Abstract
Objective
To characterize epidemiologic and clinical features of red primary amelanotic melanomas, an atypical presentation of melanoma that is underemphasized in patient and physician education.
Patients and methods
Review of a prospectively collected melanoma database identified 46 patients with red amelanotic melanomas, whose clinical features were compared with 329 patients with pigmented melanomas from the same database and same timeframe of January 1964 and September 2005.
Results
Red amelanotic melanomas represented 3.9% of all melanomas recorded in our database, and accounted for nearly 70% of amelanotic melanomas. Melanoma was included in the clinical differential diagnosis in 32% of red amelanotic melanomas, vs. 94% of pigmented melanomas (p<0.001). Red amelanotic melanomas more commonly underwent shave biopsy (55% vs. 12%, p<0.001), and more likely had positive deep margins (35% vs. 9%, p<0.001), but had comparable risks of metastasis and mortality.
Conclusion
Red amelanotic melanomas are often misdiagnosed clinically but carry mortality risk comparable to pigmented melanomas. Clinicians screening for melanoma should be more vigilant in considering melanoma in the differential diagnosis of red skin lesions.
Keywords: melanoma, diagnosis, amelanotic
INTRODUCTION
A new or changing skin lesion is the most common warning sign for melanoma. Variations in color or asymmetry of borders of a pigmented lesion are noted in the majority of patients with melanoma at the time of diagnosis. In contrast, atypical presentations of melanoma can often result in delayed diagnosis and suboptimal management. Amelanotic lesions are an atypical presentation of melanoma that may not be as easily recognized as malignant melanoma.1 Because of their lack of pigment, such lesions may be misdiagnosed as basal cell carcinoma, Bowen’s disease, eczema, keratoacanthoma, pyogenic granuloma, or extramammary Paget’s disease.1,2 In addition, diagnosis of amelanotic melanomas may be delayed until it reaches an advanced stage, when the lesion is nodular, vascular, or ulcerated.2
Amelanotic melanomas are classically described as skin-colored. However, a substantial subset of amelanotic melanomas is not skin-colored but is red, pink, or erythematous.1, 2 This finding is characteristic enough that attention to its recognition may aid in earlier diagnosis of amelanotic melanomas. Few studies have reported amelanotic melanomas presenting primarily as red lesions. Our clinical experience demonstrates that these “red amelanotic melanomas” are relatively common, but current education of physicians and of the public fails to stress this presentation of melanoma. We hypothesized that such red amelanotic melanomas may initially be misdiagnosed clinically, delaying diagnosis until a more advanced stage than pigmented melanomas. We also hypothesized that clinical outcome may differ from that of pigmented melanomas. The primary objectives of this study were to estimate the prevalence of primary cutaneous amelanotic melanomas presenting as red lesions in our patient population, and to assess whether clinical characteristics and outcomes of patients with red primary cutaneous amelanotic melanomas differ from those with classically pigmented primary cutaneous melanomas. Our secondary objective was to highlight this red feature as a useful educational and diagnostic tool.
PATIENTS AND METHODS
Study approval was obtained from the University of Virginia Institutional Review Board. We reviewed a prospectively collected database containing information on melanoma patients treated from 1991 to 2007, and identified 1170 patients with primary cutaneous melanoma diagnosed between January 1964 and September 2005.
We included lesions reported to have a red, pink, or erythematous color on presentation as “red amelanotic melanomas.” These are hereafter referred to as the “red amelanotic melanoma” group. We excluded red lesions arising in the background of a pigmented macule and lesions with red around the periphery of a pigmented lesion because we felt these were not as great a diagnostic challenge as lesions without any detectable brown or black pigmentation. For the purposes of this study, we did not describe skin-colored amelanotic melanoma, as this has been previously described in detail.1, 3–11 Other patients excluded from the study were those with ocular and mucosal primaries, multiple primary melanomas, and age under 14.
Clinical data and pathologic information were collected, including patient age, sex, presence or absence of gross pigment, presenting site, histologic classification, Breslow depth, Clark’s level, mitotic rate, and ulceration. We also collected information regarding clinical diagnosis, surgical management, recurrence and survival. The extracted data were confirmed and supplemented by individual chart reviews. Review of clinical notes and pathology reports was used to obtain clinician differential diagnosis (e.g.: “rule out melanoma vs. basal cell carcinoma”).
