Abstract
Background
We described malnutrition and the effect of age at antiretroviral therapy (ART) initiation on catch-up growth over 24 months among HIV-infected children enrolled in the IeDEA West African paediatric cohort (pWADA).
Methods
Malnutrition was defined at ART initiation (baseline) by a Z-score <-2 SD, according to three anthropometric indicators: Weight-for-age (WAZ) for underweight, Height-for-age (HAZ) for stunting, and Weight-for-Height/BMI-for-age (WHZ/BAZ) for wasting. Kaplan-Meier estimates for catch-up growth (Z-score ≥-2 SD) on ART, adjusted for gender, immunodeficiency and malnutrition at ART initiation, ART regimen, time period and country, were compared by age at ART initiation. Cox proportional hazards regression models determined predictors of catch-up growth on ART over 24 months.
Results
Between 2001 and 2012, 2004 HIV-infected children < 10 years of age were included. At ART initiation, 51% were underweight, 48% were stunted and 33% were wasted. The 24-month adjusted estimates for catch-up growth were 69% (95% confidence interval [CI]: 57;80), 61% (95%CI: 47;70), and 90% (95%CI: 76;95) for WAZ, HAZ, and WHZ/BAZ, respectively. Adjusted catch-up growth was more likely for children <5 years of age at ART initiation compared to children ≥5 years for WAZ, HAZ (P<0.001), and for WHZ/BAZ (P = 0.026).
Conclusions
Malnutrition among these children is an additional burden that has to be urgently managed. Despite a significant growth improvement after 24 months on ART, especially in children <5 years, a substantial proportion of children still never achieved catch-up growth. Nutritional care should be part of the global healthcare of HIV-infected children in sub-Saharan Africa.
Keywords: HIV, children, antiretroviral therapy, growth, malnutrition, Africa
Introduction
In 2011, 3.3 million children aged <15 years were living with Human Immunodeficiency Virus (HIV) worldwide, with more than 90% living in Sub-Saharan Africa [1]. Growth faltering has been reported in up to 50% of untreated HIV-infected children in resource-limited settings [2-4], and several studies have shown that HIV-related growth faltering can be reversed with antiretroviral therapy (ART) [3-11]. ART reduces the risk of HIV-infection progression and reduces mortality in children [12,13]. ART also has an impact on the child's nutritional status, by reducing pro-inflammatory cytokine concentration, and improving immune system functions [14]. Improvements in growth may in turn help towards a slower HIV disease progression and reduce pediatric HIV-related morbidity and mortality [15,16]. Several factors at ART initiation could influence treatment response and therefore growth reconstitution. Data suggest that children starting ART at an early age have a greater chance for catch-up growth and reaching the reference growth standards for their age and gender [17,18]. While a better growth response is found among younger children at ART initiation compared to older ones in some studies [5,6,17,19], others report that growth response after ART initiation is not associated with age [4,20]. In addition, despite an improvement of weight and height after ART initiation, restoration to a normal nutritional status is often not reached for a high proportion of children, even after two years of follow-up [3-5,18]. Most of these studies have been conducted in Eastern and Southern Africa. Few data are available on malnutrition in West-African HIV-infected children [21], and none have been described in the post-ART era.
We hypothesized that children who start ART at a younger age will have a better growth recovery than children who start ART at a later age. Our main objective was to describe malnutrition at ART initiation and study the association between age at ART initiation and time to catch-up growth within 24 months post-ART initiation among HIV-infected children enrolled in the IeDEA West African pediatric cohort.
Methods
Study population
The IeDEA network (International epidemiologic Databases to Evaluate Aids) is an international consortium collecting data on HIV infection and AIDS. The WADA collaboration (West African Database on Antiretroviral therapy) is in charge of the IeDEA program in West Africa. The pediatric WADA (pWADA) cohort is a multicenter observational prospective cohort including children from 11 pediatric clinical centers from seven countries (Benin, Burkina Faso, Côte d'Ivoire, Ghana, Mali, Senegal and Togo) of the WADA collaboration [22,23]. Eligible criteria for the pWADA cohort are the following: all HIV-infected children <16 years with a confirmed HIV-infection (positive serology in children >18 months, or positive polymerase chain reaction (PCR), whatever the age), receiving ART or not, and all HIV-exposed children born to HIV-infected mothers until their definite HIV diagnosis.
Children included in this study were those in the pWADA cohort, HIV-infected, naïve of any ART before inclusion other than Prevention of Mother to Child Transmission (PMTCT), with a documented date of birth and ART regimen, who initiated ART during follow-up in pWADA, and aged <10 years, with at least two anthropometric measurements during follow-up, including one in the first 3 months of ART and one after 6 months of ART. We excluded children who were enrolled in centers with a very small sample size (<50 children).
Variables and data management
Malnutrition is defined by several anthropometric indicators according to the Waterlow definition used by the WHO [24]. In this study, we defined standardized malnutrition using Z-scores, which quantify how many Standard Deviations (SD) child's weight and height is from the median value of a child of the same age and gender, in a reference population. Malnutrition is defined by a Z-score < -2 SD, with moderate malnutrition between -3 and -2 SD and severe malnutrition < -3 SD. The 2006 WHO growth charts were used for children <5 years [25], and the 2007 WHO growth charts for children ≥5 years [26]. We defined three anthropometric indicators: Weight-for-Age (WAZ) for underweight, Height-for-Age (HAZ) for stunting, and Weight-for-Height/BMI-for-Age (WHZ/BAZ) for wasting. The latter indicator was combined using Weight-for-Height for children until 5 years of age and BMI-for-age for older children. The Z-scores were calculated with WHO Anthro Software (version 3.2.2, January 2011) and WHO Anthro Plus. Z-scores < -10 and Z-scores > +10 SD were viewed as outliers and were not included in the catch-up growth analysis, as representing less than 2% of data.
The principal covariables were age at ART initiation (in classes: [0-2], [2-3], [3-4], [4-5] and [5-10] years), gender, ART regimen, Cotrimoxazole prophylaxis, clinical centers, and immunodeficiency for age according to the 2006 WHO guidelines [27]. CD4 was expressed in percentage for children < 5 years and in cells per μL for children ≥ 5 years. Severe immunodeficiency was defined as CD4% <15% or CD4 cells/μL <350 cells/μL, and moderate immunodeficiency as CD4% between 15-25% or CD4 count between 350-499 cells/μL.
All children on ART were seen monthly and had unrestricted free access to antiretroviral drugs. Weight and height were measured at each visit, using standardized procedures [28]. Absolute CD4 cell count and CD4% were measured every 6 months. Nutritional supplementation was not part of the standard HIV healthcare. Children detected with severe wasting were hospitalized.
Data from each center were collected prospectively from 1998 to 2012 with the formal approval of each participating clinic of pWADA, with local Institutional Review Board and NIH approval.
Statistical analysis
We first measured the prevalence of malnutrition at ART initiation (baseline) according to the three anthropometric indicators above. Baseline characteristics at ART initiation were compared according to malnutrition using the Pearson χ2 test for categorical variables and the Kruskal–Wallis test for continuous variables. To investigate the factors associated with malnutrition at ART initiation, we ran logistic regression models for each indicator. The variables with a p-value <0.25 in univariate analysis were selected for multivariate analysis and then kept in a full model. Results were adjusted for age, immunodeficiency, gender, countries and time period of ART initiation.
