex function plays a central role in the physiologic adaptation to upright posture. Afferent mechanoreceptors in the aortic arch and carotid body facilitate the vasoconstrictive (sympathetic baroreflex) and tachycardic (vagal baroreflex) responses to appropriately augment systemic vascular resistance and cardiac output to accommodate this hemodynamic challenge. Disruption of baroreflex function by microgravity has been a focus of investigation, specifically its role in post-flight orthostatic hypotension. Original post-flight studies demonstrated reductions in baroreflex sensitivity. The NEUROLAB data demonstrated that vagal baroreflex function is impaired during spaceflight. Importantly, sympathetic baroreflex function was found to be unchanged both during flight as well as after return to Earth.
Several studies have further evaluated vagal baroreflex function in space. Unfortunately, these results have been conflicting, demonstrating impaired (16), unchanged (3), or even supranormal (59) baroreflex function during short-duration spaceflight. The most robust data in terms of sample size comes from Eckberg et al, with data from a total of 13 astronauts during short-duration flights. This study measured changes to the R-R interval with graded neck pressure and suction, and showed a reduction in vagal baroreflex sensitivity, similar to that shown during the NEUROLAB mission. Data on baroreflex function during long-duration flight are limited. Early evidence of decreased vagal baroreflex function in 3 astronauts during long-duration flight, as measured during paced-breathing protocols, has been reported (11). Unfortunately, unanticipated operational problems during the mission as well as other significant confounders (including atenolol use by one cosmonaut), make these results difficult to interpret. In two contemporary studies of baroreflex function in astronauts aboard the ISS, baroreflex sensitivity was unchanged during missions of up to 6 months in duration (31, 64). Further investigation of baroreflex function during long-duration flight has not been reported.
Hemodynamics in Space
The effects of microgravity on arterial blood pressure (BP) and heart rate (HR) remain unclear. Early studies noted a reduction in both HR and BP (23). Multiple studies of inflight HR and BP, during short and long-duration flights, have been pursued since NEUROLAB (2, 3, 13, 16, 31, 45, 52, 59, 63). These results are summarized in Tables 2 and 3.
Table 2.
HR and BP data from short-duration spaceflight.
| Short-duration Flight (< 1 month) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | R-R Interval (msec) | HR (bpm) | SBP (mm Hg) | DBP (mm Hg) | 1-G position | |||||
| Pre-flight | In-flight† | Pre-flight | In-flight† | Pre-flight | In-flight† | Pre-flight | In-flight† | |||
| Eckberg (2010) | 13 | 1015 ± 72 | 898 ± 63* | 59 ± 4†† | 67 ± 5*†† | 113 ± 6 | 120 ± 5* | 67 ± 4 | 69 ± 4 | Supine |
| Beckers (2009) | 5 | 988 ± 52 | 955 ± 53 | 61 ± 3†† | 63 ± 3†† | 123 ± 4 | 118 ± 4 | 69 ± 4 | 68 ± 4 | Supine |
| Di Rienzo (2008) | 4 | 892 ± 46 | 1002 ± 54 | 67 ± 3†† | 60 ± 3†† | 116 ± 6 | 130 ± 4 (early)* | 66 ± 3 | 77 ± 2 | Seated |
| Norsk (2006) | 4 | 870 ± 50†† | 882 ± 116†† | 69 ± 4 | 68 ± 9 | 82 ± 2††† | 84 ± 4††† | — | — | Seated |
| Cox (2002) | 4 | 1053 ± 111†† | 1000 ± 67†† | 57 ± 6 | 60 ± 4 | 139 ± 16 | 151 ± 12 | 73 ± 10 | 76 ± 6 | Supine |
| Migeotte (2003) | 4 | 1051 ± 44†† | 1033 ± 56†† | 57.1 ± 2.4 | 58.1 ± 3.7 | — | — | — | — | Supine |
Unless statistically significant, the last in-flight data point is given.
HR data converted from bpm or RRI. Standard error calculated from percentage of mean.
Values listed are mean arterial pressures (MAP).
p < 0.05 compared to pre-flight values.
Table 3.
HR and BP data from long-duration spaceflight.
| Long-duration Flight (> 1 month) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | R-R Interval (msec)†† | HR (bpm) | SBP (mm Hg) | DBP (mm Hg) | 1-G position | |||||
| Pre-flight | In-flight† | Pre-flight | In-flight† | Pre-flight | In-flight† | Pre-flight | In-flight† | |||
| Verheyden (2009) | 11 | 952 ± 106 | 952 ± 136 | 63 ± 7 | 63 ± 9 | 122 ± 10 | 115 ± 10 | 69 ± 10 | 74 ± 5 | Supine |
| Baevsky (2007) | 8 | 968 ± 141 | 968 ± 141 | 62 ± 9 | 62 ± 9 | 118 ± 10 | 107 ± 9* | 76 ± 10 | 65 ± 8* | Semi-Supine |
| Hughson (2012) | 6 | 1091 ± 198 | 1034 ± 214 | 55 ± 10 | 58 ± 12 | 141 ± 11 | 133 ± 19 | 84 ± 12 | 80 ± 15 | Supine |
Unless statistically significant, the last in-flight data point is given.
