Abstract
Rationale
Phosphodiesterase-4 (PDE4) and neuroimmune signaling have been posited to regulate alcohol drinking.
Objectives
This study evaluated the involvement of PDE4 and Il22ra2 on ethanol (EtOH) intake by alcohol-preferring (P) and high-alcohol drinking (HAD1) rats.
Methods
Exp 1 determined the dose-response effects of PDE4 inhibitors, rolipram and Ro 20-1724, on 2h/day free-choice EtOH intake by adult P and HAD1 rats. Exps 2–3 examined the effects of repeated administration with the PDE4 inhibitors on EtOH or sucrose intake, and locomotor behavior. Exp 4 determined Pde4-associated gene expression differences in subregions of the extended amygdala, between high- and low-alcohol-consuming rat lines. Exp 5 evaluated the effects of infusing short hairpin RNA to knock down Il22ra2 in the nucleus accumbens (NAc) shell on 24h free-choice EtOH drinking by P rats.
Results
Administration of rolipram or Ro 20-1724 reduced EtOH intake by P rats; Ro 20-1724 reduced EtOH intake by HAD1 rats. Repeated rolipram or Ro 20-1724 exposure reduced EtOH intake by P and HAD1 rats. PDE4 inhibition induced motor impairment during the first hour of EtOH intake by P rats. Higher gene expression levels for PDE4A were found in the NAc shell of P vs. NP rats. ShRNAs targeting Il22ra2 in the NAc shell significantly reduced chronic EtOH intake.
Conclusions
PDE4 and neuroinflammatory/immune signaling pathways could represent molecular targets for the treatment of alcohol use disorders, in genetically predisposed subjects. This study underscores the importance of testing compounds over multiple days and rat lines when determining efficacy to disrupt excessive alcohol intake.
Keywords: Alcohol-preference, animal model, genetic predisposition, high-alcohol consuming, selective breeding, Interleukin 22 receptor
Introduction
Evidence suggests that 3’,5’-cyclic adenosine monophosphate (cAMP) reduces activity of pro-inflammatory cytokines, such as tumor necrosis factor α (TNF- α) and interleukin (IL)-17, and increases activity of anti-inflammatory mediators such as IL-10 (Baumer et al. 2007). Thus, cAMP regulates homeostasis in immune cell signaling (Oger et al. 2005). One manner of modulating cAMP, is through the enzymatic activity of 3’,5’-cyclic nucleotide phosphodiesterase type-IV (PDE4; Conti and Beavo 2007; Houslay and Adams 2003). PDE4 can interrupt the homeostatic immune process via its action on cAMP, altering the balance of pro- and anti-inflammatory mediators (Oger et al. 2005).
Alterations in cAMP and neuroimmune signaling are associated with elevated reward and reinforcement from drugs of abuse including cocaine, morphine, methamphetamine and alcohol (Boudreau et al. 2009; Hutchinson et al. 2007; Janes et al. 2009; Misra and Pandey 2006; Snider et al. 2013; Terwilliger et al. 1991; Thiele et al. 2000). Genetic differences (alcohol-preferring [P] vs –nonpreferring [NP] rats) in cAMP second messenger systems are associated with differences in a propensity for alcohol abuse (Pandey et al. 1999). Similarly, chronic alcohol drinking reduces cellular cAMP levels (Gobejishvili et al. 2008) and increases immune signaling and inflammatory response (Fernandez-Lizarbe et al. 2009; Gobejishvili et al. 2008; Hu et al. 2013; Qin et al. 2008). Alcohol and drug dependence are associated with complex interactions between immune and neural signaling, stress-associated activity (e.g. hypothalamic-pituitary-adrenal axis), and transcription activity within reward neurocircuitry (Allen et al. 2011; Crews and Nixon 2009; Crews et al. 2011; Frank et al. 2011; Kelley and Dantzer, 2011; Misra and Pandey 2006; Rosi, 2011; Yakovleva et al. 2011).
Innate differences in expression of genes involved in apoptosis and cell death, as well as inflammation, including Il22ra2, have been reported in the shell region of the nucleus accumbens (NAc) and in the ventral tegmental area (VTA) of some high alcohol-preferring rat lines (e.g. P and High Alcohol Drinking [HAD1 and HAD2] rats), relative to their non-alcohol-preferring counterparts (e.g. NP and Low Alcohol Drinking [LAD1 and LAD2] rats; McBride et al. 2013b). Il22ra2 encodes for IL-22 receptor α2 subunit (IL-22ra2; IL-22BP; CFR2–10), which is primarily a pro-inflammatory antagonist of IL-22 activity (Kotenko et al. 2001). Similarly, changes in expression of genes associated with cell death have been reported in the NAc shell of P rats following binge-like alcohol drinking (McBride et al. 2010, 2013a), and in the VTA of P rats following excessive binge-like alcohol drinking (McBride et al. 2013a). These findings may indicate that innate vulnerability to neuroinflammation is exacerbated by excessive ethanol (EtOH) intake, and could contribute to this high alcohol drinking phenotype.
PDE4 isoforms A, B and D are the primary mediators of cAMP activity in inflammatory cells (Page and Spina 2011). Evidence suggests that PDE4B may be a target of interest for addiction research, due to its high expression levels in brain regions associated with reward and reinforcement (e.g. NAc and central nucleus of the amygdala [CeA]; Cherry and Davis 1999; Perez-Torres et al. 2000). PDE4B expression is up-regulated following chronic alcohol exposure in vitro (Gobejishvili et al. 2008) and is heavily involved in inflammatory processes (cf. Jin et al. 2012) which are implicated in alcohol and drug dependence (Crews et al. 2011).
A recent study indicated that the non-selective PDE inhibitor ibudilast (AV-411) reduced 2h EtOH intake by P and HAD1 rats, as well as alcohol-dependent C57BL/6J mice (Bell et al. 2014a). In addition, rolipram, a selective inhibitor of PDE4 (Kenk et al. 2011), reduced EtOH-reinforced operant responding by Fawn-Hooded rats, without altering sucrose-reinforced responding (Wen et al. 2012). Similarly, rolipram and another PDE4 inhibitor Ro 20-1724 (Wachtel 1983) reduced 2-bottle choice EtOH intake in C57BL/6J mice (Hu et al. 2011).
The current study examined the effects of selective PDE4 inhibitors on binge EtOH intake, by P and HAD1 rats. Innate Pde4 gene expression differences between P vs NP and HAD1 vs LAD1 rats were also determined. Finally, the effects of microinfusing shRNAs for Il22ra2 in the NAc shell on alcohol drinking by P rats were evaluated.
Materials and Methods
Subjects
The subjects were adult, male P, NP, HAD1, and LAD1 rats and female P rats. EtOH-naïve animals were pair-housed in standard plastic tubs. Subjects given EtOH access were housed individually in hanging stainless steel wire-mesh cages (containing a Plexiglas platform). All rats received free access to standard laboratory chow and water. Male rats used for 2h scheduled access drinking were maintained on a 12/12h reverse light cycle (lights off at 1030). Female P rats used for shRNA experiments, were given 24h free-choice access to EtOH and were maintained on a 12/12h normal light cycle (lights on at 0700). Subjects were housed in a temperature- (21°C) and humidity- (50%) controlled vivarium. Animals were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All experimental procedures were approved by the Institutional Animal Care and Use Committees of the Indiana University Schools of Dentistry and Medicine (Indianapolis, IN) and are in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (Research Institute for Laboratory Animal Research 2011).
Procedures
In Exps 1 and 2, the effects of PDE4 inhibitors rolipram (Sigma-Aldrich, St. Louis, MO, USA) and Ro 20-1724 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) on EtOH intake by male P and HAD1 rats were examined during 2-hr, 3-bottle (water, and 15% and 30% EtOH) free-choice test sessions. The length of the EtOH access period was titrated as follows: two weeks of 24h access; two weeks of 4h access; then two weeks of 2h access. On the final day of access, one cohort (n = 10/rat line) was assessed for EtOH intake (g/kg) and blood EtOH concentration (BEC) 30 min into the EtOH drinking session. Briefly, animals were killed with CO2 inhalation, and then decapitated to collect blood from the trunk cavity. Blood samples were centrifuged, serum was extracted, frozen on dry ice, and stored at −80°C for subsequent analysis of BEC (Analox Instruments, Ltd., Lunenburg, MA, USA).
