Abstract
Purpose
Familial clustering of longevity is well documented and includes both genetic and other familial factors, but the specific underlying mechanisms are largely unknown. We examined whether low incidence of specific cancers is a mechanism for familial clustering of longevity.
Methods
The study-population of individuals from longevity-enriched families consisted of 3267 offspring from 610 Danish long-lived families defined by two siblings attaining an age of 90+. The offspring of the long-lived siblings were followed 1968–2009. Using high quality registry data, observed numbers of cancers were compared to expected numbers based on gender-, calendar period-, and age-specific incidence rates in the general population.
Results
During the 41 year follow-up period, a total of 423 cancers occurred in 397 individuals. The standardized incidence ratios (SIRs) (95% confidence interval) for offspring of long-lived individuals were 0.78 (0. 70, 0.86) for overall cancer; 0.66 (0.56, 0.77) for tobacco-related cancer; 0.34 (0.22, 0.51) for lung cancer; 0.88 (0.71, 1.10) for breast cancer; 0.91 (0.62, 1.34) for colon cancer.
Conclusions
The low incidence of tobacco-related cancers in long-lived families compared to non-tobacco-related cancers suggests that health behavior plays a central role in lower early cancer incidence in offspring of long-lived siblings in Denmark.
Keywords: Cancer, Familial clustering, Longevity, Cohort study, Lifestyle and ageing
Introduction
Familial clustering of longevity has been demonstrated in a number of studies in different populations. It has been shown that relatives of individuals or sib-pairs attaining high ages have a better survival than comparison groups [1–3]; that the oldest proportion of a population is more closely related than would be expected by chance [4], and that the association between long-lived probands and survival in their relatives is stronger the closer they are related [4, 5]. Several studies of Scandinavian twins found that lifespan is moderately heritable [6–8], and that the heritability is likely to increase at the highest ages [9]. Less is known about the mechanisms behind the familial clustering of longevity, but both genetic and environmental factors contribute to longevity. In cross-sectional studies, evidence has been provided for better health status in longevity-enriched families compared to control groups not enriched for longevity in terms of lower prevalence of myocardial infarction, hypertension, diabetes, cardiovascular disease, and pulmonary disease [10–13]. Interestingly, in a US and a Danish nationwide study [10, 12], no association was found between familial longevity enrichment and cancer prevalence, and in a Dutch study [14], no association between number of cancer-associated risk alleles and longevity-enriched families was found. Studies involving other measures of health such as self-rated health and physical measures [12] and measures of tasks requiring attention, working memory, and semantic processing [15] found favorable outcomes in offspring of longevity-enriched families when compared to individuals without a family history of exceptional survival.
To better understand the mechanisms that lead to these states of good health in long-lived families and to longevity itself, longitudinal studies are needed that follow members of these families over time. Some such studies exist and have found lower cause-specific mortality for and delayed onset of disease for several leading causes of death [16–19], but only one [18] found lower cancer mortality. The above-mentioned literature provides mixed evidence for a lower cancer occurrence as a mechanism for clustering of longevity in families. While some animal studies indicate a trade-off mechanism between aging and the risk of cancer [20–24], two more recent studies suggest that familial longevity enrichment is associated with lower cancer incidence [25, 26]. In the following, we take advantage of Danish population registers, and the screening of long-lived families in three nationwide studies to shed light on possible mechanisms by comparing incidence of all cancers except non-melanoma skin cancer, as well as incidence of specific common cancer types: breast cancer, colon cancer, prostate cancer, lung cancer, and tobacco-related cancer in the long-lived families, with population-based cancer incidence rates stratified for gender, age and calendar period. The offspring generation of long-lived families is thus compared to the entire Danish population using the population-based rates. Based on the Danish twin study [25], we expect to find lower cancer incidence among offspring, indicating that lower cancer occurrence is contributing to familial longevity. Also, incidence of specific cancers may provide further information about the mechanism.
Methods
Study population
For a more detailed description of the procedure for identifying and including offspring from long-lived families, see Web Appendix 1. Here follows a brief outline of the inclusion procedure: The identification of offspring from long-lived families was undertaken in three nationwide, consecutive studies in Denmark, for which recruitment ran sequentially during the years 2004 to 2009: a pilot study: Danish Oldest Siblings (DOS) pilot study, the Genetics of Healthy Aging (GeHA) study [27], and the Long Life Family Study (LLFS) [28].