RRI converted from bpm. Standard error calculated from percentage of mean.
p < 0.05 compared to pre-flight values.
For short-duration flights, the data are variable and inconsistent. Analysis of both HR and systolic blood pressure (SBP) has shown them to be increased or unchanged. Again, the most robust data in terms of sample size comes from Eckberg et al. Statistically significant increases in both HR and SBP in this cohort were consistent with NEUROLAB findings. It is difficult to interpret or compare these contemporary studies, as pre-flight postural controls are variable. However, even when comparing short-duration studies, which use a supine 1-G control (Eckberg et al and Beckers et al), their findings differ. This may be a reflection of the short-window for data collection and the small number of experimental subjects. Small sample sizes may simply not have the statistical power to show relatively small effects on HR and BP induced by microgravity. Additionally, the logistics of collecting cardiovascular data during spaceflight are difficult. Astronauts are responsible for a variety of experimental and operational tasks, often performed in close temporal proximity. Countermeasures, including exercise, centrifugation, and compression garments are often employed during these missions, though not always in a standardized fashion amongst experimental participants. Limiting and correcting for these confounding variables is an arduous if not impossible task.
HR and BP data from long-duration flights are more consistent. Though studies are limited, they demonstrate largely unchanged HR and unchanged or even reduced BP in flights of up to six months in duration. This includes results from Verheyden et al, with data from a total of 11 astronauts collected during long-duration flights, and compared to supine pre-flight values. In-flight BP was reduced in this cohort, though the results were not statistically significant. Long-term studies of HR and BP lack the variability seen in short-duration data. It is unclear if this is reflective of different cohorts, longer periods of data acquisition, in-flight countermeasures, or if neurophysiological adaptations take place during extended exposure to microgravity such that HR and BP return to, or even drop below, pre-flight values.
Mechanisms of Altered Autonomic Function in Space
There have been several proposed mechanisms to explain alterations in autonomic function in space, leading to increased sympathetic activity and depressed vagal baroreflex sensitivity. These will be addressed below and are represented in Figure 4.
Figure 4.
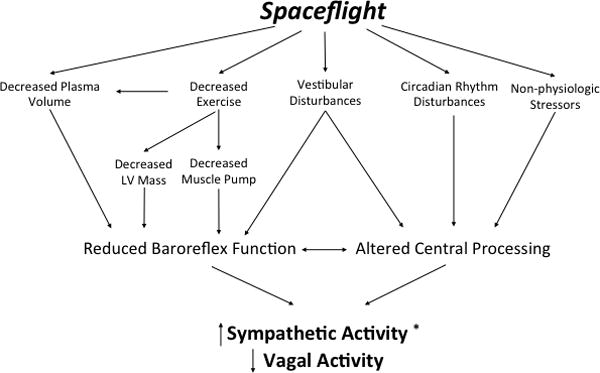
Proposed mechanisms of autonomic dysfunction during spaceflight. *Relative to the supine position on Earth.
Decreased Circulating Volume
Decreased red cell mass and plasma volume are well-established physiologic adaptations to spaceflight (14). Though decreased circulating volume during spaceflight was once believed to be a result of the diuretic response to the centralization of blood volume in microgravity, this has not been supported experimentally. Rather, reductions in plasma volume during spaceflight are the result of decreased fluid intake, fluid shifts from the intravascular to interstitial space, as well as reductions in red cell mass. In addition to cardiovascular deconditioning and reduced LV mass, these findings have been used in support of prior echocardiographic demonstration of decreased left ventricular end-diastolic volume (LVEDV) and cardiac output during flight and after landing (1, 19). Decreased circulating blood volume and depressed cardiac output decreases carotid and aortic mechanoreceptor stretch, leading to decreased afferent baroreflex stimulation. This, in turn, increases sympathetic outflow to maintain hemodynamic homeostasis in this hypovolemic or “low output” state. This mechanism is eloquently demonstrated by the near-perfect relationship between stroke volume and MSNA found during the NEUROLAB mission.
