Abstract
Rationale
Responsiveness to acute psychostimulant administration varies across ontogeny.
Objective
The purpose of the present study was to determine if age-dependent changes in D2High receptors may be responsible for the ontogeny of cocaine sensitivity in preweanling, adolescent, and adult rats.
Methods
[3H]-Domperidone/dopamine competition assays were used to determine ontogenetic changes in the proportion of D2High receptors in male and female preweanling [postnatal day (PD) 5, 10, 15, and 20], adolescent (PD 40), and adult rats (PD 80). In the behavioral experiment, responsiveness to cocaine (2.5, 5, 10, or 20 mg/kg) was assessed on PD 20, PD 40, and PD 80 for 60 min. Male and female rats were habituated to the apparatus on the two days prior to testing. Distance traveled data were presented both untransformed and as percent of saline controls.
Results
Male and female preweanling rats (PD 5–PD 20) had a significantly greater percentage of dorsal striatal D2High receptors than adolescent or adult rats. Likewise, preweanling rats (PD 20) were more sensitive to the behavioral effects of cocaine than the two older age groups. Adolescent and adult rats responded in a generally similar manner, however analysis of the untransformed locomotor activity data suggested that adolescent rats were hyporesponsive to 2.5 and 20 mg/kg cocaine when compared to adults.
Conclusions
Data from the present study are consistent with the hypothesis that ontogenetic changes in D2High receptors are responsible for age-dependent differences in psychostimulant sensitivity.
Keywords: Cocaine, Locomotor activity, Ontogeny, D2High receptors
Introduction
Psychostimulant-induced behavioral responsiveness shows maturational changes across early ontogeny, through adolescence, and into adulthood (for reviews, see Spear 1979, 2000). Although interpretation is hampered by differences in motoric ability, preweanling rats may be more sensitive to the locomotor-stimulating effects of psychostimulants than adolescent (Spear and Brick 1979) or adult rats (Campbell et al. 1969). Adolescence, on the other hand, has been described as a period of hyporesponsiveness to psychostimulant drugs. Spear (1979, 2000) has long contended that sensitivity to acute psychostimulant administration is best represented by a U-shaped curve, with preweanling and adult rats showing robust cocaine- and amphetamine-induced locomotor activity, while adolescent rats exhibit a muted response to these drugs (Spear and Brake 1983; see also Lanier and Isaacson 1977; Bolanos et al. 1998; Frantz et al. 2006). Not all findings are consistent with this conceptual framework however, as multiple reports suggest that acute psychostimulant administration causes hyperresponsiveness, rather than hyporesponsiveness, in adolescent rats and mice (Caster et al. 2005; Catlow and Kirstein 2005; Badanich et al. 2008; Kameda et al. 2011).
Difficulties associated with assessing age-dependent changes in psychostimulant responsivity are probably responsible for the lack of consistency between laboratories. Age comparisons involving preweanling rats are particularly problematic because the behavioral repertoire of the young animal, as well as general motoric ability and body size, changes across ontogeny (Moody and Spear 1992). The mechanisms of action of the different psychostimulants may also be responsible for some of the divergent results, because Walker et al. (2010) have reported that dopamine (DA) transport inhibitors (e.g., cocaine and methylphenidate) induce more locomotor activity in adolescent rats than adults, while DA releasers (e.g., amphetamine and methamphetamine) cause similar behavioral effects in the two age groups (but see Bolanos et al. 1998). Psychostimulants differentially impact the locomotor activity of male and female adult rats (Festa et al. 2004; Milesi-Hallé et al. 2005), thus it is not surprising that sex may be an important determinant affecting the ontogeny of drug responsivity (Parylak et al. 2008). Lastly, the pattern of results obtained is heavily influenced by the decision to statistically analyze untransformed locomotor activity data (Campbell et al. 1969; Spear and Brick 1979; Kameda et al. 2011) or percent of saline controls (Badanich et al. 2008; White et al. 2008; Koek et al. 2012).
The neural mechanisms responsible for the ontogeny of psychostimulant responsivity are not yet understood, although the role played by the DA transporter has been examined in some detail. It is perhaps relevant that DA release capacity increases from adolescence to adulthood, while the ratio of DA uptake to release declines (Walker and Kuhn 2008). These findings have lead Walker et al. (2010) to suggest that age-dependent changes in DA release capacity may underlie some of the behavioral differences reported in adolescent and adult rats. Whether this explanation can account for the enhanced drug sensitivity exhibited by preweanling rats is uncertain. DA receptor numbers also vary across ontogeny, but not in a simple monotonic fashion. D1 and D2 receptor sites in the dorsal striatum increase linearly in number until around the fourth postnatal week, at which time there is a dramatic overproduction of receptors and then a gradual decline of up to 35– 50% until adult-like levels are reached (for reviews, see Andersen and Teicher 2000; Tarazi and Baldessarini 2000; Andersen 2003). The transient overabundance of D1 and D2 receptors during the adolescent period may affect sensitivity to psychostimulants. In preweanling rats, however, changes in DA receptor numbers per se do not seem to correspond to alterations in psychostimulant responsivity. Instead, it is possible that age-dependent changes in high affinity D2 receptors might account for ontogenetic differences in drug sensitivity. Consistent with this explanation, manipulations that increase the percentage of D2High receptors produce a state in which rats show a supersensitive response to psychostimulant drugs (Seeman 2011). Therefore, our working hypothesis is that age-dependent alterations in the percentage of D2High receptors are at least partially responsible for ontogenetic differences in responsivity to psychostimulants.
The aim of our study was two-fold: (1) to determine the ontogeny of dorsal striatal D2High receptors during the preweanling period [postnatal day (PD) 5, PD 10, PD 15, and PD 20], adolescence (PD 40), and adulthood (PD 80); and (2) assess cocaine sensitivity in male and female preweanling, adolescent, and adult rats. Percent D2High receptors did not vary among the four preweanling ages tested, so only one of these groups was included in the behavioral experiment. In order to avoid some of the interpretive problems associated with using very young rats (e.g., limited motoric ability, poor thermoregulation, unopened eyes, etc.), PD 20 rats served as our preweanling age group. Even so, we made the testing environments more physically compatible by assessing preweanling rats in smaller sized chambers than adolescent and adult rats (see also Campbell et al. 1969; Lanier and Isaacson 1977; Shalaby and Spear 1980; Doremus-Fitzwater and Spear 2010). In all cases, rats were habituated to the testing environment for two days (60 min each day) prior to cocaine administration. Distance traveled data (a measure of horizontal locomotor activity) were presented both untransformed and as percent of same-age/same-sex saline controls from the second habituation day (White et al. 2008).
Materials and methods
Subjects
Subjects were 390 preweanling, adolescent, and adult rats. Male (N = 46) and female (N = 46) adult rats were purchased from Charles River (Hollister, CA). Adult rats were allowed to acclimate to the California State University, San Bernardino (CSUSB) vivarium for a minimum of 21 days before behavioral testing. Preweanling (males, N = 101; females, N = 100) and adolescent rats (males, N = 48; females, N = 49) were born and bred at CSUSB. Litters were culled to 10 pups on postnatal day (PD) 3 and weaned on PD 23. Preweanling rats were kept with the dam and littermates, whereas adolescent and adult rats were group housed with conspecifics. Food and water were freely available. The colony room was maintained at 22–23°C and kept under a 12 L:12 D cycle. Subjects were cared for according to the “Guide for the Care and Use of Laboratory Animals” (National Research Council 2010) under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
Apparatus
Behavioral testing was done in commercially available (Coulbourn Instruments, Allentown, PA) activity monitoring chambers, consisting of acrylic walls, a plastic floor, and an open top. Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that was used to determine distance traveled (locomotor activity) and repetitive motor movements (a measure of stereotypy). Preweanling and older rats differ substantially in terms of body weight, body length (snout to base of tail), and motoric ability. For example, male rats tested on PD 80 are longer (x̄) = 26.1 cm) and heavier (x̄ = 395.4 g) than male rats tested on PD 20 (length, x̄ = 12.7 cm; weight, x̄ = 47.2 g). Therefore, PD 20 rats were tested in smaller chambers (26 × 26 × 41 cm) than PD 40 and PD 80 rats (41 × 41 × 41 cm). In all other aspects, the different-sized chambers were identical to each other.
