Abstract
Objectives
The objective of our study was to determine whether a simple score combining indices of right ventricular (RV) function and right atrial (RA) size would offer good discrimination of outcome in patients with pulmonary arterial hypertension (PAH).
Background
Identifying a simple score of outcome could simplify risk stratification of patients with PAH and potentially lead to improved tailored monitoring or therapy.
Methods
We recruited patients from both Stanford University (derivation cohort) and VU University Medical Center (validation cohort). The composite end-point for the study was death or lung transplantation. A Cox proportional hazard with bootstrap confidence interval adjustment model was used to determine independent correlates of death or transplantation. A predictive score was developed using the β- coefficients of the multivariate models.
Results
For the derivation cohort (n=95), the majority of patients were female (79%), average age was 43±11 years, mean pulmonary arterial pressure was 54±14 mmHg, and indexed pulmonary vascular resistance was 25±12 WU m2. Over an average follow-up of 5 years, the composite end-point occurred in 34 patients consisting of 26 deaths and 8 patients undergoing lung transplantation. On multivariate analysis, RV systolic dysfunction grade [HR 3.4, 2.0 to 7.8, P<0.001], severe RA enlargement [HR 3.0, 1.3 to 8.1, P=0.009] and systemic blood pressure <110 mmHg [HR 3.3, 1.5 to 9,4, P<0.001] were independently associated with outcome. A right heart (RH) score was constructed based on these 3 parameters compared favorably to the NIH survival equation (0.88[0.79 to 0.94] vs. 0.60[0.49 to 0.710], P< 0.001) but not statistically different than the REVEAL score c-statistic of 0.80[0.69 to 0.88] with P= 0.097. In the validation cohort (n=87), the RH score remained the strongest independent correlate of outcome.
Conclusion
In patients with prevalent PAH, a simple RH score may offer good discrimination of long term outcome in PAH.
Keywords: pulmonary hypertension, heart failure, right heart, atrial function, outcome
Introduction
Pulmonary arterial hypertension (PAH) is a rare condition caused by the progressive narrowing of the small pulmonary arteries, leading to increased pulmonary vascular resistance and rightsided heart failure (1). Despite advances in therapy, the mortality remains high approaching 30 to 50% at 5 year in symptomatic patients (1,2). In recent years, right ventricular (RV) function has emerged as one of the strongest predictors of outcome in PAH (3). Hemodynamic studies have highlighted the prognostic importance of elevated right atrial pressure and decreased cardiac output while imaging studies have highlighted the importance of RV remodeling and systolic function (2,4–6). Moreover, recent scores such as the US Registry to Evaluate Early and Long- Term PAH Disease Management registry (REVEAL) score have integrate several of the clinical and functional parameters (2).
To date, only a few studies have investigated whether right atrial (RA) size or function has incremental value to RV function in predicting outcome in PAH. The importance of RA size in PAH was first suggested by Bustamente-Labarta et al. in their series of 25 patients (7). In a larger study in patients with PAH (n=81), Raymond et al. found that there was a trend for an independent association between RA area index (P=0.106) and the composite end-point of death or transplantation (8). To our knowledge, no study has of yet also investigated the prognostic value of RA function measured by active and passive emptying fractions (RAEF) in PAH.
For our study, we first hypothesized that measures of RA size or function would be independently associated with event free survival in PAH. We further hypothesized that a simple score combining quantitative measures of right heart size or function provide good discrimination of outcome in PAH.
METHODS
Study Design
Our study included first a derivation cohort at Stanford University followed by a validation cohort at the VU Medical Center. After ethics committee approval, consecutive adult patients followed at Stanford University between January 1999 and January 2009 with a confirmed diagnosis of idiopathic or drug and toxin PAH were considered for inclusion in the study. The diagnosis of PAH was based on the standard definition of a mean pulmonary arterial pressure (MPAP) ≥ 25 mmHg and a pulmonary artery wedge pressure ≤ 15 mmHg (9). We excluded patients in whom an echocardiogram was not available and patients with evidence of atrial fibrillation at baseline, left heart failure and significant parenchymal lung disease. Patients recruited at the VU Medical Center had a diagnosis of idiopathic or familial PAH and underwent cardiac magnetic resonance imaging (CMR) as part of a prospective study to evaluate the role of CMR in the management of PAH for which medical ethical consent approval was obtained.
The composite end-point of the study was death or lung transplantation. Death was verified through the National Social Security Death Index and transplantation through chart review. Data collection included demographics, the six-minute walking distance (6MWD), estimated glomerular filtration rate (eGFR), N- terminal pro B-type natriuretic peptide levels (NT-proBNP), the diffusion capacity of carbon monoxide (DLCO) and hemodynamics. Renal function was estimated using the modified diet and renal equation (10). For purposes of standardization, data was collected on the first outpatient visit after stabilization on disease modifying medication (prostanoids, endothelin receptor blockers, or phosphodiesterase inhibitors). We chose this time point for 2 reasons. First, this time point corresponds to the time when patients obtain their echocardiography, 6MWD test and laboratory testing (metabolic panel and NT-proBNP) on the same day. In addition, the baseline right heart catheterization is often obtained within a 3 to 6 month time frame of this visit.