Physical Deconditioning
Physical deconditioning that occurs in microgravity may also contribute to changes in autonomic function in space. The unloading of gravitational stressors during spaceflight results in significant muscle atrophy (20) and decreased exercise capacity (29). A growing body of evidence has emerged demonstrating a fundamental relationship between exercise and autonomic function. Prior studies have demonstrated impaired vagal baroreflex function following bed rest with head-down tilt (9, 15). A single bout of exercise has been shown to restore vagal baroreflex function following bed rest (10). Similar data have come from studies of patients with neurally mediated syncope and those suffering from the postural orthostatic tachycardia syndrome (POTS). The cardiovascular autonomic dysregulation in POTS patients is similar to those observed in returning astronauts. Reduced VO2max is present in nearly 90% of patients with POTS (55). Exercise training has been shown to improve upright hemodynamics and arterial baroreflex sensitivities in these patients (27, 28). Cardiac atrophy induced by deconditioning, as well as decreased venous return secondary to lower extremity muscle atrophy, contribute to decreases in stroke volume, and may also augment sympathetic activity via the “low output” mechanism as described above (42). Amongst a myriad of neuroendocrine effects, exercise is known to increase vagal tone and increase baroreflex sensitivity. That we see the converse in the microgravity environment, in which exercise and biomechanical stress is achieved only through complex and creative mechanisms, perhaps should not come as a surprise.
Altered Central Autonomic Processing
Augmentation of central autonomic processing may contribute to increased sympathetic activity, as well as altered baroreflex function. This has been evidenced by abnormal or subnormal efferent sympathetic responses to afferent stimulation in human and animal subjects following microgravity or simulated weightlessness. Relative epinephrine, norepinephrine, and vasopressin release in response to upright posture is altered following short-duration spaceflight (44). These findings suggest that central processing of baroreceptor afferents may be fundamentally altered with exposure to microgravity, and result in alterations in effective autonomic function despite intact afferent and efferent arms of the baroreflex arc. Similar findings have been demonstrated in animal models, with suggestions of alteration in sympathetic outflow in the rostral ventrolateral medulla (RSVM) in rats after hindlimb unloading (46, 47). Similar evaluations of central autonomic processing in humans or animals during spaceflight have not been attempted, but these studies suggest that central processing is altered by microgravity, and may contribute to observed changes in autonomic and baroreflex function during spaceflight.
Disruption of Vestibular-Autonomic Reflexes
One potential contributor to altered central autonomic processing during spaceflight is the disruption of vestibular-autonomic reflexes. Spaceflight alters the gravity-dependent sensory function of the vestibular organs of the inner ear. It particularly affects otolith function, which is an important sensor of translational motion and serves a key role in control of eye movement and posture. Studies of neuro-vestibular function both during and after spaceflight have demonstrated abnormal otolith-ocular reflexes and spatial perception both during adaptation to microgravity as well as re-adaptation after return to Earth (8). In addition to its well-established role in spatial orientation, gaze control, and neuromotor function, recent work has demonstrated the presence of vestibulo-autonomic reflexes (32, 33, 36, 56, 57, 66). These excitatory vestibulo-autonomic reflexes are the earliest stimulation of sympathetic activity in response to changes of body position. They provide a physiologic primer, in advance of the baroreflex, to support and maintain blood pressure during an orthostatic challenge. Vestibulo-autonomic reflexes have not been directly studied in space. There is evidence from terrestrial studies that impairment of the vestibulo-sympathetic reflex may contribute to orthostatic hypotension (48). Alterations and impairment of vestibular function with exposure to microgravity may similarly disrupt vestibulo-autonomic reflexes and contribute to altered autonomic nervous system activity during spaceflight, as well as post-flight orthostatic intolerance.
Alterations in visual perception, which occur during space flight may further disrupt vestibulo-autonomic function. Living in space requires adaptation to non-gravitational sensory cues, including novel visual cues, to provide a stable sense of orientation. The well-documented clinical phenomenon of space motion sickness is generally attributable to this adaptive process. While astronauts typically adapt well to this sensory challenge, they have occasionally reported illusory motion of self and their environment during flight (58). Artificial visual cues, constructed to simulate perceived (illusory) tilt, can induce autonomic responses similar to passive head-up tilt (67). Transient episodes of disorientation, which occur during spaceflight may in part be responsible for observed changes in autonomic activity.
Circadian Rhythm Disturbances
The non-24-hour light-dark cycle encountered by astronauts and cosmonauts during spaceflight represents a unique challenge to normal regulation of the circadian rhythm. Animal experiments in microgravity have shown disruption of several circadian mechanisms, with resulting alterations in normal circadian periodicity and amplitude (62). Alterations of vestibular function during spaceflight may underlie some of these changes via a vestibulo-hypothalamic pathway (25). Circadian changes in autonomic function are well known, with relatively decreased sympathetic activity during sleep, contributing to the normal nocturnal dip in blood pressure. Human studies of circadian rhythm during spaceflight are extremely limited. A study of continuous 24 hour HR monitoring in two astronauts, and BP monitoring in a single astronaut, aboard the ISS revealed subtle changes in circadian HR and BP patterns, but its interpretation is extremely limited by the small number of experimental subjects (35). Given the interaction between sleep, circadian rhythm, and autonomic function on Earth, the disruption of normal circadian mechanisms during spaceflight may contribute to alterations in autonomic function, though more in-flight human experimentation is needed.
