Abstract
A growing body of evidence suggests that complement dysregulation plays a role in the pathogenesis of preeclampsia. The kidney is one of the major organs affected in preeclampsia. Because the kidney is highly susceptible to complement activation, we hypothesized that preeclampsia is associated with renal complement activation. We performed a nationwide search for renal autopsy material in the Netherlands using a computerized database (PALGA). Renal tissue was obtained from 11 women with preeclampsia, 25 pregnant controls, and 14 non-pregnant controls with hypertension. The samples were immunostained for C4d, C1q, MBL, properdin, C3d, C5b-9, IgA, IgG, and IgM. Preeclampsia was significantly associated with renal C4d—a stable marker of complement activation—and the classical pathway marker C1q. In addition, the prevalence of IgM was significantly higher in the kidneys of the preeclamptic women. No other complement markers studied differed between the groups. Our findings in human samples were validated using a soluble fms-like tyrosine kinase 1 (sFlt-1) mouse model of preeclampsia. The kidneys in the sFlt-1–injected mice had significantly more C4 deposits than the control mice. The association between preeclampsia and renal C4d, C1q, and IgM levels suggests that the classical complement pathway is involved in the renal injury in preeclampsia. Moreover, our finding that sFlt-1–injected mice develop excess C4 deposits indicates that angiogenic dysregulation may play a role in complement activation within the kidney. We suggest that inhibiting complement activation may be beneficial for preventing the renal manifestations of preeclampsia.
Keywords: complement, preeclampsia, C4d, kidney, sFlt-1, proteinuria, hypertension
INTRODUCTION
Preeclampsia is a severe multisystem pregnancy-related complication that causes high maternal and perinatal morbidity and mortality rates worldwide.1 Preeclampsia complicates 2–8% of pregnancies and is characterized by endothelial damage, resulting in maternal hypertension and proteinuria after gestational week 20.2
Although the precise pathogenesis of preeclampsia is unknown, a growing body of evidence suggests that complement dysregulation plays a role in the development of preeclampsia.3 In support of this notion, women with preeclampsia have complement dysregulation in the placenta and elevated circulating levels of complement degradation products.4, 5 In addition, individuals with mutations in genes that encode complement regulatory proteins are predisposed to developing preeclampsia.6 Finally, in a case report, a terminal complement inhibitor was used successfully to reduce preeclampsia-associated conditions, thereby prolonging pregnancy in a patient with preeclampsia.7
In preeclampsia, the kidney is a target organ that develops severe damage leading to renal dysfunction, proteinuria, and abnormal renal histology.8 These symptoms are believed to reflect endothelial damage due to a dysregulation of proangiogenic and antiangiogenic factors.8, 9 For example, an increase in the antiangiogenic factor soluble fms-like tyrosine kinase 1 (sFlt-1) can prevent vascular endothelial growth factor (VEGF) from maintaining the renal endothelium, thereby leading to endothelial damage.9, 10 Damage to the fenestrated glomerular endothelium can activate the complement system.11–13 A recent study showed that patients with severe preeclampsia have a higher prevalence of urinary excretion of the terminal complement complex compared to controls, suggesting that the complement system may be involved in generating and/or mediating renal damage in preeclampsia.14 In addition, treating preeclamptic mice with complement inhibitors can reverse proteinuria and histopathological lesions.15 Interestingly, a case report showed glomerular C4d deposits in a patient with preeclampsia.16 We previously demonstrated that preeclampsia is associated with activation of the classical complement pathway in the placenta.4 Here, we investigated whether preeclampsia is associated with classical complement activation in the kidney. To address this question, we measured the presence of complement components in a unique cohort of renal autopsy tissue samples collected from preeclamptic patients. To validate our findings, we studied complement components in an sFlt-1–induced mouse model of preeclampsia.
METHODS
Patient selection and nationwide PALGA search for renal autopsy tissue
To study the role of the complement system in the renal pathology of preeclampsia, we performed a nationwide search for renal autopsy tissues in the Netherlands using the Dutch Pathology Registry (PALGA), a histopathology and cytopathology network and registry that includes all pathology laboratories within the Netherlands.17 The search parameters were: “autopsy”, “women”, “age between 18 and 45 years”, and “since 1990”. We included all patients who were pregnant and were confirmed cases of preeclampsia.18 In addition, we included two control groups: (1) pregnant women without a hypertensive disorder either prior to or during their pregnancy; this group was included to investigate the effect of pregnancy alone; and (2) young non-pregnant women with a medical history of chronic hypertension; this group was included to investigate the effect of hypertension alone. The search yielded paraffin-embedded kidney samples from 11 patients with preeclampsia, 25 pregnant controls, and 14 non-pregnant chronic hypertensive controls. If available, clinical characteristics were obtained from the autopsy reports. The records of the National Maternal Mortality Committee of the Dutch Society of Obstetrics and Gynecology were used to confirm the cause of death of each pregnant case.19 All tissue samples were coded and treated anonymously in accordance with Dutch national ethics guidelines (Code for Proper Secondary Use of Human Tissue, Dutch Federation of Medical Scientific Societies). This study was approved by the Medical Ethics Committee of the Leiden University Medical Center (P12.107).
sFlt-1 mouse model of preeclampsia
All animal experiments were performed at the Beth Israel Deaconess Medical Center in accordance with International Animal Care and Use Committee guidelines. Preeclampsia is a multifactorial disease in which immunological factors, genetic factors, oxidative stress, and antiangiogenic factors such as sFlt-1 are involved.20 We used the sFlt-1–induced mouse model of preeclampsia, which overexpresses the sFlt-1 protein and develops high blood pressure, proteinuria, and endotheliosis.21 In brief, on gestational day 9.5, pregnant female CD1 mice (Charles River, Wilmington, MA) received a tail vein injection of 2 × 109 pfu of either an adenovirus encoding sFlt-1 (Ad-sFlt-1) or an equivalent dose of the empty adenovirus vector CMV null (Vector Laboratories, Philadelphia). This model has been well characterized in both rats and mice and leads to hypertension, proteinuria, and glomerular endothelial damage 7–10 days after adenoviral injection of sFlt-1.9, 21–24 For our studies, mice were euthanized on gestational day 17.5, and one kidney from each mouse was frozen for immunofluorescence; the other kidney was formalin-fixed and embedded in paraffin. Paraffin-embedded kidney sections were stained with Periodic Acid Schiff (PAS) or silver using standard protocols.