Dose-dependent effects of PDE4 inhibition on EtOH intake
Two cohorts of rats were randomly assigned to treatment groups balanced for equivalent EtOH intake. For Exp 1 (within subject dose-response effects), each P and HAD1 male rat (n = 16/rat line) was administered four doses of either rolipram (0, 0.25, 0.50, and 1.00 mg/kg; i.p.) or Ro 20-1724 (0, 5, 10, and 20 mg/kg, i.p.) in a counterbalanced design across 4 consecutive days. These doses were selected to approximate those previously reported to reduce alcohol drinking in mice (Hu et al. 2011). The vehicle for all compounds was a few drops of Tween 80 in sterile saline.
Effects of repeated PDE4 inhibition on EtOH or sucrose intake
For Exp 2, P and HAD1 rats were given a single dose of one of the PDE4 inhibitors, or vehicle for 5 consecutive days. Rolipram was administered at a dose of 1 mg/kg in P and HAD1 rats (n = 8/rat line). Ro 20-1724 was administered at 10 (n = 9) or 20 (n = 6) mg/kg in P rats and 20 mg/kg in HAD1 rats (n = 8). Next, EtOH was withdrawn for 2 weeks; then rats were given two weeks (Mon-Fri, weekends off) of 2h access to 2% w/v sucrose. The following week served to assess sucrose intake, during which the rats received rolipram or Ro 20-1724. All compounds were administered i.p. 60 min prior to the drinking test sessions, which coincided with the beginning of the dark cycle. Before and after each test session, EtOH or sucrose and water intakes were assessed. Body weight was measured at least 3 days a week.
Locomotor response to repeated PDE4 inhibition
A separate cohort of male P and HAD1 rats (n = 7/drug/rat line) was tested for time-dependent effects of repeated exposure to the PDE4 inhibitors on locomotor activity (LMA) (Exp 3). Rats were provided the same EtOH drinking protocol as described above, followed by a 4-wk alcohol abstinence period. After this, subjects were injected with 1 mg/kg rolipram or 20 mg/kg Ro 20-1724, or vehicle across 5 consecutive days. On the 5th injection day, rats were placed in LMA monitoring chambers for 30 min trials starting at 60 or 120 min post-injection. These time periods reflect the beginning of the first and second hour of EtOH or sucrose access in Exps 1 and 2. Ambulatory activity and rearing were recorded (Versadat v. 3.02-127E, Accusan Instruments Inc., Columbus, OH). Food and 24h water consumption as well as body weight were measured daily, immediately prior to the start of the dark cycle, but were not available during the LMA test sessions.
Pde4 gene expression in the NAc shell, CeA, and VTA
Pde4 gene expression in the NAc shell, CeA and VTA was assessed in EtOH-naïve male P, NP, HAD1 and LAD1 rats (Exp 4). Microarray procedures to assess levels of gene expression in micro-punched brain regions were conducted as previously described (McBride et al. 2013b). CEL files were imported into Partek Genomics Suite (Partek, Inc., St. Louis, MO) for analysis.
Effects of shRNA for Il22ra2 in the NAc shell on EtOH intake of P rats
To examine a possible role for neuroimmune signaling in alcohol drinking (Exp 5), two lentiviral vector constructs (#1928, #1408) targeting Il22ra2 mRNA for knockdown in the NAc shell of adult female P rats were synthesized by the Lasek lab (University of Illinois, Chicago, IL). Sequences encoding shRNAs targeting Il22ra2 were designed and cloned into the lentiviral vector pLL3.7 and lentivirus was produced as previously described (Lasek et al. 2007). The 19 nucleotide targeting sequences for Il22ra2 were as follows: shIl22ra2-1408: 5’-GTGGCTGTCTCTAAATTAA-3’; shIl22ra2-1928: 5’-GGTGCTCCAACGTATTACT-3’. Rats were given continuous (24h), free-choice access to EtOH (15% v/v) for at least 6 weeks prior to surgery to stabilize EtOH drinking levels, then were balanced into two groups with equivalent EtOH drinking levels. Stereotaxic surgeries were performed on the rats under isoflurane anesthesia; each animal received bilateral infusions aimed at the NAc shell (AP +1.7, ML +2.4, DV −7.5, 10 ° offset from vertical; Paxinos and Watson, 1998). Animals were infused with 1.5 µl/side of lentivirus expressing Il22ra2 shRNA or a scrambled vector control (n = 7–9/group), at a flow rate of 0.2 µl/min. The infusate was allowed 10 min to diffuse. Incision sites were closed with stainless steel wound clips. Three days post-infusion, rats were given 24h free-choice access to 15% EtOH and water for 6–7 weeks. EtOH (g/kg) and water (ml/kg) intakes were assessed daily. Body weights were measured 3 days/wk. Next, EtOH access was withdrawn, and rats were given 2 weeks with 24h access to 2% w/v sucrose.
Data analyses and statistics
Fluid intakes were determined as before and after changes in the weight of glass drinking bottles. EtOH intake was converted to g of absolute EtOH consumed/kg of body weight/unit time. BECs were assessed in triplicate on the Analox alcohol analyzer and averaged. Linear regression analyses were used to assess the association between EtOH intake during the first 30 min of access and BECs.
For Exp 1, omnibus three-way (line x dose x day) mixed-model ANOVAs with repeated measures for day were used to assess 2h EtOH intake. Next, the two rat lines were analyzed separately, using two-way (dose x day) ANOVAs with repeated measures for day, to assess dose-dependent changes in EtOH and water intake and body weight during the post-treatment test periods. If a significant interaction term or overall dose effect was obtained, one-way ANOVAs, followed by Dunnett’s multiple comparisons t-tests, were conducted for each dose over the treatment period for 2h EtOH or water intake, 24h water and food intake, and body weight, to determine significant individual dose effects compared to vehicle. For Exp 2, separate two-way (treatment × day) ANOVAs with repeated measures for day, followed by one-way ANOVAs (P rats) or independent samples t-tests (HAD1 rats) were used to assess 2h EtOH or sucrose intake, 24h water and food intake, and body weight. For Exp 3, one-way ANOVAs were used to analyze the effect of treatment and treatment-test interval (i.e. 60–90 or 120–150 min; “time”) on cumulative distance traveled and rearing counts over the 30 min test sessions for each rat line separately. Dunnett’s multiple comparisons t-tests were used to compare behavior of PDE4 inhibitor-exposed groups to vehicle-values at the two post-exposure time-points.
PDE4 gene expression levels in the NAc, CeA, and VTA of P vs. NP and HAD1 vs. LAD1 rats (Exp 4) were analyzed as previously reported (McBride et al. 2013b). Robust multichip average (RMA; Bolstad, 2003) expression levels were generated for all probe sets using the RMA background correction, Quantile normalization and summarization by Median Polish. Summarized signals for each probe set were log2 transformed. Student’s t-tests were performed between each line-pair (P/NP, HAD1/LAD1) using log2 transformed signals. Fold-changes were calculated using raw expression levels.
To assess the effects of shRNAs targeting Il22ra2 on EtOH intake (Exp 5), EtOH intake was averaged by week. Two-way (treatment x week) ANOVAs with repeated measures for week were used to assess 24h EtOH or sucrose or water intake, as well as body weight. If a significant interaction term or overall treatment effect was obtained, then independent samples t-tests were conducted on each treatment week and over the treatment period for 24h EtOH or sucrose intake, 24h water intake, and body weight.
Results
BEC (30 min)
For P rats, the average EtOH intake during the first 30 min of access was 1.7 ± 0.2 g/kg, and the average 30-min BEC was 80 ± 12 mg%. For HAD1 rats, the average EtOH intake during the first 30 min of access was 2.3 ± 0.2 g/kg, and the average BEC was 50 ± 12 mg%. Linear regression analyses revealed significant associations between EtOH intake and BEC in both rat lines [F’s (1,9) ≥ 6.63, p’s < 0.05].
