Abstract
Rationale
The relatively weak reinforcing effects of nicotine in experimental studies have been attributed to possible aversive effects or the need to space nicotine administrations over time to expose reinforcing effects.
Objective
This study was designed to determine if the response-maintaining effects of nicotine are increased when availability is spaced through time, and whether nicotine is an effective punisher of remifentanil-maintained responding.
Methods
Compared to a cocaine reference dose, nicotine dose and timeout (TO) value were varied in eight rhesus monkeys responding for intravenous (i.v.) nicotine on varying fixed-ratio (FR) schedules of reinforcement. The aversive effects of nicotine were evaluated in four animals choosing between a standard dose of remifentanil alone or in combination with one of several doses of nicotine.
Results
In three of eight self-administration monkeys, 0.01 mg/kg/inj nicotine did not maintain responding at any FR value. In the other five animals, nicotine-maintained response rates increased with either FR or TO values to a certain point, and then slowed. Maximum nicotine-maintained response rates were much slower than those maintained by cocaine, and demand for nicotine was less than demand for cocaine. Nicotine was an effective punisher of remifentanil-maintained responding at doses ranging from 0.01 to 0.3 mg/kg/inj. Lower punishing dose seemed to be related to the absence of reinforcing effects within subject.
Conclusion
There are an order of magnitude individual differences in sensitivity to both the reinforcing and punishing effects of nicotine, and this drug may be unique in being a weak positive reinforcer in small doses and aversive in large doses.
Keywords: nicotine, self-administration, cocaine, rhesus monkey, time out, fixed ratio, punishment, individual differences
Introduction
The reinforcing effects of nicotine, while clearly demonstrated in several animal species and with several different schedules of response-contingent delivery, are fairly weak in most studies. In rodents (e.g. Rose and Corrigall 1997), squirrel monkeys (Spealman and Goldberg 1982), and rhesus monkeys (Mello and Newman 2011), nicotine typically produced an inverted U-shaped dose-effect curve that was indicative of the drug's reinforcing effect. When compared with cocaine, nicotine usually produced slower responding (e.g., Ator and Griffiths 1983; Mello and Newman 2011; Rose and Corrigall 1997; Spealman and Goldberg 1982).
A review by Goldberg and Henningfield (1988) considered the possibility that higher rates of nicotine-maintained responding can be generated if the opportunity for injection of the drug is spaced through time. Evidence supporting this notion included studies of nicotine self-administration under relatively rich fixed-ratio (FR) schedules with short or no post reinforcement timeout (TO), where rates of responding tended to be slow, as compared with relatively lean fixed-interval (FI) schedules or ratio schedules with longer TO values, where rates of nicotine-maintained responding tended to be higher (e.g., Corrigall and Coen 1989). Ator and Griffiths (1983), for example, compared rates of nicotine-maintained responding in baboons under a lean (FI 5′ TO 1′) and a dense (FR 2 TO 15″) schedule of reinforcement. Rates of nicotine-maintained responding were higher than rates of saline-maintained responding in one of three baboons on the FR schedule and in three of three baboons on the FI schedule, suggesting that intermittent schedules increased the likelihood that nicotine's reinforcing effects could be made evident. Nevertheless, responding was relatively fast and dose-related in only one baboon responding under either the FR or the FI condition. This study has been placed in the negative column in a review of nicotine's reinforcing effectiveness in non-human primates (Le Foll and Goldberg 2007).
Goldberg and Henningfield (1988) reviewed earlier research by their group that found that both squirrel monkeys and humans responded for nicotine at rates that were marginally above those maintained by saline when there was a 1 min TO after completion of each FR; rates were reportedly increased when a longer (4 min for monkeys; 20 min for humans) TO followed each injection. In addition, studies of nicotine self-administration in beagle dogs using a 4 min TO after each completed FR found dose-related rates of nicotine-maintained responding that were lower than those maintained by cocaine, but in patterns that indicated a reinforcing effect of the drug (Risner and Goldberg 1983).
Despite this early review that emphasized the potential importance of intermittent availability of nicotine in producing increased rates of nicotine-maintained responding, there are apparently no systematic studies of how rates of nicotine-maintained responding change as the time between reinforcer availability is increased. With other drugs that serve as reinforcers, including cocaine, alfentanil, methohexital, and nalbuphine, increasing the TO value following each reinforcer delivery leads to an initial increase in rates of responding, followed by a subsequent decrease. Importantly, as the dose per injection of the drug increases, the TO value at which maximum rates are obtained increases as well, as does the maximum rate of responding (Winger 1993; Winger et al.1996). This, along with choice procedures, is among some of the most convincing evidence that these drugs of abuse are better reinforcers (i.e., maintain faster responding) as dose increases and that there are no aversive aspects of large doses of these drugs.
Whether this is the case for nicotine is an interesting question, in part because nicotine is a relatively weak reinforcer, and because it is apparently unique among drugs of abuse in having the ability to punish responding. Spealman (1983) found that squirrel monkeys would respond to postpone injections of nicotine. Interestingly, a dose of 0.01 mg/kg/inj was only slightly more effective than saline in maintaining postponement behavior, whereas larger doses produced behavior that was much like that maintained by postponement of electric shock. This dose of 0.01 mg/kg/inj was most effective in maintaining responding in rhesus monkeys (Mello and Newman, 2011), supporting the notion that large doses of nicotine may be aversive, whereas reinforcing effects may be observed at smaller doses. Using a multiple schedule of food and food + nicotine-maintained responding, Goldberg and Spealman (1983) found that fast responding was supported by food delivery alone, and rates dropped precipitously when the first response of the FR 30 schedule was accompanied by nicotine delivery. This punishment effect was dose-related with doses of 0.3 mg/kg/inj producing complete suppression of responding. Similar results were reported by Takada et al. (1992) using an identical procedure. These investigators compared the ability of nicotine to punish food-maintained responding with that of histamine, cocaine, and β carboline. Cocaine was the only one of these drugs that did not produce a punishing effect. It suppressed responding at large doses, but the suppressant effects were equal in both the “punished” and the “unpunished” components of the schedule.