Drugs
For the receptor binding experiment, (−)-sulpiride was dissolved in a minimal amount of glacial acetic acid and diluted with distilled water. For the behavioral experiment, (−)-cocaine hydrochloride was dissolved in saline and injected intraperitoneally (IP) at a volume of 5 ml/kg (preweanling rats) or 1 ml/kg (adolescent and adult rats). Nonlabeled ligands were purchased from Sigma-Aldrich (St. Louis, MO), whereas [3H]-domperidone (25 Ci/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO).
D2 competition assays using DA and [3H]-domperidone
D2High receptors were measured in the dorsal striatum of male and female rats at four preweanling ages (PD 5, PD 10, PD 15, and PD 20), during adolescence (PD 40), and in adulthood (PD 80). Because the size of striatal samples varied according to age, samples from multiple rats from the same litter were pooled in order to provide tissue homogenates of sufficient volume. There were eight tissue homogenates at each age (four male and four female), requiring a total of 150 subjects (PD 5, n = 43; PD 10, n = 32; PD 15, n = 24; PD 20, n = 22; PD 40, n = 17; and PD 80, n = 12). On the test day, nondrug-treated rats were killed by rapid decapitation and dorsal striatal sections were dissected bilaterally on an ice-cold dissection plate and stored at −80°C. Tissue was prepared and assays were conducted as described previously (Seeman 2008; McDougall et al. 2014b). Briefly, striatal tissue was added to buffer (6 mg tissue per 1 ml buffer) consisting of 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1.5 mM CaCl2, 5 mM KCl, 120 mM NaCl, and 4 mM MgCl2. Tissue was then homogenized using a motorized Teflon-glass homogenizer.
For the competition assays, duplicate incubation tubes contained 0.15 ml of striatal homogenate, 1.2 nM [3H]-domperidone, and various concentrations of DA. Nonspecific binding was determined in the presence of 10 μM (−)-sulpiride. Total volume for each tube was 0.5 ml. The tubes were then incubated at room temperature for 2 h. Incubation was terminated by vacuum filtration over glass fiber filters (Whatman GF/C, presoaked in assay buffer). Radioactivity was measured by liquid scintillation spectrometry.
Behavioral procedures
Male and female preweanling, adolescent, and adult rats were randomly assigned to one of five different treatment conditions. During the habituation phase, which began on PD 18, PD 38, or PD 78, rats were injected with saline and placed in activity chambers where distance traveled was measured for 60 min. A total of 40 male and 40 female rats were tested at each age. The habituation phase lasted for two consecutive days. The testing phase occurred on the following day (i.e., PD 20, PD 40, or PD 80), with rats (n = 8 per group) receiving an injection of saline or cocaine (2.5, 5, 10, or 20 mg/kg, IP) immediately before being placed in the activity chambers. Data from the first 60 min of behavioral testing were statistically analyzed and graphically presented.
Data analysis
Litter effects were minimized by assigning no more than one subject from each litter to a particular group (Holson and Pearce 1992). For the homogenate ligand binding assays, 6 × 2 (age × sex) ANOVAs were used to analyze specific binding (fmol/mg wet weight tissue) and the fraction of D2 receptors in the high affinity state (i.e., D2High receptors). The percentage of D2High receptors for each [3H]-domperidone/DA competition assay was determined using the following equation for a two-site model:
where y = specific binding, min = nonspecific binding, max = total binding, F = fraction of first receptor population, and x = concentration of unbound ligand (GraphPad Software, San Diego, CA). For the behavioral experiments, repeated measures analyses of variance (ANOVAs) were used to analyze the habituation and test day data. Significant higher order interactions (e.g., Age × Drug Dose × Sex × Time Block) were further analyzed using lower order ANOVAs. When analyzing individual time blocks, the mean square error terms (i.e., MSerror) used for the Tukey calculations were acquired from separate one-way ANOVAs. When the assumption of sphericity was violated, as determined by Mauchly's test of sphericity, the Greenhouse-Geisser epsilon statistic was used to adjust degrees of freedom (Geisser and Greenhouse 1958). Corrected degrees of freedom were rounded to the nearest whole number and, in each case, are indicated by a superscripted “a” in the parenthetical statistical reports. Post hoc analysis of both the behavioral and receptor binding data was done using Tukey tests (P<0.05). A three-parameter sigmoid curve fit model (Motulsky and Christopoulos 2003) was used to determine group dose-response curves for the behavioral data. Data from preweanling rats given 20 mg/kg cocaine were not included in the regression analyses, since these data diverged from the ascending slopes. Dose-response curves were presented for descriptive purposes only, because some of the curves failed to converge and ED50 values could not be estimated (Assié et al. 2010).
Results
D2 competition assays
Specific binding
A trend analysis showed that D2 specific binding in the dorsal striatum increased linearly according to age [F1,42 =155.68, P<0.001] (Table 1). Post hoc analysis using Tukey tests showed that adolescent (PD 40) and adult rats (PD 80) had significantly more dorsal striatal D2 specific binding sites than preweanling rats (PD 5–PD 20) [Age main effect, F5,36 =36.71, P<0.001]. A separate ANOVA comparing only the preweanling age groups showed that the number of D2 specific binding sites increased significantly from PD 5 and PD 10 to PD 20 [Age main effect, F3,24 =4.86, P<0.01]. This age-dependent increase in D2 binding sites is consistent with past studies examining the preweanling period (Murrin and Zeng 1986; Rao et al. 1991). D2 specific binding in the dorsal striatum did not differ according to sex.
Table 1.
Mean D2 specific binding and percent D2High receptors in the dorsal striatum of male and female preweanling (PD 5, PD 10, PD 15, and PD 20), adolescent (PD 40), and adult rats (PD 80).
| Age | D2 Specific Binding | Percent D2High receptors | ||
|---|---|---|---|---|
|
| ||||
| Male | Female | Male | Female | |
| PD 5 | 2.70 (+0.41) ab | 3.01 (+0.29) ab | 53.5% (+6.3) a | 44.3% (+3.3) a |
| PD 10 | 2.95 (+0.23) ab | 3.20 (+0.33) ab | 47.4% (+4.9) a | 59.6% (+1.5) a |
| PD 15 | 3.32 (+0.30) a | 3.43 (+0.12) a | 45.2% (+5.3) a | 45.2% (+8.2) a |
| PD 20 | 4.02 (+0.47) a | 3.94 (+0.15) a | 45.4% (+8.0) a | 49.5% (+4.5) a |
| PD 40 | 6.91 (+0.95) | 8.37 (+0.83) | 18.7% (+3.6) | 29.4% (+5.6) |
| PD 80 | 8.46 (+0.75) | 7.65 (+0.86) | 24.3% (+1.0) | 24.5% (+3.1) |
Specific binding (mean ± SEM) is expressed as fmol/mg wet weight tissue.
Significantly different from PD 40 and PD 80 rats (P<0.05).
Significantly different from PD 20 rats (P<0.05).
Percent D2High receptors
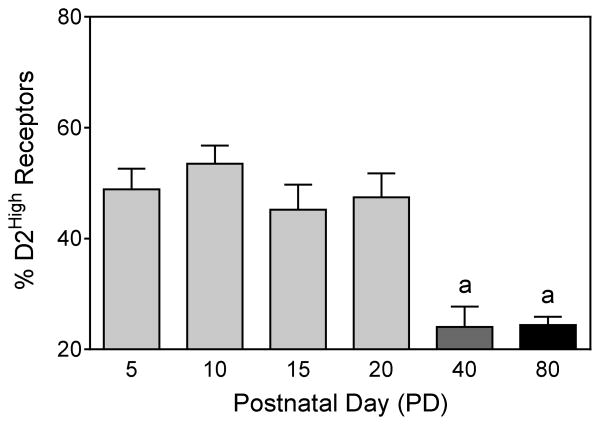
The percentage of D2High receptors in the dorsal striatum of male and female preweanling, adolescent, and adult rats are shown in Fig. 1, while representative competition curves are presented in Fig. 2. Although the percentage of high affinity receptors did not differ among the four youngest age groups tested (PD 5–PD 20), preweanling rats had a significantly greater percentage of dorsal striatal D2High receptors than adolescent or adult rats [Age main effect, F5,36 =13.02, P<0.001]. The percentage of D2High receptors in the dorsal striatum did not vary between male and female rats (Table 1).
