Abstract
Targeted inhibitors elicit heterogeneous clinical responses in genetically stratified groups of patients. While most studies focus on tumor intrinsic properties, factors in the tumor microenvironment were recently found to modulate the response to inhibitors. Here, we show that in cutaneous BRAF V600E melanoma, the cytokine TNFα blocks RAF-inhibitor-induced apoptosis via activation of nuclear factor κB (NFκB). Several NFκB-dependent factors are up-regulated following TNFα and RAF inhibitor treatment. Of these factors, we show that death receptor inhibitor cellular caspase 8 (FLICE)-like inhibitory protein (c-FLIP) is required for TNFα-induced protection against RAF inhibitor. Overexpression of c-FLIP_S or c-FLIP_L isoform decreased RAF inhibitor-induced apoptosis in the absence of TNFα. Importantly, targeting NFκB enhances response to RAF inhibitor in vitro and in vivo. Together, our results show mechanistic evidence for cytokine-mediated resistance to RAF inhibitor and provide a preclinical rationale for the strategy of co-targeting the RAF-MEK-ERK1/2 pathway and the TNFα/NFκB axis to treat mutant BRAF melanomas.
INTRODUCTION
Targeted inhibitors, such as vemurafenib and dabrafenib, elicit strong but heterogeneous effects in mutant BRAF V600E cutaneous melanoma patients (Flaherty et al., 2010; Hauschild et al., 2012; Long et al., 2012; Sosman et al., 2012). However, approximately 50% of patients do not achieve a partial or complete response. Furthermore, many patients that achieve an objective response ultimately develop acquired resistance (Hartsough et al., 2014). Heterogeneous modes of resistance have been identified in patient samples with reactivation of extracellular signal-regulated kinase 1/2 (ERK1/2) signaling being a frequent mechanism. Simultaneously targeting multiple steps in the RAF-MEK-ERK1/2 pathway is more efficacious than single agent therapy (Flaherty et al., 2012); however, resistant tumors still emerge. These findings indicate that the efficacy of single pathway targeting is limited and strategies need to be developed to co-target alternative pathways.
The tumor microenvironment modulates the response to targeted therapies. Growth factors including hepatocyte growth factor and neuregulin 1 provide protective signals counteracting the effects of vemurafenib (Abel et al., 2013; Straussman et al., 2012; Wilson et al., 2012). Accordingly, co-targeting the corresponding receptor tyrosine kinase (RTK) pathway and RAF-MEK-ERK pathway elicited better outcome than single pathway targeting (Abel et al., 2013; Lito et al., 2012; Straussman et al., 2012; Wilson et al., 2012). However, due to the complex composition of tumor microenvironment, additional compensatory pathways will likely modulate the response to RAF inhibitors and will need to be targeted to maximize the effects of RAF and MEK inhibitors in melanoma.
Advanced stage melanoma cells often have constitutive IκB kinase (IKK) and nuclear factor κB (NFκB) activity (Meyskens et al., 1999; Yang and Richmond, 2001). NFκB is a family of protein complexes that controls the transcription of genes involved in inflammatory response and cell survival (Karin and Lin, 2002; Lawrence, 2009). Anti-apoptotic targets of NFκB include Bcl-2 (Catz and Johnson, 2001) and Bcl-xl (Chen et al., 2000)), the inhibitor of apoptosis family proteins (c-IAP1 (Stehlik et al., 1998a), c-IAP2 (Stehlik et al., 1998a), XIAP (Stehlik et al., 1998b), survivin (Kawakami et al., 2005)) and death receptor inhibitor cellular caspase-8 (FLICE)-like inhibitory protein (c-FLIP) (Kreuz et al., 2001) and, therefore, NFκB is implicated in radiation- and chemo-resistance in many cancers (Magne et al., 2006; Wang et al., 1999).
In tumor microenvironment, tumor-associated macrophages (TAM) produce cytokines such as TNFα and IL-1β, which are potent inducers of NFκB pathway. Studies have shown that TAM infiltration is associated with melanoma stage and angiogenesis (Torisu et al., 2000) and may serve as prognostic marker for AJCC stage I/II melanoma (Jensen et al., 2009). Furthermore, targeting the tumor NFκB pathway or blocking TNFα signaling in TAMs sensitized melanoma cells to chemo- or radiation-therapies (Amiri et al., 2004; Meng et al., 2010). TNFα also blocks apoptosis induced by MEK inhibitor or BRAF depletion in mutant BRAF melanoma cells, although the underlying mechanism remains unclear (Gray-Schopfer et al., 2007). Here, we tested the role of NFκB pathway in the responses of mutant BRAF melanoma cells to RAF inhibition. We show that activation of NFκB pathway by TNFα protects melanoma cells against the RAF inhibitor via up-regulation of the anti-apoptotic protein c-FLIP. Importantly, targeting the NFκB pathway sensitized melanoma cells to the RAF inhibitor in vitro and in vivo. Our data provide the rationale for co-targeting the RAF-MEK-ERK and NFκB pathways to treat mutant BRAF melanoma.