Dose-dependent effects of PDE4 inhibition on EtOH intake
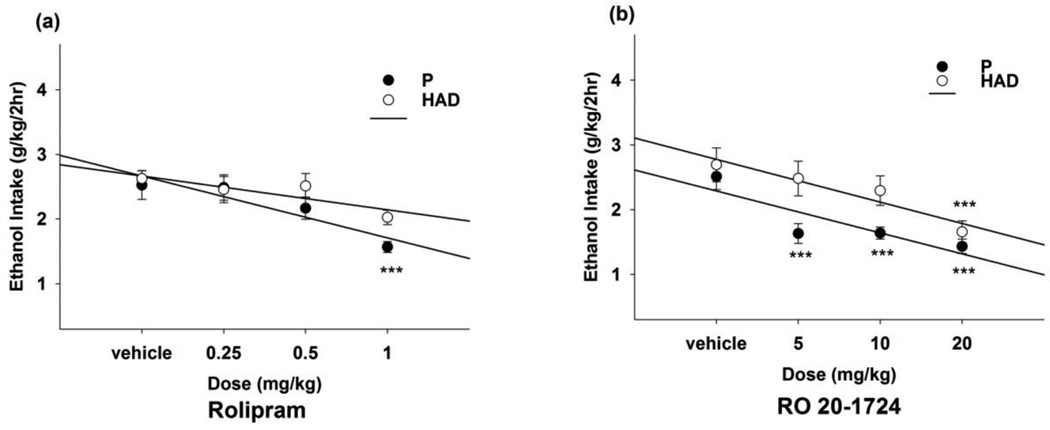
The omnibus mixed-model ANOVA in P and HAD1 rats revealed a significant main effect of dose [F(3,82) = 5.19, p < 0.01] of rolipram (Fig. 1a). Separate two-way ANOVAs revealed a significant main effect of dose in P rats [F(3,45) = 7.18, p < 0.001]. The univariate ANOVA, followed by Dunnett’s multiple comparisons t-tests, revealed that 1 mg/kg rolipram reduced (~40%) 2h EtOH intake in P rats, relative to vehicle (p < 0.001). For HAD1 rats, rolipram did not alter 2h EtOH intake significantly, relative to vehicle, although there was a trend toward a reduction.
Fig. 1.
EtOH intake (g/kg) during Exp 1 for EtOH-experienced P and HAD1 rats given 2h limited, free-choice access to 15% and 30% v/v EtOH and water, available concurrently. Each subject was administered (a) rolipram (0.25, 0.50, and 1.00 mg/kg) or (b) Ro 20-1724 (5, 10, and 20 mg/kg), and vehicle (i.p.), in a counterbalanced design, with a different dose delivered on each of 4 consecutive days. At 60 min following the injections, rats were given 2h access to 15% and 30% EtOH. ***, p < 0.001 vs. vehicle.
The omnibus mixed-model ANOVA in P and HAD1 rats revealed significant line by dose [F(3,82) = 2.77, p = 0.047] and dose by day [F(9,58) = 3.12, p = 0.004] interactions, as well as significant main effects of line and dose [F’s (1–3,82) ≥ 16.4, p’s < 0.001]for Ro 20-1724 (Fig. 1b). Separate two-way ANOVAs revealed significant main effects of dose in P and HAD1 rats [F’s (3,38) ≥ 11.0, p’s < 0.001]. One-way ANOVAs, followed by Dunnett’s t-tests, revealed that all Ro 20-1724 doses reduced (~45%) 2h EtOH intake by P rats, and 20 mg/kg Ro 20-1724 reduced (~40%) 2h EtOH intake by HAD1 rats (p’s < 0.001).
Effects of repeated PDE4 inhibition on EtOH or sucrose intake
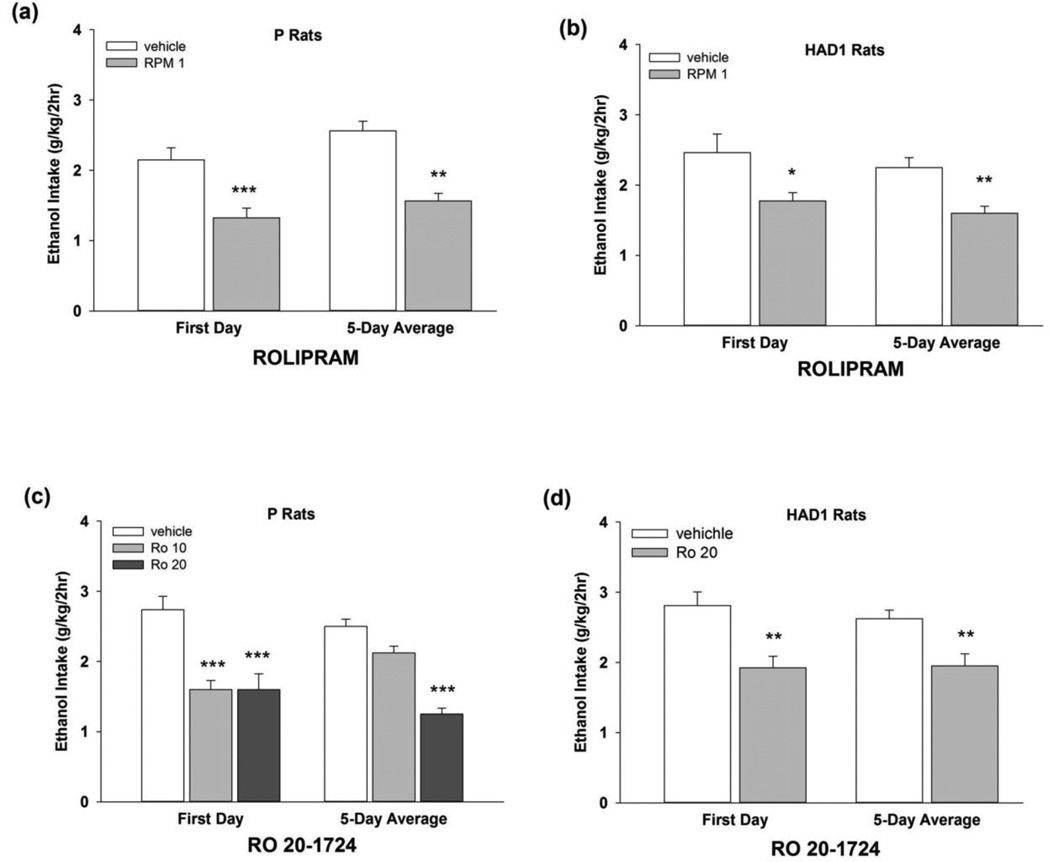
In Exp 2, following repeated administration of 1.0 mg/kg rolipram (Figs. 2a, b), an omnibus ANOVA revealed a significant main effect of treatment [F(1,14) = 36.2, p < 0.001]. Separate two-way ANOVAs revealed significant main effects of treatment in P and HAD1 rats [F’s(1,14) ≥ 10.2, p’s ≤ 0.01]. Independent samples t-tests revealed that 1 mg/kg rolipram reduced 2h EtOH intake in HAD1 and P rats (~30–40%, respectively) on all test days, with the exception of Day 5 in HAD1 rats, as well as over the 5-day test period [t’s (14) ≥ 2.24, p’s < 0.05]. For Ro 20-1724, in P rats (Fig. 2c) the two-way ANOVA revealed a significant dose by day interaction [F(8,88) = 3.79, p = 0.001], as well as a significant main effect of dose [F(2,22)= 15.9, p < 0.001]. Individual one-way ANOVAs for each test day revealed that 10 and 20 mg/kg Ro 20-1724 reduced EtOH intake on the first day. The 20 mg/kg dose also reduced (~50%) EtOH intake by P rats on the 2nd, 3rd, and 4th days, and over the 5-day period (p’s ≤ 0.01). For HAD1 rats (Fig. 2d), the two-way ANOVA revealed a significant main effect of treatment [F(1,14) = 10.1, p < 0.01]. Independent-samples t-tests for each test day revealed that 20 mg/kg Ro 20-1724 reduced (~30%) EtOH intake on the 1st, 2nd, 3rd and 5th days, as well as over the 5-day period (p’s < 0.05).
Fig. 2.
EtOH intake (g/kg) during Exp 2 for EtOH-experienced (a, c) P and (b, d) HAD1 rats given 2h limited, free-choice access to 15% and 30% v/v EtOH and water, available concurrently. Each subject was administered rolipram (1 mg/kg; upper panels) or Ro 20-1724 (10 or 20 mg/kg; lower panels), or vehicle (i.p.), for 5 consecutive test days, using a between-subjects design. At 60 min following the injections, rats were given 2h access to 15% and 30% EtOH. *, p < 0.05 vs. vehicle; **, p < 0.01 vs. vehicle; ***, p < 0.001 vs. vehicle.