Here, we evaluated the ability of nicotine both to maintain responding across increasing TO values and to punish responding maintained by remifentanil in a choice situation similar to that described by Woolverton (2003).
Method
Subjects
Eight rhesus monkeys (one female: RO) were subjects in this study. All monkeys were experienced in self-administration of a variety of drugs, including cocaine. The animals were housed individually in stainless steel cages measuring 83.3 cm high × 76.2 cm wide × 91.4 cm. A metal panel (20 cm high × 28 cm wide) was mounted on one side of each cage and contained three response levers with a stimulus light located 5 cm above each lever. Levers were positioned 5 cm above the bottom of the panel and were spaced 2.5 cm apart. The stimulus lights were spaced 5 cm apart; the two lights over the side levers could be independently illuminated red or yellow whereas the light over the center lever could be illuminated green.
The monkeys each had i.v. catheters implanted in an accessible vein under sterile conditions and surgical anesthesia (10 mg/kg ketamine i.m. and 2 mg/kg xylazine i.m.). The catheter passed subcutaneously to the animal's back where it exited in the midscapular region and proceeded through a flexible tubular metal tether to the outside rear of the cage. Monkeys wore a Teflon jacket (Lomir, Quebec, Canada) to protect the catheter and to attach the tether to the animal. One or two infusion pumps (Watson-Marlow model SCI-Q 400, Wilmington, MA), were located behind each cage along with a failsafe device to stop the pump if a computer malfunction did not stop it automatically at 5 s. Drugs were contained in plastic infusion bags made up in the concentration indicated for the drug dose and the weight of the monkey. The monkeys' intravenous catheters were connected to this system by way of a 0.22 μm Millipore filter.
The University of Michigan is accredited by the American Association for the Accreditation of Laboratory Animal Care; procedures used in this experiment were conducted in accordance with the National Research Council Guide for the use and care of laboratory animals, and approved by the University Committee on Care and Use of Animals.
Procedures
All eight monkeys (AN, BR, DA, LI, RO, ST, FO, and WA) participated in the study of the effects of increasing FR and TO variables on the reinforcing effects of nicotine, and four monkeys (BR, DA, FO, and WA) participated in the study of the punishing effects of nicotine. Of the four monkeys that were evaluated for both the positive reinforcing and punishing effects of nicotine. Two (DA, FO), were exposed to the punishment phase of the study prior to evaluation of nicotine as a reinforcer, and two (WA, BR), evaluated nicotine first as a positive reinforcer.
Nicotine as a reinforcing stimulus
Subjects were given access to i.v. drug infusions during twice-daily 2-hr sessions (starting at 6am and 12pm). The start of each session was signaled by the onset of a red stimulus light over the right lever. No priming injections were given. At the initiation of each dose and ratio or timeout condition, responses on this lever resulted in a drug infusion under a FR 10 TO 10″ schedule. Drug infusions always lasted for 5 s and were accompanied by the offset of the right red stimulus light and the onset of the center green stimulus light. A limit of 100 injections was placed on 0.03 mg/kg cocaine to prevent toxic effects. During the timeout period following each infusion, all lights were extinguished and responses had no scheduled consequence.
Rates of responding were initially measured using 0.03 mg/kg/inj cocaine as the reinforcer. After several sessions under the baseline FR 10 TO 10″ schedule, the FR value was increased in half or quarter log unit increments from 10 to 1000. Some monkeys showed high rates of responding at FR 1000, and testing continued to values of 1780 or 3200. Each value was maintained for two consecutive sessions (either on the same day, or on the afternoon of one day and the morning of the next), and exposure to the different FR values was always increasing. Once cocaine had been evaluated across increasing ratios, the baseline schedule of reinforcement was returned for two to four sessions and TO values then were increased in the same way. Half or quarter log unit increments in TO values, from 10 to as high as 3200, were used. Cocaine was not made available after FR and TO values were studied. A dose of 0.01 mg/kg/inj nicotine was then substituted for cocaine for 3 to 12 sessions under the baseline conditions until there was no clear decreasing trend in number of injections, and the experiment was repeated. This dose was selected because it maintains maximum or near maximum rates of responding in rhesus monkeys (Mello and Newman, 2011). Both FR and then TO values were incremented using this dose of nicotine as the response-contingent stimulus. In the four monkeys that showed some evidence of increased rates of responding as TO increased, a smaller (0.003 mg/kg/inj) and two larger (0.03 and 0.1 mg/kg/inj) doses were evaluated as well. As these doses were evaluated, the TO following delivery of each reinforcer was routinely increased as described above; the fixed ratio value remained at 10 throughout the determination of the effect of increasing timeout values.
Nicotine as a punishing stimulus
As was the case for studies of the positive reinforcing effects of nicotine, the 2-hr punishment sessions were scheduled twice daily, with one exception noted below. Two levers were operative throughout evaluation of punishing effects of drugs.
The start of each session was indicated by the onset of two stimulus lights, each located over a response lever in the monkey's cage. The light over one lever was red and the light over the other lever was yellow. Responses on the lever under the red light always resulted in i.v. administration of 0.0003 mg/kg/inj remifentanil. Responses on the lever under the yellow light resulted in either intravenous saline administration or in administration of 0.0003 mg/kg/inj remifentanil in combination with one of four doses of nicotine (0.01, 0.03, 0.1, or 0.3 mg/kg/inj). Each dose of nicotine in combination with remifentanil was an option for several consecutive sessions during which lever selection was not visibly trending in either direction. Then, one of the following occurred: the two lever options and the associated light colors were reversed, the nicotine-remifentanil combination was replaced with saline, or the dose of nicotine in combination with remifentanil was increased.