Histology, immunohistochemistry, and immunofluorescence
Sections of human kidney samples were stained with Periodic Acid Schiff (PAS) and silver using standard protocols. To measure human renal complement activation, immunohistochemistry was performed on adjacent kidney sections. We used primary antibodies against the following proteins: C4d (Biomedica Gruppe, 1:50), a cleavage product of C4 that binds covalently to the target tissue and can arise from the classical and mannose-binding lectin (MBL) pathways; C1q (DakoCytomation, 1:800), which reflects activation of the classical complement pathway; MBL (Sigma-Aldrich Biotechnology, 1:500), which reflects activation of the lectin pathway; properdin (kindly provided by the Department of Nephrology, LUMC, 1:200), which reflects activation of the alternative complement pathway; and C3d (Abcam, 1:800) and SC5b-9 (Quidel, 1:150), both of which are formed by activation of any of the three aforementioned pathways.
To identify immunoglobulin deposits in the human glomeruli, immunofluorescence was performed for IgA, IgG, and IgM. First, the sections were treated with protease XXIV (Sigma-Aldrich) at 37°C for one hour. The sections were then incubated for one hour with FITC-labeled rabbit anti-human IgA (DakoCytomation; 1:20), FITC-labeled goat anti-human IgG (Protos Immuno Research; 1:25), or FITC-labeled rabbit anti-human IgM (DakoCytomation; 1:20).
To identify apoptotic cells, the samples were immunostained for caspase-3 (Cell Signaling Technology, Inc., 1:300). Immunohistochemistry was performed after the sections were deparaffinized and treated for antigen retrieval. Staining was visualized using the appropriate HRP-labeled secondary antibodies with diaminobenzidine as the chromogen. Finally, the sections were counterstained with hematoxylin. In addition, the TdT-mediated dUTP nick-end labeling (TUNEL) technique was used in accordance with the manufacturer’s instructions (In Situ Cell Detection Kit, Roche).
To study co-localization of complement and endothelial cells in the mouse kidneys, frozen sections were immunostained using a rat monoclonal anti-C4 antibody (Cedarlane Laboratories, 1:200), which binds to murine C4, C4b, and C4d, and a goat polyclonal antibody against von Willebrand factor (Zymed, 1:250), an endothelial marker. Staining was visualized using a FITC-conjugated rabbit anti-rat antibody and a TRITC-conjugated rabbit anti-goat antibody.
To measure IgM deposits in mouse glomeruli, frozen sections were fixed with acetone for 5 minutes, then incubated for one hour with Alexa 488-conjugated goat anti-mouse IgM (Invitrogen; 1:200).
Complement activation
Mouse C3 fragments (C3b/C3c/iC3b) were measured by sandwich ELISA using a specific rat-anti-mouse mAb for capture (clone 2/11, HM1065, Hycult Biotechnology) and biotinylated rabbit anti-mouse C3 pAb (HP8012, Hycult Biotechnology) for detection.25 Zymosan-activated CD1 mouse serum (IMSCD1-COMPL, Innovative Research) was used as a standard and set to 100 AU/ml as described previously.26
Quantification of immunohistochemistry and immunofluorescence
The human kidney sections were scored histologically by an experienced renal pathologist who was blinded with respect to the subjects’ clinical data. Each immunostained sample was evaluated and scored by two independent observers. Because the renal pathological manifestations of preeclampsia are present in the glomerulus, we scored the staining of the various markers in the glomerulus only, scoring ≥50 glomeruli per section. The immunostained complement components were scored semi-quantitatively as follows: 0 represents an absence of—or traces of—glomerular staining; 1 represents segmental glomerular staining; and 2 represents global staining of the glomeruli. If positive (score ≥1), the kidney sections were further classified as having either focal (10–50% of the glomeruli) or diffuse (>50% of the glomeruli) deposits. Caspase-3 staining was analyzed by counting the number of caspase-3–positive cells in 50 glomeruli and comparing the number of positive cells between the study groups. TUNEL staining was scored as absent or present. For immunofluorescence, the slides were analyzed for either the absence or presence of immune deposits in the glomeruli using both a fluorescence microscope (DM5500B, Leica Instruments) and a confocal laser-scanning microscope (LSM 700, Zeiss).
Statistical analysis
Continuous variables and the frequencies of categorical variables were analyzed using the Student’s t-test or the chi-square test, as appropriate. Differences in quantitative parameters between groups were analyzed using a one-way ANOVA (for normally distributed data) or the non-parametric Kruskal-Wallis test (for non-normally distributed data). Correlations between ordinal data and numerical data were calculated using the Spearman or Pearson coefficient, respectively. All analyses were performed using the SPSS statistical software package (version 20.0; IBM Corp.). Differences with p<0.05 were considered to be statistically significant.
RESULTS
Clinical data
The clinical characteristics of the human subjects included in the study were previously described.27 In brief, for the preeclamptic women, the median age was 32.5 years (interquartile range: 29–36), median gestational age was 35.7 weeks (interquartile range: 34–39), mean parity was 0.6 children, and median proteinuria was 0.36 g/24 hours (interquartile range: 0.3–6.1). The hypertensive control group was significantly older than the other two study groups (p<0.05), and the preeclamptic women had significantly higher systolic and diastolic blood pressure (p<0.05) than pregnant controls; no other significant differences were observed with respect to the remaining clinical characteristics. Median death-autopsy interval in the preeclamptic women was 18.0 hours (interquartile range: 6.0–32.3), which did not differ significantly from the control groups (24.0 hours, interquartile range: 16.5–24.0). The preeclamptic women and control subjects had no previously reported renal disease. The cause of death was preeclampsia-associated complications in all preeclamptic cases; the cause of death in the majority of controls included thromboembolism, aortic dissection, or infection.
Histology
The renal histology of this cohort was previously published.27 In brief, the majority (82%) of preeclamptic women had prominent glomerular lesions, including various degrees of endotheliosis, podocyte swelling, and tram tracking (i.e., double contours of the glomerular basement membrane). The prevalence of endotheliosis in the preeclamptic women (55%) was higher than in the pregnant (12%; p<0.05) and hypertensive controls (15%; p<0.05). Tram tracking (36%) and podocyte swelling (18%) were present exclusively in the preeclamptic women (p<0.05 versus both control groups).
