Abstract
Children with hypodiploid acute lymphoblastic leukemia (ALL) have inferior outcomes despite intensive risk adapted chemotherapy regimens. We describe 78 children with hypodiploid ALL who underwent hematopoietic stem cell transplant (HSCT) between 1990 and 2010. Thirty nine (50%) patients had ≤ 43 chromosomes, 12 (15%) had 44 chromosomes and 27 (35%) had 45 chromosomes. Forty three (55%) patients were transplanted in first remission (CR1) while 35 (45%) were transplanted in ≥CR2. Twenty nine patients (37%) received a graft from a related donor and 49 (63%) from an unrelated donor. All patients received a myeloablative conditioning regimen. The 5-year probabilities of leukemia-free survival (LFS), overall survival (OS), relapse, and treatment related mortality (TRM) for the entire cohort were 51%, 56%, 27% and 22% respectively. Multivariate analysis confirmed that mortality risks were higher for patients transplanted in CR2 (HR 2.16, p=0.05), with chromosome number ≤43 (HR 2.15, p=0.05) and for those transplanted in the first decade of the study period (HR 2.60, p=0.01). Similarly, treatment failure risks were higher with chromosome number ≤43 (HR 2.28, p=0.04) and the earlier transplant period (HR 2.51, p=0.01). Although survival is better with advances in donor selection and supportive care, disease-related risk factors significantly influence transplantation outcomes.
Keywords: Hypodiploid ALL, HSCT
Introduction
Chemotherapy regimens have improved significantly over the last 50 years, and now more than 80% of children with ALL are cured with chemotherapy (1–7). Children with hypodiploid ALL however, continue to have inferior outcomes despite risk adapted intensive chemotherapy treatment. An early report from Children’s Cancer Group (CCG) analyzed a total of 4,986 children treated between 1988 and 1995 on CCG studies. Among these, 1,880 cases had centrally reviewed and accepted cytogenetic data and 110 cases (6%) were classified as hypodiploid. Six-year event-free survival (EFS) was worse in hypodiploid cases than non-hypodiploid patients (58% vs 76% respectively P<0.0001). Six-year EFS estimates for patients with 45 chromosomes were 65%, 33 to 44 chromosomes 40%, and 24 to 28 chromosomes 25% respectively (log rank, P <0.002). Of note, only 23 patients had fewer than 45 chromosomes (8). More recently, a case series of pediatric hypodiploid ALL patients with <45 chromosomes (n=139) treated by 10 different national ALL study groups between 1986 and 1996 (9), reported an 8-year event free survival (EFS) of 38.5%, and overall survival (OS) of 49.8%. Patients with fewer than 44 chromosomes fared significantly worse than those with 44 chromosomes (EFS of 30% vs. 52%, p=0.01, OS 37% vs 69%, p=0.017). Most of the patients received treatment on higher-risk regimens and notably, there were no induction failures, but relapses tended to occur early (within 2 years).
A similar report from the Medical Research Council (MRC) included 226 children and adults treated with chemotherapy between 1990 and 2002 (10). In that report, patients with ≤45 chromosomes were considered hypodiploid. One hundred and twenty one patients had 42–45 chromosomes and had acceptable survival with chemotherapy at 66%. The majority (n=114) of these patients had 45 chromosomes, with only 7 children in the 42–44 chromosome group. In contrast, patients with 25–39 chromosomes had 29% survival at 3 years (p=0.002,) and all but one of 14 near haploid patients died.
The goals of the present study were to describe the outcome of children undergoing related or unrelated donor hematopoietic cell transplantation for hypodiploid ALL (defined as 45 or fewer chromosomes) and to identify disease-related prognostic factors that may affect overall and leukemia-free survival post-transplant.
Patients and Methods
Patients
The Center for International Blood and Marrow Transplant Research is a voluntary working group of more than 450 transplant centers worldwide. Participating centers are required to report all consecutive transplants and compliance was ensured by on site audits. Patients are followed longitudinally until death or lost to follow-up. Patients or their guardians provided written informed consent. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Endpoints
The primary end point was leukemia-free survival, defined as being alive and without leukemia recurrence. Death from any cause or relapse was considered an event (treatment failure). Other outcomes studied included: neutrophil recovery, defined as achieving an absolute neutrophil count ≥ 0.5 × 109/L for 3 consecutive measurements; and platelet recovery defined as platelets ≥ 20 × 109/L without transfusion for 7 days. Diagnoses of grades 2–4 acute graft-versus-host disease (GVHD) and chronic GVHD were based on published criteria (11, 12). Treatment related mortality (TRM) was defined as death not attributed to relapse, and relapse was defined as morphologic recurrence of leukemia. Surviving patients were censored at last follow-up and death from any cause was considered an event.
