Abstract
Vascular inflammation plays a critical role in the pathogenesis of cerebral aneurysms. PPARγ protects against vascular inflammation and atherosclerosis, whereas dominant-negative mutations in PPARγ promote atherosclerosis and vascular dysfunction. We tested the role of PPARγ in aneurysm formation and rupture. Aneurysms were induced with a combination of systemic infusion of angiotensin-II and local injection of elastase, in: 1) mice that received the PPARγ antagonist GW9662 or the PPARγ agonist pioglitazone, 2) mice carrying dominant-negative PPARγ mutations in endothelial or smooth muscle cells, and 3) mice that received the Cullin inhibitor MLN4924. Incidence of aneurysm formation, rupture, and mortality were quantified. Cerebral arteries were analyzed for expression of Cullin3, Keap1, Nrf2, NQO-1 and inflammatory marker mRNAs. Neither pioglitazone nor GW9662 altered the incidence of aneurysm formation. GW9662 significantly increased the incidence of aneurysm rupture, whereas pioglitazone tended to decrease the incidence of rupture. Dominant-negative endothelial-specific PPARγ did not alter the incidence of aneurysm formation or rupture. In contrast, dominant-negative smooth muscle-specific PPARγ resulted in an increase in aneurysm formation (p<0.05) and rupture (P=0.05). Dominant-negative smooth muscle-specific PPARγ, but not dominant-negative endothelial-specific PPARγ, resulted in significant decreases in expression of genes encoding Cullin3, Keap1, and Nrf2, along with significant increases in TNF-α, MCP-1, Cxcl1, CD68, MMP-3, -9, and -13. MLN4924 did not alter incidence of aneurysm formation, but increased the incidence of rupture (p<0.05). In summary, endogenous PPARγ, specifically smooth muscle PPARγ, plays an important role in protecting from formation and rupture of experimental cerebral aneurysms in mice.
Keywords: Aneurysm, Cullin3, Inflammation, Peroxisome proliferator-activated receptor γ (PPARγ), Pioglitazone, Smooth muscle cells, Subarachnoid hemorrhage
Introduction
Subarachnoid hemorrhage (SAH) from intracranial aneurysm rupture results in significant morbidity and mortality.1,2 Microsurgical or endovascular interventions are among the therapeutic options for intracranial aneurysms, but may also result in severe complications. As such, observation without surgical intervention is a reasonable option for patients with cerebral aneurysms that are associated with a low risk of rupture or a high risk of treatment-induced morbidity. A significant number of these patients will still require intervention for aneurysm progression or hemorrhage.2,3 Currently, there are no medical therapies to stabilize aneurysm progression or rupture. Developing such therapies could be a beneficial treatment option for a large number of these patients.
Local vascular inflammation is thought to be a critical element of intracranial aneurysm formation and rupture.2 The ligand-activated nuclear hormone receptor PPARγ protects against vascular inflammation and atherosclerosis.4-6 Both endothelial and smooth muscle PPARγ have been shown to play important roles in the cerebral vasculature.5,7,8 The effect of pharmacological or genetic manipulation of PPARγ on intracranial aneurysm formation and rupture in mice has not previously been assessed. Although PPARγ is expressed in key cells involved in aneurysm pathogenesis [endothelial cells, smooth muscle cells (SMC), and macrophages], the cell-specific contributions of PPARγ in cerebral aneurysm formation and rupture remain unclear. The hypotheses of this study are that in mice: 1) endogenous PPARγ plays a critical role in the formation and rupture of cerebral aneurysms, and 2) that endothelial or SMC expression of dominant negative mutations in PPARγ increases cerebral aneurysm formation and rupture.
Methods
Genotyping, pharmacological protocols, measurement of blood pressure, and gene expression analysis are provided in the expanded methods section available in the online-only data supplement.
Transgenic mouse models
Generation and characterization of transgenic mice expressing dominant negative PPARγ specifically in endothelium (E-V290M) or SMC (S-P467L) was described previously.4,5 Care of the mice used in the experiments met the standards set forth by the National Institutes of Health (NIH) guidelines for the care and use of experimental animals. All procedures were approved by the University of Iowa Institutional Animal Care and Use Committee.
Intracranial aneurysm induction
Cerebral aneurysms were induced using previously published methods as described in detail.9-12 In brief, mice were anesthetized with ketamine (87.5 mg/kg) /xylazine (12.5 mg/kg) and a longitudinal incision was made in the scalp. A 1 mm hole was drilled in the skull and a stereotactic injection of elastase (20 mU in 2.5 μl) was performed using the following coordinates: 2.7 mm posterior to the bregma, 1 mm to the right of the midline, depth of 6.3 mm from the skull. In the pioglitazone group and its control, we used elastase at a higher concentration of 35 mU in 2.5 μl. This approach was used because we anticipated that pioglitazone would exert protective effects in the model. Immediately after injection of elastase, an osmotic mini-pump that delivered a pressor dose of Ang-II (1000 ng/kg/min) for three weeks was implanted subcutaneously.
Tissue collection and aneurysm analysis
Immediately after euthanasia, the chest and abdomen of each mouse was exposed and examined for major bleeding or aneurysms of the aorta. Mice that were found to have aortic aneurysms were not included in the analysis for survival or incidence of cerebral aneurysms and SAH (see Supplemental Table S1). Mice were perfused transcardially at physiological pressures with 10-15 ml of ice-cold physiologic saline solution containing papaverine (100 μM) to produce systemic vasodilation, followed by infusion of 2 mg/ml of bromophenol blue dye and 8% in gelatin saline to facilitate visualization of arteries and small vessels. The brain was then dissected and inspected for the presence of intracranial aneurysms and/or subarachnoid hemorrhages.
Aneurysms were defined as a localized out pouch arising from any cerebral arteries whose diameter was ≥ 1.5 times the parent artery diameter by two independent observers blinded to the animal cohort (Figure S1 A-D).10-12 Animal cohorts were not revealed until all experimental groups had been sacrificed. A survival curve was made according to the time of euthanasia or death. Due to the very small size of the aneurysms formed during these experiments and the small number of mice available to perform dissections (mice that survived), we were unable to dissect aneurysms and perform immunostaining.
