Abstract
Background
Having a positive family history of alcohol use disorders (FHP), as well as aberrant reward circuitry, has been implicated in the initiation of substance use during adolescence. This study explored the relationship between FHP status and reward circuitry in substance naïve youth to better understand future risky behaviors.
Methods
Participants were 49 FHP and 45 demographically matched family history negative (FHN) substance-naïve 12–14 year-olds (54 % female). Subjects underwent structural magnetic resonance imaging, including diffusion tensor imaging. Nucleus accumbens and orbitofrontal cortex volumes were derived using FreeSurfer, and FSL probabilistic tractography probed structural connectivity and differences in white matter diffusivity estimates (e.g. fractional anisotropy, and mean, radial, and axial diffusivity) between fiber tracts connecting these regions.
Result
FHP and FHN youth did not differ on nucleus accumbens or orbitofrontal cortex volumes, white matter tract volumes, or percentages of streamlines (a proxy for fiber tract count) connecting these regions. However, within white matter tracts connecting the nucleus accumbens to the orbitofrontal cortex, FHP youth had significantly lower mean and radial diffusivity (ps < 0.03) than FHN youth.
Discussion
While white matter macrostructure between salience and reward regions did not differ between FHP and FHN youth, FHP youth showed greater white matter coherence within these tracts than FHN youth. Aberrant connectivity between reward regions in FHP youth could be linked to an increased risk for substance use initiation.
Keywords: Adolescence, Cognitive control, Family history of alcohol use disorders, Nucleus accumbens, Orbitofrontal cortex, Probabilistic tractography, Reward circuitry, Substance use disorders, White matter integrity, Diffusion tensor imaging (DTI), Structural connectivity
Introduction
Youth with a positive family history of alcohol use disorders (FHP) are at increased risk for earlier initiation of alcohol use (Hill and Yuan 1999; McGue et al. 2001), greater rates of alcohol-related problems and alcohol use disorders (AUD; Elliott et al. 2012; Milberger et al. 1999), and higher rates of other illicit substance use (Elliott et al. 2012), when compared to family history negative (FHN) youth. Recent findings suggest these negative consequences could be due to underlying neural traits that exist before youth initiate substance use.
Reward circuitry has been implicated in both the initiation and maintenance of substance use problems during adolescence (Berridge et al. 2009; Geier 2013; Nixon and McClain 2010; Spear 2011; Windle et al. 2008). The nucleus accumbens, a key brain region for reward processing (Knutson et al. 2001), has specifically been related to greater reward salience during adolescence (Urošević et al. 2012), and has extensive functional connections to other reward-related regions, including the orbitofrontal cortex (Cauda et al. 2011), which is sensitive to pleasantness and reinforcer devaluation in humans (Gottfried et al. 2003). Recent findings suggest FHP youth have less integration between the nucleus accumbens and other reward-related brain areas, such as the orbitofrontal cortex, which is believed to increase their vulnerability to initiate problematic drinking (Cservenka et al. 2014). Specifically, earlier development of the accumbens relative to orbitofrontal cortex may underlie risk-taking behavior in adolescents (Galvan et al. 2006). Thus, understanding connectivity between reward processing regions like the nucleus accumbens and orbitofrontal cortex is important for identifying preexisting neural vulnerabilities that may contribute to risk for addiction.
One way to examine structural connectivity between reward regions is by using diffusion tensor imaging (DTI) analytic techniques such as probabilistic tractography (Behrens et al. 2003). This fairly new technique has been used to investigate the relative strength of white matter connections among gray matter structures (Forstmann et al. 2012). One measure of white matter integrity derived from DTI is fractional anisotropy (FA), which reflects white matter coherence by indexing the diffusion of water molecules in brain structures (Basser and Pierpaoli 1996; Le Bihan et al. 2001). Additional measures of white matter integrity obtained from DTI data include the following: mean diffusivity (MD), a measure of the overall magnitude of diffusional motion; radial diffusivity (RD), a quantification of the magnitude of diffusion perpendicular to the main fiber axis; and axial diffusivity (AD), the magnitude of diffusion parallel to the fiber axis (Lebel et al. 2012). High FA suggests strong fiber regularity and organization, but values may also reflect myelination and structural characteristics of the axon, while low MD values reflect greater white matter density (Roberts and Schwartz 2007; Schmithorst et al. 2002). Increases in FA and decreases in MD typically occur in white matter during adolescence (Giorgio et al. 2008), which are often associated with decreases in RD. In a recent study by our group, we found that substance-naïve FHP youth had higher FA and AD, and lower MD and RD, than FHN in 19 different white matter tracts throughout the brain, suggesting that FHP youth have more mature neural features than FHN youth (Squeglia et al. 2014). These findings were in contrast to the previously reported neural deficits found in FHP youth (Herting et al. 2010), but consistent with recent findings showing higher FA values in at-risk adolescents (Berns et al. 2009; Li et al. 2010; Sarkar et al. 2013), compared to controls.