Statistical Methods
The probabilities of neutrophil and platelet recovery, acute and chronic GVHD, TRM, and relapse were calculated with the use of the cumulative-incidence function method (13). For TRM, relapse was the competing event and for relapse, TRM, the competing event. The probabilities of leukemia-free and overall survival were calculated using the Kaplan-Meier estimator (14). Cox regression multivariate models were built for LFS, OS, TRM and relapse (15). Due to the relatively modest sample size only variables known to influence relapse, TRM, leukemia-free and overall survival were tested: number of chromosomes (43 or less vs. 44 or 45); disease status (CR2/3 vs. CR1); with t(9;22) (yes vs. no); year of transplant (2000–2010 vs. 1990–1999). A p-value of 0.05 or less was considered statistically significant. All p-values are 2-sided, and analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patients, Disease and Transplant Characteristics
Patients and disease characteristics of the 78 patients aged ≤18 years with hypodiploid ALL who received a transplant from HLA-matched siblings, HLA-mismatched relatives, HLA-matched or HLA-mismatched unrelated donors between 1990 and 2010 are shown in Table 1. Twenty-nine of 78 patients received grafts from a related donor and 49 patients received their grafts from an unrelated donor. Median age at transplantation was 10 years (range, 3–18). Fifty percent of patients had 43 or fewer chromosomes, 15% had 44 chromosomes and 35%, 45 chromosomes. Nine of 78 patients were reported to have a Philadelphia chromosome, but only 2 of these patients had 43 or fewer chromosomes. Fifty-five percent of transplantations occurred in CR1 and 38%, in CR2. Among patients transplanted in CR2, 19 of 26 (66%) had a short duration CR1 (CR1 ≤36 months). The median follow-up of surviving patients is 80 (14–240) months.
Table 1.
Patient and Disease Characteristics
| Characteristics | Number (%) |
|---|---|
| Number of patients | 78 |
| Number of centers | 52 |
| Age at transplant (years) | |
| ≤5 | 11 (14) |
| 6–10 | 30 (38) |
| 11–15 | 22 (28) |
| 16–18 | 15 (19) |
| Sex | |
| Male | 47 (60) |
| Female | 31 (40) |
| National Cancer Institute risk group | |
| Good risk | 27 (35) |
| Poor risk | 44 (56) |
| Unknown | 7 (9) |
| Additional cytogenetic abnormalities* | |
| t(9;22) abnormality | 9 (12) |
| t(4,11) | 1 (1) |
| Monosomy 7 | 25 (32) |
| Monosomy 5/del 5q- | 10 (13) |
| Other monosomies | 11(14) |
| Trisomy 8/14 | 12 (15) |
| Lansky performance score prior to transplant | |
| <90 | 9 (12) |
| 90 – 100 | 66 (85) |
| Unknown | 3 (4) |
| Number of chromosomes | |
| ≤ 43 chromosomes | 39 (50) |
| 44 chromosomes | 12 (15) |
| 45 chromosomes | 27 (35) |
| Disease status at transplant | |
| First complete remission | 43 (55) |
| Second complete remission | 29 (38) |
| Third complete remission | 6 (7) |
| Recipient cytomegalovirus serostatus | |
| Negative | 43 (55) |
| Positive | 35 (44) |
Not mutually exclusive. Most patients had more than one cytogenetic abnormality.
