Abstract
Thin melanomas with partial or complete regression may provide clues about antitumor immunity, but their management remains controversial. We have characterized the management and clinical outcomes of regressed thin (<1mm) T1a melanomas, and hypothesize that regression increases risk of regional metastases when compared to nonregressed thin melanomas.
A prospectively collected clinical database was reviewed, and T1a melanomas with regression were identified. Histology, surgical approach, outcome and survival were evaluated. Primary outcome measures were sentinel node positivity, subsequent lymph node metastasis, and survival.
75 patients with T1a or in situ melanomas were grouped into three subsets. Group 1: Thirty-five underwent sentinel node biopsy (SNBx), none of which were positive. No patients developed nodal recurrence. 5-year survival of this group was 93% with median followup of 52 months. Group 2: Thirty-one were followed, without SNBx; two developed regional nodal disease (5.8%) neither of whom died of subsequent distant disease. 5-year survival was 89% with median followup of 38 months. There was no significant difference in survival between groups 1 and 2. Group 3: Nine patients presented with metastatic disease concurrent with a regressed thin melanoma. These patients had a median survival of 2.3 years and a 4-year survival estimate of 22%.
Regression should not be used as an indication for SNBx in T1a melanomas; we recommend that such patients be managed with wide local excision and long-term clinical followup. The poor prognosis of thin regressed primary melanoma with simultaneous metastatic disease may indicate the existence of immune escape phenotypes supporting melanoma progression.
INTRODUCTION
Spontaneous regressions of malignant melanoma have been described for decades, including isolated partial or complete regressions of cutaneous lesions and complete and durable regressions of systemic metastatic disease.[1–8] These regressions are commonly attributed to immunologic events, though the biology remains incompletely characterized.[9] Patients with thin melanomas (AJCC stage IA) have a good prognosis, [10] but a minority of patients with thin melanomas develops metastases, and some die of metastatic melanoma. In some studies, histologic evidence of regression has had a negative prognostic impact, [4,11–16] whereas in other studies, regression did not have independent negative prognostic significance.[17–24] Because of these discordant data on the prognostic significance of regression in thin malignant melanomas, there are no standard guidelines for performing sentinel node biopsy (SNBx) for thin lesions with regression.
Based on prior experience of one author (C.L.S.) that melanoma with severe regression is associated with a higher risk of recurrence [13], our practice has been to offer SNBx to patients with histologic evidence of partial or complete regression in thin melanomas and clinically negative nodes. In this study, we identify a subset of patients with thin melanoma and histologic evidence for regression. By determining the rate of SNBx positivity, and evaluating patterns of recurrence and metastasis, we hope to understand whether regression is a prognostic indicator for sentinel node positivity. We hypothesize that patients with thin melanomas displaying regression will have an increased likelihood of sentinel lymph node positivity, and increased rates of local and distant recurrence as compared to published rates of thin melanomas. We have also observed a different clinical presentation of regression, in which patients present with clinical evidence of metastatic melanoma concurrent with the diagnosis of a partially or completely regressed primary lesion.[25] Our secondary aim is to understand the difference in biology of patients who present with regression and thin melanoma only as compared to patients who present with regression concurrent with distant disease.
PATIENTS AND METHODS
Study approval was obtained from the University of Virginia Institutional Review Board. We reviewed a prospectively collected database and identified patients diagnosed with thin (<1mm) malignant melanoma, including melanoma in situ, at a single institution. Patients were excluded if they had other adverse prognostic factors considered indications for SNBx including Clark level >3, positive deep margin on initial biopsy, microscopic satellites, and ulceration. Histologic data collected for this study included regression, ulceration, Breslow depth, Clark’s level, growth phase, microscopic satellites, and radial or vertical growth pattern of the primary melanoma. Information regarding surgical management of the primary lesion and nodes, as well as outcomes including recurrence, metastasis, and survival were also collected. Subsequent review of individual charts, as well as the Social Security Death Index, provided additional detail.
All patients meeting the inclusion criteria had their pathology slides evaluated by a dermatopathologist (J.W.P.) and were considered to have histologic evidence for regression based on the following criteria: dermal fibroplasia, pigmentary incontinence, vascular proliferation, and lymphocytic infiltrate.[26] Additionally, there was often effacement of the overlying rete ridge pattern. Inflammatory infiltrates alone were not considered regression for the purposes of this study. The extent of regression was not formally quantified but was described in several categories based on radial extent: complete (defined as absence of residual melanoma cells in the presence of dermal fibroplasia, vasodilatation, perivascular inflammation, and/or dermal pigment deposition), partial (defined as extensive, significant, or marked areas of the specimen with fibrosis, inflammation, and/or pigmentary incontinence), or focal (defined as zones or areas of fibrosis, inflammatory infiltrate, and/or pigmentary incontinence).[27] Additionally, previously established criteria for completely regressed melanoma with lymph node metastases were used as a basis for defining such patients.[26] Clinical criteria for diagnosis of complete regression can be found in Table 1. Patients referred after development of metastasis represent a subset of patients with an unknown denominator; thus, this manuscript excludes patients referred for management of metastasis following diagnosis of a thin melanoma elsewhere.
Table 1.
