Abstract
Objectives
Our objective was to estimate the risk of developing rheumatoid arthritis (RA) associated with a family history of non-RA arthritis-related diseases. This familial co-aggregation is of clinical interest since it is often encountered when assessing family history of RA specifically, but also informative on the genetic overlap between these diseases. Since anticitrullinated peptide antibodies/rheumatoid factor (RF)-positive and RF-negative RA have both specific and shared genetic factors, the familial co-aggregation was assessed separately for seropositive and seronegative disease.
Methods
Nested case-control study in prospectively recorded Swedish total population data. The Multi-Generation Register identified first-degree relatives. RA and arthritis-related diseases were ascertained through the nationwide patient register. RA serology was based on International Classification of Diseases tenth revision coded diagnoses, mainly reflecting RF. Familial risks were calculated using conditional logistic regression. Results were replicated using the Swedish rheumatology register.
Results
Familial co-aggregation was found between RA and every studied arthritis-related disease, but the magnitude varied widely, from juvenile idiopathic arthritis (JIA) (seropositive RA OR=3.98 (3.01 to 5.26); seronegative RA OR=5.70 (3.47 to 9.36)) to osteoarthritis (seropositive RA OR=1.03 (1.00 to 1.06); seronegative RA OR=1.05 (1.00 to 1.09)). The familial co-aggregation pattern of non-RA arthritis-related diseases was overall similar for seropositive and seronegative RA. Among those with family history of RA, relatives’ other arthritis-related diseases conferred little or no additional risk.
Conclusions
Although family history of several arthritis-related diseases may be useful to predict RA (eg, lupus and JIA), others (eg, osteoarthritis and arthralgia) are less useful. Seropositive and seronegative RA had rather similar familial co-aggregation patterns with arthritis-related diseases, suggesting that the two RA subsets are similar in the genetic factors that overlap with these diseases.
INTRODUCTION
Having a first-degree relative (FDR) with rheumatoid arthritis (RA) is one of the strongest risk factors for developing RA, associated with a twofold to fivefold increase in risk.1–3 Accordingly, the clinical work-up for RA includes questions on relatives’ disease history. Although less studied, it is also known that other autoimmune diseases (eg, psoriasis and systemic lupus erythematosus (SLE)) demonstrate some degree of familial co-aggregation with RA,4 and many genetic factors, including polymorphisms in PTPN22,5 increase risk for multiple immune-mediated diseases.6,7 When asked in the clinic, patients often report a family history of such inflammatory diseases and of noninflammatory arthritis-related conditions such as osteoarthritis and arthralgia. It remains uncertain, however, whether family history of such diseases is associated with increased risk of RA or whether they may add to the risk associated with a family history of RA per se.
Recent molecular studies suggest that anticitrullinated peptide antibodies (ACPA)-positive and ACPA-negative RA have partly overlapping genetic risk factors, with some polymorphisms preferentially associated to one RA subtype and others to both.8 This has been studied in detail for the human leucocyte antigen (HLA) region, where the classical HLA-DRB1 shared epitope alleles are primarily associated with ACPA-positive RA, and some polymorphisms seem protective for ACPA-positive RA while increasing risk for ACPA-negative RA.9 Most genetic studies have only included ACPA-positive RA, however, and the focus on individual genetic markers has not made it possible to estimate the overall genetic difference between the two disease subsets. Although a study combining two twin samples suggested that the heritability of ACPA-positive RA and ACPA-negative RA is the same (~65%),10 and a recent twin study found similar heritability for ACPA-positive RA and overall RA (40%),11 both studies were limited by low power. In contrast, a recent, large study on non-twin relatives found a marked difference with ACPA-positive RA being more heritable than ACPA-negative RA (~50% vs ~20%).1 It would thus seem likely that the impact of family history of specific arthritis-related diseases would be different for subtypes of RA. Beyond the clinical importance for diagnosing RA, such differences in familial co-aggregation patterns would carry information on the degree and nature of the genetic difference between seropositive and seronegative RA.
To inform clinical practice and to explore the aetiological difference between the RA subsets, we linked several Swedish nationwide registers and compared the familial co-aggregation of different arthritis-related diseases with seropositive and seronegative RA.