For comparison, a control group of patients with “classic” presentations of melanoma (those with grossly pigmented lesions) was identified by performing a database search for pigmented lesions. Such lesions have documented evidence of black or brown colors within a melanoma lesion. The final data set for control patients included 329 people (7:1) with grossly pigmented melanomas on presentation. This was not a formally matched control group but represented a comparable group, treated over the same interval by the same physicians. Overall mortality was determined using follow-up information obtained from the melanoma database, the patients’ clinical charts, the University of Virginia Cancer Registry, and the Social Security Death Index.
Descriptive and comparative statistics were performed using the chi-square test of association and the Wilcoxon rank-sum test. Kaplan-Meier survival functions were calculated for survival, disease-free survival (from date of diagnosis to date of last follow up), and time to metastasis for both the red and pigmented groups. The log-rank test was used to assess statistical significance. Statistical analyses were performed in SAS 9.1 and SPSS-17.0.
RESULTS
Of the 1170 melanoma patients isolated from the clinical database, 445 (38%) had details of pigmentation available. 287 of these were described as “pigmented,” 88 as red, 33 as black, and 19 as brown, while 3 were described as skin-colored, and 15 as amelanotic without distinguishing color. Thirty-six of the 88 patients with red lesions actually had either a red nodule arising in a pigmented macule or a combination of red and pigmented lesions, and 4 “red melanomas” were red around the periphery only. Two patients had multiple primary red lesions on presentation. Thus, from the 88 patients identified as having red lesions on presentation, a final data set included 46 patients (3.9% of total melanoma patients, 10.3% of those with color descriptions) with primary cutaneous lesions without clinically evident melanin pigmentation. These were considered primary red amelanotic melanomas. In addition, as detailed above, there were 18 patients with lesions described as “skin-colored” or “amelanotic, not otherwise specified”. Thus, 46 of 64 (72%) of amelanotic melanomas presented as red amelanotic melanomas.
Within the pigmented group, exclusions were made for the following patients: 6 with mucosal melanoma, 2 with ocular melanoma, 1 infant, and 1 with multiple primaries on presentation. A final data set of 329 lesions described as pigmented, black, or brown were confirmed to have classic melanin-type pigmentation, and were evaluated as the “pigmented” lesion control group. Figure 1 compares the clinical appearance of pigmented melanoma (Figure 1A) and red amelanotic melanomas (Figures 1B and 1C).
Figure 1.
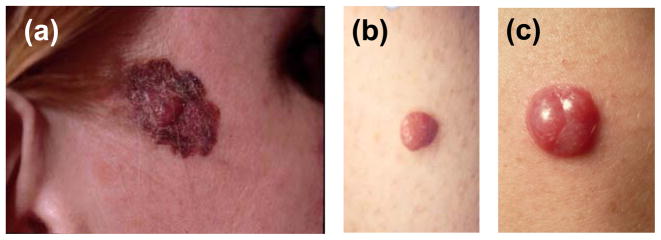
Clinical appearance of red and pigmented melanomas. (A) Primary pigmented melanoma. Although this patient has a pigmented melanoma, note the raised central papule, which is largely red in color, and a radial growth phase component that has varied pigmentation. (B) Primary red melanoma that was included in our study. Clinically this lesion lacks a radial growth phase component and lacks apparent melanin pigment. (C) Primary red melanoma, presenting as a pink-red nodule. This patient was misdiagnosed by three different dermatologists before having a diagnostic biopsy.
Demographic Data
The red amelanotic melanoma group was comprised of slightly more females than males (54% vs. 46%) while the pigmented group was more male than female (55% vs. 45%) (p=0.27). The mean age at diagnosis was 58 years (range 22–86 years) for red amelanotic melanomas and 53 years (range 14–89 years) for pigmented melanomas (p=0.07). Within both groups, the large majority of melanomas were diagnosed in Caucasian patients (Table I).
Table 1.
