Abstract
Introduction
Tuberculosis (TB) remains a primary public health problem worldwide. The number of multidrug-resistant tuberculosis (MDR TB) cases has increased in recent years in Colombia. Knowledge of M. tuberculosis genotypes defined by spoligotyping can help determine the circulation of genotypes that must be controlled to prevent the spread of TB.
Objective
To describe the genotypes of M. tuberculosis using spoligotyping in resistant and drug-sensitive isolates and their possible associations with susceptibility to first-line drugs.
Methods
An analytical observational study was conducted that included 741 isolates of M. tuberculosis from patients. The isolates originated from 31 departments and were obtained by systematic surveillance between 1999 and 2012.
Results
In total 61.94% of the isolates were resistant to 1 or more drugs, and 147 isolates were MDR. In total, 170 genotypes were found in the population structure of Colombian M. tuberculosis isolates. The isolates were mainly represented by four families: LAM (39.9%), Haarlem (19%), Orphan (17%) and T (9%). The SIT42 (LAM 9) was the most common genotype and contained 24.7% of the isolates, followed by the genotypes SIT62 (Haarlem1), SIT53 (T1), and SIT50 (H3). A high clustering of isolates was evident with 79.8% of the isolates classified into 32 groups. The Beijing family was associated with resistant isolates, whereas the Haarlem and T families were associated with sensitive isolates. The Haarlem family was also associated with grouped isolates (p = 0.031).
Conclusions
A high proportion (approximately 80%) of isolates was found in clusters; these clusters were not associated with resistance to first-line drugs. The Beijing family was associated with drug resistance, whereas the T and Haarlem families were associated with susceptibility in the Colombian isolates studied.
Introduction
Tuberculosis (TB) remains a primary public health problem and is the second leading cause of death from infectious diseases in the world. According to the World Health Organization (WHO), 8.6 million individuals worldwide were infected with TB in 2012, and although TB is a curable disease, 1.3 million individuals died from TB infections [1].
A steady trend in the incidence of tuberculosis has been observed in Colombia since 1999, with an average of 25 cases per 100,000 inhabitants and 11,000 new cases reported each year [2]. Regarding the cases resistant to first-line anti-TB drugs, the latest national surveillance study of resistance conducted in 2004 and 2005 showed a prevalence of 2.38% (95% CI: 1.58–3.57) of multidrug-resistant tuberculosis (MDR TB) in untreated patients. Although this increase was not statistically significant relative to previous studies, it may have epidemiological value and constitutes a serious threat to TB control [3].
Spoligotyping is a molecular technique based on the characterization of the polymorphisms of the direct repeat (DR) locus found exclusively in members of the M. tuberculosis complex, and it is a simple, rapid and inexpensive typification method with results that can be compared among laboratories worldwide. Additionally, the SpolDB4 database (http://pasteur-guadeloupe.fr:8081/SITVITDemo) includes classifications for spoligotypes and descriptions of the genetic families of M. tuberculosis for 62,582 isolates from 153 countries; these isolates contain 7105 patterns of spoligotyping that are grouped into 2740 SIT (Shared International Type) codes [4]. The characterization of isolates can be used to identify patients with identical genotypes, which may be potentially associated with the same transmission path, in contrast to ungrouped genotypes that originate from reactivation or latent infection [5]. Molecular epidemiology information is useful in the context of epidemic events and the transmission of tuberculosis. Some studies have reported the establishment of optimal treatment schemes for patients with identical isolates identified by spoligotyping compared with other grouped strains that were previously associated with MDR TB [6].
The evolution of the DR locus has enabled the analysis of population structures, and this approach can be used to classify M. tuberculosis complex in lineages or families [7–10]. The DR-based approach clearly reveals two major lineages (1 and 2) that contain various families or sublineages; for lineage 1, the families are: African (Uganda, Cameroon and S), Asian (Beijing and CAS), Latin American-Mediterranean and African-European (X, Ghana and Haarlem); for lineage 2, only the EAI family affects humans, whereas the M. bovis, M. caprae and M. microti families primarily affect animals [11].
This study aimed to describe the genetic diversity of M. tuberculosis by spoligotyping 741 clinical isolates obtained from 1999 to 2012 and to determine their possible associations with transmission and susceptibility to first line drugs in Colombia.
Materials and Methods
Type of study
An analytical observational study was conducted to evaluate the possible association of M. tuberculosis genotypes identified by spoligotyping a group of Colombian isolates with susceptibility to first-line drugs (rifampicin, isoniazid, streptomycin and ethambutol); similarly, demographic, clinical and epidemiological variables were assessed. This study included isolates belonging to the M. tuberculosis complex that were obtained from 31 departments in Colombia between 1999 and 2012; these isolates were collected through systematic surveillance performed by the National Institute of Health, the INS.
Sample
In total, 741 cultures of M. tuberculosis complex were collected between 1999 and 2012 from patients with or without prior treatment history from 31 departments in Colombia; these isolates were obtained through systematic surveillance conducted by the INS. The isolates were stored in the mycobacteria group biobank. For analysis, the sample was divided into two equal 7-year periods for determining the change in genotypes. In total, 410 isolates were included in the first period, and 331 isolates were included in the second period.
