Abstract

Hypersecretion of norepinephrine (NE) and angiotensin II (AngII) is a hallmark of major prevalent cardiovascular diseases that contribute to cardiac pathophysiology and morbidity. Herein, we explore whether heterodimerization of presynaptic AngII AT1 receptor (AT1-R) and NE α2C-adrenergic receptor (α2C-AR) could underlie their functional cross-talk to control NE secretion. Multiple bioluminescence resonance energy transfer and protein complementation assays allowed us to accurately probe the structures and functions of the α2C-AR–AT1-R dimer promoted by ligand binding to individual protomers. We found that dual agonist occupancy resulted in a conformation of the heterodimer different from that induced by active individual protomers and triggered atypical Gs-cAMP–PKA signaling. This specific pharmacological signaling unit was identified in vivo to promote not only NE hypersecretion in sympathetic neurons but also sympathetic hyperactivity in mice. Thus, we uncovered a new process by which GPCR heterodimerization creates an original functional pharmacological entity and that could constitute a promising new target in cardiovascular therapeutics.
The pathogenesis of arterial hypertension (HT) and heart failure (HF) is multifactorial, but hyperactivity of both the sympathetic nervous system (SNS) and the renin-angiotensin-aldosterone system is fundamental in their development1,2. Both systems are functionally interconnected as NE secretion through the SNS stimulates AngII production and vice versa. Consequently, HT and HF management is currently based on the use of molecules from similar pharmacological classes. However, the efficacy of drugs reducing renin-angiotensin-aldosterone activity, such as AngII AT1-R antagonists, on morbidity and mortality remains uncertain. Moreover, although centrally acting drugs such as α2-adrenergic agonists efficiently reduce SNS activity, they have never proven to effectively reduce morbidity and mortality3. Therefore, a better understanding of the molecular mechanisms regulating NE secretion could help to develop new efficient therapeutics.
NE secretion by SNS endings is controlled by a balance between inhibitory and stimulatory presynaptic G protein–coupled receptors (GPCRs). The α2-adrenergic receptors (α2A-AR and α2C-AR), comprising the main NE inhibitory secretion system, have a prominent role in controlling sympathetic neural output, and their dysfunction constitutes a central mechanism for sympathetic hyperactivity in HT and HF4,5. In contrast, AT1-R facilitates NE secretion6. Several ex vivo studies have highlighted the existence of functional crosstalk between the α2 and AT1 receptors in the modulation of NE secretion but led to inconsistent results7–9. It has been suggested that α2-AT1 cross-talk relies on intracellular communication involving Gβγ from Gi or Go proteins coupled to the α2-AR9. However, it is now well established that GPCRs can exist both as monomeric and oligomeric entities and that GPCR dimerization, specifically hetero-dimerization, has impacts on different functions of each receptor protomer including traffic, ligand binding and signaling10. Recently, GPCR heterodimers were suggested to have a role in physiology and pathophysiology11–15. Numerous GPCRs have been shown to dimerize, and α2-AR or AT1-R are no exception15–18. However, AT1-R–α2-AR heterodimerization is not reported. In this context, we hypothesized that the cross-talk between AT1-R and α2-AR for the regulation of NE release could rely on the existence of AT1-R–α2-AR heterodimers. The fact that AT1-R stimulates NE secretion and that α2C-AR subtype does not influence NE secretion per se but acts as a regulator of α2A-AR–promoted NE secretion4 prompted us to examine the heterodimerization of AT1-R with α2C-AR in the context of neurohumoral activation.
In this study, we used different biophysical and newly developed technical approaches, combined with in vitro and in vivo functional assays, to provide an exhaustive characterization of the structure and function of the α2C-AR–AT1-R heterodimer. We demonstrated that NE-AngII costimulation creates the α2C-AR–AT1-R heterodimer, a new pharmacological entity, that signals through a unique Gs-cAMP-PKA signaling pathway and is associated with both NE hypersecretion and sympathetic nerve hyperactivity in vivo. These results present the α2C-AR–AT1-R heterodimer as a promising potential new pharmacological target for the management of HT- and HF-associated sympathetic hyperactivity that will need to be explored in the future.
RESULTS
α2C-AR and AT1-R form constitutive heterodimers
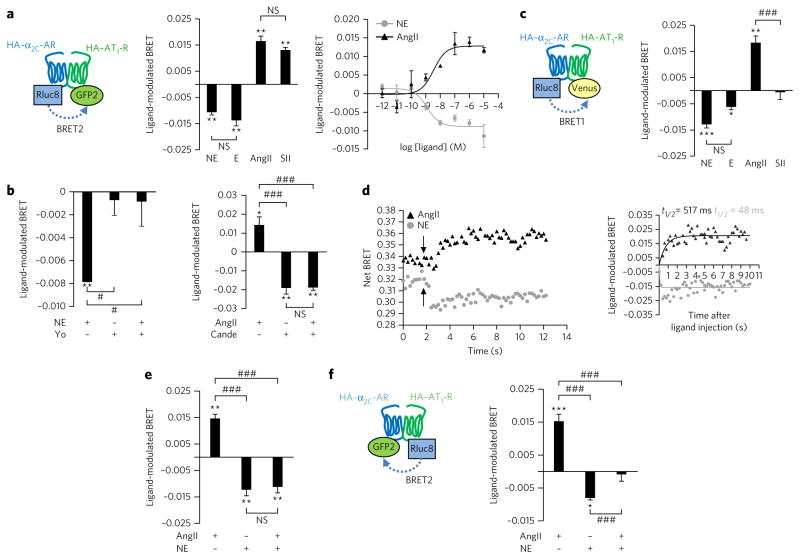
The interaction between α2C-AR and AT1-R was assessed in human embryonic kidney (HEK293T) cells by bioluminescence resonance energy transfer (BRET). Saturating BRET signals between AT1-R and α2C-AR (Fig. 1a) highlighted constitutive and specific association between both receptors. The specificity of interaction was confirmed by weak BRET signals not linearly increasing with the amount of interacting probes when measured between AT1-R–RLuc8 or α2C-AR–RLuc8 and CD8-GFP2, an unrelated transmembrane protein (Fig. 1a). Subcellular localization of α2C-AR–AT1-R heterodimers was investigated using bimolecular fluorescence complementation (BiFC). Each receptor was fused to complementary nonfluorescent halves of a Venus fluorophore (V1 and V2) at its C terminus as previously described19, resulting in receptor fusion constructs preserving their cell surface trafficking (Supplementary Results, Supplementary Fig. 1a) and their cognate G-protein activation (Supplementary Fig. 1b). Hemagglutinin (HA)–AT1-R–V1 coexpression with HA–α2C-AR–V2 generated a functional fluorescent Venus (Fig. 1b) colocalizing with cell surface HA staining, indicating that the α2C-AR–AT1-R heterodimer was expressed at the plasma membrane. In contrast, no fluorescence was detected when coexpressing HA–AT1-R–V1 or HA–α2C-AR–V2 with CD8-V2 or CD8-V1, whereas Venus fluorescence was observed at the cell surface and in intracellular compartments following coexpression of CD8-V1 and CD8-V2 (Fig. 1b). The expression of α2C-AR–AT1-R heterodimers using BiFC was also observed at the cell surface of sympathetic-like neurons from nerve growth factor (NGF)-differentiated PC12 neurites (Supplementary Fig. 2).
Figure 1. Characterization of the heterodimerization between AT1-R and α2C-AR in HEK293T cells.
(a) BRET was measured in HEK293T cells coexpressing a fixed amount of the indicated Rluc8-tagged receptor and increasing amounts of the indicated GFP2-tagged receptors. Results were analyzed by nonlinear regression on a pooled data set from three independent experiments, assuming a model with one-site binding (GraphPad Prism 4.0). (b) HEK293T cells were transiently cotransfected with HA–AT1-R–V1 and HA–α2C-AR–V2 (upper panels). Specificity was controlled in cells expressing the HA–AT1-R–V1 and CD8-V2 receptor (middle panel), the HA–α2C-AR–V2 and CD8-V1 receptor or CD8-V1 and CD8-V2, as indicated. The green channel illustrates the expression of Venus complementation (V1 + V2), whereas the red channel shows cell surface anti-HA staining, highlighting only HA–AT1-R and/or HA–α2C-AR present at the cell surface. Cell surface membranes and nuclei were visualized by Texas Red–wheat germ agglutinin staining (WGA; red) or DAPI staining (blue) respectively. Scale bars, 10 μm.
