Abstract
Cord blood (CB) leukocytes have inherent telomere length (TL) variation, and CB hematopoietic stem cells (HSC) can maintain high telomerase levels preventing telomere attrition in vitro. We evaluated TL changes in 13 adult double unit CB transplantation (CBT) recipients. In the 26 units, we observed a marked variation in CB TL at thaw (median 9.99 kb, range 6.85-13.5). All 13 patients engrafted. Of 11 engrafting with one unit, there was no correlation between unit dominance and TL (mean dominant unit TL 8.84 kb ± 1.76, mean non-engrafting unit TL 10.3 kb ± 1.81, P = 0.77). Serial measurements of TL up to 1-year post-CBT demonstrated an overall mean 3.04 kb ± 0.16 TL decrease with only one patient exhibiting telomere maintenance. In summary, initial TL does not predict CB unit dominance. Moreover, our analysis suggests neonatal hematopoiesis makes a transition to an HSC characterized by changes in average TL and potentially low telomerase asymmetric cell division in adult CBT recipients. Further investigation of alterations in telomere length and its clinical implications post-transplant of this observation are indicated.
Keywords: Allogeneic transplantation, cord blood, telomere length
Introduction
Cord blood (CB) is an alternative source of allogeneic hematopoietic stem cells (HSC) for the transplantation of patients lacking suitable human leukocyte antigen (HLA)-matched adult donors. Although CB transplantation (CBT) has the advantage of a reduced stringency of required HLA-match, it is limited by the low progenitor cell dose resulting in impaired engraftment and restricting the application of CBT in larger children and adults. One strategy to extend transplant access in adult patients is to combine two units in a double unit graft1. Intriguingly, a single unit mediates sustained donor hematopoiesis in the majority of patients. However, the mechanisms of unit dominance have not been fully elucidated. Furthermore, although healthy long-term survivors of CBT are documented, the long-term effects of transplanting a limited number of HSC from a CB unit into adult recipients have not been fully established.
It is known that CB progenitors have a significantly higher replicative potential than adult HSC2, although there is an inherent biological variation at birth. We have previously shown that CB demonstrates maintenance of telomere length (TL) in vitro due to persisting levels of telomerase activity for 4-5 months3. The effect of replicative stress in vivo on TL, however, is unknown. We, therefore, investigated the extent of TL variability in clinical CB units, the influence of TL upon unit dominance after CBT, and the effect of the in vivo microenvironment on the degree of neonatal TL changes during the first post-transplant year in adult double unit CBT recipients.
Study Design
Thirteen patients with high-risk hematologic malignancies were transplanted with 4-6/6 HLA-A, -B antigen, -DRB1 allele matched unrelated donor double unit CB grafts after myeloablative or non-myeloablative conditioning as previously described4-6. All patients provided informed consent for transplantation in accordance with the Declaration of Helsinki and signed Institutional Review Board-approved consent for the laboratory analysis of each CB unit and serial peripheral blood samples. Each CB unit was analyzed on the same day as clinical transplantation. Mononuclear cells (MNCs) were isolated from each unit and from peripheral blood at days 28, 100, 180 and 1-year post-CBT by density gradient separation with Ficoll-Hypaque. DNA was then isolated from MNC pellets and quantified using a BioTek Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, VT, U.S.A). Southern Blot was performed using the Roche TeloTAGGG Telomere Length Assay (Roche Diagnostics GmBH, Mannheim, Germany) as per the manufacturer’s instructions. Clinical engraftment was evaluated after transplantation using quantitative polymerase chain reaction (PCR) for informative short tandem repeat regions that distinguished each donor and the recipient7. Two-sided t tests were utilized to determine potential correlations between TL and unit dominance.