RESULTS
The NFκB agonist, TNFα, protects melanoma cells from RAF-inhibitor induced apoptosis
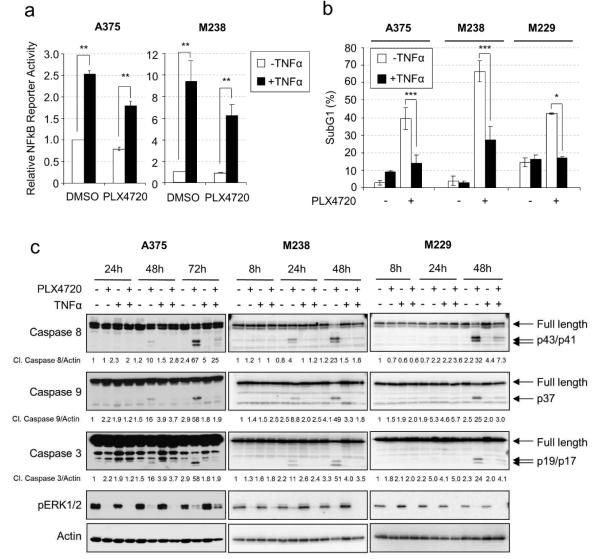
To test the role of the NFκB pathway in the response melanoma cells to RAF inhibitor, we treated three mutant BRAF melanoma cells with TNFα, and examined their response to PLX4720, the tool compound for vemurafenib (Tsai et al., 2008). Treatment with TNFα significantly enhanced NFκB reporter activity in both untreated and RAF inhibitor-treated cells, although activation was slightly lower in the presence of PLX4720 (Fig. 1a). Consistent with previous observations (Shao and Aplin, 2010), PLX4720 elicited strong cytotoxic effects in BRAF V600E melanoma cells (Fig. 1b-c, Fig. S1). TNFα treatment alone slightly increased the basal level of cell death in A375 but not M238 and M229 cells. Importantly, TNFα strongly protected multiple BRAF V600E melanoma cells against the cytotoxic effect of PLX4720 but had little impact on cell cycle progression (Fig. 1b-c, Fig. S1). TNFα was also protective against the RAF plus MEK inhibitor combination but did not reduce death induced by etoposide (Fig. S1c). Cleaved caspase 3 was detected 24-48 hours following PLX4720 treatment and strongly inhibited by TNFα stimulation (Fig. 1c). Furthermore, activation of both intrinsic (caspase 9) and extrinsic (caspase 8) apoptosis pathways was detected following PLX4720 treatment and was inhibited by TNFα (Fig. 1c). Cleavage of caspase 3 and 9 was first detected at ~18 hours following PLX4720 treatment while caspase 8 cleavage showed a delay of 2-4 hours (Fig. S2a), suggesting caspase 9 activation is an upstream event in RAF inhibitor-induced apoptosis. Interestingly, caspase 8 depletion reduced cleavage of caspase 3 and 9 (Fig. S2b), suggesting crosstalk between these caspases. Together, these results show that TNFα protects melanoma cells from RAF-inhibitor induced apoptosis and counteracts the activation of caspase 3, 8 and 9.
Figure 1. TNFα protects melanoma cells from RAF inhibitor-induced apoptosis.
a) Luciferase assays were performed as described in Material and Methods. Cells were treated with −/+ 50 ng/ml TNFα and −/+ 5 µM PLX4720 for 24 hours. Average relative luciferase activities from three experiments are shown. Error bars represent standard deviation. ** p<0.01. b) Cells were treated with −/+ 50 ng/ml TNFα and either −/+ 10 µM PLX4720 for 72 hours (A375) or −/+ 5 µM PLX4720 for 48 hours (M238, M229). Cells stained with PI for cell cycle analysis. Average percentages of sub-G1 populations from three experiments were quantified. * p<0.05; ** p<0.01; *** p<0.001. c) A375, M238 and M229 cells were treated with −/+ 50 ng/ml TNFα, −/+ 5 µM PLX4720 for 8-72 hours. Cells lysates were analyzed by Western blotting. Arrows indicate full length or cleaved products of caspases. Relative levels of cleaved caspases are indicated below the corresponding blots.