Criteria for complete regression of primary cutaneous melanoma.*
|
All patients in this series were treated with a wide local excision with a 1-cm margin. Addressing the draining nodal basin was varied during the period studied. We began recommending SNBx in 1995, and thus patients treated before 1995 were not offered SNBx. From 1995 through the time period reported in this manuscript, our recommendation for performing sentinel node biopsy in patients with melanomas <= 1 mm in thickness was based on the following criteria: T1b (Clark’s level 4 or greater, or ulceration) or a positive deep margin at biopsy. These melanomas were not included in this study. In addition, from 1995–2006, we also recommended SNBx for thin melanomas with severe regression. We also performed some SNBx for thin melanomas solely upon patient request. Additionally, there were some patients who met criteria for SLNB but did not undergo the procedure due to overall health status or patient preference.
Patients meeting inclusion criteria were divided into three groups for analysis: (1) those managed with SNBx, (2) those followed clinically without surgical management of the draining nodes, and (3) those who presented with metastatic disease synchronous with the diagnosis of a regressed melanoma. Outcomes of patients in groups 1 and 2 were compared to reported outcomes for those patients with AJCC Stage IA melanoma without regression.[28,29]
Pearson’s Chi square test was used to compare categorical data, and ANOVA used to compare continuous data. Kaplan Meier survival functions were calculated for disease free and overall survival. The Log Rank Mantel-Cox test was used to assess statistical significance. P values < 0.05 were considered significant. To estimate an upper bound for the rate of positive SNBx, a one-sided exact binomial confidence interval was calculated. Statistical analyses were performed in SPSS version 17.0.
RESULTS
We identified 1505 patients treated for malignant melanoma at a single institution between 1991 and 2006. This included 275 patients with thin primary melanomas, of which 75 were included in this study. These 75 were patients with thin melanoma and regression without other indications for SNBx (Clark IV or V, positive deep margin on biopsy, microscopic satellites, ulceration, and no clinical evidence of metastases at presentation). These patients included 35 patients managed with SNBx (group 1) and 31 patients managed with clinical evaluation of nodes only (group 2). These groups form the basis for evaluation of the impact of regression on SNBx and recurrence in this study. Additionally, we identified nine patients presenting with clinical evidence of metastatic melanoma concurrent with the diagnosis of a partially or completely regressed primary lesion (group 3).
Demographics
Overall 65% of patients were male. (Table 2) Group 1 was 71% male, Group 2 was 52% male and Group 3 was 89% male (p=0.07). Mean age was 53 years for Group 1, 54 for group 2 (range: 19–73), and 50 years for Group 3 (range: 31–82) (p=0.78). Overall the majority of lesions were found on the trunk (51%). In Groups 1 and 2, 54% and 48% of patients had trunk primaries, respectively, while and 40 and 48% had extremity primaries. A significantly greater proportion of Group 3 patients (3 of 9, or 33%) had head and neck primaries (p=0.046). (Table 2)
Table 2.
Demographic characteristics of patients with thin melanomas and regression
| All (n=75) | Group 1: SNBx performed (n=35) | Group 2: LN staging not done (n=31) | Group 3: Metastatic disease at presentation (n=9) | P value | |
|---|---|---|---|---|---|
|
| |||||
| Gender | |||||
| Male [no. (%)] | 49 (65) | 25 (71) | 16 (52) | 8 (89) | 0.07 |
|
| |||||
| Age at diagnosis | |||||
| (mean, yrs) | 53 | 54 | 53 | 50 | NS |
|
| |||||
| Location of primary [no. (%)] | |||||
| Trunk | 38 (51) | 19 (54) | 15 (48) | 4 (44) | NS |
| Extremity | 31 (41) | 14 (40) | 15 (48) | 2 (22) | NS |
| Head/Neck | 6 (8) | 2 (6) | 1 (3) | 3 (33) | 0.046 |
SNBx=Sentinel lymph node biopsy; NS= not significant
Histologic Information
Histologic subtype was similar in Groups 1 and 2. Superficial spreading melanoma (SSM) was the most common (31%), followed by melanoma in situ (MIS) and lentiginous subtype (LMM) (Table 3). The majority of MIS was further subtyped histologically, and the most common subtype identified was lentigenous (5 patients). 39% of patients had no listed histologic subtype. Partial regression was the most common pattern seen in Groups 1 and 2 (Table 3). As expected, group 3 had significantly more lesions with complete regression (p=0.008). Clark level 3 was the most common level in both group 1 and 2, at 40% and 48% respectively. There was not sufficient Clark level data in group 3 for analysis. 26 patients had radial growth phase (RGP) lesions, and 17 had vertical growth phase (VGP) lesions. There was no difference between group 1 and 2 with respect to growth phase (p=0.39). Group 3 had insufficient data for analysis. In group 3, the primary lesions were either diagnosed within 3 weeks prior to the identification of metastatic disease or within 4 months after identification of metastatic disease. The majority (8) presented with lymph node metastasis, and a regressed melanoma was subsequently identified in an area expected to drain to the tumor-involved node(s). The other patient presented with a brain metastasis that was resected and found to be melanoma, and a completely regressed primary lesion was subsequently identified.
Table 3.