The database closing date was October 30th 2012. Children were lost to follow-up (LTFU) when the last clinical contact was more than 6 months. Catch-up growth was defined by transition from a Z-score < -2 SD to a Z-score ≥ -2 SD. A sensitivity analysis was then conducted defining catch-up growth by transition to a Z-score ≥ -1 SD. Among children malnourished at ART initiation, the probability for catch-up growth, for each growth outcome separately, within the first 24 months on ART, was described using an adjusted Kaplan-Meier estimator. Children were followed up from ART initiation to time of first growth recovery, or death, date of last clinical contact, transfer out or the database closing date, whichever came first. Additionally, because wasting is defined only until 10 years, children were censored on their 10th birthday. A Cox proportional hazards regression model was used to study predictors associated with time to catch-up growth. Analyses were adjusted for age, gender, immunodeficiency status at ART initiation, ART regimen, time period of ART initiation, countries and severity of baseline malnutrition (moderate vs. severe).
Results
Baseline and follow-up characteristics of the study population
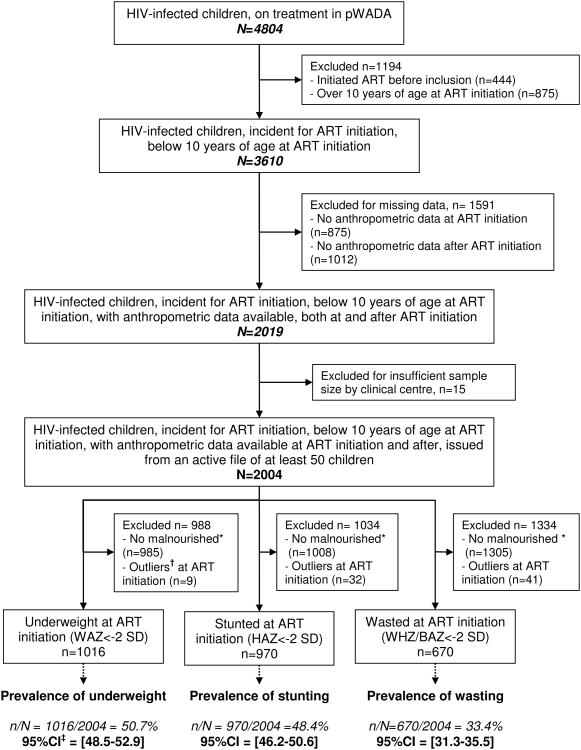
Among the 4804 HIV-infected children on ART included in the pWADA cohort, 3610 initiated ART< 10 years between 2001 and 2012 (Figure 1). Among them, 2004 (56%) fitted the inclusion criteria. At ART initiation, the median age was 4.1 years (interquartile range (IQR)=[2.1;6.7]), and 46% were girls. Median baseline WAZ, HAZ and WHZ/BAZ were -2.05 (IQR=[-3.27,-1.08]), -1.98 (IQR=[-2.98;-0.97]), and -1.31 (IQR=[-2.58;-0.32]), respectively. The first-line ART regimen was based on protease inhibitors (PI) for 17% of children. Data on Cotrimoxazole use were missing for most children, slightly more for malnourished children. Forty-one percent were classified at WHO clinical stage III or IV, and 37% were severely immunedeficient for age. Fifty-three percent of children initiated ART between 2005 and 2008 (Table 1). Overall, 50.7% were underweight (95% Confidence Interval (95%CI)=[48.5;52.9]), 48.4% were stunted (95%CI=[46.2;50.6]), and 33.4% were wasted (95%CI=[31.3;35.5]), (Figure 1). Among malnourished children, according to each type of malnutrition, 58.8% were severely underweight, 50.1% were severely stunted and 57.0% were severely wasted.
Figure 1.
Cohort profile of the study sample selection and prevalence of malnutrition with their 95% confidence Intervals according to Weight-for-Age, Height-for-Age and Weight-for-Height/Body Mass Index-for-Age Z-scores (WAZ, HAZ, WHZ/BMI). * Z-score ≥ -2 Standard Deviations (SD) † Z-score < -10 or >10 SD, ‡CI = Confidence Intervals. IeDEA West African paediatric cohort
Table 1.
Socio-demographic and clinical characteristics at ART initiation according to the prevalence of malnutrition (Z-score < -2 SD) for each anthropometric indicator in HIV-infected children. IeDEA West African paediatric cohort (n=2004)
| Variables | Underweight: WAZ< -2 SD* | Stunting: HAZ< -2 SD* | Wasting: WHZ/BAZ< -2 SD* | Total
study population |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| Yes (N=1016) | No (N=988) | P- value† |
Yes (N=970) | No (N=1034) | P- value† |
Yes (N=670) | No (N=1334) | P- value† |
|||||||||
|
|
|
|
|||||||||||||||
| Median | (IQR) | Median | (IQR) | Median | (IQR) | Median | (IQR) | Median | (IQR) | Median | (IQR) | Median | (IQR) | ||||
|
|
|
|
|
||||||||||||||
| Age (years) | 3.5 | (1.7-6.5) | 4.5 | (2.6-6.8) | <0.001 | 3.4 | (1.9-5.9) | 4.9 | (2.5-7.2) | <0.001 | 3.1 | (1.6-6.6) | 4.4 | (2.5-6.7) | <0.001 | 4.1 | (2.1-6.7) |
| CD4 % for children under 5 years (n=623) | 14.0 | (8.4-19.1) | 14.2 | (11.0-20.0) | 0.033 | 13.7 | (9.0-18.0) | 15.0 | (10.0-21.0) | 0.011 | 14.0 | (8.0-20.0) | 14.0 | (10.0-19.0) | 0.397 | 14 | (9.2-19.9) |
| CD4 cell count/mL for children upper 5 years (n=721) | 233 | (37-456) | 383 | (203-652) | <0.001 | 255 | (64-466) | 357 | (139-620) | <0.001 | 200 | (26-468) | 335 | (175-595) | <0.001 | 309 | (113-561) |
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||||
|
|
|
|
|
||||||||||||||
| Age groups (years) | <0.001 | <0.001 | <0.001 | ||||||||||||||
| [0-2] | 306 | 30.1 | 158 | 16.0 | 264 | 27.2 | 200 | 19.3 | 241 | 36.0 | 223 | 16.7 | 464 | 23.2 | |||
| [2-3] | 152 | 15.0 | 133 | 13.5 | 175 | 18.0 | 110 | 10.6 | 90 | 13.4 | 195 | 14.6 | 285 | 14.2 | |||
| [3-4] | 89 | 8.8 | 128 | 13.0 | 115 | 11.9 | 102 | 9.9 | 48 | 7.2 | 169 | 12.7 | 217 | 10.8 | |||
| [4-5] | 86 | 8.5 | 126 | 12.8 | 98 | 10.1 | 114 | 11.0 | 48 | 7.2 | 164 | 12.3 | 212 | 10.6 | |||
| [5-10] | 383 | 37.7 | 443 | 44.8 | 318 | 32.8 | 508 | 49.1 | 243 | 36.3 | 583 | 43.7 | 826 | 41.2 | |||
| Gender | 0.049 | 0.001 | 0.115 | ||||||||||||||
| Boy | 570 | 56.1 | 511 | 51.7 | 560 | 57.7 | 521 | 50.4 | 378 | 56.4 | 703 | 52.7 | 1081 | 53.9 | |||
| Girl | 446 | 43.9 | 477 | 48.3 | 410 | 42.3 | 513 | 49.6 | 292 | 43.6 | 631 | 47.3 | 923 | 46.1 | |||
| WHO clinical stage | <0.001 | <0.001 | <0.001 | ||||||||||||||