Investigating the status of the white matter microstructure in terms of FA and diffusivity measures can show potentially disrupted development of such pathways. However, it is unclear if the reported differences in FA values accompany differences in the strength of the structural connectivity within frontal white matter pathways or whether differences in FA may be due to relative differences in the underlying complexity of white matter architecture between FHP and FHN youth. Thus, this study will examine structural connectivity between reward regions using probabilistic tractography (Behrens et al. 2003), focusing on the relative strength of connections among reward-related gray matter structures (Forstmann et al. 2012).
Therefore, the goal of this study was to expand previous findings from our group (Squeglia et al. 2014), by specifically examining the relationship between reward circuitry in at-risk youth. We were particularly interested in examining differences in brain volume in the nucleus accumbens (Urošević et al. 2012) and orbitofrontal cortex (Berridge et al. 2009; Gottfried et al. 2003), and the structural connectivity between these two regions, by combining cortical and subcortical parcellation procedures (Dale et al. 1999; Fischl and Dale 2000; Fischl et al. 1999; Fischl et al. 2004) with probabilistic tractography (Behrens et al. 2003). Based on the previous literature, we hypothesized that FHP youth would exhibit less structural connectivity between reward regions (i.e., the nucleus accumbens and orbitofrontal cortex; Cservenka et al. 2014), when compared to FHN youth.
Methods
Participants
Participants were 94 healthy 12–14 year-olds (54 % female) recruited through flyers sent to households of students attending San Diego area public middle schools (Squeglia et al. 2013; Squeglia et al. 2009). Participants were the same group described in a previous study (Squeglia et al. 2014), except for one FHN adolescent who was excluded in this study due to abnormal nucleus accumbens parcellations. We also included one additional family history positive youth, so the final sample was comprised of 49 FHP and 45 FHN youth. Extensive screening and background information were obtained from the youth, their biological parent, and one other parent or close relative. The study protocol was executed in accordance with the standards approved by the University of California, San Diego Human Research Protections Program.
Exclusionary criteria included the following: any neurological or DSM-IV (American Psychiatric Association 1994) Axis I disorder, determined by the NIMH Diagnostic Interview Schedule for Children-version 4.0 (Shaffer et al. 2000); any history of head trauma or loss of consciousness (>2 min); history of chronic medical illness; learning disability or mental retardation; use of medications potentially affecting the brain; premature birth (i.e., born prior to 35th gestational week); any suggestion of prenatal alcohol (>2 drinks during a given week) or illicit drug exposure; experience with alcohol or drugs, defined as >2 total days in their life on which drinking had occurred, or >1 drink consumed on an occasion; and any history of other substance use, including marijuana or cigarette use (Squeglia et al. 2012; Squeglia et al. 2009; Wetherill et al. 2013); contraindication to magnetic resonance imaging (MRI; e.g., braces); inadequate comprehension of English; non-correctable sensory problems; and clinically abnormal brain anatomy as determined by neuroradiologist review. The final sample of 94 adolescents were typically in 7th grade, with modal family socioeconomic status in the Hollingshead (Hollingshead 1965) 11–15 range, and had high average estimated IQ and school grades (see Table 1). Of the 94 participants, two had previously had one drink on one occasion, and another had one drink on two separate occasions, leaving this a mostly substance-naïve sample.
Table 1.
Demographic information for 94 alcohol-naïve adolescents
| FH Negative n = 45 Mean (SD) |
FH Positive n = 49 Mean (SD) |
|
|---|---|---|
| Age (range, 12–14) | 13.51 (0.61) |
13.64 (0.70) |
| Gender (% Females) | 62 % | 49 % |
| Race (% Caucasian)a | 78 % | 64 % |
| FHAM diagnoses (≥2 AUD criteria endorsed) | ||
| Biological parents | n = 0 | n = 11 |
| Aunts/uncles | n = 0 | n = 26 |
| Grandparents | n = 0 | n = 27 |
| Family history density score* | 0.00 (0.00) | 0.34 (0.39) |
| Lifetime alcohol use occasions (range, 0–2 days) | 0.00 (0.00) | 0.08 (0.34) |
| Hollingshead Index of Social Position scoreb | 20.71 (11.76) |
25.51 (15.46) |
| Years of education | 6.82 (0.77) | 7.02 (0.77) |
| Pubertal Development Scale—girls | 2.97 (0.62) | 3.24 (0.62) |
| Pubertal Development Scale—boys | 2.13 (0.45) | 2.28 (0.57) |
| Grade point average | 3.62 (0.47) | 3.54 (0.58) |
| WASI-IV Vocabulary T-score | 57.66 (8.18) |
57.90 (7.78) |
| Conduct Disorder Questionnaire total # problems endorsed |
0.51 (1.08) | 1.08 (1.77) |
| Child Behavior Checklist externalizing symptoms T-score | 39.36 (6.60) |
41.25 (7.67) |
| Behavioral Inhibition System (BIS) total score | 18.73 (2.30) |
18.16 (2.28) |
| Behavioral Activation System (BAS) subscales | ||
| BAS-Drive | 9.64 (2.48) | 10.08 (2.15) |