Ethics statement
All study procedures were approved by the Ethics Committee in Research (ECR). This study did not require informed consent. The isolates were obtained from the biobank of the mycobacteria group and used directly because of the surveillance function of the INS, which is the highest public health authority in Colombia.
Methods for the microbiological study
Culture and identification
Isolates grown on Ogawa-Kudoh medium were sent to the Departmental Secretaries of Health of Colombia and analyzed for the species identification following the methodology described in the procedural handbook of the National Reference Laboratory of the INS [12] and the Centers for Disease Control and Prevention (CDC) guidelines [13].
Susceptibility testing for first-line drugs
The susceptibility testing for first-line drugs was performed using the simplified methodology of multiple proportions of Canetti Rist and Grosset [14]; the automated Bactec MGIT 960 Beckton Dickinson USA methodology was used.
Study methods for molecular epidemiology
DNA extraction
The isolates identified as M. tuberculosis complex were reseeded in Lowenstein Jensen medium and incubated for 15 days at 37°C. DNA extraction was then performed as described by Van Soolingen et al. [15].
Genotyping (spoligotyping) of the DR locus
The DR locus was genotyped (spoligotyped) following the standard methodology described by Kamerbeer et al. [16].
The genotypes obtained by spoligotyping were translated into a binary code, and the octal code was compared with the SPOLDB4 international database of the Pasteur Institute of la Guadalupe (http://www.pasteur-guadeloupe.fr:8081/SITVITDemo online version) to determine the SIT (spoligo international type), family and international location [4].
The genotypes obtained were subjected to a grouping analysis using Bionumerics version 6.0 software (Applied Maths). A grouping was defined as three or more isolates having an identical pattern.
Statistical analysis
A descriptive analysis of each variable was performed during each of the periods. The measures of central tendency and their 95% confidence intervals were calculated and compared to observe their tendencies. A bivariate analysis was performed, and the associations among the variables, the phenotypes of drug susceptibility, and the genotypes of the DR loci or families were determined using Epi Info 7.0 (CDC, public domain). The prevalence ratios and their 95% confidence intervals were estimated using Pearson’s chi-squared test or Fisher’s exact test. p<0.05 was considered statistically significant.
Results
Demographic and epidemiological descriptions
Sex and age
In total, 39.14% (n = 290) of the patients were female with an age range of 6 to 92 years, whereas 60.86% (n = 451) of the patients were male with an age range of 4 to 95 years. In the overall study population, 1.52% (n = 11) of the patients were between the ages of 1 and 15 years, 36.33% (n = 263) were between the ages of 16 and 30 years, 31.49% (n = 228) were between the ages of 31 and 45 years, 21.13% (n = 153) were between the ages of 46 and 60 years, and 9.53% (n = 69) were older than 60 years. However, no data were available on 2.29% (n = 17) of the patients.
Origin
The isolates included in the study were from patients from all of the departments in Colombia except San Andrés and Vaupés. The Department of Valle del Cauca contributed 33.6% of the isolates in this study S1 Fig.
State of TB treatment
In total, 22.4% (n = 166) of the isolates were from previously treated patients, whereas the remaining 77.6% (n = 575) were from patients who did not have a previous history of treatment.
Phenotype of isolates susceptible to first-line drugs
In total, 61.94% (n = 460) of the isolates were resistant to one or more drugs, and 33.33% (n = 246) of the isolates were susceptible. However, susceptibility information was not available for 4.72% (n = 35) of the isolates. The MDR phenotype was found in 19.83% (n = 147) of the isolates. The patterns of resistance to first-line drugs are described in Table 1.
Table 1. Distribution of patterns of susceptibility to first-line drugs.
| Resistance Pattern | N | % |
|---|---|---|
| H+R+S+E | 66 | 8.91 |
| H+R+S | 59 | 7.96 |
| H+R+E | 5 | 0.67 |
| H+R | 17 | 2.29 |
| H+S+E | 12 | 1.62 |
| H+S | 90 | 12.15 |
| H+E | 4 | 0.54 |
| R+E | 1 | 0.13 |
| S+E | 1 | 0.13 |
| S+R | 1 | 0.13 |
| E | 8 | 1.08 |
| H | 78 | 10.53 |
| R | 3 | 0.4 |
| S | 115 | 15.52 |
| Sensitive | 246 | 33.2 |
| No data | 35 | 4.72 |
| TOTAL | 741 | 100 |
H: Isoniazid, R: Rifampicin; S: Streptomycin: E: Ethambutol.
