Abstract
Purpose of Review
Several mutations in the apoB, PCSK9 and MTP genes result in low or absent levels of apoB and LDL-C in plasma which cause familial hypobetalipoproteinemia (FHBL) and abetalipoproteinemia (ABL). Mutations in the ANGPTL3 gene cause familial combined hypolipidemia. Clinical manifestations range from none to severe, debilitating and life-threatening disorders. This review summarizes recent genetic, metabolic and clinical findings and presents an update on management strategies.
Recent findings
Cases of cirrhosis and hepatocellular carcinoma have now been identified in heterozygous FHBL probably due to decreased triglyceride transport capacity from the liver. ANGPTL3 mutations cause low levels of LDL-C and low HDL-C in compound heterozygotes and homozygous subjects, decrease reverse cholesterol transport and lower glucose levels. The effect on atherosclerosis is unknown; however, severe fatty liver has been identified. Loss of function mutations in PCSK-9 cause FHBL which appears to lower risk for CAD and have no adverse sequelae. Phase III clinical trials are now underway examining the effect of PCSK-9 inhibitors on cardiovascular events in combination with statin drugs.
Summary
Mutations causing low LDL-C and apoB have provided insight into lipid metabolism, disease associations and the basis for drug development to lower LDL-C in disorders causing high levels of cholesterol. Early diagnosis and treatment is necessary to prevent adverse sequelae from FHBL and ABL.
Keywords: Hypobetalipoproteinemia, abetalipoproteinemia, combined hypolipidemia, angiopoietin-like 3 protein, PCSK9
Introduction
Cholesterol (C) and triglycerides (TG) are almost insoluble in plasma; therefore, they are transported in spherical lipoprotein particles which contain a central core of varying amounts of nonpolar lipids, TG and cholesterol ester (CE), covered on the surface by polar lipids comprised of phospholipids, one or more apolipoproteins (apo) and unesterified cholesterol [1]. ApoB exists in two isoforms in plasma, apoB-100 and apoB-48, both of which are products of the same structural gene on chromosome 2p24-p23 [2). ApoB-100 is synthesized by the liver and secreted in the form of very low-density lipoprotein (VLDL), a TG-rich-lipoprotein [3]. Produced in the intestine in response to dietary fat, chylomicrons (CM) contain apoB-48, the amino terminal 48% of apoB-100, which is produced by a premature stop codon at the apoB-100 codon 2153 by tissue specific mRNA processing in the intestine [3]. Microsomal triglyceride transfer protein (MTP) transfers TGs from the cytosol to the endoplasmic reticulum containing nascent apoB during the assembly of CM and VLDL in enterocytes and hepatocytes, respectively [4]. In the plasma, both CM and VLDL particles adhere to glycosaminoglycan molecules on endothelial cells of capillaries, primarily in muscle, lung and adipose tissue [5], where interaction with lipoprotein lipase (LPL) and glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 results in hydr olysis of VLDL and CM TGs to free fatty acids and glycerol, a process forming smaller particles termed “VLDL remnants” or intermediate density lipoprotein (IDL]) and CM remnants, respectively [6,7]. IDL is further delipidated to form CE-rich, low-density lipoprotein (LDL), the major cholesterol carrying lipoprotein in normal human plasma. ApoB-100 is the main structural protein of LDL and contains the LDL-receptor-binding domain; therefore, LDL is removed from the circulation by binding mainly to hepatic LDL receptors [2]. Proprotein convertase subtilisin kexin 9 (PCSK9) is a secreted serine protease that enhances the degradation of the LDL-receptor and thus increases levels of LDL-C in plasma. Angiopoietin-like protein 3 (ANGPTL3) inhibits lipoprotein and endothelial lipases, thus decreasing levels of triglyceride, LDL-C and HDL (high density lipoprotein)-C in the plasma.
