1 Introduction
Despite representing only 2% of the total body weight, the adult brain consumes more than 20% of the body’s total energy [1, 2]. Brain function depends critically on an adequate energy supply, requiring both oxygen and glucose provided by the cerebral blood flow, which is largely used to maintain presynaptic and postsynaptic ion gradients for glutamate neurotransmission, as well as sustain neuron resting potentials [2, 3]. Brain glucose metabolism as determined from positron emission tomography imaging has demonstrated a cerebral metabolic rate of glucose (CMRg) of 120–130 g glucose per day in healthy adult humans [4]. To date, studies of CMRg during aging have given inconsistent results, showing either no decline or up to 50% decline with age [5, 6]. The oxidation of glucose via glycolysis followed by oxidative phosphorylation (OXPHOS) accounts for almost all ATP generated in the brain [7].
Mitochondria are dynamic organelles responsible for ATP production through OXPHOS and play an essential role in a diverse array of metabolic processes; therefore, altered glucose metabolism during aging likely involves dysfunctional mitochondria. In fact, functional deficits have been reported in aged brain mitochondria including activation of the permeability transition pore, loss of membrane potential, and reduced respiration [8–10]. Interestingly, the pattern and extent of changes in respiratory complex activity in aged brain mitochondria varies and several studies, including those by our group, have demonstrated an increase in respiration and complex activity with age [11–13]. In addition to the variability observed in mitochondrial function, studies examining mRNA and protein expression levels of the respiratory chain subunits or other mitochondrial proteins in aged brain are similarly limited and inconsistent [14–17]. Therefore, a comprehensive evaluation of mitochondrial protein expression in conjunction with functional studies is vital to further the understanding of mitochondrial alterations associated with brain aging.
To date, several studies have examined subcellular proteomes during rat brain development [18] and age-related proteomic changes in the rodent brain [19–23]; however, global age-related mitochondrial proteomic changes in the brain have not been characterized. Additionally, apart from our studies of synaptic mitochondria, the simultaneous characterization of age-related brain mitochondrial proteomic changes and functional alterations has not been performed. Therefore, in this study, we have used quantitative mass-spectrometry based proteomics to examine the alterations in protein expression and bioenergetic analysis to examine the functional changes in mitochondria isolated from the brains of mature (5 months old), old (12 months old), and aged (24 months old) mice [24]. Our data revealed significant age-associated changes in proteins important for mitochondrial energy metabolism; however, we observed that aged mice do not exhibit functional alterations in brain mitochondrial respiration, suggesting that the observed mitochondrial proteomic changes may counteract any functional defects that arise with age. In fact, our results provide insight into the mitochondrial proteomic changes that occur during aging and indicate that brain mitochondria might be more resistant to the aging process than previously thought.
2 Materials and Methods
2.1 Animals
C57BL/6 male mice (27 total) from the National Institute on Aging representing three age groups (9 mice per comparative age group): mature (5 months old), old (12 months old), and aged (24 months old), using the Neuroscience Information Framework (NIF; http://neuinfo.org) [24] age classification standards were used in this study. Four mice per age group were used for immunoblotting (n = 4), three of these mice per age group were used for LC-MS/MS (n = 3), and the remaining five mice per age group were used for Seahorse XF24 bioenergetic analysis (n = 5). All procedures were conducted in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center.
2.2 Isolation of brain mitochondria for proteomics
Brain mitochondria were isolated from 5, 12, and 24 month old C57BL/6 mice using differential centrifugation (modified from MitoSciences Mitochondria Isolation Kit for Tissue). Briefly, following decapitation, fore/midbrains (hindbrain and olfactory bulbs removed) were dounce homogenized (10 strokes) in isolation medium (IM) containing 225 mM sucrose, 75 mM mannitol, 1 mM EGTA, 5 mM HEPES, and cOmplete Mini, EDTA-free protease inhibitor cocktail (Roche Diagnostics) adjusted to pH 7.4. The homogenate was centrifuged at 1,000 × g for 10 min, the supernatant collected and the pellet was resuspended in IM. Following a second centrifugation at 1,000 × g for 10 min, the pooled supernatants were centrifuged at 8,000 × g for 10 min. The pellet containing the mitochondria was further purified using anti-TOM22 immunomagnetic affinity isolation (Miltenyi Biotech). Purified mitochondria were lysed in 100 mM Tris-HCl with 4% (w/v) SDS and 0.1M DTT adjusted to pH 7.6 using brief sonication and incubation at 95°C for 5 min. The Pierce 660 nm Protein Assay was used for protein quantification (Thermo Scientific).
2.3 Electron Microscopy of Isolated Mitochondria
Brain mitochondria were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4 at 4°C overnight, postfixed with 1% cacodylate-buffered osmium tetraoxide at room temperature for 2 h, dehydrated in a graded series of ethanol, and then briefly transferred to propylene oxide and embedded in Epon-Araldite. Sections were stained with uranyl acetate and lead citrate, then randomly selected noncontiguous, nonoverlapping, digitized images of each mitochondrial section (11,000x and 52,000x magnification) were captured on a FEI Tecnai G2 Spirit transmission electron microscope as previously described [25].
2.4 Protein digestion
Unlabeled protein lysates from the isolated brain mitochondria were combined in a 1:1 protein ratio with our previously described mitochondrial super-SILAC mix (50 μg of each) [13, 25]. Briefly, the mouse cell lines Neuro-2a, CATH.a, NB41A3, and C8-D1A were SILAC-labeled by culturing in Advanced DMEM/F-12 (Invitrogen) containing heavy isotope-labeled amino acids, (U-13C615N4)-L-arginine (Arg-10) and (U-13C6)-L-lysine (Lys-6) in place of natural arginine and lysine for at least seven generations to obtain complete labeling. Mitochondria were isolated from heavy labeled cells by sequential differential centrifugation (Mitosciences) followed by anti-TOM22 immunomagnetic purification. Based on protein concentration, equal amounts of the heavy mitochondrial lysates from each of the four cell lines were mixed together creating the mitochondrial super-SILAC mix. For trypsin digestion of the brain mitochondria lysates (unlabeled) combined with the mitochondrial super-SILAC mix (labeled), filter-aided sample preparation (FASP) [26] was performed and the resultant peptides were cleaned with an Oasis mixed-mode weak cation exchange cartridge (Waters). Samples were dehydrated and resuspended in 0.1% formic acid for LC-MS/MS analysis. Peptides were quantified using a NanoDrop 2000 UV-Vis Spectrophotometer (Thermo Scientific) in conjunction with the Scopes method for protein quantification [27]. Three mice from each comparative age group (biological replicates, n = 3) were used for independent brain mitochondria preparations and LC-MS/MS.