Distinct active conformations of the α2C-AR–AT1-R dimer
Few studies have reported how ligand binding affects protomer association in a dimer, and this has led to conflicting interpretations20,21, most likely due to the use of poor-resolution RET techniques21. Also, to assess ligand modulation of the α2C-AR–AT1-R heterodimer, we used BRET2 with high spectral resolution and sensitivity21,22. The natural α2C-AR agonists NE and epinephrine (E) decreased the BRET between HA–α2C-AR–Rluc8 and HA–AT1-R–GFP2, whereas the AT1-R–specific agonists AngII and SII increased it (Fig. 2a). NE- and AngII-dependent BRET modulation correlated with G-protein activation promoted by wild-type HA–AT1-R or Myc–α2C-AR when expressed alone (NE half-maximum effective concentration (EC50) = 1.2 ± 0.4 nM; AngII EC50 = 3.4 ± 2.2 nM (s.e.m.); Fig. 2a and Supplementary Fig. 1b). The specificity of ligand-promoted BRET changes was confirmed by pretreatment with 10 μM of specific α2C-AR (yohimbine) or AT1-R (candesartan) antagonists, which completely blocked the NE-mediated decrease or AngII-mediated increase in BRET (Fig. 2b). The agonist-induced changes in BRET could indicate enhanced or decreased physical association or dissociation between α2C-AR and AT1-R but could also reflect conformational rearrangements within protein complexes as this measure relies on the distance and dipole orientation of the energy donor and acceptor21,23,24. To distinguish between these two possibilities, we manipulated the distance and dipole orientation by exchanging the GFP2 energy acceptor fused to AT1-R for Venus and measured BRET1 between HA–α2C-AR–Rluc8 and HA–AT1-R–Venus. In this configuration, NE and E still decreased the BRET signal, whereas AngII but not SII promoted an increase in BRET (Fig. 2c). As the BRET changes are consistent with conformational changes within the dimer, it seems that α2C-AR and AT1-R agonists stabilize two different conformations of the HA–α2C-AR–HA–AT1-R heterodimer. Moreover, the AT1-R agonists AngII and SII also induce different dimer conformations, in agreement with our previous demonstration that SII stabilizes a different AT1-R entity to that stabilized by AngII24. Further evidence for NE and AngII promoting different conformations of the HA–α2C-AR–HA–AT1-R heterodimer was provided by real-time BRET2 kinetics, which revealed a faster NE-induced decrease in BRET than the AngII-mediated increase in BRET (AngII t1/2 = 517 ± 6 ms (s.e.m.) versus NE t1/2 < 200 ms; Fig. 2d).
Figure 2. Characterization of individual agonist effects on the AT1-R–α2C-AR heterodimer.
(a,b,d,e) BRET in HEK293T cells coexpressing HA-α2C-Rluc8 and HA–AT1-R–GFP2. (a) Cells were stimulated or not for 1 min with 10 μM or increasing concentrations of the indicated compounds. SII, [1Sar4Ile8Ile]angiotensin IIS. (b) Cells were first pretreated with 10 μM specific antagonist (yohimbine (Yo) or candesartan (Cande)) for 10 min before agonist incubation. Cells were stimulated or not for 1 min with 10 μM of the indicated compounds. (d) BRET kinetics were measured every 200 ms for 12 s before and after 1 μM injection of AngII (top arrow) or NE (bottom arrow). Agonists were injected 2 s after the beginning of the reading. Inset, kinetics of net agonist-promoted BRET using data from d. Results were analyzed by nonlinear regression assuming a model with one-phase exponential association (GraphPad Prism 4.0). The figure is representative of one experiment performed twice in triplicate. (e) Cells were stimulated or not for 1 min with 10 μM of the indicated compounds alone or in combination. (c) BRET in HEK293T cells coexpressing HA–α2C-AR–Rluc8 and HA–AT1-R–Venus, stimulated or not for 1 min with 10 μM of the indicated compounds. (f) BRET in HEK293T cells coexpressing HA-α2C-GFP2 and HA–AT1-R–Rluc8, stimulated or not for 1 min with 10 μM of the indicated compounds. In all panels, results are expressed as the difference in BRET signals measured in the presence and absence of agonist. Data represent the mean ± s.e.m. of at least four independent experiments. The statistical significance between stimulated and unstimulated cells was assessed using a paired Student’s t-test (*P < 0.05, **P < 0.01, ***P < 0.001), and statistical difference between ligand treatments was assessed using one-way analysis of variance followed by a Tukey’s test (#P < 0.05, ###P < 0.001). NS, not statistically significant.
NE and AngII hypersecretion observed in some cardiac diseases results in high concentrations of both agonists at sympathetic nerve endings, where they can bind simultaneously on putative α2C-AR–AT1-R heterodimers. Thus, we tested whether the simultaneous presence of the natural agonists of each receptor protomer would further modify the conformation of the α2C-AR–AT1-R heterodimer. When measuring BRET2 between HA–α2C-R–Rluc8 and HA–AT1-R–GFP2, NE and AngII respectively increased and decreased BRET, and costimulation with both agonists reduced the BRET to a level similar to that resulting from stimulation with NE alone (Fig. 2e). When the experiment was repeated following energy donor-acceptor exchange between the two receptors, NE- and AngII-promoted BRET modulation remained unchanged, but costimulation had no effect (Fig. 2f). These experiments not only suggest that ligand-modulated BRET results from conformational changes in the α2C-AR–AT1-R heterodimer but also show that stimulation with AngII, NE and AngII plus NE stabilize three different conformational states of the heterodimer. This was further supported by fluorescence anisotropy measurements of complemented Venus fluorophore. In HEK cells coexpressing HA–AT1-R–V1 and HA–α2C-AR–V2, the mean Venus anisotropy was different in AngII-treated cells compared to that in cells treated with NE and NE plus AngII (Supplementary Fig. 3), indicating that AngII promoted a conformation of the AT1-R–α2C-AR dimer different than that stabilized by NE or AngII plus NE. Sequential addition of AngII and NE (Supplementary Fig. 4a–d) did not modify BRET modulation between AT1-R and α2C-AR (Fig. 2e,f) for each energy donor-acceptor combination. Thus, contrary to previous reports10, these results are consistent with a model in which the ligand occupancy of one protomer does not necessarily influence the binding properties of the other. In line with this assumption, neither the binding affinity nor the maximal binding capacity of AngII or NE were modified in the presence of the other agonist in HEK cells coexpressing α2C-AR and AT1-R (Supplementary Table 1), suggesting that the ligand binding pocket was not affected, as previously reported for allosteric communication between protomers25.
Distinct heterodimer-effector complex conformations
We next explored the consequences of the different α2C-AR–AT1-R dimer conformations by analyzing the interactions of the heterodimer with G proteins and β-arrestin. To specifically isolate ligand effects arising from the heterodimer, we used complemented donor-acceptor resonance energy transfer (CODA-RET)26 combining BiFC and BRET. Thus, HA–α2C-AR and HA–AT1-R were fused at their C termini to an N-terminal (L1) or C-terminal fragment (L2) of Rluc8 and to an N-terminal (V1) or C-terminal fragment (V2) of Venus, resulting in fusion receptors with preserved cell surface expression and function (Supplementary Fig. 1). Analysis of fluorescence or luciferase complementation for α2C-α2C, AT1-AT1 and α2C-AT1 receptors showed that all of the combinations demonstrated fluorescence or luminescence reconstitution (Supplementary Fig. 5), suggesting that α2C-AR and AT1-R have a similar propensity for forming homo- or heterodimers.
When HA–AT1-R–V1 was coexpressed with HA–AT1-R–V2 in the presence of Rluc–β-arrestin 2, real-time BRET1 measurements showed β-arrestin 2 recruitment to the AT1-R–AT1-R homodimer only in the presence of AngII (Supplementary Fig. 6a). Similarly, with α2C-α2C homodimers, no β-arrestin recruitment was measured in the presence of NE or AngII (Supplementary Fig. 6b), despite cell surface expression of the homodimer (Supplementary Fig. 5). These results agree with previous reports for β-arrestin 2 recruitment to AT1-R24 but not to α2C-AR18 (Supplementary Fig. 7). Notably, individual stimulation of the α2C-AR–AT1-R heterodimer with AngII or NE reproduced the same β-arrestin 2 recruitment profile as that obtained with the cognate receptor homodimers or monomers (Fig. 3a), suggesting that heterodimerization does not affect the functionality of each receptor protomer in the β-arrestin 2 pathway. However, costimulation with AngII and NE significantly (P = 0.0002) potentiated maximal AngII-induced β-arrestin 2 recruitment (AngII plus NE BRETmax = 121.9% ± 2.3; AngII BRETmax = 100.0% ± 2.0 (s.e.m.)). When similar experiments were performed using β-arrestin 2 fused to the Rluc probe at its C-terminal (β-arrestin 2–Rluc), NE was still unable to recruit β-arrestin 2, whereas stimulation with AngII or AngII plus NE led to significant but similar maximal β-arrestin 2 recruitment (AngII plus NE BRETmax = 86.1% ± 10.9; AngII BRETmax = 98.6% ± 14.4 (s.e.m.); Fig. 3a). This suggests that different α2C-AT1 heterodimer conformations stabilized by AngII, NE or AngII plus NE translate into three distinct heterodimer–β-arrestin 2 conformational changes rather than modifications of β-arrestin 2 levels associated to the receptor.
Figure 3. CoDA-RET characterization of β-arrestin 2 and G-protein recruitment to heteromeric α2C and AT1 receptors.