METHODS
We performed a nested case-control study in the Swedish total population. Cases with seropositive or seronegative RA were identified from the National Patient Register (NPR), which contains date and diagnosis assigned in inpatient (since 1964) and non-primary outpatient care (since 2001). We restricted our study to 2001–2009 since many of the studied conditions are primarily treated in outpatient care. Chart reviews have confirmed that approximately 90% of patients with RA, whether hospitalised or with an outpatient visit, fulfil American College of Rheumatology 1987 (ACR87) criteria for RA.12,13 To further improve this, we required two separate visits to be considered an RA case, at least one with a specialist in rheumatology or internal medicine (see table 1). Serology was based on International Classification of Diseases tenth revision (ICD10) codes, which specifically address rheumatoid factor (RF) rather than ACPA. Attempting to increase specificity for seronegative RA, patients registered with both types of RA were only considered to have seropositive RA. For each case, five general population controls were randomly selected from the Swedish Total Population Register, matched on sex, birth year, residential area and marital status. Controls were sampled among those who were at risk but had not been registered with RA at the case’s first registered diagnosis with RA. Relatives of cases and controls were identified through the Swedish national Multi-generation Register, which contains data on parents of Swedish residents born after 1931 and ever registered as living in Sweden since 1961. Siblings were identified as individuals sharing biological parents, and children had the index person registered as biological parent. For individuals born in Sweden since 1961, the register has an almost complete coverage.14
Table 1.
Algorithm used to define different rheumatic and/or inflammatory conditions in the cases and in their first-grade relatives
| Group of conditions | ICD10 codes | Criteria | Exclusion criteria |
|---|---|---|---|
| Seropositive RA | M05, M08.0 | Two or more visits, with at least one with specialist at a department of internal medicine or rheumatology | |
| Seronegative RA | M06, M08.3 | Two or more visits, with at least one with specialist at a department of internal medicine or rheumatology | Seropositive RA |
| JIA | M08.2, M08.4, M08.8, M08.9 | Two or more visits before age 18, or two or more visits, with at least one with specialist at a department of internal medicine or rheumatology | Seropositive or seronegative RA |
| Other inflammatory arthritis | M13 | Any visit | Seropositive/negative RA or JIA |
| Spondyloarthropathies* | M45, M46, M02, M03, M07.4-6, M08.1, K50, K51, H20 | Any visit | |
| Psoriasis /psoriatic arthritis | M07.0-3, L40, M09 | ICD code L40 counted only if recorded at dermatology, internal medicine or rheumatology departments | |
| Lupust | M32, L93 | Two or more visits, with at least one with specialist at a department of internal medicine, rheumatology or nephrology | |
| Connective tissue disease‡ | M33, M34, M35.0, M35.1, K73.2 | Two or more visits, with at least one with specialist at a department of internal medicine, rheumatology or nephrology | |
| Arthralgia | M25.5 | Any visit | If any diagnosis falling in any other group |
| Osteoarthritis | M15, M16, M17, M18, M19 | Any visit | If any diagnosis falling in any other group |
Ankylosing spondylitis, spondyloarthritis unspecified, reactive arthritis, juvenile spondyloarthritis, enteropathic arthritis/spondylitis, uveitis, Crohn’s disease and ulcerative colitis.
Systemic and/or discoid lupus erythematosus.
Dermatomyositis, polymyositis, systemic sclerosis, Sjögren’s syndrome and autoimmune hepatitis.
ICD10, International Classification of Diseases tenth revision; JIA, juvenile idiopathic arthritis; RA rheumatoid arthritis.
To assess the validity of the register-based RA serology, we linked our study to the EIRA study, a Swedish case-control study of incident RA, which has been described in more detail previously.15 In EIRA, RF was measured using nephelometry with cut-off for seropositivity specified at each respective laboratory. Levels of ACPA were measured using the Immunoscan RA (Mark2) anti-CCP2 ELISA, and concentrations >25 units/mL were considered positive.16
As a sensitivity analysis or replication, we redid all analyses using cases from the Swedish Rheumatology Quality register (SRQ; N=24 256). SRQ is a clinical RA register run by the Swedish Rheumatology Association, containing baseline information and longitudinal information on disease progress. Although most patients in the SRQ are also captured by the NPR, SRQ has higher diagnostic validity, and the difference in size makes the results largely independent.
Arthritis-related diseases
We defined 10 groups of arthritis-related diseases in relatives, based on ICD10 codes from the NPR 2001–2009, according to the algorithm described in table 1. Note that the definition of seropositive and seronegative RA was identical for relatives and cases. Hierarchical exclusions were made to increase diagnostic specificity: individuals with seropositive RA were not allowed to also have seronegative RA, individuals with RA could not have juvenile idiopathic arthritis (JIA), individuals with RA or JIA were excluded from ‘other inflammatory arthritis’ and individuals could only have arthralgia or osteoarthritis if they had never had a diagnosis from another category.