NFκB signaling is required for TNFα-mediated protection from RAF inhibitor-induced apoptosis
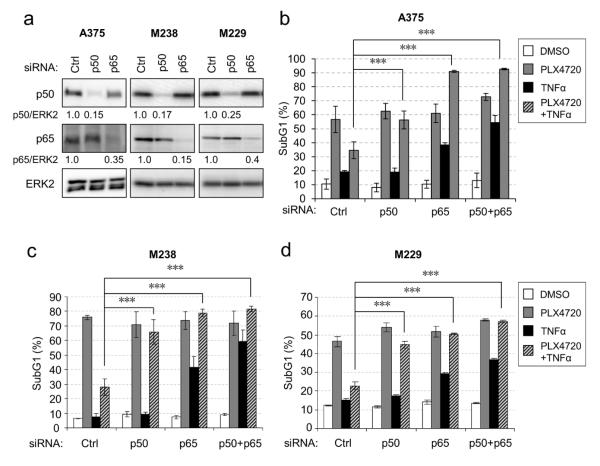
Next, we tested the requirement for NFκB in TNFα-mediated protection. We selectively depleted the NFκB components, p50 and p65, alone and in combination, as confirmed by Western blot (Fig. 2a). Depletion of p50 and p65 individually or in combination completely reversed the TNFα-mediated protection against the RAF inhibitor cytotoxicity (Fig. 2b-d). Knockdown of NFκB components also sensitized melanoma cells to TNFα treatment, indicating that NFκB may confer natural resistance of melanoma cells to TNFα-induced apoptosis. In the case of A375 cells, TNFα treatment in p65 and p65 plus p50 knockdowns further augmented the apoptotic effect of PLX4720 (Fig. 2b). These data indicate that TNFα-mediated protection against RAF inhibitor requires NFκB. By contrast, NFκB p65 and p50 knockdowns had little impact on the sensitivity of melanoma cells to non-targeted chemotherapeutics such as etoposide and cisplatin (Fig. S3a and 3b).
Figure 2. TNFα-induced protection from RAF inhibitors is mediated by NFκB.
a) Melanoma cells were transfected with control (Ctrl), p50 or p65 siRNAs for 72 hours. Cells lysates analyzed by Western blotting. Knockdown efficiency was estimated by band intensity quantitation and shown below the blots. b-d) Melanoma cells were transfected with indicated siRNAs for 72 hours and treated with −/+ 50 ng/ml TNFα and −/+ 5 µM PLX4720 for 48 (M229 and M238) or 72 (A375) hours before harvested for PI cell cycle analysis. Average percentage of sub-G1 population from three experiments was plotted for each condition. Error bars represent standard deviation. For statistics, one-way ANOVA analyses followed by Bonferroni test were performed on TNFα + PLX4720 treatment groups. * p<0.05; ** p<0.01; *** p<0.001.
c-IAP2 and c-FLIP are up-regulated upon TNFα and PLX4720 treatment in an NFκB-dependent manner
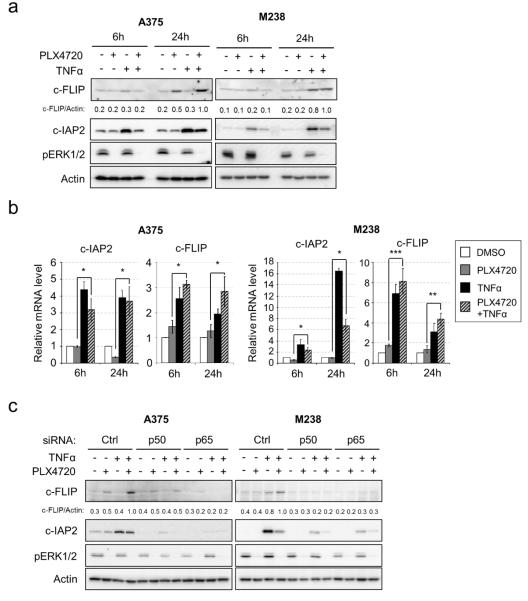
To identify the downstream effectors of NFκB that may account for the protection against PLX4720, we analyzed the expression of NFκB target proteins known to regulate apoptosis. We focused on differences in target expression in cells treated with PLX4720 alone and with a combination of PLX4720 and TNFα. Up-regulation of the BH3-only proteins, Bim-EL and Bmf, and down-regulation of the Bcl-2 family protein, Mcl-1, by PLX4720 (Boisvert-Adamo et al., 2009; Shao and Aplin, 2010) were not significantly altered by TNFα (Fig. S4a). Furthermore, no apparent TNFα-regulated differences were observed in other Bcl-2 family proteins and most IAP family proteins (XIAP, survivin, livin and c-IAP1) (Fig. S4a). By contrast, two anti-apoptotic proteins, c-IAP2 and c-FLIP, were up-regulated by TNFα and PLX4720 when compared to PLX4720 alone (Fig. 3a)
Figure 3. c-IAP2 and c-FLIP are up-regulated by TNFα and PLX4720 co-treatment.