Demographic characteristics of 46 red melanoma patients and 329 pigmented melanoma patients evaluated in melanoma clinic at the University of Virginia, 1993–2005.
| Red (n=46) No. (%) |
Pigmented (n=329) No. (%) |
P values | |
|---|---|---|---|
| Gender | 0.25 | ||
| Male | 21 (46) | 180 (54) | |
| Female | 25 (55) | 149 (45) | |
| Age, median, years | 58 | 53 | 0.07 |
| Race | NP* | ||
| Caucasian | 45 (97.8) | 292 (97.7) | |
| African American | 0 (0) | 3 (1.0) | |
| Asian | 0 (0) | 1 (0.3) | |
| Hispanic | 1 (2.2) | 3 (1.0) | |
| Not recorded | 0 | 30 |
NP=Not performed
Clinical Data
The majority of red amelanotic melanomas (61%) were located on the extremities (this includes the shoulder, arm, forearm, wrist, hand, finger, groin, thigh, knee, leg, ankle, foot, and toe), while the remainder was located on the back, trunk (includes chest, breast, abdomen, and flank), and head/neck (Table II). The largest proportion (48%) of the pigmented lesions was also located on the extremities, with similar distributions to the red amelanotic melanomas on the back and trunk. There was no significant difference between groups with regards to anatomic site of primary (p=0.36). A numerically higher percentage of pigmented lesions (13%) were observed on the head and neck than red amelanotic melanomas (4%), (p=0.09). The maximum gross diameter of the red lesions ranged from 2 to 22 mm (mean 7.7 mm), while the diameter of the pigmented lesions ranged from 1 to 70 mm (mean 12.8 mm), (p<0.001) (Table II).
Table 2.
Clinical characteristics.
| Red (n=46) No. (%) |
Pigmented (n=329) No. (%) |
P values * | |
|---|---|---|---|
|
| |||
| Color | |||
| Red | 23 (50) | 0 (0) | |
| Pink | 16 (35) | 0 (0) | |
| Erythematous | 7 (15) | 0 (0) | |
| Pigmented, | 0 (0) | 329 (100) | |
| Black | 0 (0) | 31 (9) | |
| Brown | 0 (0) | 19 (6) | |
| NOS | 0 (0) | 279 (85) | |
|
| |||
| Anatomic Site | 0.36 | ||
| Back | 11 (24) | 84 (26) | |
| Extremity | 28 (61) | 158 (48) | 0.10@ |
| Head/Neck | 2 (4) | 43 (13) | 0.09# |
| Trunk | 5 (11) | 42 (13) | |
| Other | 0 (0) | 2 (0.6) | |
|
| |||
| Lesion diameter, mm | <0.001 | ||
| Range (mean) | 2–22 (7.7) | 1–70 (12.8) | |
| 0–5 | 10 (33.3) | 46 (19.7) | |
| 5.01–10 | 17 (56.7) | 81 (34.8) | |
| 10.01–15 | 2 (6.7) | 52 (22.3) | |
| >15 | 1 (3.3) | 54 (23.2) | |
| Not reported | 16 | 96 | |
Chi-square tests were used for comparisons regarding anatomic site; Wilcoxon rank sum test was used for lesion diameter.
Extremity vs. all other categories
Head/Neck vs. all other categories
Histologic Data
Histologic specimens were classified into subtypes based on currently accepted and utilized classification criteria.12 The majority of pigmented lesions were classified as superficial spreading melanoma (SSM), while most red lesions were divided equally between nodular melanoma (NOD) and SSM (Table III). There was a statistically significant difference in histologic subtypes between the two groups (p=0.005) In addition, there was a higher proportion of desmoplastic melanomas in the red group. (Table III).
Table 3.