Statistical analysis
Familial risks were estimated as ORs through conditional logistic regression, keeping case-control clusters intact. Occurrence of disease in FDRs was assessed during the entire follow-up (2001–2009), irrespective of the case’s date of RA onset, and modelled as a binary exposure variable. Associations were estimated by treating each relationship pair separately, with each individual potentially contributing multiple observations, one for each relative. We have previously shown that cases and controls do not differ in number of FDRs.1 To correct CIs for the correlated data structure, robust SEs were calculated with the sandwich covariance estimator,17 implemented in Proc Phreg, SAS V.9.4.
RESULTS
We identified 54 515 RA cases with a total of 203 141 FDRs. Descriptive statistics for these individuals and the control population are shown in table 2. Seronegative and seropositive patients with RA were similar in age at inclusion, sex and in average number of FDRs.
Table 2.
Descriptive statistics of rheumatoid arthritis (RA) cases ascertained through the Swedish National Patient Register, their matched controls and index individuals’ first-degree relatives
| Seropositive RA
|
Seronegative RA
|
|||
|---|---|---|---|---|
| Index individuals | Cases | Controls | Cases | Controls |
| N | 41 061 | 195 095 | 13 454 | 63 395 |
| Men, % | 27.7 | 27.3 | 29.4 | 29.3 |
| Age at inclusion, years, median (1st–3rd quartile) | 62 (52–72) | 61 (52–71) | 63 (50–76) | 62 (49–74) |
| Their first-degree relatives | ||||
| N | 153 566 | 742 482 | 49 575 | 238 059 |
| Men, % | 51.0 | 51.6 | 51.2 | 51.8 |
| Birth year, median (1st–3rd quartile) | 1966 (1955–1977) | 1967 (1957–1978) | 1965 (1954–1978) | 1966 (1955–1979) |
| Diseases among the first-degree relatives | ||||
| Seropositive RA | 3447 (2.24%) | 4312 (0.58%) | 689 (1.39%) | 1360 (0.57%) |
| Seronegative RA | 720 (0.47%) | 1407 (0.19%) | 257 (0.52%) | 452 (0.19%) |
| Juvenile idiopathic arthritis | 120 (0.08%) | 159 (0.02%) | 68 (0.14%) | 57 (0.02%) |
| Other inflammatory arthritis | 1319 (0.86%) | 3979 (0.54%) | 418 (0.84%) | 1296 (0.54%) |
| Spondyloarthropathies | 4485 (2.92%) | 17 983 (2.42%) | 1586 (3.20%) | 5808 (2.44%) |
| Psoriasis/psoriatic arthritis | 2722 (1.77%) | 10 663(1.44%) | 1006 (2.03%) | 3404 (1.43%) |
| Lupus | 273 (0.18%) | 686 (0.09%) | 73 (0.15%) | 209 (0.09%) |
| Connective tissue diseases | 506 (0.33%) | 1194 (0.16%) | 138 (0.28%) | 380 (0.16%) |
| Arthralgia | 2994 (1.95%) | 11 781 (1.59%) | 972 (1.96%) | 3753 (1.58%) |
| Osteoarthritis | 8293 (5.40%) | 38 972 (5.25%) | 2797 (5.64%) | 12 565 (5.28%) |
Validity of RA serostatus
Most EIRA cases (2539/2781) were also identified in the NPR, making it possible to check how well the diagnosis-based serology corresponded to measured serology in EIRA (see online supplementary table S1). In this overlap, 80% of those categorised as seropositive RA were RF-positive and 77% ACPA-positive; of seronegative 93% were RF-negative and 84% ACPA-negative.
Disease-specific familial risks
Figure 1 and table 3 show associations of seropositive and seronegative RA to each arthritis-related disease in FDRs. Statistically significant associations with seropositive and seronegative RA were found for every disease but estimates ranged widely, with strongest associations for family history of JIA and RA, and lowest for osteoarthritis. With the exception of family history of seropositive RA and possibly JIA, the ORs were similar for seropositive and seronegative RA. The difference between seropositive and seronegative RA was significant for only two of the non-RA diseases; spondyloarthropathies and psoriasis/psoriatic arthritis (PsA). There were no marked differences in familial risk when stratified by type of FDR, that is, siblings compared with parents and offspring (see online supplementary table S2), or when stratified by sex (see online supplementary table S3).