Immunohistochemistry
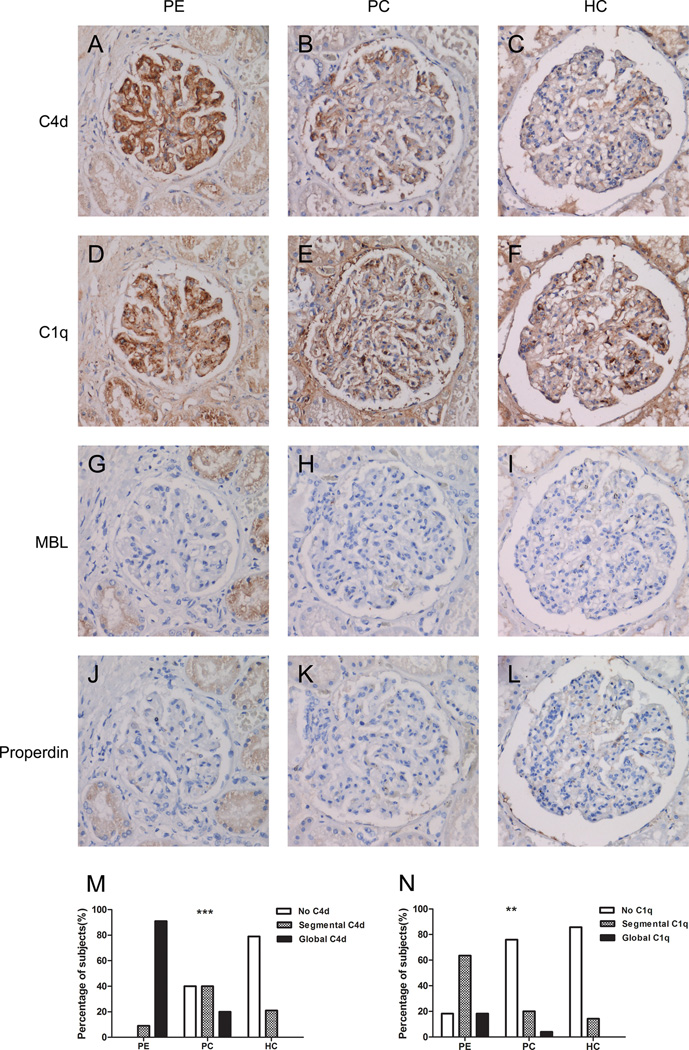
Figure 1 shows typical examples of immunostained adjacent kidney sections from a patient with preeclampsia, a pregnant control, and a hypertensive control. The glomeruli in all 11 preeclamptic women (100%) were positive for C4d; in contrast, only 15/25 (60%) pregnant controls and 3/14 (21%) non-pregnant hypertensive controls had C4d staining in the glomeruli. Positive C4d staining was strongly associated with preeclampsia (p<0.001), and global C4d staining was more prevalent in the preeclamptic women (91%) than in the controls (13%; p<0.001). The presence of C4d was correlated significantly with endotheliosis and tram tracking (p<0.05). C1q staining was observed in the glomeruli of 82% of the preeclamptic women; in contrast, C1q was detected in only 24% of the pregnant controls and 14% of the non-pregnant hypertensive controls. Positive C1q staining was significantly associated with both preeclampsia (p<0.01) and C4d staining (p<0.001). MBL was present in one preeclamptic woman and one pregnant control; in both samples, the staining pattern was segmental; no significant differences were found between cases and controls with respect to MBL staining (p=0.515). Properdin was not detected in any of the kidney samples. C3d staining was typically observed in a segmental staining pattern and was present in the glomeruli of 45% of preeclamptic women, 8% of the pregnant controls, and 14% of the non-pregnant hypertensive controls. The prevalence of glomerular C3d was significantly higher in the preeclamptic women than the controls (p<0.05); however, no significant correlation was found between C4d and C3d staining (p=0.184). The most abundant C5b-9 staining was detected in sclerotic glomeruli; C5b-9 was only present segmentally in functioning glomeruli of three autopsy samples (one from each group). Figure S1 shows typical staining patterns of C3d and C5b-9. All positive immunostained sections had a diffuse staining pattern; none of the samples had a focal staining pattern.
Figure 1. Immunohistochemistry of human kidney sections.
Adjacent sections of glomeruli were immunostained for C4d (A–C), C1q (D–F), mannose-binding lectin (G–I), or properdin (J–L). Each column contains adjacent sections and shows a single glomerulus. The left column shows a glomerulus from a patient with preeclampsia (PE), with global C4d staining. The middle column shows a glomerulus from a pregnant control (PC), with segmental C4d staining. The right column shows a C4d-negative glomerulus from a hypertensive control (HC). C1q staining was present in C4d-positive glomeruli (D) but also in C4d-negative glomeruli. In C4d-positive glomeruli, co-localization of C1q and C4d was observed (A and D). MBL was rarely observed (G–I) and properdin was never observed (J–L). Summary of the prevalence of each C4d (M) and C1q (N) staining pattern in the three groups. Kidney sections from all preeclamptic patients were positive for C4d, with global staining in the majority of the kidney sections. In contrast, the majority of the pregnant and hypertensive controls showed a segmental or negative C4d staining pattern. Overall comparison revealed that C4d was significantly increased in preeclampsia (p<0.001). Panel N shows the staining patterns for C1q; C1q was significantly increased in preeclampsia (p<0.01). **p<0.01, ***p<0.001.
No significant difference was found between the patient and control groups with respect to caspase-3 staining (p=0.529). Specifically, the samples from the preeclamptic women had an average of 0.05 caspase-3–positive cells/glomerulus, and the samples from the pregnant controls and hypertensive controls had an average of 0.02 and 0.12 caspase-3–positive cells/glomerulus, respectively. TUNEL staining revealed similar results (data not shown).