Non-Physiologic Stressors
The environment of space not only provides a novel physiologic stress to astronauts, but missions themselves can be emotionally and psychologically stimulating and stressful as well. Personal and family stressors, in addition to professional expectations to perform at a high level, undoubtedly result in physiologic stress responses and alterations in neuro-hormonal mechanisms, including the autonomic nervous system. Acute changes in HR and BP have been noted during particularly stressful mission situations, including live television broadcasts (34). The contributions of non-physiologic stressors to changes in autonomic function during spaceflight are difficult, if not impossible, to quantify. They may contribute to the observed hypertensive and tachycardic response seen during short-duration flight, and resolution of these stressors may underlie the normalization of HR and BP during long-duration flight. Non-physiologic stressors should be expected to diminish with time as astronauts psychologically adapt to the space environment, though certainly the demands of working in space may continue to contribute to mental and emotional stress in excess of that typically encountered on Earth. Further evaluation of autonomic function during long-duration flight is essential to minimizing these potentially confounding effects.
In-Flight Countermeasures
In-flight countermeasures such as fluid loading, weight-bearing exercise, and centrifugation may be essential in combating the neurophysiologic consequences of spaceflight. Isotonic fluid loading was one of the first countermeasures employed during flight, and decreases post-flight tachycardia and preserves post-flight mean arterial blood pressure (5). Structured in-flight exercise has become an important component of both short- and long-duration missions, and includes cycle ergometry, treadmill exercise, and resistance training. In-flight exercise aims to prevent skeletal and cardiac muscle atrophy, preserving venous return and cardiac stroke volume, as well as general cardiovascular fitness. Artificial gravity by centrifugation was tested during the NEUROLAB mission, exposing astronauts to experimental periods of continuous linear acceleration to simulate the downward force of gravity (49). Centrifugation provides orthostatic stress while additionally engaging postural muscles and vestibular sensory organs. Use of intermittent centrifugation during flight may prevent plasma volume loss, maintain baroreflex function, and preserve vestibular autonomic reflexes. Additional study is needed to explore the impact of centrifugation on autonomic function during spaceflight.
While post-flight orthostatic intolerance was common in early returning astronauts and cosmonauts, particularly those returning from long-duration flights, contemporary studies suggest this may be less prevalent. In a recent study of 18 cosmonauts returning from long-duration flights, only three reported mild orthostatic symptoms during 10 minute standing (61). Neither orthostatic hypotension nor presyncope were observed in any subject. However, testing was only performed 3-5 days after return from flight, which limits comparison to earlier post-flight studies. It does suggest that current countermeasures during long-duration flights aboard the ISS may mitigate, at least in part, the cardiovascular and autonomic changes that occur during flight.
Conclusions
Invasive and comprehensive study of autonomic function in space during the NEUROLAB mission, and subsequent non-invasive studies, have provided strong evidence that sympathetic activity is increased during short-duration spaceflight relative to 1-G supine controls. Other studies that demonstrate vasorelaxation during spaceflight relative to upright controls are not contradictory, but rather help define the magnitude of the orthostatic stress of microgravity. Spaceflight represents an effectual orthostatic stress roughly between that of the supine and upright posture on Earth. Though there is evidence that vagal baroreflex function is impaired during spaceflight, recent studies have been inconsistent. Alterations in autonomic function during spaceflight are likely multifactorial in etiology. Underlying mechanisms include: hypovolemia, cardiovascular deconditioning, as well as altered central processing secondary to disruption of vestibular-autonomic reflexes, circadian rhythm disturbances, and non-physiologic stressors. Data from long-duration missions have been limited, but demonstrates cardiovascular and autonomic stability. The current utilization of in-flight countermeasures, including fluid loading, resistance and aerobic exercise, as well as short-arm centrifugation, are important in maintaining neurophysiologic stability during long-duration flights. They have also likely contributed to the decreased incidence of post-flight orthostatic intolerance in contemporary astronauts and cosmonauts. The physiologic stress of microgravity is less than that of upright positioning in 1-G, and the response of the autonomic nervous system during spaceflight appears physiologic and appropriate. With the use of appropriate in-flight countermeasures, long-duration spaceflight appears safe in terms of cardiovascular and autonomic function.
Synopsis.
Available in-flight autonomic and hemodynamic data demonstrate increased sympathetic nervous system activity, and suggests impaired baroreflex function, during short-duration flight when compared to supine pre-flight controls. When compared to upright pre-flight controls, studies suggest that relative vasorelaxation occurs during spaceflight. These conflicting results have contributed to controversy regarding the response of the autonomic nervous system to spaceflight. When these results are taken together, they demonstrate that the effectual autonomic challenge of spaceflight is small, and represents an orthostatic stress less than that of upright posture on Earth. With the use of in-flight countermeasures, autonomic function and cardiovascular physiology remain stable during long-duration spaceflight.