There was no sampling period (no forced choice) at the start of each session. The first response on either lever turned the light out over the alternate lever and responses on this alternate lever had no further programmed consequence until a reinforcer had been earned as a consequence of responding on the lever that was originally selected. Reinforcement was contingent on responding on a random ratio 32 schedule and a 2 min TO followed delivery of each reinforcer. Following the TO, lights over both levers were again illuminated. The position of the lights and the consequences of responding on each lever remained the same throughout each session and typically for several consecutive sessions.
Because all but one of the monkeys developed a strong lever-side bias, regardless of whether this responding produced remifentanil alone or remifentanil + nicotine, saline alone was occasionally made available as an option for the standard dose of remifentanil, on the preferred side. The monkeys quickly shifted their responding to the previously non-preferred lever. Replacing the saline (on the preferred side) with remifentanil + nicotine at the same or larger dose usually resulted in a return to the preferred lever, even though this resulted in administration of nicotine in combination with remifentanil. When this occurred, the dose per injection of nicotine was increased systematically, as described above, until the monkeys shifted their responding to the non-preferred side, the one that delivered remifentanil alone.
For one monkey (BR) the side bias resulted in self-administration of 0.3 mg/kg/inj nicotine + remifentanil, a dose of nicotine that produced vomiting. Testing sessions were reduced to once per day in this monkey when this dose of nicotine was available.
Drugs
Remifentanil (Ultiva; a lyophilized powder) was purchased from the University of Michigan pharmacy. Nicotine was purchased from Sigma-Aldrich and was prepared as the base. Both drugs were administered in a 0.9% saline vehicle.
Data Analysis
Nicotine as a reinforcing stimulus
Rates of responding, calculated as the number of responses made in the presence of the red light stimulus, divided by the number of seconds the red light was illuminated, were determined across increasing FR or TO values. Because of the marked individual differences among animals, data are shown for each of the monkeys individually.
Comparison of nicotine and cocaine demand
The number of injections self-administered by each subject were normalized for each drug separately by dividing obtained injections at each FR for each monkey and dividing by the group mean at the FR 10. Unit price was calculated by dividing the product of FR value and consumption at FR 10 by 100. These normalized values were then fit to the equation
| (1) |
where P is the normalized price, Q is consumption (injections received) at P price, Q0 represents the level of consumption as P approaches 0 (set to Q0 = 100 for these normalized data), k is the span of the function between maximum and minimum consumption in log10 units, and α represents demand elasticity (Hursh and Silberburg 2008). Demand functions for nicotine and cocaine were analyzed separately for monkeys that self-administered nicotine in the FR and TO experiments and those that did not in one analysis and together in a second analysis. The α and k parameters are free parameters, although the k parameter was fit as a common parameter across the conditions (fitted k = 1.58). This left only the α parameter to vary which was compared across drugs with a nonlinear regression F test in GraphPad Prism 6 (La Jolla, CA, USA). A significant group F test was followed by pairwise F tests comparing each condition to each other. To maintain a familywise error rate of α .05, p values from these comparisons were assessed for significance using the Holm-Bonferroni alpha correction procedure (Holm, 1979).
Nicotine as a punishing stimulus
The number of injections earned as a consequence of responding on each of the two levers was the primary data collected. The monkeys showed strong side preferences, consistently selecting either the right or the left lever. The data were therefore calculated as the dose of nicotine required to shift responding to the non-preferred lever. Lever selection on the last five stable sessions when each dose of nicotine + remifentanil was available for responding on the preferred lever was calculated with a standard deviation of this mean. Individual differences again encouraged showing each animals' data separately.
Results
Nicotine as a reinforcing stimulus
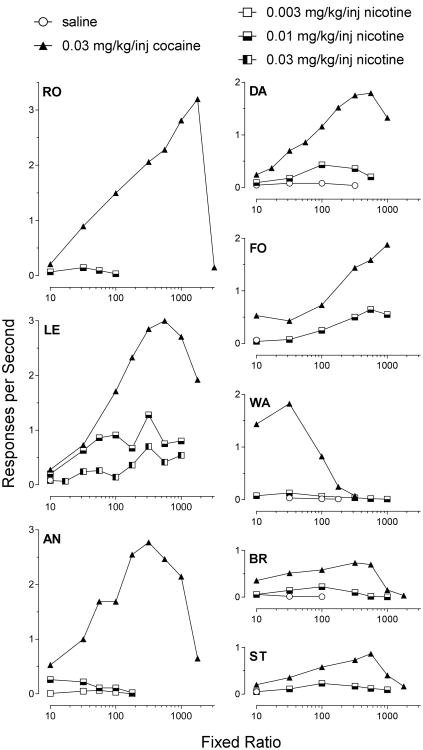
A dose of 0.01 mg/kg/inj nicotine maintained very low rates of responding in each of the seven monkeys. As shown in Figure 1, on visual inspection, as FR value increased, rates of responding increased in three of the eight monkeys (DA, LE, FO). Interestingly, two of the monkeys (BR, ST) that showed little indication of a reinforcing effect of nicotine, also showed relatively low rates of responding for 0.03 mg/kg/inj cocaine across the increasing FR values, suggesting that they might be less sensitive to drug reinforcement in general than the other two monkeys. The three remaining monkeys (WR, RO, AN), despite responding at moderate to high rates when cocaine was response-contingent, did not demonstrate any increase in response rates for nicotine as FR values were increased.
Figure 1.
Rates of responding for 0.03 mg/kg/inj cocaine (▲) or 0.003 to 0.03 mg/kg/inj nicotine (■), and saline (○) in eight monkeys as a function of the size of the fixed ratio schedule of drug reinforcement.