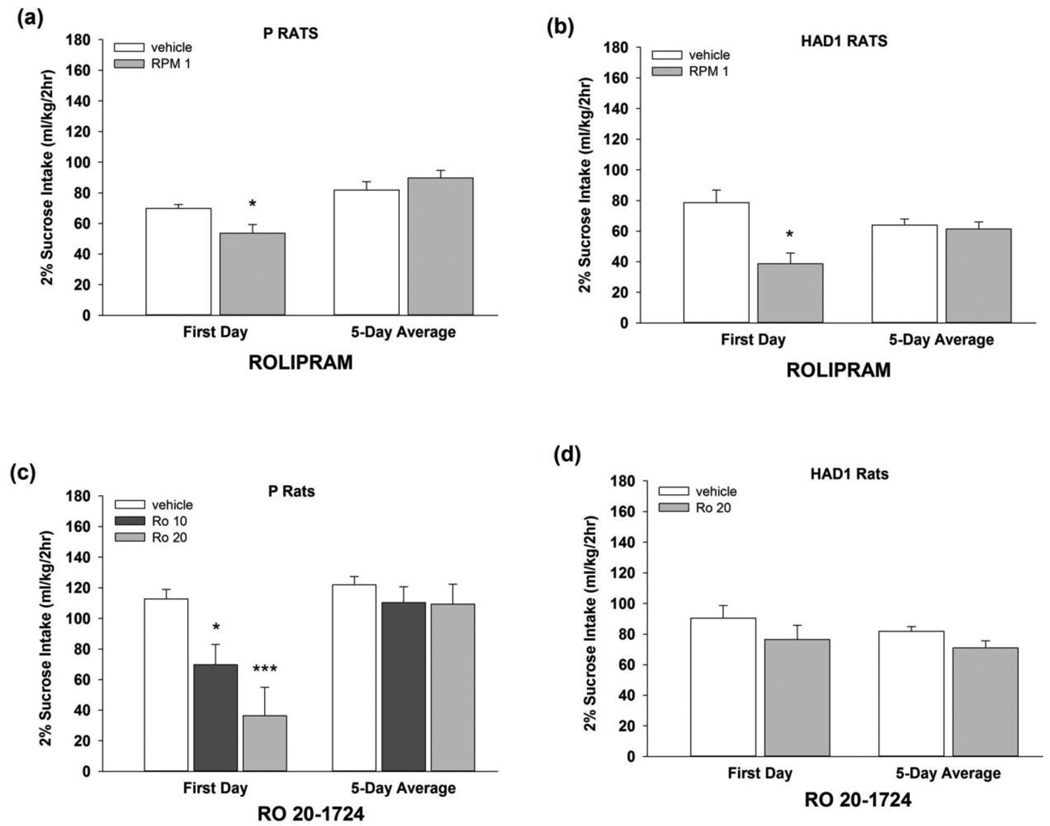
An omnibus three-way ANOVA revealed a significant treatment by day interaction [F(4,112) = 12.0, p < 0.001], and a significant main effect of line [F(1,28) = 22.8, p < 0.001] on the effect of rolipram on sucrose intake (Figs. 3a, b). Separate two-way ANOVAs revealed significant treatment by day interactions for both rat lines [F’s (4,56) ≥ 5.39, p’s < 0.001]. Independent samples t-tests revealed that rolipram treatment reduced 2h sucrose intake on the 1st test day [t’s (14) ≥ 2.61; p’s < 0.05], and increased sucrose intake on the 4th test day [t’s (14) ≥ 2.29; p’s < 0.05] in both rat lines, relative to vehicle. Rolipram did not alter sucrose intake over the 5-day test period in either rat line. For Ro 20-1724effects on sucrose intake, for P rats (Fig. 3c), the two-way ANOVA revealed a significant treatment by day interaction [F(8,84) = 3.07, p < 0.01]. Individual one-way ANOVAs for each day revealed that 10 and 20 mg/kg Ro 20-1724 reduced sucrose drinking on the first day (p’s < 0.05), but did not alter sucrose intake across the 5-day test period. Ro 20-1724 did not alter sucrose intake of HAD1 rats (Fig. 3d).
Fig. 3.
Sucrose intake (2% w/v; ml/kg) during Exp 2 for (a, c) P and (b, d) HAD1 rats with a history of EtOH drinking followed by 4 weeks without EtOH. Each subject was administered rolipram (1 mg/kg; upper panels) or Ro 20-1724 (10 or 20 mg/kg; lower panels), or vehicle (i.p.), on 5 consecutive test days, using a between-subjects design. At 60 min following the injections, rats were given 2h access to sucrose. *, p < 0.05 vs. vehicle; ***, p < 0.001 vs. vehicle.
In Exp 1, HAD1 rats given 1 mg/kg rolipram were the only animals to exhibit changes (~30% increase) in 24h water intake. No other groups treated with the PDE4 inhibitors differed from vehicle in water or food intake, or body weights (Tables 1 and 2). Following repeated exposure, rolipram increased 24h water intake in P rats. Ro 20-1724 (20 mg/kg) reduced water drinking by P rats, while increasing water intake by HAD1 rats (Table 3).
Table 1.
Dose-dependent 24h intake of water (ml/kg) and food (g/kg), and body weights (g) during Exp 1 by P and HAD1 rats across the 4 days of rolipram treatment
| Rat Line | Dose (mg/kg) |
24h Water (ml/kg) |
24h Food (g/kg) |
Body Weight (g) |
|---|---|---|---|---|
| P | 0.00 | 40.0 ± 2.6 | 42.0 ± 3.1 | 613.5 ± 12.2 |
| 0.25 | 34.7 ± 3.6 | 38.8 ± 5.0 | 613.5 ± 12.2 | |
| 0.50 | 41.0 ± 3.5 | 40.0 ± 2.8 | 611.9 ± 12.6 | |
| 1.00 | 43.3 ± 3.9 | 38.4 ± 3.2 | 613.4 ± 12.2 | |
| HAD1 | ||||
| 0.00 | 65.8 ± 4.4 | 55.5 ± 2.0 | 308.6 ± 7.7 | |
| 0.25 | 78.6 ± 4.0 | 54.8 ± 3.5 | 307.6 ± 7.6 | |
| 0.50 | 77.2 ± 5.7 | 53.0 ± 2.8 | 306.7 ± 7.6 | |
| 1.00 | 84.5 ± 5.1* | 53.3 ± 3.1 | 310.4 ± 7.4 |
Within-subjects design (i.p., vehicle [saline + a few drops of Tween 80], or 0.25, 0.50, 1.00 mg/kg rolipram).
Indicates that the respective dose differed from vehicle (Dunnett’s t-test, p < 0.05).
Table 2.
Dose-dependent 24h intake of water (ml/kg) and food (g/kg), and body weights (g) during Exp 1 by P and HAD1 rats across the 4 days of Ro 20-1724 treatment
| Rat Line | Dose (mg/kg) |
24h Water (ml/kg) |
24h Food (g/kg) |
Body Weight (g) |
|---|---|---|---|---|
| P | 0 | 68.2 ± 8.9 | 56.1 ± 1.6 | 435.7 ± 10.4 |
| 5 | 60.8 ± 3.4 | 53.9 ± 1.3 | 437.4 ± 10.8 | |
| 10 | 63.1 ± 2.6 | 54.7 ± 1.3 | 437.6 ± 10.8 | |
| 20 | 56.7 ± 3.1 | 51.1 ± 1.7 | 437.1 ± 10.3 | |
| HAD1 | ||||
| 0 | 104.6 ± 8.3 | 70.2 ± 3.8 | 271.7 ± 4.3 | |
| 5 | 118.9 ± 7.8 | 74.9 ± 3.3 | 273.7 ± 4.2 | |
| 10 | 116.9 ± 6.2 | 74.2 ± 3.2 | 271.0 ± 3.8 | |
| 20 | 113.7 ± 8.6 | 63.9 ± 3.9 | 273.4 ± 4.1 |
Within-subjects design (i.p., vehicle [saline + a few drops of Tween 80], or 5, 10, 20 mg/kg Ro 20-1724).
Table 3.