Initially, all individuals born before April 2, 1918, and alive in 2004 were identified in the Danish Civil Registration System (DCRS), which covers all persons alive and living in Denmark on or after April 2nd, 1968 [29]. Identification of long-lived sib pairs from the DCRS and church records is described in the web appendix. In all, 1511 siblings from 659 families were enrolled in either DOS, GeHA, or LLFS, with 246 siblings from 114 families in DOS, 1000 siblings from 469 families in GeHA, and 265 siblings from 76 families in LLFS. To further ensure reliable family information and that the families in this study were strongly enriched for longevity, we restricted our study population to the offspring of those siblings who 1) participated in an interview in either DOS, GEHA or LLFS 2) survived to age 90+ before July 1, 2010, and 3) had at least another participating sibling surviving to age 90+ before July 1, 2010. This means that the population under study consisted of the offspring of those sets of siblings who survived to age 90+ and participated in an interview (DOS, GEHA or LLFS). A total of 1405 siblings from 628 families (99 families in the DOS, 454 families in the GeHA study, and 75 families in the LLFS) fulfilled these criteria. Of the 1405 siblings, 264 had no offspring, so of the remaining 1141 siblings from 611 families, 3297 offspring were identified. Of these offspring, six had unknown vital status in the DCRS, a further 17 had a status as emigrants at end of study in 2009 but with an unknown date of emigration, and one with emigration status had emigrated before April 2, 1968; four offspring had died at an unknown date, and two had died before April 2, 1968. The remaining 3267 offspring from 610 families comprise our study population.
Cancer incidence
In order to study cancer incidence in the long-lived families, we used the personal identification number to link the study population to the Danish Cancer Registry (DCR) [30]. This registry is population-based and contains records of all incidences of malignant neoplasms in the Danish population from 1943 onwards. The register is considered almost complete: in a comparison with independent and redundant data from the Hospital Discharge Registry system, death certificates, and a pathology register, the validity and completeness of the DCR was found to be 95–98% [30–32]. Moreover, with a proportion of 89% of all tumors having been morphologically verified, it has a high degree of validity.
The classification of cancer in the DCR before 1977 was based on the modified ICD-7 classification, between 1978 and 2003 cancer was also classified according to the ICD-O-1 classification, and from 2004 onwards, the ICD-10 and ICD-O-3 classifications have both been used. Furthermore, for the period 1978–2003 the classification was converted from modified ICD-7 to ICD-10, and from ICD-O-1 to ICD-O-3 [30]. To allow for comparison of cancer incidence across periods of different classification systems, cancer diagnoses were grouped into 41 entities of specific cancers following the methodology in the trans-Nordic cancer study collaboration NORDCAN [33, 34]. We studied overall incidence as well as breast, colon, prostate, lung and combined tobacco-related cancer. In the study of all cancers, we excluded non-melanoma skin cancer, and in the study of overall as well as tobacco-related cancer, we permitted an individual to have several primary cancers while adhering to the IARC/IACR rules of counting multiple cancers in the same site as one primary cancer only [35]. Consequently, prevalent cases do not contribute with new cancers to the cancer site for which they are prevalent, nor do they contribute with risk time for the cancer incidence of that specific site. The category of tobacco-related cancers consisted of the pooling of the following 18 NORDCAN cancer sites (using NORDCAN terminology): lip, tongue, mouth, salivary glands, pharynx, oesophagus, stomach, colon, rectum and anus, pancreas, nose and sinuses, larynx, lung, cervix uteri, ovary etc., kidney, bladder etc., and acute leukaemia [36]. The exact ICD-10 and ICD-7 codes for the NORDCAN cancer sites have been published in Acta Oncologica (Table II) [34].
Table 2.
Cancer Incidence in Offspring of Danish Long-lived Families From 1968 to 2009, Male and Female Combined, Stratified on Age.
| Ageband | Risktime | Observed number of cancers |
Expected number of cancers |
SIR (obs/exp) |
95% CI | ||
|---|---|---|---|---|---|---|---|
| Person-years | Percent | Count | Percent | ||||
| 0 – 14 | 1,284.7 | 1.0% | 0 | 0.0% | 0.1 | 0 | |
| 15 – 29 | 22,229.0 | 16.9% | 6 | 1.4% | 7.2 | 0.83 | 0.37, 1.85 |
| 30 – 34 | 14,962.9 | 11.4% | 9 | 2.1% | 10.6 | 0.85 | 0.44, 1.62 |
| 35 – 39 | 15,854.5 | 12.0% | 14 | 3.3% | 18.7 | 0.75 | 0.43, 1.31 |
| 40 – 44 | 15,902.2 | 12.1% | 17 | 4.0% | 31.1 | 0.55 | 0.33, 0.90 |
| 45 – 49 | 15,681.1 | 11.9% | 33 | 7.8% | 50.2 | 0.66 | 0.47, 0.91 |
| 50 – 54 | 15,094.0 | 11.5% | 55 | 13.0% | 74.8 | 0.74 | 0.55, 0.98 |
| 55 – 59 | 13,442.7 | 10.2% | 85 | 20.1% | 102.3 | 0.83 | 0.67, 1.03 |
| 60 – 64 | 9,992.8 | 7.6% | 96 | 22.7% | 117.1 | 0.82 | 0.66, 1.02 |
| 65 – 69 | 5,205.8 | 4.0% | 64 | 15.1% | 87.5 | 0.73 | 0.57, 0.94 |
| 70 – 74 | 1,690.4 | 1.3% | 39 | 9.2% | 36.6 | 1.07 | 0.77, 1.49 |
| 75 – 79 | 313.1 | 0.2% | 5 | 1.2% | 8.2 | 0.61 | 0.22, 1.65 |
| 80+ | 42.9 | 0.0% | 0 | 0.0% | 1.2 | 0 | |
| Total | 131,696.0 | 100.0% | 423 | 100.0% | 545.7 | 0.78 | 0.70, 0.86 |
Abbreviations: CI, confidence interval; exp, expected number of cancers; obs, observed number of cancers; SIR, Standardized Incidence Ratio.
