Abstract
Rationale
Like other monoamine releasers such as d-amphetamine, chronic treatment with phenmetrazine can attenuate cocaine self-administration in monkeys.
Objectives
The present studies extended this finding to rodents and to cocaine-primed reinstatement, a putative laboratory animal model of relapse.
Methods
In Experiment 1, rats self-administered food pellets or injections of 0.19 mg/kg cocaine (i.v.) under a progressive-ratio schedule. When responding was stable, subcutaneous osmotic pumps were implanted containing saline or (+)-phenmetrazine (25 or 50 mg/kg per day). In Experiment 2, rats self-administered injections of 0.75 mg/kg cocaine under a fixed-ratio 1 schedule in daily 6-hr sessions. When responding was stable, rats were removed from the self-administration environment for 7 days and treated continuously with saline, 5 mg/kg per day d-amphetamine or phenmetrazine (25 or 50 mg/kg per day) via osmotic pumps. Rats were then returned to the self-administration context while treatment continued, and responding was extinguished by removing response-contingent stimulus changes and cocaine injections. Once responding was extinguished, reinstatement tests were conducted using cocaine injections (10 mg/kg i.p.).
Results
Phenmetrazine decreased self-administration of cocaine, but not food pellets, during the 14-day treatment period; effects persisted for several days after treatment was discontinued. Moreover, cocaine-induced increases in responding during the reinstatement test were attenuated by d-amphetamine and both phenmetrazine doses.
Conclusions
These results extend the study of the effects of phenmetrazine on cocaine self-administration to a rodent model, and provide further support for the use of monoamine releasers as agonist medications for cocaine abuse.
Although cocaine abuse persists as a major public health problem, no medications have demonstrated sufficient safety and efficacy to gain approval of the US Food & Drug Administration. The success of methadone and nicotine replacement therapies in the treatment of opiate and nicotine addiction, respectively, has encouraged efforts to develop an indirect dopamine agonist medication to treat stimulant abuse (Grabowski et al. 2004a; Rush and Stoops 2012; Negus and Henningfield 2014). Preclinical and clinical data have accumulated to suggest that drugs that release neuronal monoamines—particularly dopamine and norepinephrine—can decrease cocaine use (e.g. Negus et al. 2007). For example, preclinical experiments in rodents and nonhuman primates have demonstrated the ability of d-amphetamine to decrease cocaine self-administration under a variety of conditions (e.g. Negus 2003; Negus and Mello 2003a,b; Chiodo et al. 2008; Chiodo and Roberts 2009; Czoty et al. 2010, 2011; Thomsen et al. 2013). Moreover, multiple double-blind, placebo-controlled studies have demonstrated the efficacy of a sustained-release preparation in reducing cocaine use (Grabowski et al. 2001, 2004b; Shearer et al. 2003). Similar clinical success has been reported with another monoamine releaser, methamphetamine (Mooney et al. 2009).
Although preclinical and clinical results with d-amphetamine and methamphetamine are encouraging, their high abuse liability and corresponding Schedule II status presents a significant obstacle to their clinical use in cocaine-dependent individuals; abuse-deterrent strategies are needed to prevent widespread misuse and diversion. One such strategy is the use of a pro-drug which itself is inert, lacking physiological and abuse-related effects if injected or insufflated, but is slowly converted to an active compound following oral ingestion (Huttune et al. 2011; Rush and Stoops 2012). For example, phendimetrazine (PDM), used clinically as an anorectic for over 50 years (Cass 1961), is categorized as a Schedule III compound, reflecting its low abuse liability in humans and lack of reinforcing effects in laboratory animals under most conditions (Jain et al. 1979; Corwin et al. 1987). When taken orally, PDM is metabolized in the liver to phenmetrazine, an amphetamine-like releaser of dopamine and norepinephrine (Rothman et al. 2002; Negus et al. 2009; Banks et al. 2013b). Orderly pharmacokinetics and behavioral effects have been observed over several weeks of repeated intravenous and chronic oral administration, respectively, of PDM (Banks et al. 2013a,b,c), suggesting a consistent metabolism of PDM to phenmetrazine over long-term treatment. Importantly, parenterally administered phenmetrazine has cocaine-like discriminative stimulus effects (Negus 2009; Banks et al. 2011) and can decrease cocaine self-administration in monkeys at doses that do not alter food-maintained responding (e.g. Negus et al. 2009; Banks et al. 2013d).
The primary aim of the present studies was to determine the effects of continuous phenmetrazine treatment on the reinforcing strength of cocaine and, for comparison, food pellets in rats. A progressive-ratio (PR) schedule was used to facilitate comparison of the results with previous studies of continuous d-amphetamine administration (Chiodo et al. 2008; Chiodo and Roberts 2009). As in those experiments, phenmetrazine was administered for 14 days via a subcutaneous osmotic pump. Chronic drug administration was studied to better reflect the manner in which drugs would be used clinically. This is particularly important for monoamine releasers because data obtained after acute and chronic drug treatment may differ. For example, although chronic d-amphetamine treatment attenuates the reinforcing effects of cocaine (described above), acute d-amphetamine administration can increase cocaine self-administration and reinstatement (Gerber and Stretch 1975; de Wit and Stewart 1981; Barrett et al. 2004).