Figure 1.
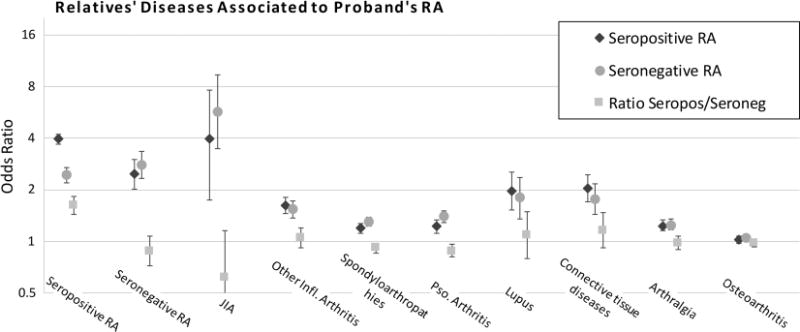
ORs and 95% CIs for arthritis-related diseases in first-degree relatives associated to index person’s rheumatoid arthritis (RA) ascertained through the National Patient Register, by serology, and the ratio of the association to seropositive and seronegative RA.
Table 3.
Arthritis-related diseases in first-degree relatives associated to proband’s rheumatoid arthritis (RA), by proband’s serostatus
| Disease in relative | Seropositive RA, OR (95% CI) | Seronegative RA, OR (95% CI) | Ratio of ORs (95% CI) |
|---|---|---|---|
| Seropositive RA | 3.95 (3.72 to 4.19) | 2.44 (2.20 to 2.71) | 1.62 (1.44 to 1.82) |
| Seronegative RA | 2.47 (2.24 to 2.72) | 2.80 (2.34 to 3.35) | 0.88 (0.72 to 1.08) |
| Juvenile idiopathic arthritis | 3.98 (3.01 to 5.26) | 5.70 (3.47 to 9.36) | 0.70 (0.39 to 1.23) |
| Other inflammation arthritis | 1.62 (1.52 to 1.73) | 1.54 (1.37 to 1.73) | 1.05 (0.92 to 1.20) |
| Spondyloarthropathies | 1.20 (1.16 to 1.25) | 1.31 (1.23 to 1.39) | 0.92 (0.85 to 0.98) |
| Psoriasis/psoriatic arthritis | 1.23 (1.18 to 1.29) | 1.40 (1.29 to 1.51) | 0.88 (0.81 to 0.96) |
| Lupus | 1.96 (1.68 to 2.28) | 1.80 (1.36 to 2.37) | 1.09 (0.79 to 1.49) |
| Connective tissue diseases | 2.04 (1.83 to 2.29) | 1.76 (1.43 to 2.17) | 1.16 (0.92 to 1.47) |
| Arthralgia | 1.23 (1.18 to 1.28) | 1.25 (1.17 to 1.35) | 0.98 (0.90 to 1.07) |
| Osteoarthritis | 1.03 (1.00 to 1.06) | 1.05 (1.00 to 1.09) | 0.98 (0.93 to 1.03) |
Parallel analyses in the SRQ (a partly overlapping, smaller register with more reliable diagnoses, described in online supplementary figure S1 and table S4) gave virtually identical results (see online supplementary table S5). The ratio of OR in SRQ to NPR was between 1.1 and 0.9 for all associations except JIA (seropositive RA: 2.78 (1.88 to 4.10) vs 3.95 (3.72 to 4.19); seronegative: 4.54 (2.53 to 8.14) vs 5.70 (3.47 to 9.36)); inflammatory arthritis to seropositive (1.44 (1.30 to 1.59) vs 1.62 (1.52 to 1.73)); and connective tissue diseases to seronegative RA (1.34 (0.97 to 1.85) vs 1.76 (1.43 to 2.17)).
Combinations of diseases
Having two or more FDRs with RA or JIA was associated with highest risk for both seropositive and seronegative RA (figure 2 and online supplementary table S6). Having at least two affected FDRs generally increased the risk of RA compared with having one FDR with the disease in question, but while this effect was strong for inflammatory arthritis and connective tissue disease, it was weaker for spondyloarthropathies and psoriasis/PsA, and negligible for arthralgia and osteoarthritis. As before, the non-RA arthritis-related diseases conferred similar risks for seropositive and seronegative RA. With the exceptions of RA, JIA and inflammatory arthritis, having an FDR with an arthritis-related disease in addition to an FDR with RA was not associated with further increase in risk for RA compared with only having an FDR with RA (cf. figure 1).