Echocardiography
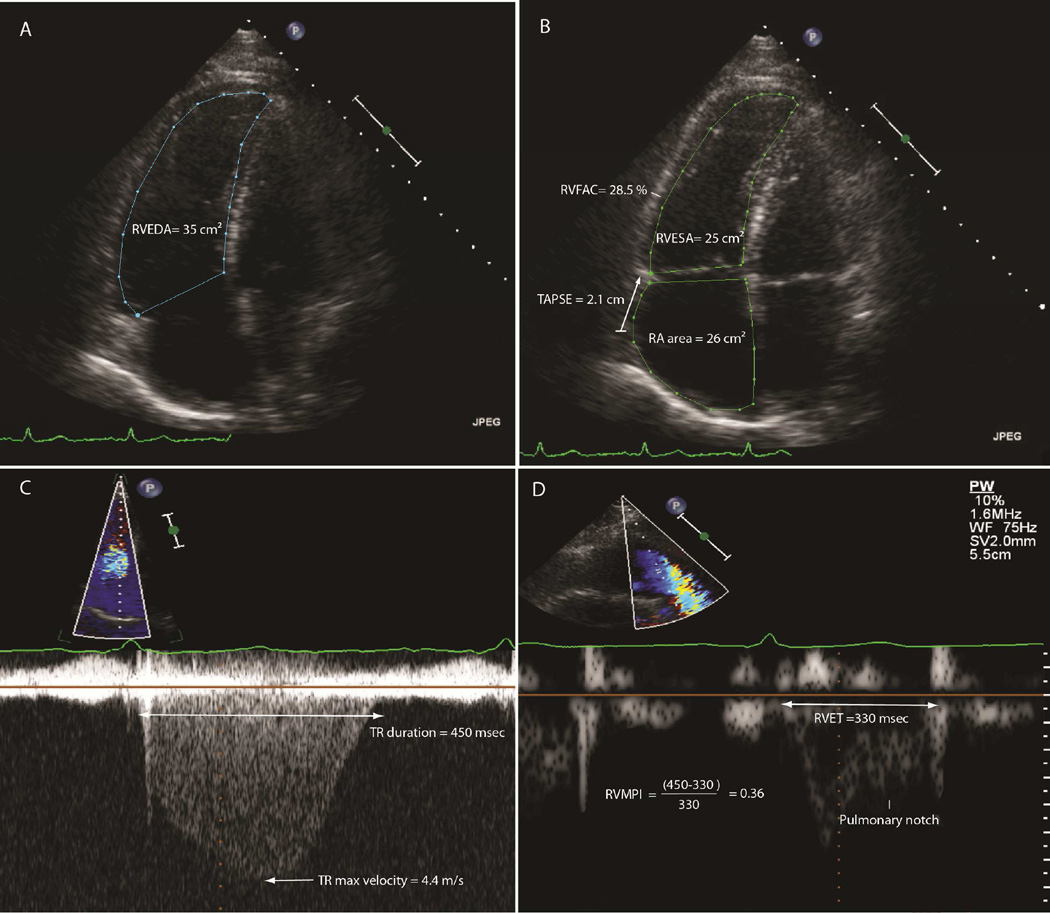
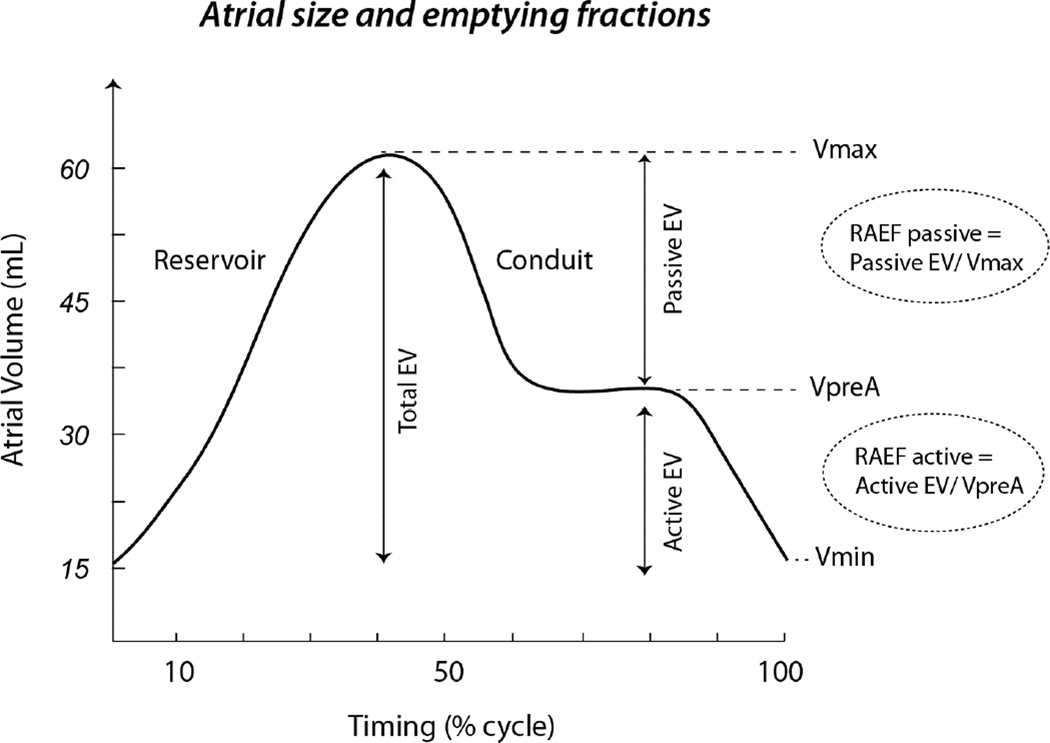
Digitized echocardiographic studies were analyzed by Stanford Cardiovascular Institute Biomarker and Imaging Core Laboratory in accordance with the published guidelines of the American Society of Echocardiography [ASE] (11). All measures were averaged over 3 cycles and RV or RA size measures were indexed to body surface area. RV end-diastolic and endsystolic areas as well as RA size were measured from the apical 4-chamber view (Figure 1). RV function was quantified using RV fractional area change (RVFAC), tricuspid annular systolic excursion (TAPSE) and RV myocardial performance index (RVMPI) as previously described (11–13). RA size was measured at end-systole (RAmax), pre-atrial contraction (RApre-A) and at end-diastole (RAmin) (Figure 2) and total, passive and active emptying fractions (RAEF) were calculated as follow: RAEFtotal = (RAmax − RAmin)/RAmax; RAEFpassive = (RAmax − RApre-A)/RAmax and RAEFactive = (RA pre-A size −RA min-size)/RA pre-A.
Figure 1. Representative measures of right heart size and functional parameters.
Section A shows measures of RVEDA, section B measures of RVESA and 2D TAPSE, section C and D measures of TR duration and RV ejection time respectively.
Figure 2. Right atrial emptying fractions.
The figure depicts the different concepts related to right atrial volumes and the related concepts of total, passive and RA emptying fractions.
Reference values for the right heart remodeling and function
RV systolic dysfunction was classified as mild, moderate or severe dysfunction if RVFAC was between 25 to 35%, 18 and 24% and ≤ 17% respectively (11). For indexed values of right atrial size and function, since no values referenced in the ASE guidelines, we used 95% of the upper limit of a prospectively recruited age and sex matched 95 healthy controls based on a 50 point questionnaire. Dimensions were categorized using similar thresholds as the left atrial volumes as < 18% from reference value increase for mild increase and > 40% increase for severe increase. For indexed RA area and RVEDA, the upper limit of normal was 11cm2/m2 and for indexed RVESA, the upper limit was 7.5 cm2/m2.