Fig. 1.
Percent D2High receptors in the dorsal striatum of preweanling (PD 5, PD 10, PD 15, and PD 20), adolescent (PD 40), and adult rats (PD 80). ‘a’ Significantly different from the preweanling age groups (PD 5–PD 20).
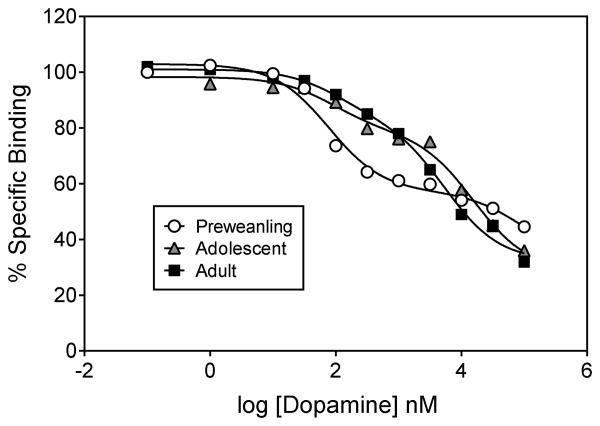
Fig. 2.
Representative competition curves from [3H]-domperidone/DA assays.
Locomotor activity of preweanling, adolescent and adult rats during the habituation phase
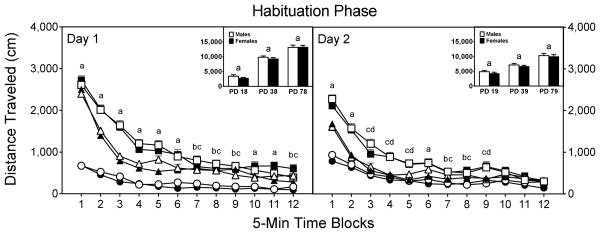
Basal locomotor activity of preweanling, adolescent, and adult rats differed according to both habituation day and time block [aAge × Day × Time Block interaction, F17,1973 =5.99, P<0.001], as distance traveled scores were greater on the first day of habituation than on the second (Fig. 3) [Day main effect, F1,237 =54.09, P<0.001]. Neither the main effect nor interactions involving the sex variable were statistically significant. As a consequence of these initial statistical analyses, distance traveled scores were collapsed across the sex variable and the two habituation days were analyzed separately.
Fig. 3.
Mean distance traveled scores (±SEM) of male and female rats (n = 8 per group) on the two habituation days. Open circle = male preweanling rats; filled circle = female preweanling rats; open triangle = male adolescent rats; filled triangle = female adolescent rats; open square = male adult rats; open square = female adult rats. ‘a’ Significant difference among all three age groups. ‘b’ Significant difference between preweanling and adolescent rats. ‘c’ Significant difference between preweanling and adult rats. ‘d’ Significant difference between adolescent and adult rats.
On Day 1 of the habituation phase (Fig. 3, left graph), Tukey tests revealed that adult rats (PD 78) had significantly greater distance traveled scores than preweanling rats (PD 18), with adolescent rats (PD 38) being intermediate between the two other age groups and significantly different from both [Age main effect, F2,237 =182.58, P<0.001]. Adult and adolescent rats locomoted more than preweanling rats on all 12 time blocks, whereas adult rats exhibited more locomotor activity than adolescent rats on time blocks 1–6, 10, and 11 [aAge × Time Block interaction, F17,2038 =37.11, P<0.001]. A similar pattern of effects was apparent on the second habituation day (Fig. 3, right graph), as adults locomoted more than preweanling (time blocks 1–9) and adolescent rats (time blocks 1–6 and 9), while adolescent rats locomoted more than preweanling rats (time blocks 1, 2, and 6–8) [Age main effect, F2,237 =49.00, P<0.001; aAge × Time Block interaction, F17,2028 =19.02, P<0.001].
Cocaine-induced locomotor activity of preweanling, adolescent, and adult rats
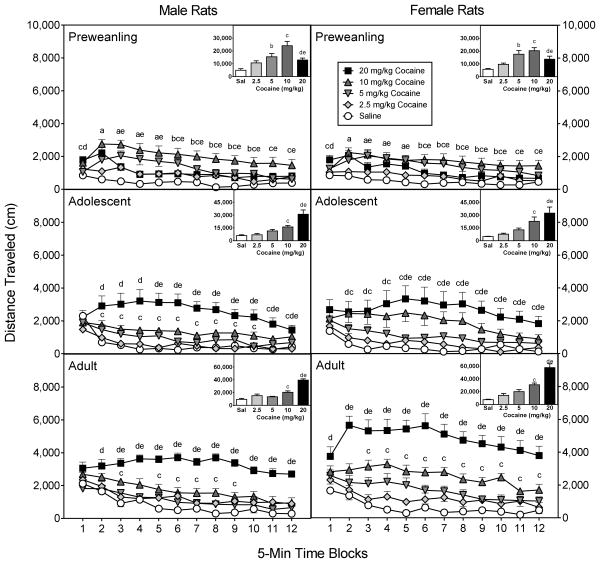
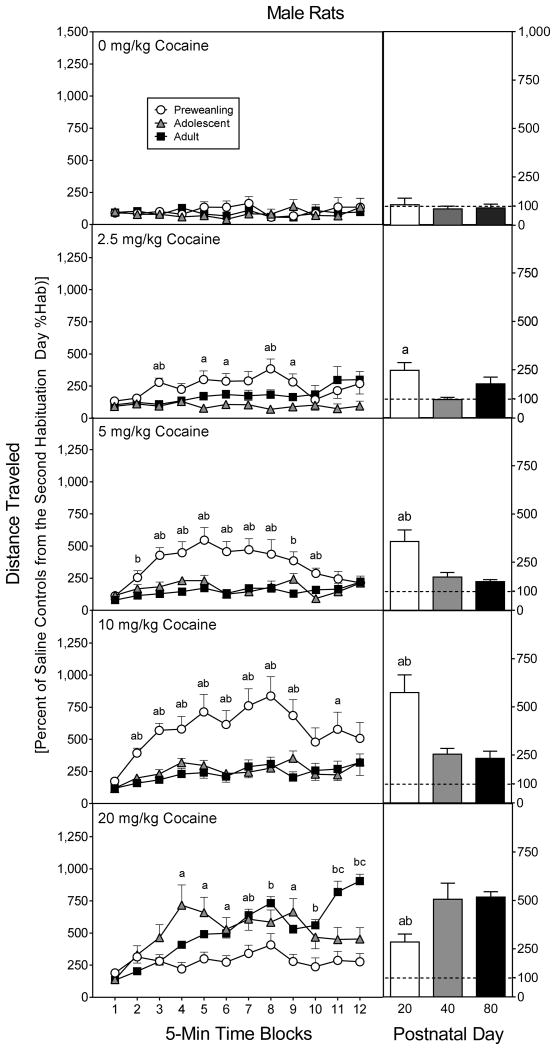
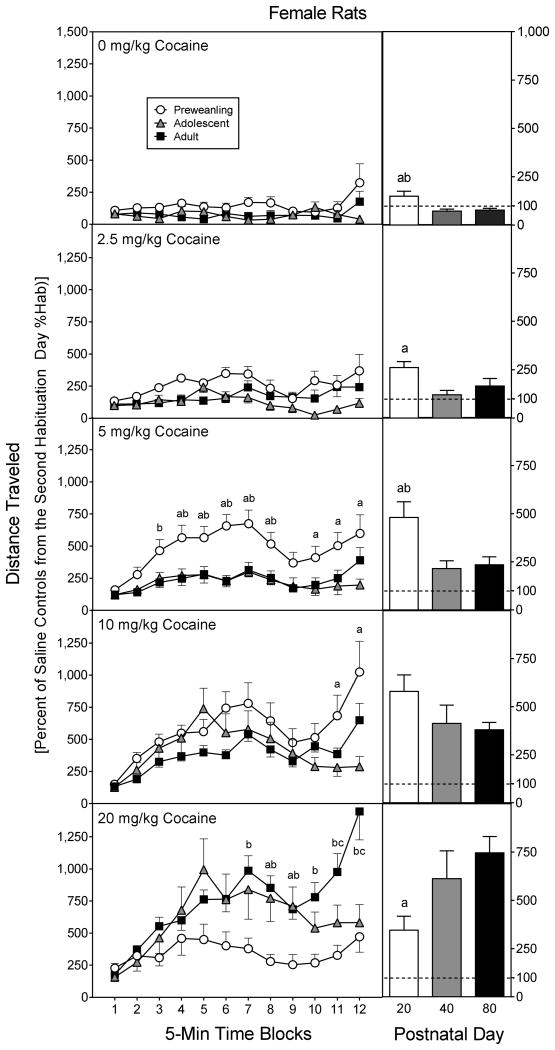
Overall, there was a progressive increase in distance traveled scores according to cocaine dose [Drug Dose main effect, F4,210 =69.61, P<0.001], but this effect was moderated by sex, age, and time block (Fig. 4) [Sex main effect, F1,210 =6.50, P<0.05; aAge × Drug Dose × Time Block interaction, F42,1094 =3.11, P<0.001]. To break apart significant higher order interactions, the distance traveled scores of the various age groups were analyzed separately.