a) A375 and M238 cells were treated with −/+ 50 ng/ml TNFα and −/+ 5 µM PLX4720 for 6 or 24 hours before analysis by Western blotting. Normalized c-FLIP/Actin band intensity ratios were shown. b) A375 and M238 cells were treated as in a) and total RNA isolated and qRT-PCR performed on c-IAP2 and c-FLIP genes. Housekeeping genes, actin or EEFA1, were internal controls. Average results from three independent experiments are shown. Error bars represent standard deviation. * p<0.05; ** p<0.01; *** p<0.001. c) A375 and M238 cells were transfected with indicated siRNAs for 72 hours and then treated with −/+ 50 ng/ml TNFα and −/+ 5 µM PLX4720 for additional 24 hours. Cells were lysed for Western blotting analysis on indicated proteins. Normalized c-FLIP/Actin band intensity ratios are indicated.
c-IAP2 was up-regulated by TNFα alone in A375 and M238 cells and co-treatment with PLX4720 weakened this up-regulation, possibly due to a decreased NFκB activity upon RAF inhibitor treatment (Fig. 3a). The mRNA levels of c-IAP2 were regulated in a similar pattern (Fig. 3b). Regulation of c-FLIP was more complex. TNFα alone induced c-FLIP protein expression in M238 cells, while PLX4720 treatment alone slightly enhanced c-FLIP expression in A375 cells (Fig. 3a and Fig. S5). Multiple forms of c-FLIP provide protection from death-receptor-mediated apoptosis (Golks et al., 2005; Krueger et al., 2001; Scaffidi et al., 1999) and we detected up-regulation of the long isoform, c-FLIP_L, in our studies. The mRNA levels of c-FLIP were up-regulated by TNFα alone or TNFα with PLX4720 in A375 and M238 cells (Fig. 3b). Despite this complexity, a consistent observation was that c-FLIP expression was up-regulated in the combined TNFα and PLX4720 treated cells versus PLX4720 alone treated cells (Fig. 3a, and quantitated in Fig. S5). This observation was also confirmed in M229 cells (Fig. S4b-c).
To confirm that the regulation of c-IAP2 and c-FLIP by TNFα are dependent on NFκB, we analyzed their expression in A375 and M238 cells depleted of NFκB subunits (Fig. 3c). Consistent with data above, in control siRNA-treated A375 and M238 cells, PLX4720 and TNFα combination treatment enhanced the expression of c-IAP2 and c-FLIP when compared to PLX4720 alone treatment. However, the up-regulation of both proteins was blunted in p50 or p65-depleted cells indicating that the regulation of c-IAP2 and c-FLIP is NFκB-dependent.
c-FLIP but not c-IAP2 is required for TNFα-induced protection against PLX4720
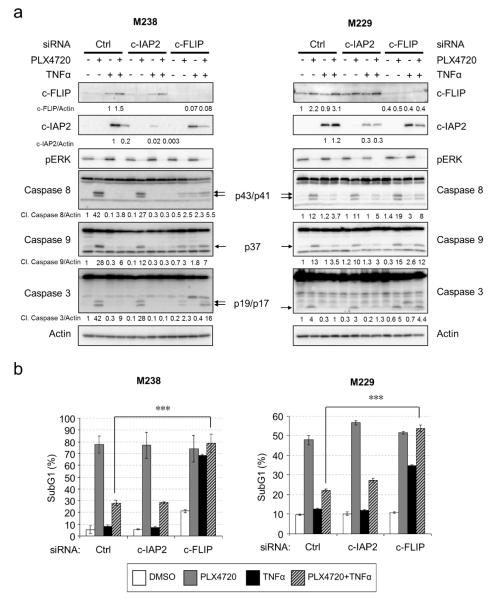
Since both c-IAP2 and c-FLIP play anti-apoptotic roles (Liston et al., 1996; Safa and Pollok, 2011), we tested individually the requirement for these two proteins in TNFα-induced protection against PLX4720. Selective siRNAs effectively reduced the induction of c-IAP2 (>90% and 75% down-regulation in M238 and M229, respectively) and c-FLIP (>90% and 87% down-regulation in M238 and M229, respectively) by TNFα and PLX4720 (Fig. 4a). Surprisingly, cells depleted of c-IAP2 were still protected by TNFα against PLX4720-induced apoptosis to a level comparable to control transfected cells (Fig. 4b). By contrast, depletion of c-FLIP completely restored cell death in the presence of TNFα and PLX4720 (Fig. 4b and Fig. S6). Similarly, the cleavage of caspase 3, 8 and 9 in the TNFα plus PLX4720 treatment samples was recovered, at least partially, to the level of PLX4720 treatment alone samples in c-FLIP-depleted but not c-IAP2-depleted M238 and M229 cells (Fig. 4a). c-FLIP depletion had no impact on cell death induced by ERK1/2 pathway-independent drugs such as etoposide and cisplatin (Fig. S3). These results suggest that c-FLIP but not c-IAP2 is required for TNFα-mediated protection against RAF inhibitor.