A second objective of these experiments was to extend our studies of the effects of monoamine releasers on active cocaine self-administration to the effects of these drugs on cocaine-primed reinstatement of extinguished responding previously maintained by cocaine. This “extinction-reinstatement” procedure is a putative model of relapse to cocaine use (e.g. de Wit and Stewart, 1981; Stewart 2000; Shaham et al. 2003). Thus, a second group of rats was trained to self-administer cocaine under a 1-response fixed-ratio schedule (FR 1) and allowed to self-administer an unlimited number of cocaine injections during daily 6-hr sessions. After 14 days of cocaine self-administration, we determined the effects of d-amphetamine or phenmetrazine on rate and extent of extinction and on cocaine prime-induced reinstatement. Unlike the vast majority of experiments using the extinction-reinstatement paradigm which assess acute administration of putative pharmacotherapies, animals were exposed to d-amphetamine or phenmetrazine for one week in an environment different from the self-administration chamber prior to being returned to the self-administration setting to undergo extinction and the reinstatement test. By examining effects on active cocaine self-administration and extinction/reinstatement in the same set of studies, we were able to assess whether monoamine releasers are able to reduce responding in models of two phases of the addiction cycle. Although a medication that blocks the reinforcing effects of cocaine will not necessarily also be efficacious in maintaining abstinence in the face of relapse triggers, a dual action would enhance the value of the pharmacotherapy and simplify treatment.
Methods
Subjects
72 male Sprague-Dawley rats (Harlan Industries Inc., Indianapolis, IN, USA), weighing 300–350 g at the start of the experiment, were used as subjects. Rats were maintained on a 12-h reverse light/dark cycle in a temperature and humidity controlled facility with food and water available ad libitum. All rats were habituated to this schedule for at least 7 days before entering the experiment. Animals that self-administered food were pair-housed for 7 days after entry into the facility, and singly housed thereafter. Upon being singly housed animals were reduced by 90% of free-feeding by food restriction. Food-self-administration occurred in commercially available operant chambers contained within sound- and light-attenuating cubicles that contained a ventilator fan (Med Associates Inc., St. Albans, VT). Each operant chamber contained a pellet dispenser for 45-mg food pellets, a food trough located in the center of one wall, one retractable response lever located to the left of the food trough, one standard response lever located to the right of the food trough, and one stimulus light located above each lever. Animals that self-administered cocaine were housed individually in custom-made stainless steel experimental chambers (30 × 30 × 30 cm) 24 h per day, 7 days per week. When removed from experimental chambers in Experiment 2, animals were singly housed in polycarbonate cages. Animal housing and handling and all experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council 2011) and were approved by the Animal Care and Use Committee of Wake Forest University.
Catheter implantation
Rats were anesthetized with ketamine (100 mg/kg i.p.) and xylazine (8 mg/kg, i.p.) and implanted with a chronically indwelling Silastic jugular catheter (outer diameter = 0.635, inner diameter = 0.305 mm; CamCaths, Cambridgeshire, UK) as described previously (Chiodo et al. 2008). Briefly, the catheter was attached to a subcutaneous plastic anchor that exited through the skin on the dorsal surface in the region of the scapulae. Tygon tubing, enclosed by a stainless steel protective tether, connected the plastic anchor to a counterbalanced fluid swivel (Instech Laboratories, Inc. Plymouth Meeting, PA) mounted above the experimental chamber. An infusion pump (Razel Scientific Instruments, Inc. Stamford, CT) located outside of the self-administration chamber was connected to the swivel by Tygon tubing. Rats were given a 3–5 day recovery period before starting self-administration. Catheters were flushed daily with heparinized saline to maintain patency. In the rare case that response rates declined rapidly during cocaine self-administration, loss of patency was confirmed by an inability to flush heparanized saline and the rat was excluded from the study. This occurred only four times; no data from these rats were used and they are not included in the count of subjects.
Osmotic pump implantation
Because pump implantation surgeries were relatively short in duration, rats were anesthetized with a gas containing oxygen, nitrogen and isoflurane (5% to induce anesthesia and 3% for the remainder of the surgery) rather than the more long-lasting ketamine/xylazine cocktail used for catheter implantations. An osmotic pump (Alzet Model 2001; Durect, Cupertino, CA) containing saline, phenmetrazine (25 or 50 mg/kg per day; Experiments 1 and 2) or 5 mg/kg per day amphetamine (Experiment 2) was implanted subcutaneously, rostral to the plastic catheter anchor with the flow moderator pointing rostrally. Pumps were removed after 7 days and replaced with a new pump delivering the same treatment. In Experiment 1, pumps were replaced immediately following that day’s cocaine self-administration session or 3 hr after completion of the food self-administration session. This ensured that subjects had 18 h of recovery from the implantation procedure before the following day’s self-administration session without a decrease in circulating phenmetrazine concentrations. In Experiment 2, pumps were implanted the morning after the final cocaine self-administration session to avoid potential interactions between anesthesia and high circulating concentrations of cocaine.
Cocaine self-administration training
Daily sessions began at 0900 (6 h into the dark phase) and were signaled by extension of a single active lever into the experimental chamber. During training, cocaine (1.5 mg/kg per infusion) was available on an FR 1 schedule of reinforcement and was injected over approximately 5 sec (depending on body weight), followed by a 20-sec timeout during which the lever was retracted and a cue light above the lever was illuminated. Each training session lasted until animals had self-administered the maximum number of injections available (40) within the 6-h session for five consecutive days, while maintaining consistent post-infusion pauses in responding between injections. Previous data have shown that these training conditions leads to subsequent stable responding on the PR schedule (e.g. Morgan et al. 2005).
Food self-administration training
Sixteen rats were initially trained under an FR 1 schedule in which each press on the left lever was reinforced by delivery of a single 45-mg chocolate-flavored purified rat chow pellet (Bio-Serv Inc., Frenchtown, NJ). Pellet availability was signaled by illumination of the stimulus light above the left lever. The left lever was retracted following each response for 1 sec and the stimulus light was turned off. After 1 sec the left lever was extended and the left stimulus light was illuminated. Responses on the right lever were recorded but had no programmed consequences. Sessions lasted until rats earned 100 pellets or 30 min elapsed, whichever occurred first. After 5 days of training on the FR schedule, the PR schedule was introduced and responses on the left lever were reinforced with a single 45-mg chocolate-flavored pellet.