Figure 2.
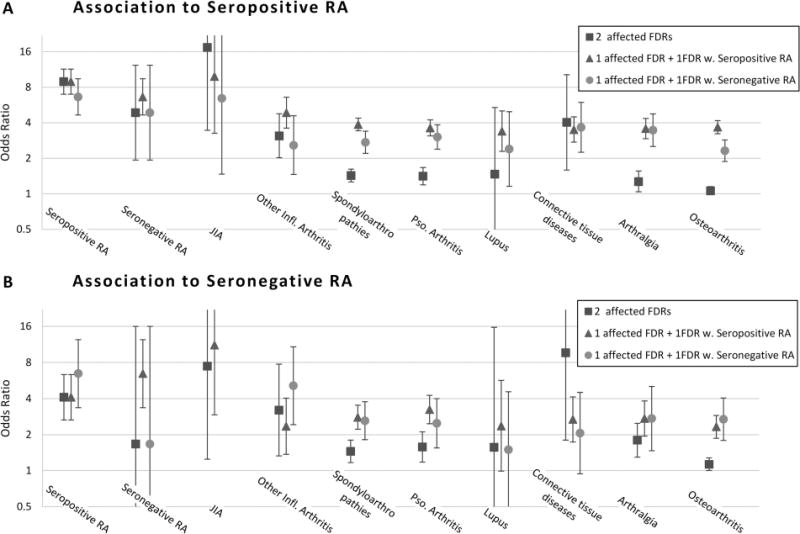
ORs and 95% CIs for combinations of arthritis-related diseases in first-degree relatives associated to proband’s rheumatoid arthritis (RA) ascertained through the National Patient Register, by serology. Affected first-degree relative (FDR) should be read as having an FDR affected by the condition on the axis. Sparse data made it impossible to estimate the risk for juvenile idiopathic arthritis (JIA) associated with one FDR with JIA and another with seronegative RA.
Results comparing RF with ACPA
Seropositive RA in FDRs was a slightly stronger predictor of ACPA-positive RA compared with RF-positive RA in proband, and a weaker predictor of ACPA-negative RA compared with RF-negative RA (table 4). The effect was stronger when comparing RA-positive for both serologies to RA-negative for both (ratio of ORs changed from 2.21 to 2.88). In contrast, the ratio of ORs was similar when comparing RF-negative RA with combined ACPA and RF-negative RA (ratio changed from 0.92 to 0.89). Note that serology in the NPR and SRQ primarily reflect RF, and we could only compare familial risks by ACPA and RF in the EIRA sample, where the sample size made it uninformative to address conditions other than RA.
Table 4.
Assessing the difference in familial aggregation of RA by ACPA versus RF serology: cases from the EIRA study
| RA in FDR | Serology in proband | Seropositive RA in case
|
Seronegative RA in case
|
Ratio of ORs (95% CI) | ||
|---|---|---|---|---|---|---|
| FDRs with RA, N (%) Cases/controls | OR (95% CI) | FDRs with RA, N (%) Cases/controls | OR (95% CI) | |||
| Seropositive | RF | 155 (2.0)/185 (0.5) | 4.27 (3.28 to 5.55) | 48 (1.2)/114 (0.6) | 1.93 (1.35 to 2.77) | 2.21 (1.42 to 3.44) |
| Seropositive | ACPA | 166 (2.2)/198 (06) | 4.29 (3.30 to 5.57) | 38 (0.9)/101 (0.5) | 1.71 (1.17 to 2.49) | 2.51 (1.59 to 3.98) |
| Seropositive | RF and ACPA | 143 (2.3)/165 (0.6) | 4.45 (3.35 to 5.91) | 28 (1.0)/80 (0.6) | 1.54 (0.99 to 2.41) | 2.88 (1.70 to 4.88) |
| Seronegative | RF | 24 (0.3)/61 (0.2) | 2.07 (1.25 to 3.41) | 15 (0.4)/35 (0.2) | 2.24 (1.20 to 4.17) | 0.92 (0.42 to 2.05) |
| Seronegative | ACPA | 26 (0.4)/63 (0.2) | 2.24 (1.38 to 3.64) | 15 (0.4)/35 (0.2) | 2.15 (1.16 to 3.98) | 1.04 (0.48 to 2.28) |
| Seronegative | RF and ACPA | 21 (0.3)/51 (0.2) | 2.26 (1.32 to 3.87) | 12 (0.4)/25 (0.2) | 2.52 (1.26 to 5.04) | 0.89 (0.37 to 2.15) |
ACPA, anticitrullinated peptide antibodies; FDR, first-degree relative; RA, rheumatoid arthritis; RF, rheumatoid factor.
