Abstract
Rationale
Previous research suggests both genetic and environmental influences on substance abuse vulnerability.
Objectives
The current work sought to investigate the interaction of genes and environment on the acquisition of amphetamine self-administration, as well as amphetamine-stimulated dopamine (DA) release in nucleus accumbens shell using in vivo microdialysis.
Methods
Inbred Lewis (LEW) and Fischer (F344) rat strains were raised in either an enriched condition (EC), social condition (SC), or isolated condition (IC). Acquisition of amphetamine self-administration (0.1 mg/kg/infusion) was determined across an incrementing daily fixed ratio (FR) schedule. In a separate cohort of rats, extracellular DA and the metabolite dihydroxyphenylacetic acid (DOPAC) were measured in the nucleus accumbens shell following an acute amphetamine injection (1 mg/kg).
Results
“Addiction-prone” LEW had greater acquisition of amphetamine self-administration on a FR1 schedule compared to “addiction-resistant” F344 when raised in the SC environment. These genetic differences were negated in both the EC and IC environments, with enrichment buffering against self-administration and isolation enhancing self-administration in both strains. On a FR5 schedule, the isolation-induced increase in amphetamine self-administration was greater in F344 than LEW. While no group differences were obtained in extracellular DA, gene x environment differences were obtained in extracellular levels of the metabolite DOPAC. In IC rats only, LEW showed an attenuation in the amphetamine-induced decrease in DOPAC compared to F344. IC LEW rats also had an attenuated DOPAC response to amphetamine compared to EC LEW.
Conclusions
The current results demonstrate gene x environment interactions in amphetamine self-administration and amphetamine-induced changes in extracellular DOPAC in NAc shell. However, the behavioral and neurochemical differences were not related directly, indicating that mechanisms independent of DA metabolism in NAc shell likely mediate the gene x environment effects in amphetamine self-administration.
Keywords: Gene x Environment Interactions, Self-Administration, Microdialysis, Amphetamine, Environmental Enrichment, Inbred Rat Strains, Lewis, Fischer 344, Dopamine
Introduction
Evidence indicates that risk for substance use and abuse is, at least in part, heritable. Human twin studies have established a genetic link for alcohol abuse and dependence, as well as for abuse of cannabis, sedatives, stimulants, cocaine, opiates, hallucinogens, and tobacco smoking (Kendler et al. 1999; 2000; 2006; Kendler and Prescott 1998a; 1998b; Lynskey et al. 2002; Maes et al. 2004; Prescott and Kendler 1999). However, non-genetic factors also play a role in substance use. Shared environmental effects such as substance, age, religiosity, gender, parental monitoring, quality of the home environment, and sociocultural factors moderate substance initiation and the magnitude of genetic influence (Harden et al. 2008; Hopfer et al. 2003; Kendler et al. 2000; Kendler and Prescott 1998a; 1998b; Legrand et al. 1999; Lynskey et al. 2002; Maes et al. 2004).
Gene x environment interactions in substance abuse have been shown in both human and non-human primates (Barr et al. 2003; 2004; Nilsson et al. 2007a; 2007b; 2010; Sluyter et al. 2000); however, less is known based on rodent models. In one study, a gene x environment interaction was demonstrated in apomorphine susceptible and nonsusceptible rats tested in the presence or absence of stress (van der Kam et al. 2005). Rodent models offer many advantages for these investigations, as they offer the greatest control over both environmental and genetic manipulations. Inbred rat strains offer a diversity of characterized phenotypes (traits), which are useful in analyzing critical determinants of behavior (Crabbe 2008; Crabbe and Belknap 1992; Marley et al. 1992). They are especially useful for the study of gene x environment interactions. By holding environmental factors constant in behavioral experiments, between strain differences in behavioral responses may be attributed to differences in genotype between strains (Crabbe 2008; Crabbe and Belknap 1992; Marley et al. 1992). Within inbred strains, individual differences are not allelic, and can be attributed to environmental factors (Crabbe 2008).
Among various commercially available inbred rat strains, Lewis (LEW) and Fischer (F344) inbred strains differ on a wide variety of behavioral and physiological measures, as well as sensitivity to drugs of abuse. Some reports have shown that LEW have greater locomotor activity than F344 following acute doses of amphetamine (Gulley et al. 2007; Miserendino et al. 2003). In contrast, others have shown that LEW have a lower locomotor response to amphetamine compared to F344, as well as a lower response to the rewarding effects of amphetamine as assessed by conditioned place preference (George et al. 1991; Stohr et al. 1998). Despite these inconsistencies, compared to F344, LEW are characterized as “addiction-prone” due to LEW acquiring cocaine, methamphetamine, amphetamine and food self-administration more rapidly and at higher rates than F344 (Kearns et al. 2006; Kosten et al. 1997; Kruzich and Xi 2006; Meyer et al. 2010).
For examining potential gene x environment interactions in LEW and F344 strains, the environmental enrichment procedure may be useful. In this model, post-weaning rats (21 days of age) are raised in either an enriched condition (EC), with social cohorts and novel objects, or an isolated condition (IC) without cohorts or objects (Stairs and Bardo 2009). A third social condition (SC) is often included, where rats are group housed with no objects. Similar to studies using LEW and F344, differences between EC and IC rats in acquisition of drug self-administration have been investigated. At low unit doses, EC rats self-administer less amphetamine and cocaine compared to IC rats (Bardo et al. 2001; Green et al. 2002; 2010). EC rats also self-administer oral alcohol at lower rates than IC rats (Deehan et al. 2007). While these results indicate that enrichment protects against drug self-administration, it is not known if this environmental influence affects LEW and F344 differentially.
Given the strain- and environment-dependent differences in behavioral response to drugs of abuse, a number of studies have attempted to identify neurochemical differences between LEW and F344 and EC and IC rats. The mesocorticolimbic dopamine (DA) system is known to be associated with brain reward circuitry, and thus has been studied intensely to understand the mechanisms of drug reward (Wise and Rompre 1989). In outbred rat strains, both amphetamine and methamphetamine increase extracellular DA levels in nucleus accumbens (NAc) and medial prefrontal cortex (mPFC), while decreasing the DA metabolites 3,4-dihydroxy-phenylacetic acid (DOPAC) and homovanillic acid (HVA) in both structures (During et al. 1992; Maisonneuve et al. 1990; Moghaddam et al. 1990; Shoblock et al. 2003). Both strain- and environment-dependent differences in extracellular DA have been investigated. In LEW and F344, no strain differences in basal levels of DA have been found in NAc (Cadoni and Di Chiara 2007; Fernandez et al., 2003; Mocsary and Bradberry 1996; and Strecker et al.,1995). When challenged with amphetamine (0.25–1.0 mg/kg, s.c.), however, increases in NAc core DA were significantly higher in LEW than in F344 at all doses tested (0.25, 0.5 and 1.0 mg/kg, s.c.), whereas increases in NAc shell DA were higher in LEW compared to F344 at the lowest dose (0.25mg/kg) only (Cadoni and Di Chiara 2007). In EC and IC rats, EC rats show greater basal levels of DA in NAc compared to IC rats (Segovia et al., 2010). EC rats also show greater amphetamine-induced DA release in NAc (Bardo et al. 1999). However, it is not known if environmental influences affect amphetamine-stimulated neurochemical mechanisms in LEW and F344 differentially.