2.5 LC-MS/MS and data analysis
The peptide samples from each independent biological replicate (n = 3) for each of the three comparative age groups were analyzed in duplicate (two technical replicates) resulting in a total of 18 LC-MS/MS runs using an Eksigent ultra nano-HPLC with a cHiPLC system connected to an AB SCIEX TripleTOF (TTOF) 5600 mass spectrometer as previously described [13]. Samples (6 μg) were loaded onto a trap column (200 μm × 6 mm ChromXP C18-CL 3 μm 120 Å), washed with 98:2 HPLC water with 1% formic acid for 10 min and then eluted through an analytical column (200 μm × 15 cm ChromXP C18-CL 3 μm 120 Å) using a 200 min linear gradient of 0–60% acetonitrile at a rate of 300 nL/min. The ProteinPilot version 4.5 (Paragon Algorithm: 4.5.0.0, 1654) software, which uses the Paragon search algorithm [28], was used for peptide matching, protein identification, and relative protein quantitation (ratios of the amount of heavy-to-light (H:L) (labeled-to-unlabeled) peptide). The search parameters were set as a maximum of two missed cleavages, 95% confidence for the detected protein threshold, trypsin digestion, carbamidomethyl (C) as fixed modification, N-acetyl (protein) and oxidation (M) as variable modifications, top 6 MS/MS peaks per 100 Da, and MS/MS mass tolerance of 0.5 Da. Exclusion criteria to remove proteins from analysis were as follows: FDR of 0.05 for both peptides and proteins, peptides must contain at least 6 amino acids, contaminants as identified through the database search and proteins identified as being in the reverse database. The additional cutoff values of Unused ProtScore ≥ 1.3 and number of unique peptides ≥ 2 were applied to the data. The mass spectrometry proteomics data have been deposited to the ProteomeXchange consortium [29] (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository with the dataset identifier PXD001370.
The H:L ratios were normalized to the heavy super-SILAC mix and differentially expressed proteins were determined using a Bayesian regularized t-test method and multiple tests correction in the Cyber-T Web server (http://cybert.ics.uci.edu/) [30]. Posterior Probabilities of Differential Expression (PPDE) values ≥ 0.95 and Benjamini-Hochberg (BH) corrected q values (FDR) ≤ 0.05 were used as cutoffs for significantly differentially expressed proteins. The multiple experiment viewer (Dana-Faber Cancer Institute) was used to generate heat maps, which represent the change in protein expression. Linear fitting and statistical analyses were performed using Prism (GraphPad Software) and Excel (Microsoft).
2.6 Bioinformatic analysis
Partek Genomics Suite 6.6 version 6.12.0713 software (Partek Inc.) was used for hierarchical clustering. The protein annotation through evolutionary relationship (PANTHER: http://www.pantherdb.org/) classification system (version 9.0) statistical enrichment test tool was used to determine which pathways have numeric values that are non-randomly distributed with respect to our entire list of proteomic values. The Database for Annotation, Visualization and Integrated Discovery (DAVID: david.abcc.ncifcrf.gov) Bioinformatics Resources 6.7 was used for Gene Ontology annotation for biological process and molecular function. Ingenuity Pathway Analysis (IPA: Ingenuity Systems; http://www.ingenuity.com/) Upstream Analysis tool was used to visualize the activation z-scores (-log of the p-value derived from the Fisher’s Exact test across all observations).
2.7 Immunoblotting
Equal amounts of brain mitochondrial protein lysates (10 μg) from four mice at each of the three ages (n = 4) were loaded onto 4–12% Bis-Tris gels, transferred to nitrocellulose membranes and confirmed using Ponceau staining, blocked, and incubated with the following primary antibodies overnight at 4°C: the oxidative phosphorylation (OXPHOS) panel (1:2000) (MS604; Mitoscences) and transcription factor A, mitochondrial (TFAM) (1:4000) (LS-C30495; LifeSpan Biosciences). The OXPHOS antibody panel is a mix of antibodies that include: NADH dehydrogenase (ubiquinone) 1 beta subcomplex subunit 8 (NDUFB8), cytochrome b-c1 complex subunit 2 (UQCRC2), cytochrome c oxidase subunit 1 (MTCO1), and ATP synthase subunit alpha (ATP5A1). The secondary antibodies used were HRP-conjugated goat anti-mouse (1:20,000) (31430; Thermo Scientific) and goat anti-rabbit (1:20,000) (31460; Thermo Scientific). Coomassie staining was performed to confirm equal protein loading (Supporting Information Figure S1) since commonly used mitochondrial protein loading controls (voltage-dependent anion-selective channel protein 1 (VDAC1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) show differential expression during aging and dependence on cellular conditions [13, 31, 32]. Chemiluminescent bands were visualized with a LI-COR C-DiGit Blot Scanner and analyzed using Image Studio software (both from LI-COR Biotech). Statistical analysis was conducted in Prism using one-way ANOVA and post-test with Tukey’s multiple comparison tests.
2.8 Isolation and bioenergetic analysis of brain mitochondria
Brain mitochondria were isolated as described above in methods section 2.2 with the following modifications. Briefly, homogenization was performed in mitochondrial isolation buffer (70 mM sucrose, 210 mM mannitol, 5 mM HEPES, 1 mM EGTA and 0.5% (w/v) fatty acid free BSA) and the anti-TOM22 immunomagnetic affinity isolation purification step was excluded to maintain functional integrity as magnetic bead purified mitochondria exhibit lower respiratory control ratio (RCR) values (state 3/4o) indicating increased proton leak [33]. Mitochondria were resuspended in isolation buffer and concentration of protein was determined by the BCA method. Isolated mitochondria were used immediately for bioenergetic analysis.
The Seahorse XF24 Flux Analyzer (Seahorse) bioenergetics assays were optimized using a modified protocol of Rogers et al. [34] as described previously [13]. Isolated brain mitochondrial amounts greater than 10 μg per well have a non-linear state 3 rate (Supporting Information Figure S2); therefore, 10 μg of mitochondria were used for all subsequent experiments. The coupling and electron flow assays were run in 3–4 technical replicate wells for each independent biological replicate (coupling and electron flow assay, n = 5). XF24 data was calculated using the algorithm previously described and used by the Seahorse software package [35]. Statistical analysis was conducted in Prism using one-way ANOVA and post-test with Tukey’s multiple comparison tests.