Results
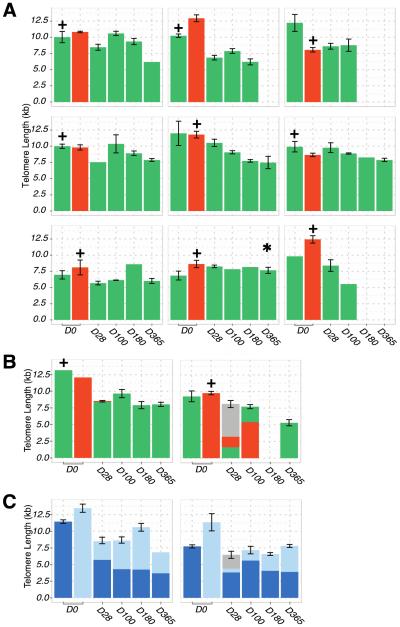
Twenty-six CB units were used to transplant 13 patients (median age 52 years, range 23-61). We observed a marked variation in CB TL at thaw: median 9.99 kilobases (kb) ± 1.86 (range 6.85-13.5). Day 0 values are shown in Figure 1. The median infused total nucleated cell (TNC) dose was 2.9 × 107/kg (range 2.0-4.4) in the larger unit and 2.0 × 107/kg (range 1.7-2.7) in the smaller unit. All 13 patients engrafted at a median of 22 days (range 15-28) in 9 myeloablative recipients and 9-13 days post-CBT in 4 non-myeloablative recipients.
Figure 1.
Bar chart to show the TL in kb for each CB unit of 13 patients from the day of transplantation (Day 0) to last follow-up Patients were analyzed up to 1-year post-transplant or death (3 patients died prior to one year). The unit with the higher infused viable CD34+ dose per kilogram recipient body weight is indicated with a + sign above the unit on day zero. 1A. In 9 patients demonstrating complete single unit dominance as from 28 days after CBT, the unit responsible for sustained donor hematopoiesis is indicated in green whereas non-engrafting units are shown in red. Only one patient with complete unit dominance demonstrated TL maintenance post-transplant with a mean percentage TL gain of 11.5% (indicated with the asterix). 1B. Unit TL in 2 patients who displayed initial mixed chimerism but ultimate unit dominance (indicated in green). The proportions represent the contribution of each unit at that time-point with the grey indicating a transient contribution of host hematopoiesis. 1C. TL in 2 patients with complete donor engraftment but persistent mixed chimerism at 1-year post-CBT. Given the units co-engrafted and clear unit dominance could not assigned they are indicated in light and dark blue.
One CB unit mediated donor hematopoiesis in 11 patients (dominant unit in green, Figures 1A and 1B) whereas 2 patients showed mixed chimerism up to 1 year (Figure 1C). In the 11 patients with unit dominance, there was no significant association between the infused TNC dose, viable CD34+ cell dose, CD3+ cell dose, nor the percentage of viable CD34+ cells post-thaw of each unit and unit dominance (all p = NS). Specifically, the infused viable CD34+ cell dose of the dominant unit was 0.88 × 105/kg (range 0.44-3.46) and that of the non-dominant unit was 1.25 × 105/kg (range 0.22-3.14). Of these 11 patients, 5 patients engrafted with the unit that had the longer TL (median 11.0 kb, range 9.93-13.2) whereas 6 engrafted with the shorter TL unit (median 9.55 kb, range 6.8-10.2), p = NS. Overall, there was no relationship between TL and unit dominance: dominant unit mean TL 8.84 kb ± 1.76 and non-engrafting unit mean 10.3 kb ± 1.81, p = 0.77.
Serial measurements of TL over time (from transplant day to 1 year) demonstrated an overall decrease in TL of 3.04 kb ± 0.16. Only one patient with complete unit dominance demonstrated TL maintenance post-transplant with a mean percentage TL gain of 11.5% (indicated with asterix, Figure 1A). The 10 remaining patients with complete unit dominance demonstrated a TL decrease with an overall mean percentage loss of −32.7% ± 3.5. TL also shortened in the 2 remaining patients with sustained co-engraftment of both units (Figure 1C). When we evaluated the changes in TL over time in the patient peripheral blood the initial average TL was 8.19 kb ± 1.41 at day 28 and 6.49 kb ± 0.96 at 1-year post-transplant or last follow-up (p = 0.09) demonstrating an overall trend toward telomere shortening.