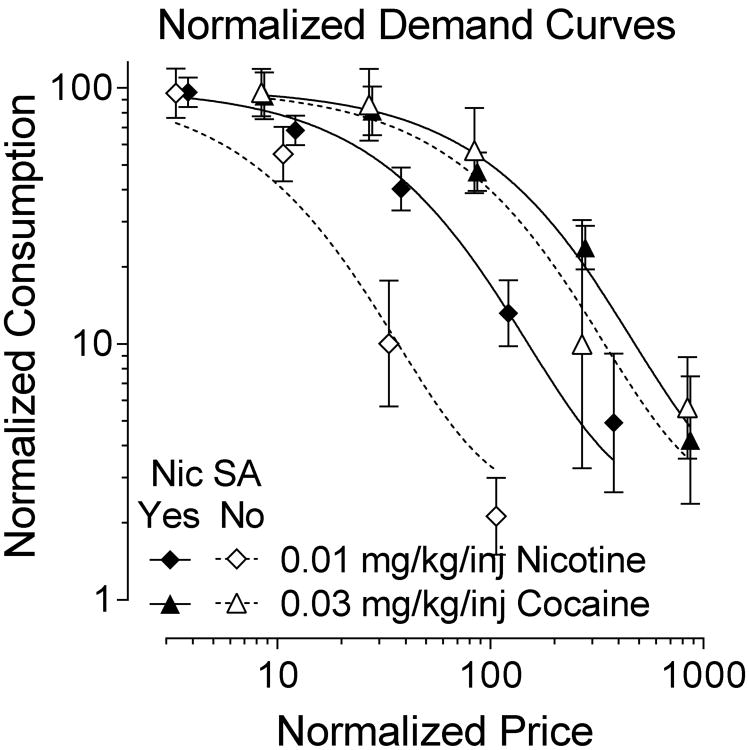
Information on the number of injections of nicotine and cocaine are presented in Table 1 for each of the eight monkeys across the various FR values. When these data on nicotine and cocaine self-administration were subjected to a demand curve analysis, nicotine yielded a significantly (F1,70 = 40.2, p < .001) higher α value (0.0114, SEM = 0.0019) than did cocaine (0.0023, SEM = 0.0004). These α values correspond to Pmax, or the price that supports the most responding, values of 36.5 for nicotine and 179.4 for cocaine. This indicates that nicotine has a reduced essential value relative to cocaine. Figure 2 shows normalized demand curves for nicotine and cocaine, with those animals that self-administered nicotine in the FR and TO experiments (ST, BR, LE, FO, and DA) analyzed separately from those that did not (RO, AN, and WA). After a significant effect of group among these four conditions (F3,68 = 25.7, p < .001), Holm-Bonferroni corrected pairwise comparisons revealed that each curve differed significantly from each other (ps from .01 to < .001) except the two cocaine curves which were not significantly different (p = .3).
Table 1.
The weight of the monkeys and the number of injections of 0.03 mg/kg/inj cocaine and 0.01 mg/kg/inj nicotine self-administered by each monkey at each FR value.
| Number of Injections | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Monkey | Weight (kg) | Drug | FR 10 | FR 32 | FR 100 | FR 320 | FR 1000 |
| RO | 9.5 | Cocaine | 100 | 100 | 90 | 44 | 7.5 |
| Nicotine | 21 | 20.5 | 10 | 1 | |||
|
| |||||||
| LE | 8.5 | Cocaine | 35 | 30.5 | 24 | 14 | 4.5 |
| Nicotine | 27.5 | 27 | 11.5 | 5.5 | 1 | ||
|
| |||||||
| AN | 12.7 | Cocaine | 52.5 | 38 | 24 | 13.5 | 3 |
| Nicotine | 34 | 11.5 | 1.5 | 0 | |||
|
| |||||||
| DA | 8.8 | Cocaine | 100 | 99.5 | 70.5 | 36 | 9 |
| Nicotine | 60 | 36.5 | 29.5 | 7.5 | 0 | ||
|
| |||||||
| WA | 11.2 | Cocaine | 100 | 100 | 51 | 1 | |
| Nicotine | 45 | 26.5 | 2.5 | 0.5 | |||
|
| |||||||
| BR | 12.3 | Cocaine | 100 | 92 | 38.5 | 15.5 | 0.5 |
| Nicotine | 37 | 30 | 15.5 | 2 | 0 | ||
|
| |||||||
| ST | 11.1 | Cocaine | 100 | 67.5 | 38 | 16 | 2.5 |
| Nicotine | 34 | 24 | 9.5 | 3.5 | 0 | ||
|
| |||||||
| FO | 10.8 | Cocaine | 100 | 94 | 47 | 30.5 | 13 |
| Nicotine | 31.5 | 16.5 | 17 | 11 | 3.5 | ||
Figure 2.
Normalized demand curves for 0.01 mg/kg/inj nicotine and 0.03 mg/kg/inj cocaine. The abscissa is the unit price for the drugs, and the ordinate is the number of injections taken at each ratio value as percent of the number of injections taken at FR 10 (± SEM). Demand curves are plotted separately for nicotine (diamonds), cocaine (triangles), those monkeys that self-administered nicotine in the FR or TO experiments (solid symbols and solid lines), and those monkeys that did not self-administer nicotine (open symbols and dashed lines).
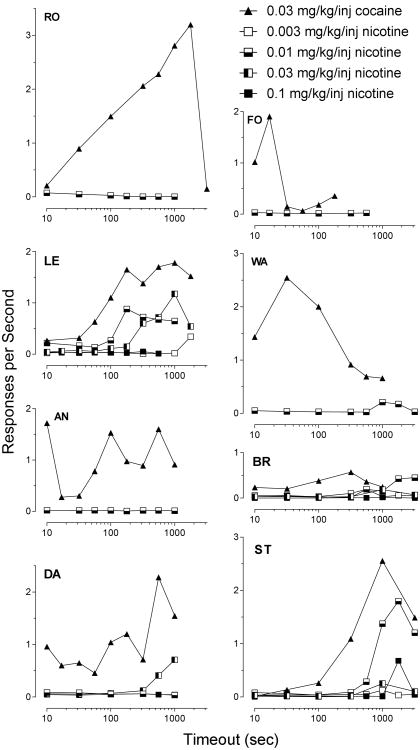
Information on the number of injections of nicotine and cocaine are presented in Table 1 for each of the eight monkeys across the various FR values. Figure 3 shows the effect of increasing TO value for several doses of nicotine in the eight monkeys. Monkey ST (and monkey DA to a lesser extent) responded at higher rates for both cocaine and nicotine when TO values were increased and the FR was held constant at 10. Monkeys BR and LE responded in a similar fashion as either FR or TO values increased, with LE responding more rapidly than BR (Fig 1). In all cases, the fastest responding occurred at intermediate doses of nicotine: 0.01 or 0.03 mg/kg/inj. Smaller (0.003 mg/kg/inj) and larger (0.1 mg/kg/inj) doses of nicotine maintained the slowest responding. Monkeys RO, AN, and WA, that had shown no evidence for a reinforcing effect of nicotine as FR increased, similarly maintained very low rates of nicotine-maintained responding across increases in TO.. Monkey FO is interesting because he showed a reinforcing effect of nicotine as FR increased, but did not do so as TO increased.