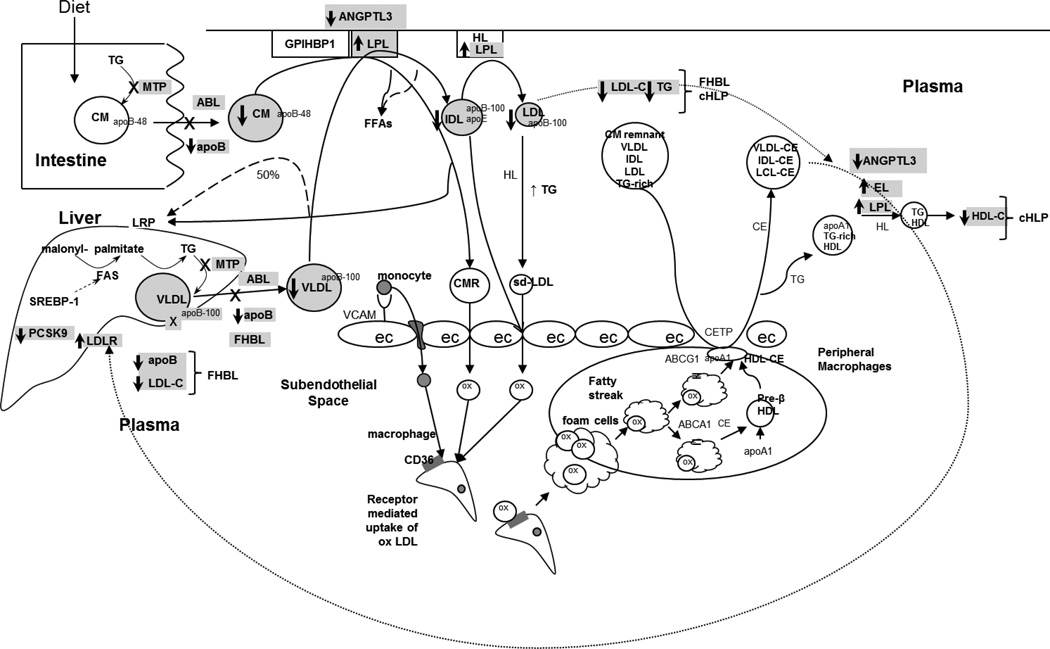
Several mutations in the apoB, MTP and PCSK9 genes result in low or absent levels of apoB and LDL-C in plasma and cause familial hypobetalipoproteinemia (FHBL) and abetalipoproteinemia (ABL). Mutations in the ANGPTL3 gene cause familial combined hypolipidemia. Figure 1 provides an overview of lipid metabolism and the pathways involved in these syndromes. This review summarizes recent genetic, metabolic and clinical findings and presents an update on management strategies.
Figure 1.
Overview of lipid metabolism showing location and mechanisms of the various mutations in the apolipoprotein (apo) B, microsomal triglyceride transfer protein (MTP), proprotein convertase subtilisin kexin 9 (PCSK9) and angiopoietin-like protein 3 (ANGPTL3) genes which cause familial hypobetalipoproteinemia (FHBL), combined hypolipidemia (cHLP) and abetalipoproteinemia (ABL). Briefly, MTP catalyzes transfer of triglyceride (TG) onto the nascent apoB particle in intestinal and hepatic cells, forming chylomicrons (CM) and very low-density lipoprotein (VLDL), respectively. Mutations in MTP decrease this transfer and therefore, decrease the formation of CM and VLDL, a process leading to near absence of LDL-C and apoB in the plasma and causing ABL. Mutations in the apoB gene decrease formation and secretion of VLDL apoB-100 from the liver which leads to decreased levels of LDL-C and causes FHBL. Loss of function mutations in PCSK9 decrease the degradation of the LDL-receptor (LDL-R) and thus make more LDL-R available on the surface of the liver, thereby lowering LDL-C levels and causing FHBL. Loss of function mutations in ANGPTL3 cause increased activity of lipoprotein lipase (LPL) and endothelial lipase (EL) and thus lower the levels of LDL-C, TG and HDL-C, leading to combined hypolipidemia.
X denotes a mutation or loss of a pathway; ec: endothelial cell; CMR: chylomicron remnant; ox: oxidized; CE: cholesterol ester; CETP: cholesterol ester transfer protein.
Familial Hypobetalipoproteinemia
FHBL is an autosomal codominant disorder characterized by apoB < 5th percentile and LDL-C usually between 20–50 mg/dL [8]. Over 60 mutations producing truncations in the apoB gene ranging from apoB2 to apoB-89 have been identified as causes of FHBL [9]. Truncations are named according to the percent length of the native apoB-100 molecule, i.e., apoB-67 is the amino terminal 67% of apoB-100 [10]. Although the low levels of LDL-C would be predicted to prevent the development of coronary artery disease (CAD), CAD has been described in some individuals [11].
Although there is one normal allele in heterozygous FHBL, plasma apoB-100 levels are approximately 24% of normal rather than the predicted 50% [12]. Stable isotope studies have shown that these lower than expected levels result from a 74% lower secretion rate of VLDL apoB-100 from the liver, decreased production of LDL apoB-100, increased catabolism of VLDL and extremely low secretion of the truncated apoB [12–16]. This decreased secretion of apoB from the liver results in decreased triglyceride export from the liver, which in turn leads to the development of fatty liver [17]. Therefore, hepatic steatosis and mild elevation of liver enzymes are the main clinical manifestations of heterozygous FHBL although oral fat intolerance and intestinal fat malabsorption have been infrequently reported.