Figure 4. c-FLIP but not cIAP2 is required for TNFα-induced protection against PLX4720.
a) M238 or M229 cells were transfected with control, c-IAP2 or c-FLIP specific siRNAs for 72 hours. Cells were then treated with −/+ 50 ng/ml TNFα and −/+ 5 µM PLX4720 for additional 24 hours before lysis for Western blot analysis on indicated proteins. Arrows indicate the cleaved forms of the respective caspases. c-IAP2 and c-FLIP knockdown efficiency was determined by quantitating the band intensity and the relative levels indicated. b) M238 and M229 cells were treated with −/+ 50 ng/ml TNFα and −/+ 5 µM PLX4720 for an additional 48 hours after siRNA transfection as in a). Cells were then harvested for PI cell cycle analysis. Average percentages of sub-G1 cells from three independent experiments were shown for each knockdown condition. Error bars represent standard deviation. ** p<0.01.
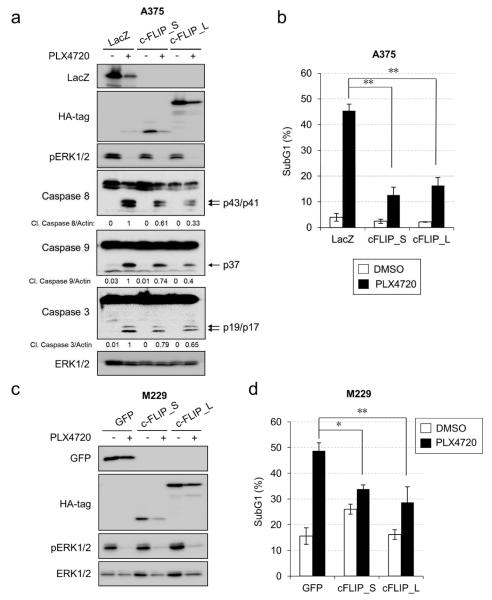
Over-expression of c-FLIP protects melanoma cells against PLX4720-induced apoptosis
To further test the role of c-FLIP as the pro-survival effector downstream of NFκB, we examined whether c-FLIP was sufficient to protect against PLX4720-induced apoptosis. We stably expressed either c-FLIP_S (short isoform), c-FLIP_L (long isoform) or LacZ (as a control) in A375 cells, as verified by Western blotting (Fig. 5a). We then tested for the response to PLX4720. PLX4720 treatment led to decreased expression of transgenes, presumably due to reduced CMV promoter activity upon ERK1/2 signaling inhibition (Chen and Stinski, 2002). Consistent with prior results, treatment of RAF inhibitor induced approximately 45% cell death in LacZ-expressing A375 cells (Fig. 5b). Ectopic expression of c-FLIP_S or c-FLIP_L decreased cell death to 12% or 16%, respectively. The reduction in cell death was associated with lower detectable levels of cleaved caspase 3, 8 and 9 (Fig. 5a). To confirm that the protective role of c-FLIP was not cell line dependent, we conducted similar experiments in M229 cells and used another negative control, GFP (Fig. 5c-d). GFP-expressing M229 cells exhibited 48% cell death after RAF inhibitor treatment, which was decreased to 34% or 28% following c-FLIP_S or c-FLIP_L expression, respectively. Therefore, c-FLIP isoforms are sufficient to protect mutant BRAF melanoma cells from PLX4720-induced cell death.