Figure 3.
Rates of responding maintained by 0.03 mg/kg/inj cocaine and as many as four doses of nicotine as a function of the TO value following each injection.
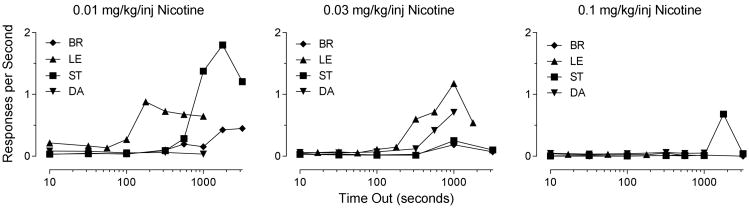
The inability of nicotine to maintain higher rates of responding as TO and dose increased is shown more directly in Figure 4 for the four monkeys that were responsive to increases in TO. As the dose of nicotine was increased, rates of responding decreased across increasing TO values, relative to smaller doses.
Figure 4.
Rates of responding maintained by each of three doses of nicotine in three monkeys. The symbols indicate data from individual monkeys
Nicotine as a punishing stimulus
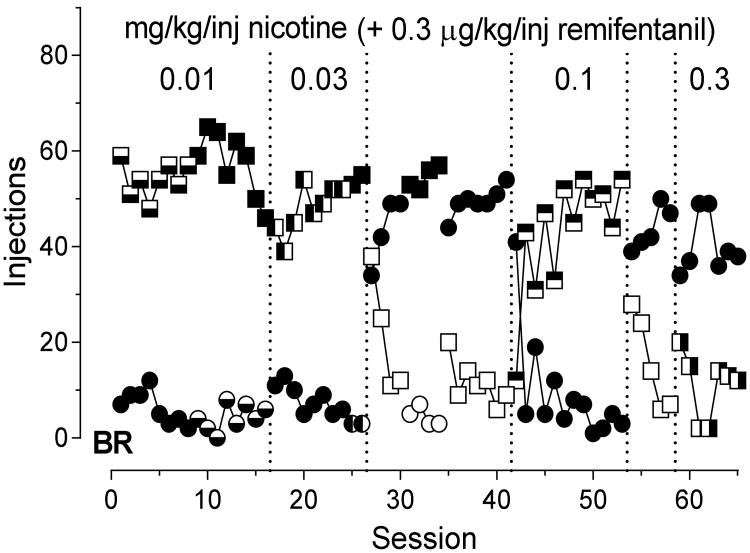
With the exception of monkey WA, who was punished by the remifentanil + nicotine combination at the smallest dose of nicotine (0.01 mg/kg/inj), all monkeys showed very strong lever side biases and selected a lever on that side regardless of whether responses there produced remifentanil alone or remifentanil in combination with 0.01 mg/kg nicotine, indicating indifference to this dose of nicotine. All subjects were sensitive to the consequences of the lever presses, however, since when saline was available on the preferred side as an option to the standard remifentanil dose, they quickly switched their responding to the non-preferred side. Responding then returned to their original preference when remifentanil + nicotine was again available on the preferred side. Reversing the contingencies and the associated light colors typically did not cause the animals to change their lever preference. An example of this choice pattern is given in Figure 5 for monkey BR. This monkey consistently selected the left lever, shown with square data points, whether responses on this lever produced remifentanil (filled points) or remifentanil + 0.01, 0.03, or 0.1 mg/kg/inj nicotine (half-filled points). Only when saline was delivered contingently on responses on the left lever did the monkey select the right lever, and he did this quickly (sessions 27-41 and 54-58). When a dose of 0.3 mg/kg/inj nicotine was combined with the standard dose of remifentanil, the monkey maintained responding on the non-preferred lever position, indicating a punishing effect of this dose of nicotine.
Figure 5.
Number of injections obtained as a consequence of responding on the left (square symbols) or right (round symbols) levers. Closed symbols indicate infusions of 0.3 μg/kg/inj remifentanil. Open symbols indicate infusions of saline. Half-closed symbols indicate infusions of 0.3 μg/kg/inj remifentanil in combination with each of four indicated doses of nicotine.
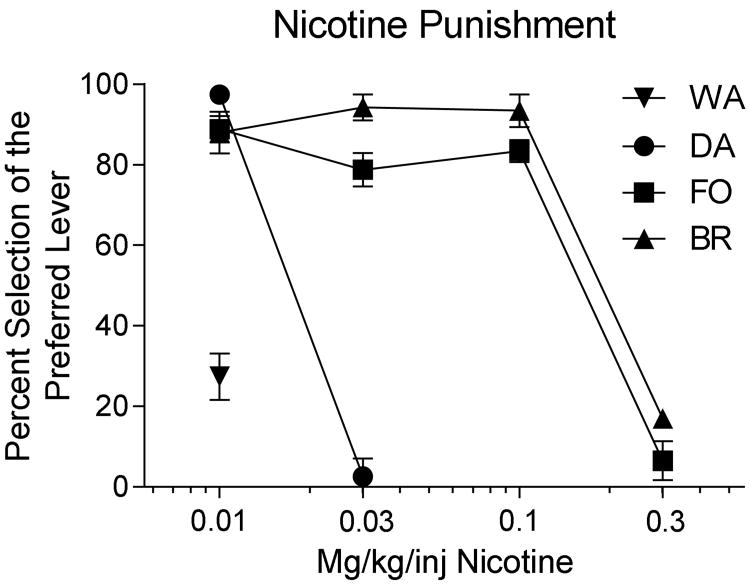
Figure 6 shows the dose of nicotine that was necessary to shift each of the animals from their preferred to their non-preferred lever position. Two monkeys (BR and FO) shifted away from their preferred side at a dose of 0.3 mg/kg/inj.; one monkey (DA) shifted at a dose of 0.03 mg/kg/inj; and the fourth monkey (WA) selected the remifentanil-alone lever from the beginning of the experiment when 0.01 mg/kg/inj nicotine was combined with remifentanil as one option.