Experiment 1: Effect of chronic treatment with phenmetrazine on cocaine or food self-administration
After training criteria were reached, animals were given access to 0.19 mg/kg per infusion cocaine or food pellets on a PR schedule for the remainder of the experiment (see Fig. 1). This dose of cocaine, which is situated on the lower end of the ascending limb of the cocaine dose-response curve, was chosen because it was the dose which was most affected by d-amphetamine in previous studies (e.g. Chiodo et al. 2008). Under the PR schedule, the number of lever presses required for a rat to receive successive cocaine injections increased along an exponential function through the following progression: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, etc. (Richardson and Roberts 1996). Sessions ended when 1 h elapsed without an injection. The primary dependent variable was the number of ratios completed during each 6-h session (i.e. the number of cocaine injections received). Stable responding was defined as 3 days of stable injections with no upward or downward trends. Numbers of injections were used as the dependent measure rather than total number of responses or final ratio values reached (i.e. “break points”) to increase homogeneity of variance (see Richardson and Roberts 1996). Immediately after animals achieved stable performance on the PR schedule, they were randomly assigned to one of three treatment groups and were implanted with a subcutaneous osmotic pump containing either saline or phenmetrazine (25 or 50 mg/kg per day). Animals continued to self-administer cocaine on the PR schedule for 14 days. The pumps were then removed and self-administration was studied for an additional 7 days (Fig. 1).
Fig. 1.
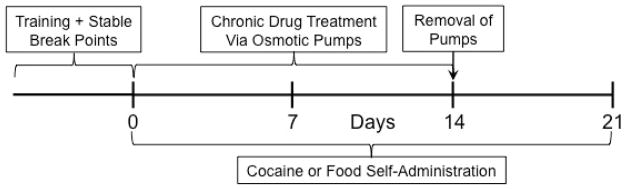
Design of Experiment 1.
The higher dose of phenmetrazine (50 mg/kg per day) was also tested in rats that self-administered food. After 11 baseline PR sessions, 8 rats were implanted with osmotic pumps containing phenmetrazine as described above and 8 rats were implanted with osmotic pumps containing an equal volume of 0.9% saline. PR food reinforcement sessions were conducted daily for the next 7 days. The pumps were then replaced and sessions were conducted for an additional 7 days. After the second week of exposure to phenmetrazine or saline, pumps were removed and sessions were conducted for a final 7 days.
Experiment 2: Effects of chronic amphetamine or phenmetrazine treatment on cocaine-primed reinstatement
Initial training sessions were conducted as described above, except that rats self-administered a maximum of 20 infusions of 0.75 mg/kg per injection cocaine. After training criteria were reached, the 20-injection maximum was removed; there was no limit to the number of infusions that could be earned over the daily 6-h session. Immediately following the 14th session, animals were removed from their experimental chambers, transferred to a separate housing room and randomly assigned to one of four groups (N=8 per group; Fig. 2). On the next day, subcutaneous osmotic pumps were implanted to deliver either saline, d-amphetamine (5 mg/kg per day) or phenmetrazine (25 or 50 mg/kg per day). Pumps were replaced every 7 days.
Fig. 2.
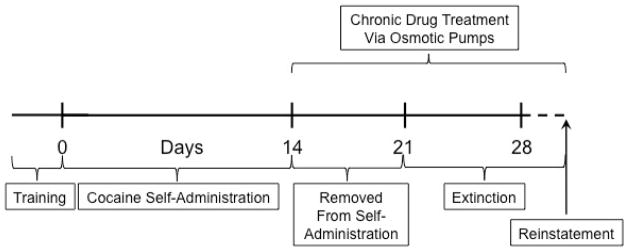
Design of Experiment 2.
On the morning of the 8th day after cessation of cocaine self-administration (day 22 in Fig. 2), animals were returned to the experimental chamber in which they had previously self-administered cocaine and underwent extinction. Extinction sessions started at the same time of day as the previous cocaine self-administration sessions (0900), were 2 h in duration and were signaled by the extension of the lever into the experimental chamber. During extinction sessions, responses on the lever were recorded, but had no programmed consequences (i.e. no stimulus light illumination, pump activation or timeout period). Daily extinction training sessions were conducted for a minimum of 7 days and until responding extinguished. Criteria for extinction were ≤25% of the average number of responses during the final two days of cocaine self-administration for two consecutive days. Osmotic pumps delivering continuous infusions of saline, amphetamine or phenmetrazine were replaced every 7 days for each animal regardless of the stage of extinction training until the end of the reinstatement session in order to maintain circulating concentrations of either amphetamine or phenmetrazine. The day after extinction criteria were met, animals were tested for cocaine-primed reinstatement. During these 2-h sessions, cocaine (10 mg/kg i.p.) was administered immediately before the start of the session and responses on the lever were recorded but had no programmed consequences.
Drugs
Cocaine HCl (National Institute of Drug Abuse, Rockville, MD), d-amphetamine sulphate (Sigma-Aldrich, St. Louis, MO) and (+)-phenmetrazine hemifumarate (RTI International, Raleigh, NC) were dissolved in sterile saline (0.9% NaCl). Cocaine solutions that were delivered intravenously were passed through a 22-μm microfilter. All doses are expressed as salts.