Statistical analysis
Analysis was performed using Sigma Plot 12.5 (Systat Software, Inc.) and Prism 6 (Graphpad, La Jolla, CA). Categorical data (incidence of aneurysms and subarachnoid hemorrhage) was compared with two-tailed Fisher’s exact test. Kaplan Meier survival analysis was carried out with comparison between cohorts with the log rank (Mantel-Cox) test. Blood pressure and gene expression data were analyzed with one-way ANOVA followed by Tukey post-hoc test. A P-value less than 0.05 was considered significant.
Results
Role of PPARγ activation and inhibition
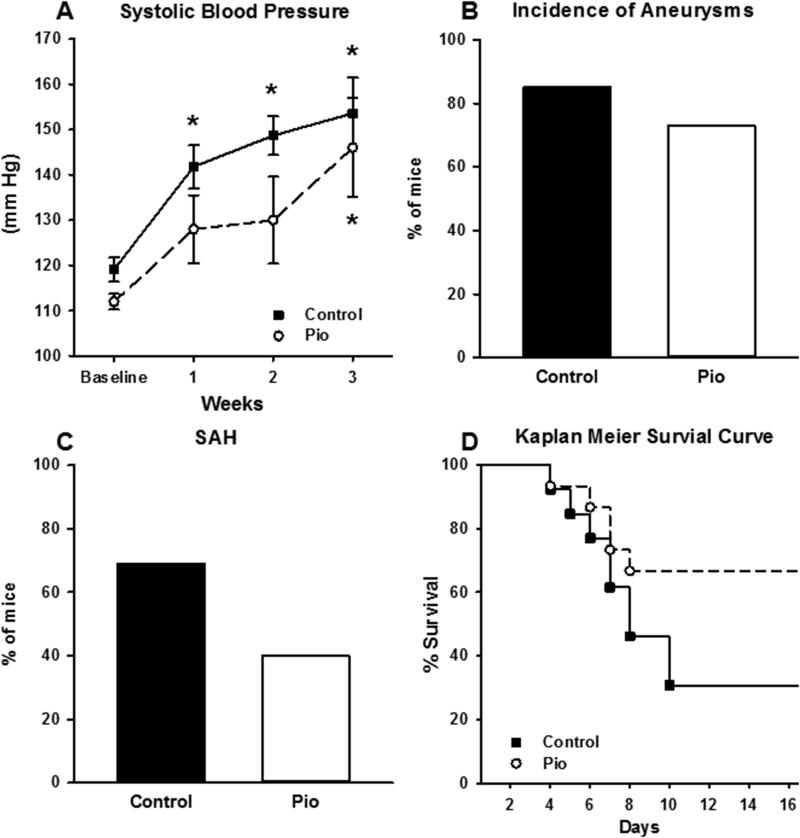
To test whether PPARγ activation by thiazolidinedione treatment (in this case pioglitazone) would decrease the incidence of cerebral aneurysm formation and/or rupture, and to test whether global inhibition of endogenous PPARγ using GW9662 would increase cerebral aneurysm formation and/or rupture, cerebral aneurysms were induced in C57BL/6 mice (pioglitazone: 15 experimental vs. 13 control mice; GW9662: 8 experimental mice vs. 12 control mice). By the end of the treatment, Ang-II increased blood pressure in all groups. There was a trend for pioglitazone treatment to reduce arterial pressure when compared to controls, particularly 2 weeks after elastase injection. This effect was sustained until 21 days. In the control group, the increase in blood pressure was significant at weeks 1-3 when compared to baseline. In the pioglitazone group, there was an increase in blood pressure but this change was not significant at weeks 1 and 2 when compared to baseline. At week three of Ang-II infusion, the increase in blood pressure was significant when compared to baseline.(Figure 1A). The incidence of cerebral aneurysm formation was similar in both the pioglitazone group and its control (p=0.7, Figure 1B). However, there was a trend for decreased SAH in the pioglitazone group (p=0.15, Figure 1C). No significant difference was noted in Kaplan Meier analysis when comparing pioglitazone to its control (p=0.09, Figure 1D).
Figure 1. Effect of PPARγ Activation.
A) Mice treated with pioglitazone and their controls had significant increases in systolic blood pressure. In the control group, the increase in blood pressure was significant at week 1, 2 and 3 when compared to baseline. In the pioglitazone group, there was an increase in blood pressure that was not significant when at week 1 and 2 when compared to baseline. However, at week 3, the increase in blood pressure was significant when compared to baseline. B) The incidence of aneurysm formation in both C57BL/6 control group and pioglitazone group was not significantly different. C) The incidence of aneurysm rupture and SAH in C57BL/6 control group and pioglitazone group. Pioglitazone had a tendency to decrease the incidence of aneurysm rupture, but this was not statistically significant. D) Kaplan Meier analysis of the survival in the pioglitazone group and its control. No significant difference was noted in Kaplan Meier analysis when comparing pioglitazone to its control (Log rank p=0.09). Pioglitazone, n=13; Control, n=15 for A-D. (* is P value <0.05).