| BAS-Fun Seeking | 12.05 (2.15) |
11.71 (2.50) |
| BAS-Reward Responsiveness | 15.05 (1.66) |
14.49 (1.73) |
FHAM family history assessment module, AUD alcohol use disorder
p < 0.01
For the full sample, race was as follows: 71 % Caucasian, 19 % multi-racial, 5 % Asian, 2 % Black, and 2 % Hawaiian/Pacific Islander. No significant differences between groups
Higher scores indicate lower socioeconomic status
Measures
Family history
The Family History Assessment Module (FHAM; Rice et al. 1995) ascertained familial alcohol use disorders (AUD) in first- and second-degree relatives, as self-reports have been found to be a reliable way to determine familial alcohol or substance use (Andreasen et al. 1986), and are valid predictors of alcohol use vulnerability and future dependence (Stoltenberg et al. 1988). Family history information was collected from the youth, one biological parent, and the other parent or (in <7 % of cases) another close relative. Informants were asked if any of the youth’s parents, aunts, uncles, and/or grandparents ever had any problems due to alcohol, such as social, academic, or occupational problems; alcohol-related arrests; negative health consequences; previous treatment for alcohol-related disorders; or if they were frequently intoxicated. Using these criteria to diagnosis FHP is more conservative than other classification schemes, valuing specificity (98 %) over sensitivity (39 %), resulting in less false positives of AUD diagnoses (Rice et al. 1995). Informants’ responses were compiled, and if any of the relatives endorsed 2 or more of the criteria, the youth was classified as family history positive for an alcohol use disorder. FHP youth (n = 49) had one or more first- or second-degree relatives with a history of alcohol use disorder, and FHN youth (n = 45) had no alcohol use disorder in any first- or second-degree relative. Additionally, family history density scores were calculated by adding 0.5 for each biological parent and 0.25 per biological grandparent (Zucker et al. 1994) endorsed by either youth or parent as having AUD. FH density scores ranged from 0 to 1.75 in the current sample.
Socioeconomic status
Socioeconomic background information (i.e., educational attainment, occupation, and salary of each parent) was obtained from parents and converted to a Hollingshead Index of Social Position score (Hollingshead 1965).
Psychopathology and personality
The Conduct Disorder Questionnaire (Brown et al. 1996) was administered to provide a continuous measure of conduct disorder behaviors based on DSM-IV criteria (American Psychiatric Association 1994) and the Child Behavior Checklist (Achenbach and Rescorla 2001) provided a parent report on level of adolescent psychopathological syndromes. The Behavioral Inhibition System/Behavioral Avoidance System (BIS/BAS) scale was given to assess sensitivity in appetitive and inhibitory systems (Carver and White 1994). The BIS scale measured responsiveness to punishment and tendencies to inhibit behavior that may result in undesirable consequences, while the BAS scale measured sensitivity to signals of reward or non-punishment, and consisted of three separate subscales including drive (BAS-D), reward responsiveness (BAS-RR), and fun seeking (BAS-FS).
Pubertal development
The Pubertal Development Scale (Petersen et al. 1988) is a reliable and valid 5-item self-report measure of pubertal maturation.
MRI acquisition
Participants were imaged in a 3T General Electric Excite MR system with an 8-channel phase-array head coil (General Electric Medical System, Milwaukee, WI, USA). A scout scan ensured good head placement and whole-brain coverage. DTI data were collected along 61 non-collinear directions determined by the electrostatic repulsion model which minimizes bias in measurements by sampling with approximately uniform distribution on a sphere (Jones et al. 1999), in addition to a reference image with no diffusion weighting (b = 0). The diffusion encoding scheme consisted of a single-shot dual spin echo excitation optimized for minimum TE and reduction of eddy current artifacts (Reese et al. 2003). The following sequence parameters were applied: TE/TR = 93/10,900 ms, FOV = 240 mm, matrix = 128 × 128, 34 contiguous slices, 3-mm slice thickness, b-value = 1500 s/mm2, one average. Two field maps were collected for unwarping to correct for signal loss and geometric distortion due to B0 field inhomogeneities (Andersson and Skare 2002; Jezzard and Balaban 1995). Total DTI scan time including field maps was 16 min and 2 s.
Structural image processing
FreeSurfer (version 5.0, surfer.nmr.mgh.harvard.edu) utilizes a series of automated imaging algorithms to produce measures of cortical volume (Dale et al. 1999; Fischl and Dale 2000; Fischl et al. 1999; Fischl et al. 2004). Following inspection, an automated parcellation procedure divided each hemisphere into independent cortical and subcortical regions based on gyral and sulcal features (Desikan et al. 2006; Fischl et al. 2004). Volume estimates from the orbitofrontal cortex (3 Desikan regions combined: lateral and medial orbitofrontal cortex and frontal pole) and the nucleus accumbens were extracted for analyses.