Magnetic resonance protocol in the validation cohort
CMR imaging was performed on a Siemens 1.5-T Sonato scanner (Siemens Medical Solutions, Erlangen, Germany), equipped with a 6-element phased array receiver coil. Short-axis images from base to apex of the ventricles were obtained with a typical slice thickness of 5 mm and an interslice gap of 5 mm were used for estimation of ventricular volumes using the Simpson method as previously described (14). The threshold chosen for the CMR categorical classification were predefined at the beginning of the study. We chose the threshold of RVEF of 35% for moderate dysfunction similar to previously established cut-off of the study of Van de Veerdonk et al. (14). In addition, based on a prior study from our group, we found that RVFAC of 25% corresponded best to an RVEF of 35% (15). We use the same threshold for RA area for both the echocardiographic and MRI study.
Statistical analysis
Continuous data are presented as mean ± SD if the Kolmogorov–Smirnov test showed a normal distribution otherwise data is presented as median ± interquartile range. Categorical variables are expressed as frequency and percentage. Comparisons between groups were performed using two-sided t tests with adjustment for unequal variance as needed. For non-normally distributed variables such as NT-pro-BNP, transformation to the common logarithm was performed prior to analysis. Linear regression analysis was used to determine independent associations between hemodynamic and structural or functional right heart parameters. The association between clinical and echocardiographic parameters and outcome was analyzed using Cox proportional hazards models. The assumption of proportional hazards was assessed by plotting the scaled Schoenfeld residuals for each independent variable against time; these correlations were found to be non-significant for all variables included in the multivariable model. We used a hierarchical modeling to determine factors independently associated with outcome and chose to include at maximum 1 co-variate per 10 events to minimize overfitting of the model. We avoided including in the model variables that were collinearly related to each other. We used bootstrapping with 5000 iterations to estimate hazard ratios and bias-corrected 95% confidence intervals (CI) for the multivariate models. For building the predictive score, the smallest absolute β coefficient was assigned a value of 0 and values for subsequent variables were assigned based on multiples of their respective β coefficients to nearest 0.5 approximation for categories with significantly different β coefficients (16). The survival c- statistic was calculated to show the discriminatory ability of the models and used to compare the predictive score and the validated REVEAL score and NIH survival equation. Intra-observer variability is assessed using the average difference in absolute measurement and the intra-class correlation coefficient (ICC). Statistical analysis wasdone using PASW statistical program (PASW 18.0 Inc, Chicago, IL).
Intervariability of echocardiographic measures
For RVFAC, the average difference in absolute measurement was 2.1 ± 1.6 % with an ICC of 0.84; for TAPSE, the average difference in absolute measurement was 0.1 ± 0.1 cm with an ICC of 0.93; for RVMPI the average difference in absolute measurement was 0.09 ± 0.11 with an ICC of 0.85. The ICCs for maximal, minimal and pre-atrial systole RA volumes were 0.95, 0.97 and 0.87, respectively. The ICC was 0.89 for total RAEF and 0.72 for active RAEF and 0.84 for passive RAEF.
RESULTS
Study population
Of the 128 patients with idiopathic and drug and toxin associated PAH who were seen during the study period, 106 were enrolled in the prospective registry. Eleven patients were excluded from the study for the following reasons: unavailable echocardiogram (2), atrial fibrillation (1), lost to follow-up (5), left heart failure (2) and restrictive lung disease (1). Table 1 summarizes the characteristics of the study population. The average follow-up time for our study was the average time of follow-up was 5.0 ± 2.4 years. The MPAP was 54 ± 14 mmHg and indexed pulmonary vascular resistance (PVRI) was 25 ± 12 Wood units m2. Forty-five percent of patients were on prostanoid therapy (n=43) and 19% (n=18) of patient were on combination therapy.
Table 1.
Patient Characteristics for the derivation cohort
| Characteristics | Value |
|---|---|
| N | 95 |
| Age (years) | 43 ± 11 |
| Women | 75 (79%) |
| Caucasian | 84 (88%) |
| Etiology of PAH | |
| Idiopathic or familial | 44 (46%) |
| Drugs and toxin (history of use) | 51 (55%) |
| Body mass index (kg/m2) | 30 ± 6 |
| Right Heart Catheterization | |
| HR (bpm) | 82 ± 14 |
| SBP (mmHg) | 120 ± 17 |
| RAP (mmHg) | 10 ± 6 |
| MPAP (mmHg) | 54 ± 14 |
| PCWP (mmHg) | 10 ± 4 |
| CI (L/min/ m2) | 2.0 ± 0.6 |
| PVRI (Wood units m2) | 25 ± 12 |
| Six minute walk distance (m) | 432 ± 117 |
| DLCO (%) | 75 ± 23 |
| Comorbid conditions | |
| CKD (eGFR<60 mL/min/ 1.73m2) | 22 (23%) |
| Hyponatremia (< 136 mEq/L) | 9 (9.5%) |
| Diabetes mellitus | 3 (3%) |
| Systemic hypertension | 4 (4%) |
| Medication | |
| Diuretics | 48(51%) |
| Prostanoid therapy | 43(45%) |
| Phophodiesterase inhibitors | 31(33%) |
| Endothelin Receptor Blockers | 39(41%) |
| Warfarin | 59(63%) |
CKD indicates chronic kidney disease; CI, cardiac index; DLCO, diffusion of carbon monoxide; HR, heart rate, MPAP, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PVRI, pulmonary vascular resistance index; RAP, right atrial pressure; SBP, systolic blood pressure,
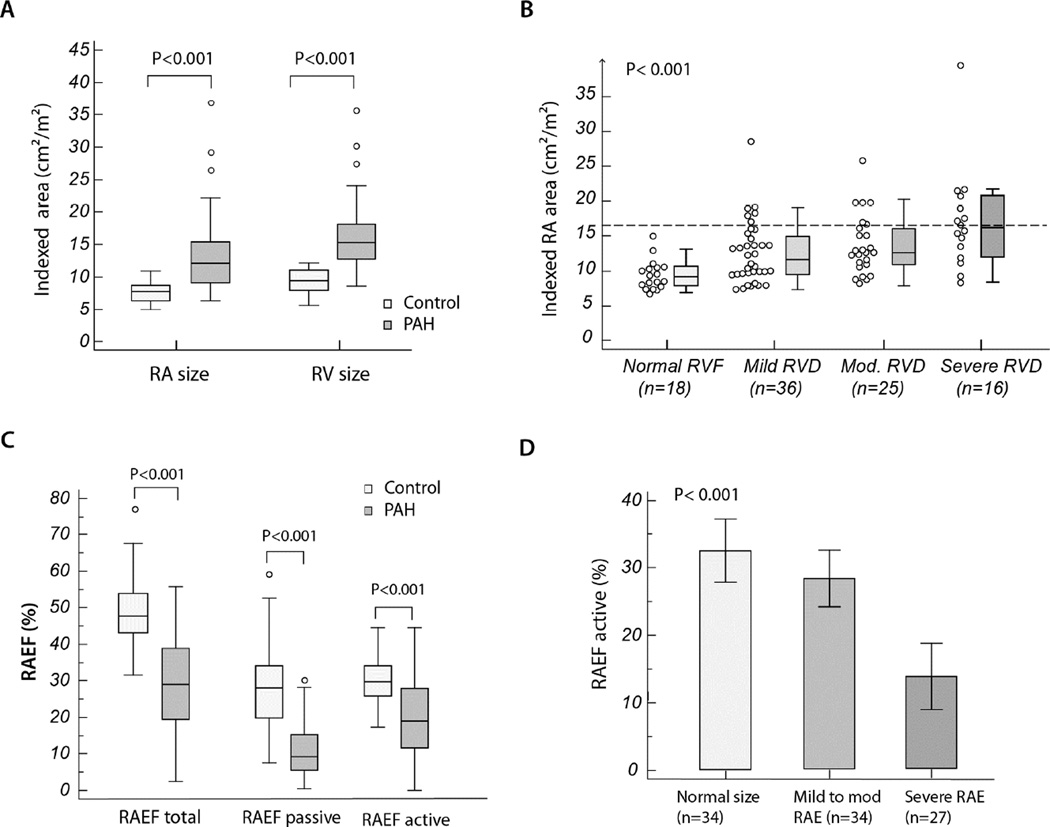
Figure 3 summarizes the relationship between RA size, emptying fractions and RV function as assessed by RVFAC. Compared to healthy controls, patients with PAH had a greater degree of RA and RV enlargement and lower emptying fractions. In general, RA enlargement (RAE) and impaired active RAEF were more common among patients with severe RV dysfunction (Figure 3b and d).