Figure 5. c-FLIP over-expression rescues melanoma cells from PLX4720-induced apoptosis.
a) A375 cells expressing the indicated transgenes were treated with −/+ 10 µM PLX4720 for 72 hours. Cells were harvested for Western blotting. b) A375 cells were treated as in a) and harvested for PI cell cycle analysis. The average percentage of sub-G1 cells from three independent experiments is shown. Error bars represent standard deviation. * p<0.05; ** p<0.01. c) M229 cells expressing the indicated transgenes were treated with −/+ 5 µM PLX4720 for 48 hours. Cells were harvested for Western blotting. d) M229 cells were treated as in c) and harvested for PI cell cycle analysis. Average percentage of sub-G1 cells from three independent experiments is shown. Error bars represent standard deviation. * p<0.05; ** p<0.01.
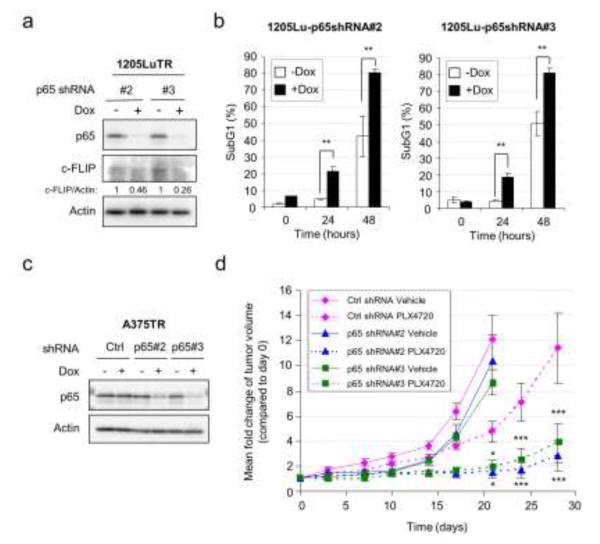
Targeting p65-NFκB enhances the response to PLX4720 in vitro and in vivo
Prompted by our observations, we hypothesized that targeting the NFκB pathway may synergize with the RAF inhibitor, PLX4720. p65 is a major component of the NFκB signaling complex; thus, we generated two 1205Lu cell lines (1205TR p65 shRNA #2 and #3) harboring a doxycycline-inducible shRNA targeting p65. In the absence of doxycycline, PLX4720 treatment induced 5-10% cell death after 24 hours and 40-50% after 48 hours in both cell lines in vitro (Fig. 6b). Treatment of doxycycline led to efficient knockdown of p65 and reduction in c-FLIP expression in both cell lines and significantly increased cell death after 24 and 48 hours of PLX4720 treatment (Fig. 6a-b).
Figure 6. NFκB targeting enhances responses to PLX4720 in vitro and in vivo.
a) 1205LuTR p65 shRNA #2 or #3 cells were grown −/+ 100 ng/ml doxycycline for 3 days and then lysed for Western blotting on p65, c-FLIP and actin. Quantitation of the c-FLIP levels is shown. b) As in a) except cells were treated after 3 days with 5 µM PLX4720 for a further 0, 24 or 48 hours and stained with PI. Error bar represents standard deviation. ** p<0.01. c) A375TR Ctrl shRNA or A375TR p65 shRNA #2 or #3 cells were cultured −/+ 100 ng/ml doxycycline for 72 hours and lysed for Western blotting. d) Growth curves of tumors formed by A375TR cells harboring control or p65 shRNAs in nude mice fed with either PLX4720 or vehicle-laced chow and doxycycline-containing water (n=6 per condition). Statistical analysis was performed on relative tumor size differences between PLX4720-treated control shRNA group and PLX4720-treated p65 shRNA#2 or #3 group. Error bars represent standard errors. * p<0.05, *** p<0.001.
We further tested this hypothesis in a mouse xenograft model using A375 cells expressing either control non-targeting or p65-specific shRNAs controlled by doxycycline. A375TR cells were used for their reproducible tumor forming ability in nude mice and efficient doxycycline inducible knockdowns (Abel et al., 2013). Doxycycline treatment significantly decreased p65 expression in A375TR p65 shRNA #2 and #3 cells (Fig. 6c). In vivo, p65 shRNA induction had marginal impact on tumor growth in the absence of the RAF inhibitor. Treatment with the RAF inhibitor alone partially inhibited tumor growth, consistent with partial resistance of this cell line to RAF inhibitor-induced cell death (Shao and Aplin, 2010). Remarkably, tumor growth was further inhibited when RAF inhibitor was combined with p65 depletion (Fig. 6d). Analysis of harvested tumors at the end of the xenograft experiment showed reduction of p65 with shRNAs (Fig. S7) although levels were variable due to the late time point of harvesting (day 28) and the presence of stromal cells. In summary, targeting the NFκB pathway enhances the response to RAF inhibitor in vivo.