3 Results and Discussion
3.1 Quantitative Proteomic Analysis of Aging Brain Mitochondria
3.1.1 Global Proteomic Alterations
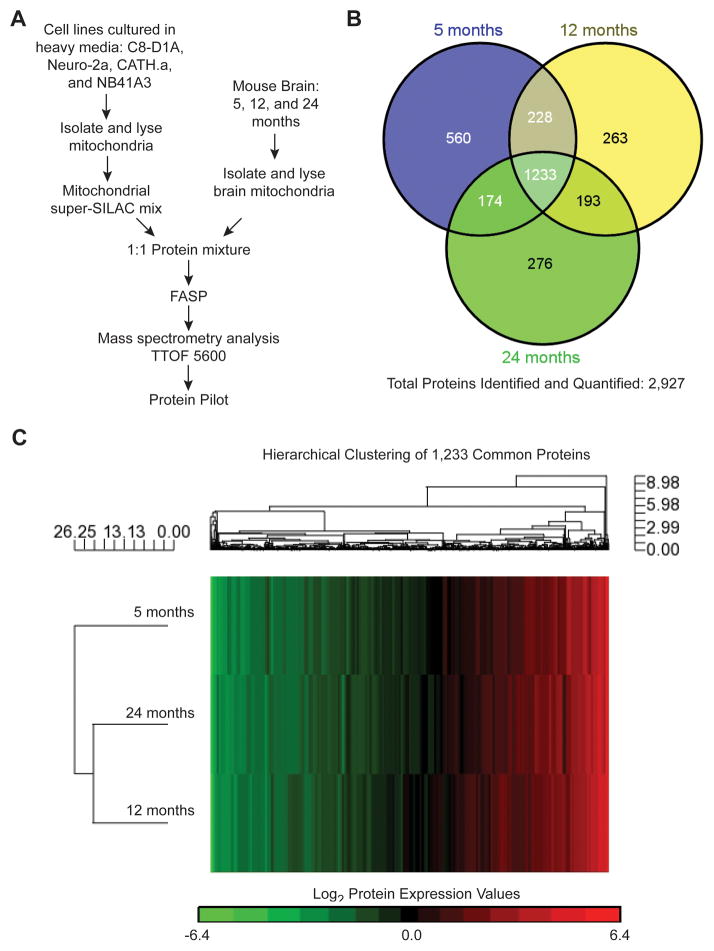
To accurately quantify the proteomic changes that occur with aging, we isolated brain mitochondria from mature (5 months old), old (12 months old), and aged (24 months old) male C57BL/6 mice. We first prepared our previously characterized mix of mitochondria isolated from multiple SILAC-labeled mouse cell lines (Neuro-2a, CATH.a, C8-D1A, and NB41A3) which each provide unique mitochondrial proteome profiles for use as internal standards for mouse brain mitochondrial proteome quantification (Figure 1A) [25], a technique termed super-SILAC [36–39]. Since one potential limitation of mass spectrometry-based quantitative proteomics is the production of an internal standard for every protein within a sample, the development of an appropriate mitochondrial super-SILAC mix to cover the mitochondrial proteome is critical. The use of our mitochondrial super-SILAC mix previously allowed for accurate quantification of the non-synaptic and synaptic mitochondrial proteomes [13, 25]. The purity of the isolated brain mitochondria was verified using immunoblotting and electron microscopy (Supporting Information Figure S3). The pure brain mitochondrial fractions were combined in a 1:1 protein ratio with our mitochondrial super-SILAC mix prior to FASP [26] and acquisition of data on a TTOF 5600 (Figure 1A). Joint analysis of the resulting LC-MS/MS files from each biological replicate (n = 3) using ProteinPilot identified and quantified a total of 2,927 proteins (Figure 1B). The complete lists of the identified and quantified proteins for the joint analysis for each brain mitochondria super-SILAC experiment (5, 12, and 24 month ages) are provided in the Supporting Information Table S1. Of the total 2,927 proteins, 1,233 (42.1% of the total) proteins were found to be common between mitochondria isolated from 5, 12, and 24 month old mice (Figure 1B). The list of the 1,233 common proteins is provided in the Supporting Information Table S1. Hierarchical clustering of the 1,233 common proteins using the protein expression values obtained from the super-SILAC heavy-to-light (labeled-to-unlabeled) ratios yielded a heat map of the global proteomic differences between brain mitochondria from each of the three ages (Figure 1C). Based on protein expression, the brain mitochondria from 12 and 24 month old mice cluster together and as depicted by the length of the dendrogram clusters, the mitochondria from 5 month old mice are slightly more dissimilar (Figure 1C). In contrast, our previous comparison of the synaptic mitochondrial proteome revealed that mitochondria from isolated nerve terminals (synaptosomes) of 5 and 24 month old mice were more similar to each other [13], suggesting that different pools of brain mitochondria may undergo distinct proteomic changes with aging. This finding is consistent with our previous work revealing proteome differences between synaptic and non-synaptic mitochondrial pools [25].
Figure 1. Brain mitochondrial proteomic changes during aging.
A) Representation of the super-SILAC experiment design. B) Venn diagram comparing the identified proteins for brain mitochondria isolated from 5, 12, and 24 month old mice. C) Dendrogram presentation of the hierarchical clustering analysis of log2 expression values for the common 1,233 identified and quantified proteins derived from super-SILAC (H:L) ratios of 5, 12, and 24 month old brain mitochondrial proteins.
3.1.2 Functional Annotation of Identified Proteins
The 1,233 common quantified proteins between the three ages were uploaded and analyzed using the PANTHER statistical enrichment test [41, 42], which uses the Mann-Whitney test [43] to determine the probability that any functional pathway has expression values that are non-randomly distributed with respect to the entire list of expression values. Based on P value, this tool revealed the top biological process terms (Table 1A) of which “respiratory electron transport chain” and “oxidative phosphorylation” contain expression value changes from 5 to 12 months that are shifted toward greater values (+) than the overall uploaded list and expression value changes from 12 to 24 months that are shifted toward smaller values (-) than the overall list. Therefore, based on protein expression, the process of OXPHOS is predicted to be upregulated from 5 to 12 months but downregulated from 12 to 24 months (Table 1A). Additionally, the similar biological process term “generation of precursor metabolites and energy” exhibits the same distribution of values as OXPHOS for each age comparison.
Table 1.
Statistical Enrichment
| A. PANTHER
| ||
|---|---|---|
| Biological Process | Over/Under | P Value |
| 5 to 12 months | ||
|
| ||
| Respiratory electron transport chain | + | 4.3E-03 |
| Oxidative phosphorylation | + | 9.3E-03 |
| Generation of precursor metabolites and energy | + | 1.5E-02 |
|
| ||
| 12 to 24 months | ||
|
| ||
| Oxidative phosphorylation | − | 2.0E-07 |
| Respiratory electron transport chain | − | 2.0E-07 |
| Generation of precursor metabolites and energy | − | 8.6E-05 |
| B. DAVID
| |
|---|---|
| 5 to 12 months | P Value |
| Gene Ontology Biological Process | |
|
| |
| Generation of precursor metabolites and energy | 1.3E-47 |
| Electron transport chain | 1.6E-31 |
| Oxidation reduction | 3.5E-28 |
| Cellular respiration | 2.7E-21 |
| Energy derivation by oxidation of organic compounds | 5.6E-17 |
|
| |
| Gene Ontology Molecular Function | |
|
| |
| Cofactor binding | 6.1E-15 |
| Coenzyme binding | 7.7E-15 |
| Electron carrier activity | 4.2E-9 |
| Hydrogen ion transmembrane transporter activity | 5.1E-9 |
| Nucleotide binding | 9.2E-9 |
|
| |
| 12 to 24 months | P value |
|
| |
| Gene Ontology Biological Process | |
|
| |
| Generation of precursor metabolites and energy | 6.1E-61 |
| Oxidation reduction | 4.6E-38 |
| Electron transport chain | 1.1E-37 |
| Cellular respiration | 1.1E-15 |
| Energy derivation by oxidation of organic compounds | 1.1E-13 |
|
| |
| Gene Ontology Molecular Function | |
|
| |
| Cofactor binding | 6.2E-15 |
| Coenzyme binding | 1.3E-14 |
| Hydrogen ion transmembrane transporter activity | 2.8E-13 |
| Monovalent inorganic cation transmembrane transporter activity | 6.5E-13 |
| NADH dehydrogenase (ubiquinone) activity | 2.3E-12 |
While the above joint analysis allows for increased numbers of protein identifications it does not allow for statistical analysis of protein expression changes between the mitochondria isolated from mice of different ages. Therefore we also performed a separate analysis of the resulting LC-MS/MS files for each biological replicate (n = 3) from each comparative age group. The complete lists of the identified and quantified proteins from each separate analysis are provided in Supporting Information Table S2 (5 month olds), Table S3 (12 month olds), and Table S4 (24 month olds). To examine the reproducibility of our super-SILAC experiments we calculated Pearson correlation coefficients (r), which indicated high values (5 month olds: r = 0.95 (mouse 1 and 2) and 0.94 (mouse 1 and 3), 12 month olds: r = 0.98 (mouse 1 and 2) and 0.95 (mouse 1 and 3), and 24 month olds: r = 0.98 (mouse 1 and 2) and 0.95 (mouse 1 and 3)) for the biological replicates at each age (Supporting Information Figure S4). In order to determine which mitochondrial proteins were differentially expressed during aging we analyzed our expression data using the Cyber-T web server, which implements a Bayesian regularized t-test and multiple tests corrections [30]. The complete lists of the differentially expressed proteins between brain mitochondria isolated from 5 and 12 month old as well as 12 to 24 month old mice are provided in Supporting Information Table S5. Comparison of the differentially expressed protein values for proteins identified using both the joint and separate analysis reveals high concordance for these two methods (Supporting Information Table S6).