Figure 3. Ventricular and atrial remodeling and function in our study population.
Section A presents the box and whisker plots of comparing indexed RA and RV areas between patients with PAH and healthy controls. Section B presents the box and whisker plots of indexed RA area according to the predefined categories of RV dysfunction. Section C presents the box and whisker plots of comparing total, active and passive RAEF between patients with PAH and healthy controls, and section D present the bar graph with 95% confidence interval for mean value for RAEF active stratified according to the pre-defined categories of RA size. In the box- and-whisker plots, the central box represents the values from the lower to upper quartile (25 to 75 percentile); the middle line represents the median and the line extends from the minimum to the maximum value, excluding outlier values.
Relationship between metrics of right heart function and hemodynamics
The different parameters of right heart size and function are not independent of each other; their interrelationship is important to consider prior to building outcome models. As expected, there was also strong co-linearity between parameters of RV function [R2 =0.61 between RVFAC and TAPSE (P<0.001) and R2 =0.51 between RVFAC and RVMPI (P<0.001)] as well as between RVEDA and RA area (R2 =0.51, P<0.001). Table 2 summarizes factors independently associated with RVFAC, RA area index, RAEF active and passive and log NT-proBNP levels. We favored including in the model factors that not only were correlates but also potential determinants. As covariates, factors considered included demographic factors (age, sex), load parameters (PVRI, RAP), functional indices (TR, TASPSE) or renal function for NT-proBNP. Among other associations, we found that pericardial effusion which was present in 17 patients was strongly related to both RAP and RA size (χ2=22, P=0.01). Systolic blood pressure was significantly correlated with cardiac output as well as the use of intravenous prostanoids (R2=0.28, P<0.011, r=0.40 with cardiac output and r= −0.28 with prostanoids).
Table 2.
Potential determinants of RV and RA indices (multivariate regression models)
| RVFAC | RAI | RAEFactive | RAEFpassive | Log-NT- proBNP |
|
|---|---|---|---|---|---|
| R2 | 0.32 | 0.61 | 0.41 | 0.27 | 0.59 |
| Correlates | PVRI (r= −0.44) Male (r= −0.30) |
RAP (r=0.44) TR (r=0.45) |
RAP (r= −0.27) TAPSE(r=0.33) Male (r= −0.27) |
Age (r= −0.35) TAPSE(r= 0.47) |
RVFAC(r=−0.48) RAI (r=0.40) eGFR (r=−0.31) Male(r=−0.24) |
eGFR indicates estimated glomerular filtration rate; NT-proBNP; N-terminal pro B-type natriuretic peptide; PVRI, pulmonary vascular resistance index; RAEF, right atrial emptying fraction; RAI, right atrial area index; RAP, right atrial pressure; RVFAC, RV fractional area change; TAPSE, tricuspid annular systolic excursion; TR, tricuspid regurgitation. The multivariate models presented all have a P< 0.001. PVRI based on the most recent right heart catheterization. r correspond to partial correlation coefficients.
Outcome analysis in the derivation cohort
The composite end-point occurred in 34 patients (36%), consisting of 26 deaths and 8 lung transplantations. Event free survival at 1, 3 and 5 years was 95%, 89% and 81%, respectively. The predicted NIH survival equation 1, 3 and 5 year survival estimates were of 66%, 44% and 33%, respectively and the revised NIH prediction scores was 91%, 71% and 63% (17).