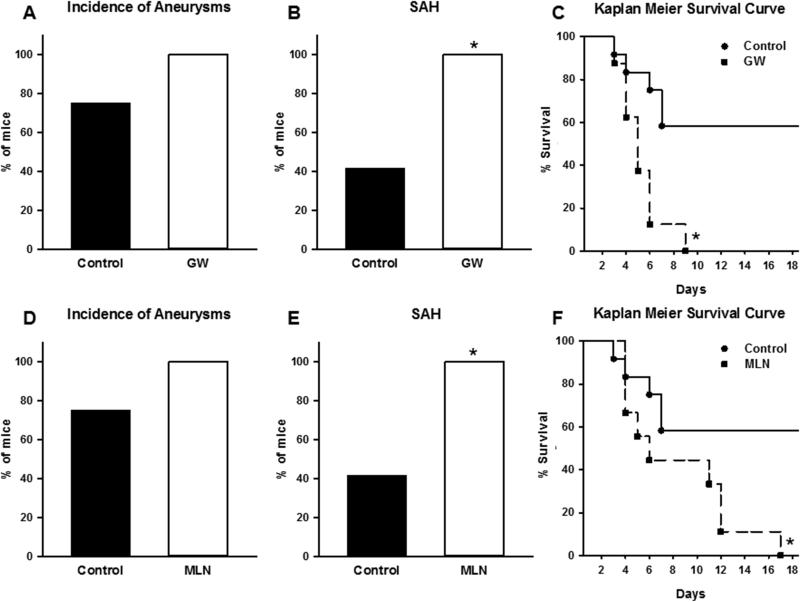
The incidence of cerebral aneurysm formation was not significantly different in mice that received GW9662 when compared to controls (Figure 2A). However, the incidence of SAH was significantly increased in the GW9662 group (Figure 2B). Kaplan Meier analysis demonstrated a significant increase in aneurysm rupture in the GW9662 cohort (Figure 2C).
Figure 2. Effect of PPARγ and Cullin Inhibition.
A) The incidence of aneurysms in both GW9662 group and its control group was not statistically different. B) The incidence of aneurysm rupture and SAH was significantly higher in GW9662 group when compared to control. C) Kaplan Meier analysis of the survival demonstrates significant difference in survival between the GW9662 group and its control (Log rank < 0.05). GW9662, n=8; Control, n=12 for A-C. (* is P value <0.05). D) The incidence of aneurysms in both MLN4924 group and its control group was not statistically significant. E) The incidence of aneurysm rupture and SAH was significantly higher in the MLN4924 group when compared to its control. F) Kaplan Meier analysis of the survival demonstrates a significant difference in survival between the MLN4924 group and its control (Log rank p< 0.05). MLN4924, n=9; Control, n=12 for A-C. (* is P value <0.05).
Specific Role of Endothelial and Smooth Muscle PPARγ
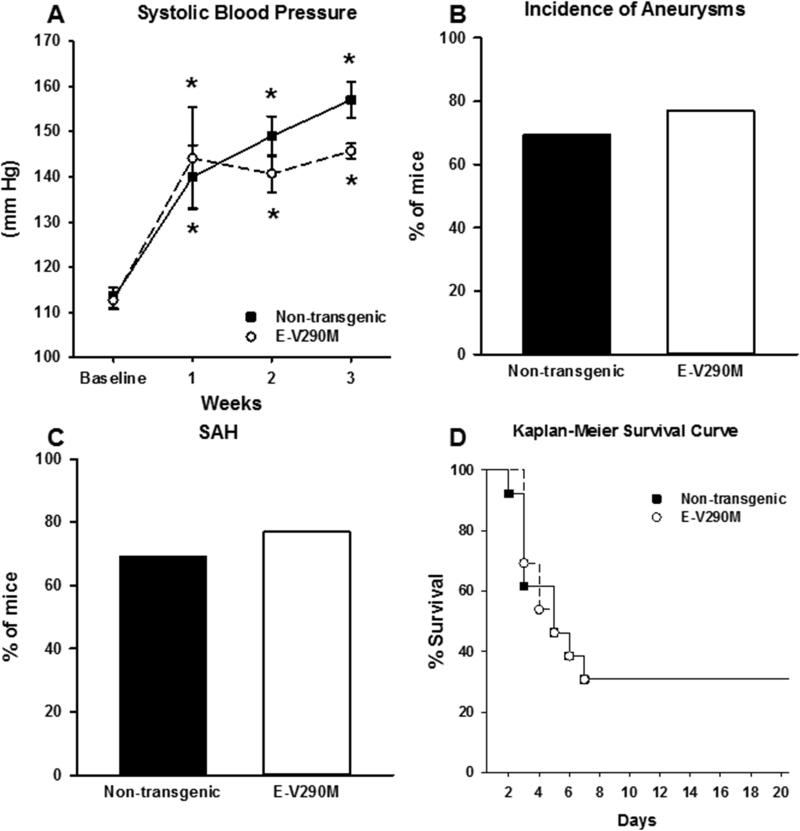
Seeing that inhibition of PPARγ increased the incidence of SAH and mortality caused by CA, we next asked if this was the result of inhibition of PPARγ in endothelium or smooth muscle. To test the role of endothelium-specific PPARγ in formation of cerebral aneurysms, aneurysms were induced in 13 E-V290M transgenic mice specifically expressing a dominant negative PPARγ in the endothelium and an equal number of non-transgenic littermates. Both E-V290M transgenic mice and non-transgenic controls had significant and sustained increases in SBP seven days after elastase injection and mini-pump implantation (Figure 3A). There were no significant differences between groups at any time point.
Figure 3. Role of Endothelial PPARγ in E-V290M Mice.
A) E-V290M mice and their non-transgenic littermates had a significant increase in systolic blood pressure seven days after elastase injection that was sustained until 21 days, but was not significantly different between cohorts at any time point. The incidence of aneurysms (B) or subarachnoid hemorrhage (C) was not significantly different in E-V290M mice versus their non-transgenic littermates. D) Kaplan Meier analysis demonstrated no significant difference in survival between cohorts. E-V290M, n=13; NT, n=13 for A-D. (* is P value <0.05)
Cerebral aneurysm formation was not significantly different in E-V290M mice versus their non-transgenic controls (Figure 3B). Similarly, there was no significant difference in incidence of subarachnoid hemorrhage or survival in either cohort (Figure 3C-D). Kaplan Meier analysis demonstrated no significant difference in the timing of aneurysm rupture between cohorts (Figure 3D).