FHBL, Hepatic Steatosis and Insulin Sensitivity
Nonalcoholic fatty liver disease is an accumulation of > 5% of total liver weight of TG in the hepatocytes and can range from simple fatty liver, which is accumulation of TGs in hepatocytes in the absence of liver damage or inflammation, to nonalcoholic steatohepatitis in which necroinflammation, fibrosis and/or cirrhosis can be present. Factors which affect hepatic fat content include nutrient intake, alcohol, abdominal obesity, dyslipidemia, insulin resistance and type 2 diabetes. FHBL also appears to be a cause of increased hepatic fat content. In 32 subjects with FHBL, average hepatic fat content measured by MR spectroscopy was 14.8% + 12.0% compared to 5.2% + 5.9%, respectively, for 33 normolipidemic controls matched for age, sex and indices of adiposity [17). Hepatic TG was 3-fold higher in subjects heterozygous for inactivating mutations in apoB compared to healthy controls [18]. Hepatic steatosis in FHBL is due to defective export of TG from the liver in the absence of other metabolic derangements; therefore, FHBL is a good model to examine whether isolated hepatic fat causes insulin resistance. Thirty children with NAFLD had statistically significantly higher insulin resistance and homeostatic model assessment of insulin resistance (HOMA) than 30 children with FHBL all of whom had hepatic steatosis but did not have obesity or other metabolic derangements [19**]. In a second study, seven men with FHBL had similar basal endogenous glucose production and glucose levels but higher insulin levels compared to seven healthy matched controls [20]. Insulin-mediated suppression of endogenous glucose production, insulin-mediated peripheral glucose uptake, fatty acids and lipolysis (glycerol turnover) were all similar. The higher insulin levels were thought related to impaired insulin clearance, a finding observed in another study of non-diabetic subjects with hepatic fat [21]. In a third study, subjects with FHBL had normal HOMA-IR [22]. Taken together, these results would suggest that intraheptic TG accumulation causing moderate to severe hepatic steatosis in FHBL does not cause hepatic or peripheral (muscle or adipose tissue) insulin resistance.
FHBL, Hepatic Cirrhosis and Hepatocarcinoma
Fatty liver in FHBL does not appear to have a benign course. In the study of children, those with FHBL had a higher fibrosis index at liver biopsy than those with NAFLD [19**]. Steatohepatitis, cirrhosis and hepatocellular carcinoma have all been described in subjects with truncated apoBs [23–25]; therefore, TG accumulation can lead to progression of liver disease.
Recently, Cefalu et al. [26**] described a novel nonsense mutation in exon 26 of the apoB gene (c.6718>T, p.K224OX) which segregated with low LDL-C in 10 members of a kindred. Of the 10 subjects with the mutation, one had liver cirrhosis, six had fatty liver, two did not have fatty liver and one had not been evaluated. Four members had died from hepatocarcinoma which segregated in a codominant fashion, but their lipids and mutation status were unknown. A member of the apoB-67 kindred recently died from hepatocellular carcinoma (personal communication Welty).
Proprotein Convertase Subtilisin Kexin 9 Gene (PCSK9) Mutations
PCSK9 is a secreted serine protease that enhances the degradation of the LDL-receptor. Loss of function mutations in the PCSK9 gene prevent the PCSK9-mediated degradation of the LDL receptor and thus increase uptake of LDL by the liver, a process leading to a 30 to 70% reduction in plasma LDL-C levels and FHBL. In contrast to apoB mutations, PCSK9 mutations are not associated with any clinical symptoms and have been associated with a decreased risk of cardiovascular disease [27]. Large scale, phase III clinical trials of humanized monoclonal antibodies to PCSK9 with major cardiovascular events as endpoints have begun enrolling subjects over the past year to determine whether PCSK9 inhibitors will have an additional benefit when added to statin drugs in decreasing CVD events [28**].
Familial Combined Hypolipidemia
Angiopoietin-like protein 3 (ANGPTL3) is a 460 amino acid protein which inhibits lipoprotein and endothelial lipases; therefore, deficiency of ANGPTL3 would be predicted to increase activity of LPL and EL, thus accelerating catabolism of VLDL and HDL, respectively, and leading to lower levels of both LDL-C and HDL-C. Loss of function mutations in the ANGPTL3 gene have been associated with recessive familial combined hypolipidemia. Heterozgotes for either of two nonsense mutations in the first exon of ANGPTL3, S17X and E129X, had intermediate levels of LDL-C and triglycerides whereas compound heterozygotes had a marked reduction in HDL-C in addition to a reduction in LDL-C and triglyceride [29].