Histologic characteristics.
| Red (n=46) No. (%) |
Pigmented (n=329) No. (%) |
P value* | |
|---|---|---|---|
|
| |||
| Histology | 0.005 | ||
| SSM | 10 (28.6) | 146 (60.6) | |
| NOD | 10 (28.6) | 34 (14.1) | 0.029@ |
| ALM | 2 (5.7) | 8 (3.3) | |
| LMM | 4 (11.4) | 16 (6.6) | |
| Desmoplastic | 5 (14.3) | 3 (1.2) | |
| Other | 4 (11.4) | 34 (14.1) | |
| Not recorded | 11 | 88 | |
|
| |||
| Breslow depth, mm** | 0.78 | ||
| Range (mean) | 0.45–12.0 (2.3) | 0.2–50.0 (2.1) | |
| 0–1 | 12 (26.1) | 97 (31.5) | |
| 1.01–2 | 17 (37.0) | 107 (34.7) | |
| 2.01–4 | 13 (28.3) | 67 (21.8) | |
| >4 | 4 (8.7) | 37 (12.0) | |
| Not recorded | 0 | 21 | |
|
| |||
| Clark’s Level | 0.02 | ||
| 1 | 0 (0.0) | 17 (5.4) | |
| 2 | 1 (2.2) | 39 (12.4) | |
| 3 | 13 (28.9) | 101 (32.1) | |
| 4 | 30 (66.7) | 139 (44.1) | |
| 5 | 1 (2.2) | 19 (6.0) | |
| Not recorded | 1 | 14 | |
|
| |||
| Ulceration | 0.66 | ||
| Yes | 9 (21.4) | 63 (24.5) | |
| No | 33 (78.6) | 194 (75.5) | |
| Not recorded | 4 | 72 | |
|
| |||
| Growth Phase | <0.001 | ||
| Vertical | 37 (100) | 191 (85) | |
| Radial | 0 (0) | 34 (15) | |
| Not recorded | 9 | 104 | |
|
| |||
| Mitoses | 0.26 | ||
| >=1/mm2 | 28 (80) | 131 (74) | |
| 0/mm2 | 7 (20) | 46 (26) | |
| Not recorded | 11 | 152 | |
Chi-square tests were used for comparisons regarding histologic subtype and ulceration; Wilcoxin rank sum test was used for Breslow depths and Clark’s level
NOD vs. all other categories
in cases where the melanoma extended to the deep margin on the original biopsy (usually due to a shave biopsy), but the wide excision specimen contained no residual melanoma or thinner residual melanoma than the thickness measured on the biopsy, the Breslow depth was recorded as the depth to tumor cells at the base of the specimen on the original biopsy.
The Breslow thicknesses of the red amelanotic melanomas ranged from 0.45 to 12 mm (mean 2.3 mm). There was no statistical difference when compared with pigmented melanomas, whose Breslow thicknesses ranged from 0.2 to 50.0 mm (mean 2.1 mm) (p=0.70) (Table III). However, a higher proportion of red amelanotic melanomas invaded to Clark’s level IV than pigmented melanomas, and patients with red amelanotic melanomas presented very rarely with Clark’s level I–II lesions (p=0.02). There was no significant difference in histologic ulceration between the two groups (Table III). All red melanomas were in vertical growth phase, while 85% of pigmented melanomas were in vertical growth phase (p<0.001). Mitoses were present in 80% of red melanomas and 74% of pigmented melanomas (p=0.26).
To characterize the neovascularity of these red amelanotic and pigmented melanomas, we examined a representative number of pathology specimens from both groups to assess the quantity of blood vessels present and the degree of dilatation of these vessels. Qualitatively, numerous, dilated blood vessels were present in both the red and pigmented lesions, and no microscopic differences existed between the two groups (data not shown).
Clinical Diagnosis
Within the red amelanotic melanoma group, melanoma was included in the clinical differential diagnosis in only 32% (12 of 38 with clinical diagnosis stated) of the cases, compared with 94% of patients in the pigmented group (p<0.001) (Table IV). Of the 26 physicians who did not include melanoma in the differential diagnosis of the red amelanotic melanomas lesions, 21 were dermatologists (primarily community based), 4 were primary care physicians, and one was unknown. Alternative clinical diagnoses were given for 20 patients, while the remaining 6 patients had either written or verbal (i.e. patient recollection) documentation that melanoma was not included in the differential diagnosis (i.e. “looks benign,” “were not worried,” “surprised by diagnosis,” “probably benign”). Basal cell carcinoma was considered a possible diagnosis in approximately 35% of the misdiagnosed red amelanotic melanomas. Other suggested clinical diagnoses included dermatofibroma, dermatitis/eczema, infection, pyogenic granuloma, and Bowen’s disease (Table IV).