DTI data processing
Datasets were visually inspected slice-by-slice for each subject, and all valid datasets were corrected for head motion, eddy current distortion, and signal loss using FSL tools (FMRIB Software Library, Oxford, United Kingdom; Smith et al. 2004). Specifically, image acquisitions for each direction were merged into a single 4D file and aligned to the first volume using affine registration with six degrees of freedom and Fourier interpolation to correct for motion (FLIRT-FMRIB’s Linear Image Registration Tool; Jenkinson et al. 2002). Each of the 61 direction files was then registered to the B0 image using a six-parameter registration in 2D to minimize eddy current distortions (FDT-FMRIB’s Diffusion Toolbox 2.0; Behrens et al. 2003). Next, phase unwrapping (PRELUDE-Phase Region Expanding Labeler for Unwrapping Discrete Estimates; Jenkinson 2003) and regularization (FUGUE-FMRIB’s Utility for Geometrically Unwarping EPIs; Jenkinson and Smith 2001) of field maps were conducted for quantifying field distortions. Resulting measurements were translated into voxel shifts, effectively assigning image intensities to correct voxel locations. FDT tools (Behrens et al. 2003) were then used to calculate FA, MD, and eigenvalues for the three ellipsoid axis by solving the diffusion tensor at each voxel for each participant’s 4D file. AD was defined as the first (i.e., greatest eigenvalue). RD was defined as the mean of the remaining two eigenvalues (Song et al. 2002).
Probabilistic tractography was performed in each participant’s diffusion space and was used to define pathways coursing between the nucleus accumbens and the orbitofrontal cortex using a robust dual-fiber model (Behrens et al. 2007). Prior to tractography, each 4D file was processed via bedpostx in FDT (Behrens et al. 2007). For tractography, at each seed point voxel, 5000 streamlines were sent. A track curvature threshold was set for 0.20 (cosine of the minimum allowable angle) which prohibited angles of greater than 80° between steps during tracking. FLIRT was used to create a registration matrix to align the FreeSurfer output (i.e., the nucleus accumbens and defined orbitofrontal cortex) to diffusion space for each participant. Each registration was visually inspected for accuracy. Next, the voxels comprising the nucleus accumbens were set as the probabilistic tractography seed points with the orbitofrontal cortex set as the waypoint, termination point, and classification target. The resultant tracks were then normalized based upon the total number of samples sent from the seed region of interest which varied per participant (i.e., track values were divided by 5000 times the number of nucleus accumbens voxels). To remove spurious connections, the tracks were then thresholded such that only those voxels with at least 0.1 % of the total sent streamlines were retained (Johansen-Berg et al. 2007). To reduce potential partial voluming effects, extraction of mean FA, MD, RD, and AD values were restricted to only voxels comprised of white matter as defined by FSL FAST segmentation output, which was registered from T1 anatomical space to DTI space using the same transformation matrix. A measure of the fiber orientation uncertainty (i.e., dispersion of the principal diffusion direction, PDD) which is calculated via the bedpostx process was also extracted (Behrens et al. 2007). This index can be used to infer whether or not changes in DTI scalars (i.e., FA), may be related to the presence or absence of additional fiber pathways within a voxel (Douaud et al. 2009). Higher dispersion values indicate a greater number of crossing fibers within a voxel, while lower numbers may reflect fewer crossing fibers.
Finally, the percentage of streamlines sent from the nucleus accumbens which then reached the orbitofrontal cortex were calculated for each subject at each hemisphere and were used as a proxy index of the strength of the structural connectivity between these two regions (Forstmann et al. 2012). All resultant tracks were inspected for accuracy by two raters (LMS and SFS) and all were deemed acceptable for the purposes of this study (i.e., each pathway was identified as appropriately coursing between the nucleus accumbens and orbitofrontal cortex and not other brain regions).
Data analyses
FHP and FHN groups were compared on demographic variables using a two-tailed Student’s t test or chi-square test in SPSS (Rel. 18.0.0. 2009. IBM, Chicago, IL). Univariate analysis compared groups on nucleus accumbens, orbitofrontal, and white matter tract volume, controlling for intracranial volume. Variations in head size were statistically controlled for by including intracranial volume (ICV) as a covariate when performing volumetric comparisons between groups. The relationship between regional volumes and ICV did not differ between groups. A proportion of ICV method was considered, but was not used since that method failed to remove all associations between region of interest volume and ICV [e.g., right orbitofrontal cortex as a proportion of ICV (r = −0.32, p < 0.01) and left nucleus accumbens as a proportion of ICV (r = −0.21, p < 0.05)]. Relative volume of reward and orbitofrontal systems (i.e., nucleus accumbens volume divided by orbitofrontal volume) was also examined between groups using univariate analysis, controlling for ICV, as differences in the nonlinear development of these two neural systems may leave adolescents distinctly vulnerable to risk taking behaviors and emotional reactivity (Galvan et al. 2007; Galvan et al. 2006; Steinberg 2004). Multivariate analysis of variance (MANOVA) examined group differences in probabilistic tractography findings (i.e., percentage of streamlines from nucleus accumbens to orbitofrontal cortex), white matter indices (i.e., FA, MD, RD, and AD values), and PDD dispersion. FA, MD, RD, and AD findings were reported for whole-brain analyses in our previous paper (Squeglia et al. 2014); these indices are examined in this study, but only for the frontostriatal white matter tract identified by the probabilistic tractography processing. To investigate possible brain-behavior relationships, bivariate Pearson correlations were run between brain structural indices that differed between groups and behavioral correlates (i.e., Conduct Disorder Questionnaire total number of problems, Child Behavior Checklist externalizing and internalizing t-scores, and BIS/BAS subscales).