Homozygous or compound heterozygotes for loss of function mutations (p.G400VfsX5, p.I19LfsX22/p.N147X) in exon 1 of ANGPTL3 had no ANGPTL3 in plasma and marked reductions of triglyceride-containing lipoproteins and HDL particles containing only apoA-I and pre-B-HDL [30*]. Moreover, reverse cholesterol efflux via ABCA1, ABCG1 and SR-BI pathways was reduced and directly correlated with the plasma HDL-C level; however, there was no clinical evidence for atherosclerosis. Of note, heterozygotes had LDL- C below the 25th percentile but normal HDL-C levels.
The prevalence of ANGPTL3 mutations causing combined hypolipidemia in patients with primary hypobetalipoproteinemia was found to be about 10% in two cohorts of 913 Italians and Americans [31*]. When the HDL-C was below the 2nd decile, no apoB gene mutations were found whereas when the HDL-C was above the 2nd decile, the prevalence of apoB mutations was 6.8% and 7.9%, respectively, in the Italian and American cohorts. These findings suggest that when HDL-C is low in FHBL, ANGPTL3 mutations are more likely whereas when HDL-C is normal, apoB mutations are more likely. Severe fatty liver was detected in one of 3 patients with ANGPTL3 mutation carriers; therefore, similar to heterozygous FHBL due to truncated apoBs, significant liver problems can occur.
ANGPTL3 S17X
Minicocci et al. [32*] identified ANGPTL3 S17X as a cause of FHBL in 9.4% of the population of Campodimele, Italy. Homozygous subjects had a 48% reduction in LDL-C, 62% reduction in TG, 46% reduction in HDL-C, 44% reduction in apoB and 48% reduction in apoA-I. Heterozygous subjects had reductions in TC and HDL-C but not LDL-C or triglyceride. No difference in elevated liver enzymes was noted in carriers compared to noncarriers. Homozygotes had a higher mean lanosterol to total cholesterol ratio, a marker of cholesterol absorption, but the lathosterol to total cholesterol ratio, a marker of cholesterol synthesis, was not different. Five S17X homozygotes had significantly lower blood glucose levels, a reduction in size and triglyceride content of VLDL and significantly higher lipoprotein lipase activity and mass in postheparin plasma than 17 heterozygotes and 22 noncarriers [33**]. Compared with heterozygotes and noncarriers, plasma free fatty acid, insulin, glucose and HOMA were significantly lower in homozygous subjects, a finding which could be due to decreased mobilization of free fatty acids from human adipose tissue, thus, resulting in a reduced supply of free fatty acids to the liver and consequently, reduced hepatic VLDL synthesis and secretion. Taken together, the ANGPTL3 S17X mutation may improve insulin sensitivity and lower glucose levels by lowering the supply of free fatty acids and increasing lipolysis of VLDL by increasing LPL activity.
Abetalipoproteinemia
ABL is a rare autosomal-recessive disease that is characterized by very low plasma concentrations of TG and cholesterol (under 30 mg/dl) and undetectable levels of LDL and apoB. Encoded by the microsomal triglyceride transfer protein (MTP) gene on chromosome 4q22-24, MTP is a heterodimer of a large 97 kDa M subunit and a 55 kDa protein disulfide isomerase resident lipid transfer protein within the endoplasmic reticulum of hepatocytes and enterocytes. MTP catalyzes the transfer of TG to the nascent apoB particle in the assembly of VLDL TG in the rough endoplasmic reticulum while apoB is being cotranslationally translocated across the endoplasmic reticulum membrane. Over 30 mutations in the MTP gene have been described for ABL [34, 35*). Inhibition of MTP leads to impairment of the assembly of VLDL TG particles, similar to FHBL, with a subsequent decrease in VLDL TG secretion from the liver and accumulation of TG in hepatic cells leading to hepatic steatosis. An inhibitor of MTP, lomitapide, is now approved as an adjunctive treatment of homozygous familial hypercholesterolemia although subjects do develop hepatic steatosis; therefore, monitoring of these patients is necessary [36*].