(a) Real-time BRET1 measurements in HEK293T cells coexpressing HA–AT1-R–V1 and HA–α2C-AR–V2 (illustrated by the diagrams) with Rluc-β-arrestin 2 (β-Arr2; top) or β-Arr2-Rluc (bottom) with or without the presence of 10 μM AngII (black), NE (gray) or both (dark gray). Data represent the BRET ratio expressed as the percentage of AngII-induced maximal β-arrestin 2 recruitment. Curves were fitted according to a one-phase hyperbolic association model and compared using two-way analysis of variance, with time and agonist as interaction factors. BRETmax and t1/2 are indicated in the table. Data represent the mean ± s.e.m. of three to seven independent experiments, as indicated in the table. ***P < 0.001; NS, not statistically significant. (b) BRET1 was measured in HEK293T cells coexpressing the indicated Gα-Venus subunits (Gαi1-Venus, Gαq-Venus or Gαs-Venus) with β1γ2 G-protein untagged subunits in the presence of HA–AT1-R–L1 and HA–α2C-AR–L2 (top) or HA–α2C-AR–L1 and HA–AT1-R–L2 (bottom), with or without stimulation by 10 μM NE (light gray), AngII (black) or both (dark gray) for 1 min. Results are expressed as the difference in the BRET signal measured in the presence and absence of agonist. Data represent the mean ± s.e.m. of three to five independent experiments. The statistical significance between stimulated and unstimulated cells was assessed using a paired Student’s t-test (*P < 0.05, **P < 0.01, ***P < 0.001).
We applied the same strategy to G-protein activity by measuring the BRET between complemented split luciferase receptor fusions and the Gα-Venus subunit of the Gαβγ heterotrimer, a BRET assay accurately sensing R-G complex conformations23,24. Consistent with the cognate G-protein coupling of each receptor, AngII increased the BRET signal between the HA–AT1-R–L1/HA–AT1-R–L2 homodimer and Gαq-Venus or Gαi1-Venus but not Gαs-Venus (Supplementary Fig. 8a), whereas a significant (P < 0.01) BRET increase was detected in the presence of NE but only between the HA–α2C-AR–L1/ HA–α2C-AR–L2 homodimer and Gαi1-Venus (Supplementary Fig. 8b). When the HA–AT1-R–L1/HA–α2C-AR–L2 heterodimer was coexpressed with the different Gα-Venus isoforms, the R-G complex exhibited distinct conformations depending on the Gα isoform and the nature of the ligand, as shown by the different BRET modulations (Fig. 3b). It is noteworthy that although NE was unable to promote α2C-AR coupling to Gαq, it significantly (P < 0.05) decreased the BRET between the AT1-R–α2C-AR heterodimer and Gαq. Remarkably, although AT1-R and α2C-AR do not usually couple to Gs, and as corroborated by the absence of conformational rearrangements between AT1-R or α2C-AR homodimer receptors and Gs (Supplementary Fig. 8a,b), costimulation with AngII and NE significantly (P < 0.05) increased the BRET between the heterodimer and Gαs (Fig. 3b), suggesting an original Gs engagement to the heterodimer. Swapping the luciferase splits between AT1-R and α2C-AR led to similar results for all G proteins except that heterodimer stimulation with AngII promoted a BRET increase instead of a decrease in the α2C-AT1–Gαq complex (Fig. 3b). This demonstrates that the AT1-R–α2C-AR–Gq complex adopts different conformations in the presence of AngII, NE or AngII plus NE, in agreement with the notion that specific ligand combinations can stabilize different heterodimer conformations.
α2C-AR–AT1-R costimulation activates Gs-cAMP-PKA signaling
We next questioned the functional consequences of the different conformations of complexes formed between the AT1-R–α2C-AR dimer and β-arrestin 2 or the Gα subunit and stabilized by the three agonist combinations.
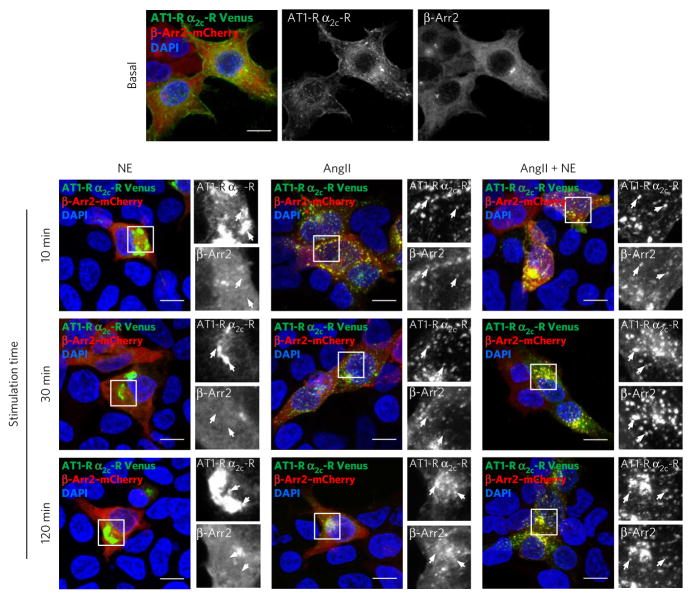
Given the central role of β-arrestin in GPCR internalization, we examined the internalization profile of the AT1-R–α2C-AR dimer in HEK 293T cells coexpressing β-arrestin 2–mCherry together with HA–AT1-R–V1 and HA–α2C-AR–V2. In agreement with the BRET assay (Fig. 3a), 120 min NE stimulation did not promote β-arrestin 2 recruitment (Fig. 4 and Supplementary Fig. 9), whereas internalization of the Venus dimer could be observed. In contrast, cell treatments with 10 μM AngII or AngII plus NE for 10 min recruited β-arrestin 2 to the AT1-R–α2C-AR dimer. However, after 30-min treatments, stimulation with AngII plus NE sustained β-arrestin 2 colocalization with the heterodimer in internalized vesicles, whereas colocalization markedly decreased with AngII (Fig. 4 and Supplementary Fig. 9) and persisted over 120 min stimulation. These results indicate that the three heterodimer conformations stabilized by the three ligand combinations traffic via distinct intracellular routes, suggesting diverse signaling outcomes.
Figure 4. The nature of ligands dictates different β-arrestin 2 trafficking pathways activated by the AT1-R–α2C-AR heterodimer.
β-Arrestin 2 (β-Arr2) recruitment to AT1-R–α2C-AR heterodimer was visualized by confocal microscopy on HEK293T cells coexpressing β-arrestin 2–mCherry (red), HA–AT1-R–V1 + HA–α2C-AR–V2 (green upon BiFC), with or without stimulation (basal) for 10 min, 30 min or 120 min with 10 μM NE, 10 μM AngII or 10 μM of both (AngII plus NE). Quadrants show 5× electronic magnification, and arrows outline two dimer-positive spots. Nuclei labeled with DAPI (blue). Scale bars, 10μm. Quantification of β-arrestin 2–mCherry and Venus (AT1-R–α2C-AR) fluorescence colocalization is shown in Supplementary Figure 9.
We next explored the G-protein activation pattern associated with the heterodimer. Indeed, α2C-AR and AT1-R coexpression in HEK293T cells will lead to the formation of monomer, dimers and higher-order oligomers, making it impossible to detect only heterodimer activity. To bypass this problem, we designed an assay based on the ligand-forcing dimerization of FKBP12 rapamycin-binding domain (FRB) (DmrC) and FK506 binding protein (FKBP) (DmrA), which has previously been used to explore protein dimerization 27–29. Thus, we engineered a chimeric α2C-AR fused to DmrA at its C terminus (Myc–α2C-AR–DmrA), whereas AT1-R was fused to DmrC (HA–AT1-R–DmrC). These fusions neither altered cell surface trafficking nor the receptor activity (Supplementary Fig. 10). We directly evaluated the activation of Gαi1, Gαq and Gαs in living cells using a BRET2 probe, which measured the increased distance between the Gα helical domain (Gα-Rluc8) and the Gγ2 N terminus (GFP10-Gγ2) during the GDP-GTP exchange and sensed a decrease in the BRET signal following receptor activation23,24. Myc–α2C-AR–DmrA and HA–AT1-R–DmrC were coexpressed in HEK293T cells with the different Gα BRET probes in the presence of NE, AngII or both. No G-protein activation was detected following 1 min of ligand stimulation in the absence of fusion receptor expression (Supplementary Fig. 11). In the presence of the Gαi1 BRET probe, NE strongly decreased the BRET signal in the absence of the A-C dimerizing agent, indicating potent Gαi1 activation consistent with action through the Gi-coupled α2C-AR (Fig. 5a). Further addition of AngII did not modify the Gαi1 activation profile, whereas AngII alone activated the Gαi1, but to a much lower extent (Fig. 5a), in agreement with lower efficiency of AngII on AT1-R–mediated Gi versus Gq coupling24. Notably, for similar receptor expression, forced dimerization of α2C-AR–AT1-R with the A-C dimerizer significantly (P < 0.05) reduced Gαi1 activation by stimulation with NE or NE plus AngII, whereas the AngII response was unchanged (Fig. 5a). This result highly suggests that in the absence of the dimerizer, Gαi1 activation most probably arises from a mixed population of active monomeric, homodimeric and heterodimeric α2C and AT1 receptors that the dimerizer disrupts to favor the heterodimeric population, resulting in a lower overall level of Gαi1 activation. In contrast, all of the ligand combinations activated Gαq, and no difference was observed when α2C-AR–AT1-R dimerization was forced (Fig. 5a). NE-induced Gαq activation was unexpected because α2C-AR is thought to exclusively couple to Gi and Go proteins24, and NE is unable to bind AT1-R. This could indicate that NE-mediated Gαq activation is triggered by the spontaneous formation of the heterodimer with expression level sufficient for Gαq activation as supplementation with the A-C dimerizer did not change the activation profile. This hypothesis agrees with our earlier results showing that NE induced conformational changes of the complex formed between α2C-AR–L1/AT1-R–L2 and Venus-Gαq (Fig. 3b). Finally, we found that only stimulation with NE plus AngII promoted Gs activation in forced α 2C-AR–AT1-R heterodimer conditions (Fig. 5a). This correlates with conformational rearrangements observed within the α2C-AR–AT1-R–Gαs complex (Fig. 3b) and demonstrates that rearrangement stabilized by dual occupancy of the heterodimer-Gs complex by AngII and NE efficiently translated into Gs activation. The lack of detectable Gs activation in the absence of the dimerizing agent could argue for production of a peculiar α2C-AR–AT1-R heterodimer entity coupled to Gs that did not spontaneously form by itself. However, BRET experiments showed that free mobile α2C and AT1 receptors can constitutively interact (Fig. 1b) and adopt a specific conformation alone (Fig. 2e,f) or in complex with Gs (Fig. 3b) in the presence of both AngII and NE. This most likely supports a model in which dual AngII and NE binding promotes Gs coupling to the α2C-AR–AT1-R heterodimer in the absence of the dimerizer, but the Gs BRET probe is not sensitive enough for detection.