In the current work, we hypothesized that enrichment would be more protective in “addiction-prone” LEW than in “addiction-resistant” F344. To assess potential underlying neural mechanisms, amphetamine-stimulated extracellular levels of DA and DOPAC also were measured in the shell of NAc in differentially reared LEW and F344 rats.
MATERIALS AND METHODS
Animals
Male 21-day old LEW and F344 inbred rats (Harlan Industries, Indianapolis, IN) had free access to food and water in their home cage and were maintained in a colony room on a 12:12-hr light/dark cycle. Behavioral testing was conducted during the light cycle. Experimental protocols followed the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Environmental Conditions
Upon arrival, rats from each strain were divided randomly into EC, IC, or SC environments. EC rats were housed together by strain in stainless steel cages (60 × 120 × 45 cm) with 14 plastic objects (commercially-available toys, plastic containers, tubes, etc.) in the cage. Seven objects were replaced daily, and objects were rearranged to create a novel configuration. IC rats were housed individually in hanging stainless steel cages (17 × 24 × 20 cm) with wire mesh floors and front panels, and solid metal walls and top. SC rats were housed together by strain in stainless steel cages (60 × 120 × 45 cm).
Drugs
d-Amphetamine sulfate (Sigma, St. Louis, MO) was prepared in 0.9% NaCl (saline). The dose was calculated based on the salt weight.
Amphetamine Self-Administration
Apparatus
Amphetamine self-administration was conducted in two-lever operant conditioning chambers (ENV-001; Med Associates, St Albans, VT). End walls of the operant chamber were aluminum, the front and back walls were clear Plexiglas, and the floor was 18 stainless steel rods. A recessed food tray was located in the bottom center of one wall, with a response lever located on each side. White cue lights (28-V) were located 6 cm above each response lever, and a 28-V white house light was centered 20 cm above the floor on the opposite wall. An infusion pump (Med Associates) delivered drug via a silastic tube attached to a swivel.
Procedure
Catheter Implantation
On PND 63–64, rats were anesthetized (80 mg/kg ketamine, 5 mg.kg diazepam, i.p.) and implanted with indwelling silastic jugular catheters secured to acrylic head mounts as described previously (Meyer et al. 2010).
Phase 1: Acquisition
Acquisition of amphetamine self-administration was initiated using an autoshaping procedure in order to ensure uniform, programmed exposure to amphetamine within and between groups, with no prior lever training (Carroll and Lac 1993). For 7 consecutive days, rats were given a 60-min autoshaping session, followed 30 min later by a 60-min contingent amphetamine self-administration session. During the autoshaping session, the house light was illuminated and an inactive lever (no programmed consequence, counterbalanced for position across rats) was extended throughout the session. For the first 15 min of each autoshaping session, an active lever was extended 10 times at random intervals and remained extended for 10 sec. If the active lever was pressed, amphetamine (0.1 mg/kg/infusion, 5.9 sec duration, 0.1 ml volume) was delivered immediately; if the active lever was not pressed, a non-contingent infusion of amphetamine was delivered when the lever retracted. For the remaining 45 min of the session, only the inactive lever was extended.
For subsequent contingent amphetamine self-administration sessions, both levers (active and inactive) were extended and infusions of amphetamine were contingent on pressing the active lever using a fixed ratio 1 (FR1); each reinforced response was followed immediately by a 20-sec interval during which both cue lights above the levers were illuminated and responding on either lever was not reinforced. Following 7 days of autoshaping and amphetamine self-administration, rats underwent 3 additional days of contingent 60-min self-administration sessions alone (FR1/20-sec timeout).
Phase 2: Incremented FR Schedule
Daily 60-min self-administration sessions continued and the schedule of reinforcement was incremented from FR1 to FR5, with rats spending 3 consecutive sessions on each FR value. The unit dose of amphetamine during this phase remained at 0.1 mg/kg/infusion (0.1 ml, 5.9 sec duration), with a 20-sec signaled time out.
Microdialysis
Apparatus
Microdialysis was conducted in individual Plexiglas chambers (25 × 44 × 38 cm) with pine bedding. A swivel and tether system (BAS, Indianapolis, IN) was attached to the side of each chamber and connected to a microsyringe pump (KD Scientific, Model KDS250).
Procedure
Cannula Implantation
For microdialysis, a separate cohort of rats underwent cannula implantation on PND 63–65. Rats were anesthetized (80 mg/kg ketamine, 5 mg/kg diazepam, i.p.) and placed in a stereotaxic apparatus (Stoelting, Wood Dale, IL). Guide cannula (MD-2251, 22 gauge, BAS) were implanted unilaterally in NAc shell (A 1.6, L 0.8 from bregma and V 5.8 from dura) according to the atlas of Paxinos and Watson (2007). To maintain guide cannula viability through the duration of the experiment, rats in the EC and SC environments remained in their group environments from 8:00 AM to 5:00 PM, but were individually housed overnight.
In Vivo Microdialysis
Microdialysis took place on PND 67–74. On each day of testing one rat from each strain and environmental condition were tested, and counterbalanced across testing chamber. Microdialysis probes (MD-2200, 2 mm active membrane, BAS) were inserted into guide cannula, connected to PE tubing (inner diameter 0.12 mm, internal volume of 1.2 µl/100 mm length), and aligned through a wire set with a collar connector and swivel assembly. Artificial cerebrospinal fluid (in mM: 145 NaCl, 2.7 KCl, 1.0 MgCl2, 1.2 CaCl2; pH adjusted to 7.3–7.4 with 2 mM sodium phosphate buffer) was perfused through the probes at a rate of 1.2 µl/min for 3 h prior to collection of baseline samples.
Baseline samples were collected into polyethylene microfuge tubes containing 5 µl 0.1 N perchloric acid at 20-min intervals for 60 min. Rats were then injected with saline (s.c.), and 20-min samples collected for another 60 min. Rats were then injected with 1.0 mg/kg of amphetamine (s.c.) and samples collected at 20-min intervals for 180 min. Samples were immediately frozen on dry ice and stored at −80 °C.