Table 4.
Rates of clinical misdiagnosis at the time of biopsy
| Red (n=46) No. (%) |
Pigmented (329) No. (%) |
P Value | |
|---|---|---|---|
|
| |||
| Melanoma in Differential | <0.001 | ||
| Diagnosis | |||
| Yes | 12 (32) | 202 (94) | |
| No | 26 (68.4) | 14 (6.5) | |
| Not reported | 8 | 113 | |
|
| |||
| Alternative Dx Provided | <0.001 | ||
| Yes | 20 (77) | 14 (100) | |
| No | 6 (23) | 0 (0) | |
|
| |||
| Alternative Dx suggested | |||
| Basal cell Ca | 7 (35) | 1 (7) | |
| Tumor NOS | 3 (15) | 0 (0) | |
| Dermatofibroma | 2 (10) | 0 (0) | |
| Squamous cell Ca | 2 (10) | 1 (7) | |
| Pyogenic granuloma | 1 (5) | 0 (0) | |
| Dermatitis | 1 (5) | 0 (0) | |
| Keratoacanthoma | 1 (5) | 0 (0) | |
| Bug bite | 1 (5) | 0 (0) | |
| Hemangioma | 1 (5) | 1 (7) | |
| Benign nevus | 1 (5) | 5 (36) | |
| Infection | 0 (0) | 1 (7) | |
| Seborrheic keratoses | 0 (0) | 5 (36) | |
Clinical Management of Primary Lesion
A higher percentage of patients with red amelanotic melanoma underwent shave biopsies (+/− curettage, cauterization and/or desiccation) on initial presentation than did their pigmented counterparts (55% vs. 12%, p<0.001). Full thickness biopsies (including excisional, incisional, and punch biopsies) were performed in the remaining patients (Table V). The initial biopsy incompletely removed the lesion in 74% of red amelanotic melanomas and 41% of pigmented melanomas (p<0.001). Many of the positive margins are original diagnosis were peripheral margins; however, deep margins were positive for 44% of red amelanotic melanomas compared with only 9% of the pigmented melanomas (p<0.001). Thus, patients with red amelanotic melanomas were more likely to have inaccurate measures of the Breslow thickness and, thus, their clinical stage. (Table V).
Table 5.
Initial biopsy techniques of patients with red and pigmented melanomas.
| Red (n=46) No (%) |
Pigmented (n=329) No (%) |
P Value* | |
|---|---|---|---|
|
| |||
| Initial biopsy technique | <0.001 | ||
| Shave | 23 (55) | 36 (12) | |
| Full Thickness | 19 (45) | 257 (88) | |
| Not reported | 4 | 36 | |
|
| |||
| Initial biopsy complete | <0.001 | ||
| Yes | 11 (26) | 183 (59) | |
| No | 31 (74) | 127 (41) | |
| Not reported | 4 | 19 | |
|
| |||
| Deep margin of initial biopsy positive | <0.001 | ||
| Yes | 20 (44) | 28 (9) | |
| No/NR* | 26 (56) | 301 (92) | |
|
| |||
| Residual melanoma in WLE | |||
| Yes | 21 (46) | 82 (25) | 0.003 |
| No/NR* | 25 (54) | 247 (75) | |
NR = not reported or not available. Assessments of positive deep margins or residual melanoma were reported specifically; negative deep margins and findings of no residual melanoma were specified or inferred in each case.
Chi square test
Outcomes
Mean followup was 48 months. Nine patients with red amelanotic melanoma developed recurrent disease following resection (20%), while 74 patients (22%) with pigmented melanoma had recurrence following initial resection (p=0.89). Thirteen patients (28%) with red amelanotic melanoma developed regional or distant metastatic disease, compared with 96 patients (29%) with pigmented melanoma (P value=0.93). There was no statistically significant difference between the two groups with regards to disease-free survival (p=0.73) or overall survival (p=0.38) (data not shown).