Acknowledgments
This work was supported in part by National Institutes of Health grants M01 RR00095, 5P01 HL56693. We thank the NEUROLAB team, especially Dr. Dwain Eckberg and Dr. Ben Levine, for the advice and support. We thank Dr. Jens Tank for providing detailed data from the ISS Pneumocard study.
Footnotes
Disclosure N/A
References
- 1.Arbeille P, Fomina G, Roumy J, Alferova I, Tobal N, Herault S. Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short- and long-term head-down tilt and spaceflights. Eur J Appl Physiol. 2001;86:157–168. doi: 10.1007/s004210100473. [DOI] [PubMed] [Google Scholar]
- 2.Baevsky RM, Baranov VM, Funtova II, Diedrich A, Pashenko AV, Chernikova AG, Drescher J, Jordan J, Tank J. Autonomic cardiovascular and respiratory control during prolonged spaceflights aboard the International Space Station. J Appl Physiol. 2007;103:156–161. doi: 10.1152/japplphysiol.00137.2007. [DOI] [PubMed] [Google Scholar]
- 3.Beckers F, Verheyden B, Liu J, Aubert AE. Cardiovascular autonomic control after short-duration spaceflights. Acta Astronaut. 2009;65:804–812. [Google Scholar]
- 4.Buckey JC, Jr, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA, Blomqvist CG. Orthostatic intolerance after spaceflight. J Appl Physiol Bethesda Md 1985. 1996;81:7–18. doi: 10.1152/jappl.1996.81.1.7. [DOI] [PubMed] [Google Scholar]
- 5.Bungo MW, Charles JB, Johnson PC., Jr Cardiovascular deconditioning during space flight and the use of saline as a countermeasure to orthostatic intolerance. Aviat Space Environ Med. 1985;56:985–990. [PubMed] [Google Scholar]
- 6.Carstensen E, Yudkin JS. Platelet catecholamine concentrations after short-term stress in normal subjects. Clin Sci Lond Engl 1979. 1994;86:35–41. doi: 10.1042/cs0860035. [DOI] [PubMed] [Google Scholar]
- 7.Christensen NJ, Heer M, Ivanova K, Norsk P. Sympathetic nervous activity decreases during head-down bed rest but not during microgravity. J Appl Physiol Bethesda Md 1985. 2005;99:1552–1557. doi: 10.1152/japplphysiol.00017.2005. [DOI] [PubMed] [Google Scholar]
- 8.Clément G, Reschke M, Wood S. Neurovestibular and sensorimotor studies in space and Earth benefits. Curr Pharm Biotechnol. 2005;6:267–283. doi: 10.2174/1389201054553716. [DOI] [PubMed] [Google Scholar]
- 9.Convertino VA, Doerr DF, Eckberg DL, Fritsch JM, Vernikos-Danellis J. Head-down bed rest impairs vagal baroreflex responses and provokes orthostatic hypotension. J Appl Physiol Bethesda Md 1985. 1990;68:1458–1464. doi: 10.1152/jappl.1990.68.4.1458. [DOI] [PubMed] [Google Scholar]
- 10.Convertino VA, Doerr DF, Guëll A, Marini JF. Effects of acute exercise on attenuated vagal baroreflex function during bed rest. Aviat Space Environ Med. 1992;63:999–1003. [PubMed] [Google Scholar]
- 11.Cooke WH, Ames JE, IV, Crossman AA, Cox JF, Kuusela TA, Tahvanainen KU, Moon LB, Drescher J, Baisch FJ, Mano T, Levine BD, Blomqvist CG, Eckberg DL. Nine months in space: effects on human autonomic cardiovascular regulation. J Appl Physiol Bethesda Md 1985. 2000;89:1039–1045. doi: 10.1152/jappl.2000.89.3.1039. [DOI] [PubMed] [Google Scholar]
- 12.Cooke WH, Cox JF, Diedrich AM, Taylor JA, Beightol LA, Ames JE, 4th, Hoag JB, Seidel H, Eckberg DL. Controlled breathing protocols probe human autonomic cardiovascular rhythms. Am J Physiol. 1998;274:H709–718. doi: 10.1152/ajpheart.1998.274.2.h709. [DOI] [PubMed] [Google Scholar]
- 13.Cox JF, Tahvanainen KU, Kuusela TA, Levine BD, Cooke WH, Mano T, Iwase S, Saito M, Sugiyama Y, Ertl AC. Influence of microgravity on astronauts' sympathetic and vagal responses to Valsalva's manoeuvre. J Physiol. 2002;538:309–320. doi: 10.1113/jphysiol.2001.012574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diedrich A, Paranjape SY, Robertson D. Plasma and blood volume in space. Am J Med Sci. 2007;334:80–85. doi: 10.1097/MAJ.0b013e318065b89b. [DOI] [PubMed] [Google Scholar]
- 15.Eckberg DL, Fritsch JM. Influence of ten-day head-down bedrest on human carotid baroreceptor-cardiac reflex function. Acta Physiol Scand Suppl. 1992;604:69–76. [PubMed] [Google Scholar]