To obtain functional insights into the differences between brain mitochondria during aging directly from our quantitative proteomics data we uploaded the lists of differentially expressed proteins between 5 and 12 months as well as 12 and 24 months to the DAVID bioinformatics database to yield Gene Ontology (GO) annotation terms for biological process and molecular function. Similar to the PANTHER analysis, biological processes involved in mitochondrial metabolic processes and energy generation were enriched with proteins that exhibit differential expression (Table 1B). Taken together these bioinformatic approaches highlighted significant alterations in the expression of mitochondrial proteins involved in energy metabolism pathways during brain aging.
3.2 Energy Metabolism during Brain Aging Assessed by Proteomics
3.2.1 Glycolysis
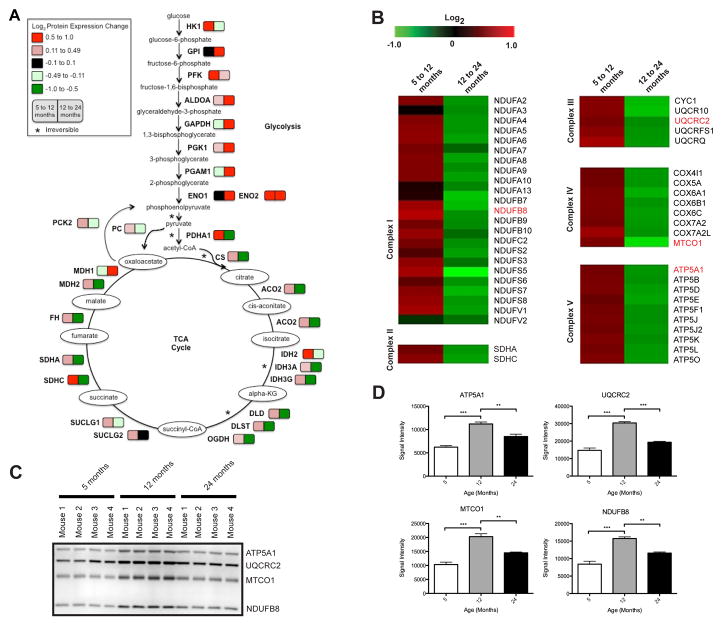
Although glycolysis is largely regarded as a cytoplasmic process, the glycolytic hexokinase (HK) enzymes associate with the mitochondrial outer membrane [44, 45]. Additionally, many glycolytic enzymes have non-glycolytic roles that are important for mitochondrial functions, such as apoptosis [46]. Given that mitochondria require pyruvate to support respiration, it has been suggested that partitioning of the glycolytic enzymes between the mitochondrial surface and cytosol could facilitate pyruvate generation at the point of demand [47]. Therefore, we further characterized the glycolytic protein expression changes identified in our proteomics experiment. As seen in Figure 2A, most of the glycolytic enzymes associated with the mitochondria increase in expression at both 12 and 24 months compared to 5 months of age, with the exception of HK1, which is decreased from 12 to 24 months, and GAPDH and phosphoglycerate mutase (PGAM1), which are decreased from 5 to 12 months. In aged rat brain, others found that the activity of most of the glycolytic enzymes were found to be unchanged with the exception of HK, which was increased [48]. Changes in the association of glycolytic proteins with mitochondria during aging may act to efficiently integrate the glycolytic pathway with mitochondrial OXPHOS via increased metabolic flux entry towards the TCA cycle.
Figure 2. Metabolic pathways affected by aging associated mitochondrial protein expression changes.
A) Glycolysis and TCA cycle. The colors represent log2 expression changes for proteins in brain mitochondria from mice at 12 versus 5 months and 24 versus 12 months of age. B–D) OXPHOS. B) The protein expression changes from 5 to 12 months and 12 to 24 months in brain mitochondria (log2 (12/5 months)) and (log2 (24/12 months)). Proteins verified orthogonally are highlighted in red. C) Immunoblot orthogonal validation of selected proteins in (B). D) Statistical analysis of immunoblot signal intensity in (C). * p ≤ 0.05, ** p ≤ 0.001, and *** p ≤ 0.0001.
3.2.2 TCA Cycle
Pyruvate generated from glycolysis is converted into acetyl-CoA and the conversion of acetyl-CoA into CO2 via the TCA cycle results in a large production of reducing equivalents for the electron transport chain (ETC) as NADH and subsequent production of ATP. Alterations at any step leading to OXPHOS may disrupt mitochondrial bioenergetics; therefore, we chose to evaluate the changes in the enzymes responsible for this process. Contrary to the changes observed in the glycolytic enzymes, the changes in the expression of the TCA cycle enzymes were almost the reverse with increased expression from 5 to 12 months but then decreased expression from 12 to 24 months (Figure 2A). These results suggest that decreased expression of TCA cycle enzymes may affect NADH levels and contribute to a decline in mitochondrial bioenergetics during aging.
3.2.3 OXPHOS
Mitochondria provide most of the energy needed for cellular functions by the metabolism of fuel molecules into ATP and these metabolic processes were identified in the PANTHER and DAVID analyses (Table 1). Therefore, we decided to investigate the expression of the subunits of the ETC protein complexes obtained from our proteomic experiments. All of the subunits of the ETC that we identified exhibit changes in protein expression during aging, specifically increased expression from 5 to 12 months and decreased expression from 12 to 24 months in brain mitochondria (Figure 2B). This result is consistent with previous reports of increased expression of mitochondrial genes for complexes I, III, IV, and V in 12 and 18 month old mice compared to 2 month olds, followed by decreased expression in 24 month olds [16]. Validation of the expression changes of a subset of the ETC complex subunits (ATP5A1, UQCRC2, MTCO1, and NDUFB8) was performed using immunoblotting (Figure 2C) and statistical analysis of the signal intensity supports our proteomic findings (ATP5A1: log2 = 0.46, BH = 0.009 (5 to 12 months) and log2 = -0.59, BH < 0.001 (12 to 24 months); UQCRC2: log2 = 0.48, BH = 0.015 (5 to 12 months) and log2 = -0.56, BH = 0.001 (12 to 24 months); NDUFB8: log2 = 0.63, BH = 0.056 (5 to 12 months) and log2 = -0.74, BH = 0.024 (12 to 24 months) of statistically significant differential expression (Figure 2D). Additionally, in the brain mitochondria studied here, the expression of the OXPHOS proteins correlates with those exhibited by the TCA cycle enzymes.