DISCUSSION
Amelanotic melanomas represent 2–8% of all melanomas.8 Although the clinical term amelanotic is defined by a lack of gross pigmentation on visual inspection,13 we have focused on a subset of patients with amelanotic melanomas that we call “red amelanotic melanomas”. The American Academy of Dermatology’s SkinCancerNet website states that: “…melanoma occasionally does not have brown or black pigmentation. An uncommon subtype called amelanotic melanoma (emphasis theirs) usually appears as a pink or red nodule.” Amelanotic melanoma presenting as red nodules is cited in literature.2,14 However, little is known about the prevalence of red coloration among amelanotic melanomas, and no studies have evaluated red melanomas in detail. Recently, a German study identified “red melanoma” as a “rare form of amelanotic melanoma” and presented two cases of a “red eczematous variety” of amelanotic melanoma.14 In addition, Bono et al reported in 2001 on a group of 15 patients whose lesions were “pink, reddish, or very light” in coloration, representing 5.5% of their melanoma patients.2 Our study of 46 patients with red melanomas suggests that this presentation may comprise 4%–10% of primary melanomas and nearly 70% of amelanotic melanomas. Given this relatively common presentation of amelanotic melanomas, we believe it is important to highlight its “red” feature as a diagnostic tool to aid in a timely diagnosis.
The present study found a higher proportion of nodular subtypes among red amelanotic melanomas compared with pigmented melanomas, which is supported in earlier studies.15 Classically pigmented melanomas have a significant radial growth phase component prior to entering a vertical growth phase.16,17 Red amelanotic melanomas have a smaller diameter than pigmented melanomas and a higher prevalence of vertical growth phase. This is potentially due to transition to a more locally invasive phenotype more quickly than pigmented lesions. This combination of a smaller diameter and nodular subtype in red amelanotic melanomas suggests a potentially more invasive pathology.
One of the most concerning findings of our study is the rate of clinical misdiagnosis of red amelanotic melanomas during screening evaluation. The majority of patients with amelanotic red melanoma in our study did not have melanoma included in the stated differential diagnosis. This is not likely due just to poor documentation, as the result is very different for patients with pigmented melanomas. A higher percentage of shave biopsies were performed within the red group (55%, vs. 12% in the pigmented group, p<0.001), leading to a significant proportion of positive deep margins and incomplete staging on histological examination. Though we did not find a significant difference in mean Breslow depth between the two groups, this high rate of deep margin positivity indicates that the red melanoma group may be understaged as a whole, and the comparison of Breslow depths between the red and pigmented groups is limited by the very high rate of positive deep margins. Other studies have determined that amelanotic melanomas are subject to misdiagnosis or delayed diagnosis, potentially leading to adverse outcomes1,4. We agree that amelanotic melanoma must be considered in the differential diagnosis of red skin lesions, and that such lesions should be considered for excisional biopsy to ensure appropriate pathological staging, especially with new or changing lesions.
Although red amelanotic melanomas were misdiagnosed at higher rates, our data do not show poorer overall survival within this group. This contrasts findings of an early study of amelanotic melanoma that found significantly lower 5-year survival as compared to classically pigmented melanoma.7 Regardless, it is likely that earlier diagnosis of such lesions may result in further improved overall mortality in patients with amelanotic melanoma. Red amelanotic melanomas are comparable in lethality to their pigmented counterparts, and we must promote enhanced screening for and diagnosis of these lesions.