Figure 5. AngII-NE costimulation of the AT1-R–α2C-AR heterodimer generates new Gs-cAMP-PKA signaling.
(a,b) For G-protein coupling measurement (a), BRET was measured in HEK293T cells coexpressing HA–AT1-R–DmrC and Myc–α2C-AR–DmrA, with GFP10-Gγ2, Gβ1 and Gαi1-Rluc8 (left); Gαq-Rluc8 (middle); or Gαs-Rluc8 (right) and with or without stimulation by 10 μM NE, AngII or both (AngII plus NE) for 1 min, whereas for PKA activation evaluation (b), BRET was measured in HEK293T cells coexpressing RIα-Rluc8 and GFP2-Cα in the presence of HA–AT1-R–DmrC and Myc–α2C-AR–DmrA, with or without stimulation for 30 min with 10 μM NE, AngII or both (AngII plus NE) with or without the presence of 10 μM propranolol, as indicated. Attached cells were pretreated (+) or not (−) with 400 nM A-C for 1 h to force AT1-R–α2C-AR before the BRET experiment. Results are expressed as the difference in the BRET signal measured in the presence and absence of agonist (upper panels in a and b) or as the difference in the G-protein activation induced by ligands in the presence and absence of A-C (lower panels in a). Data represent the mean ± s.e.m. of four to six independent experiments. The statistical significance was assessed using a paired Student’s t-test comparing ligand-stimulated and unstimulated cells (*P < 0.05, **P < 0.01, ***P < 0.001) or comparing A-C–treated and untreated cells (#P < 0.05). NS, not statistically significant.
Validation of Gs activation by dual occupancy of the α2C-AR–AT1-R heterodimer by AngII and NE was determined at the level of the cAMP-dependent protein kinase (PKA) effector. HEK293T cells were cotransfected with the BRET-based PKA biosensor, which measures the separation between RLuc8-tagged regulatory subunits and GFP2-tagged catalytic subunits of PKA as a reflection of activation24. In the absence of fusion receptor expression, NE significantly (P < 0.001) activated the PKA biosensor, and activation was completely blocked by propranolol (Supplementary Fig. 12a), suggesting the presence of an endogenous Gs-coupled β-adrenergic receptor. Although AngII stimulation alone did not promote any PKA response (Supplementary Fig. 12b), the same results were obtained in the presence of both NE and AngII (Supplementary Fig. 12c). That endogenous β-adrenergic receptor activated PKA was quite puzzling as no Gs activation could be measured after 1 min of NE stimulation in the absence of transient receptor expression (Supplementary Fig. 11c). However, when activation time was extended to 5 min, treatment with NE or NE plus AngII induced Gs activation, consistent with PKA activation in similar conditions (Supplementary Fig. 11c). In this context, the ability of the heterodimer to activate PKA was studied in the presence of propranolol when using NE. When the PKA biosensor was coexpressed with Myc–α2C-AR–DmrA and HA–AT1-R–DmrC, no PKA activation was found downstream of NE or AngII treatment, regardless of the presence of a dimerizing agent (Fig. 5b). Significant (P < 0.05) PKA activation was measured only in the presence of both NE and AngII with forced α2C-AR–AT1-R dimerization (Fig. 5b). This supports the idea that Gs is specifically activated downstream of the α2C-AR–AT1-R heterodimer.
To confirm the atypical Gs activation stimulated by the heterodimer, we directly quantified cAMP production in HEK293T cells coexpressing α2C-AR and AT1-R. Although no cAMP inhibition or production was detected in the absence of receptor expression (Supplementary Fig. 13a), NE inhibited cAMP production, consistent with activation of Gi–α2C-AR (Supplementary Fig. 13b). More unexpectedly, no cAMP production was detected in the presence of NE plus AngII, as expected on the basis of Gs-PKA biosensor activation (Fig. 5a,b). Because the cAMP production could result from the concomitant activation of both activator and inhibitory signals whose detection is highly dependent on the activation level of each pathway, we repeated the experiments in the presence of pertussis toxin (PTX) to inhibit the Gi-cAMP pathway. The inhibition of NE-mediated cAMP production was totally prevented by PTX pretreatment, but, remarkably, AngII promoted significant (P < 0.01) cAMP production, which was significantly (P < 0.001) potentiated by NE (Supplementary Fig. 13b). The potentiating effect of stimulation with AngII plus NE on cAMP production was reinforced by fluorescence resonance energy transfer (FRET)-based imaging of cAMP levels in living sympathetic-like neurons from NGF-differentiated PC12 cells coexpressing α2C-AR, AT1-R and a cAMP FRET biosensor and cocultured with H9C2 cardiomyoblasts (to establish neurocardiac synapses); in these neurons, stimulation with AngII plus NE promoted a decrease of the FRET signal, indicating cAMP production that significantly differed from FRET signals obtained with individual treatments with NE (P < 0.001) or AngII (P < 0.01) (Supplementary Fig. 14).
AngII plus NE promotes PKA-dependent SNS hyperactivity
Given the Gs coupling of the α2C-AR–AT1-R heterodimer with dual NE plus AngII occupancy and the implication of both receptors in NE secretion, we wondered whether a similar pharmacological unit could affect NE secretion. As expected, NE or AngII stimulation decreased or increased NE release in primary cultures of sympathetic neurons, respectively (Fig. 6). However, NE potentiated AngII-induced NE secretion (Fig. 6), which was completely prevented by blocking the Gs-PKA with Rp-8-cAMPS, a selective PKA inhibitor (Fig. 6a). Notably, pretreatment with candesartan selectively inhibited AngII-mediated NE release but had no effect on the potentiation of NE release induced by costimulation with NE and AngII (Fig. 6a). This is in agreement with results showing that candesartan or yohimbine, alone or in combination, are unable to block Gs stimulation promoted by treatment with NE plus AngII on the α2C-AR–AT1-R dimer (Supplementary Fig. 15) and thus suggests that an endogenous α2C-AR–AT1-R dimer could be involved in NE secretion in sympathetic neurons.
Figure 6. NE potentiates AngII-induced increase in NE release in neurons or RSNA in mice through a PKA pathway.
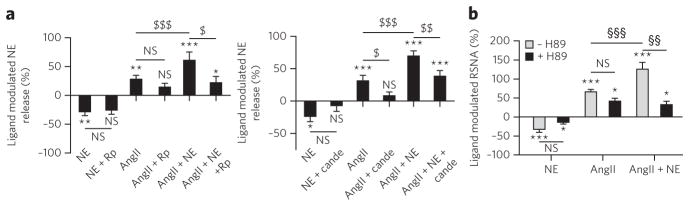
(a) NE release was assayed in superior cervical ganglion neurons (SCGN) primary cultures with or without stimulation by NE (1 μM), AngII (10 μM) or both and with or without 10-min pretreatment with the PKA antagonist Rp-cAMP (left; 100 μM, Rp) or the AT1-R antagonist candesartan (right; 10 μM, Cande). Ligand-modulated NE release is depicted as the percentage of release in the presence of drugs versus basal release (in the presence of vehicle). Data represent the mean ± s.e.m. of 10 (left panel) to 12 (right panel) independent experiments. (b) RSNA was measured after randomized intravenous injection of AngII (4 ng g−1), NE (40 ng g−1) or a combination of both (AngII + NE) without (vehicle, white bars, n = 14) or with H89 pretreatment (5 mg per kg body weight, intraperitoneal, black bars, n = 7) in hexamethonium-treated (30 μg g−1, intravenous) mice. Results are depicted as the percentage of the corresponding basal value normalized to 100% (detailed results are in Supplementary Table 2). Data represent the mean ± s.e.m. of 7–14 independent experiments. The statistical significance was assessed using one-way analysis of variance followed by the Newman-Keuls test (a) or ANOVA for repeated measures followed by Dunett’s test (b). $P < 0.05, $$ P < 0.01, $$$P < 0.001 for the indicated pairs; *P < 0.05, **P < 0.01 and ***P < 0.001 versus basal value using paired Student’s t-test; NS, not significant.