Data Analysis
For Experiment 1, the primary dependent variable for cocaine or food self-administration on the PR schedule was number of reinforcers delivered. Average baseline injections were compared between saline and phenmetrazine group(s) in both the cocaine self-administration experiment (using a one-way ANOVA) and the food self-administration experiment (using a t-test). A two-way ANOVA with repeated measures compared reinforcers delivered across groups during the 14 days of drug treatment and 7-day post-treatment period. Pre-planned pairwise comparisons were then conducted using the Holm-Sidak method. Daily body weights were also recorded for food self-administering animals and compared with a t-test. For Experiment 2, one-way ANOVAs were also conducted to determine whether there were differences between groups (saline, amphetamine, phenmetrazine) in number of days to reach extinction criteria and average number of responses during the last two days of extinction. Because individual variability in responding was observed during the last two cocaine self-administration sessions, the percent difference between responses during the extinction baseline and cocaine-primed reinstatement was calculated for each animal. A one-way ANOVA was then used to determine whether there were treatment group differences in the percent change in responses between extinction baseline and reinstatement sessions, followed by pre-planned pairwise comparisons using Bonferroni’s test. In all cases, differences were considered significant when p < 0.05.
Results
Experiment 1
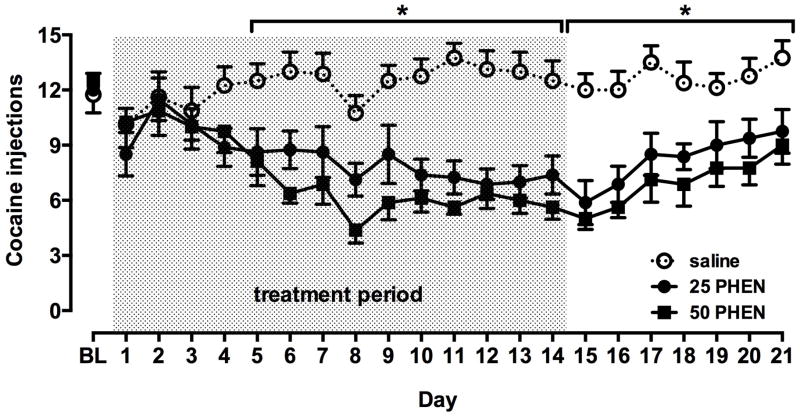
Once cocaine self-administration stabilized under PR schedule, rats self-administered on average 12.1 ± 0.5 injections of 0.19 mg/kg cocaine (Fig. 3). There were no significant differences in baseline injections across the three groups. Treatment for 14 days with saline did not alter cocaine self-administration. However, chronic phenmetrazine treatment resulted in a progressive decrease in injections received (Fig. 3). A two-way repeated measures ANOVA comparing injections across groups revealed a significant effect of treatment (F(2,273) = 11.07, p < 0.001) and day (F(13,273) = 5.64, p < 0.001) and a significant interaction (F(26,273) = 4.48, p < 0.001). A post-hoc pairwise Holm-Sidak multiple comparisons test between treatment groups across days revealed that cocaine injections were significantly lower in animals receiving chronic treatment with 25 mg/kg per day phenmetrazine compared to saline treated animals on day 5 (p < 0.05), days 6 through 9 (p < 0.01) and days 10 through 14 (p < 0.001). Cocaine injections were also significantly lower in animals receiving chronic phenmetrazine (50 mg/kg per day) compared to controls on day 5 (p < 0.01) and days 6 through 14 (p < 0.001). Decreases in cocaine self-administration were larger with the higher vs. lower dose of phenmetrazine on most days, but the difference did not reach statistical significance.
Fig 3.
Effect of continuous phenmetrazine (PHEN) infusions on self-administration of 0.19 mg/kg per injection cocaine under a PR schedule. Points represent mean (± SEM) number of injections (n=8 per group). BL = baseline. Shaded portion (days 1–14) indicates the period of treatment with saline or PHEN (25 or 50 mg/kg per day). *, number of injections in the saline group was significantly different (p < 0.05) from both PHEN groups.
Following 14 days of continuous subcutaneous administration of saline or phenmetrazine, osmotic pumps were removed and self-administration was studied for an additional 7 days (see Fig. 2). Numbers of injections received under the PR schedule gradually recovered towards baseline levels in the two groups treated with phenmetrazine (Fig 3). A two-way repeated measures ANOVA revealed a significant effect of treatment (F(2,126) = 12.65, p < 0.001) and of days (F(6,126) = 9.52, p < 0.001), but no significant interaction. A post-hoc pairwise Holm-Sidak multiple comparison test revealed that injections remained significantly lower in animals that had previously received 25 mg/kg day phenmetrazine compared to animals that had received saline on days 15 through 17 (p < 0.001), and days 18 through 21 (p < 0.01). Cocaine injections also remained lower in animals that had previously received 50 mg/kg per day phenmetrazine compared to controls, on post-treatment days 15 through 18 (p < 0.001) and days 19 through 21 (p < 0.01).
In the two groups of rats that self-administered food pellets, there was no significant difference in number of pellets received at baseline (Fig. 4). Treatment for 14 days with saline did not alter cocaine self-administration. However, chronic treatment with 50 mg/kg per day phenmetrazine resulted in increases in pellets delivered, particularly over the first week of treatment (Fig. 4). A two-way repeated-measures ANOVA comparing pellets received across groups revealed a significant effect of treatment (F(1,14) = 5.22, p < 0.05) and day (F(13,182) = 4.51, p < 0.001) and a significant interaction (F(13,182) = 1.85, p < 0.05). A post-hoc pairwise Holm-Sidak multiple comparisons test revealed that animals receiving chronic treatment with 50 mg/kg per day phenmetrazine earned more food pellets than saline-treated animals on days 1, 2, 4, 5, 6 and 12. Tolerance developed to these early increases in responding such that the number of pellets delivered at the end of the treatment period was not different from baseline. After 14 days of treatment, pumps were removed and food self-administration was studied for an additional 7 days (see Fig. 2). Following discontinuation of treatment, pellets earned by phenmetrazine-treated rats were slightly but significantly higher than saline-treated rats (Fig 4). A two-way repeated measures ANOVA revealed a significant effect of treatment (F(1,14) = 4.64, p < 0.05) but not day, and there was no significant interaction. A post-hoc pairwise Holm-Sidak multiple comparison test revealed that number of food pellets delivered was significantly higher in animals that had previously received 50 mg/kg day phenmetrazine on days 2 and 3 after pump removal (p < 0.05). Finally, although body weights increased significantly in saline-treated (paired t-test, p < 0.01), but not phenmetrazine-treated rats, average weights of the groups did not differ significantly before or after treatment.