Analysis of Extracellular DA and DOPAC using HPLC-EC
Samples were analyzed for DA and DOPAC levels using high performance liquid chromatography with electrochemical detection (HPLC-EC, ESA Inc., Chelmsford, MA) using the general methods described previously (Rahman et al. 2008), but employed an ESA 542 HPLC autosampler. Mobile phase consisted of 90 mM NaH2PO4●H2O, 50 mM citric acid, 1.7 mM 1-octanesulfonic acid, 50 µM EDTA, and 10% acetonitrile; pH 3.0 adjusted with phosphoric acid; flow rate 0.6 ml/min.
Histological Examination
Rats were sacrificed under anesthesia (150 mg/ml pentobarbital) and probe placements verified using cresyl violet stain.
Data Analysis
For acquisition and incremental FR phases of self-administration, infusions during each session were averaged across groups and analyzed by mixed factor ANOVA (strain x environment x session). Planned comparisons were performed on the final session of FR1 and the terminal FR5. For the incremental FR phase, data were averaged on the second and third session, as the first session was considered a transition day to adjust to the new schedule.
Basal DA and DOPAC levels were calculated by fitting peak heights to a concentration curve calculated from external standards. Basal levels were then averaged across groups, and analyzed using a 2 × 3 (strain x environment) factorial ANOVA. For microdialysis time course, peak height was recorded and expressed as a percent of baseline, defined as the average DA or DOPAC level in three samples prior to the first injection (normalized to 100%). Time course data were analyzed by a 2 × 3 × 15 (strain x environment x time) mixed factor ANOVA.
Extracellular DA and DOPAC levels also were assessed by determining area under the curve (AUC). Percent change in extracellular DA or DOPAC from baseline, starting from the time of amphetamine injection until the time of the last sample collected, were used in the calculation of AUC using the trapezoidal rule (GraphPad Prizm Inc., San Diego, CA). AUC values were analyzed by a 2 × 3 (strain x environment) factorial ANOVA.
For all analyses, significance was set at p < 0.05, and Bonferroni post hoc tests were used to determine differences between groups.
RESULTS
Amphetamine Self-Administration
Phase 1: Acquisition
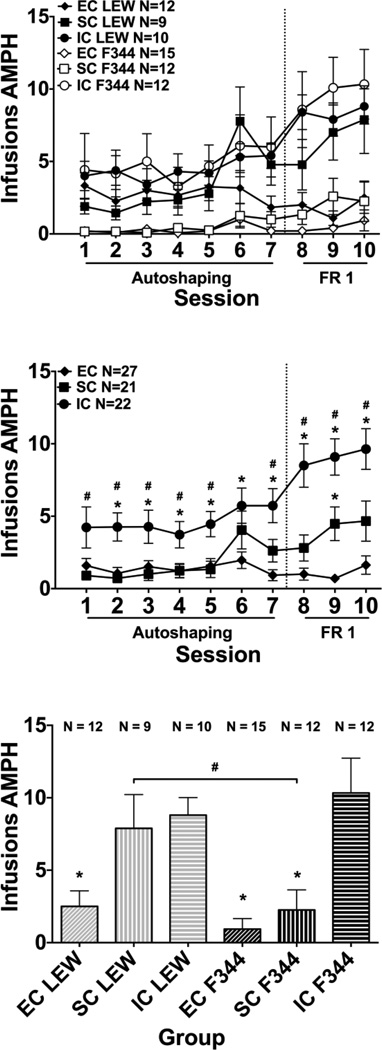
An overall ANOVA showed a significant main effect of session (F(9,576) = 13.33, p < 0.001), indicating that rats increased the number of amphetamine infusions across session (Fig. 1, top panel). There was also a significant main effect of strain (F(1,64) = 4.98, p < 0.05), with LEW rats earning more infusions overall compared to F344 rats (p < 0.05). A significant main effect of environment also was observed (F(2,64) = 14.22, p < 0.001). Post hoc analyses revealed IC rats earned more infusions overall compared to EC and SC rats (p < 0.01). A significant environment x session interaction also was revealed (F(18,576) = 5.32, p < 0.001). Collapsed across strains, post hoc analyses revealed that IC rats earned more infusions than EC rats on sessions 2 – 10 (p < 0.05; Fig. 1, middle panel). IC rats also earned more infusions than SC rats on sessions 1–5, 7, 8, 9 and 10 (p < 0.05). SC rats earned more infusions than EC rats on session 9 (p < 0.05). Planned comparisons revealed that at the end of the acquisition phase, SC LEW earned more infusions than SC F344 (p < 0.05; Fig. 1, bottom panel). IC LEW and SC LEW earned more infusions than EC LEW. Further, IC F344 earned more infusions than SC F344 and EC F344 (p < 0.05).
Fig. 1.
Amphetamine Infusions during Acquisition of Self-Administration. Top Panel: Mean (± SEM) number of infusions earned during the autoshaping phase (sessions 1–7) and after autoshaping was discontinued (sessions 8–10). Middle Panel: Mean (± SEM) number of infusions earned during contingent self-administration sessions during the autoshaping phase (sessions 1–7) and after autoshaping was discontinued (sessions 8–10), collapsed across strain. * indicates a significant difference from EC, p < 0.05; # indicates a significant difference from SC, p < 0.05. Bottom Panel: Mean (± SEM) number of infusions earned for each group during the final day of acquisition (session 10). # indicates a significant difference between LEW and F344 within the SC environment, p < 0.05; * indicates a significant difference from IC within the same strain, p < 0.05.
Phase 2: Incremented FR Schedule
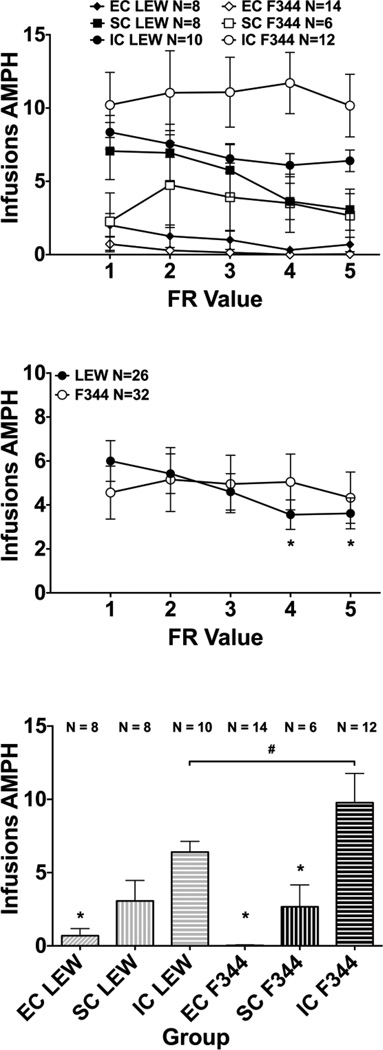
An overall ANOVA revealed significant main effects of FR value (F(4,208) = 3.02, p < 0.05), indicating that the overall number of amphetamine infusions decreased across FR values (Fig. 2, top panel). A significant main effect of environment (F(2,52) = 21.58, p < 0.001) also was observed. Post hoc analyses revealed that IC rats earned more overall infusions compared to both SC and EC rats (p < 0.05); SC rats also earned more infusions compared to EC rats (p < 0.05). A significant strain x FR value interaction also was obtained (F(4,208) = 2.75, p < 0. 05). Collapsed across environment, post hoc analyses failed to reveal significant differences between strains at any FR value (Fig. 2, middle panel); however, LEW rats showed a decrease in the number of infusions earned on the FR 4 and FR 5 sessions compared to the FR 1 session (p < 0.001), whereas the F344 rats did not show this decrease. Also, planned comparisons revealed that at the end of the incremented FR phase (Fig. 2, bottom panel), IC F344 earned more infusions than IC LEW (p < 0.05); IC LEW earned more infusions than EC LEW (p < 0.05); and IC F344 earned more infusions than SC F344 and EC F344 (p < 0.05).