| I | 80 | 7.9 | 170 | 17.2 | 75 | 7.7 | 175 | 16.9 | 56 | 8.4 | 194 | 14.5 | 250 | 12.5 | |||
| II | 131 | 12.9 | 230 | 23.3 | 137 | 14.1 | 224 | 21.7 | 88 | 13.1 | 273 | 20.5 | 361 | 18.0 | |||
| III | 318 | 31.3 | 209 | 21.2 | 283 | 29.2 | 244 | 23.6 | 207 | 30.9 | 320 | 24.0 | 527 | 26.3 | |||
| IV | 233 | 22.9 | 54 | 5.5 | 195 | 20.1 | 92 | 8.9 | 173 | 25.8 | 114 | 8.5 | 287 | 14.3 | |||
| Missing | 254 | 25.0 | 325 | 32.9 | 280 | 28.9 | 299 | 28.9 | 146 | 21.8 | 433 | 32.5 | 579 | 28.9 | |||
| Immunodeficiency status ‡ | <0.001 | <0.001 | 0.117 | ||||||||||||||
| No immunodeficient | 122 | 12.0 | 185 | 18.7 | 110 | 11.3 | 197 | 19.1 | 87 | 13.0 | 220 | 16.5 | 307 | 15.3 | |||
| Moderate | 131 | 12.9 | 166 | 16.8 | 133 | 13.7 | 164 | 15.9 | 98 | 14.6 | 199 | 14.9 | 297 | 14.8 | |||
| Severe | 414 | 40.7 | 324 | 32.8 | 388 | 40.0 | 350 | 33.8 | 266 | 39.7 | 472 | 35.4 | 738 | 36.8 | |||
| Missing | 349 | 34.4 | 313 | 31.7 | 339 | 34.9 | 323 | 31.2 | 219 | 32.7 | 443 | 33.2 | 662 | 33.0 | |||
| First line regimen§ | 0.002 | <0.001 | 0.082 | ||||||||||||||
| 2 NRTIs + 1 NNRTI | 789 | 77.7 | 824 | 83.4 | 745 | 76.8 | 868 | 83.9 | 524 | 78.2 | 1089 | 81.6 | 1613 | 80.5 | |||
| 2 NRTIs + 1 PI | 199 | 19.6 | 151 | 15.3 | 202 | 20.8 | 148 | 14.3 | 127 | 19.0 | 223 | 16.7 | 350 | 17.5 | |||
| 3 NRTIs | 28 | 2.8 | 13 | 1.3 | 23 | 2.4 | 18 | 1.7 | 19 | 2.8 | 22 | 1.6 | 41 | 2.0 | |||
| Cotrimoxazole prophylaxis | <0.001 | 0.033 | <0.001 | ||||||||||||||
| Yes | 184 | 18.1 | 208 | 21.0 | 179 | 18.5 | 213 | 20.6 | 124 | 18.5 | 268 | 20.1 | 392 | 19.6 | |||
| No | 170 | 16.7 | 217 | 22.0 | 170 | 17.5 | 217 | 21.0 | 100 | 14.9 | 287 | 21.5 | 387 | 19.3 | |||
| Missing | 662 | 65.2 | 563 | 57.0 | 621 | 64.0 | 604 | 58.4 | 446 | 66.6 | 779 | 58.4 | 1225 | 61.1 | |||
| Time period of ART initiation | 0.041 | <0.001 | 0.924 | ||||||||||||||
| 2001-2004 | 114 | 11.2 | 88 | 8.9 | 114 | 11.8 | 88 | 8.5 | 69 | 10.3 | 133 | 10.0 | 202 | 10.0 | |||
| 2005-2008 | 551 | 54.2 | 511 | 51.7 | 538 | 55.5 | 524 | 50.7 | 351 | 52.4 | 711 | 53.3 | 1062 | 53.0 | |||
| 2009-2012 | 351 | 34.5 | 389 | 39.4 | 318 | 32.8 | 422 | 40.8 | 250 | 37.3 | 490 | 36.7 | 740 | 37.0 | |||
| Countries and centres | <0.001 | <0.001 | <0.001 | ||||||||||||||
| Benin | 68 | 6.7 | 32 | 3.2 | 67 | 6.9 | 33 | 3.2 | 30 | 4.5 | 70 | 5.2 | 100 | 5.0 | |||
| Burkina Faso | 70 | 6.9 | 59 | 6.0 | 58 | 6.0 | 71 | 6.9 | 52 | 7.8 | 77 | 5.8 | 129 | 6.4 | |||
| Côte d'Ivoire | 289 | 28.4 | 396 | 40.1 | 316 | 32.6 | 369 | 35.7 | 171 | 25.5 | 514 | 38.5 | 685 | 34.2 | |||
| Site 1 | 125 | 12.3 | 177 | 17.9 | 154 | 15.9 | 148 | 14.3 | 58 | 8.7 | 244 | 18.3 | 302 | 15.1 | |||
| Site 2 | 20 | 2.0 | 39 | 3.9 | 19 | 2.0 | 40 | 3.9 | 17 | 2.5 | 42 | 3.1 | 59 | 2.9 | |||
| Site 3 | 84 | 8.3 | 96 | 9.7 | 92 | 9.5 | 88 | 8.5 | 50 | 7.5 | 130 | 9.7 | 180 | 9.0 | |||
| Site 4 | 38 | 3.7 | 49 | 5.0 | 35 | 3.6 | 52 | 5.0 | 30 | 4.5 | 57 | 4.3 | 87 | 4.3 | |||
| Site 5 | 22 | 2.2 | 35 | 3.5 | 16 | 1.6 | 41 | 4.0 | 16 | 2.4 | 41 | 3.1 | 57 | 2.8 | |||
| Ghana | 140 | 13.8 | 196 | 19.8 | 153 | 15.8 | 183 | 17.7 | 76 | 11.3 | 260 | 19.5 | 336 | 16.8 | |||
| Mali | 369 | 36.3 | 241 | 24.4 | 303 | 31.2 | 307 | 29.7 | 285 | 42.5 | 325 | 24.4 | 610 | 30.4 | |||
| Senegal | 80 | 7.9 | 64 | 6.5 | 73 | 7.5 | 71 | 6.9 | 56 | 8.4 | 88 | 6.6 | 144 | 7.2 | |||
WAZ: Weight-for-Age Z-score, HAZ: Height-for-Age Z-score, WHZ/BAZ: Weight-for-Height Z-score or Body Mass Index-for-Age Z-score, SD: Standard Deviations, IQR: InterQuartile Range
Khi-square test for qualitative variables, Kruskal-Wallis test for quantitative variables
Defined by age according to WHO 2006 guidelines
NRTI: Nucleoside Reverse-Transcriptase Inhibitor, NNRTI : Non-Nucleoside Reverse Transcriptase Inhibitor, PI : Protease Inhibitor
The median follow-up time on ART for the 2004 children was 25 months (IQR=[12;47]).Thirteen percent of children were LTFU and 3% were deceased before 24 months of ART.
The 2004 children contributed 14766 available anthropometric measurements during the first 24 months of treatment, which represents >75% of the overall available anthropometric data during the entire follow-up. The median number of observations was 4per child (IQR=[3;7]).
Compared with the selected population, children excluded for missing anthropometric data did not differ in age or gender but differed in terms of follow-up quality. Clinical variables were more often missing (p<0.001) and the proportion of death and LTFU during the entire follow-up was highest in excluded children: 11% vs. 2% and 34% vs. 24% respectively (p<0.001).
Factors associated at ART initiation, probabilities and predictors of catch-up growth at 24-month: Underweight (Weight-for-Age Z-score)
In multivariate analyses, children <2 years had significantly higher odds of being underweight at ART initiation compared to children >5 years (adjusted Odds Ratio (aOR)=2.09, 95%CI=[1.62; 2.71]). Furthermore, severely immunedeficient children had higher odds of being underweight at ART initiation compared with non immunedeficient children (aOR=1.81, 95%CI=[1.36; 2.40]) (Table 2).