One of the antiatherosclertic properties of HDL is an antiaggregatory effect on platelets. HDL particles from five patients with ABL or homozygous hypobetalipoproteinemia (HHBL) had an 11-fold higher concentration of malondialdehyde and a 40% decrease in alpha-tocopherol; therefore, the HDL particles were highly oxidized [37*]. Isolated platelets from the plasma of ABL patients aggregated 4–10 times more than platelets from control subjects. In contrast, no difference in aggregation occurred between platelet-rich plasma from ABL subjects and controls, a finding suggesting a protective factor in ABL plasma. HDL from ABL plasma inhibited agonist-induced platelet aggregation by binding to SR-BI whereas control HDL did not, a finding suggesting that ABL HDL may inhibit platelet aggregation. Taken together, these findings suggest that in vivo oxidized HDLs in ABL maintain their antiaggregatory properties despite oxidation.
Homozygous Hypobetalipoproteinemia (HHBL)
HHBL is a rare disorder in which subjects are either homozygous or compound heterozygotes for mutations in the apoB gene. The clinical presentation is similar to ABL (see below).
Diagnosis and Management
Early diagnosis of both ABL and HHBL is important in order to prevent the devastating clinical sequelae. Acanthocytosis of red blood cells on the peripheral blood smear is a distinguishing feature. During the first and second decade of life, symptoms include oral fat intolerance, steatorrhea, diarrhea, fat malabsorption, lipid accumulation in enterocytes, failure to thrive and deficiency of fat-soluble vitamins A and E. Deficiency of vitamin E leads to the most debilitating clinical manifestations which are neurological disorders which lead to progressive degeneration of the central nervous system and death. Unless treated early with vitamin E, subjects develop atypical retinitis pigmentosa, spinocerebellar degeneration with ataxia and a bleeding diathesis secondary to malabsorption of fat-soluble vitamins. Table 1 outlines diagnostic and investigative testing for ABL and HHBL, and Table 2 outlines a treatment strategy [35*]. Appropriate treatment can prevent neurological sequelae; therefore, early diagnosis is important so that treatment can be started including a low-fat diet, supplementation with essential fatty acids and high oral doses of fat soluble vitamins, vitamins A and E.
Table 1.
Abetalipoproteinemia and Homozygous Hypobetalipoproteinemia Follow-up Outline
| Clinical Evaluation- Every 6–12 months |
Laboratory investigations- Every year |
||
|---|---|---|---|
| General | • Height/weight for growth Curve | Lipids* | • Total cholesterol |
| • Triglyceride | |||
| Diet | • Adequate caloric intake | • LDL-C | |
| • Low-fat (<30 % total calories) diet with EFA supplements | • HDL-C | ||
| • MCTG intake generally not required | • apoB | ||
| • Vitamin supplementation | • apoA1 | ||
| GI | • Appetite | Hepatic | • AST |
| • Diarrhea | • ALT | ||
| • Vomiting | • GGT | ||
| • Esophagitis | • Total and direct bilirubin | ||
| • Abdominal distention | • ALP | ||
| • Hepatomegaly | • Albumin | ||
| Neurological | • Expected development for age | Vitamins | • Beta-carotene |
| • Ataxia | • 25-OH Vitamin D | ||
| • Dysarthria | • Plasma or RBC Vitamin E | ||
| • Hyporeflexia | • INR | ||
| • Proprioception loss | |||
| • Muscle pain or weakness | Other | • CBC | |
| • Vitamin B12 | |||
| • Folate | |||
| • Calcium | |||
| • Phosphate | |||
| • Uric acid | |||
| • Thyroid Stimulating Hormone | |||
| Additional investigations: Age>10 Years | |||
| Hepatic ultrasonography | Every 3 years | ||
| Neurological examination | Every 6–12 months | ||
| Ophthalmological examination | Every 6–12 months | ||
| Bone mineral density via DXA | Every 3 years | ||
| Echocardiography | Every 3 years | ||
Abbreviations: EFA, essential fatty acid;MCTG, medium chain triglyceride; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; apo, apolipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyl transferase; ALP, alkaline phosphatase; RBC, red blood cell; INR, international normalized ratio; CBC, complete blood count; DXA, dual energy X-ray Absorptiometry.
Lipid profile should be performed at baseline, but yearly follow-up is not essential as these levels typically remain stable over the long term.
Reproduced from Lee and Hegele. J Inherit Metab Dis (35*).
Table 2.
Special Dietary Counseling and Treatment
| General Lipids |
|
| Vitamins |
|
Reproduced from Lee and Hegele. J Inherit Metab Dis (35).