Figure 6.
Percent selection of the preferred lever by each of four monkeys as a function of the dose of nicotine that was delivered along with 0.3 μg/kg/inj remifentanil as a consequence of responding on that lever. Data points are the average of the last five sessions at each drug combination and the vertical bars are standard deviations of the mean of these five sessions.
Discussion
The data described here make several points. First, there were individual differences among rhesus monkeys in the reinforcing effects of nicotine. Three of eight monkeys showed no reinforcing effects of nicotine as the FR or TO values increased, whereas the other five animals indicated that nicotine had weak reinforcing effects as either the FR or the TO value increased. Demand curve analysis in the monkeys confirmed the reports of other investigators as well as these data that nicotine is a relatively weak reinforcer in some subjects and not a reinforcer in others. Since they were collected similarly, these data could be compared to previous work in which a large set of drugs was compared with demand analyses (Koffarnus et al. 2012). In that study, demand for i.v. cocaine, remifentanil, methohexital, ketamine, ethanol, and saline, along with oral sucrose pellets were compared. To facilitate comparison, the data in that study were reanalyzed with identical parameters of this study, which differed due to a different fitted k value in the two studies (2.36 vs. 1.58). The Pmax value for cocaine across all eight monkeys of the present study (179.4) was similar to the overall Pmax for cocaine in our previous study (185.4). The Pmax for nicotine in the present study (36.5) was below that of ethanol (57.3), which was the lowest Pmax observed of all drugs in that study. However, this value was still considerably higher than that for saline (20.6). Among those monkeys that showed a reinforcing effect in the FR or TO experiments, Pmax for nicotine (62.7) was very similar to that of ethanol, while for those monkeys that did not show reinforcing effects Pmax for nicotine (15.3) was similar to and slightly lower than Pmax for saline. This indicates that nicotine served as a reinforcer in the present study for a selected number of monkeys, with reinforcing efficacy similar to that of ethanol when it was a reinforcer.
Second, an inverted-U shaped dose response curve was observed across increasing FR values for all monkeys responding for 0.03 mg/kg/inj cocaine, as has been reported by us and others (Winger, 1993; Pickens et al. 1981; Kliner et al. 1988), although the monkeys differed in their maximum rate of responding. This was generally true for nicotine as well, although the curves were not as well defined as those shown by Mello and Newman (2011). The ascending limb of these curves is likely due to an increase in the time between injections enforced by the greater response requirement, and a subsequent relief of the direct rate-suppressing effects of the drugs. This is confirmed by the data showing similarly shaped curves across increasing TO values. Rates of responding maintained by cocaine, nicotine and saline, where studied, were similar at small ratio and TO values. However, the behavioral pharmacological mechanisms for these low rates may be different. Cocaine's rate maintaining effect was suppressed by high-density cocaine delivery at small ratios and short TOs. Saline maintained low response rates because it had little value as a reinforcer. Nicotine's rate-maintaining effect was likely low for reasons of both low reinforcer effectiveness and high reinforcer density, with these two factors differing in different monkeys.
Third, for four of the eight monkeys, nicotine maintained faster responding as the schedule of reinforcement allowed for less frequent drug infusions (i.e., enforced TOs). It was interesting that one monkey, FO, demonstrated a reinforcing effect of nicotine as FR was increased, but did not do so as TO was increased. The fact that this animal did not show robust increased rates of cocaine-maintained responding as TO increased may indicate that TO was more aversive (Kaufman and Baron 1968) to this animal than to the other monkeys. The finding of increased nicotine-maintained responding as TO increased supports the idea presented by Goldberg and Henningfield (1988) that the reinforcing effects of nicotine become more evident if the opportunity for self-administration is spaced through time. However, this is not unique to nicotine; other drugs of abuse, at appropriate doses, show similar inverted U-shaped curves that define the relation between rates of responding and time between injection opportunities (Winger 1993; Winger et al.1996).
Fourth, nicotine did not support faster responding at larger TO values as the dose was increased. This is in marked contrast to other drugs that serve as reinforcers such as cocaine, methohexital (Winger, 1993), remfentanil and nalbuphine (Winger et al. 1996). Each of these drugs, even those that are weak reinforcers such as methohexital and nalbuphine, showed the fastest responding at large doses and large TO values. Our interpretation of these comparisons is that a pattern of nearly monotonically increasing response rates across dose at long TOs occurs with drugs that do not have aversive effects at large doses. This pattern is not observed with nicotine, and would not be expected with a drug that has aversive effects at larger doses. Within this interpretation, these data indicate that the range of reinforcing doses of nicotine is quite limited. Doses below 0.01 mg/kg/inj are too small to support responding, and doses above 0.03 mg/kg/inj appear to be aversive.
Questions of what other environmental or historical conditions might enhance the rates of responding maintained by nicotine are not addressed by this study. We show that dose selection is critical, and that faster rates develop when density of reinforcement is decreased. Making nicotine available on another baseline, such as food or smaller doses of cocaine, or even attempting nicotine self-administration in drug-naïve monkeys may result in a different apparent reinforcing effectiveness for nicotine.