Average 24h water and food intake, and body weight during Exp 2, by alcohol-drinking P and HAD1 rats across the 5-day test of rolipram or Ro-201724 administration.
| Rat Line | Treatment-Dose (mg/kg) |
24h Water (ml/kg) |
24h Food (g/kg) |
Body Weight (g) |
|---|---|---|---|---|
| P | ||||
| RPM 0 | 33.2 ± 1.7 | 37.3 ± 1.1 | 635.2 ± 16.4 | |
| RPM 1 | 48.0 ± 2.1* | 39.4 ± 0.5 | 604.5 ± 10.1 | |
| RO 0 | 66.9 ± 2.5 | 53.3 ± 1.9 | 465.8 ± 13.2 | |
| RO 10 | 57.1 ± 1.5 | 48.3 ± 2.3 | 459.5 ± 14.0 | |
| RO 20 | 54.0 ± 5.8* | 36.6 ± 1.5* | 522.2 ± 13.1* | |
| HAD1 | ||||
| RPM 0 | 69.4 ± 5.0 | 62.6 ± 2.3 | 315.5 ± 6.7 | |
| RPM 1 | 81.9 ± 5.4 | 63.6 ± 3.4 | 311.2 ± 13.7 | |
| RO 0 | 90.4 ± 5.5 | 64.1 ± 1.5 | 297.8 ± 4.1 | |
| RO 20 | 131.8 ± 16.0* | 62.7 ± 1.5 | 277.5 ± 8.3* |
Animals were given 1 mg/kg rolipram (RPM) or 10 or 20 mg/kg Ro 20-1724 (RO) (i.p., vehicle [saline + a few drops of Tween80], using a between-subjects design.
Indicates that the respective dose differed from vehicle (p < 0.05).
Locomotor response to repeated PDE4 inhibition
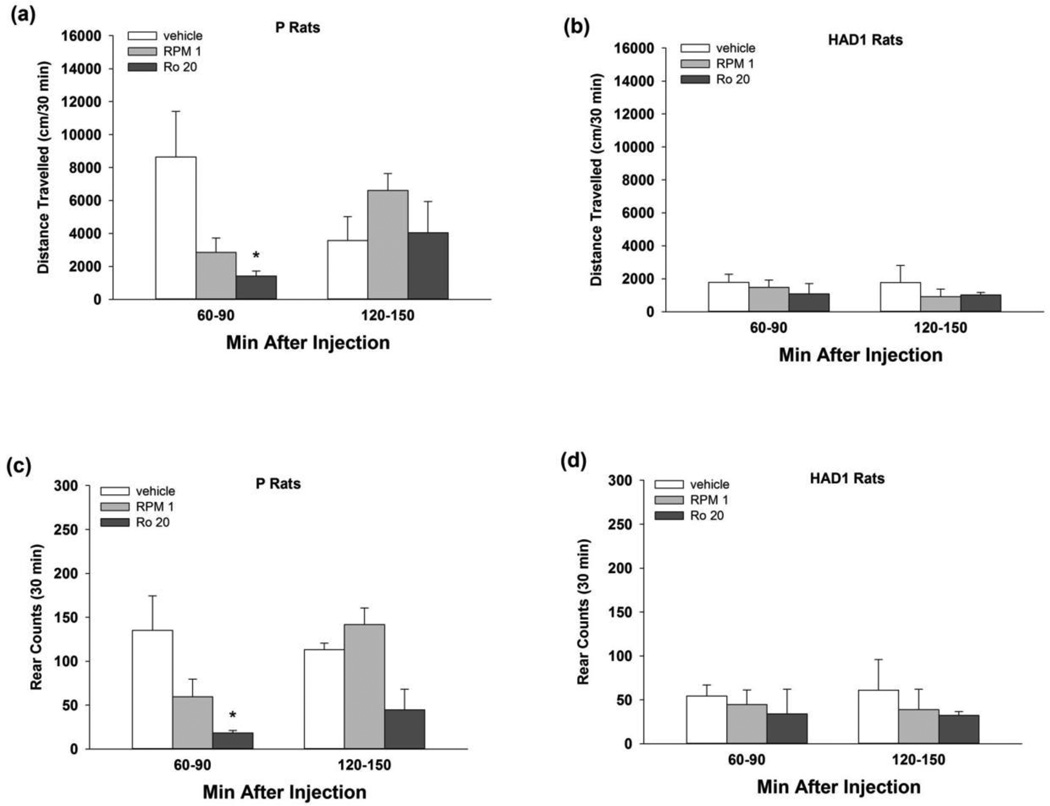
During LMA testing (Fig. 4), P rats displayed reductions in motor activity following repeated administration with the PDE4 inhibitors. In P rats, two-way ANOVAs assessing total distance travelled revealed a significant time by treatment interaction [F(4,18) = 3.03, p < 0.05] and main effect of treatment [F(2,18) = 6.50, p < 0.01]. Rolipram and Ro 20-1724 reduced ambulatory behavior by P rats at 60–90, but not 120–150, min post-injection, which corresponds to the first 30 min of EtOH or sucrose access periods, relative to vehicle (p < 0.05; Fig. 4a). For total rearing counts, two-way ANOVAs revealed a significant main effect of treatment [F(2,18) = 17.2, p < 0.001]. Rearing was reduced in P rats at 60–90, but not 120–150, min after Ro 20-1724, but not after rolipram, treatment (p < 0.05; Fig. 4c). PDE4 inhibition did not alter LMA or rearing behavior in HAD1 rats (Fig. 4b, d).
Fig. 4.
Cumulative (30 min) distance travelled (cm; upper panels) and rearing counts (lower panels) for (a, c) P and (b, d) HAD1 rats during locomotor activity test sessions (Exp 3), following 5 consecutive days of treatment with 1 mg/kg rolipram, or 20 mg/kg Ro 20-1724, or vehicle (i.p.). The 30 min test sessions occurred 6020 mg/kg Ro 20-1724, or vehicle (i.p.). The 30 min test sessions occurred 60–90 90 or 12020 mg/kg Ro 20-1724, or vehicle (i.p.). The 30 min test sessions occurred 60–90 150 min following the final injection, and coincided with the start of the first and second hrs of consummatory testing during Exp 1 and 2. *, p < 0.05 vs. vehicle.
PDE4 gene expression in the NAc shell, CeA, and VTA
Pde4 distribution was isoform-, line-, and site-specific (Table 4). In the NAc shell of P rats, Pde4a expression was higher and Pde4b expression was lower, relative to NP rats. In the CeA, Pde4b expression was also lower relative to NP rats. There were no significant differences between HAD1 and LAD1 rats in NAc shell or CeA Pde4 isoform expression. In the VTA, HAD1 rats displayed slightly higher Pde4b expression, relative to LAD1 rats. P and NP rats did not differ in VTA Pde4 isoform expression.
Table 4.
Expression levels of genes encoding for PDE4A and PDE4B in the nucleus accumbens shell, central nucleus of the amygdala, and ventral tegmental area of EtOH-naïve P vs. NP and HAD1 vs. LAD1 rats.
| P vs NP | HAD1 vs LAD1 | ||||
|---|---|---|---|---|---|
| Gene Sym | Nucleus Accumbens Shell | Fold-Change | P-value* | Fold-Change | P-value* |
| pde4a | phosphodiesterase 4A | 1.24 | 0.001 | 1.07 | 0.461 |
| pde4b1 | phosphodiesterase 4B | −1.15 | 0.117 | 1.09 | 0.454 |
| pde4b2 | phosphodiesterase 4B | −1.20 | 0.006 | −1.05 | 0.215 |
| P vs NP | HAD1 vs - LAD1 | ||||
| Sym | Central Amygdala | Fold-Change | P-value* | Fold Change | P-value* |
| pde4a | phosphodiesterase 4A | −1.06 | 0.227 | −1.04 | 0.518 |
| pde4b1 | phosphodiesterase 4B | −1.17 | 0.023 | 1.03 | 0.563 |
| pde4b2 | phosphodiesterase 4B | −1.08 | 0.072 | −1.05 | 0.574 |
| P vs NP | HAD1 vs LAD1 | ||||
| Sym | Ventral Tegmental Area | Fold-Change | P-value* | Fold-Change | P-value* |
| pde4a | phosphodiesterase 4A | 1.03 | 0.555 | −1.10 | 0.303 |
| pde4b1 | phosphodiesterase 4B | −1.02 | 0.469 | −1.01 | 0.846 |
| pde4b2 | phosphodiesterase 4B | 1.02 | 0.573 | 1.10 | 0.020 |
pde4a, Affy ID: 1368670_a_at; pde4b1, Affy ID: 1369044_a_at; pde4b2, Affy ID: 1374157_at; 1368750_a_at;
p < 0.05; Uncorrected for multiple comparisons. Parenthesized numbers indicate individual probe sets. P (Alcohol-preferring); NP (Alcohol Nonpreferring); HAD1 (High Alcohol Drinking); LAD1 (Low Alcohol Drinking) rats.