Heterozygous FHBL is often asymptomatic and not diagnosed unless a lipid profile is obtained. If significantly low levels of LDL-C and apoB are found, the lab evaluations shown in Table 1 should be considered [35*]. The recent reports of cirrhosis and hepatocellular carcinoma associated with FHBL suggest that liver enzymes should be monitored in subjects with FHBL and if elevated, hepatic imaging be considered [25, 26**]. Recently, a subject with the apoB-67 mutation died from rapidly progressive hepatocellular carcinoma. The apoB-67 kindred has at least 126 affected members. Formal liver function testing and imaging in this large kindred should provide ample opportunity for identification of factors contributing to progression of fatty liver to cirrhosis and liver cancer in FHBL.
Conclusion
Although deficiency of apoB and LDL in plasma may lower the risk of CAD, clinical manifestations include the debilitating neurological complications observed in ABL and HHBL and hepatic steatosis and hepatocellular carcinoma observed in subjects with heterozygous FHBL. Thus, early diagnosis, treatment and appropriate follow-up are essential. The various mutations causing FHBL and ABL have provided insight into aspects of lipid metabolism, disease associations and function of lipoproteins including HDL. Finally, knowledge of the mechanisms of the mutations causing low levels of apoB and LDL-C has provided the basis for drug development including the PCSK9 inhibitors, which are now in Phase III clinical trials in subjects with hypercholesterolemia, and the MTP inhibitor, lomitapide, for homozygous familial hypercholesterolemia.
Key points.
The recent reports of cirrhosis and hepatocellular carcinoma associated with FHBL are probably due to decreased TG export from the liver and suggest that liver enzymes should be monitored in subjects with FHBL and if elevated, hepatic imaging be considered
Severe fatty liver was detected in one of 3 ANGPTL3 mutation carriers; therefore, similar to heterozygous FHBL due to truncated apoBs, significant liver problems can occur.
Loss of function mutations in PCSK9 cause FHBL and appear to lower risk for CAD without adverse sequelae; therefore, phase III clinical trials are now underway examining the effect of PCSK9 inhibitors on cardiovascular events in combination with statin drugs.
In vivo oxidized HDLs in ABL maintain their antiaggregatory properties despite oxidation.
Early diagnosis of ABL and HHBL is important in order to institute treatment with Vitamins E and A to prevent devastating neurological consequences including spinocerebellar degeneration with ataxia and retinitis pigmentosa.
Acknowledgements
Dr. Welty is supported by National Institute of Health grants, NHLBI P50 HL083813 and DE018184.
Abbreviations
- C
Cholesterol
- TG
Triglyceride
- CE
Cholesterol Ester
- Apo
Apolipoprotein
- VLDL
Very Low-Density Lipoprotein
- CM
Chylomicron
- MTP
Microsomal triglyceride transfer protein
- LPL
Lipoprotein Lipase
- IDL
Intermediate Density Lipoprotein
- LDL
Low-Density Lipoprotein
- HDL
High-Density Lipoprotein
- PCSK9
Proprotein Convertase Subtilisin Kexin 9
- ANGPTL3
Angiopoietin-like protein 3
- FHBL
Familial Hypobetalipoproteinemia
- ABL
Abetalipoproteinemia
- CAD
Coronary artery disease
- HOMA
Homeostatic Model Assessment of Insulin Resistance
- HHBL
Homozygous hypobetalipoproteinemia
Footnotes
Conflict of Interest: None
References and recommended reading
- 1.Havel RJ, Kane JP. Introduction: structure and metabolism of plasma lipoproteins. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 7th ed. New York, NY: McGraw-Hill; 1995. pp. 1841–1851. [Google Scholar]
- 2.Young SG. Recent progress in understanding apolipoprotein B. Circulation. 1990;82(5):1574–1594. doi: 10.1161/01.cir.82.5.1574. [DOI] [PubMed] [Google Scholar]