Histologic characteristics of patients with thin melanomas and regression
| All patients | Group 1: SNBx performed | Group 2: LN staging not done | Group 3: Metastatic disease at presentation | P value | |
|---|---|---|---|---|---|
|
| |||||
| Histologic Subtype* [no. (%)] | |||||
| SSM | 23 (31) | 12 (34) | 10 (32) | 1 (11) | |
| MIS | 12 (16) | 6 (18) | 5 (16) | 1 (11) | |
| MIS-SSM | 3 | 2 | 2 | 0 | |
| MIS-LMM | 5 | 2 | 1 | 0 | |
| MIS-SSM/LMM | 1 | 0 | 0 | 1 | |
| MIS-ALM | 1 | 1 | 0 | 0 | |
| MIS-NR | 2 | 1 | 2 | 0 | |
| NOD | 2 (3) | 1 (3) | 1 (3) | 0 | |
| ALM | 0 | 1 (3) | 0 | 0 | |
| LMM | 8 (11) | 5 (15) | 3 (10) | 0 | |
| NR | 29 (39) | 11 (31) | 11 (35) | 7 (78) | |
|
| |||||
| Extent of Regression | |||||
| Complete | 7 (15) | 1 (3) | 2 (10) | 4 (44) | 0.008 |
| Partial | 37 (60) | 20 (59) | 13 (68) | 4 (44) | |
| Focal | 18 (29) | 13 (38) | 4 (21) | 1 (11) | |
| NR | 13 | 1 | 12 | 0 | |
|
| |||||
| Clark’s Level* | |||||
| 1 | 13 (17) | 6 (17) | 6 (19) | 1 (11) | <0.0001 |
| 2 | 22 (29) | 13 (37) | 8 (26) | 1 (11) | |
| 3 | 29 (39) | 14 (40) | 15 (48) | 0 | |
| NR | 8 | 1 | 0 | 7 | |
|
| |||||
| Growth Phase* | |||||
| Radial | 26 (60) | 15 (68) | 9 (53) | 1 (33) | 0.39 |
| Vertical | 17 (40) | 7 (32) | 8 (47) | 2 (67) | |
| NR | 22 | 11 | 10 | 1 | |
excludes patients with complete regression;
MIS- melanoma in situ; SSM- superficial spreading melanoma; NOD- nodular melanoma; ALM- acral lentiginous melanoma; LMM- lentiginous melanoma; NR- not recorded
Surgical management
Among all patients, full thickness excisional biopsies were most common, performed in 71% of cases. 11% of patients underwent shave biopsy, and 20% of patients did not have biopsy information available. 33 patients (44%) had complete removal of lesion with initial biopsy. 19% of patients had residual melanoma in the wide local excision specimen. 6 patients (11%) elected not to undergo WLE, 4 of whom had concurrent distant metastases and are included in group 3.
Group 1 patients underwent SNBx, and none had a positive SNBx (0%). (0/35, 95% upper bound 8.2%). Although 7 patients had evidence for VGP lesions, this factor was not associated with sentinel node positivity. Group 2 patients did not undergo SNBx, and instead were followed using physical exam and annual CBC and chest radiograph (Table 4). The eight Group 3 patients with regional nodal disease at diagnosis underwent a diagnostic biopsy (FNA or incisional) followed by a completion nodal dissection. Seven of these patients had one or two positive nodes in their completion dissection, and one patient had 16 of 19 positive lymph nodes.
Table 4.
Surgical management and metastatic patterns of patients with thin melanomas and regression
|
|
|||
|---|---|---|---|
| Clinically NED after primary excision
|
Group 3: Metastatic disease at presentation | ||
| Group 1: SNBx performed | Group 2: LN staging not done | ||
|
| |||
| Wide local excision [no. (%)] | |||
| Yes | 35 (100) | 29 (94) | 5 (56) |
| No | 0 (0) | 2 (6) | 4 (44) |
|
| |||
| Surgical LN evaluation* | |||
| Yes | 35 (100) | 0 (0) | 8 (89) |
| No | 0 (0) | 31 (100) | 1 (11) |
|
| |||
| Recurrence after initial diagnosis | |||
| Yes | 2 (5.7) | 3 (9.7) | |
| Local recurrence | 1 | 0 | |
| Regional LN | 0 | 2 | |
| Distant skin, nodes, or viscera | 1 | 1 | |
| No | 33 | 28 | |
|
| |||
| Median Follow-up (months) | 52 | 38 | 28 |
Refers to either SNBx (Groups 1 and 2) or lymphadenectomy (Group 3)
Outcomes
Group 1
Median followup was 52 months. Two patients (5.5%) had recurrence. One had local recurrence 19 months after original diagnosis, underwent re-excision, and is alive with no evidence of disease 48 months following re-excision. The other patient had distant recurrence 56 months after original diagnosis, and died of progressive disease. Notably, no patients had nodal recurrence. 5-year survival was 93% (SE 6%) (Figure 1).
Figure 1.