Table 2.
Factors associated with malnutrition (Z-score < -2 SD) at ART initiation, for each anthropometric indicator, adjusted logistic regression analysis (n=2004). IeDEA West African paediatric cohort
| Malnutrition at ART initiation (Zscore< -2 SD), adjusted analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Baseline variables | Underweight : WAZ* | Stunting : HAZ* | Wasting : WHZ/BAZ* | ||||||
|
|
|||||||||
| aOR† | (95% CI)† | P-value | aOR | (95% CI)† | P-value | aOR | (95% CI)† | P-value | |
| Age groups (years) | <0.001 | <0.001 | <0.001 | ||||||
| [5-10] | 1 | - | 1 | - | 1 | - | |||
| [0-2] | 2.09 | (1.62-2.71) | 2.32 | (1.80-3.00) | 2.45 | (1.89-3.17) | |||
| [2-3] | 1.19 | (0.89-1.59) | 2.68 | (1.99-3.60) | 0.99 | (0.73-1.36) | |||
| [3-4] | 0.72 | (0.52-1.00) | 1.87 | (1.35-2.58) | 0.64 | (0.44-0.93) | |||
| [4-5] | 0.75 | (0.54-1.03) | 1.44 | (1.05-1.99) | 0.70 | (0.48-1.02) | |||
| Gender | 0.122 | 0.003 | 0.320 | ||||||
| Girl | 1 | - | 1 | - | 1 | - | |||
| Boy | 1.16 | (0.96-1.39) | 1.32 | (1.10-1.58) | 1.11 | (0.91-1.35) | |||
| Immunodeficiency status‡ | <0.001 | <0.001 | 0.142 | ||||||
| No immunodeficiency | 1 | - | 1 | - | 1 | - | |||
| Moderate | 1.02 | (0.73-1.45) | 1.11 | (0.79-1.56) | 1.06 | (0.73-1.53) | |||
| Severe | 1.81 | (1.36-2.40) | 1.71 | (1.28-2.27) | 1.35 | (0.99-1.83) | |||
| Missing | 1.38 | (0.99-1.91) | 1.10 | (0.80-1.53) | 1.09 | (0.77-1.55) | |||
| Time period of ART initiation | 0.484 | 0.033 | |||||||
| 2009-2012 | 1 | - | 1 | - | - | - | |||
| 2001-2004 | 1.03 | (0.73-1.45) | 1.67 | (1.19-2.36) | - | - | |||
| 2005-2008 | 1.13 | (0.92-1.37) | 1.30 | (1.07-1.59) | - | - | |||
| Countries | <0.001 | 0.012 | <0.001 | ||||||
| Côte d'Ivoire | 1 | - | 1 | - | 1 | - | |||
| Benin | 2.85 | (1.77-4.59) | 2.20 | (1.37-3.53) | 1.30 | (0.79-2.14) | |||
| Burkina Faso | 1.59 | (1.08-2.35) | 0.89 | (0.60-1.32) | 2.05 | (1.37-3.06) | |||
| Ghana | 0.99 | (0.74-1.33) | 1.06 | (0.79-1.43) | 0.95 | (0.68-1.34) | |||
| Mali | 1.94 | (1.53-2.47) | 0.93 | (0.73-1.18) | 2.55 | (1.99-3.27) | |||
| Senegal | 1.85 | (1.27-2.69) | 1.22 | (0.83-1.77) | 2.12 | (1.43-3.14) | |||
WAZ: Weight-for-Age Z-score, HAZ: Height-for-Age Z-score, WHZ/BAZ: Weight-for-Height Z-score or Body Mass Index-for-Age Z-score.
aOR: adjusted Odds Ratio, CI: Confidence Interval
Defined by age according to WHO 2006 guidelines
For the 1016 children underweight at baseline, the median time to catch-up growth was 11.7 months (95%CI=[9.8; 12.2]). Their crude 24-month cumulative probability for catch-up growth was 70% (95%CI=[67;74]), while the adjusted rate was 69% (95%CI=[57-80]) (Figure 2). Children aged 0 to 2 years and 2 to 3 years at ART initiation had the highest probability for catch-up growth for WAZ before 24 months: the adjusted cumulative probabilities by 24 months were both 81%, (95%CI=[69; 88] and [67-89] respectively) whereas it was 53% (95%CI=[40; 63]) for children ≥5 years (p <0.001) (Figure 2). In Cox analysis, catch-up growth was more likely in all children <5 years compared to older children. Boys were more likely to catchup growth for WAZ than girls (aHR=0.80, 95%CI=[0.68; 0.95]), as well as children with severe malnutrition at baseline (aHR=0.42, 95%CI=[0.36; 0.50]) compared to moderate malnutrition (Table 3).
Figure 2.
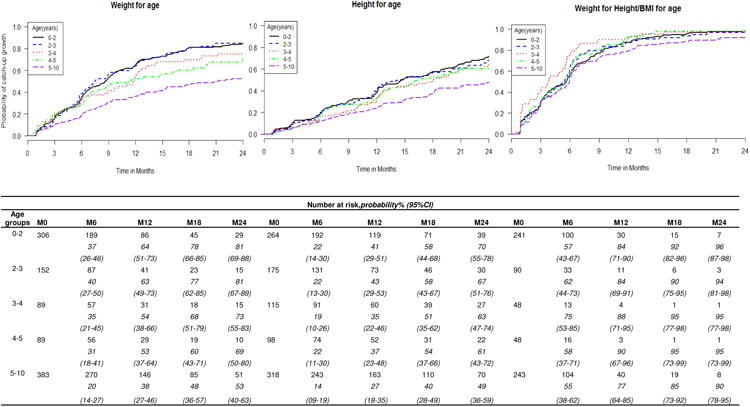
Estimated probabilities of catch-up growth (Z-score ≥ -2 SD) based on adjusted Kaplan-Meier estimates within the first 24 months on ART in HIV-infected children suffering at baseline from underweight (n=1016), stunting (n=970) and wasting (n=670) respectively, according to age at ART initiation. IeDEA West African paediatric cohort
Table 3.
Factors associated with catch-up growth (Z-score ≥ -2 SD) after ART initiation, according to Weight-for-Age, Height-for-Age, Weight-for-Height / BMI-for-age indicators among malnourished HIV-infected children (Z-score< -2SD), adjusted Cox model analysis. IeDEA West African paediatric cohort.