Transplant characteristics are summarized in Table 2. Twenty-nine percent of patients received their graft from a matched sibling, 8% from other relatives and 63% from unrelated donors. Donor-recipient pairs considered well matched were defined as having no known disparity at human leukocyte antigen (HLA) -A, -B, -C, and -DRB1; those considered mismatched were defined as having 1, 2 or more disparities (16). Among recipients of matched sibling transplants, 1 received umbilical cord blood and the remaining bone marrow (n=20) or peripheral blood (n=1). The corresponding distribution of graft type for unrelated donor transplantation was 15, 29 and 5, respectively. Over half of unrelated donor transplants were HLA-mismatched. Mismatched related donors received bone marrow (n=5) or peripheral blood (n=1). All recipients received a myeloablative preparative regimen with 95% of patients receiving total body irradiation (TBI)-containing regimens. Similarly, most (86%) received a calcineurin inhibitor containing GVHD prophylaxis.
Table 2.
Transplant Characteristics
| Characteristics | Number (%) |
|---|---|
| Donor and Graft Source | |
| Related | |
| HLA-matched sibling | 23 (29) |
| Other relative | 6 (8) |
| Unrelated | |
| Matched unrelated donor | 18 (23) |
| Mismatched unrelated donor | 16 (21) |
| Matched cord blood | 5 (6) |
| Mismatched cord blood | 10 (13) |
| Conditioning regimen | |
| Total body irradiation + cyclophosphamide | 66 (85) |
| Total body irradiation + other agents | 8 (10) |
| Busulfan + cyclophosphamide | 4 (5) |
| Anti-thymocyte globulin or alemtuzumab | |
| No | 50 (64) |
| Yes | 27 (35) |
| Not reported | 1 (1) |
| GVHD prophylaxis | |
| Ex vivo T-cell depletion | 10 (13) |
| Tacrolimus-containing | 14 (18) |
| Cyclosporine- containing | 53 (68) |
| Not reported | 1 (1) |
| Year of transplant | |
| 1990–1999 | 40 (51) |
| 2000–2010 | 38 (49) |
Outcomes
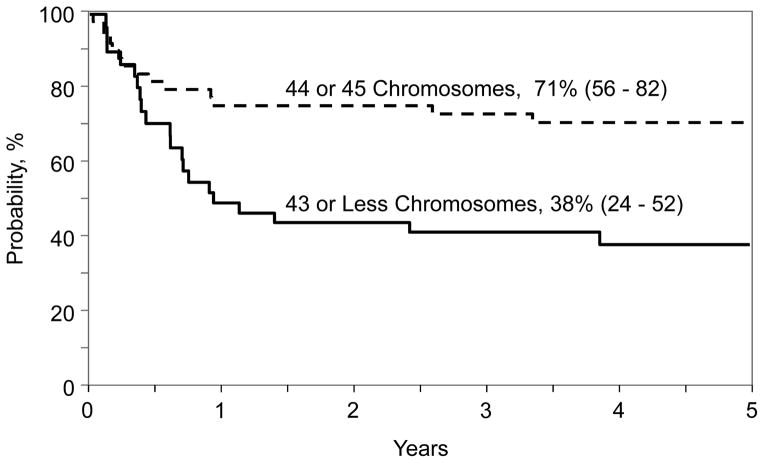
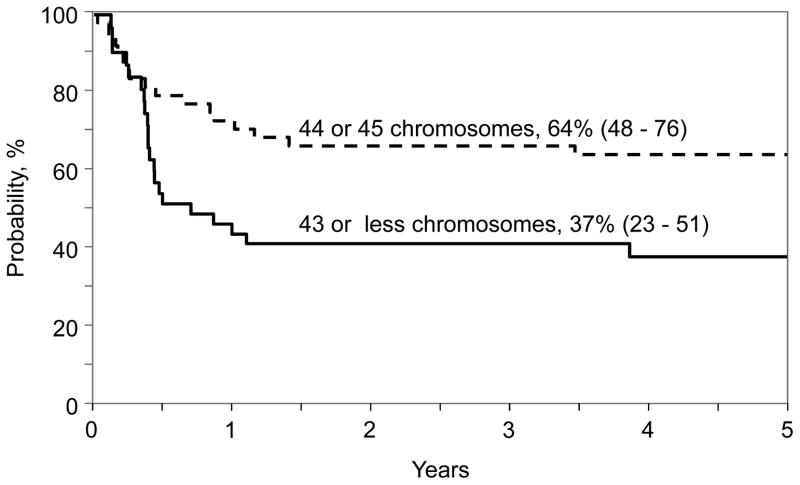
The results of univariate analysis for the entire population are shown in Table 3. The 5-year leukemia-free survival, overall survival, relapse, and TRM were 51%, 56%, 27% and 22% respectively. Results of multivariate analysis for leukemia-free survival, overall survival, relapse and TRM are presented in Table 4. Hypodiploid (chromosome number 43 or less) was associated with higher mortality and treatment failure. Other factors associated with higher mortality included transplantation in second or third CR and transplant period 1990 – 1999. The probability of 5-year overall survival adjusted for disease status and transplant period for patients with chromosome number 43 or less was 38% (95% CI 24–52) and for those with chromosome number 44 or 45, 71% (95% CI 56–82), p=0.001 (Figure 1). Treatment failure (inverse of leukemia-free survival) was higher for transplant period 1990 – 1999. The effect of disease status on treatment failure did not reach the level of significance set for this analysis. The probability of 5-year leukemia-free survival adjusted for disease status and transplant period for patients with chromosome