Discussion
We demonstrate for the first time that despite the wide variation in TL in publically banked CB units at the time of transplantation, TL does not appear to influence unit dominance in double unit CBT. Due to the small patients numbers we were not able to investigate whether TL influences the speed and success of donor-derived neutrophil engraftment. Such analyses would require large patient numbers and multivariate analysis controlling for the infused viable CD34+ cell dose of the dominant unit, an established critical determinant of engraftment after double-unit CBT7. A further significant finding is that only one patient of 13 showed TL stabilization. This patient likely had persistent telomerase activity as has been reported in CB for approximately 4-5 months after birth3. TL stabilization has also been observed in a small minority of autologous HSC recipients transplanted for the treatment of multiple myeloma (M.A.S. Moore unpublished data, 2013).
It should be acknowledged that the day 28 sample in patient 2, Figure 1B, and all time-points from both patients in Figure 1C had combinations of both units +/− a host component limiting the TL assessment of the individual CB units. Nonetheless, taken together the data from our analysis would suggest that all but one patient demonstrated an overall decrease in TL as would be expected following multiple rounds of cell division. A significant and unavoidable inherent limitation of this study was that true HSC were not able to be purified from the patient’s graft to specifically evaluate the TL in this cell sub-population. The number of HSC required to perform such an analysis would be prohibitive, and this question will likely never be able to be addressed. Additionally, telomerase activity from HSC isolated from the patient in serial samples post-transplant was also not able to be evaluated. Nonetheless we propose that evaluation of CB MNC is an acceptable surrogate for HSC TL assessment as has been suggested in analysis of adult peripheral blood
Taken together, we postulate that telomerase activity may be high but insufficient to compensate for the robust HSC expansion that is necessary for sustained engraftment and the high proliferation of more differentiated progenitors. Moreover, based on our preliminary data, we suggest that in the majority of adult CBT recipients a transition from a fetal-neonatal to adult HSC is made characterized by lower telomerase activity and asymmetric division in vivo. When this transition would naturally occur in normal human development, however, is unknown and it would be of interest to compare the natural history of TL in normal children versus patients transplanted with CB. Follow-up of adult CBT recipients who are healthy long-term survivors would suggest this observation has no clinical relevance. However, the clinical implications of telomere shortening in CBT recipients and the comparison of CBT recipients with those of adult donor allografts require further investigation.
Submission YBBMT-D-14-00582: Highlights.
Banked cord blood (CB) units have a high degree of telomere length (TL) variation.
TL does not predict unit dominance after double-unit CB transplantation.
Serial analysis of blood MNC TL is suggested in CB transplantation recipients.
Acknowledgements
This work was supported in part by the Gar Reichman Fund of the Cancer Research Institute (M.A.S.M.) and P01 CA23766 from the National Cancer Institute, National Institutes of Health (J.N.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of two partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 2.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gammaitoni L, Weisel KC, Gunetti M, et al. Elevated telomerase activity and minimal telomere loss in cord blood long-term cultures with extensive stem cell replication. Blood. 2004;103:4440–4448. doi: 10.1182/blood-2003-09-3079. [DOI] [PubMed] [Google Scholar]
- 4.Barker JN, Abboud M, Rice RD, et al. A "no-wash" albumin-dextran dilution strategy for cord blood unit thaw: high rate of engraftment and a low incidence of serious infusion reactions. Biol Blood Marrow Transplant. 2009;15:1596–1602. doi: 10.1016/j.bbmt.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponce DM, Sauter C, Devlin S, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19:799–803. doi: 10.1016/j.bbmt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponce DM, Gonzales A, Lubin M, et al. Graft-versus-Host Disease after Double-Unit Cord Blood Transplantation Has Unique Features and an Association with Engrafting Unit-to-Recipient HLA Match. Biol Blood Marrow Transplant. 2013;19:904–911. doi: 10.1016/j.bbmt.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avery S, Shi W, Lubin M, et al. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood. 2011;117:3277–3285. doi: 10.1182/blood-2010-08-300491. [DOI] [PMC free article] [PubMed] [Google Scholar]