We next assessed the in vivo relevance of costimulation with NE and AngII on the firing rate of sympathetic nerves, which correlates with NE release30. We measured renal sympathetic nerve activity (RSNA) in mice following intravenous injections of AngII, NE or both in the presence of hexamethonium to prevent baroreflex-induced changes in RSNA subsequent to changes in arterial pressure31. Hexamethonium significantly lowered mean arterial pressure (MAP; 60 ± 8 Hg versus 88 ± 6 mm (s.e.m.) Hg in controls; P < 0.001), did not modify heart rate (553 ± 19 beats min−1 versus 537 ± 19 (s.e.m.) beats min−1 in untreated controls) and decreased RSNA (14.4 ± 5.0 bursts s−1 versus 35.7 ± 7.7 (s.e.m.) bursts s−1 in controls; P < 0.001). RSNA significantly (P < 0.001) increased following AngII administration (Fig. 6b and Supplementary Table 2). In contrast, NE administration tended to decrease RSNA (Fig. 6b and Supplementary Table 2). Notably, administration of both AngII and NE markedly increased RSNA by 125.7 ± 17.8% versus 65.9 ± 6.3% (s.e.m.) with AngII alone (Fig. 6b and Supplementary Table 2). This RSNA hyperactivity induced by costimulation with AngII and NE was not related to differences in drug-induced vasoconstriction (Supplementary Table 2). Blocking the Gs-PKA pathway using the H89 PKA inhibitor completely prevented the RSNA potentiation induced by AngII plus NE (Fig. 6b and Supplementary Table 2), indicating involvement of a Gs-coupled receptor and correlating with the original Gs coupling of the α2C-AR–AT1-R dimer we identified in vitro.
DISCUSSION
Numerous studies show that GPCR dimerization modulates the pharmacological properties of individual receptor protomers through alterations in ligand binding or signaling properties32. In most cases, signaling modulation of the dimer refers to increased or decreased activation of the G protein coupled to each protomer, although in several studies, a G protein–coupling switch between promoters was reported32. Only one study suggested the creation of a new form of Gq protein coupling in the D1-D2 dopamine receptor heterodimer33. However, given the existence of multiple receptor signaling units (monomers, homodimers and heterodimers) with variable cellular expression levels in all of these studies, it is difficult to infer that the effects result from altered coupling of the heterodimer per se or from downstream signaling cross-talk.
In this context, a major finding of this study is that, using a strategy of ligand-forcing GPCR dimerization, we provide clear evidence that dual occupancy of the α2C-AT1 receptor heterodimer by two natural selective agonists generates a new Gs coupling that arises from the heterodimer only. This new active pharmacological entity, the AngII- and NE-occupied α2C-AR–AT1-R heterodimer, was confirmed by the conformational rearrangement of the receptor-receptor dimeric interface observed by BRET to the receptor heterodimer–effector complexes observed by CODA-RET. Although all homodimeric, heterodimeric and monomeric receptor species are likely to coexist simultaneously when two different receptors are coexpressed34–37, our strategy allowed us to shift this equilibrium toward the heterodimeric state. When compared to the bicomplementation approach, which provides a static snapshot of the receptor dimer immediately after its biosynthesis, FRB-FKBP–driven dimerization only occurs following addition of the dimerizing agent once all receptor species have already been expressed, thereby offering the possibility of modulating the equilibrium of different receptor species. Accordingly, we found changes in G-protein coupling following addition of the dimerizing agent, strongly supporting the notion that different receptor populations do coexist and that the net signaling output following the coexpression of different receptors most likely reflects the average signaling arising from these different receptor populations. This notion is further reinforced by the observation that cAMP production, which is generally associated with Gs coupling and arises from costimulation with AngII and NE of the α2C-AT1 dimer, could not be detected without inactivating Gi proteins (Supplementary Fig. 13b), indicating activation of dual Gs-Gi signals. It thus follows that a specific GPCR dimer function can be easily missed.
The exclusive Gs-cAMP-PKA signaling identified for the NE- and AngII-occupied α2C-AR–AT1-R heterodimer could be involved in the regulation of arterial blood pressure, but it could also be particularly relevant to the pathophysiological context of HT and HF with sustained production of both agonists. Indeed, the unusual and unexpected sympathetic hyperactivity and NE hypersecretion in sympathetic neurons measured following coadministration of AngII and NE, which are both dependent on a PKA pathway, strongly supports a specific molecular mechanism unrelated to the individual activities of presynaptic α2C-AR or AT1-R. Although this study is far from proving this, the implication of the α2C-AR–AT1-R heterodimer in the regulation of sympathetic nerve activity deserves further investigations. Permanent costimulation of the α2C-AR–AT1-R heterodimer, similar to conditions in HT and HF, could promote continuous Gs-PKA–dependent NE release by enhancing the exocytotic machinery, as previously described38. In this context, the α2C-AR–AT1-R heterodimer could constitute a potential new target for the treatment of NE hypersecretion–associated diseases. Specific pharmacological blocking of the α2C-AR–AT1-R heterodimer using bivalent ligands targeting the two protomers of the dimer or monovalent ligands specifically targeting the heterodimer, as previously shown39, could be particularly relevant.
A second major finding of this study is that the different ligand occupancy states of the α2C-AT1 receptor heterodimer stabilize distinct conformations of the dimer, which then translate into specific signaling outputs. Whereas NE bound to the α2C-AR protomer or AngII bound to the AT1-R protomer stabilized different conformations of the dimer, the concomitant presence of both agonists promoted a third conformation triggering a new form of Gs signaling. The activation of Gs by the α2C-AR–AT1-R heterodimer we describe herein differs from the asymmetric model, in which numerous class A or C GPCRs are proposed to reach their active dimeric state40–46 with one protomer controlling the activation process of the dimer and the other fine-tuning it. Contrarily, our data highlight a new model whereby the conformational change of the two protomers act in concert to create a unique structural conformation of the heterodimer, which translates into a specific G-protein response. Until now, the functional asymmetric modes described have relied essentially on receptor homodimer models. In the future, it will be interesting to see whether the new complementary mode demonstrated here is specific to the α2C-AR–AT1-R dimer or is a general feature of heterodimers, thus giving rise to a new receptor functional entity.
In summary, our finding that a HT- and HF-like neurohumoral active state of the AT1-R–α2C-AR heterodimer provides a new structurally and functionally unique pharmacological entity indicates that, in the future, GPCR heterodimer function itself should be screened without a priori knowledge of the function of the individual protomers. Thus, a larger set of effectors should be included in the evaluation. The examination of structural rearrangements within GPCR dimers and their associated signaling cascades based on the ligand-forcing FRB-FKBP dimerization approach could be applied generally to identify new pharmacological signatures. In this study, we have found a novel RSNA activity under HT- and HF-like neurohumoral conditions. It remains to be fully confirmed whether the AT1-R–α2C-AR heterodimer is specifically involved and thus contributes to HT and HF pathophysiology, yet results presented here provide evidence that the HT- and HF-like neuro-humoral active state of AT1-R–α2C-AR could constitute a promising target for future HT and HF treatment.
ONLINE METHODS
Live animals
Two-month-old male or 1- to 3-d-old C57BL/6 mice were used in accordance with the US National Institutes of Health (NIH) guidelines. In vivo experiments were approved by the Comité d’éthique d’expérimentation animale 122.