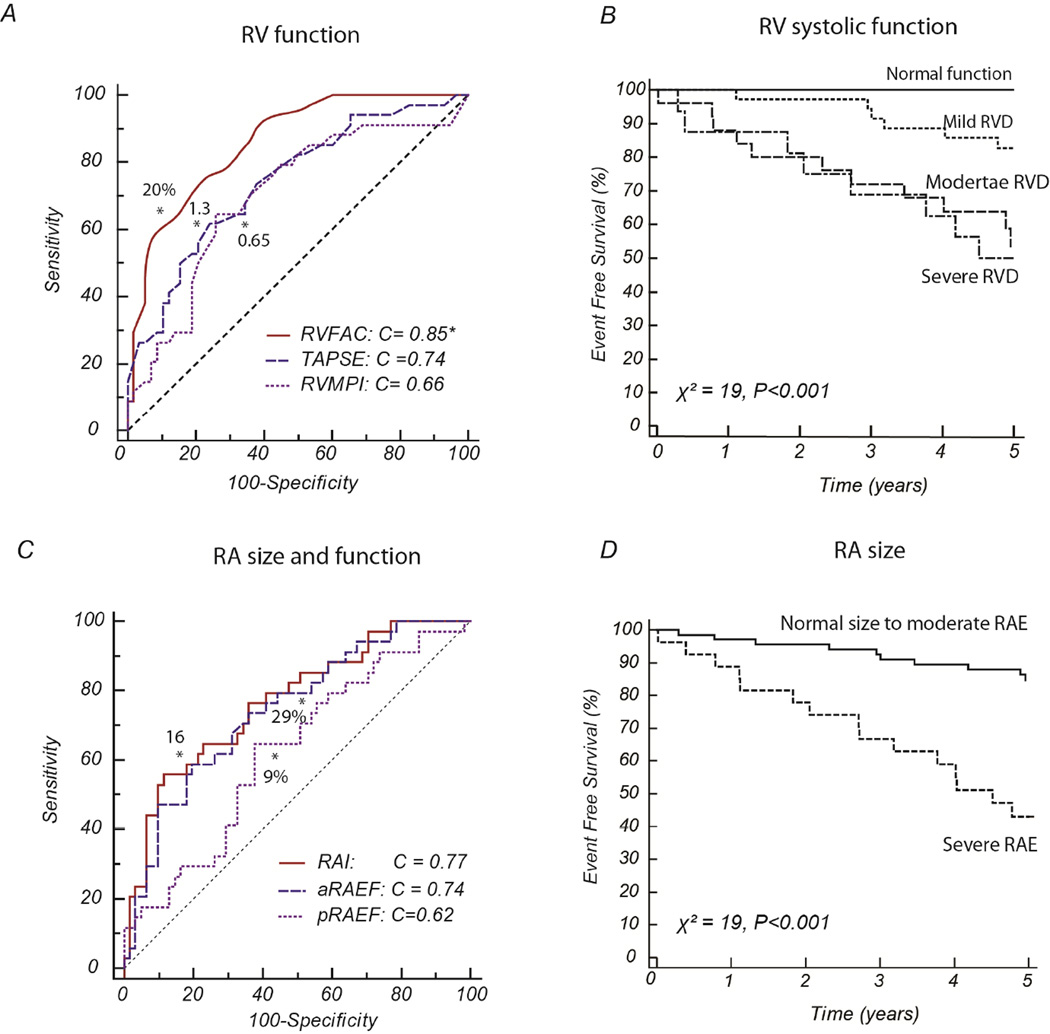
Several parameters of right heart structure and function were strongly related to outcome on univariate analysis (Table 3). The strongest relationships were found with RVEDAI, RVESAI, RVFAC, TAPSE, RA size, active RAEF and log NT-proBNP levels. In addition, NYHA functional class, resting SBP, kidney function, low cardiac index on right heart catheterization and PVRI were also associated with outcome. Figure 4 presents the c-statistic of the RV and RA parameters as well as their Kaplan-Meier survival curves from RVFAC and RAI categories. Using the area-length method, volumetric measures of RA size or RAEF were not associated with significantly different c-statistic (P=0.79 and P=0.87, respectively).
Table 3.
Univariable analysis of factors associated with the composite end-point
| HR | 95% CI | p | |
|---|---|---|---|
| Clinical | |||
| Age (per 10 years) | 0.75 | 0.54 to 1.03 | 0.082 |
| Male sex | 1.90 | 0.90 to 4.03 | 0.094 |
| DT vs. idiopathic | 0.94 | 0.47 to 1.85 | 0.84 |
| NYHA (III–IV vs. I–II) | 2.67 | 1.34 to 5.32 | 0.005* |
| Walking distance (per 100 m) | 0.73 | 0.55 to 0.96 | 0.026* |
| SBP (per 10 mmHg) | 0.73 | 0.58 to 0.92 | 0.009* |
| HR (per 10 bpm) | 1.17 | 0.89 to 1.54 | 0.26 |
| DLCO (per 10%) | 0.97 | 0.88 to 1.09 | 0.61 |
| Co-morbidities-Laboratory | |||
| CKD | 2.18 | 1.07 to 4.46 | 0.033* |
| Hyponatremia | 1.80 | 0.69 to 4.68 | 0.23 |
| Log NT-proBNP | 4.81 | 2.13 to 10.86 | <0.001* |
| Echocardiography parameters | |||
| Right ventricular | |||
| RVEDAI (per 3 cm2/m2) | 1.60 | 1.29 to 2.04 | <0.001* |
| RVESAI (per 3 cm2/m2) | 1.82 | 1.49 to 2.22 | <0.001* |
| RVFAC (per 5%) | 0.52 | 0.41 to 0.67 | <0.001* |
| TAPSE (per 0.3 cm) | 0.61 | 0.46 to 0.82 | 0.001* |
| RVMPI (per 0.3 units) | 2.06 | 1.16 to 3.69 | 0.015* |
| Right atrial | |||
| RAI per 5 cm2/m2) | 1.81 | 1.44 to 2.28 | <0.001* |
| RAEF active (per 5%) | 0.69 | 0.57 to 0.83 | <0.001* |
| RAEF passive (per 5%) | 1.27 | 1.02 to 1.58 | 0.029 |
| Septal curvature | |||
| Diastolic EI (per 0.5 units) | 1.84 | 1.19 to 2.87 | 0.007* |
| Systolic EI (per 0.5 units) | 1.33 | 1.11 to 1.57 | 0.001* |
| Tricuspid regurgitation | 1.95 | 1.30 to 2.90 | 0.002* |
| Hemodynamic | |||
| RAP (per 5mmHg) | 2.12 | 1.51 to 3.01 | < 0.001* |
| RVSP (per 10 mmHg) | 1.14 | 0.91 to 1.43 | 0.25 |
| RVSP/SBP (per 0.25) | 2.77 | 1.61 to 4.75 | <0.001* |
| SVI (per 5 mL/m2) | 0.82 | 0.70 to 0.97 | 0.019 |
| Left ventricular | |||
| LVID (per 0.5 cm) | 0.79 | 0.54 to 0.99 | 0.049* |
| LVEF (per 5%) | 0.72 | 0.60 to 0.88 | 0.001* |
| Right heart catheterization | |||
| Cardiac index < 1.8 L/min/ m2 | 2.22 | 1.09 to 4.50 | 0.025 |
| PVRI (per 10 Wood units m2) | 1.41 | 1.02 to 1.96 | 0.039 |
CKD indicates chronic kidney disease; DT, drug and toxin, eGFR, estimated glomerular filtration rate; EI, eccentricity index; HR, heart rate; LVID, left ventricular internal dimension; LVEF, left ventricular ejection fraction; NT-proBNP; N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association Class; PVRI, pulmonary vascular resistance index; RAEF, right atrial emptying fraction; RAI, right atrial area index; RAP, right atrial pressure; RVEDAI, RV end-diastolic area index; RVESAI, RV end-systolic area index; RVESP, RV end-systolic pressure; RVFAC, RV fractional area change; RVMPI, RV myocardial performance index; SBP, systolic blood pressure; SVI, stroke volume index; TAPSE, tricuspid annular systolic excursion;
Figure 4.