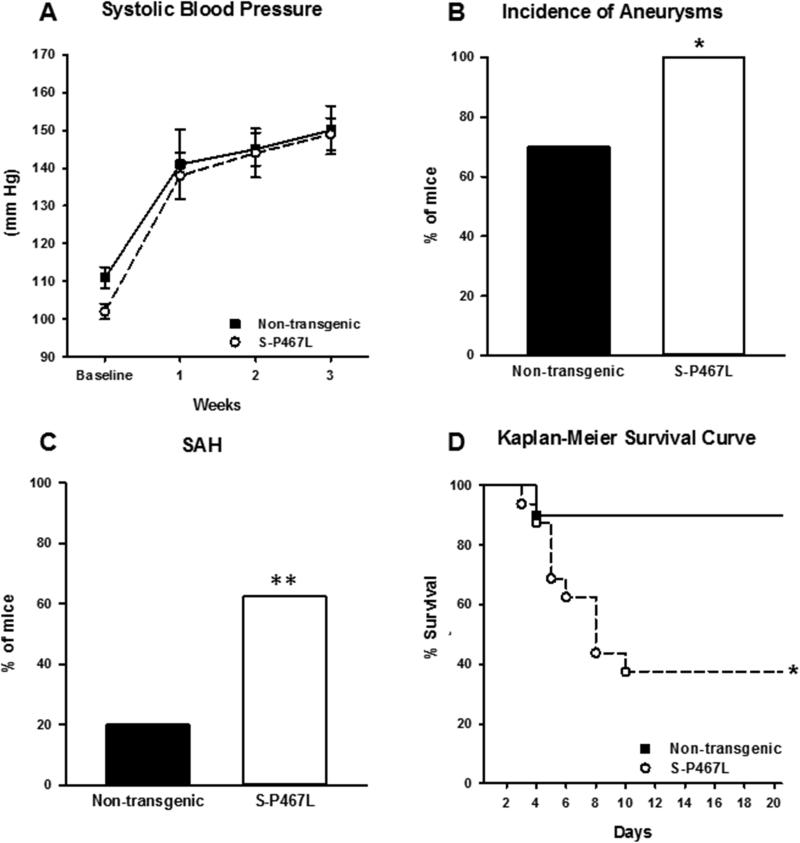
To test the role of smooth muscle cell PPARγ in formation of cerebral aneurysms, cerebral aneurysms were induced in 16 S-P467L transgenic mice expressing dominant negative PPARγ specifically in SMC, and 10 of their non-transgenic littermate controls. S-P467L transgenic mice and littermate controls had significant increases in systolic blood pressure seven days after elastase injection that were sustained until 21 days, but were not significantly different between cohorts at any time after injection (Figure 4A).
Figure 4. Role of Smooth Muscle PPARγ in S-P467L Mice.
A) S-P467L mice and their non-transgenic littermates had a significant increase in systolic blood pressure seven days after elastase injection that was sustained until 21 days, but was not significantly different between cohorts at any time point after injection. The incidence of aneurysms (B) was significantly increased (p<0.05), and subarachnoid hemorrhage (C) showed a strong trend toward increase (p=0.05) in S-P467L mice versus their non-transgenic littermates. D) Kaplan Meier analysis demonstrated a significant decrease in survival in the S-P467L cohort (Log rank p<0.05). S-P467L, n=16; NT, n=10 for A-D. (* is P value <0.05; ** is P=0.051).
The incidence of cerebral aneurysm formation (p=0.046) and SAH (p=0.05) were significantly greater in S-P467L mice compared to their non-transgenic controls (Figure 4 B-C). Kaplan Meier analysis demonstrated a significant decrease in survival due to increase in aneurysm rupture in the S-P467L cohort (Figure 4D).
Effect of PPARγ on Expression of Cullin-3, Keap1, Nfr2, and NQO1
Dominant negative mutations in PPARγ promote atherosclerosis and vascular dysfunction through distinct effects in endothelium and vascular smooth muscle.4-6 Given the increase in incidence, SAH, and mortality in the S-P467L group, we explored additional mechanisms that might be involved. In vascular smooth muscle, PPARγ regulates the activity of RhoA/Rho-kinase through its effects on the Cullin-3 pathway.4-6 Therefore, we tested the effect of inhibiting Cullin activity using MLN4924 on the incidence of formation and/or rupture of cerebral aneurysms. For this subgroup, cerebral aneurysms were induced in 9 MLN4924-treated and 12 control C57BL/6 mice. Whereas, the incidence of cerebral aneurysm formation was not significantly affected (Figure 2D), the incidence of SAH was significantly greater in mice that received MLN4924 when compared to controls (Figure 2E). This data was similar to the effect of PPARγ inhibition (Figure 2 A-C). Kaplan Meier analysis demonstrated a significant increase in aneurysm rupture in MLN4924 cohort (Figure 2F).
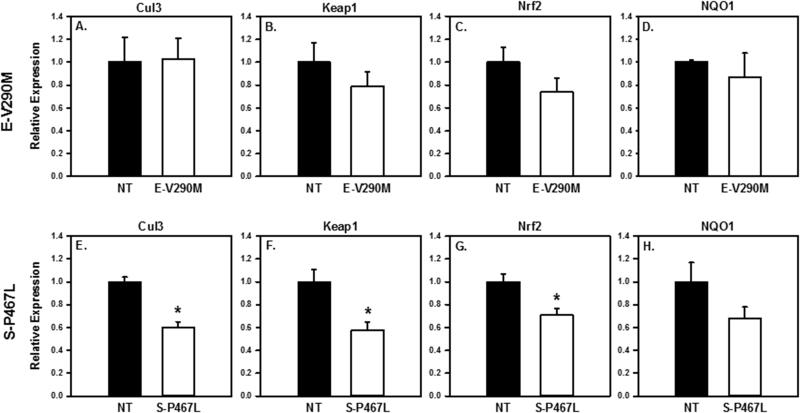
We also examined the effect of PPARγ interference in endothelial or smooth muscle cells on the expression of genes encoding Cullin-3, Kelch-Like ECH-associated protein 1(Keap1), Nuclear factor [erythroid-derived 2]-like 2 (Nrf2), and NAD(P)H dehydrogenase [quinone]1 (NQO1) in cerebral arteries. Following the aneurysm induction surgery, E-V290M mice showed no significant difference in expression of these mRNAs when compared to non-transgenic littermates (Figure 5 A-D). In contrast, there was a significant decrease in expression of Cullin-3, Keap1, and Nrf2 in cerebral arteries from S-P467L mice (Figure 5 E-G). There was no change in expression of NQO1 (Figure 5 H).