Fig. 4.
Mean distance traveled scores (±SEM) of male and female rats (n = 8 per group) on the test day. Preweanling (PD 20), adolescent (PD 40), and adult rats (PD 80) were injected with saline or cocaine (2.5, 5, 10, or 20 mg/kg) immediately before testing. The inset shows data collapsed across the 12 time blocks. ‘a’ Significant difference between the saline group and the groups injected with 5, 10, and 20 mg/kg cocaine. ‘b’ Significant difference between the saline group and rats injected with 5 mg/kg cocaine. ‘c’ Significant difference between the saline group and rats injected with 10 mg/kg cocaine. ‘d’ Significant difference between the saline group and rats injected with 20 mg/kg cocaine. ‘e’ Significant difference between the 10 mg/kg cocaine group and rats injected with 20 mg/kg cocaine.
Preweanling rats (PD 20)
Among preweanling rats, distance traveled scores did not vary according to sex (Fig, 4, upper panels). Lower doses of cocaine (2.5–10 mg/kg) caused a linear increase in distance traveled scores [F1,60 =71.02, P<0.001], with 5 and 10 mg/kg cocaine inducing significantly more locomotion than saline [Drug Dose main effect, F4,70 =17.82, P<0.001]. An even higher dose of cocaine (20 mg/kg) caused a significant reduction in distance traveled scores relative to the 10 mg/kg cocaine group. An analysis of individual time blocks using Tukey tests showed that 10 mg/kg cocaine stimulated more locomotion than saline on all 12 time blocks, while rats treated with 5 mg/kg cocaine had greater distance traveled scores than saline controls on time blocks 2–10 [aDrug Dose × Time Block interaction, F19,328 =4.11, P<0.001]. Importantly, 20 mg/kg cocaine produced less locomotion than 10 mg/kg cocaine on time blocks 3–12, and more locomotion than saline on time blocks 1–5.
Adolescent rats (PD 40)
Cocaine caused a linear and progressive increase in the distance traveled scores of adolescent rats (Fig. 4, middle panels) [F1,75 =73.06, P<0.001], with 10 and 20 mg/kg cocaine producing more locomotor activity than the lesser doses of cocaine or saline (time blocks 2–12) [Drug Dose main effect, F4,70 =19.19, P<0.001; aDrug Dose × Time Block interaction, F18,311 =4.24, P<0.001]. On time blocks 5–12, 20 mg/kg cocaine stimulated more locomotor activity than 10 mg/kg cocaine. Distance traveled scores of adolescent rats did not differ according to sex.
Adult rats (PD 80)
Adult female rats had significantly greater distance traveled scores than male rats [Sex main effect, F1,70 =12.52, P<0.001], an effect that was only apparent in groups receiving 10 or 20 mg/kg cocaine (Fig. 4, lower panels) [Sex × Drug Dose interaction, F4,70=4.12, P<0.01]. Among adult males, 20 mg/kg cocaine stimulated greater distance traveled scores than 10 mg/kg cocaine (time blocks 3–12) or saline (time blocks 2–12) [Drug Dose main effect, F4,35 =35.69, P<0.001; aDrug Dose × Time Block interaction, F18,159 =2.55, P<0.001]. The saline and 10 mg/kg cocaine groups differed significantly on time blocks 3 and 5–9. Among adult females, 20 mg/kg cocaine caused greater locomotor activity than saline on all 12 time blocks, and more locomotor activity than 10 mg/kg cocaine on time blocks 2–12 [Drug Dose main effect, F4,35 =29.48, P<0.001; aDrug Dose × Time Block interaction, F19,169 =2.71, P<0.001]. Ten mg/kg cocaine enhanced the distance traveled scores of adult females, relative to saline controls, on time blocks 3–11.
Cross-age comparisons
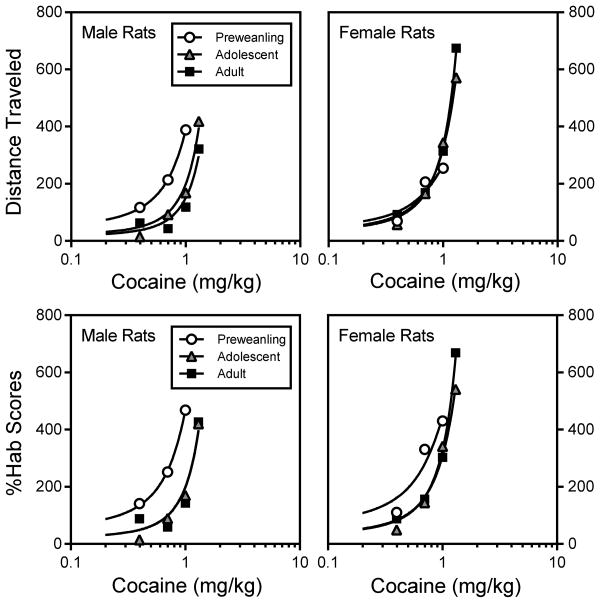
Adult rats treated with saline, 2.5 or 20 mg/kg cocaine had greater distance traveled scores than preweanling or adolescent rats (Fig. 4) [Age × Drug Dose interaction, F8,210 =13.28, P<0.001]. Preweanling rats injected with 20 mg/kg cocaine also exhibited less locomotor activity than adolescent rats. Dose-response curves representing group performance suggest a leftward shift in cocaine sensitivity among male, but not female, preweanling rats (upper graphs, Fig. 5). Distance traveled scores varied according to sex, with adult females evidencing more locomotor activity than adult males [Sex × Age interaction, F4,210 =3.63, P<0.05].
Fig. 5.
Comparison of cocaine dose-response curves for male and female preweanling, adolescent, and adult rats (n = 8 per group) on the test day. Distance traveled data (expressed as the percent of the saline control group on the test day) are shown in the upper graphs, while %Hab data [(distance traveled on the test day / distance traveled on the second habituation day) × 100] are shown in the lower graphs.