Fig 4.
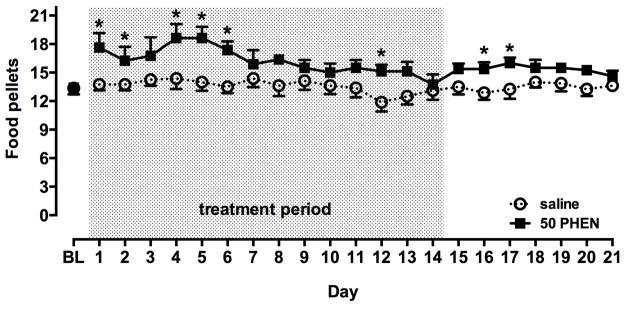
Effect of continuous phenmetrazine (PHEN) infusions on self-administration of food under a PR schedule. Points represent mean (± SEM) number of food pellets delivered (n=8 per group). BL = baseline. Shaded portion (days 1–14) indicates the period of treatment with saline or PHEN (50 mg/kg per day). *, significant difference (p < 0.05) in number of food pellets delivered on this day.
Experiment 2
After training criteria were reached, animals responded for cocaine infusions (0.75 mg/kg) on a FR 1 schedule with no limit on infusions that could be earned during the daily 6-h session (see Fig. 2). Removal of the infusion limit resulted in an increase in infusions per day which stabilized (data not shown). Mean (± SEM) cumulative cocaine intake did not differ across the four treatment groups prior to implantation of osmotic pumps (F(3,28) = 1.36, p = 0.185; saline group, 851 ± 33 mg/kg; 5 mg/kg per day d-amphetamine, 872 ± 26 mg/kg; 25 mg/kg per day phenmetrazine 957 ± 39 mg/kg, 50 mg/kg per day phenmetrazine, 905 ± 58 mg/kg).
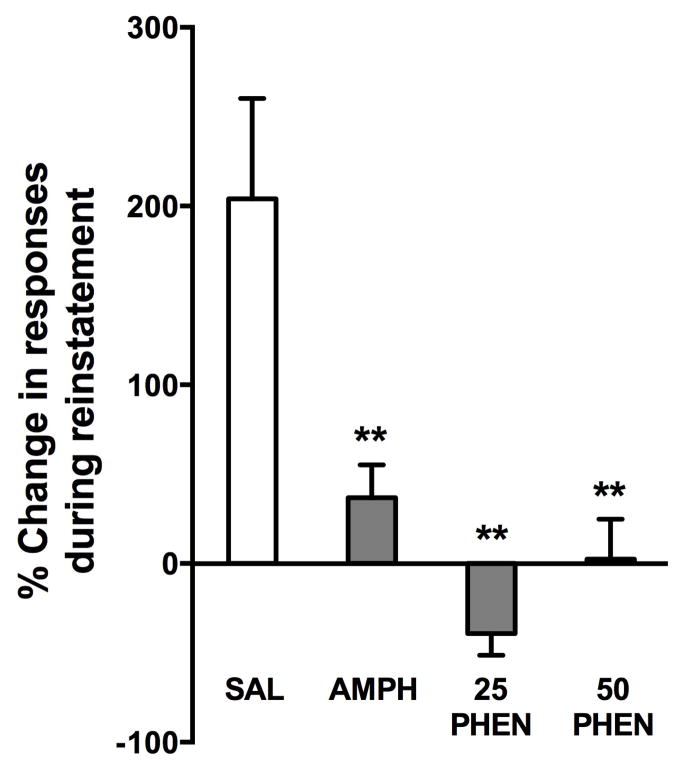
After 7 days, rats were returned to their self-administration chambers and daily extinction experiments were conducted until criteria were met. Although individual differences were observed in the number of days required to extinguish responding, there were no significant differences by group. The SAL, AMPH, 25 PHEN and 50 PHEN groups required 9.3 ± 1.0, 9.3 ± 1.3, 10.3 ± 1.7 and 8.6 ± 1.7 days to extinguish, respectively. There were also no significant differences between groups in the number of responses emitted on the last two days of extinction (16.5 ± 2.4, 15.3 ± 2.0, 15.3 ± 2.0 and 16.1 ± 2.0 responses, respectively). The day after extinction criteria were met, an injection of cocaine (10.0 mg/kg i.p.) was administered immediately before a self-administration session conducted under extinction conditions. Cocaine reinstated responding in the group that had been treated with saline, but not in the animals that had been treated with d-amphetamine or phenmetrazine (Fig. 5). A one-way ANOVA revealed a significant difference between the treatment groups (F(3,28) = 10.965, p = 0.0001), and planned multiple comparisons using Bonferroni’s test revealed significant differences between the saline group and the groups that received amphetamine, 25 and 50 mg/kg per day phenmetrazine (both p = 0.01).
Fig 5.
Mean ± SEM percent change in responses compared to extinction during the reinstatement test with 10.0 mg/kg cocaine, i.p. in rats (n=8 per group) treated with continuous saline (SAL), 5 mg/kg per day d-amphetamine (AMPH), 25 mg/kg per day phenmetrazine (25 PHEN) or 50 mg/kg per day phenmetrazine (50 PHEN) **, p < 0.01 compared to SAL group.