- 3.Chen SH, Habib G, Yang CY, et al. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987;238(4825):363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- 4.Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, et al. The role of the microsomal triglyceride transfer protein in abetalipoproteinemia. Annu Rev Nutr. 2000;20:663–697. doi: 10.1146/annurev.nutr.20.1.663. [DOI] [PubMed] [Google Scholar]
- 5.Weisgraber KH, Rall SC., Jr Human apolipoprotein B-100 heparin-binding sites. J Biol Chem. 1987;262(23):11097–11103. [PubMed] [Google Scholar]
- 6.Sehayek E, Lewin-Velvert U, Chajek-Shaul T, Eisenberg S. Lipolysis exposes unreactive endogenous apolipoprotein E-3 in human and rat plasma very low density lipoprotein. J Clin Invest. 1991;88(2):553–560. doi: 10.1172/JCI115339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigneux AP, Davies BS, Gin P, et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5(4):279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linton MF, Farese RV, Jr, Young SG. Familial hypobetalipoproteinemia. J Lipid Res. 1993;34(4):521–541. [PubMed] [Google Scholar]
- 9.Burnett JR, Bell DA, Hooper AJ, Hegel RA. Clinical utility gene card for: Familial hypobetalipoproteinaemia (APOB) Eur J Hum Genet. 2012;20 doi: 10.1038/ejhg.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welty FK, Hubl ST, Pierotti VR, Young SG. A truncated species of apolipoprotein B (B67) in a kindred with familial hypobetalipoproteinemia. J Clin Invest. 1991;87(5):1748–1754. doi: 10.1172/JCI115193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welty FK, Ordovas J, Schaefer EJ, et al. Identification and molecular analysis of two apoB gene mutations causing low plasma cholesterol levels. Circulation. 1995;92(8):2036–2040. doi: 10.1161/01.cir.92.8.2036. [DOI] [PubMed] [Google Scholar]
- 12.Welty FK, Lichtenstein AH, Barrett PH, et al. Decreased production and increased catabolism of apolipoprotein B-100 in apolipoprotein B-67/B-100 heterozygotes. Arterioscler Thromb Vasc Biol. 1997;17(5):881–888. doi: 10.1161/01.atv.17.5.881. [DOI] [PubMed] [Google Scholar]
- 13.Welty FK, Lichtenstein AH, Barrett PH, et al. Production of apolipoprotein B-67 in apolipoprotein B-67/B-100 heterozygotes: technical problems associated with leucine contamination in stable isotope studies. J Lipid Res. 1997;38(8):1535–1543. [PubMed] [Google Scholar]
- 14.Elias N, Patterson BW, Schonfeld G. Decreased production rates of VLDL triglycerides and ApoB-100 in subjects heterozygous for familial hypobetalipoproteinemia. Arterioscler Thromb Vasc Biol. 1999;19(11):2714–2721. doi: 10.1161/01.atv.19.11.2714. [DOI] [PubMed] [Google Scholar]
- 15.Aguilar-Salinas CA, Barrett PH, Parhofer KG, et al. Apoprotein B-100 production is decreased in subjects heterozygous for truncations of apoprotein B. Arterioscler Thromb Vasc Biol. 1995;15(1):71–80. doi: 10.1161/01.atv.15.1.71. [DOI] [PubMed] [Google Scholar]
- 16.Parhofer KG, Barrett PH, Aguilar-Salinas, Schonfeld G. Positive linear correlation between the length of truncated apolipoprotein B and its secretion rate: in vivo studies in human apoB89, apoB-75, apoB-54.8, and apoB-31 heterozygotes. J Lipid Res. 1996;37(4):844–852. [PubMed] [Google Scholar]
- 17.Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J Lipid Res. 2004;45(5):941–947. doi: 10.1194/jlr.M300508-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Schonfeld G, Patterson BW, Yablonskiy DA, et al. Fatty liver in familial hypobetalipoproteinemia: triglyceride assembly into VLDL particles is affected by the extent of hepatic steatosis. J Lipid Res. 2003;44(3):470–478. doi: 10.1194/jlr.M200342-JLR200. [DOI] [PubMed] [Google Scholar]
- 19. Della Corte C, Fintini D, Giordano U, et al. Fatty liver and insulin resistance in children with hypobetalipoproteinemia: the importance of aetiology. Clinical Endocrinology. 2013;79:49–54. doi: 10.1111/j.1365-2265.2012.04498.x. This study showed that fatty liver due to familial hypobetalipoproteinemia in the absence of overweight or other metabolic derangements was not associated with insulin resistance in children.