Cocaine-induced repetitive motor movements
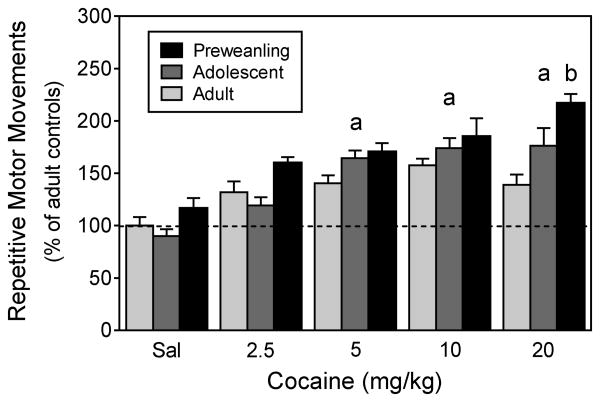
No sex differences were apparent, so repetitive motor movement data are shown collapsed across the sex variable (Fig. 6). Escalating doses of cocaine (0–20 mg/kg) caused a linear increase in repetitive motor movements [F1,235 =87.50, P<0.001], as 5, 10, and 20 mg/kg cocaine produced significantly more repetitive motor movements than saline [Drug Dose main effect, F4,210 =28.08, P<0.001]. Preweanling rats exhibited more repetitive motor movements than adolescent and adult rats [Age main effect, F2,210 =16.96, P<0.001], with this effect being particularly prominent in rats given 20 mg/kg cocaine [Age × Drug Dose interaction, F8,210 =2.17, P<0.05].
Fig. 6.
Mean repetitive motor movements (±SEM) of male and female preweanling, adolescent, and adult rats (n = 16 per group) on the test day. Data are expressed as the percent of saline-treated adult rats. ‘a’ Significantly different from the saline group. ‘b’ Significantly different from adolescent and adult rats.
Cocaine-induced locomotor activity: percent of habituation day
Basal locomotor activity differed substantially according to age, thus test day data were converted to percent of same-age/same-sex saline controls from the second habituation day [hereafter referred to as percent habituation (%Hab)]. The omnibus Age × Drug × Sex ×Time Block ANOVA indicated that the %Hab scores [(distance traveled on the test day / distance traveled on the second habituation day) × 100] of female rats were significantly elevated relative to male rats [Sex main effect, F1,210 =10.81, P<0.01]. As a consequence, drug effects involving male and female rats were analyzed separately.
Male rats
Statistical analysis of the %Hab scores of male rats showed that cocaine interacted with age and time to affect behavior [Age × Drug Dose interaction, F8,105 =7.86, P<0.001; aAge × Drug Dose × Time Block interaction F44,572 =4.15, P<0.001]. %Hab scores did not differ among saline-treated preweanling, adolescent, or adult rats (Fig. 7, top panel); however, preweanling rats injected with 2.5 mg/kg cocaine had greater %Hab scores than adolescent (time blocks 3, 5, 6, 8, and 9) or adult rats (time blocks 3 and 8) [Age main effect, F2,21 =5.94, P<0.01; aAge × Time Block interaction, F9,93 =2.58, P<0.05]. Among male rats administered 5 mg/kg cocaine, preweanling rats had significantly larger %Hab scores than adolescent and adult rats on time blocks 2–10 (Fig. 7, middle panel) [Age main effect, F2,21 =9.22, P<0.001; aAge × Time Block interaction, F10,105 =5.33, P<0.001]. When treated with 10 mg/kg cocaine (Fig. 7, second panel from the bottom), preweanling rats had greater %Hab scores than the two older age groups (time blocks 2–9 and 11) [Age main effect, F2,21 =9.22, P<0.001; aAge × Time Block interaction, F6,67 =3.55, P<0.01]. Consistent with these findings, dose-response curves based on %Hab scores reflected an enhanced sensitivity to cocaine in preweanling rats when compared to adolescents and adults (bottom left graph, Fig. 5).
Fig. 7.
Percent habituation scores (%Hab; ±SEM) of male rats (n = 8 per group) on the test day. Preweanling (PD 20), adolescent (PD 40), and adult rats (PD 80) were injected with saline or cocaine (2.5, 5, 10, or 20 mg/kg) immediately before testing. The right panels show data collapsed across the 12 time blocks. ‘a’ Significant difference between preweanling and adolescent rats. ‘b’ Significant difference between preweanling and adult rats. ‘c’ Significant difference between adolescent and adult rats.
A different pattern of effects was apparent when male rats were injected with 20 mg/kg cocaine (Fig. 7, bottom panel), as preweanling rats had smaller %Hab scores than adolescent (time blocks 4–7 and 9) and adult rats (time blocks 7, 8, and 10–12) [Age main effect, F2,21 =5.47, P<0.05; aAge × Time Block interaction, F7,73 =10.16, P<0.001]. Tukey tests revealed that adult rats had greater %Hab scores than adolescents on time blocks 11 and 12. Importantly, increasing the cocaine dose from 10 to 20 mg/kg caused a significant reduction in the %Hab scores of preweanling rats; whereas, cocaine produced a dose-dependent increase in the %Hab scores of adolescent and adult male rats [Age × Drug Dose interaction, F8,105 =7.86, P<0.001].
Female rats
When treated with saline or the lesser doses of cocaine (2.5 or 5 mg/kg), female preweanling rats had greater %Hab scores than the two older age groups (Fig. 8) [Age main effects, F2,21 =6.70, P<0.01; F2,21 =5.17, P<0.05; F2,21 =6.62, P<0.01, respectively]. Preweanling rats injected with 5 mg/kg cocaine differed from similarly treated adult rats on time blocks 3–8 and from adolescent rats on time blocks 4–8 and 10–12 [aAge × Time Block interaction, F9,95 =3.19, P<0.01]. Unlike what was observed in male rats, 10 mg/kg cocaine did not differentially affect the overall performance of the three female age groups (Fig. 8, second panel from the bottom). Only at the end of the testing session (time blocks 11 and 12) did 10 mg/kg cocaine stimulate greater %Hab scores in preweanling rats relative to adolescents [aAge × Time Block interaction, F5,57 =3.22, P<0.05]. Dose-response curves representing the %Hab scores of female rats reflect an increased sensitivity to cocaine in the youngest age group, although the effect appears to be muted when compared to male rats (bottom right graph, Fig. 5).
Fig. 8.
Percent habituation scores (%Hab; ±SEM) of female rats (n = 8 per group) on the test day. Preweanling (PD 20), adolescent (PD 40), and adult rats (PD 80) were injected with cocaine immediately before testing. The right panels show data collapsed across the 12 time blocks. ‘a’ Significant difference between preweanling and adolescent rats. ‘b’ Significant difference between preweanling and adult rats. ‘c’ Significant difference between adolescent and adult rats.
When treated with the highest dose of cocaine (20 mg/kg), female preweanling rats had significantly smaller %Hab scores than adult rats (time blocks 7–12), while adolescent rats were intermediate between the two other age groups (Fig. 8, bottom panel) [Age main effect, F2,21 =3.75, P<0.05; aAge × Time Block interaction, F6,75 =7.73, P<0.001]. Cross-dose comparisons showed that adult female rats injected with 20 mg/kg cocaine had significantly greater %Hab scores than same-age rats given any of the lesser doses of cocaine [Age × Drug Dose interaction, F8,105 =4.32, P<0.001]. In contrast, 20 mg/kg cocaine, when compared to 10 mg/kg cocaine, produced only marginally smaller %Hab scores in female preweanling rats (Tukey test, P<0.1).
Discussion
The aim of the present study was to determine whether the percentage of D2High receptors varies across ontogeny and whether these changes in receptor affinity correspond to age-dependent differences in behavioral responsiveness to cocaine. Our overarching hypothesis was that cocaine-induced increases in extracellular DA would produce heightened behavioral effects in preweanling rats by acting on an excess population of high affinity receptors (i.e., D2High receptors) more of which were responsive to DA. As expected, preweanling rats had two-fold more dorsal striatal D2High receptors than adolescent and adult rats. The percentage of D2High receptors did not vary among the four preweanling age groups tested (PD 5–PD 20), suggesting that a large proportion (∼50%) of D2 receptors stay in a high affinity state throughout early development. D2High receptors declined to adult-like levels by the mid-adolescent period (see also McDougall et al. 2014b), which contrasts with the transient overproduction of D1 and D2 receptors that occurs during adolescence (Tarazi and Baldessarini 2000; Andersen 2003). We did not observe elevated levels of D2 binding sites during adolescence; however, the [3H]-domperidone/DA competition assay is designed to measure D2High receptors, and is not as sensitive as saturation binding assays for detecting age-dependent changes in D2 binding sites.