- 16.Eckberg DL, Halliwill JR, Beightol LA, Brown TE, Taylor JA, Goble R. Human vagal baroreflex mechanisms in space. J Physiol. 2010;588:1129–1138. doi: 10.1113/jphysiol.2009.186650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ertl AC, Diedrich A, Biaggioni I, Levine BD, Robertson RM, Cox JF, Zuckerman JH, Pawelczyk JA, Ray CA, Buckey JC. Human muscle sympathetic nerve activity and plasma noradrenaline kinetics in space. J Physiol. 2002;538:321–329. doi: 10.1113/jphysiol.2001.012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esler M. Clinical application of noradrenaline spillover methodology: delineation of regional human sympathetic nervous responses. Pharmacol Toxicol. 1993;73:243–253. doi: 10.1111/j.1600-0773.1993.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 19.European Space Agency, European Symposium on Life Sciences Research in Space. Proc Third Eur Symp Life Sci Res Space. Karl Franzens Univ; Graz Austria.: Sep 14-18, 1987. Cardiovascular adaptation to zero-G during a long-term flight (237 days) on board the Salyut-VII Soviet Space Station (1984) [Google Scholar]
- 20.Fitts RH, Trappe SW, Costill DL, Gallagher PM, Creer AC, Colloton PA, Peters JR, Romatowski JG, Bain JL, Riley DA. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J Physiol. 2010;588:3567–3592. doi: 10.1113/jphysiol.2010.188508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritsch JM, Charles JB, Bennett BS, Jones MM, Eckberg DL. Short-duration spaceflight impairs human carotid baroreceptor-cardiac reflex responses. J Appl Physiol Bethesda Md 1985. 1992;73:664–671. doi: 10.1152/jappl.1992.73.2.664. [DOI] [PubMed] [Google Scholar]
- 22.Fritsch-Yelle JM, Charles JB, Jones MM, Beightol LA, Eckberg DL. Spaceflight alters autonomic regulation of arterial pressure in humans. J Appl Physiol Bethesda Md 1985. 1994;77:1776–1783. doi: 10.1152/jappl.1994.77.4.1776. [DOI] [PubMed] [Google Scholar]
- 23.Fritsch-Yelle JM, Charles JB, Jones MM, Wood ML. Microgravity decreases heart rate and arterial pressure in humans. J Appl Physiol Bethesda Md 1985. 1996;80:910–914. doi: 10.1152/jappl.1996.80.3.910. [DOI] [PubMed] [Google Scholar]
- 24.Fritsch-Yelle JM, Whitson PA, Bondar RL, Brown TE. Subnormal norepinephrine release relates to presyncope in astronauts after spaceflight. J Appl Physiol Bethesda Md 1985. 1996;81:2134–2141. doi: 10.1152/jappl.1996.81.5.2134. [DOI] [PubMed] [Google Scholar]
- 25.Fuller PM, Jones TA, Jones SM, Fuller CA. Neurovestibular modulation of circadian and homeostatic regulation: vestibulohypothalamic connection? Proc Natl Acad Sci U S A. 2002;99:15723–15728. doi: 10.1073/pnas.242251499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Q, Levine BD, Pawelczyk JA, Ertl AC, Diedrich A, Cox JF, Zuckerman JH, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Cooke WH, Robertson RM, Baisch FJ, Blomqvist CG, Eckberg DL, Robertson D, Biaggioni I. Cardiovascular and sympathetic neural responses to handgrip and cold pressor stimuli in humans before, during and after spaceflight. J Physiol. 2002;544:653–664. doi: 10.1113/jphysiol.2002.025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension. 2011;58:167–175. doi: 10.1161/HYPERTENSIONAHA.111.172262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galbreath MM, Shibata S, VanGundy TB, Okazaki K, Fu Q, Levine BD. Effects of exercise training on arterial-cardiac baroreflex function in POTS. Clin Auton Res Off J Clin Auton Res Soc. 2011;21:73–80. doi: 10.1007/s10286-010-0091-5. [DOI] [PubMed] [Google Scholar]
- 29.Hargens AR, Richardson S. Cardiovascular adaptations, fluid shifts, and countermeasures related to space flight. Respir Physiol Neurobiol. 2009;169(Suppl 1):S30–33. doi: 10.1016/j.resp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Hoffler G, Johnson R. Apollo Flight Crew Cardiovascular Evaluations. Washington, D.C.: NASA Headquarters; 1975. [Google Scholar]
- 31.Hughson RL, Shoemaker JK, Blaber AP, Arbeille P, Greaves DK, Pereira-Junior PP, Xu D. Cardiovascular regulation during long-duration spaceflights to the International Space Station. J Appl Physiol. 2012;112:719–727. doi: 10.1152/japplphysiol.01196.2011. [DOI] [PubMed] [Google Scholar]