Statistical methods
Comparison of cancer incidence between the offspring of long-lived families and the background Danish population was carried out using indirect standardized incidence ratios (SIRs) stratified on 5-year age bands, 5-year calendar periods and on sex. Since members of long-lived families were linked to the DCR by their personal identification number from the DCRS, which has only been given to persons alive and living in Denmark on or after April 2nd, 1968, we only considered cancer incidence after this date. Last follow-up date was December 31, 2009. Estimation of SIRs were done using Poisson regression with robust standard error estimates [37, 38] to adjust for the family clusters.
The study has been approved by The Regional Scientific Ethical Committees for Southern Denmark (S-VF-20030227) and The Danish Data Protection Agency (# J.nr. 2008-41-1753).
Results
A total of 3267 offspring from 610 families with 3696 identified siblings constituted the risk population of which 1641 (50.2%) were males. The total observation time was 130,000+ person years. For breast cancer in women the observation time was 64,980 person years, and for prostate cancer in men the observation time was 65,876 person years.
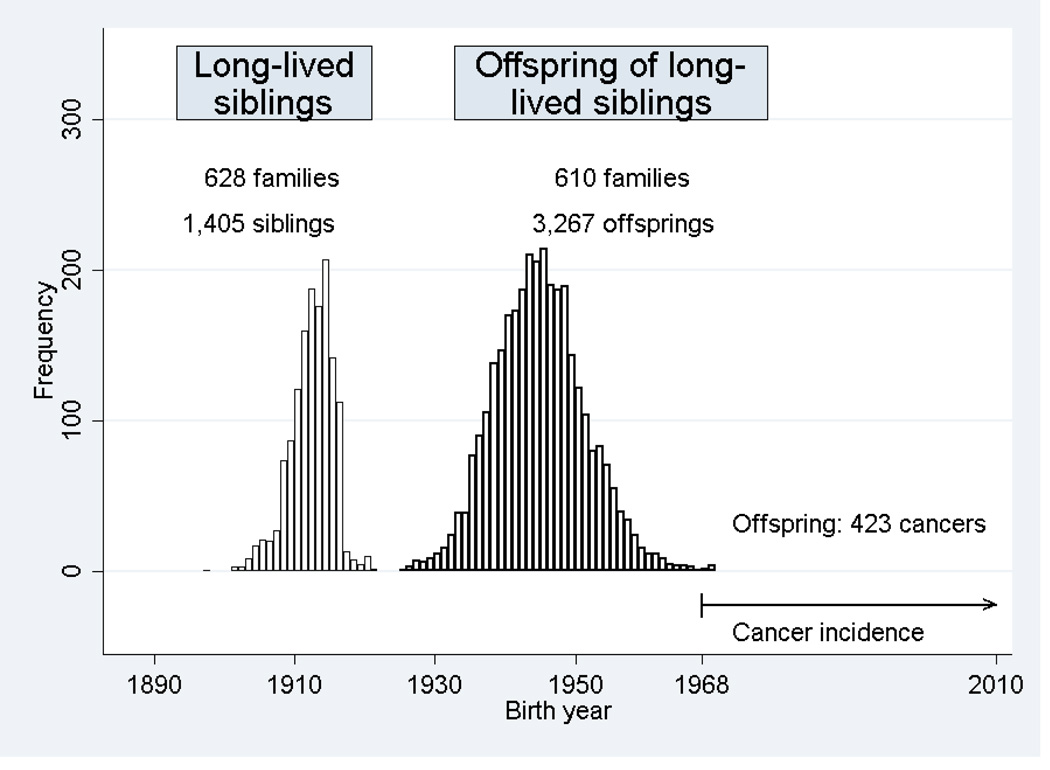
The birth year ranged from 1921 to 1970 with a median birth year of 1944 (see Figure 1). The median age at entry was 23.4 years (range: 0 to 46.6 years), and the median age at exit from study was 64.4 (range: 18.0 to 88.3 years). On December 31, 2009, at the end of study, 88.9% of the initial study population of 3267 individuals was still under observation, 9.1% were deceased, and 2.0% had out-migrated. With regard to observation time, 85.9% was in the age group 15 to 59 years, and only 13.1% was in the age group above 60 years (Tables 1 and 2). A total of 423 primary cancers were observed among 397 individuals during the study period. This corresponded to 373 individuals with one primary cancer, 22 individuals with two primary cancers and 2 individuals with three primary cancers. Of these cancers, 219 (51.8%) were diagnosed at ages 15–59, another 160 cases (37.8%) at ages 60–69, and finally 44 cases (10.4%) after age 70 (Tables 1 and 2).
Figure 1.
Danish long-lived siblings and their offspring: Birth year distribution and cancer occurrence in offspring.
Table 1.