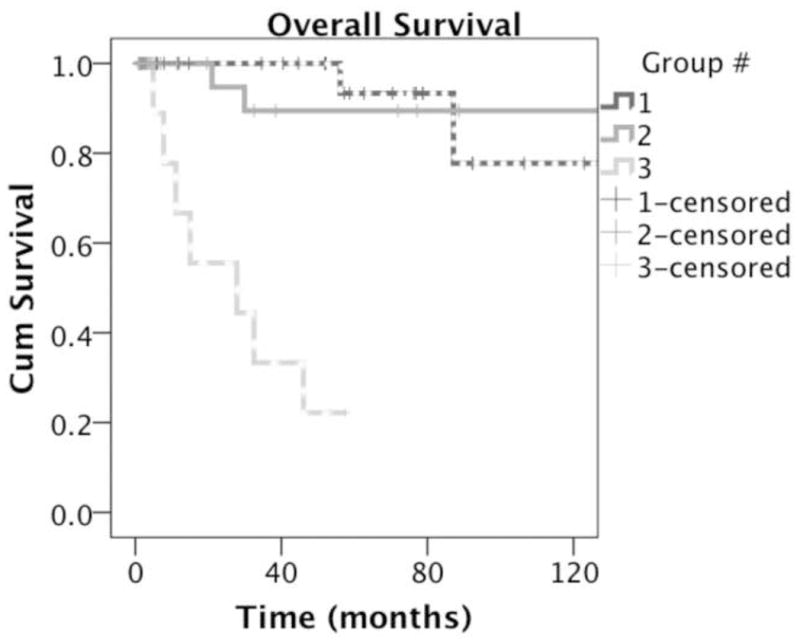
Kaplan-Meier survival curve of patients managed without sentinel node biopsy (dotted line) vs. patients managed with sentinel node biopsy (solid line) vs. patients with metastasis at diagnosis (dashed line).
Group 2
Median followup was 38 months. Four patients (11.8%) had recurrence. Two patients (6.7%) developed regional nodal recurrence, one at 10 years and one at 21 years. Both had lymphadenectomies, and both remain alive and clinically free of disease 6.26 and 7.7 years after surgical management of their lymph node metastases. Two other patients developed distant metastatic disease: one with subcutaneous metastases who is alive with disease, and one with multiple distant visceral metastases who died of progressive disease (Table 4). 5-year survival was 89% (SE 7%) (Figure 1).
Group 3
Six patients (67%) had further progression of disease. These six are deceased, one of which died of unrelated causes. One patient had no evidence of further progression during followup, but was listed as deceased on the Social Security Death Index. His disease status at time of death is unknown. The two remaining patients are alive with no evidence of disease following surgical resection of their metastases. Both were participants on vaccine clinical trials, one patient additionally underwent radiation and the other interferon therapy. The median follow up time for Group 3 was 28 months with a median survival of 2.3 years, and 4-year survival of 22% (Figure 1).
Combining groups 1 and 2, overall recurrence rate was 9.1% and nodal recurrence rate was 3.0%. 5- and 10-year overall survival was 92% (SE 4%) and 87% (SE 6%). There was no significant difference in survival between groups 1 and 2. Survival is comparable to reported 5-year survival estimates for patients with AJCC Stage IA disease (95.3% ± 0.4).[29] When all patients are combined, overall 5-year survival is 80% (SE 6%).
DISCUSSION
The prognostic significance of regression in thin melanoma is poorly defined. Our data have provided insight into two presentations of melanoma with regression, which have distinct clinical outcomes. Type 1 regression patients are those with thin melanomas and regression who do not have clinical evidence for metastatic disease on presentation. Within this group we have evaluated the role of SNBx performance and its prognostic implications. Type 2 regression patients are those who present with metastatic melanoma and in whom a regressed primary melanoma was concurrently or subsequently discovered on physical examination. The differences in outcomes for these two groups raise the possibility of differing mechanisms of tumor development, suggesting further potential for immunologic inquiry and study.
The selection of patients with thin melanomas for SNBx remains an area of debate, without consistent findings among multiple studies (Table 5).[11,13,16,22–24,30–44]
Table 5.
Current literature addressing regression in melanoma.
| First Author [citation] | Year | Number of patients | Incidence of Regression (%) | Regression as prognostic factor |
|---|---|---|---|---|
| Guitart [4] | 2002 | 43 | 42% (metastatic) 5% (nonmetastatic) |
Negative prognostic indicator for metastasis |
| Gromet [11] | 1978 | 121 | 19% | Negative prognostic indicator for metastasis |
| Paladugu [12] | 1983 | 36 | 30% | Negative prognostic indicator for metastasis |
| Sondergaard [14] | 1985 | 486 | N/A | Negative prognostic indicator for survival |
| Clark [15] | 1989 | 501 | N/A | Negative prognostic indicator for survival |
| Blessing [16] | 1990 | 26 | 50% | Difference in regression rate b/w metastatic and nonmetastatic thin melanoma |
| Slingluff [13] | 1988 | 681 | 40% (metastatic) 17%(nonmetastatic) |
Negative prognostic indicator for metastasis |
| Wanebo [21] | 1985 | 48 | 50% | Not a prognostic indicator for survival |
| Kelly [18] | 1985 | 844 | 20.4% | Not a prognostic indicator for survival |
| McGovern [19] | 1983 | 353 | 58% | Not a prognostic indicator for survival |
| Trau [20] | 1983 | 116 | 35% | Not a prognostic indicator for metastasis |
| Cooper [17] | 1985 | 48 | 23% definite 27% probable |
Not a prognostic indicator for metastasis |
| Morris [22] | 2008 | 344 | 25.5% | Not a predictor for SLN status or recurrence |
| Cecchi [23] | 2007 | 50 | 70% | Not a predictor for SLN status |
| Socrier [24] | 2010 | 397 | 23% | Not a predictor for SLN status |
| Kramkimel [45] | 2010 | 34 | 76% | Not a predictor for SLN status |
| Kaur [46] | 2008 | 146 | 48% | Not a predictor for SLN status |
Previous studies have suggested that regression is associated with poor prognosis. Several studies have associated regression with a greater risk of metastasis to lymph nodes.[11,44] Clark et al included regression as a poor prognostic indicator in their model predicting survival in stage I melanoma.[15] Blessing et al studied a group of patients who had experienced metastatic disease or died of melanoma and found that, compared to controls, the patients experiencing poor outcomes were more likely to have had regression of their tumor.[16]
In a study of prognostic factors in thin melanoma Slingluff et al found that the presence of severe regression shortened the disease free interval compared to similar lesions without regression.[13] We believe now that the negative associations of regression are explained by the inclusion of a patient population like Group 3 in this study (synchronous with the diagnosis of distant metastases). Those patients have survival outcomes that are much worse than patients with thin melanomas and Type 1 regression.