Figure 5. Expression of Cullin Pathway Genes.
Gene expression in cerebral arteries in E-V290M (A-D), S-P467L (E-H) versus their non-transgenic littermates. Cullin3, Keap1, Nrf2 were statistically lower in S-P467L (P <0.05) but not E-V290M. E-V290M n=5, NT n=5; S-P467L n=15, NT n=9.
Effect of PPARγ on Expression of Inflammatory Mediators
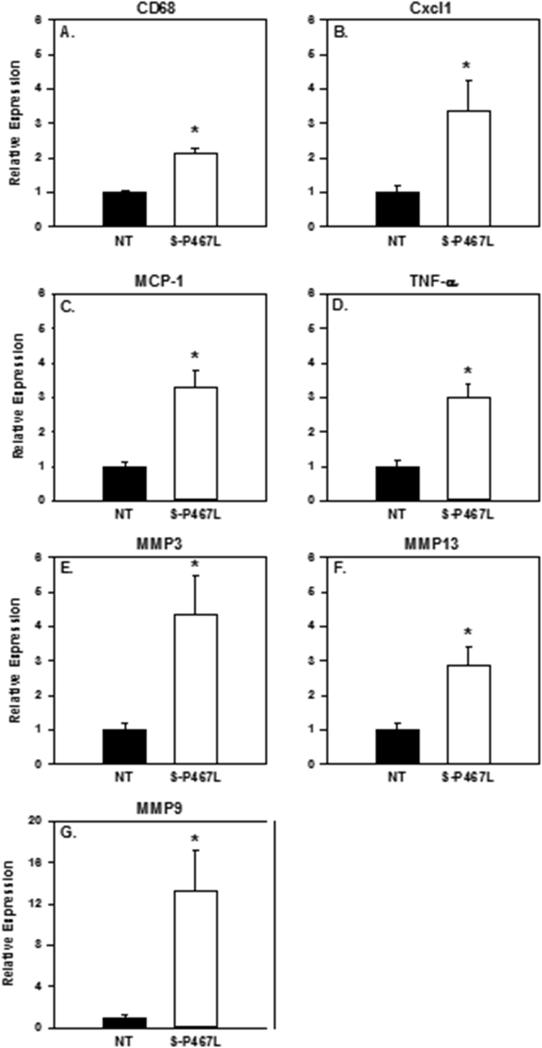
Because alterations in inflammatory mediators are associated with formation and rupture of cerebral aneurysms, we assessed the role of PPARγ in upregulation of specific inflammatory mediators during cerebral aneurysm formation and rupture.2 Following the aneurysm induction, E-V290M mice showed no significant difference in expression of multiple inflammatory mediators (CD68, Cxcl1, MCP-1, TNF-α, MMP-3, MMP-13 and MMP-9) when compared to non-transgenic littermates (Figure 2S). In contrast, there was a significant increase in the expression of genes encoding mediators of inflammation, including the chemokines MCP-1 and Cxcl1, a cytokine (TNF-α), matrix remodeling enzymes (MMP-3, -9, -13), and an inflammatory cell marker (CD68) in S-P467L mice (Figure 6).
Figure 6. Expression of Inflammatory Genes.
Gene expression in cerebral arteries in S-P467L versus their non-transgenic littermates. Cxcl1, MCP-1, TNFα, MMP3, MMP9, MMP13, and CD68 were statistically higher in S-P467L (P <0.05). S-P467L, n=15; NT, n=9.
Discussion
The major findings of this study are that 1) PPARγ activation by pioglitazone did not alter the incidence of aneurysm formation but had a tendency to decrease aneurysm rupture; 2) inhibition of endogenous PPARγ using the PPARγ antagonist GW9662 increased the incidence of aneurysm rupture and SAH; 3) endothelial-specific interference with PPARγ does not alter the incidence of aneurysm formation or rupture, whereas interference with PPARγ function specifically in SMC increases both cerebral aneurysm formation and rupture; 4) the effect seen following interference of PPARγ in SMC may have a Cullin E3 ring-ligase component as Cullin-inhibition increased risk of aneurysm rupture and SAH; and 5) there was a significant decrease in expression of Cullin-3, Keap1, and Nrf2 mRNAs, and significant increases in expression of genes encoding inflammatory mediators (TNF-α, CD68, MCP-1, Cxcl1, MMP-3, -9, and -13) in response to SMC-specific PPARγ interference. Thus, this study defines an important role for PPARγ, and more specifically PPARγ in vascular smooth muscle, in protecting from formation and rupture of experimental cerebral aneurysms in mice.
When activated by endogenous or pharmacological ligands, the nuclear hormone receptor PPARγ protects against elements of metabolic syndrome, hypertension, obesity, diabetes, and abdominal aortic aneurysms.4,13-18 One mechanism by which PPARγ and its ligands exert these protective effects is by decreasing vascular inflammation and atherosclerosis.19,20 Although atherosclerosis is likely a key aspect of cerebral aneurysm pathophysiology, our murine model does not reflect this process in aneurysm formation or rupture.2 The current approach does model the effects of hypertension and inflammation. With that in mind, the role of PPARγ activation in cerebral aneurysm formation and rupture in mice has not been investigated previously to our knowledge. In addition, although PPARγ is expressed in a wide variety of cells, cell-specific contributions of PPARγ in SMC and endothelium in the formation and rupture of cerebral aneurysms were not known prior to the current study.