Immunofluorescence
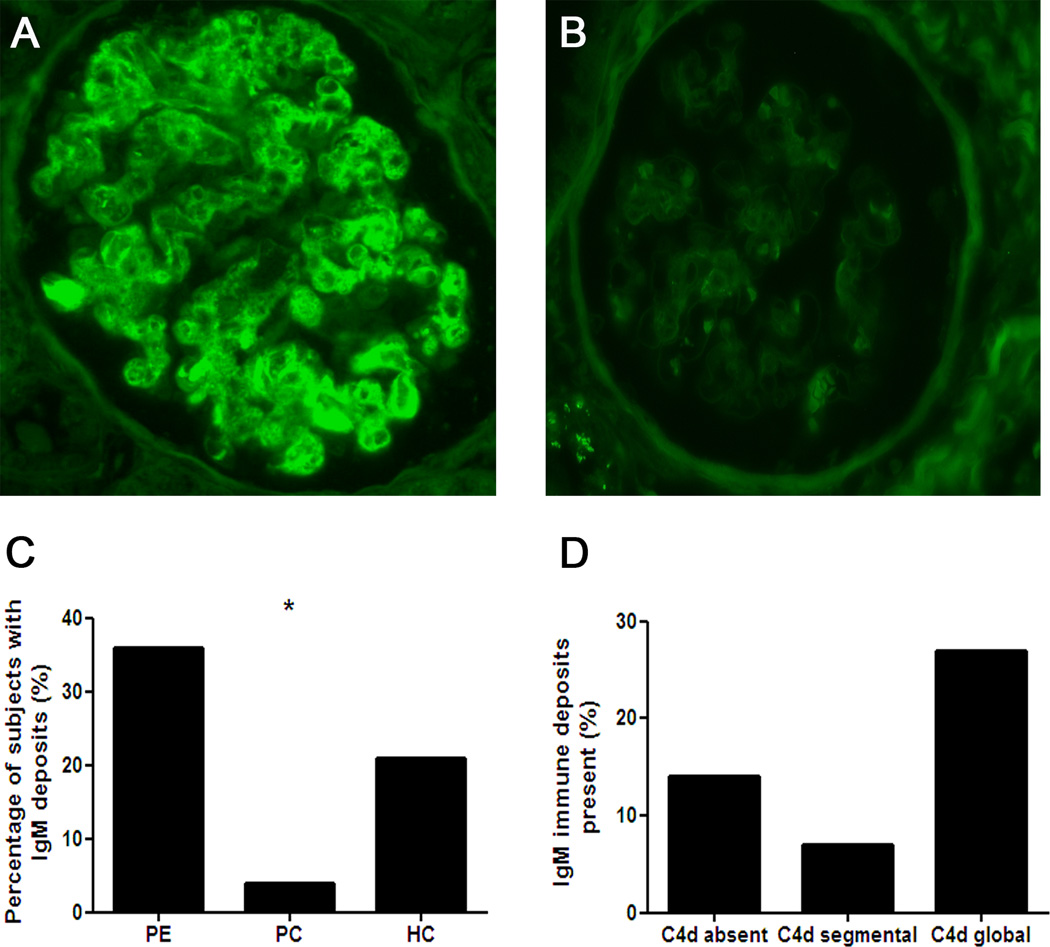
IgA was not detected in any of the samples. However, the preeclamptic patients, pregnant controls, and non-pregnant hypertensive controls had weak mesangial IgG staining (in 27%, 8%, and 21% of the subjects, respectively; p=0.265 between the groups). IgM (Figure 2) was detected in 36%, 4%, and 21% of the preeclamptic patients, pregnant controls, and non-pregnant hypertensive controls, respectively (p<0.05 between the groups). We also analyzed the prevalence of IgM staining based on whether the sections were C4d-positive or negative; 14% (3/21) C4d-negative kidney sections contained IgM deposits; 7% (1/14) kidney sections with segmental C4d staining contained IgM; and 27% (4/15) kidney sections with global C4d staining contained IgM. Although IgM deposits were more prevalent in the kidney sections with global C4d, this correlation was not statistically significant. In contrast, the presence of IgM was correlated significantly with tram tracking (p<0.001).
Figure 2. Immunofluorescent staining of IgM.
Representative images of an IgM-positive glomerulus (A) and an IgM-negative glomerulus (B). IgM deposits were significantly more prevalent in the kidney sections from the preeclamptic women compared to the two control groups (C). Distribution of the percentage of IgM-positive sections based on C4d staining pattern (D) (p>0.05). *p<0.05.
Correlation between clinical characteristics and C4d
Among the 36 samples obtained from the preeclamptic patients and pregnant controls, 10 samples were negative for C4d, 11 samples were C4d-positive with segmental staining, and 15 samples were C4d-positive with global staining. Global C4d deposits were significantly correlated with increased gestational age (p<0.05), whereas C4d-negative staining was not correlated significantly with gestational age. Neither the level of proteinuria nor peak blood pressure was correlated with the pattern of C4d staining.