Fig. 2.
Amphetamine Infusions during Incremented FR Schedule. Top Panel: Mean (± SEM) number of infusions earned, averaged across sessions 2 and 3 at each FR value. Middle Panel: Mean (± SEM) number of infusions earned, averaged across sessions 2 and 3 at each FR value, collapsed across environment. * indicates a significant difference from FR 1 of the same strain, p < 0.05. Bottom Panel: Mean (± SEM) number of infusions earned, averaged across sessions 2 and 3 at FR 5. # indicates a significant difference between LEW and F344 within the IC environment, p < 0.05; * indicates a significant difference from IC within the same strain, p < 0.05.
Microdialysis
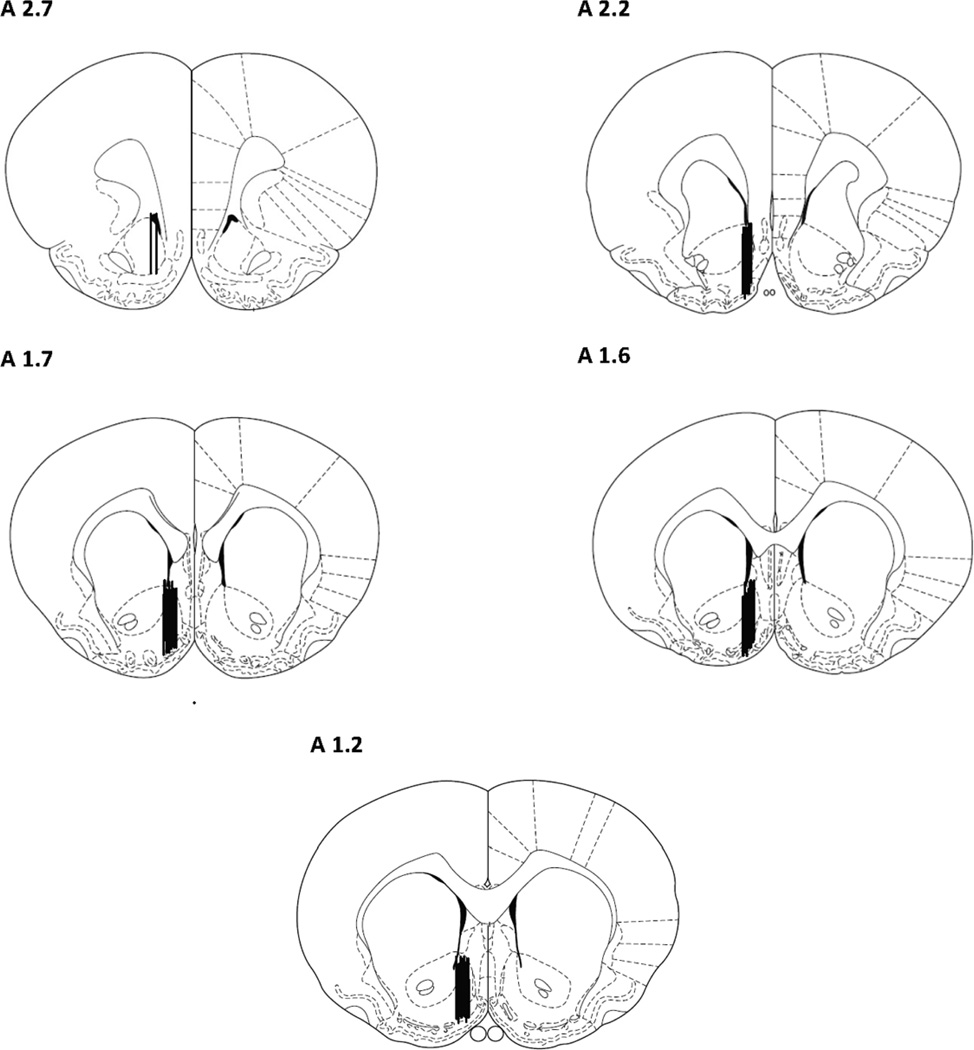
Fig. 3 shows the location of probes in the NAc shell of rats included in the analyses.
Fig. 3.
Location of Dialysis Probes in Coronal Plane. Location of the dialysis probes (membrane portion) within the NAc shell of animals included in the statistical analyses.
Extracellular DA in NAc Shell
An overall ANOVA on basal DA levels revealed no significant effect of strain, environment or strain x environment interaction (Table 1).
Table 1.
Basal Extracellular Levels of DA and DOPAC in NAc.
| DA (pg / 20 µL) | DOPAC (ng / 20 µL) | ||
|---|---|---|---|
| Group | n | Mean (± SEM) | Mean (± SEM) |
| EC-LEW | 6 | 2.18 (± 0.27) | 1.16 (± 0.17) |
| SC-LEW | 7 | 2.59 (± 0.33) | 1.03 (± 0.18) |
| IC-LEW | 7 | 2.56 (± 0.49) | 0.61 (± 0.11)# |
| EC-F344 | 8 | 2.48 (± 0.29) | 1.67 (± 0.36) |
| SC-F344 | 8 | 2.73 (± 0.37) | 1.02 (± 0.17)* |
| IC-F344 | 6 | 3.37 (± 0.46) | 2.39 (± 0.48) |
indicates a significant difference between strains within environment, p < 0.05
indicates a significant difference from IC within strain, p < 0.05
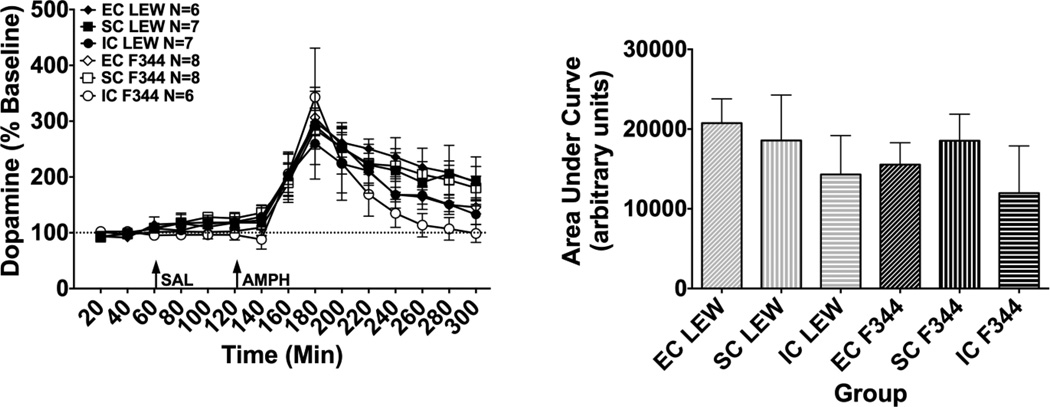
As shown in Fig. 4 (left panel), an overall ANOVA of DA revealed a significant main effect of time (F(14,504) = 47.35, p < 0.001), demonstrating an overall increase in extracellular DA following amphetamine. However, there were no significant effects of strain, environment or strain x environment interaction.