Cancer Incidence in Offspring of Danish Long-lived Families From 1968 to 2009 Stratified on Sex and Age.
| Sex | Ageband | Risktime | Observed number of cancers |
Expected number of cancers |
SIR (obs/exp) |
95% CI | ||
|---|---|---|---|---|---|---|---|---|
| Person- years |
Percent | Count | Percent | |||||
| Males | ||||||||
| 0 – 14 | 630.3 | 1.0% | 0 | 0.0% | 0.1 | 0 | ||
| 15 – 29 | 11,073.8 | 16.8% | 3 | 1.7% | 3.6 | 0.83 | 0.27, 2.54 | |
| 30 – 34 | 7,495.5 | 11.3% | 3 | 1.7% | 4.2 | 0.71 | 0.23, 2.20 | |
| 35 – 39 | 7,930.2 | 12.0% | 5 | 2.9% | 6.5 | 0.77 | 0.32, 1.84 | |
| 40 – 44 | 7,957.1 | 12.0% | 5 | 2.9% | 10.1 | 0.49 | 0.21, 1.18 | |
| 45 – 49 | 7,876.2 | 11.9% | 9 | 5.1% | 17.5 | 0.52 | 0.27, 0.98 | |
| 50 – 54 | 7,589.4 | 11.5% | 14 | 8.0% | 30.1 | 0.46 | 0.28, 0.78 | |
| 55 – 59 | 6,795.0 | 10.3% | 34 | 19.4% | 47.5 | 0.72 | 0.51, 1.01 | |
| 60 – 64 | 5,079.2 | 7.7% | 49 | 28.0% | 61.2 | 0.80 | 0.60, 1.07 | |
| 65 – 69 | 2,607.3 | 3.9% | 34 | 19.4% | 49.2 | 0.69 | 0.49, 0.98 | |
| 70 – 74 | 818.6 | 1.2% | 15 | 8.6% | 21.4 | 0.70 | 0.41, 1.20 | |
| 75 – 79 | 171.3 | 0.3% | 4 | 2.3% | 5.3 | 0.75 | 0.24, 2.39 | |
| 80+ | 28.0 | 0.0% | 0 | 0.0% | 0.9 | 0 | ||
| Total | 66,051.8 | 100.0% | 175 | 100.0% | 257.7 | 0.68 | 0.58, 0.79 | |
| Females | ||||||||
| 0 – 14 | 654.4 | 1.0% | 0 | 0.0% | 0.1 | 0 | ||
| 15 – 29 | 11,155.2 | 17.0% | 3 | 1.2% | 3.6 | 0.84 | 0.27, 2.60 | |
| 30 – 34 | 7,467.4 | 11.4% | 6 | 2.4% | 6.4 | 0.94 | 0.43, 2.08 | |
| 35 – 39 | 7,924.2 | 12.1% | 9 | 3.6% | 12.2 | 0.74 | 0.36, 1.51 | |
| 40 – 44 | 7,945.1 | 12.1% | 12 | 4.8% | 20.9 | 0.57 | 0.31, 1.05 | |
| 45 – 49 | 7,804.9 | 11.9% | 24 | 9.7% | 32.7 | 0.73 | 0.50, 1.08 | |
| 50 – 54 | 7,504.6 | 11.4% | 41 | 16.5% | 44.6 | 0.92 | 0.66, 1.28 | |
| 55 – 59 | 6,647.7 | 10.1% | 51 | 20.6% | 54.8 | 0.93 | 0.71, 1.22 | |
| 60 – 64 | 4,913.7 | 7.5% | 47 | 19.0% | 56.0 | 0.84 | 0.63, 1.12 | |
| 65 – 69 | 2,598.4 | 4.0% | 30 | 12.1% | 38.3 | 0.78 | 0.55, 1.12 | |
| 70 – 74 | 871.8 | 1.3% | 24 | 9.7% | 15.2 | 1.58 | 1.05, 2.40 | |
| 75 – 79 | 141.8 | 0.2% | 1 | 0.4% | 2.9 | 0.34 | 0.05, 2.46 | |
| 80+ | 14.9 | 0.0% | 0 | 0.0% | 0.3 | 0 | ||
| Total | 65,644.2 | 100.0% | 248 | 100.0% | 288.0 | 0.86 | 0.75, 0.98 | |
Abbreviations: CI, confidence interval; exp, expected number of cancers; obs, observed number of cancers; SIR, Standardized Incidence Ratio.
The comparison of overall cancer incidence between the offspring and the Danish population is found in Tables 1 and 2 together with age-specific SIRs. We found 22% fewer cancer cases among the offspring compared to the Danish population, corresponding to 423 observed versus 545.7 expected cancer cases. The SIR was 0.68 (95%-CI: 0.58, 0.79) for men and 0.86 (95%-CI: 0.75, 0.98) for women. For men, at ages under 29, over 80, and in intervening five-year age groups, age-specific SIRs varied between 0.46 and 0.83. Similarly for women, in the same age intervals, the SIRs ranged between 0.57 and 0.94 with two exceptions, one at ages 70–74 for which the SIR was 1.58 (95% CI: 1.05, 2.40) and the other at ages 75–79 with a SIR of 0.34 (95% CI: 0.05, 2.46).