Interestingly, these findings are the exact opposite of what we reported previously for synaptic mitochondria, where we observed decreased expression from 5 to 12 months and increased expression from 12 to 24 months [13]. However, relative to presynaptic mitochondria (those isolated from synaptosomes), the brain mitochondria examined in this study would be expected to be more heterogeneous, given the variety of cell types in the brain, including different glia in addition to neurons. Taken with our previous work revealing that non-synaptic mitochondria exhibit a different proteomic profile than synaptic mitochondria [25] these findings reflect the overall mitochondrial proteomic changes that occur during brain aging, and not limited to those at the synapse, where the mitochondria may be specialized to meet the functional requirements of this structure. Since, the maintenance of bioenergetic function via compensatory mechanisms including alterations in mitochondrial remodeling have recently been shown to contribute to longevity despite decreases in complex I and IV proteins [49], we next chose to address whether the overall complex subunit abundance correlates with the functional respiratory capacity of brain mitochondria during aging.
3.3 Functional Bioenergetics Analysis of Aging Brain Mitochondria
3.3.1 Coupling
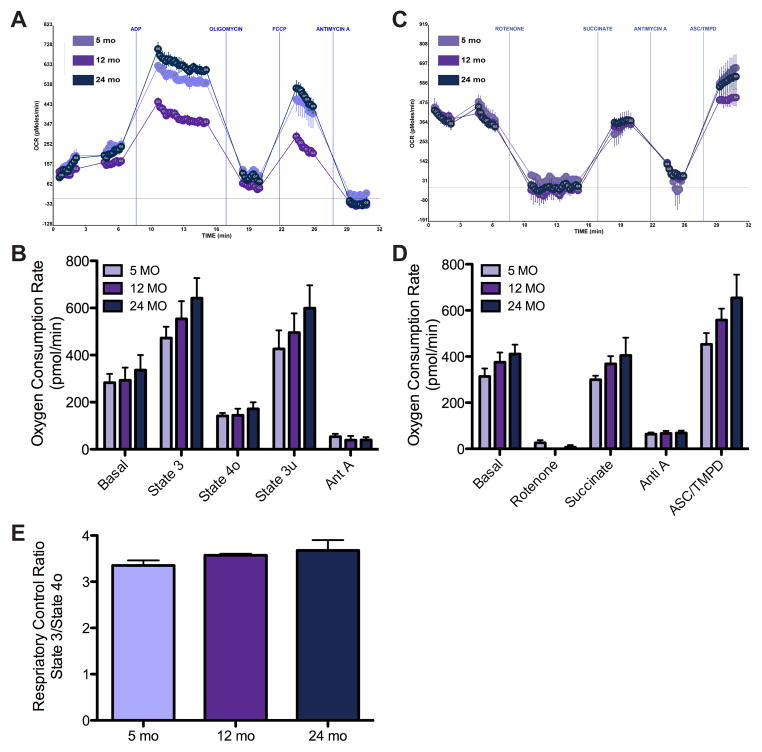
The oxygen consumption rate (OCR) of brain mitochondria during aging was assessed using the Seahorse XF24 machine [34]. Respiration (using succinate as the substrate) was sequentially measured in a coupled state for basal complex II respiration followed by phosphorylating ADP-stimulated respiration (state 3), then non-phosphorylating respiration (state 4o) was induced by adding oligomycin, an ATP synthase inhibitor, and maximal uncoupled respiration (state 3u) was measured after addition of FCCP. Finally, all mitochondrial respiration was stopped by the addition of antimycin A. Compared to brain mitochondria isolated from 5 month old mice, those from 12 and 24 month old mice showed a trend towards an increased rate of complex II driven basal respiration and ADP-driven state 3 respiration as well as an increased maximum uncoupled rate induced by FCCP, although none of these reached significance (Figure 3A and B). Although, reduced activities of ETC complexes I and IV have been observed with aging in mouse brain [50, 51], the respiratory rate of mouse brain mitochondria appeared unchanged with aging in our study.
Figure 3. Brain mitochondria show no significant change in bioenergetics with age.
A) Representative OCR curves of brain mitochondria coupling assay with the substrate succinate and sequential additions of ADP, oligomycin, FCCP, and antimycin A. B) Bar graph summary of basal (complex II), state 3, state 4o, and state 3u respiration, n = 5. C) Representative OCR graph output of the electron flow assay with substrates pyruvate and malate and sequential additions of rotenone, succinate, antimycin A, and ACS/TMPD. D) Bar graph representation of rotenone inhibition of complex I, succinate driven complex II, antimycin A inhibition of complex III, and ASC/TMPD driven complex IV respiration, n = 5. E) RCR (state 3/4o) reveals no change between isolated brain mitochondria at 5, 12, and 24 months.
3.3.2 Electron Flow
Next an electron flow experiment was performed in the presence of FCCP using pyruvate and malate to drive complex I respiration. We found that compared to 5 months, brain mitochondria from 12 and 24 month old mice showed a trend toward increased complex I basal respiration (Figure 3C and D). Rotenone, an inhibitor of complex I driven respiration, was able to equally inhibit mitochondria from all ages. Again using succinate revealed that similar to the coupling assay there was a trend toward increased complex II driven respiration in brain mitochondria with age. Inhibition of complex III with antimycin A decreased the respiratory rate similarly between all ages as expected. Finally, evaluation of complex IV driven respiration with ASC/TMPD revealed a trend towards increased oxygen consumption with age. Despite the trend toward increased respiratory rates, the changes were not significant suggesting maintenance of function during aging.
3.3.3 Respiratory Control Ratio
Although trends hinting at increased mitochondrial respiratory rates in the coupling and electron flow assays were found with brain aging, it is notable that the RCR values (state3/4o), which are an overall indicator of mitochondrial integrity, were not found to be significantly different between ages (Figure 3E). In contrast, we previously found that synaptic mitochondria exhibit hyperfunction with aging [13]. As the mammalian brain can be subdivided into more than 100 anatomically and functionally distinct regions, each containing multiple cell types, including various classes of neurons and glia, we propose that the functional alterations of distinct populations of mitochondria could be masked or diluted due to the presence of other mitochondrial populations in the heterogeneous pool of mitochondria studied here. Thus, future studies of other brain mitochondrial pools such as non-synaptic mitochondria in the absence of synaptic mitochondria may reveal the subtle functional changes that are being masked leading to a trend for an increase in respiration versus the significant hyperfunction observed in the synaptic terminal isolated mitochondria.
These findings suggest that during aging, as synaptic mitochondrial respiratory function is increased, non-synaptic mitochondrial function may slightly decrease. A limitation is that brain region specific changes cannot be determined from this study. Thus, future studies of mitochondria isolated from specific brain regions and cellular locations (i.e. presynaptic terminals) are needed to shed light on the heterogeneity of mitochondrial populations and differential effects of aging. A caveat of studying isolated mitochondria functionally is that several important aspects of mitochondrial biology are lost during isolation including mitochondrial localization and network formation. This would be currently impossible to study in situ in the brain, and further complicating its analysis is that the successful culturing of viable primary cells from adult animals has been historically difficult, if not impossible, thus limiting studies on cell specific age-related mitochondrial changes and their impact on function.