In general, the ontogeny of dorsal striatal D2High receptors corresponds to age-dependent changes in cocaine sensitivity. When locomotor responsiveness was assessed in terms of percent of same-age/same-sex saline controls (%Hab), male and female preweanling rats were significantly more sensitive to low (2.5 and 5 mg/kg) and moderate (males only, 10 mg/kg) doses of cocaine than older rats. A high dose of cocaine (20 mg/kg) caused a significant reduction in the %Hab scores of preweanling rats and a corresponding increase in repetitive motor movements (a measure of stereotypy). Conversely, the same high dose of cocaine (20 mg/kg) further enhanced the locomotor activity of adolescent and adult rats. This pattern of effects indicates that the PD 20 age group had a greater sensitivity to cocaine, because the transition from predominately nonstereotyped to stereotyped behavior occurred at a lower dose in preweanling rats than adults (for a discussion of psychostimulants and “behavioral competition,” see Morelli et al. 1980; Bordi et al. 1989; Varela et al. 2014). Comparisons between adolescent and adult rats showed that cocaine sensitivity, at least in terms of %Hab scores, varied only slightly according to age. For example, adult rats treated with 20 mg/kg cocaine had greater %Hab scores than adolescent rats on time blocks 11 and 12. In sum, the receptor binding and %Hab data appear to be linked at each age, since D2High receptors and cocaine sensitivity were simultaneously elevated in preweanling rats and correspondingly reduced in adolescent and adult rats.
When untransformed data were statistically analyzed a somewhat different pattern of cocaine-induced effects was obtained as adolescent rats, when compared to adults, exhibited a hypoactive response to cocaine (2.5 and 20 mg/kg). Conversely, analysis of the untransformed data showed once again that preweanling rats were hyperresponsive to cocaine when compared to the two older age groups. As evidence, 5 mg/kg cocaine only increased the locomotor activity of preweanling rats and not older rats; whereas, 2.5–10 mg/kg cocaine caused a progressive increase in the cocaine-induced locomotor activity of preweanling rats, while an even higher dose of cocaine (20 mg/kg) produced both a blunted locomotor response and increased repetitive motor movements. This pattern of results suggests that stereotypy competed with, and partially masked, the locomotor response in preweanling rats. Although not as obvious as when data were transformed to percent of saline controls (%Hab), these results indicate that preweanling rats were more sensitive to cocaine than adolescent and adult rats.
Considering the present results in light of past studies examining the ontogeny of responsivity to psychostimulants, the most obvious explanation is that rats from the late preweanling period (i.e., PD 20) are more sensitive to cocaine than older rats. Campbell et al. (1969) came to the same conclusion when comparing even younger (PD 15) amphetamine-treated rats to adults. Data interpretation involving the older age groups is more difficult because one set of results suggested that adolescents and adult are equally sensitive to cocaine (%Hab scores), while analysis of untransformed data indicated that adolescent rats were hyporesponsive to 2.5 and 20 mg/kg cocaine. A similar hyporesponsiveness to psychostimulants has been reported many times before in adolescent rats and mice (Lanier and Isaacson 1977; Spear and Brake 1983; Bolanos et al. 1998; Frantz et al. 2006; Mathews et al. 2009). As mentioned in the Introduction, however, there are probably an equal number of studies showing that psychostimulants either induce hyperresponsiveness during adolescence (Caster et al. 2005; Catlow and Kirstein 2005; Badanich et al. 2008; Walker et al. 2010; Kameda et al. 2011) or do not differentially affect the behavior of adolescent and adult rats (Niculescu et al. 2005; Walker et al. 2010). In regard to these discrepant results, it seems likely that methodological differences among the various laboratories are responsible (for a fuller discussion, see Frantz et al. 2007; Kameda et al. 2011). For example, different results are often obtained depending on whether testing occurs during early, middle, or late adolescence. Indeed, Badanich et al. (2008) have reported that early adolescent male rats injected with 20 mg/kg cocaine exhibited more locomotor activity than late adolescent rats or young adults (see also Badanich et al. 2006; Caster et al. 2007). Until the various factors and situational constraints controlling the ontogeny of psychostimulant sensitivity are better understood, undue speculation about the translational relevance of these types of findings is premature.
In addition to providing information about cocaine sensitivity, the design of the present study allowed basal locomotor activity to be assessed across three ontogenetic periods. Regardless of habituation day, adult rats exhibited more locomotor activity than preweanling rats, with adolescent rats being intermediate between and significantly different from the younger and older age groups. The finding that preweanling rats locomoted less than adults has been observed before in both habituated and nonhabituated animals (McDougall et al. 2007, 2014a; but see Campbell et al. 1969). Less clear is the relationship between adolescents and adults, because adult rats and mice were found to exhibit more (Adriani and Laviola 2000; Koek et al. 2012; McDougall et al. 2014a), less (Lanier and Isaacson 1977; Spear and Brake 1983), or similar levels of basal locomotor activity when compared to adolescent rodents (White and Holtzman 2005; Zombeck et al. 2010). Procedural differences, especially involving the amount of habituation provided, probably account for the inconsistent results (Koek et al. 2012).
Although sex was included as a factor in the statistical analysis of the behavioral and neurochemical data, very few sex differences were observed. Specifically, D2High receptors did not vary according to sex (see also McDougall et al. 2014b), nor did the basal locomotor activity of male and female rats differ at any of the ages tested. The absence of sex differences involving basal locomotor activity has often been reported with preweanling (Frantz et al. 1996; Sobrian et al. 2003), adolescent (Slob et al. 1986; Brown et al. 2012), and adult rats (Schindler and Carmona 2002; Festa et al. 2004). Even so, adolescent and adult female rats will occasionally show more basal locomotor activity than males (Slob et al. 1986; Parylak et al. 2008). Among preweanling and adolescent rats, the sex variable did not influence cocaine-induced locomotor activity (see also Snyder et al. 1998; Kozanian et al. 2012); however, adult female rats injected with 10 or 20 mg/kg cocaine did show more locomotor activity than similarly-treated adult males. This is not a novel finding since many psychostimulants (e.g., cocaine, amphetamine, and methamphetamine) induce an excess of locomotor activity in adult female rats and mice (Schindler and Carmona 2002; Festa et al. 2004; Milesi-Hallé et al. 2005, 2007). Importantly, changes in the number of high affinity D2 receptors cannot account for sex-related differences in cocaine sensitivity, because the heightened locomotor response shown by female rats treated with 20 mg/kg cocaine was not matched by an increase in D2High receptors. Lastly, hormonal cycling of female rats was left uncontrolled in the present study; therefore, it is not known what impact circulating estrogen levels had on D2High receptors or cocaine responsivity. D2 binding site density and cocaine-induced locomotion vary during the different stages of the estrous cycle (Bazzett and Becker 1994; Quiñones-Jenab et al. 1999; Sell et al. 2000), so it is possible that our results were impacted by this uncontrolled variable.
The reason why young rats have more D2High receptors than adolescent or adult rats has not been established, but one possibility is that low DA levels during the preweanling period, or a reduced DA release rate, may cause a greater proportion of D2 receptors to be in a high affinity state. Consistent with this explanation, total striatal DA content is reduced in preweanling rats relative to adults (Giorgi et al. 1987; Broaddus and Bennett 1990). Alternatively, the percentage of D2High receptors may be elevated in order to compensate for the relatively low number of D2 receptors present during early ontogeny. Two pieces of evidence support this explanation as D2 receptors are present in reduced numbers during the preweanling period (Murrin and Zeng 1986; Rao et al. 1991), and drug-induced inactivation of D2 receptors causes a possible compensatory increase in the percentage of D2High receptors in preweanling rats (McDougall et al. 2014b). A third explanation involves calcium/calmodulin-dependent kinase II (CaMKII), as CaMKIIα knock-out mice show an almost 3-fold increase in D2High receptors. Novak and Seeman (2010) suggest that the ratio of CaMKII α and β subunits is a critical regulator of D2High states. Interestingly, CaMKIIα levels are significantly reduced during the preweanling period (Moreiera et al. 2010), thus potentially accounting for the excess number of D2High receptors found during early ontogeny. Arguing against all three of these explanations, however, is the lack of age-dependent differences in D2High receptors among our four preweanling age groups. Specifically, DA content, D2 receptor numbers, and CaMKIIα levels increase linearly across the preweanling period, yet the percentage of D2High receptors stayed at a high, stable rate in rats tested on PD 5, PD 10, PD 15, and PD 20.