Fig. 4.
DA Microdialysis. Left Panel: Mean (± SEM) percent baseline of the DA peak height for each time point following administration of saline (SAL) and amphetamine (AMPH, 1.0 mg/kg, s.c.). The arrows indicate time of treatment. Right Panel: Mean (± SEM) DA expressed as area under the curve (AUC) for each treatment group.
As shown in Fig. 4 (right panel), an overall ANOVA of AUC values for DA revealed no significant effects of strain, environment or strain x environment interaction.
Extracellular DOPAC in NAc Shell
An overall ANOVA of basal DOPAC revealed a significant main effect of strain (F(1,36) = 11.75, p < 0.01), as well as a significant interaction of strain x environment (F(2,36) = 5.64, p < 0.01; Table 1). Post hoc analyses on strain revealed that F344 had greater overall basal DOPAC compared to LEW (p < 0.01) and that IC F344 had greater basal DOPAC compared to SC F344 and IC LEW (p < 0.05).
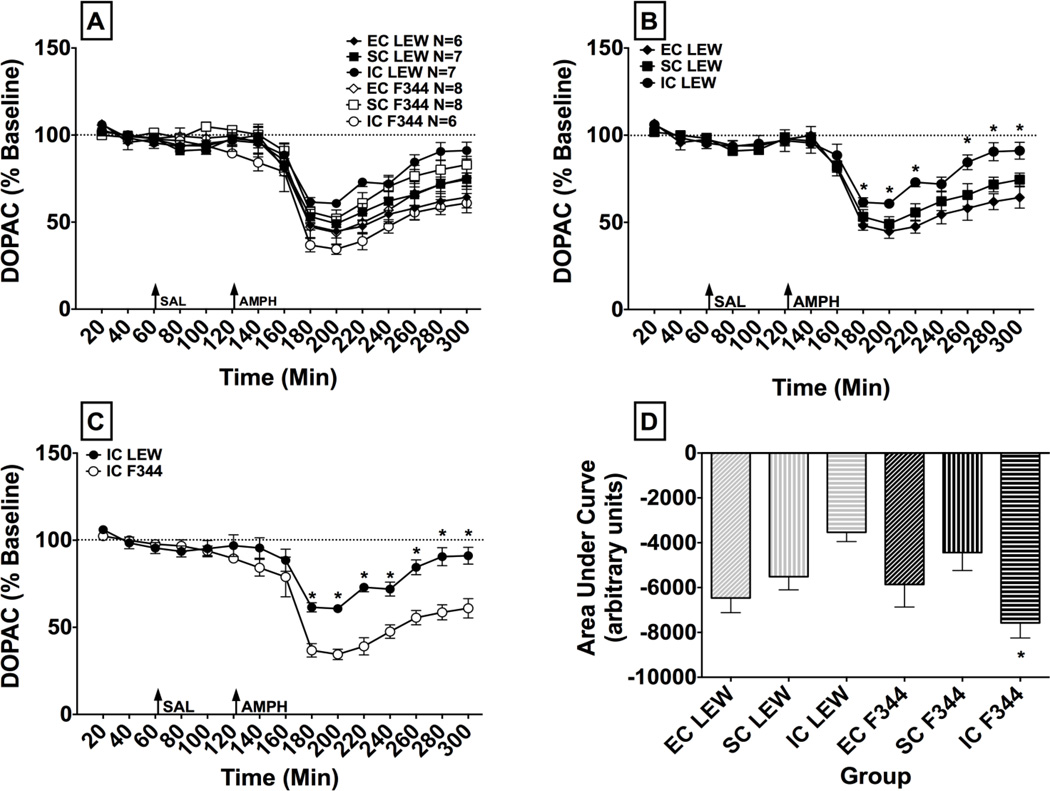
As shown in Fig. 5A, an overall ANOVA revealed a significant main effect of time (F(14, 504) = 170.16, p < 0.001), demonstrating an overall decrease in extracellular DOPAC following amphetamine treatment (1.0 mg/kg, s.c.). There were also significant interactions of strain x environment (F(2,36) = 6.15, p < 0.01), and strain x environment x time (F(28,504) = 2.97, p < 0.001).
Fig. 5.
DOPAC Microdialysis. Panel A: Mean (± SEM) percent baseline of the DOPAC peak height for each time point following administration of saline (SAL) and amphetamine (AMPH, 1.0 mg/kg, s.c.). The arrows indicate time of treatment. Panel B: Mean (± SEM) percent baseline of the DOPAC peak height for each time point following administration of saline and amphetamine (1.0 mg/kg, s.c.), in EC, SC and IC LEW rats. The arrows indicate time of treatment. * indicates a significant difference from EC rats at the same time point, p < 0.05. Panel C: Mean (± SEM) percent baseline of the DOPAC peak height for each time point following administration of saline and amphetamine (1.0 mg/kg, s.c.) in IC LEW and F344. The arrows indicate time of treatment. * indicates a significant difference from IC F344 at the same time point p < 0.05. Panel D: Mean (± SEM) DOPAC expressed as area under the curve (AUC) for each treatment group. * indicates a significant difference from IC LEW, p < 0.05.
To clarify the strain x environment x time interaction, individual ANOVAs were conducted for each strain. For LEW, the analysis revealed significant main effects of time (F(14,238) = 75.71, p < 0.001) and environment (F(2,17) = 5.98, p < 0.05). Post hoc analyses revealed that overall, IC LEW showed greater DOPAC than EC LEW. A significant environment x time interaction also was observed (F(28,238) = 2.97, p < 0.001; Fig. 5B). Post hoc comparisons revealed that EC LEW had a greater amphetamine-induced decrease in DOPAC compared to IC LEW at the following time points: 180, 200, 220, 260, 280, and 300 min (p < 0.01). For F344, the ANOVA revealed only a significant main effect of time (F(14,266) = 97.10, p < 0.001), with no significant main effect or interaction involving the environment factor.