Genotypes by spoligotyping and drug susceptibility
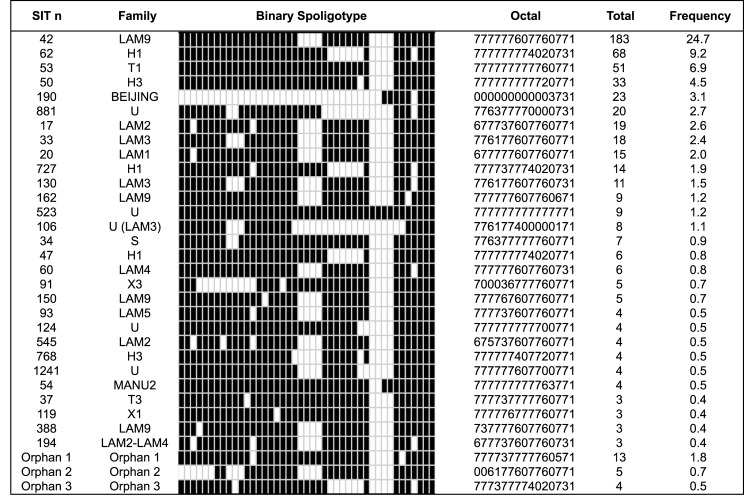
In total, 170 genotypes were identified; the SIT42 (LAM 9) was the most frequent genotype and included 24.7% (n = 183) of the isolates. The distribution of the families identified, their frequency and the susceptible phenotypes are presented in Table 2.
Table 2. Distribution of the identified genotypes by family and frequency among the drug-susceptible phenotypes.
| FAMILY | SIT | Total | Resistant | Sensitive | No data | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | ||
| Beijing | 190 | 23 | 3.1 | 23 | 100 | 0 | 0 | |
| Beijing | 1 | 1 | 0.13 | 1 | 100 | 0 | 0 | |
| CAS1_DELHI | 26 | 1 | 0.13 | 0 | 0 | 1 | 100 | |
| H | 62 | 68 | 9.18 | 34 | 50 | 34 | 50 | |
| H | 50 | 33 | 4.45 | 18 | 54.55 | 14 | 42.42 | 1 |
| H | OTHERS | 28 | 3.78 | 19 | 67.86 | 9 | 32.14 | |
| H | 727 | 14 | 1.89 | 5 | 35.71 | 9 | 64.29 | |
| Orphan | NR | 126 | 17 | 91 | 72.22 | 32 | 25.4 | 3 |
| LAM | 42 | 183 | 24.7 | 116 | 63.39 | 63 | 34.43 | 4 |
| LAM | OTHERS | 45 | 6.07 | 27 | 60 | 15 | 33.33 | 3 |
| LAM | 33 | 18 | 2.43 | 11 | 61.11 | 7 | 38.89 | |
| LAM | 20 | 15 | 2.02 | 11 | 73.33 | 4 | 26.67 | |
| LAM | 17 | 19 | 2.56 | 16 | 84.21 | 3 | 15.79 | |
| LAM | 130 | 11 | 1.48 | 9 | 81.82 | 2 | 18.18 | |
| LAM | 162 | 9 | 1.21 | 7 | 77.78 | 2 | 22.22 | |
| M bovis | 820 | 1 | 0.13 | 0 | 0 | 1 | 100 | |
| MANU S | 54 | 4 | 0.54 | 0 | 0 | 0 | 0 | 4 |
| S | 34 | 7 | 0.94 | 4 | 57.14 | 3 | 42.86 | |
| S | 831 | 1 | 0.13 | 1 | 100 | 0 | 0 | |
| T | 53 | 51 | 6.88 | 17 | 33.33 | 23 | 45.1 | 11 |
| T | OTHERS | 23 | 3.1 | 10 | 43.48 | 13 | 56.52 | |
| U | 881 | 20 | 2.7 | 14 | 70 | 6 | 30 | |
| U | OTHERS | 10 | 1.35 | 9 | 90 | 1 | 10 | |
| U | 106 | 8 | 1.08 | 7 | 87.5 | 1 | 12.5 | |
| U | 523 | 9 | 1.21 | 0 | 0 | 0 | 0 | 9 |
| X | 91 | 5 | 0.67 | 2 | 40 | 3 | 60 | |
| X | OTHERS | 8 | 1.08 | 7 | 87.5 | 1 | 12.5 | |
| TOTAL | 741 | 100 | 460 | 62.08 | 246 | 33.2 | 35 | |
Description of groupings by spoligotyping
There were 32 groupings that consisted of 3–183 isolates from the total population; therefore, 79.8% (n = 591) of the isolates were grouped. The major grouping genotypes were SIT42 (LAM9), SIT62 (H1), SIT53 (T1) and SIT50 (H3) (Fig 1).
Fig 1. Major genotypes identified according to family and frequency in Colombia, 1999–2012.
For both study periods analyzed (1999–2005 and 2006–2012), a high proportion of grouped isolates was found, (78.78% (n = 323) and 80.97% (n = 268), respectively).
In total, 80.4% (n = 369) of the resistant isolates were grouped together, whereas 77.7% (n = 192) of the susceptible isolates were grouped together. The two populations showed no significant differences in terms of active or recent transmission (p = 0.229).
Bivariate analyses and associations
The results from analyzing the variables according to the presence of grouping isolates are shown in Table 3. The variable genotypic family, specifically the Haarlem family, was associated with grouping isolates (p = 0.031), whereas the T, X and Orphan families were associated with the non-grouped isolates (p < 0.001).