- 32.Hume KM, Ray CA. Sympathetic responses to head-down rotations in humans. J Appl Physiol Bethesda Md 1985. 1999;86:1971–1976. doi: 10.1152/jappl.1999.86.6.1971. [DOI] [PubMed] [Google Scholar]
- 33.Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ. Effects of bilateral vestibular lesions on orthostatic tolerance in awake cats. J Appl Physiol Bethesda Md 1985. 1999;86:1552–1560. doi: 10.1152/jappl.1999.86.5.1552. [DOI] [PubMed] [Google Scholar]
- 34.Karemaker JM, Berecki-Gisolf J. 24-h blood pressure in Space: The dark side of being an astronaut. Respir Physiol Neurobiol. 2009;169:S55–S58. doi: 10.1016/j.resp.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Karemaker JM, Gisolf J, Stok WJ, van Montfrans GA. 24-hr blood pressure in HDT-bed rest and short-lasting space flight. J Gravitational Physiol J Int Soc Gravitational Physiol. 2007;14:P49–50. [PubMed] [Google Scholar]
- 36.Kaufmann H, Biaggioni I, Voustianiouk A, Diedrich A, Costa F, Clarke R, Gizzi M, Raphan T, Cohen B. Vestibular control of sympathetic activity: An otolith-sympathetic reflex in humans. Exp Brain Res. 2002;143:463–469. doi: 10.1007/s00221-002-1002-3. [DOI] [PubMed] [Google Scholar]
- 37.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 38.Kvetnansky R, Noskov VB, Blazicek P, Gharib C, Popova IA, Gauquelin G, Macho L, Guell A, Grigoriev AI. Activity of the sympathoadrenal system in cosmonauts during 25-day space flight on station Mir. Acta Astronaut. 1991;23:109–116. doi: 10.1016/0094-5765(91)90106-f. [DOI] [PubMed] [Google Scholar]
- 39.Leach CS, Alfrey CP, Suki WN, Leonard JI, Rambaut PC, Inners LD, Smith SM, Lane HW, Krauhs JM. Regulation of body fluid compartments during short-term spaceflight. J Appl Physiol Bethesda Md 1985. 1996;81:105–116. doi: 10.1152/jappl.1996.81.1.105. [DOI] [PubMed] [Google Scholar]
- 40.Leach CS, Altchuler SI, Cintron-Trevino NM. The endocrine and metabolic responses to space flight. Med Sci Sports Exerc. 1983;15:432–440. [PubMed] [Google Scholar]
- 41.Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol. 2002;538:331–340. doi: 10.1113/jphysiol.2001.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96:517–525. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- 43.Malliani A, Lombardi F, Pagani M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J. 1994;71:1. doi: 10.1136/hrt.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meck JV, Waters WW, Ziegler MG, deBlock HF, Mills PJ, Robertson D, Huang PL. Mechanisms of postspaceflight orthostatic hypotension: low alpha1-adrenergic receptor responses before flight and central autonomic dysregulation postflight. Am J Physiol Heart Circ Physiol. 2004;286:H1486–1495. doi: 10.1152/ajpheart.00740.2003. [DOI] [PubMed] [Google Scholar]
- 45.Migeotte PF, Prisk GK, Paiva M. Microgravity alters respiratory sinus arrhythmia and short-term heart rate variability in humans. Am J Physiol Heart Circ Physiol. 2003;284:H1995–2006. doi: 10.1152/ajpheart.00409.2002. [DOI] [PubMed] [Google Scholar]
- 46.Moffitt JA, Heesch CM, Hasser EM. Increased GABA(A) inhibition of the RVLM after hindlimb unloading in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R604–614. doi: 10.1152/ajpregu.00341.2001. [DOI] [PubMed] [Google Scholar]
- 47.Moffitt JA, Schadt JC, Hasser EM. Altered central nervous system processing of baroreceptor input following hindlimb unloading in rats. Am J Physiol. 1999;277:H2272–2279. doi: 10.1152/ajpheart.1999.277.6.h2272. [DOI] [PubMed] [Google Scholar]
- 48.Monahan KD, Ray CA. Vestibulosympathetic reflex during orthostatic challenge in aging humans. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1027–1032. doi: 10.1152/ajpregu.00298.2002. [DOI] [PubMed] [Google Scholar]
- 49.Moore ST, Diedrich A, Biaggioni I, Kaufmann H, Raphan T, Cohen B. Artificial gravity: a possible countermeasure for post-flight orthostatic intolerance. Acta Astronaut. 2005;56:867–876. doi: 10.1016/j.actaastro.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Murray A, Ewing DJ, Campbell IW, Neilson JM, Clarke BF. RR interval variations in young male diabetics. Br Heart J. 1975;37:882–885. doi: 10.1136/hrt.37.8.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norsk P. Blood pressure regulation IV: adaptive responses to weightlessness. Eur J Appl Physiol. 2014;114:481–497. doi: 10.1007/s00421-013-2797-2. [DOI] [PubMed] [Google Scholar]