sFlt-1 mouse model of preeclampsia
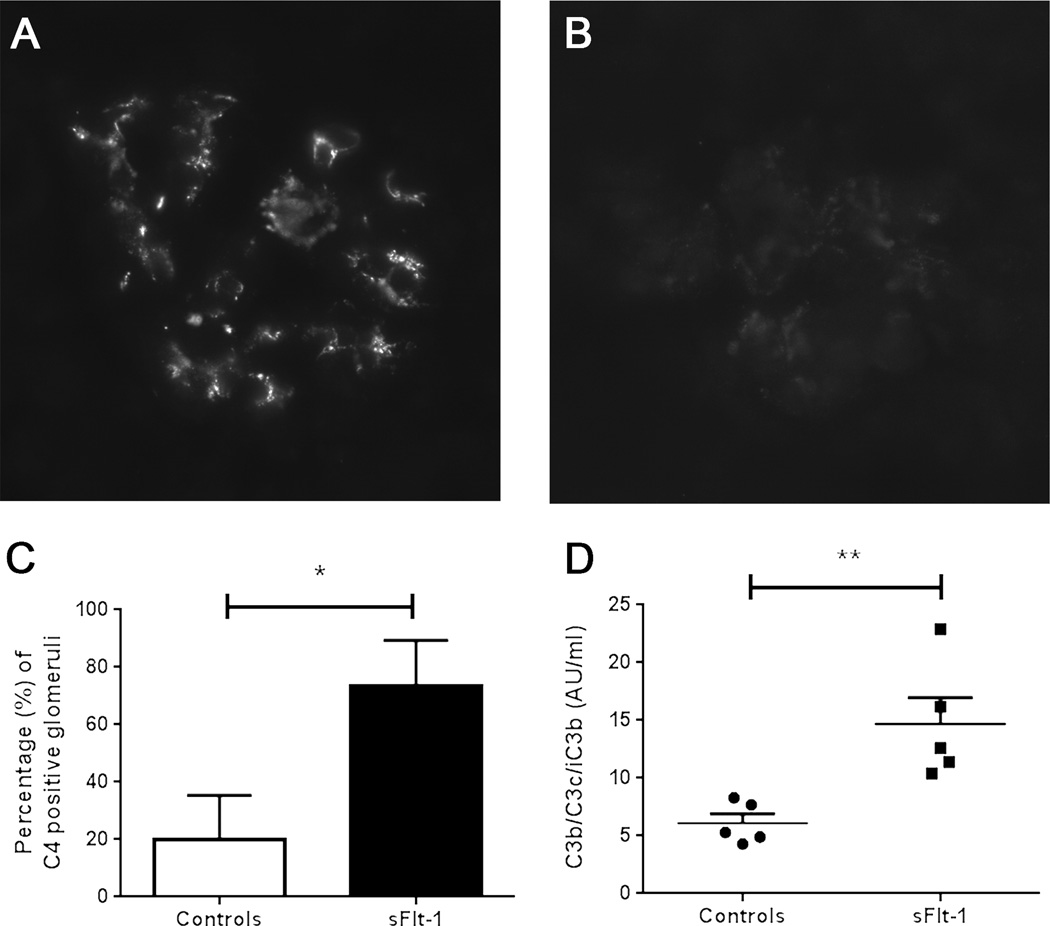
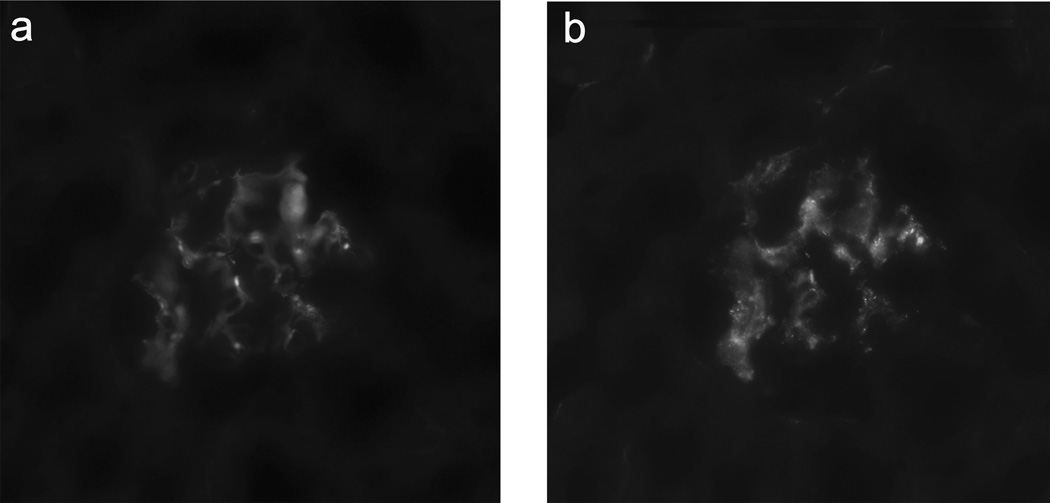
Next, we validated our findings in an sFlt-1 mouse model that develops endothelial dysfunction and manifests preeclampsia-like signs and symptoms.9, 21–23 As reported previously, injecting sFlt-1 into the tail vein caused a preeclampsia-like phenotype, with significantly elevated blood pressure, urinary albumin secretion, and endotheliosis (measured using open capillary volume).9, 28, 29 The sFlt-1–injected mice (n=6) had significantly more C4-positive glomeruli (p<0.05) and significantly higher levels of circulating activated C3 fragment in serum (p<0.01) compared to control-treated mice (n=5), indicating increased complement activation (Figure 3). IgM was present in the glomeruli of 100% and 60% of sFlt-1–injected and control-treated mice, respectively (p=0.151). C4 deposits were present on the endothelial cells, as demonstrated by the co-localization of C4 and von Willebrand factor in double-stained sections (Figure 4).
Figure 3. Complement activation in the kidneys of sFlt-1–injected mice as a model of preeclampsia.
Representative images of C4 deposits in a glomerulus from an sFlt-1–injected (A) and a control-treated (B) mouse. The average (±SD) percentage of C4-positive glomeruli (C) is significantly higher in the kidneys of sFlt-1–injected mice (N=6 mice) than control-treated mice (N=5 mice). sFlt-1–injected mice had significantly higher levels of activated C3 fragments in the serum compared to control-treated mice (D). *p<0.05, **p<0.01. AU = arbitrary units.
Figure 4. Double-staining for C4 and von Willebrand factor.
Mice injected with sFlt-1 have co-localization of complement factor C4 (A) and von Willebrand factor (B).
DISCUSSION
The mechanisms that underlie the renal pathology in preeclampsia are poorly understood. Here, we report that the glomeruli of all preeclamptic women in our study were positive for C4d deposits, with a predominantly global staining pattern. In contrast, C4d deposits were significantly less prevalent in the two control groups, which were comprised of non-hypertensive pregnant women and non-pregnant women with chronic hypertension. Importantly, C4d was correlated with C1q, whereas C4d was not correlated with MBL, and properdin was not observed; thus, the complement system appears to be activated via the classical pathway but not via the lectin or alternative pathways. In sFlt-1-injected mice, an established model of preeclampsia, the prevalence of C4-positive glomeruli and the levels of activated C3 fragment in the serum were significantly higher than in control-treated mice. Taken together, these findings suggest that preeclampsia is associated with activation of the classical complement pathway in the kidney.
We previously described a similar relationship between preeclampsia and activation of the classical complement pathway in the placenta.4 Both the previous study and our current study raise the question of what drives activation of the classical complement pathway in the setting of preeclampsia.