In the analyses of specific cancer types (Table 3), without correcting the significance level for multiple testing, we found a markedly and significantly lower incidence of lung cancer in the offspring for both sexes. The SIR estimate was 0.20 (95%-CI: 0.10, 0.40) for men and 0.50 (95%-CI: 0.30, 0.84) for women. Similarly, for tobacco-related cancer (including lung cancer) the SIR was 0.53 (95%-CI: 0.43, 0.66) for men and 0.82 (95%-CI: 0.66, 1.02) for women. For tobacco-related cancer, we observed 172 cancers which compares to an expected number of 260.7 and a difference of 88.7 cancers. This leaves 33.9 fewer cases of tobacco-unrelated cancer among offspring when comparing the observed 251 tobacco-unrelated cancers to an expected number of 284.9 tobacco-unrelated cancers in a Danish population with similar sex, age and birth year distribution, corresponding to a SIR of 0.88 (95%-CI: 0.77, 1.00). Considering even tobacco-related cancers excluding lung cancer, we observed 148 cases compared to an expected 189.4 cases corresponding to a SIR of 0.78 (95%-CI: 0.66, 0.93).
Table 3.
Cancer Incidence of Overall and Specific Cancers in Offspring of Danish Long-lived Families From 1968 to 2009, Stratified on Sex.
| Cancer type | Observed number of cancers |
Expected number of cancers |
SIR (obs/exp) |
95% CI | |
|---|---|---|---|---|---|
| Overall | Males | 175 | 257.7 | 0.68 | 0.58, 0.79 |
| Females | 248 | 288.0 | 0.86 | 0.75, 0.98 | |
| Males+Females | 423 | 545.7 | 0.78 | 0.70, 0.86 | |
| Breast | Females | 84 | 95.2 | 0.88 | 0.71, 1.10 |
| Colon | Males | 12 | 17.9 | 0.67 | 0.38, 1.17 |
| Females | 19 | 16.2 | 1.17 | 0.71, 1.94 | |
| Males+Females | 31 | 34.1 | 0.91 | 0.62, 1.34 | |
| Prostate | Males | 43 | 38.2 | 1.13 | 0.84, 1.52 |
| Lung | Males | 8 | 39.5 | 0.20 | 0.10, 0.40 |
| Females | 16 | 31.7 | 0.50 | 0.30, 0.84 | |
| Males+Females | 24 | 71.2 | 0.34 | 0.22, 0.51 | |
| Tobacco-related (including lung) | Males | 76 | 143.4 | 0.53 | 0.43, 0.66 |
| Females | 96 | 117.3 | 0.82 | 0.66, 1.02 | |
| Males+Females | 172 | 260.7 | 0.66 | 0.56, 0.77 | |
| Tobacco-unrelated | Males | 99 | 114.3 | 0.87 | 0.71, 1.06 |
| Females | 152 | 170.6 | 0.89 | 0.75, 1.05 | |
| Males+Females | 251 | 284.9 | 0.88 | 0.77, 1.00 |
Abbreviations: CI, confidence interval; exp, expected number of cancers; obs, observed number of cancers; SIR, Standardized Incidence Ratio.
Generally, the observed SIRs were lower in males than in females, most pronounced for tobacco-related cancers: 0.53(0.43, 0.66) vs. 0.82 (0.66, 1.02). Of the 88.7 “missing” tobacco-related cancers among the longevity-enriched offspring, males accounted for 67.4 (76%).
In these results, multiple testing problems may be an issue, and a conservative way to adjust for this is to use a Bonferroni correction. Basing the correction on 12 individual hypotheses, the SIRs for overall cancer and lung cancer were no longer significantly below 1 among females, but among males the SIRs for overall cancer, lung cancer, and tobacco-related cancer remained significantly well below 1.
In our analyses, we have excluded 17 emigrants with unknown date of migration, 6 individuals with unknown vital status, and 4 individuals with unknown date of death. Due to the access of all Danish citizens to a high quality, free health care system and, given the recruitment of the study, to the presumably Danish family network of the offspring, it is unlikely that migrants should have higher cancer incidence than other offspring. However, a possible bias toward too low SIR estimates as a consequence of the exclusion cannot be ruled out. To assess an upper bound of the possible impact this exclusion could have on biasing our SIR estimates, we performed a sensitivity analysis for the overall cancer SIRs, specifying that all 27 individuals had cancer, uniformly diagnosed over the study period and with subsequent death 7 days after cancer diagnosis. As expected, the SIRs of the sensitivity analysis were slightly larger than those from our main analysis: 0.73 (95%-CI: 0.63, 0.85) for males and 0.90 (95%-CI: 0.79, 1.03) for females.
The reduced occurrence of tobacco-related cancer prompted an analysis of the smoking behavior in the offspring of the families from the LLFS, since for these families questionnaire data on smoking habits in offspring were available. Among 627 interviewed offspring in LLFS 19.1% (95%-CI: 15.7, 23.1%) were current smokers and 9.6% (95%-CI: 7.3, 12.6%) were current heavy smokers. These prevalences were 24% respectively 17% lower on a relative scale than predicted from age and sex specific smoking prevalences in the Danish population (www.sundhedsprofil2010.dk).