Individual ANOVAs also were conducted for each environment. For EC and SC rats, the ANOVAs revealed only a significant main effect of time (F(14,168) = 61.67, p < 0.001 and F(14,182) = 59.16, p < 0.001, respectively). For IC rats, however, the ANOVA revealed significant main effects of time (F(14,154) = 51.02, p < 0.001) and strain (F(1,11) = 18.43, p < 0.001), as well as a significant strain x time interaction (F(14,154) = 6.75, p < 0.001; Fig. 5C). Post hoc comparisons revealed that IC F344 had a significantly greater amphetamine-induced decrease in DOPAC compared IC LEW at the following time points: 180, 200, 220, 240, 260, 280, 300 min (p < 0.01).
An overall ANOVA of AUC values for DOPAC failed to reveal any significant main effects of strain or environment; however, a significant strain x environment interaction was observed (F(2,36) = 6.88, p < 0.01; Fig. 5D). Post hoc comparisons revealed that IC F344 had a greater amphetamine-induced decrease in DOPAC compared to IC LEW (p < 0.05).
DISCUSSION
The current experiments are the first to investigate the effect of differential rearing environments with inbred LEW and F344 rat strains. A gene x environment interaction was observed for amphetamine self-administration, as well as for extracellular DOPAC levels in response to acute amphetamine. Behaviorally, LEW self-administered more amphetamine than F344 when raised in a social environment, but not when raised in either an isolated or enriched environment. Neurochemically, LEW also showed an attenuation in the amphetamine-induced decrease in DOPAC than F344 when raised in isolation, but not when raised in either a social or enriched condition. However, since strain differences in behavior occurred in social housing only, whereas strain differences in neurochemistry occurred in isolate housing only, these results suggest no direct relation between amphetamine reinforcement and accumbal DA metabolism.
Consistent with previous research, during acquisition, LEW self-administered overall more amphetamine compared to F344 (Kearns et al. 2006; Kosten et al. 1997; Kruzich and Xi 2006; Meyer et al. 2010) and IC rats self-administered overall more amphetamine infusions compared to both SC and EC rats (Bardo et al. 2001; Deehan et al. 2007; Green et al. 2002; 2010). More important, between-strain differences were apparent only in the SC environment, with SC LEW self-administering more amphetamine infusions than SC F344 at the end of the acquisition phase. Thus, these results demonstrate a gene x environment interaction in amphetamine self-administration because LEW and F344 were differentially affected by the environmental housing condition.
During the incremented FR phase, the difference between SC LEW and SC F344 seen in acquisition was attenuated across increasing ratio requirements. Overall, LEW exhibited a decrease in responding across incrementing FR schedules, whereas F344 did not. One explanation for the decrease in responding in LEW rats is that the amphetamine unit dose may not have been sufficiently rewarding in LEW to maintain responding as the FR requirement increased. In contrast, F344 rats may have regulated their drug intake across an incrementing FR schedule by increasing the number of responses on the active lever in order to reach their optimal drug level, as described previously by Lynch and Carroll (2001). Alternatively, it is possible that LEW rats sensitized more rapidly to the reinforcing effect of amphetamine, thus reaching an optimal drug level with fewer infusions. Consistent with this latter possibility, locomotor sensitization to cocaine occurs more quickly and at lower doses in LEW compared to F344 (Kosten et al. 1994).
The gene x environment interaction observed with amphetamine self-administration may reflect pharmacokinetic differences. While the pharmacokinetic profile of amphetamine has not been investigated in LEW and F344, it is possible that environmental housing modulated the brain bioavailability of amphetamine in these inbred strains. While further work is needed to address this possibility directly, at least one study found that environmental differences do not alter brain availability of [3H]-amphetamine in outbred rats (Bardo et al. 1999), suggesting that pharmacokinetics may not readily explain the gene x environment interaction observed here.
Results from the self-administration study also suggest that manipulations in environmental housing had a greater effect in F344 than in LEW. At the end of the acquisition phase, IC F344 self-administered significantly more amphetamine infusions compared to both SC and EC F344. At the terminal FR5 of the incremented FR phase, IC F344 continued to earn significantly more amphetamine infusions compared to both SC and EC F344, as well as compared to IC LEW. These findings support previous work suggesting that F344 may be more susceptible to conditioning and adaptations from environmental and biochemical stimuli. F344 show greater context conditioning, fear conditioning and shock avoidance than LEW (Haile et al. 2001; Katzev and Mill 1974). F344 also maintain cocaine self-administration at higher levels than LEW, and it is thought this may be due to greater conditioning to cues promoting lever pressing (Kosten et al. 2007). These behavioral effects may reflect genetic differences in mesolimbic DA function because chronic cocaine treatment in F344, but not in LEW, reduces tyrosine hydroxylase in NAc and increases tyrosine hydroxylase and glial fibrillary acidic protein immunoreactivity in the ventral tegmental area (Haile et al. 2001).
Results from the microdialysis experiment failed to find any differences in basal DA in NAc shell across strains or environments. While no previous studies have examined the effect of differential rearing environments on basal and amphetamine-stimulated extracellular DA in LEW and F344 rats, these results corroborate previous research finding no basal DA differences between LEW and F344 (Cadoni and Di Chiara 2007; Fernandez et al. 2003; Mocsary and Bradberry 1996) or between EC and IC rats (Bardo et al. 1995; Bowling et al. 1993; Jones et al. 1992).
Although amphetamine produced an increase in extracellular DA (~300% of baseline) across all groups, no significant differences in amphetamine-stimulated extracellular DA were observed between strains or rearing environments. These results were unexpected, as previous studies found differences between LEW and F344 (Cadoni and Di Chiara 2007), between EC and IC (Bardo et al. 1999), and between SC and IC (Jones et al. 1992) in extracellular DA following acute amphetamine. However, the amphetamine doses used in prior studies differed from the current dose (1 mg/kg). Higher doses were not used in the current study because they may not be behaviorally relevant. While two studies that investigated LEW and F344 or EC and IC used 1 mg/kg of amphetamine and found no strain- or environment-dependent differences (Bardo et al. 1999; Cadoni and Di Chiara 2007), this does not exclude the possibility that other doses may have undercovered gene x environment differences.
Consistent with previous research, the current study found that F344 rats had greater basal extracellular DOPAC compared to LEW rats (Strecker et al. 1995). This strain difference was seen only within the IC environment, but not within either the EC or SC environments. Following amphetamine administration, between-strain differences in DOPAC also were found, with IC LEW showing an attenuation in the amphetamine-induced decrease in DOPAC compared to IC F344. IC LEW also showed an attenuation in the amphetamine-induced decrease in DOPAC compared to EC LEW, which is consistent with results from a previous report in outbred rats showing that IC rats have less amphetamine-induced decrease in DOPAC compared to EC rats (Bowling et al. 1993). In contrast, the current report found no differences in DOPAC across environments in F344. Taken together, these results demonstrate a gene x environment interaction in accumbal DA metabolism.