Results
Demographics
FHP (n = 49) and FHN (n = 45) youth were well-matched at the group level on age, gender, race, lifetime alcohol use, socioeconomic status, years of education, pubertal development, academic achievement, externalizing symptoms, verbal intelligence, conduct disorder symptoms, and BIS/BAS scales (see Table 1). As expected, FHP youth had higher family history density scores than FHN youth. Groups did not differ significantly on any other demographic variable, or any item on the PDS.
Nucleus accumbens and orbitofrontal volume
No differences between FHP and FHN youth were observed in the left (p = 0.77) or right (p = 0.44) nucleus accumbens or orbitofrontal cortex volumes (ps > 0.32), controlling for intracranial volume (see Table 2 and Fig. 1). Relative volume of nucleus accumbens and orbitofrontal cortex (i.e., nucleus accumbens volume divided by orbitofrontal volume) did not differ between groups (ps > 0.34). Relative volume of nucleus accumbens to the separate subsections of the orbitofrontal cortex (lateral and medial orbitofrontal cortex and frontal pole) did not differ between groups either (ps > 0.26).
Table 2.
Volume and white matter data from 94 alcohol-naïve adolescents
| FH Negative n = 45 Mean (SD) |
FH Positive n = 49 Mean (SD) |
p value |
|
|---|---|---|---|
| Gray matter volume (mm3)a | |||
| L NAcc | 765.63 (139.11) | 802.06 (120.94) | 0.77 |
| R NAcc | 794.77 (147.66) | 819.38 (108.28) | 0.44 |
| L lateral OFC | 9288.29 (1280.92) | 9561.80 (899.24) | 0.36 |
| L medial OFC | 6283.16 (931.59) | 6608.22 (918.77) | 0.85 |
| L frontal pole | 1077.67 (167.26) | 1068.63 (191.67) | 0.32 |
| R lateral OFC | 9406.40 (1140.48) | 9740.57 (975.37) | 0.78 |
| R medial OFC | 6344.27 (828.38) | 6529.88 (719.56) | 0.40 |
| R frontal pole | 1412.38 (221.53) | 1492.51 (216.77) | 0.48 |
| White matter tract volume (mm3)a | |||
| L NAcc to OFC white matter volume |
3532.54 (883.11) | 3705.18 (939.70) | 0.79 |
| R NAcc to OFC white matter volume |
3965.45 (878.61) | 4411.01 (788.76) | 0.14 |
| ProbtrackX findings | |||
| %age streamlines from L NAcc to OFC |
0.46 (0.15) | 0.48 (0.11) | 0.59 |
| %age streamlines from R NAcc to OFC |
0.42 (0.12) | 0.38 (0.10) | 0.23 |
| White matter integrity indices in NAcc-OFC tract | |||
| L FA | 0.35 (0.03) | 0.36 (0.03) | 0.07 |
| R FA | 0.36 (0.03) | 0.36 (0.02) | 0.10 |
| L MD | 7.75 × 10−4 (2.29 × 10−5) |
7.62 × 10−4 (2.67 × 10−5) |
0.01 |
| R MD | 7.72 × 10−4 (2.15 × 10−5) |
7.62 × 10−4 (2.35 × 10−5) |
0.03 |
| L RD | 6.21 × 10−4 (2.58 × 10−5) |
6.06 × 10−4 (3.18 × 10−5) |
0.01 |
| R RD | 6.17 × 10−4 (2.47 × 10−5) |
6.05 × 10−4 (3.18 × 10−5) |
0.02 |
| L AD | 1.08 × 10−3 (3.60 × 10−5) |
1.07 × 10−3 (3.04 × 10−5) |
0.16 |
| R AD | 1.08 × 10−3 (3.18 × 10−5) |
1.07 × 10−3 (3.22 × 10−5) |
0.23 |
| Principal diffusion direction dispersion | |||
| L PDD | 0.03 (0.01) | 0.03 (0.01) | 0.27 |
| R PDD | 0.03 (0.01) | 0.03 (0.01) | 0.43 |
Lateral OFC, medial OFC, and frontal pole were combined for ProbtrackX analyses to comprise the “orbitofrontal pole”. Separate Desikan atlas defined regions are presented in this table.
L left, R right, NAcc nucleus accumbens, OFC orbitofrontal cortex, FA fractional anisotropy, MD mean diffusivity, RD radial diffusivity, AD axial diffusivity, PDD principal diffusion direction
Controlled for total intracranial volume in group analyses
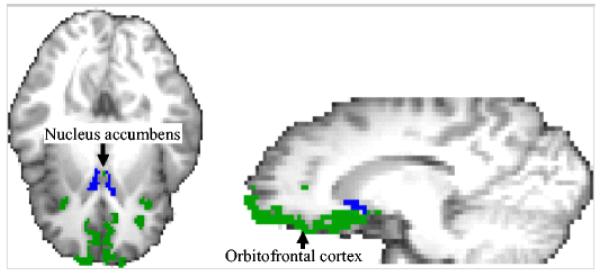
Fig. 1.