Discussion
Our findings of lower cancer occurrence in the offspring of long-lived individuals confirm the findings of a Danish twin study [25] that indicates that longer lifespan of one co-twin is associated with lower cancer incidence in the other. Also, our findings are in agreement with those of the Health and Retirement Study (HRS) cohort [26], which is a US representative cohort study that showed that having one or two long-lived parents is associated with lower cancer incidence compared to having two parents attaining an intermediate age. The novelty of the finding in our study is that the lower cancer incidence in long-lived families can largely be attributed to lower tobacco-related cancer incidence. Two factors might underlie our finding of reduced tobacco-related cancers in individuals with long-lived parents. First, genetic factors contributing to longevity might overlap genes that protect the host from developing cancer, given exposure. Second, genetic factors contributing to longevity might overlap genes related to smoking exposure.
Scandinavian twin studies have estimated that 20–30% of the variation in lifespan can be attributed to genetic factors, and that this heritability increases with age, whereas essentially no effect of shared environment has been found. At a first glance, a finding of familial clustering of low smoking prevalence could be seen as a contradiction to twin studies that have not been able to demonstrate an effect of shared familial environment as a mechanism for familial clustering of longevity [6–9]. However, it is quite possible that genes contributing to longevity express themselves through an affinity for healthy lifestyles: e.g. lifestyles in mid and late life, such as smoking and physical activities, have a small to moderate degree of heritability [39]. The choice of lifestyles is a decision of the individual that may be influenced by genetically heritable personality traits and abilities, so that, for instance, physically adept individuals seek out environments with a high degree of physical activity. The lower tobacco-related cancer incidence in offspring of long-lived siblings may be explained by genetic loci in the genome of the longevity families contributing to longevity by protecting smokers from developing cancer. Alternatively, the families may display beneficial smoking habits due to the influence of such loci or shared environmental factors. The latter explanation seems most likely considering the lower smoking frequency found among the offspring in the LLFS.
The overall cancer SIR of 0.75 in our study was remarkably similar to the hazard ratio estimate of 0.76 for cancer incidence in the HRS [26] that compared offspring having at least one long-lived parent to those having two intermediate-lived parents, and where the offspring were followed from about age 55 to about age 75. The hazard ratio estimate was, however, adjusted for environmental factors, including smoking, but this only minimally modified the unadjusted hazard ratio. To the extent that familial longevity is associated with less smoking, it seems reasonable to expect that cancer-specific mortality should be lower in longevity-enriched families, as was found in the New England Centenarian Study [18] but not in the Utah Population Database study [17]. In the former study, the centenarian offspring had lower smoking prevalence than the controls, whereas in the latter study of a Utah population, no information on smoking habits was presented. However, Utah has one of the lowest smoking prevalences in the US, so longevity-enriched families in Utah are perhaps not discriminated by their smoking habits. Moreover, in the Utah study, the main analysis was adjusted for a family history of cancer, and without adjustment there was in fact a moderately lower cancer mortality associated with familial longevity.
Of the specific cancer types, lung cancer stood out as having a very low incidence in the offspring, which, given the strong association between smoking and lung cancer, further supports the finding that smoking prevalence contributes to familial clustering of longevity. Also, we found lower SIRs for males than females, most pronounced for tobacco-related cancers, so lower smoking prevalence among the offspring, and in particular the male offspring, is likely to be the major factor resulting in the low cancer incidence. Solid organ cancers such as breast, colon or prostate cancer did not show marked incidence differences between the offspring and the Danish population, although an observed SIR for breast cancer below one was slightly opposite of what would be expected if longevity-enriched families were associated with a higher socioeconomic status, as a lower smoking prevalence could suggest.
The strength in this study lies in the large number of individuals from longevity-enriched families (close to 3300) and the comparison group of the entire Danish population (about 5 million), both groups followed for more than four decades. The comparison ensures that the association between the groups and cancer incidence is not partially due to higher than normal incidence in the group not enriched for longevity, as might be the case if offspring of short-lived parents were selected as comparison group. Moreover, the high-quality registry data on demographic and cancer variables have ensured the virtually complete information on ages and cancer incidence with no loss to follow-up before death or end of study, except for 2% of out-migrants, and they allowed for adjustment for age, calendar period and sex. Also, the initiation of the DCR in 1943 has meant that probably no prevalent cancer cases are registered as incident cases after 1968, as they would otherwise have remained undetected for more than 25 years. A weakness of our study is the unavailability of response rates in the recruitment of nonagenarian siblings, which makes it difficult to assess the possible extent of a response bias, e.g. a tendency to recruit siblings with safer behavior than other long-lived siblings, and potentially with safer health behaviors in the next generation. Another weakness is that the observational power is rather small after the age of 70. Hence, the association of familial longevity with e.g. prostate cancer incidence could not be fully tested in the present sample.
Conclusion
This study suggests lower tobacco-related cancer incidence as a contributing mechanism for lower early cancer incidence in offspring of long-lived siblings.