More recent studies investigating the use of SNBx in the setting of regression have concluded that regression is not an indication for SLN Bx in the setting of thin melanoma.[22–24,45] Morris et al evaluated 344 patients with regression in their primary melanoma, and found a consistently lower incidence of positive SLN Bx as compared to primary melanomas without regression at all Breslow depths.[22] Cecchi et al retrospectively evaluated 59 patients with thin melanoma, and found no correlation between the presence of regression and the outcome of SLN Bx.[23] Two studies found a lower incidence of SLN positivity in patients with regression than patients without regression, and concluded that regression is not an independent risk factor for SLN metastasis.[24,46] The present study agrees that regression is not an unfavorable prognostic factor, and advocate that thin melanomas with regression and no other negative prognostic factor do not require SNBx.
For those patients with thin melanomas and Type 1 regression, SNBx has routinely been performed at this institution. In the present study, 3% of patients (2/66) with T1a melanomas with regression had clinically or histologically evident metastases to regional nodes. This is comparable to the sentinel node positivity rate for all T1 melanomas and the regional nodal metastasis rate of T1a patients not undergoing SNBx.[13,37–39,47,48] Thus, Type 1 regression in thin melanomas does not appear to be associated with increased risk of nodal metastasis. Based upon these data, we no longer recommend SNBx for patients with T1a melanomas solely based on histologic evidence of regression. It is appropriate, however, for these patients to be followed regularly for at least 20 years after excision of their primary tumor, maintaining high vigilance for clinically detectable metastases.
It is possible that patients with occult lymph nodes may not present with clinically evident metastases for a decade or more after diagnosis and excision of a thin melanoma; so the incidence of regional node metastasis observed in patients managed only with WLE may slightly underestimate the true risk of nodal metastases. Similarly, histological evaluation of sentinel nodes may miss a few clinically significant micrometastases, or may identify very small metastases that will not evolve in to clinically evident disease. Thus, assessment of lymph node metastatic risk by either SNBx or observation may be criticized as imperfect. However, the very low rates of regional recurrence in both groups 1 and 2 supports the general conclusion that regression of a primary melanoma is associated with low rates of regional node metastasis. The conflicting data and opinions of the significance of regression may be related to the difficulty in identifying and categorizing regression in histological study. The recent studies have cited vastly different rates of regression (23%–70%), perhaps indicating that there is a lack of consensus in the definition of regression.[22–24] Requena et al advocate dividing regression into two phases: an early “regressing” phase (which is primarily inflammatory and a late “regressed” phase (primarily scarring).[49] Because any lesion showing melanoma with early regression will be resected it is impossible to know if “regressing” melanoma progresses inexorably to “regressed” melanoma. This raises the possibility that early and late regression may be biologically distinct entities with distinct prognostic values. Combining these two phases into a single category may account for the disparity among studies.
The exact biological nature of regression is poorly understood, and some believe it is immune mediated.[8,50] It is thought that progressive inflammation within the tumor induces destruction of malignant cells, causing fibrous tissue underlying or replacing malignant cells.[51] Stimulation by tumor-associated antigens initiates a cell-mediated immune response, ultimately causing destruction of tumor cells by cytotoxic CD8+ lymphocytes.[51–53] In theory, this immune response would represent a favorable prognostic indicator. A fascinating theory is that lymph node metastases are a prerequisite for regression of the primary melanoma.[33] Shaw et al identified 28 patients with thin melanomas (<0.76 mm) who presented with simultaneous clinically detectable lymph node metastasis. All 28 of these patients had primary lesions which showed histological evidence of regression (defined as “early”, “intermediate”, and “late”), with all but two having evidence for late regression.[54] They hypothesized that regional spread of the melanoma to lymph nodes increased the immune response against the primary melanoma by increasing the activity of cytotoxic T-cells at the primary site while activating suppressor T-cells within the lymph node itself (thus explaining why the lymph node metastases evade destruction).[54]
We suspect that immune responses occur in regional nodes for most or all patients with regressed melanomas, and that patients in Group 1 and 2 have had successful eradication both of the primary lesion and any metastases to the nodes, while those in Group 3 have tumor cells in the nodes that escape immune recognition and progress while the primary lesion is controlled by the immune response generated in the nodes. Further characterization of the specificity of the T cell response in nodes and in the primary site is needed to test or to modify this hypothesis. Immunologic studies of lymphocytes in regressed primaries may identify factors that predispose patients to allow tumor escape. In their review of tumor escape Poggi and Zocchi suggest that immunoselective pressure can favor tumor cells that have become resistant via loss of HLA alleles, and evaded the immune response.[55] If this were the case it is possible that the immune response leading to regression could in certain cases paradoxically increase the malignant potential of the tumor.