| Baseline variables | Adjusted model WAZ* | Adjusted model HAZ* | Adjusted model WHZ/BAZ* | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| aHR† | (95% CI) | P-value | aHR† | (95% CI) | P-value | aHR† | (95% CI) | P-value | |
| Age groups (years) | <0.001 | <0.001 | 0.026 | ||||||
| [5-10] | 1 | - | 1 | - | 1 | - | |||
| [0-2] | 2.34 | (1.87-2.94) | 2.03 | (1.58-2.60) | 1.21 | (0.96-1.51) | |||
| [2-3] | 2.35 | (1.82-3.03) | 1.98 | (1.52-2.56) | 1.25 | (0.95-1.65) | |||
| [3-4] | 1.80 | (1.33-2.44) | 1.71 | (1.29-2.28) | 1.69 | (1.21-2.36) | |||
| [4-5] | 1.60 | (1.18-2.18) | 1.53 | (1.12-2.09) | 1.37 | (0.98-1.91) | |||
| Gender | 0.008 | 0.497 | 0.016 | ||||||
| Girl | 1 | - | 1 | - | 1 | - | |||
| Boy | 0.80 | (0.68-0.95) | 0.94 | (0.79-1.12) | 0.81 | (0.68-0.96) | |||
| Immunodeficiency status‡ | 0.005 | 0.118 | 0.233 | ||||||
| No immunodeficiency | 1 | - | 1 | - | 1 | - | |||
| Moderate | 1.18 | (0.85-1.63) | 1.00 | (0.75-1.34) | 0.97 | (0.70-1.34) | |||
| Severe | 1.31 | (0.99-1.72) | 1.04 | (0.75-1.46) | 1.21 | (0.92-1.59) | |||
| Missing | 0.90 | (0.66-1.23) | 0.78 | (0.56-1.08) | 1.04 | (0.75-1.43) | |||
| Time period of ART initiation | 0.940 | 0.031 | |||||||
| 2009-2012 | 1 | - | - | - | 1 | - | |||
| 2001-2004 | 0.95 | (0.70-1.29) | - | - | 0.66 | (0.48-0.91) | |||
| 2005-2008 | 1.00 | (0.83-1.19) | - | - | 0.87 | (0.73-1.04) | |||
| Malnutrition | <0.001 | <0.001 | <0.001 | ||||||
| Moderate (-3 ≤ Z-score < -2) | 1 | - | 1 | - | 1 | - | |||
| Severe (Z-score < -3) | 0.42 | (0.36-0.50) | 0.33 | (0.27-0.39) | 0.52 | (0.44-0.62) | |||
| First line regimen§ | 0.851 | 0.222 | |||||||
| 2 NRTIs + 1 NNRTI or 3 NRTIs | 1 | - | 1 | - | 1 | - | 0.846 | ||
| 2 NRTIs + 1 PI | 1.02 | (0.83-1.26) | 0.87 | (0.70-1.09) | 1.02 | (0.81-1.29) | |||
| Countries | <0.001 | <0.001 | <0.001 | ||||||
| Côte d'Ivoire | 1 | - | 1 | - | 1 | - | |||
| Benin | 0.98 | (0.65-1.47) | 0.92 | (0.53-1.61) | 0.92 | (0.59-1.42) | |||
| Burkina Faso | 1.16 | (0.82-1.64) | 1.80 | (1.26-2.57) | 0.46 | (0.32-0.67) | |||
| Ghana | 1.98 | (0.96-1.49) | 1.84 | (1.36-2.49) | 2.10 | (1.51-2.91) | |||
| Mali | 1.20 | (0.96-1.49) | 1.64 | (1.31-2.06) | 1.16 | (0.93-1.46) | |||
| Senegal | 2.16 | (1.58-2.97) | 1.83 | (1.31-2.06) | 1.28 | (0.93-1.78) | |||
WAZ: Weight-for-Age Z-score, HAZ: Height-for-Age Z-score, WHZ/BAZ: Weight-for-Height Z-score or Body Mass Index-for-Age Z-score
HR: Hazard Ratio, aHR: adjusted Hazard Ratio, CI: Confidence Interval,
Defined by age according to WHO 2006 guidelines,
NRTI: Nucleoside Reverse-Transcriptase Inhibitor, NNRTI : Non-Nucleoside Reverse Transcriptase Inhibitor, PI : Protease Inhibitor.
Stunting (Height-for-Age Z-score)
Adjusted for all co-variables, children <5 years suffered more from stunting at ART initiation than children ≥5 years (p<0.001). Boys had higher odds of being stunted at ART initiation than girls (aOR=1.32, 95%CI=[1.10; 1.58]), as well as severely immunedeficient children compared to those not (aOR=1.71, 95%CI=[1.28; 2.27]), and children initiating ART in the 2001-2004 and the 2005-2008 periods, compared with children initiating ART between 2009 and 2012 (aOR=1.67, 95%CI=[1.19;2.36] and aOR=1.30, 95%CI=[1.07-1.59] respectively) (Table 2).
For the 970 children stunted at baseline, the median time to catch-up growth was 17.7 months (95%CI=[15.4; 19.3]).Their crude 24-month cumulative probability for catch-up was 61% (95%CI=[58; 65]), while the adjusted rate was 61% (95%CI=[47-70]) (Figure 2). Children aged ≥5 years had a significantly lower 24-month probability for catch-up growth (40% (95%CI=[28;49]) compared to younger children, where this probability reached 58% (95%CI=[44;68])(p<0.001) (Figure 2).In Cox analysis, catch-up growth was more likely in children <5 years, and less likely in children with severe compared to moderate malnutrition at ART initiation(0.33, 95%CI=[0.27; 0.39]). The time period of ART initiation did not have an effect on catch-up growth for HAZ (Table 3).
Wasting (Weight-for-Height / BMI-for-age Z-score)
Compared to children ≥5 years, children <2 years had higher odds of being wasted at ART initiation (aOR=2.45, 95%CI=[1.89;3.17]), whereas we observed an opposite trend in children aged 3 to 4 years (aOR=0.64, 95%CI=[0.44;0.93]). For other age groups, there was no significant difference, though there was trend towards lower risks in 4-5 years as well (Table 1). For the 670 children wasted at baseline, the median time to catch-up growth was 5.5 months (95%CI=[4.8;5.7]). The overall crude cumulative probability for catch-up growth by 24 months was 94% (95%CI=[91;96]), while the adjusted rate was 90% (95%CI=[76-95]) (Figure 2). The probability for catch-up growth for WHZ/BAZ differed according to age at ART initiation (p=0.026).In Cox analysis, there was a significant association between age at ART initiation and catch-up growth; children aged 3-4 years had higher odds compared to children ≥5 years (aOR=1.69, 95%CI=[1.21-2.36].As in previous analyses, children with severe malnutrition at baseline were less likely to catchup growth (aHR=0.52, 95%CI=[0.44;0.62]), as were boys (aHR=0.81, 95%CI=[0.68; 0.96]). Catch-up growth tended to be more likely in children initiating ART between 2001 and 2004 than in children initiating ART after 2009 (aOR=0.66, 95%CI=[0.48-0.91])(Table 3).
Sensitivity analyses: catch-up growth at a Z-score ≥ -1 SD
Using a higher threshold for the definition of catch-up growth, the adjusted 24-month cumulative probabilities for catch-up growth were much lower than previously: 28% (95%CI=[16;38]), 31% (95%CI=[14-44]), 61% (95%CI=[43-73]) for WAZ, HAZ and WHZ/BAZ respectively, with significant differences by age group (p<0.001). In Cox analyses, the association with age at ART initiation was more pronounced, for each anthropometric indicator, younger children were more likely to catch up growth.
Discussion
This large sample-sized prospective cohort study of 2004 HIV-infected children allows providing an original estimation of the prevalence of malnutrition among children at ART initiation in West Africa, and assessing their rates and predictors to catch-up of normal growth for age during the first 24 months on ART. ART was initiated at a late age (median: 4.1 years) and the prevalence of malnutrition at that time was already high according to all three indicators (51% underweight, 48% stunted, and 33% wasted, with a higher prevalence in the younger children and those severely immunedeficient). After 24 months on ART, malnourished children had a substantial probability of reaching their population age norms, varying from 61% for HAZ, 70% for WAZ, to 94% for WHZ/BAZ. In adjusted analysis, the rates for catch-up growth differed by age at ART initiation for Weight-for-age and Height-for-age indicators: children <5 years were more likely to catchup growth compared to older children. Furthermore, children aged 3-4 years were more likely to catch-up growth according to Weight-for-Height / BMI-for-age compared to children ≥5 years.