The main limitation of this study is that the relationship between age-dependent changes in D2High receptors and cocaine sensitivity is correlative. Unfortunately, most manipulations known to increase the percentage of D2High receptors (e.g., repeated psychostimulant or antipsychotic treatment, ethanol withdrawal, hippocampal lesions, etc.; Seeman et al. 2005) produce a range of neurochemical and behavioral effects that are either incompatible with assessing cocaine sensitivity or have actions (e.g., increasing D2 receptor levels) that hopelessly confound data interpretation. One exception involves the DA receptor alkylating agent N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ). In adolescent and adult rats, EEDQ minimally elevates the percentage of D2High receptors while attenuating DA agonist-induced behaviors (Der Ghazarian et al. 2012; McDougall et al. 2014b). Microinjecting EEDQ into the striatum of preweanling rats, on the other hand, significantly reduces D2 receptor densities, increases the percentage of D2High receptors, and potentiates cocaine-induced locomotor activity (Der Ghazarian et al. 2012, 2014; McDougall et al. 2014b). Thus, EEDQ-induced increases in D2High receptors appear to enhance behavioral sensitivity to cocaine and other DA agonists. This past research is consistent with our current explanation that ontogenetic changes in the percentage of D2High receptors are responsible for age-dependent differences in cocaine sensitivity. Even so, additional research is necessary before a causal relationship between maturational changes in D2High receptors and cocaine sensitivity can be established.
In conclusion, manipulations producing DA supersensitivity (e.g., repeated amphetamine treatment) invariably increase the proportion of dorsal striatal D2High receptors (Seeman et al. 2005). This has led some authors to suggest that an excess of D2High receptors is an important neural mechanism underlying DA supersensitivity and, perhaps, schizophrenia (Seeman 2011; Seeman and Seeman 2014). The normal or aberrant ontogeny of D2High receptors could have long-term behavioral implications. In the case of schizophrenia, early prodromal states might be associated with prolonged alterations in the percentage of D2High receptors (see also Kuepper et al. 2012). Consistent with this idea, Andersen (2003) proposed that perturbations in the ontogeny of DA receptors may underlie various psychopathologies in humans. In any event, results from the present study suggest that ontogenetic changes in the percentage of D2High receptors may be responsible for age-dependent differences in the sensitivity to cocaine. Determining whether a causal relationship exists between D2High receptors and psychostimulant sensitivity will require additional study.
Acknowledgments
Funding sources: This research was supported by NIMH research grant MH102930 (SAM).
Footnotes
Conflict of interest: All authors declare no conflict of interest.
References
- Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–346. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity. Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Assié MB, Bardin L, Auclair AL, Carilla-Durand E, Depoortère R, Koek W, Kleven MS, Colpaert F, Vacher B, Newman-Tancredi A. F15599, a highly selective post-synaptic 5-HT1A receptor agonist: in-vivo profile in behavioural models of antidepressant and serotonergic activity. Int J Neuropsychopharmacol. 2010;13:1285–1298. doi: 10.1017/S1461145709991222. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. Early adolescents show enhanced acute cocaine-induced locomotor activity in comparison to late adolescent and adult rats. Dev Psychobiol. 2008;50:127–133. doi: 10.1002/dev.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Bordi F, Carr KD, Meller E. Stereotypies elicited by injection of N-propylnorapomorphine into striatal subregions and nucleus accumbens. Brain Res. 1989;489:205–215. doi: 10.1016/0006-8993(89)90852-4. [DOI] [PubMed] [Google Scholar]
- Broaddus WC, Bennett JP. Postnatal development of striatal dopamine function. I. An examination of D1 and D2 receptors, adenylate cyclase regulation and presynaptic dopamine markers. Dev Brain Res. 1990;52:265–271. doi: 10.1016/0165-3806(90)90244-s. [DOI] [PubMed] [Google Scholar]
- Brown RW, Hughes BA, Hughes AB, Sheppard AB, Perna MK, Ragsdale WL, Roeding RL, Pond BB. Sex and dose-related differences in methylphenidate adolescent locomotor sensitization and effects on brain-derived neurotrophic factor. J Psychopharmacol. 2012;26:1480–1488. doi: 10.1177/0269881112454227. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Lytle LD, Fibiger HC. Ontogeny of adrenergic arousal and cholinergic inhibitory mechanisms in the rat. Science. 1969;166:635–637. doi: 10.1126/science.166.3905.635. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology (Berl) 2005;183:218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. A single high dose of cocaine induces differential sensitization to specific behaviors across adolescence. Psychopharmacology (Berl) 2007;193:247–260. doi: 10.1007/s00213-007-0764-5. [DOI] [PubMed] [Google Scholar]
- Catlow BJ, Kirstein CL. Heightened cocaine-induced locomotor activity in adolescent compared to adult female rats. J Psychopharmacol. 2005;19:443–447. doi: 10.1177/0269881105056518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Ghazarian T, Gutierrez A, Varela FA, Herbert MS, Amodeo LR, Charntikov S, Crawford CA, McDougall SA. Dopamine receptor inactivation in the caudate-putamen differentially affects the behavior of preweanling and adult rats. Neuroscience. 2012;226:427–440. doi: 10.1016/j.neuroscience.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Ghazarian T, Widarma CB, Gutierrez A, Amodeo LR, Valentine JM, Humphrey DE, Gonzalez AE, Crawford CA, McDougall SA. Dopamine receptor inactivation in the caudate-putamen potentiates dopamine agonist-induced locomotor activity in preweanling rats: role of the D2 receptor. Psychopharmacology (Berl) 2014;231:651–662. doi: 10.1007/s00213-013-3280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Age-related differences in amphetamine sensitization: effects of prior drug or stress history on stimulant sensitization in juvenile and adult rats. Pharmacol Biochem Behav. 2010;96:198–205. doi: 10.1016/j.pbb.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46:672–687. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Frantz K, Babcock D, Van Hartesveldt C. The locomotor effects of a putative dopamine D3 receptor agonist in developing rats. Eur J Pharmacol. 1996;302:1–6. doi: 10.1016/0014-2999(96)00014-3. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O'Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2006;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Geisser S, Greenhouse SW. An extension of Box's results on the use of the F distribution in multivariate analysis. Ann Math Statist. 1958;29:885–891. [Google Scholar]
- Giorgi O, De Montis G, Porceddu ML, Mele S, Calderini G, Toffano G, Biggio G. Developmental and age-related changes in D1-dopamine receptors and dopamine content in the rat striatum. Dev Brain Res. 1987;35:283–290. doi: 10.1016/0165-3806(87)90053-8. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Kameda SR, Fukushiro DF, Trombin TF, Procópio-Souza R, Patti CL, Hollais AW, Calzavara MB, Abílio VC, Ribeiro RA, Tufik S, D'Almeida V, Frussa-Filho R. Adolescent mice are more vulnerable than adults to single injection-induced behavioral sensitization to amphetamine. Pharmacol Biochem Behav. 2011;98:320–324. doi: 10.1016/j.pbb.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Koek W, France CP, Javors MA. Morphine-induced motor stimulation, motor incoordination, and hypothermia in adolescent and adult mice. Psychopharmacology (Berl) 2012;219:1027–1037. doi: 10.1007/s00213-011-2432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozanian OO, Gutierrez A, Mohd-Yusof A, McDougall SA. Ontogeny of methamphetamine- and cocaine-induced one-trial behavioral sensitization in preweanling and adolescent rats. Behav Pharmacol. 2012;23:367–379. doi: 10.1097/FBP.0b013e32835651c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuepper R, Skinbjerg M, Abi-Dargham A. The dopamine dysfunction in schizophrenia revisited: new insights into topography. In: Gross G, Geyer MA, editors. Handbook of experimental pharmacology: Current antipsychotics. Springer; Berlin: 2012. pp. 1–26. [DOI] [PubMed] [Google Scholar]