Discussion
In previous studies in rats and monkeys, continuous treatment with d-amphetamine produced decreases in cocaine self-administration that persisted beyond termination of d-amphetamine treatment (Negus and Mello 2003a,b; Chiodo et al. 2008; Chiodo and Roberts 2009; Czoty et al. 2010, 2011). Results of Experiment 1 demonstrate that phenmetrazine has a similar effect, further supporting monoamine releasers as promising candidates for pharmacotherapies to treat cocaine addiction. Phenmetrazine is of particular interest because, with respect to its effects on food versus cocaine self-administration, its 37-fold selectivity in potency to release dopamine versus serotonin is near the value determined to be optimal for monoamine releasers (~30; Negus et al. 2007, 2009). In other studies in monkeys, phenmetrazine had cocaine-like discriminative stimulus effects (Negus 2009; Banks et al. 2013b) and decreased cocaine self-administration under a second-order schedule at doses that did not alter food-maintained responding (Negus et al. 2009). Moreover, phenmetrazine decreased the reinforcing strength of cocaine relative to food under a choice procedure (Banks et al. 2011, 2013d). In the present studies, the higher dose of phenmetrazine produced only increases in responding maintained by a non-drug reinforcer, food pellets—effects similar to, but of lesser magnitude than, d-amphetamine (Chiodo et al. 2008). It is noteworthy that the number of cocaine injections delivered at baseline was not different from the number of food pellets delivered at baseline,, suggesting that the two reinforcers used in the present study are of comparable reinforcing strength. Taken together, the data indicate that phenmetrazine-induced decreases cocaine self-administration are not due to a general suppression of responding.
The present studies also examined the effects of phenmetrazine and d-amphetamine in a putative animal model of relapse. The traditional extinction-reinstatement model was used with two important alterations. First, whereas most reinstatement experiments examine acute drug treatment, in Experiment 2, as in Experiment 1, drugs were given continuously via osmotic pump. Second, d-amphetamine or phenmetrazine was administered for one week before responding was extinguished. These features were incorporated to better reflect a clinical situation in which an individual may be able to abstain from cocaine and avoid relapse triggers for a brief period of time after initiation of pharmacotherapy, for example due to incarceration, inpatient treatment or through brief voluntary abstinence. Chronic treatment with phenmetrazine or d-amphetamine did not affect the time course of extinction, and responding during extinction declined to similar levels, on average, regardless of drug or saline treatment. This suggests that continuous administration of d-amphetamine or phenmetrazine did not alter the rats’ ability to emit responses or the neuroadaptations and learning processes active during extinction (e.g. Myers and Davis 2002; Bouton 2004). Following extinction, cocaine reinstated responding in rats treated with saline, but not in those treated chronically with d-amphetamine or phenmetrazine. Thus, the findings that acute and chronic d-amphetamine have opposite effects on cocaine self-administration (see Introduction), extends to drug-primed reinstatement of extinguished cocaine seeking. Moreover, the results suggest that, in addition to facilitating cessation of cocaine use, chronic treatment with monoamine releasers may also be effective in preventing relapse, enhancing its utility as a pharmacotherapy.
While the specific behavioral and pharmacological mechanisms underlying these effects remain to be completely elucidated, one possibility is that chronic treatment with monoamine releasers resulted in neurobiological adaptations that produced cross-tolerance to the reinforcing (Experiment 1) and discriminative-stimulus (Experiment 2) effects of cocaine. Regarding cocaine self-administration, a previous study suggests that cross-tolerance is not sufficient to fully explain the decreases in behavior. In that experiment (Chiodo et al. 2008), chronic d-amphetamine treatment decreased cocaine-self-administration in rats that self-administered cocaine daily during d-amphetamine treatment, but not in rats who did not receive cocaine during the treatment period. Moreover, effects were observed at low cocaine doses, but not at higher cocaine doses at which tolerance might be expected to be more evident. These data indicate that d-amphetamine and cocaine must be present concurrently for the decrease in reinforcing effects to occur; some extinction process or other learning mechanism may be necessary (for a more extensive discussion see Chiodo and Roberts 2009). Cross-tolerance to discriminative stimulus effects may play a role in the effects of phenmetrazine and d-amphetamine in Experiment 2, as discriminative stimulus effects likely play a prominent role in the response-reinstating effect of the cocaine prime (Odum and Shahan 2004; Banks et al. 2007; Keiflin et al. 2008). There is ample evidence indicating that chronic treatment with cocaine or monoamine releasers decreases each others’ cocaine-like discriminative stimulus effects in a manner consistent with cross-tolerance that is likely to be mediated through changes in brain dopamine systems (e.g. Wood and Emmett-Oglesby 1987, 1988; Wood et al. 1987; Peltier et al. 1996). Importantly, the present study shows that the ability of phenmetrazine to decrease cocaine self-administration (Negus et al. 2009; Banks et al. 2013d) extends to rodents. This observation will enable a more detailed investigation of the specific alterations in dopamine receptor and transporter densities and function underlying these effects in future studies.
A comment on the doses of phenmetrazine that were studied is warranted. Doses were chosen based on the expectation that phenmetrazine would be approximately 5 times less potent than d-amphetamine. For example, EC50 values for d-amphetamine and phenmetrazine to release dopamine are 24.8 nM and 131 nM, respectively (Rothman and Bauman 2003). Moreover, similar response rates were maintained by injections of 0.25 mg/kg d-amphetamine and 1.0 mg/kg phenmetrazine under an FR 50 schedule (Gotestam and Andersson 1975). Thus, since 5.0 mg/kg per day d-amphetamine was effective in reducing cocaine self-administration in this paradigm (Chiodo et al. 2008; Chiodo and Roberts 2009), we initially tested 25 mg/kg per day phenmetrazine, then doubled the dose. Ongoing studies testing other phenmetrazine doses have found that 10 mg/kg per day phenmetrazine produces small, transient decreases in cocaine self-administration (MJ Ferris, personal communication).