Table 3. Bivariate analysis of the variables associated with grouping isolates.
| Variables | Grouping | p* | ||||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| n | % | n | % | |||
| Sex | ||||||
| Male | 357 | 79.3 | 93 | 20.6 | ||
| Female | 234 | 80.4 | 57 | 19.6 | 0.397 | |
| Age | ||||||
| 0–15 years | 10 | 90.9 | 1 | 9.1 | 0.21 | |
| 16–30 years | 215 | 83 | 44 | 71 | 0.137 | |
| 31–45 years | 167 | 76.6 | 51 | 23.4 | 0.385 | |
| 46–60 years | 118 | 80.3 | 29 | 19.7 | 0.243 | |
| 61–75 years | 28 | 65.1 | 15 | 34.9 | 0.379 | |
| >76 years | 18 | 72 | 7 | 28 | ref | |
| Status of treatment | ||||||
| PT | 132 | 81.9 | 29 | 18.1 | ||
| NT | 459 | 79.1 | 121 | 20.8 | 0.24 | |
| Susceptibility to first-line drugs | ||||||
| R | 369 | 80.4 | 90 | 19.6 | ||
| S | 192 | 77.7 | 55 | 22.3 | ||
| ND | 30 | 85.7 | 5 | 14.3 | 0.229 | |
| MDR | ||||||
| Yes | 125 | 85 | 22 | 14.9 | ||
| No | 466 | 78.4 | 128 | 21.5 | 0.045 | |
| Period of the study | ||||||
| 1999–2005 | 323 | 78.8 | 87 | 21.2 | ||
| 2006–2012 | 268 | 77.7 | 63 | 19 | 0.26 | |
| Family | ||||||
| LAM | 280 | 93.3 | 20 | 6.7 | ref | |
| Beijing | 23 | 95.8 | 1 | 4.8 | 0.527 | |
| U | 45 | 95.7 | 2 | 4.3 | 0.51 | |
| S | 7 | 87.5 | 1 | 12.5 | 0.435 | |
| H | 125 | 87.4 | 18 | 12.6 | 0.031 | |
| X | 8 | 61.5 | 5 | 38.5 | 0.001 | |
| T | 54 | 72.9 | 20 | 27.1 | <0.001 | |
| Orphan | 45 | 35.7 | 81 | 64.3 | <0.001 | |
| MANU | 4 | 100 | 0 | 0 | NA | |
| M. bovis | 0 | 0 | 1 | 100 | NA | |
| CAS1_Delhi | 0 | 0 | 1 | 100 | NA | |
* Test of significance: Fisher’s Exact
Moreover, a statistically significant association was found between the MDR isolates and the non-grouped isolates (p = 0.045).
The variables analyzed regarding the resistance to first-line drugs are shown in Table 4. The Beijing family was strongly associated with drug-resistant isolates, whereas the Haarlem (p = 0.003) and T (p < 0.001) families were associated with susceptibility to first-line drugs.
Table 4. Bivariate analysis of the variables associated with drug resistance.
| Variables | Drug resistance | p* | ||||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| n | % | n | % | |||
| Sex | ||||||
| Male | 276 | 65.7 | 144 | 34.3 | ||
| Female | 182 | 63.9 | 103 | 36.1 | 0.334 | |
| Age | ||||||
| 0–15 years | 8 | 72.7 | 3 | 27.3 | 0.285 | |
| 16–30 years | 156 | 61.7 | 97 | 38.3 | 0.363 | |
| 31–45 years | 141 | 66.2 | 72 | 33.8 | 0.212 | |
| 46–60 years | 95 | 66 | 49 | 34 | 0.229 | |
| 61–75 years | 32 | 74.4 | 11 | 25.6 | 0.098 | |
| >76 years | 14 | 56 | 11 | 44 | ref | |
| Status of treatment | ||||||
| PT | 146 | 90.7 | 15 | 9.3 | ||
| NT | 313 | 57.4 | 232 | 42.6 | <0.001 | |
| Grouping | ||||||
| Yes | 369 | 65.8 | 192 | 34.2 | ||
| No | 90 | 62.1 | 55 | 37.9 | 0.229 | |
| Period of the study | ||||||
| 1999–2005 | 326 | 79.9 | 82 | 20.1 | ||
| 2006–2012 | 133 | 44.6 | 165 | 55.4 | <0.001 | |
| Family | ||||||
| LAM | 197 | 67.2 | 96 | 32.8 | ref | |
| Beijing | 9 | 69.2 | 4 | 30.8 | 0.527 | |
| U | 5 | 62.5 | 3 | 37.5 | 0.522 | |
| S | 91 | 73.9 | 32 | 26.1 | 0.105 | |
| H | 30 | 78.9 | 8 | 21.1 | 0.098 | |
| X | 76 | 53.5 | 66 | 46.5 | 0.003 | |
| T | 27 | 42.9 | 36 | 57.1 | <0.001 | |
| Orphan | 24 | 100 | 0 | 0 | ||
| MANU | 0 | 100 | 1 | 0 | NA | |
| M. bovis | 0 | 0 | 1 | 100 | NA | |
| CAS1_Delhi | 0 | 0 | 1 | 100 | NA | |
* Test of significance: Fisher’s Exact
Moreover, the isolates from the second study period were associated with drug sensitivity (p < 0.001), and the resistant isolates were associated with patients who had been previously treated (p < 0.001).