In general, complement imbalance can be caused by excessive activation and/or inadequate regulation of the complement system. Excessive complement activation could result from endothelial damage caused by angiogenic dysregulation. Angiogenic dysregulation is believed to cause the initial preeclampsia-related renal injury due to increased sFlt-1 levels preventing vascular endothelial growth factor (VEGF) from maintaining the renal endothelium.9, 10 In our study, the presence of the IgM isotype was significantly associated with preeclampsia. Although glomerular IgM deposits have been observed in a wide range of renal diseases, the role of these deposits has remained elusive, suggesting that these pentameric IgM deposits might not be involved in the pathogenesis of these diseases, but rather represent non-specific IgM entrapment.30, 31 However, the presence of IgM deposits might have other explanations.
First, the presence of IgM antibodies and the activation of the classical complement pathway in the kidneys of preeclamptic women could have resulted from autoantibodies such as angiotensin II type 1 receptor agonistic antibodies (AT1-AA),32, 33 anti-phospholipid auto-antibodies,34 and/or anti-laminin auto-antibodies.35 In the context of preeclampsia, complement activation could result from these autoantibodies binding to glomerular structures or by the deposition of circulating antibody–antigen immune complexes and their subsequent entrapment in renal tissue. In our study, although we observed glomerular immunoglobulins in preeclamptic patients and in some controls, IgM was the only immunoglobulin isotype that was significantly more prevalent in the patients with preeclampsia. If immunoglobulin deposits had resulted from auto-antibodies, we would have expected to find increased IgG deposits in the kidneys of these women. Therefore, based on our observations, it is unlikely that the glomerular complement deposits in the kidneys of the preeclamptic women were caused by autoantibodies.
Second, the presence of IgM deposits could reflect the binding of IgM antibodies to damaged endothelium. Natural IgM antibodies play a major role in the clearance of damaged cells36, 37, and they can bind to both hypoxic38 and apoptotic cells30, 39 through intracellular antigens that become externalized under these conditions. The binding of IgM antibodies to either hypoxic or apoptotic cells activates the complement system.30, 38, 39 Taken together, these previously reported findings suggest that the initial endothelial damage—mediated via high sFlt-1 levels in the kidneys of preeclamptic women—might trigger the binding of IgM antibodies, thereby activating the complement system. Our finding of classical complement pathway components in the glomeruli of preeclamptic women—combined with excess deposits of C4 on glomerular endothelial cells in our sFlt-1–injected mice—supports this hypothesis. However, complement activation in preeclampsia could also result from other factors, including inflammation, immune complexes, oxidative stress, and/or ischemia-reperfusion damage.40, 41
In addition, inadequate regulation of the complement system may have caused glomerular complement activation. High levels of complement regulatory proteins are expressed in the kidney,42–44 suggesting the presence of renal complement regulation. However, in our study, we found no correlation between late complement cascade components and preeclampsia, suggesting that the complement cascade does not become activated—at least to a detectable degree—beyond the level of C3; adequate complement regulation may be responsible for our observation. Nevertheless, the association between preeclampsia and mutations in genes that encode complement regulatory proteins suggests that inadequate complement regulation is involved in the pathogenesis of preeclampsia.6 Mutations in factor H, a regulator of the alternative and classical complement pathways, have been observed in relation to preeclampsia, and reduced levels of factor H have been related to angiogenic imbalance within the kidney.45, 46
Interestingly, IgM, IgG, and C3 deposits were reported by Tribe et al. in the glomeruli of relatively few preeclamptic women and by Petrucco et al. in the glomeruli of severe cases (almost exclusively in the afferent and efferent arterioles).47, 48 Others have reported fibrin deposits, but failed to detect antibody or complement deposits.49, 50 In our study, C4d was observed in the glomeruli of all preeclamptic women; the pattern was predominantly global. In contrast, IgM, IgG and C3d were less prevalent than C4d deposits. The high prevalence of C4d compared to other complement factors could be explained by the ability of C4d to bind covalently, even after the pathway-initiating factors have dissociated.51 Because C4d was present in all preeclamptic women and was primarily present in a global staining pattern, C4d was not correlated with clinical data such as proteinuria or peak blood pressure, suggesting that the presence of C4d does not necessarily reflect disease severity in preeclampsia.
Our study has several limitations that warrant discussion. First, because we studied autopsy material, we cannot exclude the possibility that post-mortem changes may have influenced our results. Furthermore, the post-mortem death-autopsy interval in our study was longer than in the cohort studied by Sheehan, who examined the renal histopathology of preeclamptic women who were autopsied within two hours of death.52 Therefore, our observations might differ from findings in living individuals or other autopsy studies. Nevertheless, in our study, the death-autopsy interval was similar between cases and controls. In addition, we found no significant difference in death-autopsy interval with respect to the presence or absence of histologic parameters, immunoglobulins, or complement deposits (data not shown). Second, this study was an association study. Complement activation may be a cause of damage in preeclampsia in some patients, which is supported by the association with mutations in complement regulatory genes, the efficacy of eculizumab during active clinical disease, and the efficacy of inhibiting complement activation in a mouse model of preeclampsia.6, 7, 15 Complement activation may also be a result of damage, as supported by this study.
In summary, complement activation is involved in the renal damage that occurs in women with preeclampsia. Future studies that systematically manipulate complement factors will likely determine whether complement activation is a cause and/or consequence of renal damage in preeclampsia. Future studies should also be designed to determine whether inhibiting complement activation is a viable option for treating the renal manifestations of preeclampsia.
PERSPECTIVES
Here, we report the extensive activation of the classical complement pathway in the kidneys of preeclamptic women. The presence of excess C4 deposits in our sFlt-1–induced preeclampsia mouse model strongly supports the notion that preeclampsia-related renal complement activation is initiated by endothelial damage. Our results suggest that complement activation might contribute to renal injury in preeclampsia. Moreover, our findings suggest that inhibiting the complement system might reduce both the renal and placental manifestations of preeclampsia.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
The kidney sections obtained from all of the preeclamptic women in our study contained C4d deposits in the glomeruli, indicating that preeclampsia is associated with renal activation of the classical complement pathway.
Our hypothesis that angiogenic dysregulation plays an important role in triggering complement activation in the kidney is supported by our finding that the prevalence of C4-positive glomeruli and the serum levels of activated C3 fragments were significantly increased in sFlt-1–injected mice, an established model of preeclampsia.
What is relevant?