- 52.Norsk P, Damgaard M, Petersen L, Gybel M, Pump B, Gabrielsen A, Christensen NJ. Vasorelaxation in Space. Hypertension. 2006;47:69–73. doi: 10.1161/01.HYP.0000194332.98674.57. [DOI] [PubMed] [Google Scholar]
- 53.Norsk P, Drummer C, Röcker L, Strollo F, Christensen NJ, Warberg J, Bie P, Stadeager C, Johansen LB, Heer M. Renal and endocrine responses in humans to isotonic saline infusion during microgravity. J Appl Physiol Bethesda Md 1985. 1995;78:2253–2259. doi: 10.1152/jappl.1995.78.6.2253. [DOI] [PubMed] [Google Scholar]
- 54.Pagani M, Montano N, Porta A, Malliani A, Abboud FM, Birkett C, Somers VK. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–1448. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- 55.Parsaik A, Allison TG, Singer W, Sletten DM, Joyner MJ, Benarroch EE, Low PA, Sandroni P. Deconditioning in patients with orthostatic intolerance. Neurology. 2012;79:1435–1439. doi: 10.1212/WNL.0b013e31826d5f95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radtke A, Popov K, Bronstein AM, Gresty MA. Evidence for a vestibulo-cardiac reflex in man. Lancet. 2000;356:736–737. doi: 10.1016/S0140-6736(00)02635-0. [DOI] [PubMed] [Google Scholar]
- 57.Ray CA, Hume KM. Neck afferents and muscle sympathetic activity in humans: implications for the vestibulosympathetic reflex. J Appl Physiol Bethesda Md 1985. 1998;84:450–453. doi: 10.1152/jappl.1998.84.2.450. [DOI] [PubMed] [Google Scholar]
- 58.Reschke MF, Bloomberg JJ, Harm DL, Paloski WH, Layne C, McDonald V. Posture, locomotion, spatial orientation, and motion sickness as a function of space flight. Brain Res Brain Res Rev. 1998;28:102–117. doi: 10.1016/s0165-0173(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 59.Di Rienzo M, Castiglioni P, Iellamo F, Volterrani M, Pagani M, Mancia G, Karemaker JM, Parati G. Dynamic adaptation of cardiac baroreflex sensitivity to prolonged exposure to microgravity: data from a 16-day spaceflight. J Appl Physiol. 2008;105:1569–1575. doi: 10.1152/japplphysiol.90625.2008. [DOI] [PubMed] [Google Scholar]
- 60.Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, Boutouyrie P, Somers VK, Narkiewicz K. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens. 2010;28:979–984. doi: 10.1097/hjh.0b013e328336ed9a. [DOI] [PubMed] [Google Scholar]
- 61.Tank J, Baevsky RM, Funtova II, Diedrich A, Slepchenkova IN, Jordan J. Orthostatic heart rate responses after prolonged space flights. Clin Auton Res. 2011;21:121–124. doi: 10.1007/s10286-010-0106-2. [DOI] [PubMed] [Google Scholar]
- 62.Tosini G, Aguzzi J. Effect of space flight on circadian rhythms. Adv Space Biol Med. 2005;10:165–174. doi: 10.1016/s1569-2574(05)10006-9. [DOI] [PubMed] [Google Scholar]
- 63.Verheyden B, Liu J, Beckers F, Aubert AE. Adaptation of heart rate and blood pressure to short and long duration space missions. Respir Physiol Neurobiol. 2009;169:S13–S16. doi: 10.1016/j.resp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Verheyden B, Liu J, Beckers F, Aubert AE. Operational point of neural cardiovascular regulation in humans up to 6 months in space. J Appl Physiol Bethesda Md 1985. 2010;108:646–654. doi: 10.1152/japplphysiol.00883.2009. [DOI] [PubMed] [Google Scholar]
- 65.Watenpaugh DE, Buckey JC, Lane LD, Gaffney FA, Levine BD, Moore WE, Wright SJ, Blomqvist CG. Effects of spaceflight on human calf hemodynamics. J Appl Physiol. 2001;90:1552–1558. doi: 10.1152/jappl.2001.90.4.1552. [DOI] [PubMed] [Google Scholar]
- 66.Woodring SF, Rossiter CD, Yates BJ. Pressor response elicited by nose-up vestibular stimulation in cats. Exp Brain Res. 1997;113:165–168. doi: 10.1007/BF02454153. [DOI] [PubMed] [Google Scholar]
- 67.Wood SJ, Ramsdell CD, Mullen TJ, Oman CM, Harm DL, Paloski WH. Transient cardio-respiratory responses to visually induced tilt illusions. Brain Res Bull. 2000;53:25–31. doi: 10.1016/s0361-9230(00)00305-1. [DOI] [PubMed] [Google Scholar]