C-statistics and Kaplan-Meier curves for selected parameters of RV and RA function. Section A illustrates the c-statistic between indices of RV function. Section B represents the 5-year Kaplan-Meier curves of RV systolic dysfunction based on RVFAC. Section C illustrates the c-statistic curves between indices of RA indices and section D shows the associated 5-year Kaplan-Meier curves and severe RA enlargement.
To minimize over fitting the multivariate Cox proportional-hazard model, we only include 4 variables in the initial analysis, i.e. RVFAC, RAI, resting SBP and NYHA class III–IV vs. I–II. The choice of variables was based on the following rationale: (a) RVFAC was more strongly associated with outcome than other RV functional parameters and was not co-linearly related to RA size in contrast to RVEDA or RVESA, (b) RA size was more reproducible than aRAEF in our study population, (c) SBP was not was not co-linearly related to RVFAC; in contrast, there was a moderate relationship between RVSP or relative RVSP and RVFAC (r=0.45, P<0.001 and r=0.48, P<0.001) and (4) NYHA class was related to outcome in many previous studies. On multivariate analysis, RVFAC, RA size and SBP were strongly and independently associated with outcome as shown in Table 4 (both in continuous and categorical analysis). In the subgroup of patients in whom NT-proBNP was available (n=79), NT-proBNP was not retained in the multivariate model.
Table 4.
Independent correlates of the composite end-point in the derivation cohort
| HR | 95% CI | p | Overall χ2 | |
|---|---|---|---|---|
| Multivariate model -continuous | ||||
| RVFAC per 5% | 0.6 | 0.4 to 0.7 | <0.001 | 44 |
| RAI (per 5 cm2/m2) | 1.4 | 1.1 to 2.8 | 0.021 | - |
| SBP baseline (per 10 mmHg) | 0.7 | 0.5 to 0.9 | 0.007 | - |
| Multivariate model -categorical | ||||
| RV systolic dysfunction per grade* | 3.4 | 2.0 to 7.75 | < 0.001 | 47 |
| Severe RAE (> 16 cm2/m2) | 3.0 | 1.3 to 8.1 | 0.009 | - |
| SBP < 110 mmHg | 3.3 | 1.5 to 9.4 | 0.002 | - |
| RH score (categorical) | ||||
| RH Score (per grade) | 3.2 | 2.3 to 5.4 | <0.001 | 47 |
RAI indicates right atrial area index; RAE, right atrial enlargement; RVFAC, RV fractional area change; SBP, systolic blood pressure
RV dysfunction was classified into normal (no dysfunction), mild or moderate to severe according to the ASE criteria. The 95% confidence intervals are reported afte5 5000 iteration of the bootstrap procedure. Model were age and sex adjusted.
Right Heart Score and other validated scores
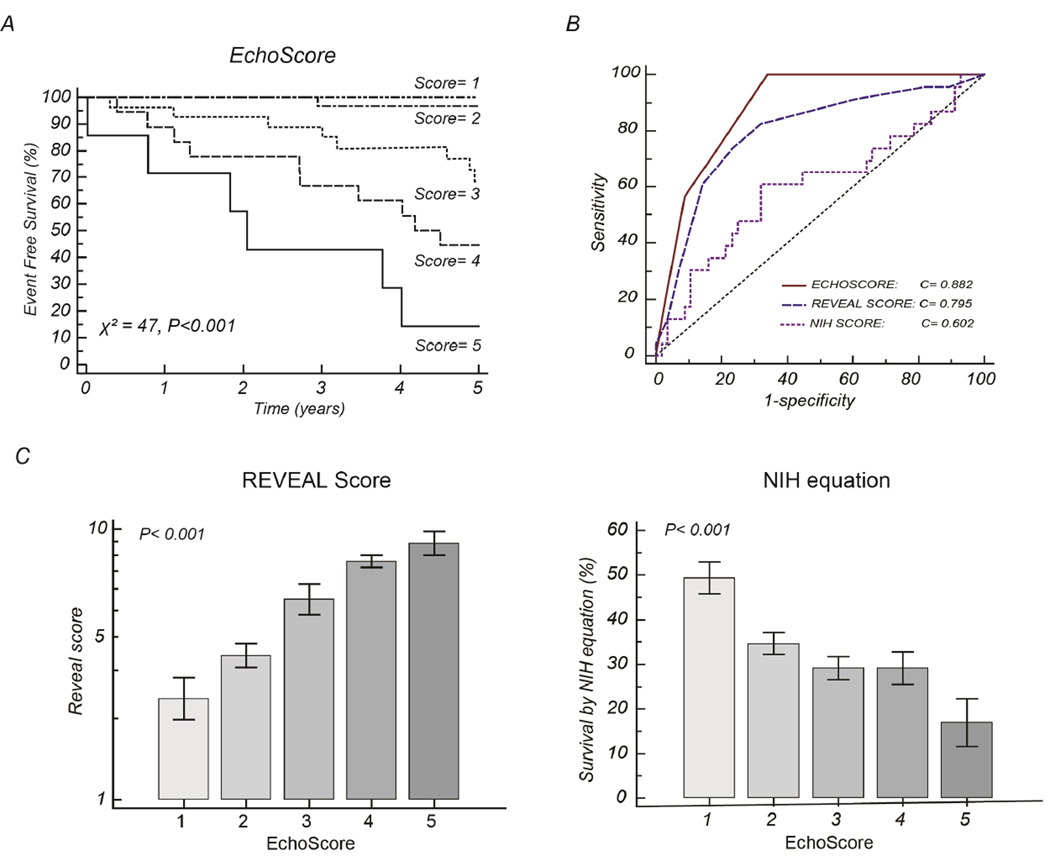
A right heart (RH) score was built based on the β-coefficients of the multivariate model assigning a baseline value of 1 and additional points for each category of risk (Table 5). The RH score had a c-statistic of 0.88 [0.79–0.94], the REVEAL score had a c-statistic of 0.80 [0.69–0.88] and the NIH survival equation had a c-statistic of 0.60 [0.49–0.71]. Using the DeLong method, both the RH score and the REVEAL score had significantly higher c-statistic than the NIH survival equation (P< 0.001 and P=0.013, respectively). There was no statistical difference between the RH score and the REVEAL score in the cohort (P=0.097). Figure 5 illustrates the Kaplan-Meier survival curves associated with the RH score as well as its relationship with other scores.