number 43 or less was 37% (95% CI 23–51) and for those with chromosome number 44 or 45, 64% (95% CI 48–76), p=0.01 (Figure 2). None of the variables tested attained the level of significance set for this analyses for TRM and relapse. At the last follow-up (median 80 months; range, 14–240 months), 35 (45%) patients had died. Seventeen (22%) died of recurrent disease, the predominant cause of treatment failure. Other causes of death include GVHD (n=5), infection (n=7), organ failure (n=4) and other causes (n=2). Two patients developed EBV associated lymphoma at 1.5 months and 10 years after transplantation. The patient who developed EBV associated lymphoma at 1.5 months died from an infection at 3 months after transplantation and the other patient is alive, 16 years after transplantation. There were 3 reported second malignant neoplasms: 1 patient with tumor of the parotid gland, 1 with brain tumor and 1 with cancer of the thyroid gland at 5.5 years, 7.5 years and 3.3 years respectively after transplantation. Two of these patients are alive and the patient with brain tumor died a year after diagnosis.
Table 3.
Unadjusted probabilities of outcomes after transplantation for the entire cohort
| Probability (95% confidence interval) | |
|---|---|
| Day-28 neutrophil recovery | 79% (70–87) |
| Day-100 platelet recovery | 78% (67–87) |
| Day-100 grade 2–4 Acute graft vs. host disease | 56% (45–67) |
| 5-year chronic graft vs. host disease | 31% (22–42) |
| 5-year relapse | 27% (18–38) |
| 5-year transplant-related mortality | 22% (14–32) |
| 5-year leukemia-free survival | 51% (40–62) |
| 5-year overall survival | 56% (44–67) |
Table 4.
Results of multivariate analysis
| Hazard ratio (95% confidence interval) | p-value | |
|---|---|---|
| Treatment Related Mortality | ||
| Disease status | ||
| First complete remission | 1.00 | |
| Second/third complete remission | 1.84 (0.60 – 5.60) | 0.28 |
| Number of chromosomes | ||
| 44 or 45 | 1.00 | |
| 43 or less | 1.81 (0.59 – 5.57) | 0.30 |
| Year of transplant | ||
| 2000 – 2010 | 1.00 | |
| 1990 – 1999 | 2.68 (0.93 – 7.75) | 0.06 |
| Relapse | ||
| Disease status | ||
| First complete remission | 1.00 | |
| Second/third complete remission | 2.23 (0.76 – 6.56) | 0.14 |
| Number of chromosomes | ||
| 44 or 45 | 1.00 | |
| 43 or less | 2.82 (0.92 – 8.61) | 0.07 |
| Year of transplant | ||
| 2000 – 2010 | 1.00 | |
| 1990 – 1999 | 2.46 (0.98 – 1.08) | 0.06 |
| Treatment Failure | ||
| Disease status | ||
| First complete remission | 1.00 | |
| Second/third complete remission | 2.02 (0.93 – 4.36) | 0.07 |
| Number of chromosomes | ||
| 44 or 45 | 1.00 | |
| 43 or less | 2.28 (1.04 – 5.01) | 0.04 |
| Year of transplant | ||
| 2000 – 2010 | 1.00 | |
| 1990 – 1999 | 2.51 (1.27 – 5.00) | 0.01 |
| Overall Mortality | ||
| Disease status | ||
| First complete remission | 1.00 | |
| Second/third complete remission | 2.28 (1.02 – 5.10) | 0.04 |
| Number of chromosomes | ||
| 44 or 45 | 1.00 | |
| 43 or less | 2.69 (1.17 – 6.15) | 0.02 |
| Year of transplant | ||
| 2000 – 2010 | 1.00 | |
| 1990 – 1999 | 2.60 (1.27 – 5.31) | 0.01 |
Figure 1.