3.4 Mitochondrial Protein Transcriptional Regulation
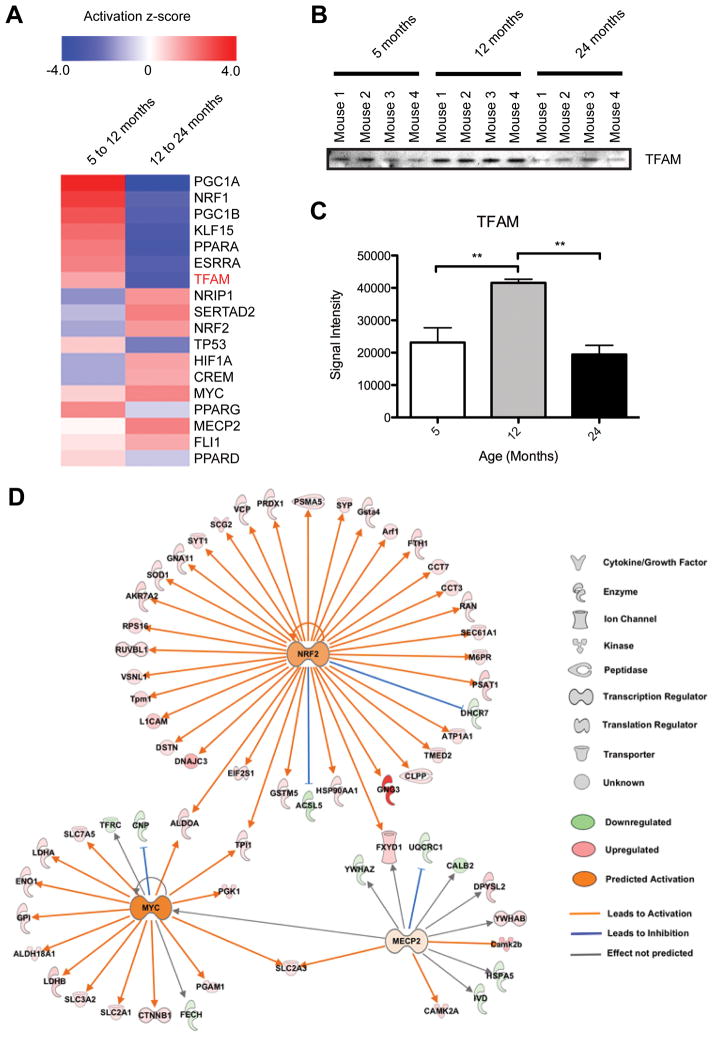
Despite the proteomic changes in mitochondrial metabolic pathways, we observed no significant change in mitochondrial respiration in aged mouse brain, similar to studies in aged rat brain [52]. This highlights the importance of coordination between the mitochondrial and nuclear genomes to regulate the expression of proteins responsible for mitochondrial function and biogenesis, which may contribute to the overall health of mitochondria during brain aging. Although using isolated mitochondria allowed us to study proteomic and functional changes during mouse brain aging, as above, one important limitation is that the cellular context is lost. To obtain a measure of cellular changes we therefore used IPA upstream analysis to examine the changes during aging in transcriptional control pathways responsible for the regulation of mitochondrial biogenesis and energy metabolism predicted based on our proteomic data. As seen in Figure 4A, many relevant transcriptional regulators are predicted to display differential activation during the transition from mature (5 months) to old (12 months) age as compared to the progression from old (12 months) to aged (24 months). Specifically, the main regulators of mitochondrial biogenesis and energy homeostasis, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1A) and mitochondrial transcription factor A (TFAM) [53], show the highest degree of change based on IPA activation z-score predictions using our proteomics data. These transcriptional regulators exhibit a dramatic shift from high activation z-score (activated) from 5 to 12 months to a low activation z-score (inhibited activity) from 12 to 24 months, which is consistent with the observed expression changes for the OXPHOS subunit proteins (Figure 2). Since TFAM was not quantified in all biological replicates, we used immunoblotting (Figure 4B) and statistical analysis of the signal intensity (Figure 4C) to show that the TFAM expression change is consistent with the IPA prediction. Additionally, estrogen receptor-like 1 (ESRRA) is an effector of PGC1A and thus also regulates the expression of genes involved in OXPHOS and mitochondrial biogenesis. Nuclear receptor-interacting protein 1 (NRIP1) affects oxidative metabolism and mitochondrial biogenesis by negatively regulating mitochondrial pathways regulated by PGC1A; so increased NRIP1 would be expected to decrease PGC1A [54] as observed. Although these changes suggest that mitochondrial OXPHOS would increase from 5 to 12 months and decrease from 12 to 24 months we observed maintenance of function.
Figure 4. Age-associated alterations in transcriptional regulators predicted from brain mitochondria quantitative proteomic data.
A) IPA generated heat map showing predicted activation z-scores for several transcriptional regulators important for mitochondrial biogenesis and function. Protein verified orthogonally is highlighted in red. B) Immunoblot orthogonal validation of TFAM in (A). C) Statistical analysis of TFAM immunoblot signal intensity in (B). ** p ≤ 0.001. D) IPA generated NRF2, MYC, and MECP2 upstream regulator networks overlaid with our proteomic expression data for brain mitochondria from 5 to 24 month old mice. All proteins shown (except the nuclear proteins NRF2, MYC, and MECP2) were found in the proteomic analysis.
Based on our proteomic expression data, the transcriptional regulators MYC, methyl CpG binding protein 2 (MECP2), and nuclear factor (erythroid-derived 2)-like 2 (NRF2) have predicted activation at 24 compared to 5 months of age (Figure 4D). MYC expression stimulates nuclear encoded mitochondrial genes and mitochondrial biogenesis. In fact, induction of MYC has been shown to increase oxygen consumption as well as mitochondrial mass [55]. The observed increase in MECP2 may also contribute to maintenance of bioenergetics function since MECP2 null mouse brain mitochondria exhibit abnormal respiration and reduced activity in respiratory complexes [56]. The NRF2 signaling pathway is induced by cellular stress including ROS, which would be expected to increase with age, and has been shown to protect against many age-related diseases including cancer and neurodegeneration [57]. NRF2 directly regulates mitochondrial bioenergetics and its activation has been shown to improve OXPHOS efficiency and increase both the rate of respiration and ATP levels [58]. Additionally, NRF2 has been proposed to play a role in longevity and healthspan by activating pathways important for cellular integrity [59]. Since NRF2 has been shown to impact bioenergetics by controlling substrate availability for mitochondrial respiration, we propose that activation of this factor in combination with MYC and MECP2 may counteract the effects of inhibition of the other factors important for mitochondrial biogenesis and function (i.e. PGC1A and TFAM).
In addition to evaluating the predicted age-dependent changes in transcriptional regulation, we used DAVID GO annotation to evaluate the proteins that were significantly differentially expressed between 5 and 24 months of age (Supporting Information Table S6). The GO biological process terms “cellular macromolecular complex assembly”, “macromolecular complex assembly”, “cellular macromolecular complex subunit organization”, “macromolecular complex subunit organization”, and “cellular protein complex assembly”, five of the top ten terms, were enriched suggesting the potential for aging related alterations of supramolecular assembly (supercomplexes) of respiratory complexes, which could promote sustained respiratory function [60] during brain aging.
4 Concluding Remarks
In the present study, we evaluated the proteomic profile of isolated brain mitochondria during aging as well as the functional bioenergetic correlates. Our quantitative proteomics revealed age-related changes in proteins related to mitochondrial energy metabolism that coincided with maintenance of bioenergetic function. These findings suggest that changes in mitochondrial protein expression with age may act as a compensatory mechanism allowing for preservation of function. This is in line with other studies that have shown maintenance of primary mitochondrial function even in advanced aging. Our results characterize the mitochondrial proteomic changes associated with aging and may serve as resource for others studying mitochondria in aging.