Table 5.
Example of RH score and point allocation
| Value | |
|---|---|
| Baseline value | 1 + |
| RV function | |
| Normal | 0 |
| Mild | 1 |
| Moderate-severe | 2 |
| Less than severe | 0 |
| RAE | 1 |
| Severe RAE | |
| SBP > 110 mmHg | 0 |
| SBP< 110 mmHg | 1 |
| RH score | 1 to 5 |
RAE, right atrial enlargement; SBP, systolic blood pressure
RV dysfunction was classified into normal (no dysfunction), mild or moderate to severe according to the ASE criteria.
Figure 5.
The Right heart (RH) score in relation to the REVEAL and NIH scores. Section A shows the 5-year Kaplan-Meier curves based on the Right heart score; Section B compares the c-statistic of the right heart score with the REVEAL score and the 5 year predicted NIH survival. Section C and D illustrates the strong relationship between the right heart score and the REVEAL and NIH scores, respectively with 95% confidence interval for mean value.
Validation Cohort
The validation cohort included 87 patients with idiopathic or familial PAH followed at VUMC between 2001 and 2012. The average age was 47.8±16 years, the majority of patients were female (75%), baseline PVR was 11.2±5 Wood units and baseline 6MWD of 407±127 m. All patients were on disease modifying therapy, the average time between MRI and diagnosis was 1.5±1.5 years and the average follow-up time was 4.2±3.2 years. The composite end-point occurred in 29 patients consisting of 23 deaths and 6 lung transplantation. On univariate analysis, the strongest correlates of outcome included RVEF (χ2 = 12, P<0.001), RAI (χ2 = 11, P<0.001), RVESVI (χ2 = 9, P<0.001), the RH score (χ2 = 14, P<0.001) and more weakly 6MWD (χ2 = 5, P=0.03). On multivariate analysis, RH score (HR of 1.9 per grade, 95% CI 1.4 to 2.6) and age (HR of 1.3 per grade, 95% CI 0.98 to 1.69) were the only 2 variables independently associated with outcome with a χ2 = 19, P<0.001. The c-statistic for the RH score in the validation cohort was 0.76 [0.66–0.84] was significantly different from the c-statistic for the NIH survival equation which was at 0.59 [0.48–0.70], P=0.030. Because, NT-proBNP levels and the percentage predicted DLCO were not systematically available, the derived REVEAL score could not be calculated in the majority of patients at the time of follow-up.
Discussion
Our study is the first to demonstrate that a simple score combining measures of RV systolic function, RA size and systolic blood pressure offers a good discrimination of outcome in patients with established PAH. Consistent with other studies, our results of our study highlight that the quantitative metrics of right heart remodeling or function may simplify the risk stratification of patients with PAH (3,18).
The REVEAL score and the NIH survival equation represent the two most validated survival scores in PAH (2,4). The NIH registry score relies on hemodynamic parameters while the REVEAL registry score incorporates clinical, functional and imaging parameters. Although our sample size was small, confidence in our results can be provided by the fact that the RH score correlated well with established outcome score, that the findings were validated in an independent cohort and that the results were consistent using different imaging modalities. In a recent publication, in a large series of patients with PAH, Fine et al. has shown that RV global longitudinal strain (RVGLS), log-NT-proBNP levels and NYHA class were independent correlates of clinical deterioration in patients with PAH. Consistent with the study of Fine et al., our study also highlights the importance of right heart function. In contrast, NYHA functional class and log NT-proBNP did not emerge as independently correlates of outcome due to their strong relationship with RV function and RA size; alternatively our study may have been underpowered to assess their incremental value. In the Reveal registry score, qualitative assessment of RV function were considered but did not emerge in the multivariate model; one can theorize, although not yet proven, that this may reflect the inter-laboratory variability in assessing RV function and the multiple grades of dysfunction considered (5 classes).
Using echocardiography, different metrics of RV systolic function are considered including RVFAC, TAPSE, RVMPI and more recently RVGLS (3,12,13,18). In our study, RVFAC emerged as a stronger correlate of outcome than either TAPSE or RVMPI. In a recent study, we have shown than RVFAC is more closely related to RVEF than TAPSE (19). Moreover, we have shown that a RVFAC of 25% corresponds best to a RVEF of 35%, a commonly chosen threshold for moderate RV dysfunction in CMR studies of patients with PAH (14,15). In comparison to RVFAC, TAPSE has the advantage of reproducibility but does not take into account the radial component of RV contraction (20). Although RVMPI combines information of both systolic and diastolic function, in different studies it does not appear to carry stronger prognostic value than RVFAC, TAPSE or RVGLS (18,21). Although not yet proven, this can be in part due to pseudonormalization of RVMPI values that can occur in patients with severe dysfunction. As pointed out by the recent study of Fine et al., RVGLS emerged as the best metric of RV function when compared to RVFAC and TAPSE in PAH; ongoing studies are currently validating the findings in independent cohorts (18).
One of the most important contributions of our study was to prove the independent contribution to right atrial size (22). In fact, in contrast to studies on atrial remodeling in left heart failure, there has been a limited number of studies addressing atrial remodeling or atrial function in PAH (7,8,23). Bustamante-Labarta et al. were the first to suggest an association between RA size and outcome in 25 patients with PAH (7). In the study of Raymond et al., on 81 patients with NYHA class III or IV PAH, there was a trend for an independent association between RA area indexed to height and the composite end-point of death or transplantation (P=0.106) (8). In the recent study of Kane et al., severe RAE assessed qualitatively were also predictive of survival when corrected for age, sex and the functional class (23). Mechanistically, right atrial size is strongly associated with right atrial pressure and tricuspid regurgitation severity can therefore provide important information on adverse ventricular remodeling. Further studies are however needed to provide better normative indexed threshold of RA size.
In addition to changes in RA remodeling, we have shown that right atrial function was significantly impaired in patients with PAH. While the change affected both passive and atrial components of atrial function, better prognostic information was provided by active atrial emptying. The association between active RAEF and RAP as well as TAPSE is not surprising as RAP may be an indirect metric of RA afterload and TAPSE may limit the extent of active RAEF as the atria cannot contract if the ventricular has a very limit annular excursion. As a marker of outcome, active RAEF has the potential disadvantage of lower reproducibility when compared to maximal RA size as is more co-linearly related with metrics of RV systolic function which may limit its incremental value in multivariate models. Conversely, RA size was more related to RV end-systolic dimension which may limit their incremental values if considered together as covariates. The sex differences related to active RAEF will require further study and validation. The association that we found between SBP and outcome is consistent with the findings of the REVEAL registry and may reflect lower cardiac output or the use of prostanoid therapy.