The 5-year probability of overall survival by chromosome number and adjusted for disease status at transplantation and transplantation period
Figure 2.
The 5-year probability of leukemia-free survival by chromosome number and adjusted for disease status at transplantation and transplantation period
DISCUSSION
The remarkable progress achieved in the treatment of childhood ALL is the result of a series of large-scale clinical studies conducted by cooperative clinical trials groups. In addition, biologic investigations have improved risk group identification (17) and allowed administration of risk-adapted therapy. These advances combined with a better understanding of disease mechanisms and the ability to better target chemotherapy have occurred in parallel with important changes in transplantation techniques, donor availability, donor-recipient HLA match and other supportive care measures. Together, these events have led to changes in indications for, transplantation for childhood ALL as well as associated outcomes. Perhaps the best example of this is the use of the tyrosine kinase inhibitor imatinib in childhood ALL that has led to current data suggesting that transplant is routinely not needed for Philadelphia positive ALL in CR1 (18).
Our goal in the current study was to identify factors that predict for better overall and leukemia-free survival in children with hypodiploid ALL undergoing allogeneic HSCT. Data describing outcomes after transplantation for these patients are sparse. A previous Children’s Oncology group (COG) study described 9 patients who underwent HSCT during the study period of 10 years (9). Patients underwent transplantation in first remission, and 5 had an adverse event after transplantation. The current report with its larger population identified three prognostic factors; 43 or fewer chromosomes, second or subsequent CR and transplantation prior to 2000 as predictive for lower overall survival and 43 or fewer chromosomes and transplantation prior to 2000 predictive for lower leukemia-free survival. Although the risks of leukemia recurrence were higher for patients with 43 or fewer chromosomes, second or subsequent CR and transplantation prior to 2000, this did not reach the level of significance set for the current analysis. The lack of a statistically significant association between these factors and leukemia recurrence is likely due to the modest sample size.
A limitation of the current analysis is that we report transplantation outcomes and therefore applicable only to those patients who attained remission and proceeded to transplantation. The series on hypodiploid ALL from the COG include patients treated on their chemotherapy trials and thereby capturing events beginning from time of diagnosis. Therefore it is not possible to compare the survival and leukemia-free survival rates from the current analysis to the COG studies. To be able to assess whether transplantation is indeed superior to intensive chemotherapy, would require a carefully planned clinical trial that randomly allocates those beyond CR1 with 43 or fewer chromosomes to HSCT or continuing chemotherapy. Such a trial will only be feasible with the cooperation of several international pediatric consortia such as the COG and others. In two recent COG reviews which included patients with hypodiploid ALL enrolled on their trials, minimal residual (MRD) ≥ 0.01% at the end of induction was associated with inferior outcome. We are unable to evaluate the effect of MRD status at end of induction therapy or pre-transplant, as this was not systematically tested for in our cohort (19, 20).
A recent publication by Holmfeldt et al. reported novel genomic properties of childhood hypodiploid ALL (21). Marked enrichment for Ras-pathway, RB1 and TP53 alterations were seen in patients with hypodiploid ALL. A high frequency of TP53 alterations in both pediatric and adult low-hypodiploid ALL (91.2% and 90.9%, respectively) were seen, suggesting that mutation of TP53 is a significant event in the pathogenesis of low-hypodiploid ALL. Furthermore, almost half of the TP53 mutations identified in pediatric low-hypodiploid ALL were present as heterozygous mutations in remission bone marrow or peripheral blood and in purified normal T-cell populations, and most of these are known Li-Fraumeni syndrome-associated mutations (22). The frequency of secondary malignancy in our cohort was significant and raises concern that possible excess of malignancy will be seen on longitudinal follow-up. Future studies should look directly for TP53 mutations, and longer follow-up will better define the risk of subsequent cancers.