Materials
Angiotensin II (AngII), L-(–)-norepinephrine (NE), (–)-epinephrine (E), 3-isobutyl-1-methylxanthine (IBMX) and yohimbine were purchased from Sigma-Aldrich. Candesartan was kindly provided by J.L. Hansen. Coelenterazine 400a and h were purchased from Biotum. Levo-[7-3H]norepinephrine and [125I]Tyr4-angiotensin II were purchased from Perkin-Elmer.
cDNA expression vectors
Plasmids encoding rat HA–AT1-R and HA–AT1-R– GFP2 were a generous gift from J.L. Hansen. All receptors were fused in-frame at their C terminus to either Rluc8, GFP2 or Rluc8 or Venus splits. Human HA–α2C-AR–GFP2 was obtained by inserting the receptor coding sequence lacking its stop codon into the humanized pGFP2-N1 vector (Perkin Elmer, Life Science). HA–α2C-AR–Rluc8 was obtained from pGFP2-MCS-Rluc8 (customized) vector by replacing the GFP2-MCS sequence with the receptor coding sequence of α2C-AR. HA–AT1-R–V1, HA–AT1-R–V2, HA–α2C-AR–V1 and HA–α2C-AR–V2 as well as HA–AT1-R–L1, HA–AT1-R–L2, HA–α2C-AR–L1 and HA–α2C-AR–L2 were constructed from HA–AT1-R–GFP2 and HA–α2C-AR–GFP2 by replacing GFP2 with the coding sequence of the different Venus fragments (from CD8-V1 and CD8-V2 (ref. 19), kindly provided by J.A. Javitch) or Rluc8 fragments (from D2-L1 and D2-L2, kindly provided by J.A. Javitch). Plasmids encoding HA–AT1-R–Venus, αi1-91Rluc8, αq-97Rluc8, αs-113Rluc8, GFP10-Gγ2, Gβ1, Rluc-βarr2, RIα-Rluc8 and GFP2-Cα were previously described24. αi1-Venus and αs-Venus were a generous gift from J.A. Javitch. αq-Venus was obtained from αq-97Rluc8 by replacing Rluc8 with the coding sequence of Venus. HA–AT1-R–DmrC and Myc–α2C-AR–DmrA were generated from HA–AT1-R–V1 and Myc–α2C-AR–V1, respectively, by replacing V1 with the encoding sequence of FRB (DmrC) or FKBP12 (DmrA), respectively (obtained by PCR from pHet-1 and pHet-Nuc1 vectors using iDimerize Inducible Heterodimer System, Clontech). All generated constructs were confirmed by DNA sequencing. Rluc-Barr2 and β-arrestin 2–cherry vectors were kindly provided by S. Marullo and were already described47,48. Pm-Epac2-camps was a generous gift from V. Nikolaev.
Cell culture and transfection
Human embryonic kidney 293 cells (HEK293T) cells were cultured in DMEM Glutamax supplemented with 10% (v/v) FBS and 100 U/ml penicillin/streptomycin at 37 °C in a humidified atmosphere at 5% CO2. Transient transfections were performed 24 h after cell seeding using polyethylenimine (PEI, Polysciences Inc.) or Lipofectamine 2000 (Invitrogen), according to the manufacturer’s protocol. Mouse superior cervical ganglion neurons were cultured as previously described49. Briefly, superior cervical ganglions were dissected from 1- to 3-d-old C57BL/6J mice and dissociated for 20 min in 3 mg/ml collagenase with 0.5 mg/ml trypsin, followed by washing (10% FBS in DMEM). Cells were plated and incubated with 3% FBS in UltraCulture medium containing NGF for 2 h at 37 °C to allow fibroblasts to adhere. The supernatant medium (containing SCG cells) was centrifuged, resuspended in medium supplemented with FBS and NGF and cultured on poly-D-lysine– and collagen-coated glass-bottom plates for 14 d before experiments (treating with 1 μM 5-fluoro-5-deoxyuridine).
Immunofluorescence microscopy
HEK293T cells were seeded and transfected using Lipofectamine 2000 in six-well plates containing glass cover slips precoated with poly-L-lysine (1 mg/ml; Sigma). Forty-eight hours after transfection, cells were washed twice with PBS, fixed with 4% paraformaldehyde for 15 min, blocked in PBS-0.2% BSA and incubated for 2 h with a mouse anti-HA antibody (16B12, Covance) or a mouse anti-Myc tag (9E10, Santa Cruz). Immunoreactivity was revealed using an Oregon Green– or Texas Red–conjugated secondary goat anti-mouse antibody (Molecular Probes). Cell membranes and nuclei were respectively stained with Texas Red–conjugated wheat germ agglutinin (WGA; Molecular Probes) and DAPI (Sigma). Fluorescent images were acquired with an Axio Observer Z.1m inverted microscope associated with ApoTome (Carl Zeiss Jena, Germany) and a 63× objective lens (Plan-Apochromat, 1.4 Oil DIC). Colocalization was assessed by overlay using Zeiss microscopy software AxioVision Rel. 4.8. Confocal imaging was performed using a LSM 780 microscope, piloted by manufacturer software, with a 63× Plan-Neofluar objective (Carl Zeiss), and the colocalization parameter (correlation coefficient) was calculated for each cell using ZEN software.
FRET imaging
PC12 cells were electroporated with the Epac2-pm cAMP FRET sensor together with HA–AT1-AR and Myc–α2C-AR according the Neon Transfection kit (Invitrogen) recommendation. Twenty four hours after transfection, cells were placed in a differentiation medium (RPMI 1% HS, 70 ng/ml NGF, Pen/Strep), and H9C2 cells were added into the culture 8h after. Cells were imaged 4 to 6 d after differentiation using a confocal LSM 780 microscope piloted by ZEN software, with a 63× Plan-Neofluar objective (Carl Zeiss). Contact between PC12 and H9C2 was imaged with the following parameters: electronic zoom 2, confocal pinhole 1.5 Airy units no frame averaging, 6 slices Z-stack. CFP and EYFP were excited using 452-nm and 51-nm laser lines (from the 488 nm Argon laser), and emitted light was collected at 475–510 nm and 520–555 nm (CFP and EYFP or FRET imaging, respectively). A first image with bright field, CFP and YFP excitation was acquired to choose the cell; then, to study agonist-induced changes in FRET, we acquired the data in the CFP channel and in the FRET channel only with CFP excitation. Ten images before stimulation and 20 images after stimulation were acquired, with 10-s intervals. At least 25 cells were analyzed per condition over two experiments. After background subtraction (bleed through correction), net FRET was calculated as the ratio of the FRET channel over the CFP channel using ImageJ. The difference in net FRET between stimulated and unstimulated for 1–5 ROIs (regions of interest) was then expressed as a percentage and plotted over time.
BRET
Receptors, G-protein subunits or β-arrestin–encoding vectors were transiently transfected into HEK293T cells. Forty-eight hours after transfection, cells were washed with PBS, detached in PBS/5 mM EDTA and resuspended in PBS/0.1% (w/v) glucose at room temperature. Cells were then distributed (80 μg of protein per well) into a 96-well microplate (Wallac, PerkinElmer Life and Analytical Sciences) and incubated in the presence of the different ligands for 1 min or 5 min, except for the kinetics study (Fig. 2d) and PKA activation (Fig. 5b and Supplementary Fig. 10). BRET2 between native or complemented Rluc8 and GFP10 or complemented GFP2 was measured after the addition of the Rluc substrate coelenterazine 400a (5 μM, Interchim). For BRET1 experiments, BRET between Rluc and native or reconstituted Venus was measured following the addition of the Rluc substrate coelenterazine h (5 μM, Interchim). Where indicated in Figure 2d, BRET signal values were corrected by subtracting the background signal detected when an Rluc8/Rluc-tagged construct was expressed alone from the BRET signal detected in cells coexpressing both Rluc8/Rluc-tagged and GFP2/Venus constructs (Net BRET).
For titration experiments (Fig. 1a), the expression level of each tagged protein was determined by direct measurement of total fluorescence and luminescence in aliquots of the transfected cells. Total fluorescence was first measured with an excitation filter at 400 nm and an emission filter at 510 nm for GFP2. Then, the same sample was incubated for 8 min with 5 μM coelenterazine h, and the total luminescence was measured using a modified Infinite F500 (Tecan Group Ltd).
For kinetics analysis of α2C-AR–AT1-R interactions (Fig. 2d), coelenterazine 400a was added before injection of the ligand. Readings were then collected at 200-ms intervals for 12 s. Ligands were injected 2 s after the beginning of the reading to allow baseline recording followed by real-time recording of the BRET changes. Net BRET signals were determined for each time point by calculating the ratio of the light emitted by GFP10 over that emitted by RLuc8.
BRET readings were collected using a modified Infinite F500 (Tecan Group Ltd). The BRET signal was calculated by the ratio of GFP10/GFP2 (510–540 nm) to Rluc/Rluc8 (370–450 nm) for BRET2 readings or Venus (520–570 nm) to RLuc/RLuc8 (370–480 nm) for BRET1 readings.
Anisotropy measurements
Anisotropy measurements were performed on a LSM 710 confocal microscope (Zeiss, Germany) implemented for anisotropy imaging. Two consecutive images were recorded, corresponding to the orientation of polarizers in the emission path of the microscope set first to parallel and then to perpendicular, with respect to the excitation polarization. Images were recorded through a 10×/0.30 objective to prevent mixing of different polarization components. Venus dye was excited with a 488-nm argon laser line, and fluorescence emission was recorded from 500 nm to 600 nm. Anisotropy images were calculated from raw data with ImageJ (NIH) using equation (1) and a custom-built macro. G-factor was calculated with equation (2) from the measurement of an isotropic solution of fluorescein.
| (1) |
| (2) |
Histograms of frequency versus anisotropy values were calculated for each individual anisotropy image and fitted with a Gaussian model to accurately estimate the mean anisotropy value.