Extrapolating from the literature on abdominal aortic aneurysm (AAA), where treatment with PPARγ ligands (pioglitazone and rosiglitazone) reduce the incidence of AAAs and rupture using a murine model,17 and deletion of SMC PPARγ promoted AAA,14 we tested the hypothesis that PPARγ plays a critical role in formation and rupture of cerebral aneurysms in mice. These findings and others provided rationale for a role of altered SMC function within cerebral aneurysms leading to local inflammation, infiltration of macrophages, and matrix remodeling, contributing to SMC apoptosis, thinning of the vascular wall, and aneurysm rupture. In our study, treatment with pioglitazone did not affect the incidence of aneurysm formation but tended to decrease the risk of aneurysm rupture. This result could be potentially explained by the reduction in blood pressure produced by pioglitazone when compared to controls. In this murine model of cerebral aneurysm, elastase injection in the basal cistern leads to fragmentation of the internal elastic membrane and formation of cerebral aneurysms. Based on previous studies using the same model, increases in arterial pressure will increase the risk of aneurysm rupture and SAH.9-12 It is notable that inhibition of PPARγ either globally (with GW9662) or cell-specifically did not further augment the increased blood pressure induced by Ang-II, suggesting the increase incidence of aneurysms or aneurysm rupture was not due to a worsening of hypertension in the model.
In SMC-specific PPARγ dominant negative (S-P467L) mice, expression of Cullin-3, Keap1, and Nrf2 was significantly decreased, an effect not observed in endothelial-specific PPARγ dominant negative (E-V290M) mice. This observation is novel and suggests that PPARγ may play a role in the regulation of expression of these molecules in SMCs, but perhaps not endothelial cells. Of course, we cannot rule out the possibility that the decrease in expression of these genes is due to direct effects of Ang-II, or the resultant hypertension, and not the consequences of dominant negative PPARγ. Because Cullin-3, Keap1, and Nrf2 regulate many inflammatory cytokines and oxidative stress related molecules,21 we then tested for changes in expression of several inflammatory mediators (TNF-α, CD68, MCP-1, Cxcl1, and MMP-3, -9 and -13) which were significantly increased in SMC-specific PPARγ dominant negative mice, but not in endothelial-specific PPARγ dominant negative mice. In a separate study, we showed that Rho-kinase activity was increased in mice expressing dominant negative PPARγ in SMC.22 The increase in Rho kinase activity was due to the loss of a specific PPARγ target gene, RhoBTB1, proposed to be a component of the Cullin-3 RING E3 ubiquitin ligase (CRL3) complex. We proposed a mechanism by which interference with PPARγ in vascular SMC led to decreased expression of RhoBTB1, subsequent loss of Cullin-3 CRL3 activity leading to increased levels of the Cullin-3 substrate RhoA, which could be activated in response to contractile agonists. Thus, one could hypothesize that in our model, increased RhoA could increase the risk of cerebral aneurysm formation and rupture. In this regard, it is interesting that fasudil (a Rho-kinase inhibitor) attenuates induction and progression of experimental cerebral aneurysms.23 These findings suggest that interference with PPARγ in smooth muscle cells leads to aneurysm formation and rupture by 1) decreasing Cullin-3 which in turn leads to increased Rho kinase activity and 2) decreased Keap1 and Nrf2 through an unclear mechanism (Figure 3S). The finding of decreased expression of Cullin-3, Keap1 and Nrf2 in the aneurysm could be viewed as counterintuitive as it might be expected that decreased Cullin-3 activity would lead to accumulation of Nrf2. Under conditions of oxidative stress, Nrf2 is stabilized by inhibition of Keap1-dependent CRL3 complex. This normal regulatory impairment of the CRL3 complex results from oxidant-dependent modification of multiple cysteine residues in Keap1, which can prevent Keap1 interaction with Cul3 or disrupt the interaction between Keap1 and Nrf2.22,24 A potential limitation of this study is that we examined expression of Cullin-3, Keap1 and Nrf2 mRNA, and neither protein nor CRL3 activity. However, that inhibiting all members of the Cullin family with MLN4924 increased the incidence of aneurysm rupture and SAH, combined with decreased expression of Cullin-3 in the aorta of S-P467L mice and in the aneurysm is consistent with an effect mediated by inhibition of Cullin-3.22 Inhibition of PPARγ and the lack of Nrf2 activation may both independently lead to increased inflammatory markers and oxidative stress.21,23
Several lines of evidence suggest that increased oxidative stress may contribute to the pathogenesis of aneurysms in both humans and some experimental models.25-27 In relation to other vascular disease endpoints, previous studies of E-V290M and S-P467L mice suggest that genetic interference with PPARγ in endothelium promotes oxidative stress, while the same intervention in vascular muscle produced vascular abnormalities that are independent of oxidative stress.5-8 Thus, while is it possible that oxidative stress was present in the vasculature in the current experiments, E-V290M mice did not exhibit increased aneurysm formation or rupture. In contrast, formation and rupture of aneurysms was altered in S-P467L mice, a model in which previous studies implicated non-oxidant-dependent mechanisms, at least in relation to other vascular endpoints. Collectively, these findings make it difficult predict whether there is a causal relationship between PPARγ and oxidative stress in the current models of cerebral aneurysm formation and progression.
Recent studies in cultured SMC’s, in vivo models of cerebral aneurysm formation, and human cerebral aneurysms have demonstrated that TNF-α can induce alterations in SMC function, which may contribute to cerebral aneurysm pathophysiology through epigenetic alterations in inflammatory genes.28-30 Genetic or pharmacological inhibition of TNF-α has been found to decrease the incidence of experimental cerebral aneurysm formation, progression, and rupture.9,18,29,30 Through activation of chemoattractants (MCP-1 and Cxcl1), there is further influx of CD68 lineage macrophages which also secrete TNF-α.21,28 Specifically, MCP-1 is increased in aneurysm walls and MCP-1 knockout mice have decreased expression of MMPs, and a lower incidence of aneurysm formation.10 TNF-α activates inflammatory SMC and macrophages to release additional matrix remodeling genes and enzymes including MMP-3, -9 & -13. MMPs degrade vascular extracellular matrix and are upregulated in human cerebral aneurysms.31,32 Inhibition of MMPs also decreases the incidence of aneurysm formation and progression in animals.11,12 Although there are likely multiple mediators of formation and rupture of cerebral aneurysms, altered SMC-specific PPARγ represents a potential pathway to protect against local vascular injury, inflammation, and apoptosis within aneurysms. These findings present a potential mechanistic pathway by which altered SMC-specific PPARγ increases the risk of formation and rupture of cerebral aneurysms in mice (Figure 3S).