DISCUSSION
By linking Swedish nationwide registers, we performed the largest study to date of the familial co-aggregation of arthritis-related diseases and RA, and the first to study the familial co-aggregation stratified by RA serostatus. Although statistically significant familial co-aggregation was found for RA to every non-RA arthritis-related disease group, interestingly with no pronounced difference between seropositive and seronegative RA, there was no clinically meaningful association between relatives’ arthralgia or osteoarthritis and an individual’s onset of RA.
Family history as predictors of RA
Familial risks of several diseases were of such strength that they may prove useful for clinical disease prediction. For instance, family history of JIA was as informative as a family history of RA. Similarly, an FDR with lupus or connective tissue disease was associated with almost doubled odds for RA, an increase similar to that of ever versus never smoking,18 and not much lower than the risk for seropositive RA associated with relatives’ seronegative RA. This may seem surprisingly strong but is weaker than a previous population-based estimate of the familial co-aggregation using hospitalised (possibly more severe) RA and SLE (OR=2.8 (1.4 to 5.5)).19 The familial co-aggregation of RA to psoriasis/PsA and spondyloarthropathies was quite weak (OR~1.2 to 1.4), and thus less likely to be useful for predicting RA. However, we compared risks in patients with RA versus the general population, whereas in a clinical context, a family history of these diseases may potentially still help differentiate between, for instance, seronegative RA and PsA. Although a statistically significant predictor of RA, FDRs’ diagnosis of non-RA inflammatory arthritis (OR~1.5) was substantially weaker than FDRs’ RA, as was arthralgia (OR~1.2) and osteoarthritis (OR~1.05). We think these weak associations send an important clinical message, for example regarding which patients should be referred to early arthritis clinics, since it stresses the importance of verifying that a patient’s reported family history is actually RA, as family history of other pain or arthritis is common yet not very informative on the development of RA.
When combinations of relatives’ diseases were examined, no disease provided information beyond that conferred by an FDR with RA. In other words, while a family history of lupus is informative, there is no additional information in it if the patient is already known to have a family history of RA.
Aetiology of RA
Many recent studies focus on aetiological differences between ACPA-positive and ACPA-negative RA. The HLA-DRB1 shared epitope alleles and smoking are predominantly risk factors for ACPA-positive disease, particularly when the risk factors are combined.20 Studies have also found both shared genetic factors and those unique to ACPA-positive or ACPA-negative RA, across the genome,8 and specifically in the HLA region.9 As noted earlier, we also find a notable difference in that seropositive RA has a stronger familial aggregation than seronegative RA does.1 Despite this, our overall finding was that the familial co-aggregation with non-RA arthritis-related diseases did not differ materially by serology, and we replicated previous findings of a substantial familial co-aggregation of seropositive with seronegative RA.1 Thus, at least with regards to genetic or family environmental factors influencing other arthritis-related diseases, the aetiological similarity of the two RA subsets seems greater than their difference.
Seronegative RA is often considered a subset of RA where some cases may be similar to seropositive RA but with undetected antibodies (eg, only a specific ACPA not covered by commercially available assays), whereas others may have different disease aetiology, with other, or no, antibodies. Our results are compatible with this view, but to explain the strong co-aggregation of seropositive and seronegative RA, and the similarity in the familial co-aggregation pattern, the diseases grouped as seronegative RA must share a core set of genetic factors that are also shared with seropositive RA. Alternatively, a major part of the seronegative group would need to be genetically very similar to the seropositive group, mixed with smaller proportions of other groups. One such group may be patients with a disease that aetiologically has more in common with PsA or spondyloarthropathies, explaining their higher familial co-aggregation with seronegative RA. However, an alternative explanation for this higher co-aggregation may be that patients diagnosed with seronegative RA may in clinical practice more often also be diagnosed as spondyloarthropathy or PsA or the reverse, a diagnostic ambiguity less likely for seropositive RA.