Trend analysis
Families
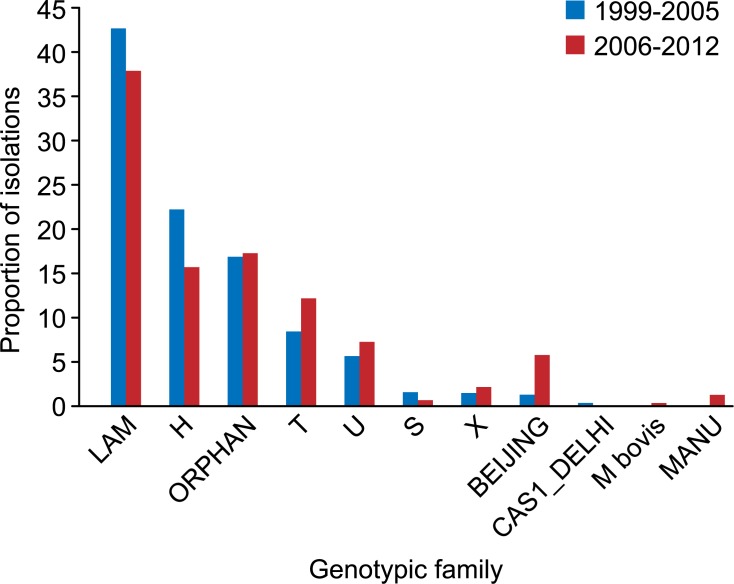
The evaluation of the dynamics of presentation of the genotypic families in the total study population showed a significant increase in the Beijing family from the first to the second study period (p < 0.001). Similarly, the T family showed a significant increase during the second period (p = 0.03) (Fig 2).
Fig 2. Distribution of the genotypic families according to the isolation period.
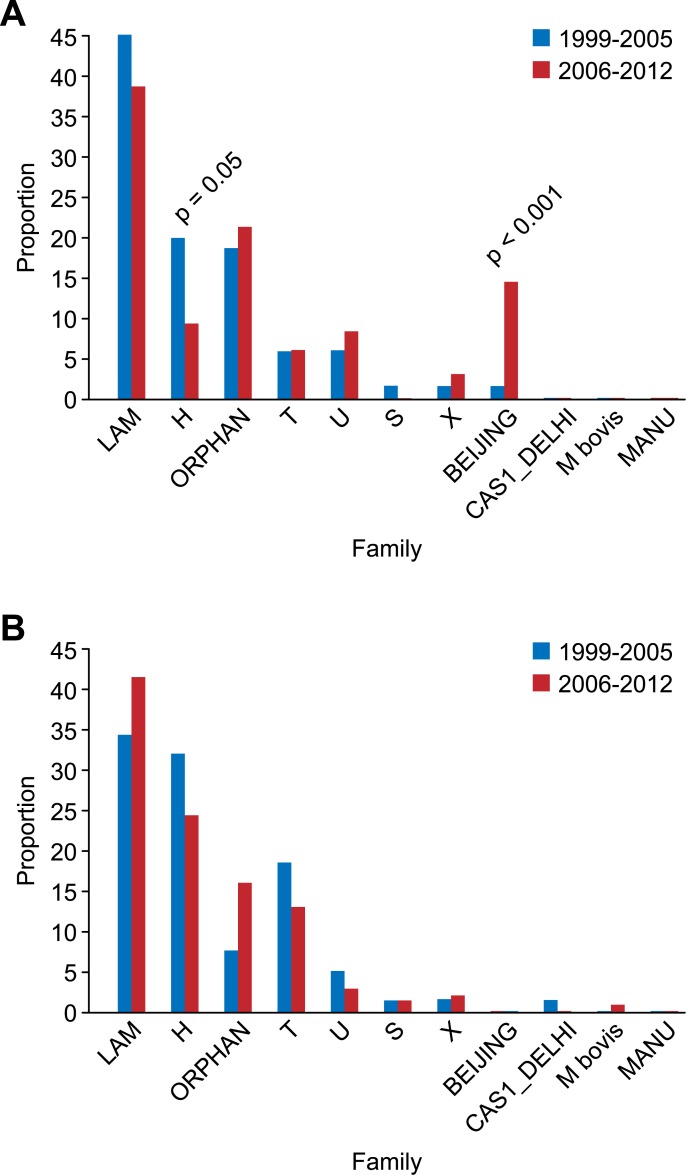
The proportion of drug-resistant isolates of the Beijing family increased significantly in the second study period compared with the first study period (p < 0.001); conversely, the Haarlem family proportion was significantly reduced (p = 0.05) during the same period. This behavior was most likely determined by the MDR isolates (Fig 3A). Significant variation among the susceptible isolates was not observed in any of the circulating families in Colombia when comparing the two periods (Fig 3B).
Fig 3. Transmission dynamics of genotypes in isolates resistant (A) and sensitive (B) to first-line drugs.
Grouped isolates
No significant difference was found in the total population when the proportion of grouped isolates from the first period was compared with that from the second period (p = 0.260).
No significant differences were found (p = 0.44) in the drug-resistant population, when comparing the proportion of grouped isolates from the first study period (80.1%, n = 261) with that from the second period (81.2%, n = 108).