Radioligand binding assay
For AT1-R, whole-cell radioligand binding studies were performed as previously described50. Competition studies were done with HEK293T cells transiently transfected with either 1 μg AT1-R–Rluc8 or 1 μg AT1-R–Rluc8 plus 6 μg HA–α2C-AR–GFP2, in the presence of 10-10 M [125I]Tyr4 AngII ([125I]AngII, Perkin-Elmer) and increasing concentrations (from 10−12 M to 10−6 M) of unlabeled AngII in the presence or absence of 10 μM NE.
For α2C-AR, competition experiments were done as previously described51 on crude membrane from HEK293T cells transiently transfected with either 10 μg HA–α2C-AR–Rluc8 or 10μg HA–α2C-AR–Rluc8 plus 10 μg HA–AT1-R–GFP2, using 10−8 M [3H](-)norepinephrine ([3H]NE, Perkin-Elmer) as a tracer and increasing concentrations of unlabeled NE (from 10−12 to 10−6 M) as a competitor agent, in the presence or absence of 10 μM AngII.
Each experiment was realized in triplicate, and competition curves were fitted using GraphPad Prism (version 5.04, GraphPad Software, USA) using a one-site competition model, followed by pKi (-log Ki) calculation.
cAMP quantification
Quantification of cAMP levels was performed using the homogeneous time-resolved fluorescence (HTRF) assay (femto cAMP kit (Cisbio, Bedford, USA), based on a competitive immunoassay using the Lumi4-TbTM cryptate-labeled antibodies anti-cAMP and d2-labeled cAMP). For that purpose, HEK293 cells were transiently cotransfected with HA-AT1R and Myc-α2CR using X-tremeGENE 9 DNA transfection reagent (Roche). Twenty-four hours after transfection, cells were pretreated overnight with PTX (100 ng/ml) or vehicle. The day of the experiment, cells were washed and resuspended in PBS/5 mM glucose/2 mM IBMX. Then, 35,000 cells/well (384 wells per plate) were plated and stimulated for 1 h at room temperature with 10 μM NE, AngII or NE plus AngII in the presence of 10 μM propranolol in a final volume of 10 μl. Cells were then lysed using 5 μl of lysis buffer containing d2-labeled cAMP and 5μl of Lumi4-TbTM cryptate-labeled anti-cAMP. The signal was measured after 1 h room temperature incubation using a modified Infinite F500 (Tecan Group Ltd). The RET signal was calculated by the ratio of d2-cAMP/Lumi4-TbTM (665 nm/620 nm), the specific signal being inversely proportional to the concentration of cAMP in the sample. For each experiment, a calibration curve was established with cAMP standards allowing the quantification of cAMP levels by regression.
Culture of mouse superior cervical ganglion neurons
Mouse superior cervical ganglion neurons were cultured as previously described49. Briefly, superior cervical ganglions were dissected from 1- to 3-d-old C57BL/6J mice and dissociated for 20 min in 3 mg/ml collagenase and 0.5 mg/ml trypsin, followed by washing (10% FBS in DMEM). Cells were plated and incubated with 3% FBS in UltraCulture medium containing NGF for 2 h at 37 °C to allow fibroblasts to adhere. Supernatant medium (containing SCG cells) was centrifuged, resuspended in medium supplemented with FBS and NGF and cultured on poly-D-lysine– and collagen-coated glass-bottom plates for 14 d before experiments (treating with 1 μM 5-fluoro-5-deoxyuridine).
Determination of [3H]noradrenaline release
Neurons cultures were washed, incubated in culture medium with 0.2 uM [3H]NE (2 h) and rinsed with fresh culture medium. Subsequently, cultures were washed (1 h) in a buffer containing NaCl (120 mM), KCl (6.0 mM), CaCl2 (2.0 mM), MgCl2 (2.0 mM), glucose (10 mM), HEPES (10 mM), ascorbic acid (0.50 mM) and bovine serum albumin (1%) and adjusted to pH 7.4 with NaOH. Cultures were treated with 10 μM propranolol and 10 μM cocaine (10 min) in above buffer. Inhibitors (candesartan or Rp-cAMP) or vehicle were added during this preincubation period. Cultured were treated with drugs in above buffer (10 min (37 °C), including inhibitors) and tritium content was measured by scintillation counting. Cultures were extracted using 0.1% SDS, and remaining radioactivity was evaluated by scintillation counting.
In vivo measurement of RSNA
Experiments were performed on adult C57BL/6J (12–17 weeks old, 25–35 g) male mice housed in a temperature-controlled room with a 12 h/12 h light/dark cycle with free access to standard laboratory chow and tap water.
Mice were anesthetized with isoflurane gas administration (induction: 3%, maintenance: 1.5%). Body temperature was continuously monitored using rectal probe and maintained within a normal range using a heating pad. The trachea was cannulated with PE-90 polyethylene tubing and, until the end of the surgical procedure, the mice spontaneously breathed isoflurane gas using a mask connected to the cannula. The right jugular vein and left carotid artery were cannulated with PE-10 polyethylene catheters for drug administration and measurement of arterial pressure, respectively. The arterial pressure catheter was connected to a pressure transducer allowing signal amplification (hydraulic pressure; Millar Instruments, Houston, Texas, USA). The left kidney was exposed retroperitoneally through a left flank incision. Under the dissecting microscope, the left renal sympathetic nerve was isolated from the surrounding connective tissue and mounted on a bipolar 36-gauge platinum-iridium electrode (Cooner Wire Co, Chadsworth, CA). Once optimal recording parameters were established, the nerve was fixed to the electrode with silicone gel (Kwik-Sil; World Precision Instruments Inc., Sarasota, FL). The nerve electrode was attached to a high-impedance probe (HIP-511; Grass Instruments Co, Quincy, MA). The signal was amplified 105 times with a Grass P5 AC preamplifier and filtered at both low- (100 Hz) and high-frequency (1,000 Hz) cut-off. To prevent baroreflex-induced decrease in RSNA subsequent to the increase in arterial pressure induced by NE or AngII, a ganglionic blocker (hexamethonium, 30 μg.g-1, intravenous) was administered, and mice were artificially ventilated (MiniVent ventilator, Hugo Sachs Electronik, Harvard Apparatus, USA). Then, mice received in a randomized order intravenous injections of AngII (4 ng g−1) or NE (NE, 40 ng g−1) alone or in combination. Arterial pressure and RSNA were sampled at 2,000 Hz and sent to a PowerLab analog digital converter (Ad Instruments, Castle Hill, New South Wales, Australia) for recording on a PC computer and off-line data analysis (LabChart 7 Pro, Ad Instruments, Castle Hill, New South Wales, Australia). Heart rate (HR) was extrapolated from phasic arterial pressure. Mean arterial pressure (MAP; mm Hg), HR (beats per min, b.p.m.) and RSNA (bursts/s) were averaged over a 1-min period before drug injection (baseline) and over a 5-s period 20–30 s after injections, i.e., at the time of the maximal pressure effect of injected drugs. The mean time interval between each drug injection (5 min) was defined in preliminary experiments as the time allowed for MAP to return to baseline values. RSNA was analyzed as previously described subtracting the integrated voltage after death (background noise) from the total integrated voltage.
Data and statistical analysis
Results are expressed as mean values ± s.e.m. of at least three independent experiments. Statistical analysis was carried out using GraphPad Prism 4 software (GraphPad Software Inc.). Statistical tests used are indicated in the figure legends.
Acknowledgments
M.B. was supported by a doctoral fellow fellowship from the Fondation pour la Recherche Médicale. J.-M.S. and C.G. are supported by the Institut National de la Santé et de la Recherche Médicale and by grants from La Société Française d’Hypertension Artérielle and the Fondation Bettencourt Schueller. H.J.M. was supported by a US National Institutes of Health grant (grant number DA038058-01).
Footnotes
Author contributions
M.B. designed and performed most of the experiments, analyzed and interpreted data and helped to write the manuscript. S.G. and C.B. helped in the anisotropy experiments and performed β-arrestin and cAMP experiments, analyzed data and helped to write the manuscript. H.J.M. performed NE-release experiments in primary cultures of sympathetic neurons and analyzed the data. F.D. designed, performed and analyzed mouse microneurography experiments. C.D. helped in the binding studies, the design of most experiments and the interpretation of the data. J.J. helped with construction of the Venus and Luciferase fusion BRET probes, the interpretation of the data and the editing of the manuscript. S.M. performed anisotropy experiments and analyzed the data. V.P. and M.-H.S. established some plasmid constructs. S.J.S. and J.L.H. performed some binding studies and analyzed and interpreted data. A.P. assisted in data processing and analysis and with manuscript preparation. A.G. supervised the neuron experiments and wrote the manuscript. J.-M.S. supervised some aspects of the project, analyzed the data and wrote the manuscript. C.G. conceived and supervised the project, performed data analysis and wrote the manuscript.
Competing financial interests
The authors declare competing financial interests: details accompany the online version of the paper.