Some limitations of these studies should be mentioned. In Experiment 1, the effects of phenmetrazine and d-amphetamine were studied in combination with only one dose of cocaine (0.19 mg/kg per infusion). This relatively low dose was chosen for evaluation of phenmetrazine because the effects of d-amphetamine were most prominent on responding maintained by injections of this cocaine dose (Chiodo et al., 2008). In that study, the effect of d-amphetamine diminished as the cocaine dose increased, an effect also observed in previous studies in nonhuman primates (Negus and Mello 2003b). That is, the d-amphetamine-induced decreases in cocaine self-administration were overcome by raising the cocaine dose. Although it is unknown whether phenmetrazine would also decrease self-administration of higher cocaine doses, that effect would not be predicted based on the d-amphetamine results. The clinical implication of this pattern of effects would be that monoamine releasers may be more effective in individuals with less severe cocaine dependence. Finally, it should be acknowledged that despite the widespread use of the extinction/reinstatement paradigm as a model of relapse, some incongruities exist (see Katz and Higgins 2003), particularly regarding the manner of terminating cocaine use, which is vastly different in the preclinical (extinction) and clinical setting (voluntary abstinence).
Like d-amphetamine and other anorectics, phenmetrazine is self-administered by humans and nonhuman primates (Wilson et al. 1971; Griffiths et al. 1978, 1979; Chait et al. 1987); due to its abuse liability phenmetrazine is no longer in clinical use in the United States. In general, abuse liability represents a significant obstacle to the widespread clinical use of monoamine releasers, particularly in stimulant-dependent patients. Pro-drugs and other clinically available abuse-deterrent formulations of monoamine releasers may provide a way to realize the therapeutic effects of monoamine releasers while reducing abuse liability (e.g. Rush and Stoops 2012; Negus and Henningfield 2014). For example, lisdexamfetamine, a pro-drug that consists of d-amphetamine conjugated to the amino acid L-lysine, is currently used to treat attention-deficit hyperactivity disorder (Steer et al. 2012). Lisdexamfetamine is enzymatically converted to d-amphetamine in red blood cells, resulting in a slower time to peak d-amphetamine blood concentrations. Both increases in striatal dopamine concentrations and locomotor-stimulant effects are of lower magnitude and slower to peak after lisdexamfetamine compared to d-amphetamine, and lisdexamfetamine was not self-administered by rats when substituted for cocaine under a FR 2 schedule (Heal et al. 2013; reviewed in Hutson et al. 2014). Although these results are encouraging, the fact that lisdexamfetamine, like d-amphetamine, is currently a Schedule II compound may limit the willingness of physicians to prescribe it to stimulant-dependent individuals. The present results with phenmetrazine provide proof-of-concept data to support the evaluation of its pro-drug, the Schedule III compound PDM. As described above (see Introduction) PDM, which has been used clinically anorectic for over 50 years, can attenuate the reinforcing effects of cocaine in monkeys under multiple experimental conditions and possesses low abuse liability (Jain et al. 1979; Corwin et al. 1987; Banks et al. 2013a,c). PDM is therefore a promising candidate to provide the selective attenuation of cocaine self-administration observed with monoamine releasers without the high abuse liability inherent in Schedule II drugs. The present studies demonstrate that chronic treatment with the active metabolite, phenmetrazine, can decrease cocaine self-administration in rats as has been demonstrated in nonhuman primates (Negus 2009; Banks et al. 2011, 2013d). This result and the demonstration that cocaine-primed reinstatement is blocked after chronic phenmetrazine treatment suggests that its pro-drug PDM merits further evaluation as a pharmacotherapy for cocaine dependence.
Acknowledgments
This research was supported by NIDA grant P50 DA 06634.
Footnotes
The authors have no conflicts of interest to declare.
References
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Effects of phendimetrazine treatment on cocaine vs. food choice and extended-access cocaine consumption in rhesus monkeys. Neuropsychopharmacology. 2013a;38:2698–2707. doi: 10.1038/npp.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Role of phenmetrazine as an active metabolite of phendimetrazine: evidence from studies of drug discrimination and pharmacokinetics in rhesus monkeys. Drug Alcohol Depend. 2013b;130:158–166. doi: 10.1016/j.drugalcdep.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of 14-day treatment with the schedule III anorectic phendimetrazine on choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2013c;131:204–213. doi: 10.1016/j.drugalcdep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Interaction between behavioral and pharmacological treatment strategies to decrease cocaine choice in rhesus monkeys. Neuropsychopharmacology. 2013d;38:395–404. doi: 10.1038/npp.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav Pharmacol. 2011;22:824–836. doi: 10.1097/FBP.0b013e32834d63ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Czoty PW, Nader MA. The influence of reinforcing effects of cocaine on cocaine-induced increases in extinguished responding in cynomolgus monkeys. Psychopharmacology. 2007;192:449–456. doi: 10.1007/s00213-007-0732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropsychopharmacology. 2004;47(Suppl 1):256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Cass LJ. Evaluation of phendimetrazine bitartarate as an appetite suppressant. Can Med Assoc J. 1961;84:1114–1116. [PMC free article] [PubMed] [Google Scholar]
- Chait LD, Uhlenhuth EH, Johanson CE. Reinforcing and subjective effects of several anorectics in normal human volunteers. J Pharmacol Exp Ther. 1987;242:777–783. [PubMed] [Google Scholar]