The lower level of basal extracellular DOPAC and attenuated amphetamine-induced decrease in extracellular DOPAC in IC LEW compared to IC F344 may be due to differences in metabolism, synthesis and/or uptake of DA. In support of this, LEW have lower levels of tyrosine hydroxylase in NAc compared to F344 (Beitner-Johnson et al. 1991). Since tyrosine hydroxylase is the rate-limiting enzyme in DA biosynthesis (Cooper et al. 2003), LEW may have less intracellular DA for catabolism to DOPAC by monoamine oxidase (MAO), thus attenuating basal DOPAC overflow compared to IC F344. Moreover, since amphetamine inhibits MAO (Sulzer et al. 2005), between-strain differences in MAO activity may explain why IC LEW showed an attenuated neurochemical response to amphetamine compared to IC F344. Further work is needed to elucidate the precise role of biosynthetic, catabolic and uptake processes on the gene x environment differences in extracellular DOPAC observed here.
While gene x environment interactions were determined in both the self-administration and microdiaylsis experiments, the neurochemical data does not fully explain the differences in amphetamine self-administration. This may be due to how the amphetamine was administered across experiments, as well as the amount of amphetamine exposure. In the self-administration experiment, rats received repeated IV infusions of amphetamine contingent on lever presses, whereas in the microdialysis experiment, rats received an acute, non-contingent amphetamine injection delivered subcutaneously. These differences in route of administration, contingency, and repeated exposure are a limitation of the current results. Lecca et al. (2007a;b) have shown differences in DA response in NAc shell following response contingent and response noncontingent cocaine and heroin. Future studies could investigate the gene x environment interaction by examining DA transmission during the amphetamine self-administration sessions.
In conclusion, the current study demonstrates gene x environment interactions using “addiction-prone” LEW and “addiction-resistant” F344 strains. In the case of amphetamine self-administration, strain differences were observed in standard housing conditions (SC), but were negated by more extreme environmental conditions, i.e., stimulus deprivation (IC) or stimulus enrichment (EC). Specifically, the IC environment made both strains “addiction-prone”, whereas the EC environment made both strains “addiction-resistant”. In the case of microdialysis, the strain differences observed in extracellular DOPAC in the IC environment were negated by both the social and enriched environments. Although the differences in amphetamine self-administration were not associated directly with DOPAC changes, the overall finding that strain differences did not occur in EC rats suggests that enriched environments buffer against the genetic risk for drug abuse.
Acknowledgements
We thank Emily Denehy, Travis McCuddy, and Luke Holderfield for technical assistance. This work was supported by National Institute on Drug Abuse grants DA12964 and DA05312. These experiments were completed as part of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the University of Kentucky.
Footnotes
Conflict of Interest
The authors have no financial conflicts of interest to disclose related to this work.
REFERENCES
- Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Valone JM, Robinet PM, Shaw WB, Dwoskin LP. Environmental enrichment enhances the stimulant effect of intravenous amphetamine: search for a cellular mechanism in the nucleus accumbens. Psychobiology. 1999;27:292–299. [Google Scholar]
- Barr CS, Newman TK, Becker ML, Chamoux M, Lesch KP, Suomi SJ, et al. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcohol Clin Exp Res. 2003;27:812–817. doi: 10.1097/01.ALC.0000067976.62827.ED. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, et al. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry. 2004;61:1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ. Dopaminergic brain reward regions of Lewis and Fischer rats display different levels of tyrosine hydroxylase and other morphine- and cocaine-regulated phosphoproteins. Brain Res. 1991;561:147–150. doi: 10.1016/0006-8993(91)90759-o. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–93. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Differences in dopamine responsiveness to drugs of abuse in the nucleus accumbens shell and core of Lewis and Fischer F344 rats. J Neurochem. 2007;103:487–499. doi: 10.1111/j.1471-4159.2007.04795.x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Autoshaping i.v. cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology (Berl) 1993;110:5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. 8th edn. New York: Oxford University Press; 2003. [Google Scholar]
- Crabbe JC. Neurogenetic studies of alcohol addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3201–3211. doi: 10.1098/rstb.2008.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK. Genetic approaches to drug dependence. Trends Pharmacol Sci. 1992;13:212–219. doi: 10.1016/0165-6147(92)90066-f. [DOI] [PubMed] [Google Scholar]
- Deehan GA, Jr, Cain ME, Kiefer SW. Differential rearing conditions alter operant responding for ethanol in outbred rats. Alcohol Clin Exper Res. 2007;31:1692–1698. doi: 10.1111/j.1530-0277.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- During MJ, Bean AJ, Roth RH. Effects of CNS stimulants on the in vivo release of the colocalized transmitters, dopamine and neurotensin, from rat prefrontal cortex. Neurosci Lett. 1992;140:129–133. doi: 10.1016/0304-3940(92)90698-7. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Porras G, Mormède P, Spampinato U, Chaouloff F. Effects of 3,4-methylenedioxymethamphetamine on locomotor activity and extracellular dopamine in the nucleus accumbens of Fischer 344 and Lewis rats. Neurosci Lett. 2003;335:212–216. doi: 10.1016/s0304-3940(02)01180-1. [DOI] [PubMed] [Google Scholar]
- George FR, Porrino LJ, Ritz MC, Goldberg SR. Inbred rat strain comparisons indicate different sites of action for cocaine and amphetamine locomotor stimulant effect. Psychopharmacology (Berl) 1991;104:457–462. doi: 10.1007/BF02245649. [DOI] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DEH, Birnbaum SG, et al. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in nucleus accumbens. Biol Psychiatry. 2010;67:28–35. doi: 10.1016/j.biopsych.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Everett CV, Zahniser NR. Inbred Lewis and Fischer 344 rat strains differ not only in novelty- and amphetamine-induced behaviors, but also in dopamine transporter activity in vivo . Brain Research. 2007;1151:32–45. doi: 10.1016/j.brainres.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Hiroi N, Nestler EJ, Kosten TA. Differential behavioral responses to cocaine are associated with dynamics of mesolimbic dopamine proteins in Lewis and Fischer 344 rats. Synapse. 2001;41:179–190. doi: 10.1002/syn.1073. [DOI] [PubMed] [Google Scholar]