Fifth, larger doses of nicotine are aversive, and there are individual differences among the monkeys with respect to the dose of nicotine required to suppress remifentanil-maintained responding. Although too few monkeys were tested to make this point with confidence, the data are consistent with the notion that monkeys that show a reinforcing effect of nicotine are less sensitive to the punishing effects of the drug. Although one monkey (DA) indicated that a dose of 0.03 nicotine was both reinforcing and aversive, the reinforcing effects were demonstrated when there was no opportunity for rapid drug administration (drug available every 17 min), whereas the aversive effects were evidenced when drug could be administered as often as every 2 min.
Because nicotine does serve as a reinforcer in some monkeys, it is possible that the combination of remifentanil + nicotine would have been preferred over remifentanil alone at some nicotine doses. This was never seen; most animals were indifferent to the addition of nicotine to remifentanil until a certain nicotine dose was delivered, at which point they selected the remifentanil-alone option. This approach, which looks for a change is preference, is somewhat different from the typical punishment procedure, in which the ability of a stimulus to suppress responding maintained by a positive reinforcer is evaluated. As noted by Freeman et al. (2014), choice procedures provide stable baseline measures relatively quickly, and the dose-related effects of the punisher can be observed fairly quickly. This procedures is also likely to be more sensitive to the aversive properties of a drug stimulus as noted by Negus (2005) and Woolverton (2003), and it has the advantage of providing a control for the direct rate-decreasing effect of the two drugs in combination. Simple suppression of responding by a combination of nicotine and remifentanil would be difficult to interpret. Interestingly, none of the monkeys tested fail to show a punishing effect of nicotine at some dose, and this aversive effect of nicotine may account for the unique and consistent inability of this drug to maintain higher response rates at larger doses as the timeout after drug administration is increased.
Many of these points (nicotine can both support and suppress responding, depending on the environmental conditions) were made by Goldberg et al. (1983) over 30 years ago, although this earlier work did not report the individual differences that we observed in this study. Since that time, a great many studies have been conducted on the stimulus properties of nicotine, with the majority of studies indicating that the drug can function as a positive reinforcer in rats and non-human primates. The reinforcing effects of nicotine have been demonstrated in previous studies with rhesus monkeys. Most recently, Mello and Newman (2011) observed that each of 5 monkeys showed inverted U-shaped dose-response curves with both nicotine and cocaine and with a combination of these two drugs as reinforcers. Although responding was consistently slower for nicotine than for cocaine, the differential between behaviors supported by the two drugs was not as great in their study as that shown by many of the monkeys in the current study, and less individual difference was observed. Mello and Newman used a second-order schedule of drug-maintained responding in which the stimulus that accompanied drug delivery was delivered alone as well as paired with either cocaine or nicotine contingently on responding on a variable ratio schedule. The distinct capacity of nicotine to enhance the ability of other stimuli to serve as reinforcers was suggested by Goldberg and Henningfield (1988) and demonstrated by Cagguila et al. 2009.. Studies in squirrel monkeys have also shown high rates of nicotine-maintained responding using second-order schedules. It is possible that behavior maintained under a second order schedule is more likely to occur at higher rates than behavior maintained under a simple FR schedule, even if frequency of reinforcer delivery is similar. This may be even more likely when the stimulus in question has been paired with cocaine as well as nicotine. It should be noted that in the current experiment the same stimulus light accompanied delivery of both response-contingent nicotine and response-contingent cocaine. It is impossible to know without further experiments how much of the apparent nicotine-maintained responding shown here was actually the consequence of nicotine increasing the reinforcing effectiveness of the cocaine-paired stimulus.
The ability of nicotine to punish behavior has also been observed in squirrel monkeys (Goldberg and Spealman, 1983; Takada et al. 1992). In both of these studies, behavior was maintained by food under a multiple schedule; in one component of the schedule, nicotine as well as food was response contingent. There was no indication in either of these publications that there were individual differences in sensitivity of the monkeys to the punishing effects of nicotine. Woolverton (2003), however, evaluated the punishing effects of histamine under a paradigm very similar to the one shown here, but with food rather than drug serving to maintain responding. He found that four rhesus monkeys were differentially sensitive to the punishing effects of histamine in much the same way as shown in the current paper with nicotine. In a more recent study from this laboratory (Freeman et al., 2014), striking individual differences among monkeys were found in the punishing effects of histamine on cocaine-reinforced responding.
Although Goldberg and Spealman (1983) suggested that the punishing effects of nicotine might not be unique because it is possible for any stimulus to serve as a reinforcer or a punisher if appropriate behavioral histories are established, Takada et al. (1992) found that cocaine did not serve as a punisher in a paradigm in which histamine, nicotine and β carboline did serve as punishing stimuli. There is no evidence that either histamine or β carboline can act as reinforcing stimuli, leaving nicotine as the only drug studied thus far that appears to have both reinforcing and punishing stimulus effects. The mechanism whereby a single drug can serve as both a punisher and a positive reinforcer may be straightforward. Studies of the discriminative stimulus effects of nicotine in rats showed that, whereas small doses of nicotine act on the α4β2 receptor to produce their stimulus effects, larger doses act on this and one or more other nicotinic receptor(s) (Jutkiewicz et al. 2011). Studies of the punishing effects of nicotine in rats indicated that this effect is antagonized by the non-selective nicotine antagonist, mecamylamine, but not by the α4β2-selective antagonist, dihydroβerythoydin (Truong 2014, Unpublished observations). These differential effects of nicotine at high and low doses may account for the findings reported here. A dose of 0.01 mg/kg/inj nicotine is likely to produce reinforcing effects through an action at α4β2 receptors; larger doses may act on other nicotinic receptors (e.g., α5 receptor) to produce an aversive effect that prevents the reinforcing effects of nicotine from being demonstrated (Tuesta et al., 2011). Even by considerable spacing of the opportunities for drug administration, there is little evidence that a dose of 0.3 mg/kg/inj nicotine has any reinforcing effectiveness, suggesting that a single injection of this dose is aversive.