- Chiodo KA, Lack CM, Roberts DC. Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous D-amphetamine treatment in rats. Psychopharmacology. 2008;200:465–473. doi: 10.1007/s00213-008-1222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo KA, Roberts DC. Decreased reinforcing effects of cocaine following 2 weeks of continuous d-amphetamine treatment in rats. Psychopharmacology. 2009;206:447–456. doi: 10.1007/s00213-009-1622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Woolverton WL, Schuster CR, Johanson CE. Anorectics: effects on food intake and self-administration in rhesus monkeys. Alcohol Drug Res. 1987;7:351–361. [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Nader MA. Effects of chronic d-amphetamine on the reinforcing strength of cocaine in rhesus monkeys. Psychopharmacology. 2010;209:375–382. doi: 10.1007/s00213-010-1807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Martelle JL, Nader MA. Prolonged attenuation of the reinforcing strength of cocaine by chronic d-amphetamine in rhesus monkeys. Neuropsychopharmacology. 2011;36:539–547. doi: 10.1038/npp.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacol Biochem Behav. 1975;3:1055–1061. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Gotestam KG, Andersson BE. Self-administration of amphetamine analogues in rats. Pharmacol Biochem Behav. 1975;3:229–233. doi: 10.1016/0091-3057(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacology. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004a;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004b;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Brady JV, Snall JD. Relationship between anorectic and reinforcing properties of appetite suppressant drugs: Implications for assessment of abuse liability. Biol Psychiatry. 1978;13:283–290. [PubMed] [Google Scholar]
- Griffiths RR, Brady JV, Bradford LD. Predicting the abuse liability of drugs with animal drug self-administration procedures: psychomotor stimulants and hallucinogens. In: Thompson T, Dews PB, editors. Advances in Behavioral Pharmacology. Vol. 2. New York: Academic Press; 1979. pp. 163–208. [Google Scholar]
- Heal DJ, Buckley NW, Gosden J, Slater N, France CP, Hackett D. A preclinical evaluation of the discriminative and reinforcing properties of lisdexamfetamine in comparison to D-amfetamine, methylphenidate and modafinil. Neuropharmacology. 2013;73:348–358. doi: 10.1016/j.neuropharm.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Hutsan PH, Pennick M, Secker R. Preclinical pharmacokinetics, pharmacology and toxicology of lisdexamfetamine: A novel d-amphetamine pro-drug. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.02.014. in press. [DOI] [PubMed] [Google Scholar]
- Huttune KM, Raunio H, Rautio J. Prodrugs—from serendipity to rational design. Pharmacol Rev. 2011;63:750–771. doi: 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- Jain NC, Budd RD, Sneath TC. Frequency of use or abuse of amphetamine-related drugs. Am J Drug Alcohol Abuse. 1979;6:53–57. doi: 10.3109/00952997909007032. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Smith MA, Roberts DC. Binge self-administration and deprivation produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology. 2005;178:309–316. doi: 10.1007/s00213-004-1992-6. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:909–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Baumann MH, Rothman RB, Mello NK, Blough BE. Selective suppression of cocaine-versus food-maintained responding by monoamine releasers in rhesus monkeys: benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine. J Pharmacol Exp Ther. 2009;329:272–281. doi: 10.1124/jpet.108.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Henningfield J. Agonist medications for the treatment of cocaine use disorder. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology. 2003a;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003b;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Odum AL, Shahan TA. D-amphetamine reinstates behavior previously maintained by food: importance of context. Behav Pharmacol. 2004;15:513–516. doi: 10.1097/00008877-200411000-00007. [DOI] [PubMed] [Google Scholar]
- Peltier RL, Li DH, Lytle D, Taylor CM, Emmett-Oglesby MW. Chronic d-amphetamine or methamphetamine produces cross-tolerance to the discriminative and reinforcing stimulus effects of cocaine. J Pharmacol Exp Ther. 1996;277:212–218. [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Katsnelson M, Vu N, Partilla JS, Dersch CM, Blough BE, Baumann MH. Interaction of the anorectic medication, phendimetrazine, and its metabolites with monoamine transporters in rat brain. Eur J Pharmacol. 2002;447:51–57. doi: 10.1016/s0014-2999(02)01830-7. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW. Agonist replacement therapy for cocaine dependence: a translational review. Future Med Chem. 2012;4:245–265. doi: 10.4155/fmc.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98:1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Steer C, Froelich J, Soutullo CA, Johnson M, Shaw M. Lisdexamfetamine dimesylate: a new therapeutic option for attention-deficit hyperactivity disorder. CNS Drugs. 2012;26:691–705. doi: 10.2165/11634340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Fink-Jensen A, Woldbye DP, Wortwein DP, Sagen TN, Holm R, Pepe LM, Caine SB. Effects of acute and chronic aripiprazole treatment on choice between cocaine self-administration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology. 2008;201:43–53. doi: 10.1007/s00213-008-1245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MC, Hitomi M, Schuster CR. Psychomotor stimulant self-administration as a function of dosage per injection in the rhesus monkeys. Psychopharmacologia. 1971;22:271–281. doi: 10.1007/BF00401789. [DOI] [PubMed] [Google Scholar]
- Wood DM, Emmett-Oglesby MW. Evidence for dopaminergic involvement in tolerance to the discriminative stimulus properties of cocaine. Eur J Pharmacol. 1987;138:155–157. doi: 10.1016/0014-2999(87)90354-2. [DOI] [PubMed] [Google Scholar]
- Wood DM, Emmett-Oglesby MW. Substitution and cross-tolerance profiles of anorectic drugs in rats trained to detect the discriminative stimulus properties of cocaine. Psychopharmacology. 1988;95:364–368. doi: 10.1007/BF00181948. [DOI] [PubMed] [Google Scholar]
- Wood DM, Retz KC, Emmett-Oglesby MW. Evidence of a central mechanism mediating tolerance to the discriminative stimulus properties of cocaine. Pharmacol Biochem Behav. 1987;28:4001–406. doi: 10.1016/0091-3057(87)90461-8. [DOI] [PubMed] [Google Scholar]