Our study has 3 main clinical implications. First, a simple RH score can be useful for stratified randomization strategies in phase II clinical trials as matching based only on NYHA may not capture the complexity of the disease process and all variables from the REVEAL registry may not be available. Second, a simple RH score can serve as a “benchmark” against which the incremental value of novel biomarker can be assessed. Third, empirically patients with higher scores could be monitoring more closely clinically as they are at higher risk of clinical deterioration. It is however important to mention that our study was not designed to provide comparison with well validated scores such as the REVEAL registry score and should by no means be considered interchangeable. Our study does however suggest as did the study of Fine et al. that quantitative assessment of right heart function and remodeling may simplify risk assessment in patients with PAH.
The study has several limitations. First, the still small sample size limits the number of variables that we can consider in the multivariate model. The strong relationship with the REVEAL registry and NIH survival equation however brings indirect external validation to our findings as does the validation cohort. Second, we did not include more complex imaging modalities in our study such as strain imaging. Finally, it is important to emphasize that our study focuses on prevalent cases of patients with PAH rather than incident treatment naive patients.
Conclusion
In this study, we have shown that in patients with idiopathic, familial or drug and toxin-prevalent PAH, a simple right heart score combining indices of right heart remodeling and function could predict long-term outcome. If further validated, this simple score may significantly improve the evaluation of novel biomarkers and help guide stratified randomization in clinical trials.
Clinical Perspectives.
Competency in Medical Knowledge
Imaging cardiovascular biomarkers have diagnostic and prognostic value and are useful in guiding clinical management in patients with pulmonary arterial hypertension (PAH). Finding the best combination of biomarkers is essential in order to translate into better diagnostic or predictive tools. In this study, we identify right ventricular function by conventional echo, right atrial enlargement, and systemic systolic blood pressure as key factors determining outcome, and a score derived from these simple three parameters had prognostic power superior to an established PAH score.
Translational Outlook
Additional clinical studies are needed to validate the incremental prognostic value of simplified imaging scores in patients with pulmonary arterial hypertension.
Acknowledgement
We want to thank the Vera Moulton Wall Center, Stanford Cardiovascular Institute and Pai Chan Lee research fund at Stanford University for their support.
Source of financial support: This work was supported by the Vera Moulton Wall Center for Pulmonary Vascular Disease at Stanford University, Stanford Cardiovascular Institute and the Pai Chan Lee Research Fund
LIST OF ABBREVIATIONS
- PAH
pulmonary arterial hypertension
- RAI
right atrial area index
- RAEF
right atrial emptying fraction
- RAP
right atrial pressure
- RV
right ventricular
- RVEDAI
RV end-diastolic area index
- RVESAI
RV end-systolic area index
- RVFAC
RV fractional area change
- RVMPI
RV myocardial performance index
- RVSP
right ventricular systolic pressure
- SBP
systolic blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure and relation to industry: None
Conflict of Interest: None
References
- 1.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 2.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 3.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. Journal of the American College of Cardiology. 2013;62:D22–D33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 4.D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Annals of internal medicine. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 5.van Wolferen SA, van de Veerdonk MC, Mauritz GJ, et al. Clinically significant change in stroke volume in pulmonary hypertension. Chest. 2011;139:1003–1009. doi: 10.1378/chest.10-1066. [DOI] [PubMed] [Google Scholar]
- 6.Bossone E, D'Andrea A, D'Alto M, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2013;26:1–14. doi: 10.1016/j.echo.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Bustamante-Labarta M, Perrone S, De La Fuente RL, et al. Right atrial size and tricuspid regurgitation severity predict mortality or transplantation in primary pulmonary hypertension. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2002;15:1160–1164. doi: 10.1067/mje.2002.123962. [DOI] [PubMed] [Google Scholar]
- 8.Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. Journal of the American College of Cardiology. 2002;39:1214–1219. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 9.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. Journal of the American Society of Nephrology : JASN. 2005;16:459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 11.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786-8. [DOI] [PubMed] [Google Scholar]
- 12.Yeo TC, Dujardin KS, Tei C, Mahoney DW, McGoon MD, Seward JB. Value of a Doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. The American journal of cardiology. 1998;81:1157–1161. doi: 10.1016/s0002-9149(98)00140-4. [DOI] [PubMed] [Google Scholar]
- 13.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. American journal of respiratory and critical care medicine. 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 14.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. Journal of the American College of Cardiology. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 15.Shiran H, Zamanian RT, McConnell MV, et al. Relationship between Echocardiographic and Magnetic Resonance Derived Measures of Right Ventricular Size and Function in Patients with Pulmonary Hypertension. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2014 doi: 10.1016/j.echo.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA : the journal of the American Medical Association. 1997;277:488–494. [PubMed] [Google Scholar]
- 17.Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2010;35:1079–1087. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine NM, Chen L, Bastiansen PM, et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circulation Cardiovascular imaging. 2013;6:711–721. doi: 10.1161/CIRCIMAGING.113.000640. [DOI] [PubMed] [Google Scholar]
- 19.Shiran H, Haddad F, Miller DC, Liang D. Comparison of aortic root diameter to left ventricular outflow diameter versus body surface area in patients with marfan syndrome. The American journal of cardiology. 2012;110:1518–1522. doi: 10.1016/j.amjcard.2012.06.062. [DOI] [PubMed] [Google Scholar]
- 20.Kind T, Mauritz GJ, Marcus JT, van de Veerdonk M, Westerhof N, Vonk-Noordegraaf A. Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2010;12:35. doi: 10.1186/1532-429X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghio S, Klersy C, Magrini G, et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. International journal of cardiology. 2010;140:272–278. doi: 10.1016/j.ijcard.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 22.Grunig E, Henn P, D'Andrea A, et al. Reference values for and determinants of right atrial area in healthy adults by 2-dimensional echocardiography. Circulation Cardiovascular imaging. 2013;6:117–124. doi: 10.1161/CIRCIMAGING.112.978031. [DOI] [PubMed] [Google Scholar]
- 23.Kane GC, Maradit-Kremers H, Slusser JP, Scott CG, Frantz RP, McGoon MD. Integration of clinical and hemodynamic parameters in the prediction of long-term survival in patients with pulmonary arterial hypertension. Chest. 2011;139:1285–1293. doi: 10.1378/chest.10-1293. [DOI] [PubMed] [Google Scholar]