Although the current analyses has several limitations, including its modest sample size, transplantation period that spanned over two decades and our inability to determine why some transplantations occurred in first CR and others in second or subsequent CR; the analyses confirms a worse outcome for patients with chromosome number 43 or less compared to chromosome number 44 or 45 and transplantation. This information is relevant for physicians, patients and their families, and for future clinical trial planning to determine a role for allogeneic transplant in the era of risk adapted therapy for ALL.
Highlights.
Treatment failure and mortality after HSCT are higher with ≤43 chromosomes
Higher mortality in CR2 compared to CR1, independent of chromosome number
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from * Actinium Pharmaceuticals; Allos Therapeutics, Inc.; * Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; * Blue Cross and Blue Shield Association; * Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; * Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;* Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; * Milliman USA, Inc.; * Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; * Remedy Informatics; * Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; * Tarix Pharmaceuticals; * TerumoBCT; * Teva Neuroscience, Inc.; * THERAKOS, Inc.; University of Minnesota; University of Utah; and * Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Corporate Members
Financial Disclosure Statement: The authors do not have any conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conter V, Arico M, Basso G, et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:255–264. doi: 10.1038/leu.2009.250. [DOI] [PubMed] [Google Scholar]
- 2.Moricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 3.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veerman AJ, Kamps WA, van den Berg H, et al. Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997–2004) Lancet Oncol. 2009;10:957–966. doi: 10.1016/S1470-2045(09)70228-1. [DOI] [PubMed] [Google Scholar]
- 5.Schmiegelow K, Heyman M, Gustafsson G, et al. The degree of myelosuppression during maintenance therapy of adolescents with B-lineage intermediate risk acute lymphoblastic leukemia predicts risk of relapse. Leukemia. 2010;24:715–720. doi: 10.1038/leu.2009.303. [DOI] [PubMed] [Google Scholar]
- 6.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell C, Richards S, Harrison CJ, Eden T. Long-term follow-up of the United Kingdom medical research council protocols for childhood acute lymphoblastic leukaemia, 1980–2001. Leukemia. 2010;24:406–418. doi: 10.1038/leu.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heerema NA, Nachman JB, Sather HN, et al. Hypodiploidy with less than 45 chromosomes confers adverse risk in childhood acute lymphoblastic leukemia: a report from the children’s cancer group. Blood. 1999;94:4036–4045. [PubMed] [Google Scholar]
- 9.Nachman JB, Heerema NA, Sather H, et al. Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood. 2007;110:1112–1115. doi: 10.1182/blood-2006-07-038299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison CJ, Moorman AV, Broadfield ZJ, et al. Three distinct subgroups of hypodiploidy in acute lymphoblastic leukaemia. Br J Haematol. 2004;125:552–559. doi: 10.1111/j.1365-2141.2004.04948.x. [DOI] [PubMed] [Google Scholar]
- 11.Przepiorka D, Chan KW, Champlin RE, et al. Prevention of graft-versus-host disease with anti-CD5 ricin A chain immunotoxin after CD3-depleted HLA-nonidentical marrow transplantation in pediatric leukemia patients. Bone Marrow Transplant. 1995;16:737–741. [PubMed] [Google Scholar]
- 12.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13:1091–1112. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 13.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 15.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B-Statistical Methodology. 1972;34:187–200. [Google Scholar]
- 16.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz KR, Pullen DJ, Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) Blood. 2007;109:926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devidas M, Raetz E, Loh M, et al. Pediatric Blood and Cancer. Wiley Blackwell; 2013. Outcome For Children with Hypodiploid Acute Lymphoblastic Leukemia (ALL) on Contemporary Children’s Oncology Group (COG) Clinical Trial; pp. 10–10. [Google Scholar]
- 20.Schultz KR, Devidas M, Bowman WP, et al. Philadelphia chromosome-negative very high-risk acute lymphoblastic leukemia in children and adolescents: results from Children’s Oncology Group Study AALL0031. Leukemia. 2014;28:964–967. doi: 10.1038/leu.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmfeldt L, Wei L, Diaz-Flores E, et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet. 2013;45:242–252. doi: 10.1038/ng.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petitjean A, Mathe E, Kato S, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]