Supplementary Material
Acknowledgments
We would like to acknowledge the following University of Nebraska Medical Center Core facilities for their assistance: the Mass Spectrometry and Proteomics Core Facility particularly Dr. Pawel Ciborowski and Melinda Wojtkiewicz; and the Electron Microscopy Core Facility especially Tom Barger (Department of Genetics, Cell Biology, and Anatomy). We are grateful to Dr. Robert Lewis and Dr. Mario Fernandez (UNMC Eppley Cancer Institute) for their assistance with the Seahorse XF24 analyzer and the PRIDE Team for their support during deposition of our data.
Funding
This research was supported by the NIH grants P30 MH06221 and R01 MH073490.
Abbreviations
- ATP5A1
ATP synthase subunit alpha
- BH
Benjamini-Hocberg
- CMRg
cerebral metabolic rate of glucose
- DAVID
Database for Annotation, Visualization and Integrated Discovery
- ESRRA
estrogen receptor-like 1
- ETC
electron transport chain
- FASP
filter-aided sample preparation
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GO
gene ontology
- HK
hexokinase
- H:L
heavy-to-light
- IM
isolation media
- IPA
Ingenuity Pathway Analysis
- MECP2
methyl CpG binding protein 2
- MTCO1
cytochrome c oxidase subunit 1
- NDUFB8
NADH dehydrogenase (ubiquinone) 1 beta subcomplex 8
- NRF2
nuclear factor (erythroid-derived 2)-like 2
- NRIP1
nuclear receptor-interacting protein 1
- OCR
oxygen consumption rate
- OXPHOS
oxidative phosphorylation
- PANTHER
protein annotation through evolutionary relationship
- PGAM1
phosphoglycerate mutase
- PGC1A
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PPDE
Posterior Probabilities of Differential Expression
- RCR
respiratory control ratio
- TCA
tricarboxylic acid cycle
- TFAM
transcription factor A, mitochondrial
- UQCRC2
cytochrome b-c1 complex subunit 2
- VDAC
voltage-dependent anion-selective channel 1
Footnotes
Conflict of Interest Statement
The authors declare no competing financial/commercial interest.
References
- 1.Holliday MA. Metabolic rate and organ size during growth from infancy to maturity and during late gastation and early infancy. Pediatrics. 1971;47(Suppl 2):169+. [PubMed] [Google Scholar]
- 2.Sokoloff L. Energetics of functional activation in neural tissues. Neurochemical research. 1999;24:321–329. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- 3.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends in neurosciences. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 4.Owen OE, Morgan AP, Kemp HG, Sullivan JM, et al. Brain metabolism during fasting. The Journal of clinical investigation. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kety SS. Human cerebral blood flow and oxygen consumption as related to aging. Research publications - Association for Research in Nervous and Mental Disease. 1956;35:31–45. [PubMed] [Google Scholar]
- 6.Duara R, Margolin RA, Robertson-Tchabo EA, London ED, et al. Cerebral glucose utilization, as measured with positron emission tomography in 21 resting healthy men between the ages of 21 and 83 years. Brain: a journal of neurology. 1983;106(Pt 3):761–775. doi: 10.1093/brain/106.3.761. [DOI] [PubMed] [Google Scholar]
- 7.Hall CN, Klein-Flugge C, Attwell D. Oxidative phosphorylation, not glycolysis, powers pre- and postsynaptic mechanisms underlying brain information processing. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:8940–8951. doi: 10.1523/JNEUROSCI.0026-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen T, Sen N, Jana S, Khan FH, et al. Depolarization and cardiolipin depletion in aged rat brain mitochondria: relationship with oxidative stress and electron transport chain activity. Neurochemistry international. 2007;50:719–725. doi: 10.1016/j.neuint.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Bagh MB, Thakurta IG, Biswas M, Behera P, Chakrabarti S. Age-related oxidative decline of mitochondrial functions in rat brain is prevented by long term oral antioxidant supplementation. Biogerontology. 2011;12:119–131. doi: 10.1007/s10522-010-9301-8. [DOI] [PubMed] [Google Scholar]
- 10.Ferrandiz ML, Martinez M, De Juan E, Diez A, et al. Impairment of mitochondrial oxidative phosphorylation in the brain of aged mice. Brain research. 1994;644:335–338. doi: 10.1016/0006-8993(94)91699-3. [DOI] [PubMed] [Google Scholar]
- 11.Sharman EH, Bondy SC. Effects of age and dietary antioxidants on cerebral electron transport chain activity. Neurobiology of aging. 2001;22:629–634. doi: 10.1016/s0197-4580(01)00226-3. [DOI] [PubMed] [Google Scholar]
- 12.Takai D, Inoue K, Shisa H, Kagawa Y, Hayashi J. Age-associated changes of mitochondrial translation and respiratory function in mouse brain. Biochemical and biophysical research communications. 1995;217:668–674. doi: 10.1006/bbrc.1995.2826. [DOI] [PubMed] [Google Scholar]
- 13.Stauch KL, Purnell PR, Fox HS. Aging synaptic mitochondria exhibit dynamic proteomic changes while maintaining bioenergetic function. Aging. 2014;6:320–334. doi: 10.18632/aging.100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fattoretti P, Bertoni-Freddari C, Giorgetti B, Balietti M. Increased mitochondrial and nuclear gene expression of cytochrome oxidase subunits I and IV in neuronal aging. Annals of the New York Academy of Sciences. 2004;1030:303–309. doi: 10.1196/annals.1329.038. [DOI] [PubMed] [Google Scholar]
- 15.Nicoletti VG, Marino VM, Cuppari C, Licciardello D, et al. Effect of antioxidant diets on mitochondrial gene expression in rat brain during aging. Neurochemical research. 2005;30:737–752. doi: 10.1007/s11064-005-6867-7. [DOI] [PubMed] [Google Scholar]
- 16.Manczak M, Jung Y, Park BS, Partovi D, Reddy PH. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J Neurochem. 2005;92:494–504. doi: 10.1111/j.1471-4159.2004.02884.x. [DOI] [PubMed] [Google Scholar]
- 17.Calabrese V, Scapagnini G, Ravagna A, Colombrita C, et al. Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mechanisms of ageing and development. 2004;125:325–335. doi: 10.1016/j.mad.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 18.McClatchy DB, Liao L, Lee JH, Park SK, Yates JR. 3rd, Dynamics of subcellular proteomes during brain development. Journal of proteome research. 2012;11:2467–2479. doi: 10.1021/pr201176v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poon HF, Vaishnav RA, Getchell TV, Getchell ML, Butterfield DA. Quantitative proteomics analysis of differential protein expression and oxidative modification of specific proteins in the brains of old mice. Neurobiology of aging. 2006;27:1010–1019. doi: 10.1016/j.neurobiolaging.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Mehan ND, Strauss KI. Combined age- and trauma-related proteomic changes in rat neocortex: a basis for brain vulnerability. Neurobiology of aging. 2012;33:1857–1873. doi: 10.1016/j.neurobiolaging.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perluigi M, Di Domenico F, Giorgi A, Schinina ME, et al. Redox proteomics in aging rat brain: involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. Journal of neuroscience research. 2010;88:3498–3507. doi: 10.1002/jnr.22500. [DOI] [PubMed] [Google Scholar]
- 22.Yang S, Liu T, Li S, Zhang X, et al. Comparative proteomic analysis of brains of naturally aging mice. Neuroscience. 2008;154:1107–1120. doi: 10.1016/j.neuroscience.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Carrette O, Burkhard PR, Hochstrasser DF, Sanchez JC. Age-related proteome analysis of the mouse brain: a 2-DE study. Proteomics. 2006;6:4940–4949. doi: 10.1002/pmic.200600084. [DOI] [PubMed] [Google Scholar]