Effects of microinfusing shRNAs for Il22ra2 into the NAc shell on EtOH intake by P rats
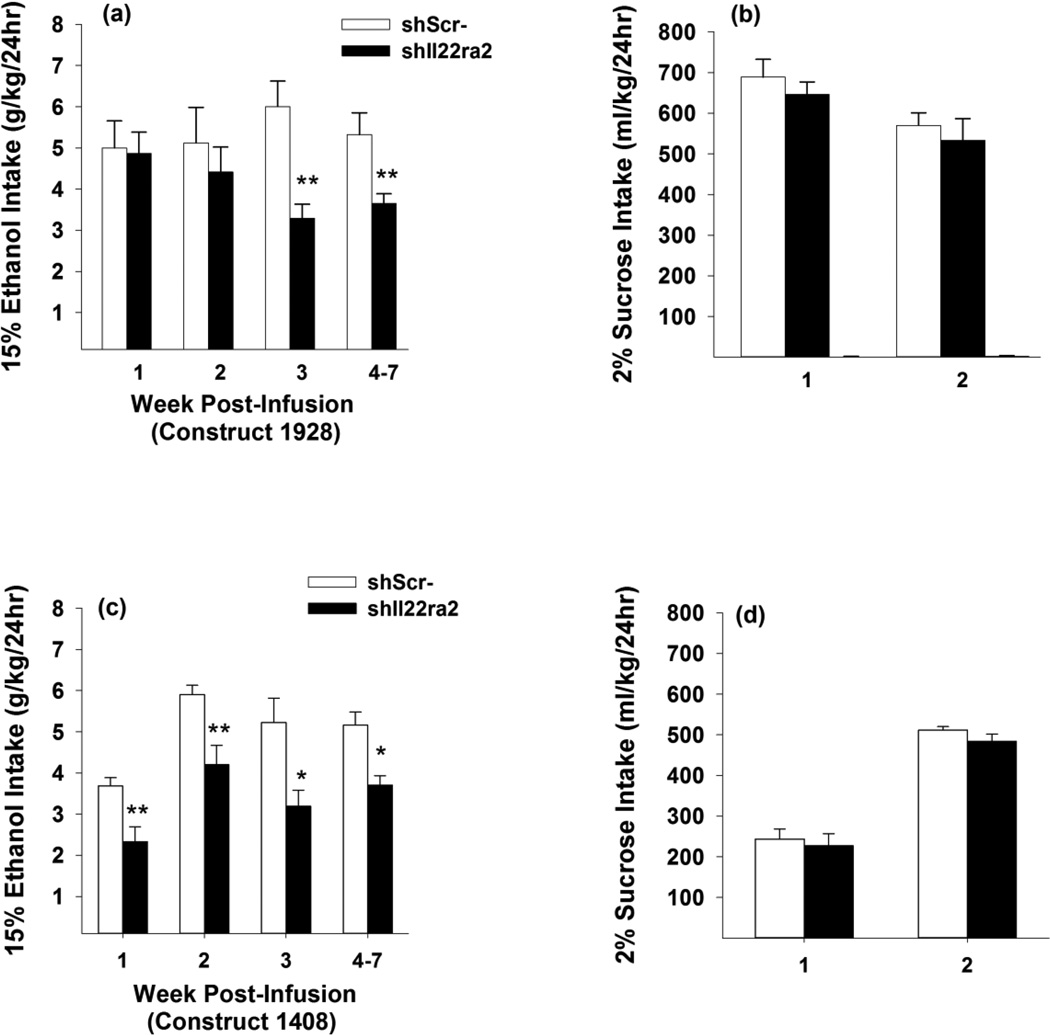
Infusion of shRNA construct #1928 targeting Il22ra2 (shIl22ra2) in the NAc shell reduced (~35%) EtOH intake during the final 5 weeks of EtOH access compared to expression of a non-targeting control shRNA (shScr) (Fig. 5a). The two-way ANOVA revealed a significant treatment by week interaction [F(6,78) = 2.64, p < 0.05], as well as a significant main effect of treatment [F(1,13) = 6.51, p < 0.05]. Independent samples t-tests revealed that EtOH intake was reduced in Weeks 3–7, in subjects infused with shIl22ra2, relative to shScr [t’s (13) > 2.88; p’s < 0.05]. Similar results were obtained following the test of a second shIl22ra2 construct #1408, which reduced EtOH intake (~5.2 to ~3.7 g/kg/day) over the final 4 weeks of EtOH access ( p’s < 0.05). Neither shIll22ra2 construct reduced sucrose (Fig. 5b & 5d), water intakes (data not shown) or body weights (data not shown).
Fig. 5.
Weekly 15% v/v EtOH (g/kg) and 2% w/v sucrose (ml/kg) per 24h for EtOH-experienced female P rats infused with lentiviral vector constructs (#1928 or #1408) expressing Il22ra2 shRNA (shIl22ra2; black bars) or a scrambled control vector (shScr-; white bars) into the NAc shell. Following surgery, rats were given continuous, free-choice access to EtOH (a, c) and water, and monitored daily (Mon-Fri) over 620 mg/kg Ro 20-1724, or vehicle (i.p.). The 30 min test sessions occurred 60–90 7 weeks. Rats were then provided continuous, free-choice access to sucrose (b, d) and water, and monitored daily (Mon-Fri) over two additional weeks (right side of panels). **, p < 0.01
Discussion
The present study tested the hypothesis that inhibition of PDE4 would reduce 2h binge EtOH intake by P and HAD1 rats. In the dose-response experiment, both rolipram and Ro 20-1724 appeared to be more effective in reducing 2h EtOH intake by P compared to HAD1 rats (Figs. 1 and 2). Disparities in the effects of PDE4 inhibitors to alter EtOH drinking in the two rat lines could be associated with their distinct genetic backgrounds (cf. Bell et al. 2012; McBride et al. 2014). Thus, the innately higher expression levels of Pde4a and Pde4b in the NAc shell of P vs NP, but not HAD1 vs LAD1, rats observed in Exp 4 of the present study (Table 4) may indicate that the PDE4 system is more involved in EtOH intake by P vs HAD1 rats. These findings highlight the need to evaluate target compounds in more than one genetic model. With repeated exposure, 1 mg/kg rolipram or 20 mg/kg Ro 20-1724 consistently reduced alcohol drinking in both rat lines (Fig. 2), suggesting no apparent loss in their effectiveness under repeated administration. These results indicate the importance of giving repeated treatments in preclinical studies since some effects may not be observed with a single treatment or may disappear across consecutive treatments.
PDE inhibition has been reported to reduce alcohol drinking in rodents displaying elevated EtOH intake (5–6 g/kg/day) levels (e.g. Hu et al. 2011; Wen et al. 2012). In another study, Bell et al. (2014a) reported that repeated injections with the nonselective PDE inhibitor ibudilast reduced 2h intake of 15% EtOH in P and HAD1 rats from 2 to 1 g/kg/2hr. Evidence indicates that ibudilast acts as a fairly potent inhibitor of PDE4 (Souness et al. 1994); however, its lack of specificity precludes the identification of a specific PDE subfamily that mediates its alcohol-reducing effects. With regard to PDE4 inhibition, Wen et al. (2012) reported that acute rolipram exposure (0.025 and 0.05 mg/kg, s.c.) reduced 5% EtOH-reinforced operant responding, and 0.1 and 0.2 mg/kg, s.c. reduced voluntary EtOH intake under continuous (5% v/v EtOH) and intermittent (every 3–4 days; 10% EtOH) 2-bottle choice conditions in Fawn-Hooded rats. In contrast, our findings indicate that a similar rolipram dose (0.25 mg/kg, i.p.) did not reduce EtOH intake in P or HAD1 rats (Fig. 1). Possible explanations for this difference include the route of compound administration, the genetic background of the subjects, and operant vs. home-cage drinking parameters. Wen et al. (2012) found that four days of exposure to 0.2 mg/kg, rolipram (s.c., 3x per day) decreased (~50%) intake of 5% v/v EtOH, under 24h access conditions. Similarly, Hu et al. (2011) reported that rolipram (0.25 and 0.50 mg/kg, i.p.) and Ro 20-1724 (10 mg/kg, b.i.d.), reduced 24h EtOH drinking of C57BL/6J mice from approximately 6 to 1 g/kg. The success of low doses in these two experiments may indicate that rolipram’s short (1–3h) half-life and rapid clearance (Krause and Kuhne 1988) require multiple daily exposures to observe behavioral changes when examining 24h drinking.
The reductions reported by Hu et al. (2011) exceed those in the present study following repeated injections of 1 mg/kg rolipram or 20 mg/kg Ro 20-1724. However, experimental differences, including the number and schedule of compound administrations, likely contributed to these differences. The present results indicate that the effects on EtOH and sucrose drinking may differ between the first test day and the overall test period average (i.e. the dose by day interaction; Fig. 2c and Fig. 3a–c). In addition, the current experiments, examining the effects of PDE4 inhibitors to alter drinking behaviors, utilized binge-like drinking conditions, which may produce different results compared to 24 hr free-choice drinking.