Our study suggests that initial endothelial damage mediated via high sFlt-1 levels in the kidneys of preeclamptic women can trigger the binding of IgM antibodies, thereby activating the complement system.
Complement activation may contribute to renal injury in preeclampsia.
Our findings provide compelling evidence that inhibiting the complement system could significantly reduce the renal manifestations of preeclampsia.
Summary
The clear association between preeclampsia and renal C4d, C1q, and IgM levels suggests that the classical complement pathway plays a role in the pathogenesis of renal injury in preeclampsia. Moreover, our finding that mice injected with sFlt-1 develop excess C4 deposits and increased levels of circulating activated C3 indicates that angiogenic dysregulation may play an important role in complement activation within the kidney. Based on these findings, inhibiting complement activation may help prevent the renal manifestations of preeclampsia.
ACKNOWLEDGMENTS
None
SOURCES OF FUNDING
E.K is supported by NIH KO8 award and S.A.K. is supported by Howard Hughes Medical Institute.
Footnotes
DISCLOSURES
S.A.K is a co-inventor on patents related to angiogenic markers in preeclampsia and is a consultant to Siemens and Aggamin. The other authors report no conflicts of interest.
REFERENCES
- 1.Khan KS, Wojdyla D, Say L, Gülmezoglu aM, Van Look PFa. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 2.Steegers EaP, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 3.Haeger M, Unander M, Norder-Hansson B, Tylman M, Bengtsson A. Complement, neutrophil, and macrophage activation in women with severe preeclampsia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 1992;79:19–26. [PubMed] [Google Scholar]
- 4.Buurma A, Cohen D, Veraar K, Schonkeren D, Claas FH, Bruijn JA, Bloemenkamp KW, Baelde HJ. Preeclampsia is characterized by placental complement dysregulation. Hypertension. 2012;60:1332–1337. doi: 10.1161/HYPERTENSIONAHA.112.194324. [DOI] [PubMed] [Google Scholar]
- 5.Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, Salmon JE, Holers VM. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008;198:385 e381–389 e381. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmon JE, Heuser C, Triebwasser M, Liszewski MK, Kavanagh D, Roumenina L, Branch DW, Goodship T, Fremeaux-Bacchi V, Atkinson JP. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS medicine. 2011;8:e1001013–e1001013. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burwick RM, Feinberg BB. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta. 2013;34:201–203. doi: 10.1016/j.placenta.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Karumanchi SA, Maynard SE, Stillman IE, Epstein FH, Sukhatme VP. Preeclampsia: a renal perspective. Kidney international. 2005;67:2101–2113. doi: 10.1111/j.1523-1755.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 9.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SH, Jeong HJ. Glomerular C4d deposition indicates in situ classic complement pathway activation, but is not a marker for lupus nephritis activity. Yonsei Med J. 2003;44:75–80. doi: 10.3349/ymj.2003.44.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Mauiyyedi S, Crespo M, Collins aB, Schneeberger EE, Pascual Ma, Saidman SL, Tolkoff-Rubin NE, Williams WW, Delmonico FL, Cosimi aB, Colvin RB. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. Journal of the American Society of Nephrology. 2002;13:779–787. doi: 10.1681/ASN.V133779. [DOI] [PubMed] [Google Scholar]
- 13.Savage CO. The biology of the glomerulus: endothelial cells. Kidney Int. 1994;45:314–319. doi: 10.1038/ki.1994.40. [DOI] [PubMed] [Google Scholar]
- 14.Burwick RM, Fichorova RN, Dawood HY, Yamamoto HS, Feinberg BB. Urinary excretion of C5b-9 in severe preeclampsia: tipping the balance of complement activation in pregnancy. Hypertension. 2013;62:1040–1045. doi: 10.1161/HYPERTENSIONAHA.113.01420. [DOI] [PubMed] [Google Scholar]
- 15.Qing X, Redecha PB, Burmeister Ma, Tomlinson S, D'Agati VD, Davisson RL, Salmon JE. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney international. 2011;79:331–339. doi: 10.1038/ki.2010.393. [DOI] [PubMed] [Google Scholar]
- 16.Joyama S, Yoshida T, Koshikawa M, Sawai K, Yokoi H, Tanaka A, Gotoh M, Ueda S, Sugawara A, Kuwahara T. C4d and C4bp deposition along the glomerular capillary walls in a patient with preeclampsia. Am J Kidney Dis. 2001;37:E6. doi: 10.1016/s0272-6386(01)90003-4. [DOI] [PubMed] [Google Scholar]
- 17.Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, Meijer GA. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29:19–24. doi: 10.1155/2007/971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 19.Schutte JM, Steegers EA, Schuitemaker NW, Santema JG, de Boer K, Pel M, Vermeulen G, Visser W, van Roosmalen J. Rise in maternal mortality in the Netherlands. BJOG. 2010;117:399–406. doi: 10.1111/j.1471-0528.2009.02382.x. [DOI] [PubMed] [Google Scholar]
- 20.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F, Hagaman JR, Kim HS, Maeda N, Jennette JC, Faber JE, Karumanchi SA, Smithies O, Takahashi N. eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype. J Am Soc Nephrol. 2012;23:652–660. doi: 10.1681/ASN.2011040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves A, Grone HJ, Ahmed A, Weich HA. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2010;14:1857–1867. doi: 10.1111/j.1582-4934.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mateus J, Bytautiene E, Lu F, Tamayo EH, Betancourt A, Hankins GD, Longo M, Saade GR. Endothelial growth factor therapy improves preeclampsia-like manifestations in a murine model induced by overexpression of sVEGFR-1. Am J Physiol Heart Circ Physiol. 2011;301:H1781–H1787. doi: 10.1152/ajpheart.00373.2011. [DOI] [PubMed] [Google Scholar]