“ABCD” criteria (Asymmetry, Border irregularity, Color-variegation, Diameter >6mm) are widely taught and used in diagnosing and screening for malignant melanoma.18,19 However, there is recent evidence to suggest that dermatologists are abandoning this mnemonic in favor of assessing overall patterns of nevi, relying on the “ugly duckling” sign (comparisons among nevi), and eliciting a history of recent change within a nevus.20 To this end, Kelly et al have proposed the addition of “EFG” (Elevated, Firm and Growing progressively for more than a month) to the “ABCD” acronym to aid in clinical diagnosis of melanomas.21 We agree that the “ABCD” rule does not appropriately include presentations of red amelanotic melanomas. With so much focus on irregular shape and color variegation of pigmented lesions, patients and clinicians are not uniformly alert to suspicious nonpigmented lesions. As a result, red amelanotic melanomas are often overlooked and misdiagnosed.4
We propose the need to expand the ABCD mnemonic, or develop separate criteria to aid in the clinical recognition and diagnosis of red amelanotic melanomas. “Redness” and “elevation” are the only features we identified in this review to serve as criteria for screening for such lesions. Presumably, these lesions also exhibited change or growth over time. Thus, we propose a second mnemonic for consideration: Red, Raised lesion, with Recent change (the 3 Rs). We hope that this information will be considered in developing educational tools to categorize red amelanotic melanomas during skin screening examinations.
Additionally, these features of red primary amelanotic melanomas are useful tools when following patients for recurrence, as some dermal metastases of melanoma can have the same clinical appearance (see Figure 1 C). Thus, the finding of a red, raised skin lesion that is new or changing should be assessed as possible melanoma, especially in a patient at high risk for recurrence, even if the original melanoma was pigmented.
We recognize the limitations of our study. Not all of the patients in our database had color descriptions available. This additional information may vary the percentage of patients evaluated with red lesions. As stated, the prevalence ranges from 4% to 10%, depending on the denominator. Some of our information was based upon patient recall, which is not universally reliable. Dermatologists or local surgeons typically perform definitive management of thin melanomas and MIS. The referral pattern of academic institutions such as ours may increase the proportion of patients in our practice who have thicker melanomas. Thus, the similar thickness of red and pigmented melanoma groups may reflect the fact that many patients with thin, pigmented melanomas are not referred to a tertiary melanoma center.
Nonetheless, red melanoma represents a relatively common presentation of amelanotic melanoma. These lesions are misdiagnosed at a high rate, often evade standard melanoma screening practices, and are comparable in lethality to classically pigmented lesions. Therefore, practitioners screening for melanoma should be vigilant in examining red, elevated cutaneous lesions, and should develop a low threshold for performing full thickness biopsies of such lesions, especially if they are changing or if their history is uncertain.
Acknowledgments
Financial support: There were no external sources of funding for this study.
ABBREVIATION AND ACRONYMS
- NOS
Not otherwise specified
- SSM
Superficial spreading melanoma
- NOD
Nodular melanoma
- ALM
Acral lentiginous melanoma
- LMM
Lentigo maligna melanoma
- Ca
Cancer
- WLE
Wide local excision
- SLN
Sentinel lymph node
- SNBx
Sentinel lymph node biopsy
- ELND
Elective lymph node dissection
- LN
Lymph node
- CLND
Complete lymph node dissection
- MIS
Melanoma in situ
- L/S/I
Local/satellite/in transit metastasis
- DM
Distant metastasis
- ABCDE
Asymmetry, Border irregularity, Color variegation, Diameter >6mm, Elevation
- AAD
American Academy of Dermatology
- ACS
American Cancer Society
Footnotes
Disclosure:The authors have no conflict of interest to disclose.