Growth reconstitution on ART was more effective and occurred sooner in wasted children compared to those underweight or stunted: it is easier to recover from acute malnutrition than from chronic malnutrition, where damages can be irreversible. Similar results were observed in a Kenyan study where catch-up growth was defined by a Z-score ≥ 0 SD [17]. In a study in Southern Africa, the percentage of children with a Z-score > -2 SD at different times after ART initiation was similar for Weight-for-age and Weight-for-Height, and higher than for Height-for-age [18].
Children <5 years at ART initiation presented a better growth reconstitution than older children. This is consistent with other studies [5,6,17,29], but not all [4]. This could be explained by metabolic and nutritional disorders occurring before ART initiation that obviously would not last as long in the youngest children compared to the older ones, if we assume that all these children were perinatally infected. Indeed, HIV-infected children suffer from opportunistic infections such as persistent diarrhea, malabsorption, and have pro-inflammatory effects which can cause intestinal dysfunction [30] and affect height and weight. Early ART initiation could prevent these disorders, but its beneficial effects may be too late for older children.
Overall, the population selection process could have led to an underestimation of the prevalence of malnutrition among HIV-infected children initiating ART. Indeed, the children excluded for missing anthropometric data represented 45% of the targeted population, and were more often LTFU or deceased, possibly because they suffered from malnutrition. Multiple imputation could be a solution, however, we felt we had two very different populations here and chose not to impute for these reasons. More efforts are needed to improve the quality of the follow-up and data collection. In addition, our study is not representative of all children on ART in these regions as most of the data were gathered from urban sites, in which the standard of care may be higher than in rural areas.
Our prospective cohort design allowed controlling the temporality of the events, observing the development of malnutrition in children after ART initiation over 24 months. Unfortunately, we could only include few explanatory variables to study associated factors to malnutrition and catch-up growth for data availability issues. Data on viral load were not collected routinely, neither were data on nutritional supplementation practices during the follow-up period, which could be critical to further explore this question.
All the results observed at and after ART initiation should be interpreted with caution keeping in mind that children were not issued from a representative birth-cohort, with a high risk of death in children before the age of two years [31,32]. In this context of difficult access to HIV-care, the younger children are also the more symptomatic which is what brings them to care, leading to an indication bias. However, this study offers a unique opportunity to describe the problem of malnutrition in this pediatric population in its context and its evolution under ART given the currents healthcare practices.
In our study, children started ART late, at an advanced stage of the disease, reflecting the difficulties for early detection of HIV-infected children in resource-limited settings [33]. The prevalence of malnutrition in HIV-infected children at ART initiation in our study was higher than that in the general child population in West Africa (22% of underweight, 36% of stunting and 10% of wasting) [34], underlining the increasing effect of HIV-infection on malnutrition among children in this region.
There was no association between baseline immunodeficiency and growth recovery, as reported in previous studies [35]. However, it has been previously reported that height is correlated with CD4% at baseline in children on ART [36].For wasted and underweight children, some studies also observed an association with immunodeficiency at ART initiation [18,29]. Among malnourished children at ART initiation, those who were severely immunedeficient could have nutritional problems directly due to HIV-infection. Thus, after ART initiation, immune system disorders can be reversed and associated nutritional problems can be reduced. We report elsewhere in the same population that the initiation of ART at the earliest age before 5 years and before any severe immunodeficiency occurred is needed to optimize the 24-month immune recovery on ART [37].
Our cohort represents a decade of pediatric HIV care, but we observed little differences in the prevalence of malnutrition and the probabilities of catch-up growth according to the time period of ART initiation. Despite a better access to ART and HIV care, nutritional care does not seem to have evolved. Few studies have investigated nutritional supplementation among HIV-infected children. Despite a trend to beneficial effects of these supplements for the development of HIV-infected children, the level of proof remains low [38,39].
In conclusion, malnutrition among HIV-infected children in West Africa is highly prevalent at ART initiation, representing an additional burden that needs to be urgently managed. In resource-limited settings, pediatric HIV infection is still detected too late, and ART initiation is delayed [31,33]. Initiation of ART before children develop growth failure, especially stunting, should be encouraged. The present study highlights age at ART initiation as a predictor of better catch-up growth, with significantly improved chances for catch-up growth among those starting ART before the age of 5 years. These findings support ART initiation at the earliest convenience before the age of 5 years and the presence of growth failure, independently of baseline immunodeficiency. This is in line with the revised WHO guidelines recommending ART initiation in all children aged <5 years [40]. Despite a significant growth improvement after ART initiation, a substantial proportion of children still did not achieve catch-up growth after two years on ART, even in the age groups with the better growth responses, and when defining catch-up growth with the lowest possible threshold. Further research is needed to better understand the growth reconstitution in ART treated children and to investigate nutritional interventions to improve growth on ART. This will lead to the optimization of the ART response of HIV-infected children in West Africa, improving their survival and quality of life.
Acknowledgments
The authors would like to thank all the participating children and their families, as well as all the members of the hospital teams of the sites involved in the IeDEA West Africa paediatric cohort.
Source of Funding: The National Cancer Institute (NCI), the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health (NIH), as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) [U01AI069919]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Sophie Desmonde was a fellow of the Ecole des Hautes Etudes en Santé Publique (EHESP), Rennes, France.
Footnotes
Conflict of interest and Source of Funding: The authors do not have a commercial or other association that might pose conflict of interest.
The IeDEA West Africa Collaboration Study Group (as of April 29, 2013): Participating sites (*members of the Steering Committee, §members of the Executive Committee):
Benin, Cotonou:
Adults: Djimon Marcel Zannou*, Carin Ahouada, Jocelyn Akakpo, Christelle Ahomadegbé, Jules Bashi, Alice Gougounon-Houéto, Angèle Azon-Kouanou, Fabien Houngbé, Jean Sehonou (CNHU Hubert Maga).
Pediatrics: Sikiratou Koumakpaï*§, Florence Alihonou, Marcelline d'Almeida, Irvine Hodonou, Ghislaine Hounhoui, Gracien Sagbo, Leïla Tossa-Bagnan, Herman Adjide (CNHU Hubert Maga).
Burkina Faso:
Adults: Joseph Drabo*, René Bognounou, Arnaud Dienderé, Eliezer Traore, Lassane Zoungrana, Béatrice Zerbo (CHU Yalgado, Ouagadougou), Adrien Bruno Sawadogo*§, Jacques Zoungrana, Arsène Héma, Ibrahim Soré, Guillaume Bado, Achille Tapsoba (CHU Souro Sanou, Bobo Dioulasso)
Pediatrics: Diarra Yé*, FlaKouéta, Sylvie Ouedraogo, Rasmata Ouédraogo, William Hiembo, Mady Gansonré (CH Charles de Gaulle, Ouagadougou).
Côte d'Ivoire, Abidjan:
Adults: Eugène Messou*, Joachim Charles Gnokoro, Mamadou Koné, Guillaume Martial Kouakou, (ACONDA-CePReF); Clarisse Amani Bosse*, Kouakou Brou, AchiIsidore Assi (ACONDA-MTCT-Plus); Henri Chenal*, Denise Hawerlander, Franck Soppi (CIRBA); Albert Minga*, Yao Abo, Jean-Michel Yoboue (CMSDS/CNTS); Serge Paul Eholié*§, Mensah Deborah Noelly Amego, Viviane Andavi, Zelica Diallo, Frédéric Ello, Aristophane Koffi Tanon (SMIT, CHU de Treichville), Serge Olivier Koule*, Koffi Charles Anzan, Calixte Guehi (USAC, CHU de Treichville);.