Moreover, when comparing the proportion of grouped isolates from the first study period (65%, n = 61) with those from the second period (66%, n = 36), no significant differences were found (p = 0.5) in the MDR population. Similarly, when comparing the proportion of grouped isolates from the first study period (73.1%, n = 60) with those from the second period (80.00%, n = 132), no significant differences (p = 0.146) were found in the susceptible population.
Discussion
Since the introduction of spoligotyping in 1997, this method has become one of the most widely used tools worldwide for typing of isolates of Mycobacterium tuberculosis because it adds discriminatory power to previously existing tools, such as RFLP IS6110 (reference method), used for molecular epidemiology studies of tuberculosis [17–19].
Given the evolutionary mechanisms of the DR region (i.e., the sequential loss of spacers without the ability to recover lost spaces), the unambiguous phylogenetic classification of strains according to patterns or spoligotypes enables the strains to be related to specific phenotypes of individual clinical isolates. This method has increased the understanding of the population genetics of Mycobacterium tuberculosis, its evolutionary history and transmission in different regions. Identical genotypes are considered to be isolates that cause active transmission, whose quantification enables the measurement of the effect of strategies for tuberculosis control programs with the aim of reducing and controlling disease transmission.
In this framework, the present study demonstrated that in Colombia between 1999 and 2012 approximately 80% of the isolates belonged to groups suggesting that the program strategies to control TB probably was slightly affected; this situation should be corroborated using the combination of highly discriminating methods, this high grouping is a much higher proportion than that reported in other countries where there are effective control programs. A national study in the United States reported that 34.4% of isolates were grouped during the period of 2008 to 2010 [20]. Similar proportions of grouped isolates were reported in this study and in countries with a high burden of disease, including some of the African countries belonging to the group of 22 countries selected by the WHO to emphasize control strategies for their high levels of TB [21]. Consistent with our data, previous studies in Colombia have reported a high proportion of M. tuberculosis groupings using genetic methods for different regions; in particular, grouping proportions between 20 and 74% have been reported [22–24]. Each region must expand the number of isolates characterized to determine the own genotypes. One strength of this work is the characterization of a large number of isolates (741) from 31 of the 33 departments in Colombia over a long study period.
A comparison of the two seven-year periods emphasizes that the status of TB transmission has not changed; this observation agrees with reports of classical epidemiology in which no variation was observed in the number of new cases diagnosed over time in Colombia [1].
There were no significant differences in the MDR or sensitive isolates in the two periods studied with respect to the groupings, which may indicate that the level of active transmission is not decreasing in Colombia.
The Haarlem family was associated with grouped isolates, whereas the T, X and Orphan families were associated with the non-grouped isolates (p <0.001), which most likely represented endogenous reactivation or latent tuberculosis.
In this study, a high genetic diversity of M. tuberculosis was reported, with 170 different genotypes present that were mainly represented by four families: LAM (39.9%), Haarlem (19%), Orphan (17%) and T (9%). The isolates of type SIT42 were the most common isolates belonging to the LAM9 family, which was found in all the departments included in this study. The SIT62 (H1) was the second most common type of isolate Fig 1. The LAM family has been described as prevalent in other countries including Paraguay and Venezuela and in countries in the Americas, Europe and the Caribbean [25, 26]. Previous studies that genotyped isolates in Colombia using spoligotyping reported that the LAM family was the most frequent family in regional circulation, followed by the Haarlem family [24–27]. Moreover, in this study the MANU family is first report in Colombian isolates from individuals coinfected with HIV.
In total, 83 genotypes were found that had not been previously reported (orphans). These genotypes were likely native from Colombia, and 72.2% of the newly discovered genotypes were resistant to one or more drugs Fig 3A and 3B. The high proportion of patterns and orphan spoligotypes detected in this study, particularly those belonging to new cases, indicates that these genotypes should be monitored and investigated further because they may have been generated by recent developments in pre-existing genotypes.
Regarding the association of families with phenotypes susceptible to first-line drugs, it was shown that an isolate of the Beijing family was a predictor of drug-resistant insulation; the frequency of these isolates increased significantly during the second period of the study. Previous studies showed that some isolates of the Beijing family are sensitive to drugs [28] and that in the Latin American population, Beijing family isolates are rare [29]. It is important to emphasize that all of our isolates belonging to the Beijing family were resistant to first-line drugs and were exclusively obtained from the municipality of Buenaventura, Valle del Cauca, which has an African-American population [30]. It is known that certain ethnic characteristics confer susceptibility to human hosts for infection and disease development by strains of this family, which was most likely determined by the co-evolution of the pathogen and the population group [31].
The genotypes SIT53 (H) (p = 0.003) and SIT727 (T) (p <0.001) were clearly associated with isolates sensitive to first-line drugs. This finding has also been documented in recent studies from Taiwan [32,33]; therefore, it would be useful to continue monitoring the presentation of these genotypes over time to predict the success of the treatment schemes used in Colombia. It is necessary to intensify to the epidemiological surveillance of drug-resistant tuberculosis in Colombia, because we find 31.9% of isolates that were MDR, 44.6% of the isolates were mono-resistant and 20.9% of the isolates were bi-resistant during this 14-year period. Because the treatment schemes used in Colombia are conjugated, it is assumed that the isolates with mono- and bi-resistance to first-line drugs would have been eliminated by these schemes; therefore, it is believed that these isolates reflect unfinished treatments and dropouts resulting from lack of adherence to treatment by Colombian patients.