Supplementary Material
Acknowledgments
This work is supported by the Danish Agency for Science, Technology and Innovation/The Danish Council for Independent Research [grant number 11-107308] and by the National Institute on Aging [grant number P01 AG08761]. The LLFS study is funded by the US National Institute on Aging / National Institutes of Health [NIA/NIH cooperative agreements U01AG023712, U01AG23744, U01AG023746, U01AG023749, U01AG023755]. The work is based on the EU GEHA (GEnetics of Healthy Ageing) Project [contract number LSHM-CT-2004-503-270] and the Odense University Hospital AgeCare program (Academy of Geriatric Cancer Research). The Danish Aging Research Center is supported by a grant from the VELUX Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perls TT, Bubrick E, Wager CG, Vijg J, Kruglyak L. Siblings of centenarians live longer. Lancet. 1998;351(9115):1560. doi: 10.1016/S0140-6736(05)61126-9. [DOI] [PubMed] [Google Scholar]
- 2.Schoenmaker M, de Craen AJ, de Meijer PH, Beekman M, Blauw GJ, Slagboom PE, et al. Evidence of genetic enrichment for exceptional survival using a family approach: the Leiden Longevity Study. European journal of human genetics : EJHG. 2006;14(1):79–84. doi: 10.1038/sj.ejhg.5201508. [DOI] [PubMed] [Google Scholar]
- 3.Willcox BJ, Willcox DC, He Q, Curb JD, Suzuki M. Siblings of Okinawan centenarians share lifelong mortality advantages. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61(4):345–354. doi: 10.1093/gerona/61.4.345. [DOI] [PubMed] [Google Scholar]
- 4.Gudmundsson H, Gudbjartsson DF, Frigge M, Gulcher JR, Stefansson K. Inheritance of human longevity in Iceland. European journal of human genetics : EJHG. 2000;8(10):743–749. doi: 10.1038/sj.ejhg.5200527. [DOI] [PubMed] [Google Scholar]
- 5.Kerber RA, O'Brien E, Smith KR, Cawthon RM. Familial excess longevity in Utah genealogies. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56(3):B130–B139. doi: 10.1093/gerona/56.3.b130. [DOI] [PubMed] [Google Scholar]
- 6.Herskind AM, McGue M, Holm NV, Sorensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Human genetics. 1996;97(3):319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- 7.Ljungquist B, Berg S, Lanke J, McClearn GE, Pedersen NL. The effect of genetic factors for longevity: a comparison of identical and fraternal twins in the Swedish Twin Registry. The journals of gerontology Series A, Biological sciences and medical sciences. 1998;53(6):M441–M446. doi: 10.1093/gerona/53a.6.m441. [DOI] [PubMed] [Google Scholar]
- 8.Skytthe A, Pedersen NL, Kaprio J, Stazi MA, Hjelmborg JV, Iachine I, et al. Longevity studies in GenomEUtwin. Twin research : the official journal of the International Society for Twin Studies. 2003;6(5):448–454. doi: 10.1375/136905203770326457. [DOI] [PubMed] [Google Scholar]
- 9.Hjelmborg Jv, Iachine I, Skytthe A, Vaupel JW, McGue M, Koskenvuo M, et al. Genetic influence on human lifespan and longevity. Human genetics. 2006;119(3):312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 10.Terry DF, Wilcox M, McCormick MA, Lawler E, Perls TT. Cardiovascular advantages among the offspring of centenarians. The journals of gerontology Series A, Biological sciences and medical sciences. 2003;58(5):M425–M431. doi: 10.1093/gerona/58.5.m425. [DOI] [PubMed] [Google Scholar]
- 11.Westendorp RG, van Heemst D, Rozing MP, Frolich M, Mooijaart SP, Blauw GJ, et al. Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: The Leiden Longevity Study. Journal of the American Geriatrics Society. 2009;57(9):1634–1637. doi: 10.1111/j.1532-5415.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- 12.Frederiksen H, McGue M, Jeune B, Gaist D, Nybo H, Skytthe A, et al. Do children of long-lived parents age more successfully? Epidemiology. 2002;13(3):334–339. doi: 10.1097/00001648-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Newman AB, Glynn NW, Taylor CA, Sebastiani P, Perls TT, Mayeux R, et al. Health and function of participants in the Long Life Family Study: A comparison with other cohorts. Aging. 2011;3(1):63–76. doi: 10.18632/aging.100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beekman M, Nederstigt C, Suchiman HE, Kremer D, van der Breggen R, Lakenberg N, et al. Genome-wide association study (GWAS)-identified disease risk alleles do not compromise human longevity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(42):18046–18049. doi: 10.1073/pnas.1003540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barral S, Cosentino S, Costa R, Matteini A, Christensen K, Andersen SL, et al. Cognitive function in families with exceptional survival. Neurobiology of aging. 2012;33(3):619 e1–619 e7. doi: 10.1016/j.neurobiolaging.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Florez H, Ma Y, Crandall JP, Perreault L, Marcovina SM, Bray GA, et al. Parental longevity and diabetes risk in the Diabetes Prevention Program. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66(11):1211–1217. doi: 10.1093/gerona/glr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien E, Kerber R, Smith K, Mineau G, Boucher K, Reed DL. Familial mortality in the Utah population database: characterizing a human aging phenotype. The journals of gerontology Series A, Biological sciences and medical sciences. 2007;62(8):803–812. doi: 10.1093/gerona/62.8.803. [DOI] [PubMed] [Google Scholar]