Limitations
The model of intra-cranial aneurysm formation used in these experiments (including the use of hypertension and elastase) has both merits and limitations when compared to other models: A) side-wall aneurysms with elastase injection,33 and B) ligation of left common carotid arteries and posterior branches of bilateral renal arteries with high salt diet.34 The elastase model facilitates aneurysm formation and rupture over a relatively short-time interval with the formation of large aneurysms that can be detected and isolated. One possible limitation of this approach is that elastase chemically alters and fragments the internal elastic membrane and thus may induce inflammation, which may alter the natural course of aneurysm formation. Therefore, the rapidity of aneurysm formation and the use of exogenous elastase to induce aneurysms may activate different mechanisms than those underlying the natural progression of aneurysm formation in humans.35 However, histological analysis suggests that the cellular processes are similar in humans and the current mouse model in terms of inflammatory cell infiltration, fragmentation of internal elastic membrane, and morphological changes in the endothelium and smooth muscle cells.9-12,33 Thus, the results of this study employing an experimental model of CAs along with the use of selectively targeted cell-specific interference with PPARγ may not apply directly to humans, particularly with respect to potential use of these pharmaceutical agents to modify and halt the progression of human cerebral aneurysm to rupture.
Perspectives
We demonstrated that neither pioglitazone nor GW9662 altered the incidence of aneurysm formation. However, GW9662 increased the incidence of aneurysm rupture and pioglitazone had a strong tendency in decreasing this incidence. Dominant negative endothelial-specific PPARγ did not alter the incidence of aneurysm formation or rupture. In contrast, dominant negative SMC-specific PPARγ resulted in a significant increase in cerebral aneurysm formation and rupture. Dominant negative SMC-specific PPARγ, but not dominant negative endothelial-specific PPARγ, resulted in a significant decrease in expression of genes encoding Cullin3, Keap1, and Nrf2, and significant increase in TNF-α, MCP-1, Cxcl1, CD68, MMP-3, -9, & -13. MLN4924 did not alter the incidence of aneurysm formation, but did increase the incidence of aneurysm rupture.
Conclusion
This study supports the concept that endogenous PPARγ, specifically smooth muscle PPARγ, plays an important role in protecting from formation and rupture of experimental cerebral aneurysms. There appears to be fundamental differences in the importance of endothelial versus smooth muscle PPARγ in mediating this process, possibly via effects of Cullin-3 and inflammation.
Supplementary Material
Novelty and Significance.
What is New?
This study provides the first evidence for a protective role of PPARγ in cerebral aneurysms.
There appears to be fundamental differences in the importance of endothelial versus smooth muscle PPARγ in mediating this process, possibly via effects of Cullin-3 and inflammation.
What is Relevant?
Pharmacological activation of PPARγ may have therapeutic benefit by halting progression of cerebral aneurysms and preventing rupture of unstable aneurysms.
Summary.
Endogenous PPARγ, specifically smooth muscle PPARγ, plays an important role in protecting from formation and rupture of cerebral aneurysms, possibly via effects of Cullin-3 and inflammation.
Acknowledgments
Source of Funding: This work was supported by the National Institute of Health [grants to DMH (R03NS079227, K08NS082363), DDH (NS72628, HL62984), FMF (HL62984 and HL113863) and CDS (HL084207, HL048058, and HL062984)], the Department of Veterans Affairs (BX001399 to FMF), and the Fondation Leducq (FMF).
Footnotes
Disclosures: The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, Rischmiller J. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8:427–433. doi: 10.1016/S1474-4422(09)70080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiebers DO, Whisnant JP, Huston J, III, Meissner I, Brown RD, Jr., Piepgras DG, Forbes GS, Thielen K, Nichols D, O'Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 3.Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–2482. doi: 10.1056/NEJMoa1113260. [DOI] [PubMed] [Google Scholar]
- 4.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPARγ Function in smooth muscle causes vascular dysfunction and hypertension. Cell Metabolism. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyer AM, de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM, Sigmund CD. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circ Res. 2008;103:654–661. doi: 10.1161/CIRCRESAHA.108.176339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelham CJ, Keen HL, Lentz SR, Sigmund CD. Dominant negative PPARγ promotes atherosclerosis, vascular dysfunction, and hypertension through distinct effects in endothelium and vascular muscle. Am J Physiol Regul Integr Comp Physiol. 2013;304:R690–R701. doi: 10.1152/ajpregu.00607.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Silva TM, Modrick ML, Ketsawatsomkron P, Lynch C, Chu Y, Pelham CJ, Sigmund CD, Faraci FM. Role of peroxisome proliferator-activated receptor-gamma in vascular muscle in the cerebral circulation. Hypertension. 2014;64:1088–1093. doi: 10.1161/HYPERTENSIONAHA.114.03935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Silva TM, Ketsawatsomkron P, Pelham C, Sigmund CD, Faraci FM. Genetic interference with peroxisome proliferator-activated receptor gamma in smooth muscle enhances myogenic tone in the cerebrovasculature via A Rho kinase-dependent mechanism. Hypertension. 2015;65:345–351. doi: 10.1161/HYPERTENSIONAHA.114.04541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starke RM, Chalouhi N, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Wada K, Shimada K, Hasan DM, Greig NH, Owens GK, Dumont AS. Critical role of TNF-alpha in cerebral aneurysm formation and progression to rupture. J Neuroinflammation. 