The 24h EtOH intake reported by Hu et al. (2011) and Wen et al. (2012) was in the range of 5–6 g/kg using C57Bl/6J mice and Fawn-Hooded rats, respectively, which is similar to levels of 24h EtOH intake reported previously for P and HAD1 rats (Bell et al. 2014b; McBride et al. 2014). In the present study, P rats surpassed 80 mg% BEC within the first 30 min, whereas HAD1 rats displayed an average BEC of 50 mg% after 30 min of access. BEC collected from a separate group of HAD1 rats following the 2h drinking sessions exceeded 100 mg% (data not shown), which is binge-like alcohol drinking (e.g. Bell et al. 2011, 2014b). Therefore, differences in response to PDE4 inhibitors between the present studies and those previously reported may be due to a combination of BECs attained and species/strain differences.
PDE4 inhibition only had a transient effect on sucrose intake in either rat line (Fig. 3). Reductions were no longer present after the first test day. These findings indicate that the present reductions in EtOH intake following repeated PDE4 inhibition do not reflect a general reduction in the consumption of rewarding fluids.
To assess the effects of repeated exposure with these PDE4 inhibitors on motor impairment, LMA tests were conducted at times post-injection coinciding with the time of EtOH or sucrose access (i.e. 60–150 min post-injection). Although other studies with mice (Hu et al. 2011; Smith 1990) or rats (Wen et al. 2012; Wachtel 1983) reported reduced LMA following PDE4 inhibition, the present findings did not observe significant reductions in LMA of HAD1 rats. In addition, PDE4 inhibition did not consistently reduce sucrose intake suggesting that the reductions in EtOH intake are not attributable to motor-impairment.
Growing evidence supports a role for the anti-immune and anti-inflammatory properties of PDE4 inhibition in the treatment of drug (Hutchinson et al. 2007; Narita et al. 2006) and alcohol (Fernandez-Lizarbe et al. 2009; Gobejishvili et al. 2008; He and Crews 2008) use disorders. CNS evidence from several research arenas suggests that some of the neuro-protectant qualities of rolipram result from region-specific enhancements in anti-inflammatory activity and reductions in pro-inflammatory activity (e.g. Schaal et al. 2012; Wang et al. 2012).
In the NAc shell, Pde4a expression was higher and Pde4b expression was lower in P rats, relative to NP rats. In the CeA, Pde4b expression was also lower relative to NP rats. Differences in Pde4 isoform expression between HAD1 and LAD1 rats were only observed in the VTA (Table 4). In general, these findings suggest the influence of PDE4 on EtOH intake may reside in the extended amygdala of P rats, whereas this influence may be localized in the mesolimbic dopamine system of HAD1 rats.
In support of a potential involvement of the neuroinflammatory processes in mediating alcohol drinking behavior, micro-infusion into the NAc shell of shRNAs interfering with the expression of Il22ra2 significantly reduced EtOH intake by P rats (Fig. 5). IL-22ra2 binds and neutralizes IL-22, thus blocking its interactions with cell surface receptor complexes and limiting its activity (Kotenko et al. 2001). IL-22 is a member of the IL-10 cytokine superfamily that can exert both pro-inflammatory and anti-apoptotic effects (Sanjabi et al. 2009). Since inhibition of PDE4 will result in the reduction of inflammatory and apoptotic responses (Genain et al. 1995; Sekut et al. 1995; Souness et al. 2000; Teixeira et al. 1997; Sousa et al. 2010), the findings suggest pro-inflammatory and apoptotic neuro-immune system responses to EtOH exposure may be factors contributing to high alcohol drinking by the P and HAD1 lines of rats.
Stereotaxic coordinates were verified in a separate group of female P rats prior to the microinfusions. In addition, these coordinates have been used successfully in previous experiments that employ similar surgical techniques to those in the current study (Engleman et al., 2009). Although there may be vector diffusion into the NAc core, the majority of the shIl22ra2 vector would likely have infected the shell. Lack of a specific antibody prevented the validation of a reduction in IL22ra2 protein levels.
PDE4 inhibition has been reported to inhibit hyperlocomotion associated with methamphetamine and morphine (Mori et al. 2000), cocaine sensitization (Janes et al. 2009), self-administration (Knapp et al. 1999), and conditioned place preference (Thompson et al. 2004). It is plausible that PDE4 inhibitors could reduce alcohol and drug use indirectly through reducing the DA response to these substances.
Overall, the current findings support the contribution of neuroimmune signaling to high EtOH intake and the potential therapeutic use of PDE4 inhibitors to reduce EtOH intake in individuals with AUDs.
Acknowledgments
This research was supported in part by the National Institute of Alcohol Abuse and Alcoholism (NIAAA) grants AA07611, AA013522, AA016654 and NIAAA contract HHSN267200700037C. Kelle Franklin and Sheketha Hauser share first authorship on this manuscript. The authors would like to thank Thomas H. Ewing, Jason D. Pope, and Ian S. Roberts for their technical support with this research. None of the authors has a conflict of interest associated with this research. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of NIAAA or NIH.
References
- Allen CD, Lee S, Koob GF, Rivier C. Immediate and prolonged effects of alcohol exposure on the activity of the hypothalamic-pituitary-adrenal axis in adult and adolescent rats. Brain Behav Immun. 2011;25:S50–S60. doi: 10.1016/j.bbi.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer W, Hoppmann J, Rundfeldt C, Kietzmann M. Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm Allergy Drug Targets. 2007;6:17–26. doi: 10.2174/187152807780077318. [DOI] [PubMed] [Google Scholar]
- Bell RL, Lopez MF, Cui C, Egli M, Johnson KW, Franklin KM, Becker HC. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol. 2014a doi: 10.1111/adb.12106. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Engleman EA, Toalston JE, McBride WJ. Scheduled access alcohol drinking by alcohol-preferring (P) and high alcohol-drinking (HAD) rats: Modeling adolescent and adult binge-like drinking. Alcohol. 2014b;48:225–234. doi: 10.1016/j.alcohol.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, McBride WJ. Modeling bingelike EtOH drinking by peri-adolescent and adult P rats. Pharmacol Biochem Behav. 2011;100:90–97. doi: 10.1016/j.pbb.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol Biochem Behav. 2012;103:119–155. doi: 10.1016/j.pbb.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics (Oxford, England) 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Ferrario CR, Glucksman MJ, Wolf ME. Signaling pathway adaptations and novel protein kinase A substrates related to behavioral sensitization to cocaine. J Neurochem. 2009;110:363–377. doi: 10.1111/j.1471-4159.2009.06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JA, Davis RL. Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J Comp Neurol. 1999;407:287–301. [PubMed] [Google Scholar]
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25:S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin.Exp.Res. 2009;33:2162–2171. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by EtOH. J Immunol. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF. Stress- and glucocorticoid-induced priming of neuroinflammatory responses: Potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun. 2011;25:S21–S28. doi: 10.1016/j.bbi.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genain CP, Roberts T, Davis RL, Nguyen MH, Uccelli A, Faulds D, Li Y, Hedgpeth J, Hauser SL. Prevention of autoimmune demyelination in non-human primates by a cAMP-specific phospodiesterase inhibitor. Proceedings of the National Academy of Sciences USA. 1995;92:3601–3605. doi: 10.1073/pnas.92.8.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobejishvili L, Barve S, Joshi-Barve S, McClain C. Enhanced PDE4B expression augments LPS-inducible TNF expression in EtOH-primed monocytes: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2008;295:G718–G724. doi: 10.1152/ajpgi.90232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu TM, Lee RP, Lee CJ, Subeq YM, Lin NT, Hsu BG. Heavy EtOH intoxication increases proinflammatory cytokines and aggravates hemorrhagic shock-induced organ damage in rats. Mediators Inflamm. 2013;2013:121786. doi: 10.1155/2013/121786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Lu T, Chen A, Huang Y, Hansen R, Chandler LJ, Zhang HT. Inhibition of phosphodiesterase-4 decreases EtOH intake in mice. Psychopharmacology (Berl) 2011;218:331–339. doi: 10.1007/s00213-011-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. Scientific World Journal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Kantak KM, Cherry JA. The involvement of type IV phosphodiesterases in cocaine-induced sensitization and subsequent pERK expression in the mouse nucleus accumbens. Psychopharmacology (Berl) 2009;206:177–185. doi: 10.1007/s00213-009-1594-4. [DOI] [PubMed] [Google Scholar]