Example from one participant showing the FreeSurfer derived parcellations for the nucleus accumbens (blue) and orbitofrontal cortex (green). Each participant’s nucleus accumbens and orbitofrontal cortex was derived individually. ProbtrackX used the nucleus accumbens as the seed region and the orbitofrontal cortex as the target region to determine the percentage of streamlines that connected the two regions
White matter tract volume
There were no differences between FHP and FHN youth in the left or right white matter tract volumes connecting the nucleus accumbens and orbitofrontal cortex, after controlling for intracranial volume (ps > 0.79). See Table 2.
Percentage of streamlines from the nucleus accumbens to the orbitofrontal cortex
No group differences were observed between FHP and FHN youth in the percentage of streamlines coursing between the nucleus accumbens and the orbitofrontal cortex in the left or right hemisphere (ps > 0.23), see Fig. 2 and Table 2.
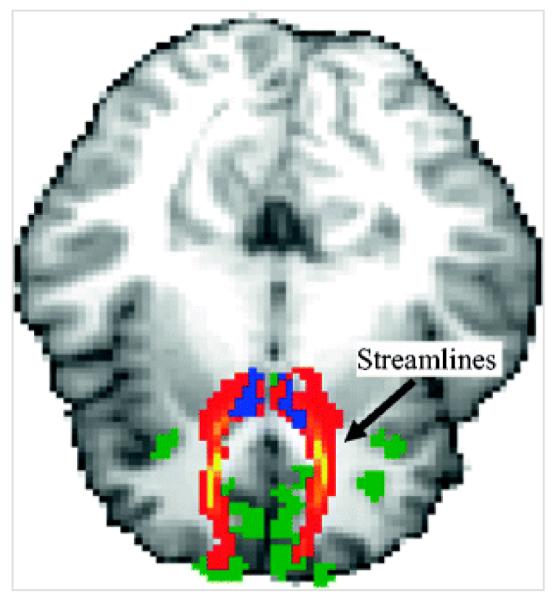
Fig. 2.
Example of one participant’s ProbtrackX results. Areas in yellow-red indicate the streamlines extending from the nucleus accumbens (blue) to the orbitofrontal cortex (green). These results were derived individually for each participant
White matter integrity indices
FHP youth had significantly lower MD and RD (ps < 0.03) than FHN youth in both the left and right white matter tracts connecting the nucleus accumbens and the orbitofrontal cortex. There was a trend (ps < 0.10) for FHP youth having higher FA than FHN youth in left and right white matter tracts connecting the nucleus accumbens and orbitofrontal cortex, see Table 2.
Crossing fibers
There were no group differences in the left or right PDD dispersion values (ps > 0.27), see Table 2.
Behavioral correlates
There were no significant correlations between white matter indices that differed between groups (i.e., MD and RD) and behavioral correlates (i.e., Conduct Disorder Questionnaire total number of problems, Child Behavior Checklist externalizing and internalizing t-scores, and BIS/BAS subscales).
Discussion
This study combined cortical and subcortical parcellation procedures with probabilistic tractography (Behrens et al. 2003) to probe salience and reward systems in substance naïve FHP and FHN youth. Findings suggest that the pathways connecting the nucleus accumbens and the orbitofrontal cortex are the same between FHP and FHN youth in terms of volume, track strength, and architectural complexity. Thus, the foundations of the cortico-subcortical reward circuitry network do not differ by FHP status. However, the microstructure of the pathways within this network showed irregularities within the FHP group relative to those with negative family histories, which could potentially affect behavior and relate to normal adolescent white matter development (Bava et al. 2010; Yap et al. 2013).
The finding that FHP youth had lower RD and MD (i.e., generally considered better white matter integrity) than FHN youth is consistent with our previous findings (Squeglia et al. 2014), which was expected considering the highly overlapping samples. However, the current study was focused specifically on white matter tracts involved in reward circuitry, extending our previous findings that examined whole-brain differences unrestricted to specific tracts of interest.
Improper functional connectivity has been found in FHP youth between the nucleus accumbens and orbitofrontal cortex (Cservenka et al. 2014). The authors suggest that irregular reward valuation due to aberrant connectivity between these regions could increase risky behaviors in FHP youth. Our findings suggest that while there are no macrostructural differences between the nucleus accumbens and orbitofrontal cortex underlying connectivity irregularities, differences in white matter microstructure may help explain observed aberrant functional connectivity in FHP youth (Cservenka et al. 2014).