In summary, based on the results of this study, molecular markers such as MIRU-VNTR [34] should be used to increase the power of discrimination and to identify the real proportions of groupings associated with active transmission in Colombia while recognizing the benefits of the knowledge of the genotypes circulating in Colombia by spoligotyping. This information can help the National Tuberculosis Control Program intensify its intervention strategies to achieve early detection and timely establishment of treatment for cases of active tuberculosis because the delay in treatment is a key factor of disease transmission. This action is proposed because the drug-resistant isolates have not been shown to be responsible for the active transmission of TB in Colombia.
This study provided an overview of the population structure of M. tuberculosis in all regions of Colombia and may be the first national study of genetic diversity identified by spoligotyping and its association with susceptibility and the active/recent transmission of tuberculosis in Colombia.
As Colombia strives to eliminate tuberculosis, surveillance of genotypes may lead to earlier detection of micro-epidemics and outbreaks, resulting in continuous improvement of TB control activities and maximizing the use of the limited resources of the state public health system both locally and nationally.
Supporting Information
(EPS)
Acknowledgments
We thank all the members of the National Network of Laboratories of Colombia for their contributions in collecting samples and conducting phenotypic tests for identification and drug susceptibility.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was fully funded by the National Institute of Health.
References
- 1. World Health Organization. Global tuberculosis control: WHO Report 2012. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 2.Organización Panamericana de la Salud, Instituto Nacional de Salud, Ministerio de la Protección social. Plan Estratégico Colombia Libre de Tuberculosis 2010–2015. Para la Expansión y Fortalecimiento de la Estrategia Alto a la TB. Bogotá: Ducal; 2009.
- 3.Instituto Nacional de Salud, Dirección de Vigilancia y Análisis del Riesgo en Salud Pública. Informe del evento tuberculosis farmacorresistente periodo epidemiológico VII del año 2014. 2014. Available: http://www.ins.gov.co/lineaseaccion/SubdireccionVigilancia/Informe%20de%20Evento%20Epidemiolgico/TUBERCULOSIS%20%20FARMACORRESISTENTE%20Periodo%20VII%202014.pdf. Accessed 10 July 2014.
- 4. Demay C, Liens B, Burguière T, Hill V, Couvin D, Millet J, et al. SITVITWEB—A publicly available international multimarker database for studying Mycobacterium tuberculosis genetic diversity and molecular epidemiology. Infect Genet Evol. 2012;12: 755–766. 10.1016/j.meegid.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 5. Guernier V, Sola C, Brudey K, Guégan JF, Rastogi N. Use of cluster-graphs from spoligotyping data to study genotype similarities and a comparison of three indices to quantify recent tuberculosis transmission among culture positive cases in French Guiana during a eight year period. BMC Infect Dis. 2008. 10.1186/1471-2334-8-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gori A, Bandera A, Marchetti G, Degli Esposti A, Catozzi L, Nardi GP, et al. Spoligotyping and Mycobacterium tuberculosis . Emerg Infect Dis. 2005;11: 1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brudey K, Driscoll JR, Rigouts L, Prodinger WM, Gori A, Al-Hajoj SA, et al. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 2006;6: 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roetzer A, Schuback S, Diel R, Gasau F, Ubben T, di Nauta A, et al. Evaluation of Mycobacterium tuberculosis typing methods in a 4-year study in Schleswig-Holstein, Northern Germany. J Clin Microbiol. 2011;49: 4173–4178. 10.1128/JCM.05293-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filliol I, Driscoll JR, van Soolingen D, Kreiswirth BN, Kremer K, Valétudie G, et al. Snapshot of moving and expanding clones of Mycobacterium tuberculosis and their global distribution assessed by spoligotyping in an international study. J Clin Microbiol. 2003;41: 1963–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sebban M, Mokrousov I, Rastogi N, Sola C. A data-mining approach to spacer oligonucleotide typing of Mycobacterium tuberculosis . Bioinformatics. 2002; 18: 235–243. [DOI] [PubMed] [Google Scholar]
- 11. Wirth T, Hildebrand F, Allix-Béguec C, Wölbeling F, Kubica T, Kremer K, et al. Origin, spread and demography of the Mycobacterium tuberculosis complex. PLoS Pathog. 2008. 10.1371/journal.ppat.1000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garzón M, Mejía G, Llerena C, Orjuela D, Bueno J (2012) Diagnostico bacteriológico de tuberculosis y Micobacteriosis. Bogotá: INS. [Google Scholar]
- 13. Kent PT, Kubica G, Centers for Disease Control (U.S.). (1985) Public health Mycobacteriology A Guide for the Level III Laboratory. Atlanta, GA: US Department of Health and Human Services, Public Health Service, Centers for Disease Control. [Google Scholar]