- Jin SL, Ding SL, Lin SC. Phosphodiesterase 4 and its inhibitors in inflammatory diseases. Chang Gung Med J. 2012;35:197–210. doi: 10.4103/2319-4170.106152. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Dantzer R. Alcoholism and inflammation: Neuroimmunology of behavioral and mood disorders. Brain Behav Immun. 2011;25:S13–S20. doi: 10.1016/j.bbi.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenk M, Thomas A, Lortie M, Dekemp R, Beanlands RS, Dasilva JN. PET measurements of cAMP-mediated phosphodiesterase-4 with (R)-[11C]rolipram. Curr Radiopharm. 2011;4:44–58. doi: 10.2174/1874471011104010044. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Foye MM, Ciraulo DA, Kornetsky C. The type IV phosphodiesterase inhibitors, Ro 20-1724 and rolipram, block the initiation of cocaine self-administration. Pharmacol Biochem Behav. 1999;62:151–158. doi: 10.1016/s0091-3057(98)00154-3. [DOI] [PubMed] [Google Scholar]
- Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. Journal of Immunology. 2001;166:7096–7103. doi: 10.4049/jimmunol.166.12.7096. [DOI] [PubMed] [Google Scholar]
- Krause W, Kuhne G. Pharmacokinetics of rolipram in the rhesus and cynomolgus monkeys, the rat and the rabbit. Studies on species differences. Xenobiotica. 1988;18:561–571. doi: 10.3109/00498258809041693. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, McClintick JN, Ding ZM, Hauser SR, Edenberg HJ, Bell RL, Rodd ZA. Changes in gene expression within the ventral tegmental area following repeated excessive binge-like alcohol drinking by alcohol-preferring (P) rats. Alcohol. 2013a;47:367–380. doi: 10.1016/j.alcohol.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, McClintick JN, Ding ZM, Hyytia P, Colombo G, Liang T, Edenberg HJ, Lumeng L, Bell RL. Gene expression within the extended amygdala of 5 pairs of rat lines selectively bred for high or low EtOH consumption. Alcohol. 2013b;47:517–529. doi: 10.1016/j.alcohol.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li T-K. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats—Animal models of alcoholism. Alcohol. 2014;48:209–215. doi: 10.1016/j.alcohol.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra K, Pandey SC. The decreased cyclic-AMP dependent-protein kinase A function in the nucleus accumbens: a role in alcohol drinking but not in anxiety-like behaviors in rats. Neuropsychopharmacology. 2006;31:1406–1419. doi: 10.1038/sj.npp.1300900. [DOI] [PubMed] [Google Scholar]
- Mori T, Baba J, Ichimaru Y, Suzuki T. Effects of rolipram, a selective inhibitor of phosphodiesterase 4, on hyperlocomotion induced by several abused drugs in mice. Jpn J Pharmacol. 2000;83:113–118. doi: 10.1254/jjp.83.113. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Oger S, Mehats C, Dallot E, Cabrol D, Leroy MJ. Evidence for a role of phosphodiesterase 4 in lipopolysaccharide-stimulated prostaglandin E2 production and matrix metalloproteinase-9 activity in human amniochorionic membranes. J Immunol. 2005;174:8082–8089. doi: 10.4049/jimmunol.174.12.8082. [DOI] [PubMed] [Google Scholar]
- Page CP, Spina D. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handb Exp Pharmacol. 2011;204:391–414. doi: 10.1007/978-3-642-17969-3_17. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Mittal N, Lumeng L, Li TK. Involvement of the cyclic AMP-responsive element binding protein gene transcription factor in genetic preference for alcohol drinking behavior. Alcohol Clin Exp Res. 1999;23:1425–1434. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Perez-Torres S, Miro X, Palacios JM, Cortes R, Puigdomenech P, Mengod G. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat. 2000;20:349–374. doi: 10.1016/s0891-0618(00)00097-1. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following EtOH treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, D.C.: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Rosi S. Neuroinflammation and the plasticity-related immediate-early gene Arc . Brain Behav Immun. 2011;25:S39–S49. doi: 10.1016/j.bbi.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9:447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaal SM, Garg MS, Ghosh M, Lovera L, Lopez M, Patel M, Louro J, Patel S, Tuesta L, Chan WM, Pearse DD. The therapeutic profile of rolipram, PDE target and mechanism of action as a neuroprotectant following spinal cord injury. PLoS One. 2012;7:e43634. doi: 10.1371/journal.pone.0043634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekut L, Yarnall D, Stimpson SA, Noel LS, Bateman-Fite R, Clark RL, Brackeen MF, Menius JA, Jr, Connolly KM. Anti-inflammatory activity of phosphodiesterase (PDE)-IV inhibitors in acute and chronic models of inflammation. Clinical & Experimental Immunology. 1995;100:126–132. doi: 10.1111/j.1365-2249.1995.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF. Effects of lithium and rolipram enantiomers on locomotor activity in inbred mice. Pharmacol Toxicol. 1990;66:142–145. doi: 10.1111/j.1600-0773.1990.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol. 2013;701:124–130. doi: 10.1016/j.ejphar.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souness JE, Aldous D, Sargent C. Immunosuppressive and anti-inflammatory effects of cyclic AMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacology. 2000;47:127–162. doi: 10.1016/s0162-3109(00)00185-5. [DOI] [PubMed] [Google Scholar]
- Souness JE, Villamil ME, Scott LC, Tomkinson A, Giembycz MA, Raeburn D. Possible role of cyclic AMP phosphodiesterases in the actions of ibudilast on eosinophil thromboxane generation and airways smooth muscle tone. Br J Pharmacol. 1994;111:1081–1088. doi: 10.1111/j.1476-5381.1994.tb14855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa LP, Lopes F, Silva DM, Tavares LP, Vieira AT, Rezende BM, Carmo AF, Russo RC, Garcia CC, Bonjardim CA, Alessandri AL, Rossi AG, Pinho V, Teixeira MM. PDE4 inhibition drives resolution of neutrophilic inflammation by inducing apoptosis in PKA-PI3K/Akt-dependent and NF-kappaB-independent manner. Journal of Leukocyte Biology. 2010;87:895–904. doi: 10.1189/jlb.0809540. [DOI] [PubMed] [Google Scholar]
- Teixeira MM, Gristwood RW, Cooper N, Hellewell PG. Phosphodiesterase (PDE)4 inhibitors: anti-inflammatory drugs of the future? Trends in Pharmacological Sciences. 1997;8:164–171. doi: 10.1016/s0165-6147(97)01049-3. [DOI] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High EtOH consumption and low sensitivity to EtOH-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BE, Sachs BD, Kantak KM, Cherry JA. The type IV phosphodiesterase inhibitor rolipram interferes with drug-induced conditioned place preference but not immediate early gene induction in mice. Eur J Neurosci. 2004;19:2561–2568. doi: 10.1111/j.0953-816X.2004.03357.x. [DOI] [PubMed] [Google Scholar]
- Wachtel H. Species differences in behavioural effects of rolipram and other adenosine cyclic 3H, 5H–monophosphate phosphodiesterase inhibitors. J Neural Transm. 1983;56:139–152. doi: 10.1007/BF01243273. [DOI] [PubMed] [Google Scholar]
- Wang C, Yang XM, Zhuo YY, Zhou H, Lin HB, Cheng YFm Xy JP, Zhang HT. The phosphodiesterase-4 inhibitor rolipram reverses Aβ-induced cognitive impairment and neuroinflammatory and apoptotic responses in rats. Int J Neuropsychopharmacol. 2012;15:749–766. doi: 10.1017/S1461145711000836. [DOI] [PubMed] [Google Scholar]
- Wen RT, Zhang M, Qin WJ, Liu Q, Wang WP, Lawrence AJ, Zhang HT, Liang JH. The phosphodiesterase-4 (PDE4) inhibitor rolipram decreases EtOH seeking and consumption in alcohol-preferring Fawn-Hooded rats. Alcohol Clin Exp Res. 2012;36:2157–2167. doi: 10.1111/j.1530-0277.2012.01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovleva T, Bazov I, Watanabe H, Hauser KF, Bakalkin G. Transcriptional control of maladaptive and protective responses in alcoholics: A role of the NF-kappaB system. Brain Behav Immun. 2011;25:S29–S38. doi: 10.1016/j.bbi.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]