Consistent with these findings, a growing number of studies have shown greater white matter integrity in frontal white matter tracts in at-risk adolescents when compared to controls. Specifically, higher FA values have been observed in youth who meet the criteria for ADHD (Li et al. 2010) and conduct disorder (Sarkar et al. 2013), as well as teens who engage in more dangerous and risky behaviors (Berns et al. 2009), when compared to demographically matched controls. Similar to FHP youth, these youth are also at risk for developing substance use disorders (Charach et al. 2011; Lee et al. 2011). Therefore, increases in white matter coherence and organization observed during adolescence (Bava et al. 2010; Yap et al. 2013) may begin earlier in FHP youth, before behavioral changes are apparent (as evidenced by the lack of findings between our white matter indices and behavioral correlates), and could be related to a range of risk-taking behaviors. This accelerated maturation may be viewed as advantageous, as it could be related to earlier autonomy, behavioral exploration, and prosocial behaviors. However, this could also be viewed as “vulnerability” for youth, increasing their likelihood of engaging in sensation-seeking behaviors at an earlier age. Neurodevelopmentally precocious youth may have a tendency to initiate and escalate risk-taking behaviors when compared to their peers, which could lead to either positive or negative risk-taking, depending on other environmental and peer influences.
Despite alcohol-naïve FHP youth showing greater white matter coherence, this advantage or possible vulnerability, appears to attenuate after youth initiate heavy substance use during adolescence. Specifically, previous studies have consistently found that heavy substance-using teens show decreasing FA compared to their non-using counterparts after alcohol and marijuana initiation (Bava et al. 2013; Jacobus et al. 2013a; Jacobus et al. 2013b; McQueeny et al. 2009), suggesting these higher FA levels are not maintained once heavy substance use begins. These findings are consistent with fMRI studies that show more mature neural response patterns appear to be a risk factor for future initiation of substance use, with advantages decreasing after alcohol use initiation (Squeglia et al. 2012; Wetherill et al. 2013). Positive family history of alcohol dependence has been shown to interact with substance use, predicting worse language and attentional functioning (Tapert and Brown 2000), as well as neural abnormalities (Hardee et al. 2014; Tapert et al. 2003). Importantly, these alcohol-related aberrations in brain structure and function are during a time when healthy non-using adolescents tend to show increasing white matter coherence and more mature neural processing (Giorgio et al. 2008; Lebel et al. 2012; Schmithorst and Yuan 2010; Stiles and Jernigan 2010; Tamnes et al. 2010). In sum, more mature neural markers may predate substance use; however, once substance use is initiated, white matter tracts needed for neurocognitive functioning such as cognitive control, may degrade. Therefore, differential processes and mechanisms may be involved in substance use initiation and maintenance.
Despite previous research suggesting FHP youth have smaller volumes in frontal and subcortical regions than FHN youth (Benegal et al. 2007; Hill et al. 2001; Hill et al. 2007), these findings were not replicated in our sample. Youth in previous studies had higher family history loadings for alcohol use disorders than youth reported in this study, which may account for the discrepant findings. It is possible that gross brain measures such as volume could mask subtle differences in brain integrity between at-risk youth, particularly within high-functioning adolescents. Utilizing sensitive techniques such as probabilistic tractography have a number of advantages, including its ability to empirically define specific fiber tracts, as well as its sensitivity to crossing fibers, resulting in greater accuracy of resultant fiber pathways. These techniques could be important in examining other hypotheses related to risk and resilience attributed to underlying brain circuitry.
In summary, FHP youth showed similar structure in brain reward regions than FHN youth. However, within these structures, white matter integrity may be more progressed in FHP youth before they ever start using alcohol or other drugs. The methodology used in this study could be of interest to other neuroimaging researchers using multimodal imaging to understand brain circuitry issues. Future longitudinal studies should prospectively link individual differences in white matter coherence to actual future substance use behaviors and examine how family history status affects brain development of circuitry within reward regions after substance use is initiated.
Acknowledgments
Special thanks to the Adolescent Brain Imaging Project lab and the participating schools in the San Diego Unified School District and their families.
Financial support Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Numbers: R01 AA13419 (PI: Tapert), U01 AA021692 (PI: Tapert), F32 AA021610 (PI: Squeglia), T32 AA013525 (Brumback), and the National Institute on Drug Abuse: F32 DA032188 (PI: Jacobus). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding support R01 AA13419, U01 AA021692 (PI: Tapert), F32 AA021610 (PI: Squeglia), F32 DA032188 (PI: Jacobus), and T32 AA013525 (Brumback).