- 24.Gardner D, Akil H, Ascoli GA, Bowden DM, et al. The neuroscience information framework: a data and knowledge environment for neuroscience. Neuroinformatics. 2008;6:149–160. doi: 10.1007/s12021-008-9024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stauch KL, Purnell PR, Fox HS. Quantitative Proteomics of Synaptic and Nonsynaptic Mitochondria: Insights for Synaptic Mitochondrial Vulnerability. Journal of proteome research. 2014 doi: 10.1021/pr500295n. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nature methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 27.Scopes RK. Measurement of protein by spectrophotometry at 205 nm. Analytical biochemistry. 1974;59:277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- 28.Shilov IV, Seymour SL, Patel AA, Loboda A, et al. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Molecular & cellular proteomics: MCP. 2007;6:1638–1655. doi: 10.1074/mcp.T600050-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Vizcaino JA, Cote RG, Csordas A, Dianes JA, et al. The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic acids research. 2013;41:D1063–1069. doi: 10.1093/nar/gks1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayala MA, Baldi P. Cyber-T web server: differential analysis of high-throughput data. Nucleic acids research. 2012;40:W553–559. doi: 10.1093/nar/gks420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuqi L, Lei G, Yang L, Zongbin L, et al. Voltage-dependent anion channel (VDAC) is involved in apoptosis of cell lines carrying the mitochondrial DNA mutation. BMC Med Genet. 2009;10:114. doi: 10.1186/1471-2350-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferguson RE, Carroll HP, Harris A, Maher ER, et al. Housekeeping proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics. 2005;5:566–571. doi: 10.1002/pmic.200400941. [DOI] [PubMed] [Google Scholar]
- 33.Franko A, Baris OR, Bergschneider E, von Toerne C, et al. Efficient isolation of pure and functional mitochondria from mouse tissues using automated tissue disruption and enrichment with anti-TOM22 magnetic beads. PloS one. 2013;8:e82392. doi: 10.1371/journal.pone.0082392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers GW, Brand MD, Petrosyan S, Ashok D, et al. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PloS one. 2011;6:e21746. doi: 10.1371/journal.pone.0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerencser AA, Neilson A, Choi SW, Edman U, et al. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boersema PJ, Geiger T, Wisniewski JR, Mann M. Quantification of the N-glycosylated secretome by super-SILAC during breast cancer progression and in human blood samples. Molecular & cellular proteomics: MCP. 2013;12:158–171. doi: 10.1074/mcp.M112.023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deeb SJ, D’Souza RC, Cox J, Schmidt-Supprian M, Mann M. Super-SILAC allows classification of diffuse large B-cell lymphoma subtypes by their protein expression profiles. Molecular & cellular proteomics: MCP. 2012;11:77–89. doi: 10.1074/mcp.M111.015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geiger T, Cox J, Ostasiewicz P, Wisniewski JR, Mann M. Super-SILAC mix for quantitative proteomics of human tumor tissue. Nature methods. 2010;7:383–385. doi: 10.1038/nmeth.1446. [DOI] [PubMed] [Google Scholar]
- 39.Geiger T, Wehner A, Schaab C, Cox J, Mann M. Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Molecular & cellular proteomics: MCP. 2012;11:M111 014050. doi: 10.1074/mcp.M111.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Little RJAaRDB. Statistical Analysis with Missing Data. New York: John Wiley; 2002. [Google Scholar]
- 41.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nature protocols. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mi H, Muruganujan A, Thomas PD. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic acids research. 2013;41:D377–386. doi: 10.1093/nar/gks1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mann HB, Whitney DR. On a test of whether one of two random variables is stochostically larger than the other. Ann Math Statist. 1947;18:50–60. [Google Scholar]
- 44.Majewski N, Nogueira V, Bhaskar P, Coy PE, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Molecular cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Sun L, Shukair S, Naik TJ, Moazed F, Ardehali H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Molecular and cellular biology. 2008;28:1007–1017. doi: 10.1128/MCB.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends in biochemical sciences. 2005;30:142–150. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Graham JW, Williams TC, Morgan M, Fernie AR, et al. Glycolytic enzymes associate dynamically with mitochondria in response to respiratory demand and support substrate channeling. The Plant cell. 2007;19:3723–3738. doi: 10.1105/tpc.107.053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Hassan A, Zubairu S, Hothersall JS, Greenbaum AL. Age-related changes in enzymes of rat brain. 1. Enzymes of glycolysis, the pentose phosphate pathway and lipogenesis. Enzyme. 1981;26:107–112. doi: 10.1159/000459157. [DOI] [PubMed] [Google Scholar]
- 49.Sgarbi G, Matarrese P, Pinti M, Lanzarini C, et al. Mitochondria hyperfusion and elevated autophagic activity are key mechanisms for cellular bioenergetic preservation in centenarians. Aging. 2014;6:296–310. doi: 10.18632/aging.100654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navarro A, Sanchez Del Pino MJ, Gomez C, Peralta JL, Boveris A. Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. American journal of physiology Regulatory, integrative and comparative physiology. 2002;282:R985–992. doi: 10.1152/ajpregu.00537.2001. [DOI] [PubMed] [Google Scholar]
- 51.Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. American journal of physiology Regulatory, integrative and comparative physiology. 2004;286:R505–511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- 52.Gilmer LK, Ansari MA, Roberts KN, Scheff SW. Age-related changes in mitochondrial respiration and oxidative damage in the cerebral cortex of the Fischer 344 rat. Mechanisms of ageing and development. 2010;131:133–143. doi: 10.1016/j.mad.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Austin S, St-Pierre J. PGC1alpha and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. Journal of cell science. 2012;125:4963–4971. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- 54.Izzo A, Manco R, Bonfiglio F, Cali G, et al. NRIP1/RIP140 siRNA-mediated attenuation counteracts mitochondrial dysfunction in Down syndrome. Human molecular genetics. 2014 doi: 10.1093/hmg/ddu157. [DOI] [PubMed] [Google Scholar]
- 55.Li F, Wang Y, Zeller KI, Potter JJ, et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Molecular and cellular biology. 2005;25:6225–6234. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kriaucionis S, Paterson A, Curtis J, Guy J, et al. Gene expression analysis exposes mitochondrial abnormalities in a mouse model of Rett syndrome. Molecular and cellular biology. 2006;26:5033–5042. doi: 10.1128/MCB.01665-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. The Journal of biological chemistry. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holmstrom KM, Baird L, Zhang Y, Hargreaves I, et al. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biology open. 2013;2:761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integrative and comparative biology. 2010;50:829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vartak R, Porras CA, Bai Y. Respiratory supercomplexes: structure, function and assembly. Protein & cell. 2013;4:582–590. doi: 10.1007/s13238-013-3032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.