- Harden KP, Hill JE, Turkheimer E, Emery RE. Gene-environment correlation and interaction in peer effects on adolescent alcohol and tobacco use. Behav Genet. 2008;38:339–347. doi: 10.1007/s10519-008-9202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. J Am Acad Child Adolesc Psychiatry. 2003;42:710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav. 1992;43:17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- Katzev RD, Mills SK. Strain differences in avoidance conditioning as a function of the classical CS-US contingency. J Comp Physiol Psychol. 1974;87:661–671. doi: 10.1037/h0036960. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Tambs K, Reichborn-Kjennerud T. Illicit psychoactive substance use, abuse and dependence in a population-based sample of Norwegian twins. Psychol Med. 2006;36:955–962. doi: 10.1017/S0033291706007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Larkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse and dependence in a U.S. population-based sample of male twins. Arch Gen Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardener CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse and dependence, in a population-based sample of female twins. Am J Psychiatry. 1998a;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cocaine use, abuse and dependence in a population-based sample of female twins. Br J Psychiatry. 1998b;173:345–350. doi: 10.1192/bjp.173.4.345. [DOI] [PubMed] [Google Scholar]
- Kearns DN, Gomez-Serrano MA, Weiss SJ, Riley AL. A comparison of Lewis and Fischer rat strains on autoshaping (sign-tracking), discrimination reversal learning and negative automaintenance. Behav Brain Res. 2006;169:193–200. doi: 10.1016/j.bbr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not locomotor activity or conditioned taste aversion. J Pharmacol Exp Ther. 1994;269:137–144. [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–429. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Haile CN. Strain differences in maintenance of cocaine self-administration and their relationship to novelty activity responses. Behave Neurosci. 2007;121:380–388. doi: 10.1037/0735-7044.121.2.380. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Xi J. Differences in extinction responding and reinstatement of methamphetamine-seeking behavior between Fischer 344 and Lewis rats. Pharmacol Biochem Behav. 2006;83:391–395. doi: 10.1016/j.pbb.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Pyschopharmacology (Berl) 2007a;191:653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- Lecca D, Valentini V, Cacciapaglia F, Acquas E, Di Chiara G. Reciprocal effects of response contingent and noncontingent intravenous heroin on in vivo nucleus accumbens shell versus core dersus core dopamine in the rat: a repeated sampling microdialysis study. Psychopharmacology (Berl) 2007b;194:103–116. doi: 10.1007/s00213-007-0815-y. [DOI] [PubMed] [Google Scholar]
- Legrand LN, McGue M, Iacono WG. Searching for interactive effects in the etiology of early-onset substance use. Behav Genet. 1999;29:433–444. doi: 10.1023/a:1021627021553. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PAF, Slutske WS, et al. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychol Med. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, et al. A twin study of genetic environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol Med. 2004;34:1251–1261. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Keller RW, Glick SD. Similar effects of d-amphetamine and cocaine on extracellular dopamine levels in medial prefrontal cortex of rats. Brain Res. 1990;535:221–226. doi: 10.1016/0006-8993(90)91604-f. [DOI] [PubMed] [Google Scholar]
- Marley RJ, Elmer GI, Goldberg SR. The use of pharmacogenetic techniques in drug abuse research. Pharmacol Ther. 1992;53:217–237. doi: 10.1016/0163-7258(92)90010-w. [DOI] [PubMed] [Google Scholar]
- Meyer AC, Rahman S, Charnigo RJ, Dwoskin LP, Crabbe JC, Bardo MT. Genetics of novelty seeking, amphetamine self-administration and reinstatement using inbred rats. Genes Brain Behav. 2010;9:790–798. doi: 10.1111/j.1601-183X.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserendino MJ, Haile CN, Kosten TA. Strain differences in response to escapable and inescapable novel environments and their ability to predict amphetamine-induced locomotor activity. Psychopharmacology (Berl) 2003;167:281–290. doi: 10.1007/s00213-003-1411-4. [DOI] [PubMed] [Google Scholar]
- Mocsary Z, Bradberry CW. Effect of ethanol on extracellular dopamine in nucleus accumbens: comparison between Lewis and Fischer 344 rat strains. Brain Res. 1996;706:194–198. doi: 10.1016/0006-8993(95)01200-1. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Roth RH, Bunney BS. Characterization of dopamine release in the rat medial prefrontal cortex as assessed by in vivo microdialysis: comparison to the striatum. Neuroscience. 1990;36:669–676. doi: 10.1016/0306-4522(90)90009-s. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Comasco E, Åslund C, Nordquist N, Leppert J, Oreland L. MAOA genotype, family relations and sexual abuse in relation to adolescent alcohol consumption. Addict Biol. 2010;16:347–355. doi: 10.1111/j.1369-1600.2010.00238.x. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Wargelius H, Sjöberg RL, Leppert J, Oreland L. The MAO-A gene, platelet MAO-B activity and psychosocial environment in adolescent female alcohol-related problem behavior. Drug Alcohol Depend. 2007;93:51–62. doi: 10.1016/j.drugalcdep.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Sjöberg RL, Wargelius H, Leppert J, Lindström L, Oreland L. The monoamine oxidase A (MAO-A) gene, family function and maltreatment as predictors of destructive behaviour during male adolescent alcohol consumption. Addiction. 2007;102:389–398. doi: 10.1111/j.1360-0443.2006.01702.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th edn. London: Academic Press; 2007. [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang Z, Papke RL, Crooks PA, Dwoskin LP, Bardo MT. Region-specific effects of N,N’-dodecane-1,12-diyl-bis-3-picolinium dibromide on nicotine-induced increase in extracellular dopamine in vivo. Br J Pharmacol. 2008;153:792–804. doi: 10.1038/sj.bjp.0707612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia G, Del Arco A, De Blas M, Garrido P, Mora F. Environmental enrichment increases the in vivo extracellular concentration of dopamine in the nucleus accumbens: a microdialysis study. J Neural Transm. 2010;117:1123–1130. doi: 10.1007/s00702-010-0447-y. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 2003;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- Sluyter F, Hof M, Ellenbroek BA, Degen SB, Cools AR. Genetic, sex, and early environmental effects on the voluntary alcohol intake in Wistar rats. Pharmacol Biochem Behav. 2000;67:801–808. doi: 10.1016/s0091-3057(00)00425-1. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav. 2009;92:377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr T, Wermeling DS, Weiner I, Feldon J. Rat strain differences in open-field behavior and the locomotor stimulating and rewading effects of amphetamine. Pharmacol Biochem Behav. 1998;59:813–818. doi: 10.1016/s0091-3057(97)00542-x. [DOI] [PubMed] [Google Scholar]
- Strecker RE, Eberle WF, Ashby CR., Jr Extracellular dopamine and its metabolites in the nucleus accumbens of Fischer and Lewis rats: basal levels and cocaine-induced changes. Life Sci. 1995;56:135–141. doi: 10.1016/0024-3205(94)00913-9. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- van der Kam EL, Ellenbroek BA, Cools AR. Gene – environment interactions determine the individual variability in cocaine self-administration. Neuropharmacology. 2005;48:685–695. doi: 10.1016/j.neuropharm.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]