Limitations of study
This study’s retrospective nature and small sample size are identified as limitations. Additionally, the tertiary referral center that sees a high volume of metastatic or high-risk patients lends a patient selection bias toward patients with thin melanomas who have metastatic disease and a less favorable prognosis. This has been resolved through inclusion of only patients who received treatment of their primary melanoma at our institution. The ability of this study to focus on a specific subset of thin regressed melanomas without other negative prognostic factors (Clark level >3, ulceration, or positive deep margin) gives it the ability to conclude that thin melanomas with regression do not have an increased likelihood of sentinel node positivity or distant metastases, and regression alone should not be a determinant in selection for sentinel node evaluation.
CONCLUSION
The current review has helped to clarify the presentation, natural history and appropriate management of T1 melanomas with regression, and suggests avenues for future research to characterize the tumor biology and immunobiology that explain the differences in these two presentations.
Contributor Information
Susannah E. McClain, Department of Dermatology, University of Maryland Medical System, Baltimore, Maryland, USA
Amber L. Shada, Department of Surgery, University of Virginia Health System, Charlottesville, Virginia, USA
Megan Barry, University of Virginia Health System, Charlottesville, Virginia, USA
James W. Patterson, Departments of Pathology and Dermatology, University of Virginia Health System, Charlottesville, Virginia, USA
Craig L. Slingluff, Jr., Division of Surgical Oncology, University of Virginia Health System, Charlottesville, Virginia, USA
References
- 1.Anstey AV, Arlett CF, Cole J, Norris PG, Hamblin AS, Limb GA, et al. Long-term survival and preservation of natural killer cell activity in a xeroderma pigmentosum patient with spontaneous regression and multiple deposits of malignant melanoma. Br J Dermatol. 1991;125:272–78. doi: 10.1111/j.1365-2133.1991.tb14754.x. [DOI] [PubMed] [Google Scholar]
- 2.Baldo M, Schiavon M, Cicogna PA, Boccato P, Mazzoleni F. Spontaneous regression of subcutaneous metastasis of cutaneous melanoma. Plast Reconstr Surg. 1992;90:1073–76. doi: 10.1097/00006534-199212000-00022. [DOI] [PubMed] [Google Scholar]
- 3.COLE WH, EVERSON TC. Spontaneous regression of cancer: preliminary report. Ann Surg. 1956;144:366–83. doi: 10.1097/00000658-195609000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guitart J, Lowe L, Piepkorn M, Prieto VG, Rabkin MS, Ronan SG, et al. Histological characteristics of metastasizing thin melanomas: a case-control study of 43 cases. Arch Dermatol. 2002;138:603–08. doi: 10.1001/archderm.138.5.603. [DOI] [PubMed] [Google Scholar]
- 5.McGovern VJ. Spontaneous regression of melanoma. Pathology. 1975;7:91–99. doi: 10.3109/00313027509092702. [DOI] [PubMed] [Google Scholar]
- 6.Wagner SN, Schultewolter T, Wagner C, Briedigkeit L, Becker JC, Kwasnicka HM, et al. Immune response against human primary malignant melanoma: a distinct cytokine mRNA profile associated with spontaneous regression. Lab Invest. 1998;78:541–50. [PubMed] [Google Scholar]
- 7.Wang TS, Lowe L, Smith JWn, Francis IR, Sondak VK, Dworzanian L, et al. Complete spontaneous regression of pulmonary metastatic melanoma. Dermatol Surg. 1998;24:915–19. doi: 10.1111/j.1524-4725.1998.tb04275.x. [DOI] [PubMed] [Google Scholar]
- 8.High WA, Stewart D, Wilbers CR, Cockerell CJ, Hoang MP, Fitzpatrick JE. Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteria. J Am Acad Dermatol. 2005;53:89–100. doi: 10.1016/j.jaad.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Printz C. Spontaneous regression of melanoma may offer insight into cancer immunology. J Natl Cancer Inst. 2001;93:1047–48. doi: 10.1093/jnci/93.14.1047. [DOI] [PubMed] [Google Scholar]
- 10.Breslow A, Macht SD. Evaluation of prognosis in Stage I cutaneous melanoma. Plast Reconstr Surg. 1978;61:342–46. doi: 10.1097/00006534-197803000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Gromet MA, Epstein WL, Blois MS. The regressing thin malignant melanoma: a distinctive lesion with metastatic potential. Cancer. 1978;42:2282–92. doi: 10.1002/1097-0142(197811)42:5<2282::aid-cncr2820420528>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 12.Paladugu RR, Yonemoto RH. Biologic behavior of thin malignant melanomas with regressive changes. Arch Surg. 1983;118:41–44. doi: 10.1001/archsurg.1983.01390010031008. [DOI] [PubMed] [Google Scholar]