- 20.Visser ME, Lammers NM, Nederveen AJ, et al. Hepatic steatosis does not cause insulin resistance in people with familial hypobetalipoproteinaemia. Diabetologia. 2011;54:2113–2121. doi: 10.1007/s00125-011-2157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotronen A, Vehkavaara S, Seppala-Lindroos A, et al. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293:E1709–E1715. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
- 22.Lonardo A, Lombardini S, Scaglioni F, et al. Hepatic steatosis and insulin resistance: does etiology make a difference? Journal of Hepatology. 2006;44:190–196. doi: 10.1016/j.jhep.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Tarugi P, Lonardo A. Heterozygous familial hypobetalipoproteinemia associated with fatty liver. Am J Gastroenterol. 1997;92:1400–1402. [PubMed] [Google Scholar]
- 24.Bonnefont-Rousselot D, Condat B, Sassolas A, et al. Cryptogenic cirrhosis in a patient with familial hypocholesterolemia due to a new truncated form of apolipoprotein B. Eur J Gastroenterol Hepatol. 2009;21:104–108. doi: 10.1097/MEG.0b013e3282ffd9f8. [DOI] [PubMed] [Google Scholar]
- 25.Lonardo A, Tarugi P, Ballarini G, Bagni A. Familial heterozygous hypobetalipoproteinemia, extrahepatic primary malignancy, and hepatocellular carcinoma. Dig Dis Sc. 1998;43:2489–2492. doi: 10.1023/a:1026646618643. [DOI] [PubMed] [Google Scholar]
- 16. Cefalu AB, Pirruccello JP, Noto D, et al. A novel apoB mutation identified by exome sequencing cosegregates with steatosis, liver cancer and hypocholesterolemia. Arterioscler Thromb Vasc Biol. 2013;33:2021–2025. doi: 10.1161/ATVBAHA.112.301101. Exome sequencing was used to identify a novel nonsense mutation, A>T in exon 26 of apoB (c.6718A>T) which cosegregates with familial hypobetalipoproteinemia, hepatic steatosis and 4 cases of hepatocarcinoma in a large kindred.
- 27.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 28. Hooper AJ, Burnett PC. Anti-PCSK9 therapies for the treatment of hypercholesterolemia. Expert Opin Biol Ther. 2013;13:429–435. doi: 10.1517/14712598.2012.748743. Excellent review summarizing the latest findings in clinical trials of PCSK9 inhibitors, including antibodies, gene silencing and small peptides highlighting PCSK9 inhibition as an effective strategy for lowering plasma LDL-cholesterol.
- 29.Musunuru K, Pirruccello JP, Do R, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010;363:2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pisciotta L, Favari E, Magnolo L, et al. Characterization of three kindreds with familial combined hypolipidemia caused by loss-of function mutations of ANGPTL3. Circ Cardiovasc Genet. 2012;5:42–50. doi: 10.1161/CIRCGENETICS.111.960674. Sera from patients with familial combined hypolipidemia had a reduced capacity for reverse cholesterol efflux, but there was no clinical evidence of atherosclerosis.
- 31. Noto D, Cefalu AB, Valenti V, et al. Prevalence of ANGPTL3 and APOB gene mutations in subjects with combined hypolipidemia. Arterioscler Thromb Vasc Biol. 2012;32:805–809. doi: 10.1161/ATVBAHA.111.238766. HDL-cholesterol levels may be used to distinguish between ANGPTL3 and APOB mutations as causes of hypocholesterolemia.
- 32. Minicocci I, Montali A, Robciuc MR, et al. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: a clinical and biochemical characterization. J Clin Endocrinol Metab. 2012;97:E1266–E1275. doi: 10.1210/jc.2012-1298. In a study of 60 subjects either heterozygous or homozygous for the ANGPTL3 S17X mutation in Campodimele, Italy, homozygosity was associated with a combined hypolipidemia whereas heterozygotes had a reduction in only total cholesterol and HDL-C compared with noncarriers.
- 33. Robciuc MR, Maranghi M, Lahikainen A, et al. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler Thromb Vasc Biol. 2013;33:1706–1713. doi: 10.1161/ATVBAHA.113.301397. This is the first study to show that ANGPTL3 mutations may be associated with low levels of glucose.
- 34.Burnett JR, Bell DA, Hooper AJ, Hegele RA. Clinical utility gene card for: Abetalipoproteinaemia. Eur J Hum Genet. 2012;20 doi: 10.1038/ejhg.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee J, Hegele RA. Abetalipoproteinemia and homozygous hypobetalipoproteinemia: a framework for diagnosis and management. J Inherit Metab Dis published online. 2013 Nov;28 doi: 10.1007/s10545-013-9665-4. This is an excellent review providing an algorithm for the diagnosis and management of ABL and HHBL.
- 36. Cuchel M, Meagher EA, du Toit TH, et al. Phase 3 HoFHLomitapide Study investigators. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–46. doi: 10.1016/S0140-6736(12)61731-0. This phase III clinical trial examined the safety and efficacy of an MTP inhibitor.
- 37. Calzada C, Vericel E, Colas R, et al. Inhibitory effects of in vivo oxidized high-density lipoproteins on platelet aggregation: evidence from patients with abetalipoproteinemia. FASEB J. 2013;27:2855–2861. doi: 10.1096/fj.12-225169. This unique study used ABL as an in vivo model to determine that oxidization of HDL does not affect the antiaggregatory properties of HDL.