- 14. Canetti G, Fox W, Khomenko A, Mahler HT, Menon NK, Mitchison DA, et al. Advances in techniques of testing mycobacterial drug sensitivity and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organization. 1969;41: 21–43. [PMC free article] [PubMed] [Google Scholar]
- 15. Van Soolingen D, De Haas PE, Kremer K (2001) Restriction fragment length polymorphism (RFLP) typing of mycobacteria. Bilthoven: National Institute of Public Health and the environment; 10.1385/1-59259-147-7:165 [DOI] [PubMed] [Google Scholar]
- 16. Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goyal M, Saunders NA, van Embden JD, Young DB, Shaw RJ. Differentiation of Mycobacterium tuberculosis isolates by spoligotyping and IS6110 restriction fragment length polymorphism. J Clin Microbiol. 1997;35: 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bauer J, Andersen AB, Kremer K, Miörner H. Usefulness of Spoligotyping to discriminate IS6110 low-copy-number Mycobacterium tuberculosis complex strains cultured in Denmark. J Clin Microbiol. 1999;37: 2602–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cronin WA, Golub JE, Magder LS, Baruch NG, Lathan MJ, Mukasa LN, et al. Epidemiologic usefulness of Spoligotyping for secondary typing of Mycobacterium tuberculosis isolates with low copy numbers of IS6110. J Clin Microbiol. 2001;39: 3709–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention (2012) Tuberculosis genotyping-United States, 2004–2010. MMWR. Morb Mortal Wkly Rep 36: 723–735. [PubMed] [Google Scholar]
- 21. Tessema B, Beer J, Merker M, Emmrich F, Sack U, Rodloff AC, et al. Molecular epidemiology and transmission dynamics of Mycobacterium tuberculosis in Northwest Ethiopia: new phylogenetic lineages found in Northwest Ethiopia. BMC Infect Dis. 2013. 10.1186/1471-2334-13-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosero DA, Del Corral H. Contribución del análisis RFLP del IS6110 de Mycobacterium tuberculosis al diseño y refinamiento de estrategias para el control de la tuberculosis en Colombia. Infectio. 2008;12: 175–191. [Google Scholar]
- 23. Hernández JE, Murcia MI, De la Hoz F. Epidemiología molecular de la tuberculosis en Bogotá en Aislados Clínicos obtenidos durante 11 Años. Rev Salud Pública. 2008; 10: 126–136. [DOI] [PubMed] [Google Scholar]
- 24. Cerezo I, Jiménez Y, Hernandez J, Zozio T, Murcia MI, Ratogi N. A first insight on the population structure of Mycobacterium tuberculosis complex as studied by spoligotyping and MIRU-VNTRs in Bogotá, Colombia. Infect Genet Evol. 2012;12: 657–663. 10.1016/j.meegid.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 25. Candia N, Lopez B, Zozio T, Carrivale M, Diaz C, Russomando G, et al. First insight into Mycobacterium tuberculosis genetic diversity in Paraguay. BMC Microbiol. 2007. 10.1186/1471-2180-7-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sequera M, Delgado V, Araque W, Torrealba M, Núñez R, Da Mata J O, et al. Mycobacterium tuberculosis: Spoligotypes in the Carabobo State, Venezuela. Rev Chil Infectol. 2008;25: 362–367. [PubMed] [Google Scholar]
- 27. Realpe T, Correa N, Rozo JC, Ferro BE, Gómez V, Zapata E, et al. Population structure among Mycobacterium tuberculosis Isolates from pulmonary tuberculosis patients in Colombia. PLoS One 9 2014. 10.1371/journal.pone.0093848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwamoto T, Grandjean L, Arikawa K, Nakanishi N, Caviedes L, Coronel J, et al. Genetic diversity and transmission characteristics of Beijing family Strains of Mycobacterium tuberculosis in Peru. PLOS One. 2012. 10.1371/journal.pone.0049651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ritacco V, López B, Cafrune PI, Ferrazoli L, Suffys PN, Candia N, et al. Mycobacterium tuberculosis strains of the Beijing genotype are rarely observed in tuberculosis patients in South America. Mem Inst Oswaldo Cruz. 2008;103: 489–492. [DOI] [PubMed] [Google Scholar]
- 30.Departamento Administrativo Nacional de Estadística. Censo Colombia 2005. 2007. Available: http://www.dane.gove.co/files/censo2005/etnia/sys/visibilidad_estadistica_etnicos.pdf.
- 31. Parwati I, van Crevel R, van Soolingen D. Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. Lancet Infect Dis. 2010;10: 103–111. 10.1016/S1473-3099(09)70330-5 [DOI] [PubMed] [Google Scholar]
- 32. Chang JR, Chen YY, Huang TS, Huang WF, Kuo SC, Tseng FC, et al. Clonal expansion of both Modern and ancient genotypes of Mycobacterium tuberculosis in Southern Taiwan. PLoS One. 2012. 10.1371/journal.pone.0043018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dou HY, Tseng FC, Lin CW, Chang JR, Sun JR, Tsai WS, et al. Molecular epidemiology and evolutionary genetics of Mycobacterium tuberculosis in Taipei. BMC Infect Dis. 2008. 10.1186/1471-2334-8-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rusch-Gerdes S, Willery E, et al. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol. 2006;44: 4498–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(EPS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.