Several other aetiological conclusions may be drawn. First, the elevated risk of JIA in relatives of patients with RA may indicate that JIA is closely related to adult RA, even though cases with juvenile RA or seronegative polyarthritis were excluded from the JIA group. As we have previously shown for RA,1 young age at onset is associated with higher familial risk, which could partly explain the increased familial risk for JIA compared with other diseases. Second, the comparatively higher risks seen for lupus and connective tissue diseases may suggest that these disease groups are genetically closer to RA than spondyloarthropathies and psoriasis/PsA, for example, through shared genetics related to adaptive immunity. However, the prevalence is also higher for the latter disease groups, and with the same genetic correlation, higher disease prevalence would yield lower familial risks.21 This should be studied further in pedigree-based models where cases were not sampled by only one of the co-aggregating conditions and/or by estimating the genetic correlation of these diseases using observed genetic relatedness, as has previously been done, for instance, among psychiatric diseases.22
Finally, we found a statistically significant (OR~1.02 to 1.07) but clinically irrelevant familial co-aggregation of osteoarthritis and RA. A limited shared aetiology between RA and osteoarthritis is possible, but we find it likely that this association has some other explanation, for example, a small degree of misclassification between the disorders. It is also likely that relatives of patients with RA are more vigilant and prone to seek medical attention for joint pain, which would also explain part of the familial co-aggregation with arthralgia and non-RA inflammatory arthritis.
Strengths and limitations
This study has several strengths. RA diagnoses in the NPR have high validity compared with chart reviews (~90% fulfil ACR criteria). By combining three sources of RA cases, we could capitalise on the statistical strength of the nationwide Swedish patient register, replicate our findings with the better validated information in SRQ and use RF and ACPA measured in the EIRA study to validate our diagnostic algorithm.
Our measure of disease in FDRs cannot be directly compared with the family history of arthritis-related diseases reported by patients in the clinical setting. The use of nationwide prospectively collected patient information removed the risk for selective participation, recall bias or misclassification due to patients’ limited or inaccurate knowledge of their relatives’ disease history. However, the assessment was truncated by the register start in 2001 and will thus underestimate the absolute rate of family history. It should also be recognised that the diagnostic value of family history in the clinical setting likely differs from the familial risks in the general population since patients are already selected by their symptoms, and where the relevant question is which of several diagnoses best describes the patient’s condition.
A limitation of this study is our lack of ACPA measurements in the NPR and SRQ, where the ICD10-based codes refer to RF. Although ACPA is preferred over RF due to higher specificity, stability and more probable role in disease onset and progression, the antibodies are highly correlated. In fact, when we validated the NPR algorithm against the EIRA, our definition of serostatus was almost equally correct compared with ACPA as to RF. A misclassification of serology would lead to underestimates of the true difference in familial co-aggregation, but since we observe virtually no differences at all, an unrealistically large misclassification would be needed to mask differences of a substantial magnitude. That the lack of difference between seropositive and seronegative RA is not simply caused by misclassification is also supported by the SRQ estimates being so similar to the NPR estimates despite the more reliable RF serology in the SRQ.
CONCLUSIONS
A family history of ‘arthritis’ may imply very different risks, and, if it is to be used as a basis for risk prediction, it is important to be as specific as possible. While family history of seropositive RA is one of the strongest risk factors for RA, a family history of osteoarthritis or unspecified joint pain is not appreciably predictive of RA. Further, in individuals with a known family history of RA, family history of other arthritis-related conditions does not further increase the RA risk. Of aetiological interest, seropositive and seronegative RA do not appear to differ much in the genetic or familial risk factors that are shared with other arthritis-related diseases. An important task is now to investigate ‘seronegative’ RA in more detail, in particular, the extent to which ‘seronegative’ RA is made up of individuals positive to specific ACPAs or antibodies against similar epitopes, and thus potentially driven by pathophysiological mechanisms similar to those for seropositive RA.
Supplementary Material
Acknowledgments
Funding This study was supported by the Swedish Foundation for Strategic Research, The COMBINE public–private research programme, Swedish Research Council, ALF, the Strategic Research Programme for Epidemiology (SFO-Epi) and EU-IMI BTCure. EIRA was supported by grants from the Swedish Medical Research Council and the Swedish Research Council for Health, Working Life and Welfare.
Footnotes
Handling editor: Tore K Kvien
Contributors All authors made substantial contributions to conception and design, data acquisition/analysis, interpretation, drafting or critical revision of manuscript and approved the manuscript for publication. They have met the International Committee of Medical Journal Editors Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals (ICMJE Recommendations 2013) recommend criteria for authorship.
Competing interests None.
Ethics approval EPN (the Regional Ethics Review Board in Stockholm).
Provenance and peer review Not commissioned; externally peer reviewed.