Pediatrics: Edmond Addi Aka*, Koffi Ladji Issouf, Jean-Claude Kouakou, Marie-Sylvie N'Gbeche, (ACONDA-CePReF); Touré Pety*, Divine Avit-Edi (ACONDA-MTCT-Plus); Kouadio Kouakou*, Magloire Moh, Valérie Andoblé Yao (CIRBA); Madeleine Amorissani Folquet*, Marie-Evelyne Dainguy, Cyrille Kouakou, Véronique Tanoh Méa-Assande, Gladys Oka-Berete, Nathalie Zobo, Patrick Acquah, Marie-Berthe Kokora (CHU Cocody); Tanoh François Eboua*, Marguerite Timité-Konan, Lucrèce Diecket Ahoussou, Julie Kebé Assouan, Mabéa Flora Sami, Clémence Kouadio (CHU Yopougon).
Ghana, Accra:
Pediatrics: Lorna Renner*§, Bamenla Goka, Jennifer Welbeck, Adziri Sackey, Seth Ntiri Owiafe (Korle Bu TH).
Guinea-Bissau:
Adults: Christian Wejse*§, Zacarias José Da Silva*, Joao Paulo (Bandim Health Project), The Bissau HIV cohort study group: Amabelia Rodrigues (Bandim Health Project), David da Silva (National HIV program Bissau), Candida Medina (Hospital National Simao Mendes, Bissau), Ines Oliviera-Souto (Bandim Health Project), Lars Østergaard (Dept of Infectious Diseases, Aarhus University Hospital), Alex Laursen (Dept of Infectious Diseases, Aarhus University Hospital), Morten Sodemann (Dept of Infectious Diseases, Odense University Hospital), Peter Aaby (Bandim Health Project), Anders Fomsgaard (Dept. of Virology, Statens Serum Institut, Copenhagen), Christian Erikstrup (Dept. of Clinical Immunology), Jesper Eugen-Olsen (Dept. of Infectious Diseases, Hvidovre Hospital, Copenhagen).
Mali, Bamako:
Adults: Moussa Y Maïga*§, Fatoumata Fofana Diakité, Abdoulaye Kalle, Drissa Katile (CH Gabriel Toure), Hamar Alassane Traore*, Daouda Minta*, Tidiani Cissé, Mamadou Dembelé, Mohammed Doumbia, Mahamadou Fomba, Assétou Soukho Kaya, Abdoulaye M Traoré, Hamady Traoré, Amadou Abathina Toure (CH Point G).
Pediatrics: Fatoumata Dicko*, Mariam Sylla, Alima Berthé, Hadizatou Coulibaly Traoré, Anta Koïta, Niaboula Koné, Clémentine N'Diaye, Safiatou Touré Coulibaly, Mamadou Traoré, Naïchata Traoré (CH Gabriel Toure).
Nigeria:
Adults: Man Charurat* (UMB/IHV), Samuel Ajayi*, Georgina Alim, Stephen Dapiap, Otu (UATH, Abuja), Festus Igbinoba (National Hospital Abuja), Okwara Benson*, Clément Adebamowo*, Jesse James, Obaseki, Philip Osakede (UBTH, Benin City), John Olasode (OATH, Ile-Ife).
Senegal, Dakar:
Adults: MoussaSeydi*, Papa Salif Sow, Bernard Diop, Noël Magloire Manga, Judicael Malick Tine§, Coumba Cissé Bassabi (SMIT, CHU Fann),
Pediatrics: Haby Signate Sy*, Abou Ba, Aida Diagne, Hélène Dior, Malick Faye, Ramatoulaye Diagne Gueye, Aminata Diack Mbaye (CH Albert Royer).
Togo, Lomé:
Adults: Akessiwe Patassi*, Awèrou Kotosso, Benjamin Goilibe Kariyare, Gafarou Gbadamassi, Agbo Komi, KankoéEdem Mensah-Zukong, Pinuwe Pakpame (CHU Tokoin/Sylvanus Olympio).
Pediatrics: Koko Lawson-Evi*§, Yawo Atakouma, Elom Takassi, Améyo Djeha, Ayoko Ephoévi-gah, Sherifa El-Hadj Djibril (CHU Tokoin/Sylvanus Olympio).
Executive Committee*: François Dabis (Principal Investigator, Bordeaux, France), Emmanuel Bissagnene (Co-Principal Investigator, Abidjan, Côte d'Ivoire), Elise Arrivé (Bordeaux, France), Patrick Coffie (Abidjan, Côte d'Ivoire), Didier Ekouevi (Abidjan, Côte d'Ivoire), Antoine Jaquet (Bordeaux, France), Valériane Leroy (Bordeaux, France), Charlotte Lewden (Bordeaux, France), Annie J Sasco (Bordeaux, France).
Operational and Statistical Team: Dieudonné Amani (Abidjan, Côte d'Ivoire), Jean-Claude Azani (Abidjan, Côte d'Ivoire), Eric Balestre (Bordeaux, France), Serge Bessekon (Abidjan, Côte d'Ivoire), Franck Bohossou (Abidjan, Côte d'Ivoire), Camille Gilbert (Bordeaux, France), Sophie Karcher (Bordeaux, France), Jules Mahan Gonsan (Abidjan, Côte d'Ivoire), Jérôme Le Carrou (Bordeaux, France), Séverin Lenaud (Abidjan, Côte d'Ivoire), Célestin Nchot (Abidjan, Côte d'Ivoire), Karen Malateste (Bordeaux, France), Amon Roseamonde Yao (Abidjan, Côte d'Ivoire), Bertine Siloué (Abidjan, Côte d'Ivoire).
Administrative Team: Gwenaelle Clouet (Bordeaux, France), Madikona Dosso (Abidjan, Côte d'Ivoire), Alexandra Doring§ (Bordeaux, France), Adrienne Kouakou (Abidjan, Côte d'Ivoire), Elodie Rabourdin (Bordeaux, France), Jean Rivenc (Pessac, France).
Consultants/ Working Groups: Xavier Anglaret (Bordeaux, France), Boubacar Ba (Bamako, Mali), Renaud Becquet (Bordeaux, France), Jean Bosco Essanin (Abidjan), Andrea Ciaranello (Boston, USA), Sébastien Datté (Abidjan, Côte d'Ivoire), Sophie Desmonde (Bordeaux, France), Jean-Serge Elvis Diby (Abidjan, Côte d'Ivoire), Geoffrey S.Gottlieb* (Seattle, USA), Apollinaire Gninlgninrin Horo (Abidjan, Côte d'Ivoire), Serge N'zoré Kangah (Abidjan, Côte d'Ivoire), Denis Malvy (Bordeaux, France), David Meless (Abidjan, Côte d'Ivoire), Aida Mounkaila-Harouna (Bordeaux, France), Camille Ndondoki (Bordeaux, France), Caroline Shiboski (San Francisco USA), Boris Tchounga (Abidjan, Côte d'Ivoire), Rodolphe Thiébaut (Bordeaux, France), Gilles Wandeler (Dakar, Senegal).