- 18.Terry DF, Wilcox MA, McCormick MA, Pennington JY, Schoenhofen EA, Andersen SL, et al. Lower all-cause, cardiovascular, and cancer mortality in centenarians' offspring. Journal of the American Geriatrics Society. 2004;52(12):2074–2076. doi: 10.1111/j.1532-5415.2004.52561.x. [DOI] [PubMed] [Google Scholar]
- 19.Terry DF, Wilcox MA, McCormick MA, Perls TT. Cardiovascular disease delay in centenarian offspring. The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59(4):385–389. doi: 10.1093/gerona/59.4.m385. [DOI] [PubMed] [Google Scholar]
- 20.Campisi J. Cancer and ageing: rival demons? Nature reviews Cancer. 2003;3(5):339–349. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- 21.Campisi J. Aging and cancer cell biology, 2008. Aging Cell. 2008;7(3):281–284. doi: 10.1111/j.1474-9726.2008.00383.x. [DOI] [PubMed] [Google Scholar]
- 22.Ukraintseva SV, Arbeev KG, Akushevich I, Kulminski A, Arbeeva L, Culminskaya I, et al. Trade-offs between cancer and other diseases: do they exist and influence longevity? Rejuvenation research. 2010;13(4):387–396. doi: 10.1089/rej.2009.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinstein BS, Ciszek D. The reserve-capacity hypothesis: evolutionary origins and modern implications of the trade-off between tumor-suppression and tissue-repair. Experimental gerontology. 2002;37(5):615–627. doi: 10.1016/s0531-5565(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 24.Yashin AI, Ukraintseva SV, Akushevich IV, Arbeev KG, Kulminski A, Akushevich L. Trade-off between cancer and aging: what role do other diseases play? Evidence from experimental and human population studies. Mechanisms of ageing and development. 2009;130(1–2):98–104. doi: 10.1016/j.mad.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen K, Pedersen JK, Hjelmborg JV, Vaupel JW, Stevnsner T, Holm NV, et al. Cancer and Longevity--Is There a Trade-off? A Study of Cooccurrence in Danish Twin Pairs Born 1900–1918. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67(5):489–494. doi: 10.1093/gerona/gls087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutta A, Henley W, Robine JM, Langa KM, Wallace RB, Melzer D. Longer lived parents: protective associations with cancer incidence and overall mortality. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(11):1409–1418. doi: 10.1093/gerona/glt061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skytthe A, Valensin S, Jeune B, Cevenini E, Balard F, Beekman M, et al. Design, recruitment, logistics, and data management of the GEHA (Genetics of Healthy Ageing) project. Experimental gerontology. 2011;46(11):934–945. doi: 10.1016/j.exger.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sebastiani P, Hadley EC, Province M, Christensen K, Rossi W, Perls TT, et al. A family longevity selection score: ranking sibships by their longevity, size, and availability for study. American journal of epidemiology. 2009;170(12):1555–1562. doi: 10.1093/aje/kwp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen CB. The Danish Civil Registration System. Scandinavian journal of public health. 2011;39(7 Suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 30.Gjerstorff ML. The Danish Cancer Registry. Scandinavian journal of public health. 2011;39(7 Suppl):42–45. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 31.Østerlind A, Jensen OM. Evaluering af cancerregistreringen i Danmark 1977: en præliminær evaluering af Cancerregistrets og Landspatientregistrets registrering af cancertilfælde. Ugeskr Læger. 1985;147:2483–2488. [PubMed] [Google Scholar]
- 32.Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish Cancer Registry--history, content, quality and use. Danish medical bulletin. 1997;44(5):535–539. [PubMed] [Google Scholar]
- 33.Engholm G, Storm HH, Ferlay J, Christensen N the Nordic Cancer Registries, on behalf of the Association of Nordic Cancer Registries (ANCR) NORDCAN. [updated December; cited 2013 April 10];2012 Available from: http://www-dep.iarc.fr/nordcan.htm. [Google Scholar]
- 34.Engholm G, Ferlay J, Christensen N, Bray F, Gjerstorff ML, Klint A, et al. NORDCAN--a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49(5):725–736. doi: 10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
- 35.International Association of Cancer Registries, International Agency for Research on Cancer, European Network of Cancer Registries. International Rules for Multiple Primary Cancers (ICD-O Third Edition) Lyon: IARC; 2004. Internal Report No. 2004 / 2. [Google Scholar]
- 36.American Cancer Society. Cancer Facts & Figurs, 2013. 2013 Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf. [Google Scholar]
- 37.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrika. 1980;48:817–838. [Google Scholar]
- 38.Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. 1967;1:221–223. [Google Scholar]
- 39.McGue M, Skytthe A, Christensen K. The nature of behavioural correlates of healthy ageing: a twin study of lifestyle in mid to late life. International journal of epidemiology. 2014 doi: 10.1093/ije/dyt210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.