Footnotes
Conflicts of interest None.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edition. American Psychiatric Association; Washington, DC: 1994. (DSM-IV) [Google Scholar]
- Andersson JL, Skare S. A model-based method for retrospective correction of geometric distortions in diffusion-weighted EPI. Neuroimage. 2002;16:177–199. doi: 10.1006/nimg.2001.1039. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Rice J, Endicott J, Reich T, Coryell W. The family history approach to diagnosis. How useful is it?. Arch Gen Psychiatry. 1986;43:421–429. doi: 10.1001/archpsyc.1986.01800050019002. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol Clin Exp Res. 2013;37:E181–E189. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Berns GS, Moore S, Capra CM. Adolescent engagement in dangerous behaviors is associated with increased white matter maturity of frontal cortex. PLoS One. 2009;4:e6773. doi: 10.1371/journal.pone.0006773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Gleghorn A, Schuckit MA, Myers MG, Mott MA. Conduct disorder among adolescent alcohol and drug abusers. J Stud Alcohol. 1996;57:314–324. doi: 10.15288/jsa.1996.57.314. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- Cauda F, Cavanna AE, D’agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 2011;23:2864–2877. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: Comparative meta-anaylses. J Am Acad Child Adolesc Psychiatry. 2011;50:9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Casimo K, Fair DA, Nagel BJ. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res. 2014;221:210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Douaud G, Behrens TE, Poupon C, Cointepas Y, Jbabdi S, Gaura V, Golestani N, Krystkowiak P, Verny C, Damier P, Bachoud-Lévi AC, Hantraye P, Remy P. In vivo evidence for the selective subcortical degeneration in Huntington’s disease. Neuroimage. 2009;46:958–966. doi: 10.1016/j.neuroimage.2009.03.044. [DOI] [PubMed] [Google Scholar]
- Elliott JC, Carey KB, Bonafide KE. Does family history of alcohol problems influence college and university drinking or substance use? A meta-analytical review. Addiction. 2012;107:1774–1785. doi: 10.1111/j.1360-0443.2012.03903.x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Keuken MC, Jahfari S, Bazin PL, Neumann J, Schäfer A, Anwander A, Turner R. Cortico-subthalamic white matter tract strength predicts interindividual efficacy in stopping a motor response. Neuroimage. 2012;60:370–375. doi: 10.1016/j.neuroimage.2011.12.044. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Dev Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Geier CF. Adolescent cognitive control and reward processing: implications for risk taking and substance use. Horm Behav. 2013;64:333–342. doi: 10.1016/j.yhbeh.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Hardee JE, Weiland BJ, Nichols TE, Welsh RC, Soules ME, Steinberg DB, Zubieta JK, Zucker RA, Heitzeg MM. Development of impulse control circuitry in children of alcoholics. Biol Psychiatry. 2014;76:708–716. doi: 10.1016/j.biopsych.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol Clin Exp Res. 2010;34:1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Yuan H. Familial density of alcoholism and onset of adolescent drinking. J Stud Alcohol. 1999;60:7–17. doi: 10.15288/jsa.1999.60.7. [DOI] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2007;61:41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. Yale University Press; New Haven: 1965. [Google Scholar]
- Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without cooccurring marijuana use: a 3-year investigation. Psychiatry Res. 2013a;214:374–381. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Thayer RE, Trim RS, Bava S, Frank LR, Tapert SF. White matter integrity, substance use, and risk taking in adolescence. Psychol Addict Behav. 2013b;27:431–442. doi: 10.1037/a0028235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007;36:T16–T21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42:515–525. [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sun J, Guo L, Zang Y, Feng Z, Huang X, Yang H, Lv Y, Huang M, Gong Q. Increased fractional anisotropy in white matter of the right frontal region in children with attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. Neuroendocrinol Lett. 2010;31:747–753. [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res. 2001;25:1166–1173. [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberger S, Faraone SV, Biederman J, Chu MP, Feighner JA. Substance use disorders in high-risk adolescent offspring. Am J Addict. 1999;8:211–219. doi: 10.1080/105504999305820. [DOI] [PubMed] [Google Scholar]
- Nixon K, McClain JA. Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Curr Opin Psychiatry. 2010;23:227–232. doi: 10.1097/YCO.0b013e32833864fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17 doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JIJ, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Roberts TP, Schwartz ES. Principles and implementation of diffusion-weighted and diffusion tensor imaging. Pediatr Radiol. 2007;37:739–748. doi: 10.1007/s00247-007-0516-z. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Craig MC, Catani M, Dell’acqua F, Fahy T, Deeley Q, Murphy DG. Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: a diffusion tensor imaging study. Psychol Med. 2013;43:401–411. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1:392–400. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. J Stud Alcohol Drugs. 2012;73:749–760. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, McKenna BS, Jacobus J, Castro N, Sorg SF, Tapert SF. BOLD response to working memory not related to cortical thickness during early adolescence. Brain Res. 2013;1537:59–68. doi: 10.1016/j.brainres.2013.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Brumback T, Meloy MJ, Tapert SF. White matter integrity in alcohol-naïve youth with a family history of alcohol use disorders. Psychol Med. 2014;44:2775–2786. doi: 10.1017/S0033291714000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann N Y Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1988;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95:1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Urošević S, Collins P, Muetzel R, Lim K, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Dev Psychol. 2012;48:1488–1500. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology (Berl) 2013;230:663–671. doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, Smith GT, Giedd J, Dahl RE. Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age. Pediatrics. 2008;121:S273–S289. doi: 10.1542/peds.2007-2243C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap QJ, Teh I, Fusar-Poli P, Sum MY, Kuswanto C, Sim K. Tracking cerebral white matter changes across the lifespan: Insights from diffusion tensor imaging studies. Journal of Neural Transmission. 2013 doi: 10.1007/s00702-013-0971-7. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE. Developmental evidence for at least two alcoholisms: I. Biopyschosocial variation among pathways into symptomatic difficulty. The New York Academy of Sciences; New York: 1994. [DOI] [PubMed] [Google Scholar]