DISCUSSION
The response in mutant BRAF melanoma patients to the RAF inhibitors is heterogeneous (Hartsough et al., 2014). While many forms of acquired resistance involve changes in the allelic frequency of mutations that re-activate the RAF-MEK-ERK1/2 pathway, factors in the tumor microenvironment are important in modulating the primary response to RAF inhibitors (Abel et al., 2013; Straussman et al., 2012; Wilson et al., 2012). In this study, we showed that TNFα modulates the apoptotic response of mutant BRAF melanoma cells to the RAF inhibitor, PLX4720, in an NFκB-dependent manner. This work builds on previous studies showing that TNFα blocks apoptosis in melanoma cells treated with MEK inhibitors/depleted for BRAF (Gray-Schopfer et al., 2007) and adds TNFα to the list of factors that provide protection against RAF inhibitor monotherapy and the RAF/MEK inhibitor combination.
Our results showed that TNFα protects melanoma cells against the RAF inhibitor-induced apoptosis by up-regulation of c-FLIP. Furthermore, ectopic expression of c-FLIP is sufficient to decrease RAF inhibitor-induced apoptosis in the absence of exogenous TNFα. The molecular details of c-FLIP-mediated protection remain unclear. c-FLIP is generally implicated in protection against extrinsic apoptosis although the long isoform, c-FLIP_L, may either promote or inhibit apoptosis depending on its expression level (Chang et al., 2002). In the extrinsic apoptosis pathway, activated death receptors induce the assembly of death-inducing signaling complex (DISC). DISC recruits the Fas-associated death domain-containing protein (FADD), which in turn recruits pro-caspase 8 via its death-effector domain (DED) for self-cleavage and activation. All three c-FLIP isoforms (c-FLIP_L, c-FLIP_S and c-FLIP_R) carry two DEDs at the N terminus, which enable them to compete with pro-caspase 8 for binding to FADD and prevent apoptosis (Irmler et al., 1997; Rasper et al., 1998). c-FLIP may contribute to RAF inhibitor resistance by preventing caspase 8 activation induced by RAF inhibitor. Indeed, over-expression of c-FLIP rescued PLX4720-induced apoptosis with a concurrent reduction in caspase 8 cleavage, as well as cleavage of caspase 3 and 9 (Fig. 5a); knockdown of c-FLIP led to the recovery of caspase 8 cleavage that was inhibited by TNFα plus PLX4720 treatment (Fig. 4a). In addition, caspase 8 interference protected melanoma cells against PLX4720 but not etoposide treatment (Fig. S8). While our data implicate the c-FLIP-caspase 8 axis in the protective role of TNFα against the RAF inhibitor, it is still possible that c-FLIP protects melanoma cells against PLX4720 through other downstream targets. For example, c-FLIP may counteract the activation of caspase 10, another caspase activated upon DISC engagement (Safa and Pollok, 2011). Previous studies have shown that c-FLIP expression is elevated in melanoma compared to benign melanocytic lesions (Bullani et al., 2001) and suppression of c-FLIP expression sensitizes melanoma cells to death ligand-induced apoptosis (Geserick et al., 2008). Thus, strategies to decrease c-FLIP expression may prove efficacious for melanoma.
Consistent with previous studies (Kreuz et al., 2001), we found in melanoma cells that TNFα up-regulated c-FLIP at the transcriptional level in an NFκB-dependent manner. The regulation of c-FLIP may be complex since it is regulated by several transcription factors (Safa et al., 2008) and post-translational regulation may also occur (Chang et al., 2006). Nevertheless, c-FLIP protein level was consistently elevated in PLX4720 and TNFα co-treated cells compared to cells treated with PLX4720 alone, supporting its protective role against PLX4720.
Studies on resistance to RAF inhibitors have mainly focused on the RAF-MEK-ERK and PI3K-AKT pathways. We present a mechanism of resistance and possible therapeutic strategies. Importantly, we identified NFκB pathway as an anti-apoptotic signal in RAF inhibitor-treated melanoma cells and co-targeting mutant BRAF and the NFκB pathway had enhanced anti-tumor effects in vitro and in vivo compared to targeting either pathway alone. The NFκB pathway is implicated in chemo-/radio-therapy resistance of many cancers and is readily targetable. Various compounds target this pathway at different levels. Bortezomib, a proteasome inhibitor, inhibits NFκB pathway by preventing the degradation of the NFκB inhibitor, IκB. The small molecule, BMS-345541, specifically inhibits the NFκB upstream activating kinase, IKKβ (Burke et al., 2003). Furthermore, NEMO-binding-domain peptides block the interaction between NEMO and IκB kinase complex (May et al., 2000). Tumor-associated macrophages (TAM) are a major source of TNFα in tumor microenvironment and TAM infiltration into tumors is enhanced by RAF and MEK inhibitor treatment (Smith et al., 2014). Targeting TAMs may be a viable alternative to direct NFκB intervention and the clinical efficacy of combined treatment of macrophage inhibitor, PLX3397, and vemurafenib on BRAF V600E melanoma patients is under investigation.