Additional information
Supplementary information is available in the online version of the paper.
References
- 1.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 2.Paulis L, Unger T. Novel therapeutic targets for hypertension. Nat Rev Cardiol. 2010;7:431–441. doi: 10.1038/nrcardio.2010.85. [DOI] [PubMed] [Google Scholar]
- 3.Perk J, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 4.Hein L, Altman JD, Kobilka BK. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- 5.Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007;13:315–323. doi: 10.1038/nm1553. [DOI] [PubMed] [Google Scholar]
- 6.Rump LC, Bohmann C, Schaible U, Schultze-Seemann W, Schollmeyer PJ. β-adrenergic, angiotensin II, and bradykinin receptors enhance neurotransmission in human kidney. Hypertension. 1995;26:445–451. doi: 10.1161/01.hyp.26.3.445. [DOI] [PubMed] [Google Scholar]
- 7.Costa M, Majewski H. Facilitation of noradrenaline release from sympathetic nerves through activation of ACTH receptors, β-adrenoceptors and angiotensin II receptors. Br J Pharmacol. 1988;95:993–1001. doi: 10.1111/j.1476-5381.1988.tb11730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox SL, Schelb V, Trendelenburg AU, Starke K. Enhancement of noradrenaline release by angiotensin II and bradykinin in mouse atria: evidence for cross-talk between Gq/11 protein– and Gi/o protein–coupled receptors. Br J Pharmacol. 2000;129:1095–1102. doi: 10.1038/sj.bjp.0703167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talaia C, Queiroz G, Pinheiro H, Moura D, Goncalves J. Involvement of G-protein βγ subunits on the influence of inhibitory α2-autoreceptors on the angiotensin AT1-receptor modulation of noradrenaline release in the rat vas deferens. Neurochem Int. 2006;49:698–707. doi: 10.1016/j.neuint.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Smith NJ, Milligan G. Allostery at G protein–coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev. 2010;62:701–725. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González S, et al. Circadian-related heteromerization of adrenergic and dopamine D4 receptors modulates melatonin synthesis and release in the pineal gland. PLoS Biol. 2012;10:e1001347. doi: 10.1371/journal.pbio.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Maeso J, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apoghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73:317–332. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivero-Müller A, et al. Rescue of defective G protein–coupled receptor function in vivo by intermolecular cooperation. Proc Natl Acad Sci USA. 2010;107:2319–2324. doi: 10.1073/pnas.0906695106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozenfeld R, et al. AT1R-CB1R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J. 2011;30:2350–2363. doi: 10.1038/emboj.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- 17.Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between μ opioid and α2A-adrenergic receptors. Mol Pharmacol. 2003;64:1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]
- 18.Small KM, et al. α2A- and α2C-adrenergic receptors form homo- and heterodimers: the heterodimeric state impairs agonist-promoted GRK phosphorylation and β-arrestin recruitment. Biochemistry. 2006;45:4760–4767. doi: 10.1021/bi052074z. [DOI] [PubMed] [Google Scholar]
- 19.Guo W, et al. Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 2008;27:2293–2304. doi: 10.1038/emboj.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalrymple MB, Pfleger KD, Eidne KA. G protein–coupled receptor dimers: functional consequences, disease states and drug targets. Pharmacol Ther. 2008;118:359–371. doi: 10.1016/j.pharmthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Lohse MJ, Nuber S, Hoffmann C. Fluorescence/bioluminescence resonance energy transfer techniques to study G-protein–coupled receptor activation and signaling. Pharmacol Rev. 2012;64:299–336. doi: 10.1124/pr.110.004309. [DOI] [PubMed] [Google Scholar]
- 22.Denis C, Sauliere A, Galandrin S, Senard JM, Gales C. Probing heterotrimeric G protein activation: applications to biased ligands. Curr Pharm Des. 2012;18:128–144. doi: 10.2174/138161212799040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galés C, et al. Probing the activation-promoted structural rearrangements in preassembled receptor–G protein complexes. Nat Struct Mol Biol. 2006;13:778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- 24.Saulière A, et al. Deciphering biased-agonism complexity reveals a new active AT1 receptor entity. Nat Chem Biol. 2012;8:622–630. doi: 10.1038/nchembio.961. [DOI] [PubMed] [Google Scholar]
- 25.Springael JY, Urizar E, Costagliola S, Vassart G, Parmentier M. Allosteric properties of G protein–coupled receptor oligomers. Pharmacol Ther. 2007;115:410–418. doi: 10.1016/j.pharmthera.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Urizar E, et al. CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat Chem Biol. 2011;7:624–630. doi: 10.1038/nchembio.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthuswamy SK, Gilman M, Brugge JS. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol Cell Biol. 1999;19:6845–6857. doi: 10.1128/mcb.19.10.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhan L, Xiang B, Muthuswamy SK. Controlled activation of ErbB1/ErbB2 heterodimers promote invasion of three-dimensional organized epithelia in an ErbB1-dependent manner: implications for progression of ErbB2-overexpressing tumors. Cancer Res. 2006;66:5201–5208. doi: 10.1158/0008-5472.CAN-05-4081. [DOI] [PubMed] [Google Scholar]
- 29.Terrillon S, Bouvier M. Receptor activity-independent recruitment of β-arrestin 2 reveals specific signalling modes. EMBO J. 2004;23:3950–3961. doi: 10.1038/sj.emboj.7600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand. 2003;177:275–284. doi: 10.1046/j.1365-201X.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Abboud FM, Chapleau MW. A novel effect of angiotensin on renal sympathetic nerve activity in mice. J Hypertens. 2001;19:609–618. doi: 10.1097/00004872-200103001-00014. [DOI] [PubMed] [Google Scholar]
- 32.Rozenfeld R, Devi LA. Exploring a role for heteromerization in GPCR signalling specificity. Biochem J. 2011;433:11–18. doi: 10.1042/BJ20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rashid AJ, et al. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fonseca JM, Lambert NA. Instability of a class a G protein–coupled receptor oligomer interface. Mol Pharmacol. 2009;75:1296–1299. doi: 10.1124/mol.108.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hern JA, et al. Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc Natl Acad Sci USA. 2010;107:2693–2698. doi: 10.1073/pnas.0907915107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasai RS, et al. Full characterization of GPCR monomer-dimer dynamic equilibrium by single molecule imaging. J Cell Biol. 2011;192:463–480. doi: 10.1083/jcb.201009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert NA. GPCR dimers fall apart. Sci Signal. 2010;3:pe12. doi: 10.1126/scisignal.3115pe12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubista H, Boehm S. Molecular mechanisms underlying the modulation of exocytotic noradrenaline release via presynaptic receptors. Pharmacol Ther. 2006;112:213–242. doi: 10.1016/j.pharmthera.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Waldhoer M, et al. A heterodimer-selective agonist shows in vivo relevance of G protein–coupled receptor dimers. Proc Natl Acad Sci USA. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goudet C, et al. Asymmetric functioning of dimeric metabotropic glutamate receptors disclosed by positive allosteric modulators. J Biol Chem. 2005;280:24380–24385. doi: 10.1074/jbc.M502642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol. 2009;5:688–695. doi: 10.1038/nchembio.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hlavackova V, et al. Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. EMBO J. 2005;24:499–509. doi: 10.1038/sj.emboj.7600557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaupmann K, et al. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 44.Maurice P, Kamal M, Jockers R. Asymmetry of GPCR oligomers supports their functional relevance. Trends Pharmacol Sci. 2011;32:514–520. doi: 10.1016/j.tips.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Pin JP, et al. Activation mechanism of the heterodimeric GABAB receptor. Biochem Pharmacol. 2004;68:1565–1572. doi: 10.1016/j.bcp.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 46.Xu H, et al. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci USA. 2004;101:14258–14263. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boularan C, et al. β-Arrestin 2 oligomerization controls the Mdm2-dependent inhibition of p53. Proc Natl Acad Sci USA. 2007;104:18061–18066. doi: 10.1073/pnas.0705550104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Storez H, et al. Homo- and hetero-oligomerization of β-arrestins in living cells. J Biol Chem. 2005;280:40210–40215. doi: 10.1074/jbc.M508001200. [DOI] [PubMed] [Google Scholar]
- 49.Matthies HJ, et al. Rab11 supports amphetamine-stimulated norepinephrine transporter trafficking. J Neurosci. 2010;30:7863–7877. doi: 10.1523/JNEUROSCI.4574-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen JL, Haunso S, Brann MR, Sheikh SP, Weiner DM. Loss-of-function polymorphic variants of the human angiotensin II type 1 receptor. Mol Pharmacol. 2004;65:770–777. doi: 10.1124/mol.65.3.770. [DOI] [PubMed] [Google Scholar]
- 51.Sénard JM, Mauriege P, Daviaud D, Paris H. α2-Adrenoceptor in HT29 human colon adenocarcinoma cell-line: study of [3H](-)-adrenaline binding. Eur J Pharmacol. 1989;162:225–236. doi: 10.1016/0014-2999(89)90285-9. [DOI] [PubMed] [Google Scholar]