Contributor Information
Susannah E. McClain, Division of Dermatology, University of Maryland, Baltimore, MD, USA
Kira B. Mayo, University of Virginia School of Medicine, Charlottesville, VA, USA
Amber L. Shada, Department of Surgery, University of Virginia Health System, Charlottesville, VA, USA
Mark E. Smolkin, Department of Public Health Sciences, University of Virginia Health System, Charlottesville, VA, USA
James W. Patterson, Departments of Pathology and Dermatology, University of Virginia Health System, Charlottesville, VA, USA
Craig L. Slingluff, Jr., Division of Surgical Oncology, University of Virginia Health System, Charlottesville, VA, USA
References
- 1.Adler MJ, White CRJ. Amelanotic malignant melanoma. Semin Cutan Med Surg. 1997;16:122–130. doi: 10.1016/s1085-5629(97)80006-5. [DOI] [PubMed] [Google Scholar]
- 2.Bono A, Maurichi A, Moglia D, et al. Clinical and dermatoscopic diagnosis of early amelanotic melanoma. Melanoma Res. 2001;11:491–494. doi: 10.1097/00008390-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Amster MS, Klaus MV. Amelanotic melanoma. Arch Dermatol. 1995;131:951–2. 954–5. doi: 10.1001/archderm.1995.01690200091018. [DOI] [PubMed] [Google Scholar]
- 4.Andersen WK, Silvers DN. ‘Melanoma? It can’t be melanoma!’ A subset of melanomas that defies clinical recognition. JAMA. 1991;266:3463–3465. [PubMed] [Google Scholar]
- 5.Ariel IM. Amelanotic melanomas: an analysis of 77 patients. Curr Surg. 1981;38:151–155. [PubMed] [Google Scholar]
- 6.BRAITMAN M. Amelanotic malignant melanoma. Postgrad Med. 1959;26:707–710. doi: 10.1080/00325481.1959.11712691. [DOI] [PubMed] [Google Scholar]
- 7.Huvos AG, Shah JP, Goldsmith HS. A clinicopathologic study of amelanotic melanoma. Surg Gynecol Obstet. 1972;135:917–920. [PubMed] [Google Scholar]
- 8.Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000;42:731–734. doi: 10.1067/mjd.2000.103981. [DOI] [PubMed] [Google Scholar]
- 9.Shah JP. Amelanotic melanoma. Prog Clin Cancer. 1975;6:195–197. [PubMed] [Google Scholar]
- 10.Thorstenson S. Amelanotic malignant melanoma. Ultrastruct Pathol. 1984;6:351–357. doi: 10.3109/01913128409018594. [DOI] [PubMed] [Google Scholar]
- 11.Yaacob HB. Malignant amelanotic melanoma--a rare tumor. J Oral Med. 1982;37:49–51. [PubMed] [Google Scholar]
- 12.Porras BH, Cockerell CJ. Cutaneous malignant melanoma: classification and clinical diagnosis. Semin Cutan Med Surg. 1997;16:88–96. doi: 10.1016/s1085-5629(97)80002-8. [DOI] [PubMed] [Google Scholar]
- 13.Beyeler M, Dummer R. Cutaneous melanoma: uncommon presentations. Clin Dermatol. 2005;23:587–592. doi: 10.1016/j.clindermatol.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Coras B, Hohenleutner S, Raff K, et al. The “red melanoma”--a rare form of amelanotic malignant melanoma. J Dtsch Dermatol Ges. 2004;2:597–600. doi: 10.1046/j.1439-0353.2004.04774.x. [DOI] [PubMed] [Google Scholar]
- 15.Gualandri L, Betti R, Crosti C. Clinical features of 36 cases of amelanotic melanomas and considerations about the relationship between histologic subtypes and diagnostic delay. J Eur Acad Dermatol Venereol. 2009;23:283–287. doi: 10.1111/j.1468-3083.2008.03041.x. [DOI] [PubMed] [Google Scholar]
- 16.Mihm MCJ, Clark WHJ, Reed RJ. The clinical diagnosis of malignant melanoma. Semin Oncol. 1975;2:105–118. [PubMed] [Google Scholar]
- 17.Koh HK. Cutaneous melanoma. N Engl J Med. 1991;325:171–182. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- 18.Weinstock MA, Goldstein MG, Dube CE, et al. Basic skin cancer triage for teaching melanoma detection. J Am Acad Dermatol. 1996;34:1063–1066. doi: 10.1016/s0190-9622(96)90287-x. [DOI] [PubMed] [Google Scholar]
- 19.Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA. 2004;292:2771–2776. doi: 10.1001/jama.292.22.2771. [DOI] [PubMed] [Google Scholar]
- 20.Gachon J, Beaulieu P, Sei JF, et al. First prospective study of the recognition process of melanoma in dermatological practice. Arch Dermatol. 2005;141:434–438. doi: 10.1001/archderm.141.4.434. [DOI] [PubMed] [Google Scholar]
- 21.Kelly JW, Chamberlain AJ, Staples MP, et al. Nodular melanoma. No longer as simple as ABC. Aust Fam Physician. 2003;32:706–709. [PubMed] [Google Scholar]