References
- 1.UNAIDS. UNAIDS report on the global AIDS. Geneva, Switzerland: UNAIDS; 2012. [Google Scholar]
- 2.Anabwani G, Navario P. Nutrition and HIV/AIDS in sub-Saharan Africa: an overview. Nutrition. 2005;21(1):96–9. doi: 10.1016/j.nut.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Bolton-Moore C, Mubiana-Mbewe M, Cantrell R, et al. Clinical outcomes and cd4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298(16):1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 4.Weigel R, Phiri S, Chiputula F, et al. Growth response to antiretroviral treatment in HIV-infected children: a cohort study from Lilongwe, Malawi. Trop Med Int Health. 2010;15(8):934–44. doi: 10.1111/j.1365-3156.2010.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutcliffe CG, Van Dijk JH, Munsanje B, et al. Weight and height z-scores improve after initiating ART among HIV-infected children in rural Zambia: a cohort study. BMC Infect Dis. 2011;11:54. doi: 10.1186/1471-2334-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yotebieng M, Van Rie A, Moultrie H, et al. Six-month gains in weight, height, and CD4 predict subsequent antiretroviral treatment responses in HIV-infected South African children. AIDS. 2010;24(1):139–46. doi: 10.1097/QAD.0b013e328332d5ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aurpibul L, Puthanakit T, Taecharoenkul S, et al. Reversal of growth failure in HIV-infected Thai children treated with non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. AIDS Patient Care STDS. 2009;23(12):1067–71. doi: 10.1089/apc.2009.0093. [DOI] [PubMed] [Google Scholar]
- 8.Reddi A, Leeper SC, Grobler AC, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr. 2007;7(1):13. doi: 10.1186/1471-2431-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chantry CJ, Cervia JS, Hughes MD, et al. Predictors of growth and body composition in HIV-infected children beginning or changing antiretroviral therapy. HIV Med. 2010;11(9):573–83. doi: 10.1111/j.1468-1293.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies MA, Keiser O, Technau K, et al. Outcomes of the South African National Antiretroviral Treatment Programme for children: The IeDEA Southern Africa collaboration. S Afr Med J. 2009;99(10):730–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Verweel G, Van Rossum AMC, Hartwig NG, et al. Treatment with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children is associated with a sustained effect on growth. Pediatrics. 2002;109(2):E25. doi: 10.1542/peds.109.2.e25. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Antiretroviral therapy for HIV infection in infants and children: Towards universal access, Recommendations for a public health approach, 2010 revision. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 13.Gortmaker S, Hughes M, Cervia, et al. Effect of Combination Therapy Including Protease Inhibitors on Mortality Among Children and Adolescents Infected With HIV-1. New England Journal of Medicine. 2001;345(21):1522–28. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 14.Cervia JS, Chantry CJ, Hughes MD, et al. Associations of proinflammatory cytokine levels with lipid profiles, growth, and body composition in HIV-infected children initiating or changing antiretroviral therapy. Pediatr Infect Dis J. 2010;29(12):1118–22. doi: 10.1097/INF.0b013e3181ed9f4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115(6):1119–28. doi: 10.1016/j.jaci.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Allard JP, Aghdassi E, Chau J, et al. Effects of vitamin E and C supplementation on oxidative stress and viral load in HIV-infected subjects. AIDS. 1998;12(13):1653–9. doi: 10.1097/00002030-199813000-00013. [DOI] [PubMed] [Google Scholar]
- 17.McGrath CJ, Chung MH, Richardson BA, et al. Younger age at HAART initiation is associated with more rapid growth reconstitution. AIDS. 2011;25(3):345–55. doi: 10.1097/QAD.0b013e32834171db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gsponer T, Weigel R, Davies MA, et al. Variability of growth in children starting antiretroviral treatment in southern Africa. Pediatrics. 2012;130(4):e966–77. doi: 10.1542/peds.2011-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musoke PM, Mudiope P, Barlow-Mosha LN, et al. Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort study. BMC Pediatr. 2010;(10):56. doi: 10.1186/1471-2431-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchacz K, Cervia JS, Lindsey JC, et al. Impact of protease inhibitor-containing combination antiretroviral therapies on height and weight growth in HIV-infected children. Pediatrics. 2001;108(4):E72. doi: 10.1542/peds.108.4.e72. [DOI] [PubMed] [Google Scholar]
- 21.Madec Y, Germanaud D, Moya-Alvarez V, et al. HIV prevalence and impact on renutrition in children hospitalised for severe malnutrition in Niger: an argument for more systematic screening. PLoS One. 2011;6(7):e22787. doi: 10.1371/journal.pone.0022787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arrivé E, Dicko F, Amghar H, et al. HIV status disclosure and retention in care in HIV-infected adolescents on antiretroviral therapy (ART) in West Africa. PLoS ONE. 2012;7(3):e33690. doi: 10.1371/journal.pone.0033690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekouevi DK, Azondekon A, Dicko F, et al. 12-month mortality and loss-to-program in antiretroviral-treated children: The IeDEA pediatric West African Database to evaluate AIDS (pWADA), 2000-2008. BMC Public Health. 2011;(11):519. doi: 10.1186/1471-2458-11-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waterlow JC. Classification and definition of protein-calorie malnutrition. Br Med J. 1972;3(5826):566–9. doi: 10.1136/bmj.3.5826.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. Multicentre Growth Reference Study Group WHO Child Growth Standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 26.de Onis M, Onyango AW, Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bulletin of the World Health Organization. 2007;85:660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 28.WHO. Training Course on Child Growth Assessment Module B: Measuring a child's growth. Geneva, Switzerland: WHO; 2008. [Accessed 9 October 2014]. Available at: http://www.who.int/childgrowth/training/module_b_measuring_growth.pdf. [Google Scholar]
- 29.Zanoni BC, Phungula T, Zanoni HM, et al. Predictors of poor CD4 and weight recovery in HIV-infected children initiating ART in South Africa. PLoS One. 2012;7(3):e33611. doi: 10.1371/journal.pone.0033611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller TL, Agostoni C, Duggan C, et al. Gastrointestinal and nutritional complications of human immunodeficiency virus infection. J Pediatr Gastro enterol Nutr. 2008;47(2):247–53. doi: 10.1097/MPG.0b013e318181b254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364(9441):1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 32.Becquet R, Marston M, Dabis F, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breast milk: a meta-analysis. PLoS ONE. 2012;7(2):e28510. doi: 10.1371/journal.pone.0028510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leroy V, Malateste K, Rabie H, et al. Outcomes of antiretroviral therapy in children in Asia and Africa: a comparative analysis of the IeDEA pediatric multiregional collaboration. J Acquir Immune Defic Syndr. 2013;62(2):208–219. doi: 10.1097/QAI.0b013e31827b70bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.United Nations Children's Fund, World Health Organization, and World Bank. UNICEF-WHO-World Bank Joint Child Malnutrition Estimates. New York, USA: UNICEF; Geneva, Switzerland: WHO; Washington DC, USA: World Bank; 2012. [Google Scholar]
- 35.Feinstein L, Yotebieng M, Moultrie H, et al. Effect of baseline immune suppression on growth recovery in HIV positive South African children receiving antiretroviral treatment. J Acquir Immune Defic Syndr. 2012;61(2):235–242. doi: 10.1097/QAI.0b013e3182634e09. [DOI] [PubMed] [Google Scholar]
- 36.Chantry CJ, Cervia JS, Hughes MD, et al. Predictors of growth and body composition in HIV-infected children beginning or changing antiretroviral therapy. HIV Med. 2010;11(9):573–583. doi: 10.1111/j.1468-1293.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desmonde S, Dicko F, Koueta F, et al. Association between age at antiretroviral therapy initiation and 24-month immune response in HIV-infected children in West Africa. AIDS. 2014 doi: 10.1097/QAD.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grobler L, Siegfried N, Visser ME, et al. Nutritional interventions for reducing morbidity and mortality in people with. HIV Cochrane Database Syst Rev. 2013;2:CD004536. doi: 10.1002/14651858.CD004536.pub3. [DOI] [PubMed] [Google Scholar]
- 39.Irlam JH, Siegfried N, Visser ME, et al. Micronutrient supplementation for children with HIV infection. Cochrane Database Syst Rev. 2013;10:CD010666. doi: 10.1002/14651858.CD010666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO. Consolidated guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. [Accessed 9 October 2014];Summary of key features and recommendations. 2013 Available at: http://www.who.int/hiv/pub/guidelines/arv2013/short_summary/en/