In conclusion, nicotine appears to be dissimilar to most abused drugs in that it has both reinforcing and punishing effects at relatively low doses, with the first active reinforcing dose similar to or only slightly lower than the first active punishing dose. Furthermore, the reinforcing effects of low doses of nicotine are often only revealed when administrations are spaced out in time. In the present paradigm, prominent individual differences exist in each of these effects, which may explain the relatively low mean reinforcing efficacy of this drug, which was similar to that of ethanol in a previous study.
Acknowledgments
This work was supported by PHS DA023992 to GW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The excellent technical assistance of Angela Lindsey, Matthew Zaks, Kathy Carey Zelenock, and Yong Gong Shi is gratefully acknowledged, as are the helpful editorial comments of JH Woods.
This research is dedicated to William L. Woolverton and Steven R Goldberg who set the standard for studies of drugs as reinforcers and as punishers, as well as for clear, concise scientific writing.
Footnotes
The authors have no conflict of interest to declare.
References
- Ator NA, Griffiths RR. Nicotine self-administration in baboons. Pharmacol Biochem Behav. 1983;19:993–1003. doi: 10.1016/0091-3057(83)90406-9. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: A dual-reinforcement model. In: Caggiula AR, Bevins RA, editors. The Motivational Impact of Nicotine and its Role in Tobacco Use Nebraska Symposium on Motivation. Vol. 55. 2009. pp. 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Freeman KB, McMaster BC, Roma PG, Woolverton WL. Assessment of the effects of contingent histamine injections on the reinforcing effectiveness of cocaine using behavioral economic and progressive-ratio designs. Psychopharmacology. 2014;231:2395–2403. doi: 10.1007/s00213-013-3396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KB, Naylor JE, Prisinzano TE, Woolverton WL. Assessment of the kappa opioid agonist, salvinorin A as a punisher of drug self-administration in monkeys. Psychopharmacology. 2014;231:2751–2758. doi: 10.1007/s00213-014-3436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Henningfield JE. Reinforcing effects of nicotine in humans and experimental animals responding under intermittent schedules of i.v.drug injection. Pharmacol Biochem Behav. 1988;30:227–234. doi: 10.1016/0091-3057(88)90450-9. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD. Suppression of behavior by intravenous injections of nicotine or by electric shocks in squirrel monkeys: effects of chlordiazepoxide and mecamylamine. J Pharmacol Exp Ther. 1983;224:334–340. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Risner ME, Henningfield JE. Control of behavior by intravenous nicotine injections in laboratory animals. Pharmacol Biochem Behav. 1983;19:1011–1020. doi: 10.1016/0091-3057(83)90408-2. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand Stat Theory. 1979;6:65–70. [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Brooks EA, Kynaston AD, Rice KC, Woods JH. Patterns of nicotinic receptor antagonism: nicotine discrimination studies. J Pharmacol Exp Ther. 2011;339:194–202. doi: 10.1124/jpet.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Baron A. Suppression of behavior by timeout punishment when suppression results in loss of positive reinforcement. J Exp Anal Behav. 1968;11:595–607. doi: 10.1901/jeab.1968.11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliner DI, Lemaire GA, Meisch RA. Interactive effects of fixed-ratio size and number of food pellets per fixed-ratio on rats' food reinforced behavior. Psychological Record. 1988;193:676–688. [Google Scholar]
- Koffarnus MN, Hall A, Winger G. Individual differences in rhesus monkeys' demand for drugs of abuse. Addict Biol. 2012;17:887–896. doi: 10.1111/j.1369-1600.2011.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFoll B, Wertheim C, Goldberg SR. High reinforcing efficacy of nicotine in non-human primates. PLoS ONE. 2007;2:e230. doi: 10.1371/journal.pone.0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Newman JL. Discriminative and reinforcing stimulus effects of nicotine, cocaine and nicotine + cocaine combinations in rhesus monkeys. Exp Clin Psychopharmacol. 2011;19:203–214. doi: 10.1037/a0023373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2005;181:244–252. doi: 10.1007/s00213-005-2266-7. [DOI] [PubMed] [Google Scholar]
- Pickens R, Muchow D, DeNoble V. Methohexital-reinforced responding in rats: effects of fixed ratio size and injection dose. J Pharmacol Exp Ther. 1981;216:205–209. [PubMed] [Google Scholar]
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: fixed ratio and progressive ratio schedules of intravenous drug infusion. J Pharmacol Exp Therap. 1983;224:319–326. [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology. 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Maintenance of behavior by postponement of scheduled injections of nicotine in squirrel monkeys. J Pharmacol Exp Ther. 1983;227:154–159. [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Maintenance of schedule-controlled responding by intravenous injections of nicotine in squirrel monkeys. J Pharmacol Exp Ther. 1982;223:402–408. [PubMed] [Google Scholar]
- Takada K, Barrett JE, Allen MS, Cook JM, Katz JL. Punishment of schedule-controlled behavior with beta-carboline injections: antagonism and comparisons with other compounds. J Pharmacol Exp Ther. 1992;261:138–145. [PubMed] [Google Scholar]
- Tuesta LM, Fowler CD, Kenny PJ. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol. 2011;82:984–995. doi: 10.1016/j.bcp.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger G. Fixed-ratio and time-out changes on behavior maintained by cocaine or methohexital in rhesus monkeys: 1. Comparison of reinforcing strength. Exp Clin Psychopharmacol. 1993;1:142–153. [Google Scholar]
- Winger G, Woods JH, Hursh SR. Behavior maintained by alfentanil or nalbuphine in rhesus monkeys: Fixed ratio and time-out changes to establish demand curves and relative reinforcing effectiveness. Exp Clin Psychopharmacol. 1996;4:131–140. [Google Scholar]
- Woolverton WL. A novel choice method for studying drugs as punishers. Pharmacol Biochem Behav. 2003;76:125–131. doi: 10.1016/s0091-3057(03)00219-3. [DOI] [PubMed] [Google Scholar]