- 13.Slingluff CLJ, Vollmer RT, Reintgen DS, Seigler HF. Lethal “thin” malignant melanoma. Identifying patients at risk. Ann Surg. 1988;208:150–61. doi: 10.1097/00000658-198808000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sondergaard K, Hou-Jensen K. Partial regression in thin primary cutaneous malignant melanomas clinical stage I. A study of 486 cases. Virchows Arch A Pathol Anat Histopathol. 1985;408:241–47. doi: 10.1007/BF00707986. [DOI] [PubMed] [Google Scholar]
- 15.Clark WHJ, Elder DE, Guerry Dt, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 16.Blessing K, McLaren KM, McLean A, Davidson P. Thin malignant melanomas (less than 1. 5 mm) with metastasis: a histological study and survival analysis. Histopathology. 1990;17:389–95. doi: 10.1111/j.1365-2559.1990.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 17.Cooper PH, Wanebo HJ, Hagar RW. Regression in thin malignant melanoma. Microscopic diagnosis and prognostic importance. Arch Dermatol. 1985;121:1127–31. [PubMed] [Google Scholar]
- 18.Kelly JW, Sagebiel RW, Blois MS. Regression in malignant melanoma. A histologic feature without independent prognostic significance. Cancer. 1985;56:2287–91. doi: 10.1002/1097-0142(19851101)56:9<2287::aid-cncr2820560924>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.McGovern VJ, Shaw HM, Milton GW. Prognosis in patients with thin malignant melanoma: influence of regression. Histopathology. 1983;7:673–80. doi: 10.1111/j.1365-2559.1983.tb02279.x. [DOI] [PubMed] [Google Scholar]
- 20.Trau H, Rigel DS, Harris MN, Kopf AW, Friedman RJ, Gumport SL, et al. Metastases of thin melanomas. Cancer. 1983;51:553–56. doi: 10.1002/1097-0142(19830201)51:3<553::aid-cncr2820510332>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 21.Wanebo HJ, Cooper PH, Hagar RW. Thin (less than or equal to 1 mm) melanomas of the extremities are biologically favorable lesions not influenced by regression. Ann Surg. 1985;201:499–504. doi: 10.1097/00000658-198504000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris KT, Busam KJ, Bero S, Patel A, Brady MS. Primary cutaneous melanoma with regression does not require a lower threshold for sentinel lymph node biopsy. Ann Surg Oncol. 2008;15:316–22. doi: 10.1245/s10434-007-9675-2. [DOI] [PubMed] [Google Scholar]
- 23.Cecchi R, Pavesi M, Buralli L, Innocenti S, De Gaudio C. Tumour regression does not increase the risk of sentinel node involvement in thin melanomas. Chir Ital. 2008;60:257–60. [PubMed] [Google Scholar]
- 24.Socrier Y, Lauwers-Cances V, Lamant L, Garrido I, Lauwers F, Lopez R, et al. Histological regression in primary melanoma: not a predictor of sentinel lymph node metastasis in a cohort of 397 patients. Br J Dermatol. 2010;162:830–34. doi: 10.1111/j.1365-2133.2009.09606.x. [DOI] [PubMed] [Google Scholar]
- 25.Abramova L, Slingluff CLJ, Patterson JW. Problems in the interpretation of apparent “radial growth phase” malignant melanomas that metastasize. J Cutan Pathol. 2002;29:407–14. doi: 10.1034/j.1600-0560.2002.290704.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith JLJ, Stehlin JSJ. Spontaneous regression of primary malignant melanomas with regional metastases. Cancer. 1965;18:1399–415. doi: 10.1002/1097-0142(196511)18:11<1399::aid-cncr2820181104>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 27.Ronan SG, Eng AM, Briele HA, Shioura NN, Das Gupta TK. Thin malignant melanomas with regression and metastases. Arch Dermatol. 1987;123:1326–30. [PubMed] [Google Scholar]
- 28.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–48. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 29.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karakousis GC, Gimotty PA, Botbyl JD, Kesmodel SB, Elder DE, Elenitsas R, et al. Predictors of regional nodal disease in patients with thin melanomas. Ann Surg Oncol. 2006;13:533–41. doi: 10.1245/ASO.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Sondak VK, Taylor JM, Sabel MS, Wang Y, Lowe L, Grover AC, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity: lessons learned from the generation of a probabilistic model. Ann Surg Oncol. 2004;11:247–58. doi: 10.1245/aso.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 32.Gimotty PA, Guerry D, Ming ME, Elenitsas R, Xu X, Czerniecki B, et al. Thin primary cutaneous malignant melanoma: a prognostic tree for 10-year metastasis is more accurate than American Joint Committee on Cancer staging. J Clin Oncol. 2004;22:3668–76. doi: 10.1200/JCO.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Azzola MF, Shaw HM, Thompson JF, Soong SJ, Scolyer RA, Watson GF, et al. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer. 2003;97:1488–98. doi: 10.1002/cncr.11196. [DOI] [PubMed] [Google Scholar]