- 24.Venditti CC, Casselman R, Young I, Karumanchi SA, Smith GN. Carbon monoxide prevents hypertension and proteinuria in an adenovirus sFlt-1 preeclampsia-like mouse model. PLoS One. 2014;9:e106502. doi: 10.1371/journal.pone.0106502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mastellos D, Prechl J, Laszlo G, Papp K, Olah E, Argyropoulos E, Franchini S, Tudoran R, Markiewski M, Lambris JD, Erdei A. Novel monoclonal antibodies against mouse C3 interfering with complement activation: description of fine specificity and applications to various immunoassays. Mol Immunol. 2004;40:1213–1221. doi: 10.1016/j.molimm.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Kotimaa JP, van Werkhoven MB, J OF, Klar-Mohamad N, van Groningen J, Schilders G, Rutjes H, Daha MR, Seelen MA, van Kooten C. Functional assessment of mouse complement pathway activities and quantification of C3b/C3c/iC3b in an experimental model of mouse renal ischemia/reperfusion injury. J Immunol Methods. 2015 doi: 10.1016/j.jim.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Penning ME, Bloemenkamp KW, van der Zon T, Zandbergen M, Schutte JM, Bruijn JA, Bajema IM, Baelde HJ. Association of preeclampsia with podocyte turnover. Clin J Am Soc Nephrol. 2014;9:1377–1385. doi: 10.2215/CJN.12811213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rana S, Karumanchi SA, Lindheimer MD. Angiogenic factors in diagnosis, management, and research in preeclampsia. Hypertension. 2014;63:198–202. doi: 10.1161/HYPERTENSIONAHA.113.02293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O'Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50:686–692. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 30.Strassheim D, Renner B, Panzer S, Fuquay R, Kulik L, Ljubanovic D, Holers VM, Thurman JM. IgM contributes to glomerular injury in FSGS. J Am Soc Nephrol. 2013;24:393–406. doi: 10.1681/ASN.2012020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz M. Focal Segmental Glomerulosclerosis. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall’s Pathology of the Kidney. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 155–204. [Google Scholar]
- 32.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Irani RA, Zhang Y, Ramin SM, Blackwell SC, Tao L, Kellems RE, Xia Y. Autoantibody-mediated complement C3a receptor activation contributes to the pathogenesis of preeclampsia. Hypertension. 2012;60:712–721. doi: 10.1161/HYPERTENSIONAHA.112.191817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briones-Garduno JC, Diaz de Leon-Ponce M, Barrios-Prieto E, Salazar-Exaire JD. IgM antiphospholipical antibodies in preeclampsia-eclampsia. Cir Cir. 2003;71:449–454. [PubMed] [Google Scholar]
- 35.Foidart JM, Hunt J, Lapiere CM, Nusgens B, De Rycker C, Bruwier M, Lambotte R, Bernard A, Mahieu P. Antibodies to laminin in preeclampsia. Kidney Int. 1986;29:1050–1057. doi: 10.1038/ki.1986.106. [DOI] [PubMed] [Google Scholar]
- 36.Fu M, Fan PS, Li W, Li CX, Xing Y, An JG, Wang G, Fan XL, Gao TW, Liu YF, Ikeda S. Identification of poly-reactive natural IgM antibody that recognizes late apoptotic cells and promotes phagocytosis of the cells. Apoptosis. 2007;12:355–362. doi: 10.1007/s10495-006-0581-z. [DOI] [PubMed] [Google Scholar]
- 37.Vollmers HP, Brandlein S. Natural human immunoglobulins in cancer immunotherapy. Immunotherapy. 2009;1:241–248. doi: 10.2217/1750743X.1.2.241. [DOI] [PubMed] [Google Scholar]
- 38.van der Pol P, Roos A, Berger SP, Daha MR, van Kooten C. Natural IgM antibodies are involved in the activation of complement by hypoxic human tubular cells. Am J Physiol Renal Physiol. 2011;300:F932–F940. doi: 10.1152/ajprenal.00509.2010. [DOI] [PubMed] [Google Scholar]
- 39.Peng Y, Kowalewski R, Kim S, Elkon KB. The role of IgM antibodies in the recognition and clearance of apoptotic cells. Mol Immunol. 2005;42:781–787. doi: 10.1016/j.molimm.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 40.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 41.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 42.Alexander JJ, Wang Y, Chang A, Jacob A, Minto AW, Karmegam M, Haas M, Quigg RJ. Mouse podocyte complement factor H: the functional analog to human complement receptor 1. J Am Soc Nephrol. 2007;18:1157–1166. doi: 10.1681/ASN.2006101125. [DOI] [PubMed] [Google Scholar]
- 43.Lin F, Emancipator SN, Salant DJ, Medof ME. Decay-accelerating factor confers protection against complement-mediated podocyte injury in acute nephrotoxic nephritis. Lab Invest. 2002;82:563–569. doi: 10.1038/labinvest.3780451. [DOI] [PubMed] [Google Scholar]
- 44.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 45.Keir LS, Welsh GI, Coward R, Richards A, Spooner RA, Saleem M. The podocyte is the initial target in the renal pathogenesis of diarrhoea-associated haemolytic uraemic syndrome. J Am Soc Nephrol. 2011;22:659A. [Abstract]. [Google Scholar]
- 46.Kishore U, Sim RB. Factor H as a regulator of the classical pathway activation. Immunobiology. 2012;217:162–168. doi: 10.1016/j.imbio.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 47.Petrucco OM, Thomson NM, Lawrence JR, Weldon MW. Immunofluorescent studies in renal biopsies in pre-eclampsia. Br Med J. 1974;1:473–476. doi: 10.1136/bmj.1.5906.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tribe CR, Smart GE, Davies DR, Mackenzie JC. A renal biopsy study in toxaemia of pregnancy. J Clin Pathol. 1979;32:681–692. doi: 10.1136/jcp.32.7.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vassalli P, Morris RH, McCluskey RT. The Pathogenic Role of Fibrin Deposition in the Glomerular Lesions of Toxemia of Pregnancy. J Exp Med. 1963;118:467–478. doi: 10.1084/jem.118.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris RH, Vassalli P, Beller FK, McCluskey RT. Immunofluorescent Studies of Renal Biopsies in the Diagnosis of Toxemia of Pregnancy. Obstet Gynecol. 1964;24:32–46. [PubMed] [Google Scholar]
- 51.Cohen D, Colvin RB, Daha MR, Drachenberg CB, Haas M, Nickeleit V, Salmon JE, Sis B, Zhao MH, Bruijn JA, Bajema IM. Pros and cons for C4d as a biomarker. Kidney Int. 2012;81:628–639. doi: 10.1038/ki.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheehan HL. Renal morphology in preeclampsia. Kidney Int. 1980;18:241–252. doi: 10.1038/ki.1980.132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.