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/annrheumdis-2014-206133).
References
- 1.Frisell T, Holmqvist M, Kallberg H, et al. Familial risks and heritability of rheumatoid arthritis: role of rheumatoid factor/anti-citrullinated protein antibody status, number and type of affected relatives, sex, and age. Arthritis Rheum. 2013;65:2773–82. doi: 10.1002/art.38097. [DOI] [PubMed] [Google Scholar]
- 2.Grant SF, Thorleifsson G, Frigge ML, et al. The inheritance of rheumatoid arthritis in Iceland. Arthritis Rheum. 2001;44:2247–54. doi: 10.1002/1529-0131(200110)44:10<2247::aid-art387>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Somers EC, Antonsen S, Pedersen L, et al. Parental history of lupus and rheumatoid arthritis and risk in offspring in a nationwide cohort study: does sex matter? Ann Rheum Dis. 2013;72:525–9. doi: 10.1136/annrheumdis-2011-201165. [DOI] [PubMed] [Google Scholar]
- 4.Cardenas-Roldan J, Rojas-Villarraga A, Anaya JM. How do autoimmune diseases cluster in families? A systematic review and meta-analysis. BMC Med. 2013;11:73. doi: 10.1186/1741-7015-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vang T, Congia M, Macis MD, et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37:1317–19. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 6.Okada Y, Wu D, Trynka G, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–81. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baranzini SE. The genetics of autoimmune diseases: a networked perspective. Curr Opin Immunol. 2009;21:596–605. doi: 10.1016/j.coi.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Eyre S, Bowes J, Diogo D, et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet. 2012;44:1336–40. doi: 10.1038/ng.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han B, Diogo D, Eyre S, et al. Fine mapping seronegative and seropositive rheumatoid arthritis to shared and distinct HLA alleles by adjusting for the effects of heterogeneity. Am J Hum Genet. 2014;94:522–32. doi: 10.1016/j.ajhg.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Woude D, Houwing-Duistermaat JJ, Toes RE, et al. Quantitative heritability of anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis. Arthritis Rheum. 2009;60:916–23. doi: 10.1002/art.24385. [DOI] [PubMed] [Google Scholar]
- 11.Haj Hensvold A, Magnusson PK, Joshua V, et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: an epidemiological investigation in twins. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203947. Published Online First: 25 November 2013. [DOI] [PubMed] [Google Scholar]
- 12.Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54:692–701. doi: 10.1002/art.21675. [DOI] [PubMed] [Google Scholar]
- 13.Knight A, Sandin S, Askling J. Increased risk of autoimmune disease in families with Wegener’s granulomatosis. J Rheumatol. 2010;37:2553–8. doi: 10.3899/jrheum.091280. [DOI] [PubMed] [Google Scholar]
- 14.Ekbom A. The Swedish multi-generation register. Methods Mol Biol. 2011;675:215–20. doi: 10.1007/978-1-59745-423-0_10. [DOI] [PubMed] [Google Scholar]
- 15.Bengtsson C, Berglund A, Serra ML, et al. Non-participation in EIRA: a population-based case-control study of rheumatoid arthritis. Scand J Rheumatol. 2010;39:344–6. doi: 10.3109/03009740903501634. [DOI] [PubMed] [Google Scholar]
- 16.Laki J, Lundstrom E, Snir O, et al. Very high levels of anti-citrullinated protein antibodies are associated with HLA-DRB1*15 non-shared epitope allele in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:2078–84. doi: 10.1002/art.34421. [DOI] [PubMed] [Google Scholar]
- 17.Lin DY, Wei LJ. The robust inference for the cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–78. [Google Scholar]
- 18.Sugiyama D, Nishimura K, Tamaki K, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010;69:70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 19.Hemminki K, Li X, Sundquist J, et al. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum. 2009;60:661–8. doi: 10.1002/art.24328. [DOI] [PubMed] [Google Scholar]
- 20.Kallberg H, Padyukov L, Plenge RM, et al. Gene-gene and gene-environment interactions involving HLA-DRB1, PTPN22, and smoking in two subsets of rheumatoid arthritis. Am J Hum Genet. 2007;80:867–75. doi: 10.1086/516736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todorov AA, Suarez BK. Genetic liability model encyclopedia of biostatistics. John Wiley & Sons, Ltd; 2005. [Google Scholar]
- 22.Lee SH, Ripke S, Neale BM, et al. Cross-Disorder Group of the Psychiatric Genomics Consortium Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