- 34.Kesmodel SB, Karakousis GC, Botbyl JD, Canter RJ, Lewis RT, Wahl PM, et al. Mitotic rate as a predictor of sentinel lymph node positivity in patients with thin melanomas. Ann Surg Oncol. 2005;12:449–58. doi: 10.1245/ASO.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 35.Thompson JF, Shaw HM. Should tumor mitotic rate and patient age, as well as tumor thickness, be used to select melanoma patients for sentinel node biopsy? Ann Surg Oncol. 2004;11:233–35. doi: 10.1245/aso.2004.01.912. [DOI] [PubMed] [Google Scholar]
- 36.Aloia TA, Gershenwald JE. Management of early-stage cutaneous melanoma. Curr Probl Surg. 2005;42:460–534. doi: 10.1067/j.cpsurg.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Bleicher RJ, Essner R, Foshag LJ, Wanek LA, Morton DL. Role of sentinel lymphadenectomy in thin invasive cutaneous melanomas. J Clin Oncol. 2003;21:1326–31. doi: 10.1200/JCO.2003.06.123. [DOI] [PubMed] [Google Scholar]
- 38.Gershenwald JE, Thompson W, Mansfield PF, Lee JE, Colome MI, Tseng CH, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976–83. doi: 10.1200/JCO.1999.17.3.976. [DOI] [PubMed] [Google Scholar]
- 39.Kalady MF, White RR, Johnson JL, Tyler DS, Seigler HF. Thin melanomas: predictive lethal characteristics from a 30-year clinical experience. Ann Surg. 2003;238:528–35. doi: 10.1097/01.sla.0000090446.63327.40. discussion 535–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nahabedian MY, Tufaro AP, Manson PN. Sentinel lymph node biopsy for the T1 (thin) melanoma: is it necessary? Ann Plast Surg. 2003;50:601–06. doi: 10.1097/01.SAP.0000069065.00486.1E. [DOI] [PubMed] [Google Scholar]
- 41.Puleo CA, Messina JL, Riker AI, Glass LF, Nelson C, Cruse CW, et al. Sentinel node biopsy for thin melanomas: which patients should be considered? Cancer Control. 2005;12:230–35. doi: 10.1177/107327480501200404. [DOI] [PubMed] [Google Scholar]
- 42.Stitzenberg KB, Groben PA, Stern SL, Thomas NE, Hensing TA, Sansbury LB, et al. Indications for lymphatic mapping and sentinel lymphadenectomy in patients with thin melanoma (Breslow thickness < or =1. 0 mm) Ann Surg Oncol. 2004;11:900–06. doi: 10.1245/ASO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Wong SL, Brady MS, Busam KJ, Coit DG. Results of sentinel lymph node biopsy in patients with thin melanoma. Ann Surg Oncol. 2006;13:302–09. doi: 10.1245/ASO.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Olah J, Gyulai R, Korom I, Varga E, Dobozy A. Tumour regression predicts higher risk of sentinel node involvement in thin cutaneous melanomas. Br J Dermatol. 2003;149(3):662–63. doi: 10.1046/j.1365-2133.2003.05502.x. [DOI] [PubMed] [Google Scholar]
- 45.Kramkimel N, Maubec E, Boitier F, Cavalcanti A, Beldi M, Mamelle G, et al. Tumour regression is not predictive for higher risk of sentinel node involvement in thin melanomas (Breslow thickness < or = 1 mm) Ann Dermatol Venereol. 2010;137:276–80. doi: 10.1016/j.annder.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Kaur C, Thomas RJ, Desai N, Green MA, Lovell D, Powell BW, et al. The correlation of regression in primary melanoma with sentinel lymph node status. J Clin Pathol. 2008;61:297–300. doi: 10.1136/jcp.2007.049411. [DOI] [PubMed] [Google Scholar]
- 47.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 48.Warycha MA, Zakrzewski J, Ni Q, Shapiro RL, Berman RS, Pavlick AC, et al. Meta-analysis of sentinel lymph node positivity in thin melanoma (<or=1 mm) Cancer. 2009;115:869–79. doi: 10.1002/cncr.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Requena C, Botella-Estrada R, Traves V, Nagore E, Almenar S, Guillen C. Problems in defining melanoma regression and prognostic implication. Actas Dermosifiliogr. 2009;100:759–66. [PubMed] [Google Scholar]
- 50.Ceballos PI, Barnhill RL. Spontaneous regression of cutaneous tumors. Adv Dermatol. 1993;8:229–61. discussion 262. [PubMed] [Google Scholar]
- 51.Cook MG. The significance of inflammation and regression in melanoma. Virchows Arch A Pathol Anat Histopathol. 1992;420:113–15. doi: 10.1007/BF02358800. [DOI] [PubMed] [Google Scholar]
- 52.Barr RJ. The many faces of completely regressed malignant melanoma. Pathology (Phila) 1994;2:359–70. [PubMed] [Google Scholar]
- 53.Tefany FJ, Barnetson RS, Halliday GM, McCarthy SW, McCarthy WH. Immunocytochemical analysis of the cellular infiltrate in primary regressing and non-regressing malignant melanoma. J Invest Dermatol. 1991;97:197–202. doi: 10.1111/1523-1747.ep12479662. [DOI] [PubMed] [Google Scholar]
- 54.Shaw HM, McCarthy SW, McCarthy WH, Thompson JF, Milton GW. Thin regressing malignant melanoma: significance of concurrent regional lymph node metastases. Histopathology. 1989;15:257–65. doi: 10.1111/j.1365-2559.1989.tb03076.x. [DOI] [PubMed] [Google Scholar]
- 55.Poggi A, Zocchi MR. Mechanisms of tumor escape: role of tumor microenvironment in inducing apoptosis of cytolytic effector cells. Arch Immunol Ther Exp (Warsz) 2006;54:323–33. doi: 10.1007/s00005-006-0038-7. [DOI] [PubMed] [Google Scholar]