2014;11:77. doi: 10.1186/1742-2094-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van RN, Lawton MT, Young WL, Liang EI, Nuki Y, Hashimoto T. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178. doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344. doi: 10.1161/HYPERTENSIONAHA.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makino H, Tada Y, Wada K, Liang EI, Chang M, Mobashery S, Kanematsu Y, Kurihara C, Palova E, Kanematsu M, Kitazato K, Hashimoto T. Pharmacological stabilization of intracranial aneurysms in mice: a feasibility study. Stroke. 2012;43:2450–2456. doi: 10.1161/STROKEAHA.112.659821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 14.Hamblin M, Chang L, Zhang H, Yang K, Zhang J, Chen YE. Vascular smooth muscle cell peroxisome proliferator-activated receptor-gamma deletion promotes abdominal aortic aneurysms. J Vasc Surg. 2010;52:984–993. doi: 10.1016/j.jvs.2010.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golledge J, Cullen B, Rush C, Moran CS, Secomb E, Wood F, Daugherty A, Campbell JH, Norman PE. Peroxisome proliferator-activated receptor ligands reduce aortic dilatation in a mouse model of aortic aneurysm. Atherosclerosis. 2010;210:51–56. doi: 10.1016/j.atherosclerosis.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian V, Golledge J, Ijaz T, Bruemmer D, Daugherty A. Pioglitazone-induced reductions in atherosclerosis occur via smooth muscle cell-specific interaction with PPARγ. Circ Res. 2010;107:953–958. doi: 10.1161/CIRCRESAHA.110.219089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones A, Deb R, Torsney E, Howe F, Dunkley M, Gnaneswaran Y, Gaze D, Nasr H, Loftus IM, Thompson MM, Cockerill GW. Rosiglitazone reduces the development and rupture of experimental aortic aneurysms. Circulation. 2009;119:3125–3132. doi: 10.1161/CIRCULATIONAHA.109.852467. [DOI] [PubMed] [Google Scholar]
- 18.Qu A, Shah YM, Manna SK, Gonzalez FJ. Disruption of endothelial peroxisome proliferator-activated receptor gamma accelerates diet-induced atherogenesis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2012;32:65–73. doi: 10.1161/ATVBAHA.111.239137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 20.Clark RB. The role of PPARs in inflammation and immunity. J Leukoc Biol. 2002;71:388–400. [PubMed] [Google Scholar]
- 21.Lv P, Xue P, Dong J, Peng H, Clewell R, Wang A, Wang Y, Peng S, Qu W, Zhang Q, Andersen ME, Pi J. Keap1 silencing boosts lipopolysaccharide-induced transcription of interleukin 6 via activation of nuclear factor kappaB in macrophages. Toxicol Appl Pharmacol. 2013;272:697–702. doi: 10.1016/j.taap.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi SR, Keen HL, Weatherford ET, Faraci FM, Sigmund CD. Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARγ and RhoA/Rho-kinase. Cell Metab. 2012;16:462–472. doi: 10.1016/j.cmet.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eldawoody H, Shimizu H, Kimura N, Saito A, Nakayama T, Takahashi A, Tominaga T. Fasudil, a Rho-kinase inhibitor, attenuates induction and progression of cerebral aneurysms: experimental study in rats using vascular corrosion casts. Neurosci Lett. 2010;470:76–80. doi: 10.1016/j.neulet.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 24.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–L529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 26.Starke RM, Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ, Dumont AS. The role of oxidative stress in cerebral aneurysm formation and rupture. Curr Neurovasc Res. 2013;10:247–255. doi: 10.2174/15672026113109990003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki T, Nishimura M, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N. Reactive oxygen species modulate growth of cerebral aneurysms: a study using the free radical scavenger edaravone and p47phox(−/−) mice. Lab Invest. 2009;89:730–741. doi: 10.1038/labinvest.2009.36. [DOI] [PubMed] [Google Scholar]
- 28.Collino M, Patel NS, Lawrence KM, Collin M, Latchman DS, Yaqoob MM, Thiemermann C. The selective PPARgamma antagonist GW9662 reverses the protection of LPS in a model of renal ischemia-reperfusion. Kidney Int. 2005;68:529–536. doi: 10.1111/j.1523-1755.2005.00430.x. [DOI] [PubMed] [Google Scholar]
- 29.Jayaraman T, Berenstein V, Li X, Mayer J, Silane M, Shin YS, Niimi Y, Kilic T, Gunel M, Berenstein A. Tumor necrosis factor alpha is a key modulator of inflammation in cerebral aneurysms. Neurosurgery. 2005;57:558–564. doi: 10.1227/01.neu.0000170439.89041.d6. [DOI] [PubMed] [Google Scholar]
- 30.Jayaraman T, Paget A, Shin YS, Li X, Mayer J, Chaudhry H, Niimi Y, Silane M, Berenstein A. TNF-alpha-mediated inflammation in cerebral aneurysms: a potential link to growth and rupture. Vasc Health Risk Manag. 2008;4:805–817. doi: 10.2147/vhrm.s2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 32.Kim SC, Singh M, Huang J, Prestigiacomo CJ, Winfree CJ, Solomon RA, Connolly ES., Jr Matrix metalloproteinase-9 in cerebral aneurysms. Neurosurgery. 1997;41:642–666. doi: 10.1097/00006123-199709000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Marbacher S, Marjamaa J, Bradacova K, von GM, Honkanen P, Abo-Ramadan U, Hernesniemi J, Niemela M, Frosen J. Loss of mural cells leads to wall degeneration, aneurysm growth, and eventual rupture in a rat aneurysm model. Stroke. 2014;45:248–254. doi: 10.1161/STROKEAHA.113.002745. [DOI] [PubMed] [Google Scholar]
- 34.Morimoto M, Miyamoto S, Mizoguchi A, Kume N, Kita T, Hashimoto N. Mouse model of cerebral aneurysm: experimental induction by renal hypertension and local hemodynamic changes. Stroke. 2002;33:1911–1915. doi: 10.1161/01.str.0000021000.19637.3d. [DOI] [PubMed] [Google Scholar]
- 35.Koffijberg H, Buskens E, Algra A, Wermer MJ, Rinkel GJ. Growth rates of intracranial aneurysms: exploring constancy. J Neurosurg. 2008;109:176–185. doi: 10.3171/JNS/2008/109/8/0176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.