- Lanier LP, Isaacson RL. Early developmental changes in the locomotor response to amphetamine and their relation to hippocampal function. Brain Res. 1977;126:567–575. doi: 10.1016/0006-8993(77)90610-2. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Waters P, McCormick CM. Changes in hyporesponsiveness to acute amphetamine and age differences in tyrosine hydroxylase immunoreactivity in the brain over adolescence in male and female rats. Dev Psychobiol. 2009;51:417–428. doi: 10.1002/dev.20381. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Baella SA, Stuebner NM, Halladay LM, Crawford CA. Cocaine-induced behavioral sensitization in preweanling and adult rats: effects of a single drug-environment pairing. Psychopharmacology (Berl) 2007;193:323–332. doi: 10.1007/s00213-007-0788-x. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Pipkin JA, Der-Ghazarian T, Cortez AM, Gutierrez A, Lee RJ, Carbajal A, Mohd-Yusof A. Age-dependent differences in the strength and persistence of psychostimulant-induced conditioned activity in rats: effects of a single environment-cocaine pairing. Behavioural Pharmacology. 2014a;25:695–704. doi: 10.1097/FBP.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Valentine JM, Gonzalez AE, Humphrey DE, Widarma CB, Crawford CA. Behavioral effects of dopamine receptor inactivation during the adolescent period: age-dependent changes in dorsal striatal D2High receptors. Psychopharmacology (Berl) 2014b;231:1637–1647. doi: 10.1007/s00213-013-3355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milesi-Hallé A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM. Sex- and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol Appl Pharmacol. 2005;209:203–213. doi: 10.1016/j.taap.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody CA, Spear LP. Ontogenetic differences in the psychopharmacological responses to separate and combined stimulation of D1 and D2 dopamine receptors during the neonatal to weanling age period. Psychopharmacology (Berl) 1992;106:161–168. doi: 10.1007/BF02801967. [DOI] [PubMed] [Google Scholar]
- Moreira JD, Knorr L, Ganzella M, Thomazi AP, de Souza CG, de Souza DG, Pitta CF, Mello e Souza T, Wofchuk S, Elisabetsky E, Vinadé L, Perry ML, Souza DO. Omega-3 fatty acids deprivation affects ontogeny of glutamatergic synapses in rats: relevance for behavior alterations. Neurochem Int. 2010;56:753–759. doi: 10.1016/j.neuint.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Morelli M, Porceddu ML, Di Chiara G. Lesions of substantia nigra by kainic acid: effects on apomorphine-induced stereotyped behaviour. Brain Res. 1980;191:67–78. doi: 10.1016/0006-8993(80)90315-7. [DOI] [PubMed] [Google Scholar]
- Motulsky H, Christopoulos A. Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. GraphPad Software Inc; San Diego: 2003. [Google Scholar]
- Murrin LC, Zeng W. Postnatal ontogeny of dopamine D2 receptors in rat striatum. Biochem Pharmacol. 1986;35:1159–1162. doi: 10.1016/0006-2952(86)90154-1. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press; Washington: 2010. [Google Scholar]
- Niculescu M, Ehrlich ME, Unterwald EM. Age-specific behavioral responses to psychostimulants in mice. Pharmacol Biochem Behav. 2005;82:280–288. doi: 10.1016/j.pbb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Novak G, Seeman P. Hyperactive mice show elevated D2High receptors, a model for schizophrenia: Calcium/calmodulin-dependent kinase II alpha knockouts. Synapse. 2010;64:794–800. doi: 10.1002/syn.20786. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008;89:314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res. 1999;101:15–20. doi: 10.1016/s0166-4328(98)00073-4. [DOI] [PubMed] [Google Scholar]
- Rao PA, Molinoff PB, Joyce JN. Ontogeny of dopamine D1 and D2 receptor subtypes in rat basal ganglia: A quantitative autoradiographic study. Dev Brain Res. 1991;60:161–177. doi: 10.1016/0165-3806(91)90045-k. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Carmona GN. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol Biochem Behav. 2002;72:857–863. doi: 10.1016/s0091-3057(02)00770-0. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine D2(High) receptors moderately elevated by bifeprunox and aripiprazole. Synapse. 2008;62:902–908. doi: 10.1002/syn.20557. [DOI] [PubMed] [Google Scholar]
- Seeman P. All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2High receptors. CNS Neurosci Ther. 2011;17:118–132. doi: 10.1111/j.1755-5949.2010.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman MV, Seeman P. Is schizophrenia a dopamine supersensitivity psychotic reaction? Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:155–160. doi: 10.1016/j.pnpbp.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O'Dowd BF, George SR, Perreault ML, Männistö PT, Robinson S, Palmiter RD, Tallerico T. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci USA. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- Shalaby IA, Spear LP. Psychopharmacological effects of low and high doses of apomorphine during ontogeny. Eur J Pharmacol. 1980;67:451–459. doi: 10.1016/0014-2999(80)90186-7. [DOI] [PubMed] [Google Scholar]
- Slob AK, Huizer T, Van der Werff ten Bosch JJ. Ontogeny of sex differences in open-field ambulation in the rat. Physiol Behav. 1986;37:313–315. doi: 10.1016/0031-9384(86)90239-8. [DOI] [PubMed] [Google Scholar]
- Snyder KJ, Katovic NM, Spear LP. Longevity of the expression of behavioral sensitization to cocaine in preweanling rats. Pharmacol Biochem Behav. 1998;60:909–914. doi: 10.1016/s0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Sobrian SK, Jones BL, Varghese S, Holson RR. Behavioral response profiles following drug challenge with dopamine receptor subtype agonists and antagonists in developing rat. Neurotoxicol Teratol. 2003;25:311–328. doi: 10.1016/s0892-0362(03)00009-6. [DOI] [PubMed] [Google Scholar]
- Spear LP. The use of psychopharmacological procedures to analyse the ontogeny of learning and retention: issues and concerns. In: Spear NE, Campbell BA, editors. Ontogeny of learning and memory. Lawrence Erlbaum Associates; Hillsdale, NJ: 1979. pp. 135–156. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brick J. Cocaine-induced behavior in the developing rat. Behav Neural Biol. 1979;26:401–415. doi: 10.1016/s0163-1047(79)91410-9. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D1, D2 and D4 receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Varela FA, Der-Ghazarian T, Lee RJ, Charntikov S, Crawford CA, McDougall SA. Repeated aripiprazole treatment causes dopamine D2 receptor up-regulation and dopamine supersensitivity in young rats. J Psychopharmacol. 2014;28:376–386. doi: 10.1177/0269881113504016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Kuhn CM. Cocaine increases stimulated dopamine release more in periadolescent than adult rats. Neurotoxicol Teratol. 2008;30:412–418. doi: 10.1016/j.ntt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Morris SE, Arrant AE, Nagel JM, Parylak S, Zhou G, Caster JM, Kuhn CM. Dopamine uptake inhibitors but not dopamine releasers induce greater increases in motor behavior and extracellular dopamine in adolescent rats than in adult male rats. J Pharmacol Exp Ther. 2010;335:124–132. doi: 10.1124/jpet.110.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Holtzman SG. Periadolescent morphine exposure alters subsequent behavioral sensitivity to morphine in adult rats. Eur J Pharmacol. 2005;528:119–123. doi: 10.1016/j.ejphar.2005.10.026. [DOI] [PubMed] [Google Scholar]
- White DA, Michaels CC, Holtzman SG. Periadolescent male but not female rats have higher motor activity in response to morphine than do adult rats. Pharmacol Biochem Behav. 2008;89:188–199. doi: 10.1016/j.pbb.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck JA, Lewicki AD, Patel K, Gupta T, Rhodes JS. Patterns of neural activity associated with differential acute locomotor stimulation to cocaine and methamphetamine in adolescent versus adult male C57BL/6J mice. Neuroscience. 2010;165:1087–1099. doi: 10.1016/j.neuroscience.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]