In summary, our findings underline the importance of the tumor microenvironment component, TNFα, to the responsiveness of BRAF V600E melanoma cells to RAF inhibitors and provide a strong rationale for the strategy of co-targeting the RAF-MEK-ERK1/2 pathway and the TNFα/NFκB axis to treat this subset of melanomas.
MATERIALS AND METHODS
Reagents
PLX4720 was provided by Dr. Gideon Bollag and Plexxikon Inc. (Berkeley, CA, USA). TNFα was purchased from Sigma-Aldrich (St. Louis, MO, USA). Doxycycline was obtained from Thermo Scientific (Rockford, IL, USA).
Cell Culture
Melanoma cells were cultured as previously described (Abel et al., 2013; Shao and Aplin, 2012). Cell lines were sequenced at multiple loci. 1205LuTR and A375TR are sublines with high Tet repressor expression (Abel et al., 2013).
Western Blotting
Western blotting was performed, as previously described (Shao and Aplin, 2010). Antibodies against Bcl-xL, phospho-ERK1/2 (Thr202/Tyr204), caspase 8, caspase 9, caspase 3, c-IAP1, cIAP2, XIAP, livin, survivin, p50 and HA-tag were purchased from Cell Signaling Technology (Beverley, MA, USA). ERK2, p65 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Additionally antibodies towards Bcl-2 and Mcl-1 (BD Biosciences, San Jose, CA, USA), anti-actin (Sigma-Aldrich), Bim (Stressgen, San Diego, CA, USA), Bmf (ENZO Life Science, Farmingdale, NY, USA), and c-FLIP (Adipogen, San Diego, CA, USA) were utilized.
Luciferase Assay
Melanoma cells were transfected with pNFκB-luc plasmid (2 µg/33 mm well) using Fugene HD reagent (Promega, Madison, WI, USA). After 24 hours, cells were harvested and sub-cultured equally into 8 wells. Next day, 4 wells were treated with −/+ TNFα and −/+ PLX4720, respectively, for another 24 hours and lysed for luciferase assay using Dual- Luciferase® Reporter Assay Kit (Promega). The other 4 wells were treated in the same manner and lysed for Bradford Assay. The luciferase readings were normalized against Bradford Assay readings accordingly and plotted against each treatment condition.
Quantitative RT-PCR
Total RNA isolation and qRT-PCR were performed as previously described (Shao and Aplin, 2010). Primer sequences are provided in the Supplemental Information.
Propidium Iodide (PI) Staining
Cells were treated with indicated drugs for 24 hours and then washed in PBS and fixed in 70% ethanol for 2 hours. Fixed cells were washed in PBS, pelleted and resuspended in 500 µl PI staining buffer (PBS with 40 µg/ml PI (Sigma-Aldrich), 100 µg/ml RNaseA (Thermo Scientific) and 0.1% Triton-X 100. Cells were stained for 30 minutes at room temperature and analyzed by flow cytometry on a FACS Calibur. Data were analyzed using Flowjo software (Three Star, Inc., Ashland, OR, USA).
RNA Interference
Cells were transfected for 72 hours with 12.5 nM small-interfering RNAs (siRNA) and Lipofectamine RNAiMAX (Invitrogen). siRNAs for p50 (GCAAUAGCCUGCCAUGUUU), p65 (GGAUUGAGGAGAAACGUAA), c-IAP2 (UCAAUGAUCUUGUGUUAGA), c-FLIP (GAUGUGUCCUCAUUAAUUU), and non-targeting siRNA control (UAGCGACUAAACACAUCAA) were purchased from Dharmacon (Lafayette, CO, USA).
Lentiviral cDNA Constructs
c-FLIP cDNA isoforms (HA-c-FLIP-L and HA-c-FLIP-S) were amplified from a melanoma cDNA pool to incorporate a HA-tag using KOD hot Start DNA polymerase kit. DNA fragments were cloned into pENTR™/D-TOPO vector (Invitrogen) and the resultant entry plasmids were then recombined with pLentipuro/TO/V5-DEST. Lentivirus were produced in 293FT cells (Shao and Aplin, 2010) and melanoma cells were infected with lentivirus for 72 hours before selection with puromycin.
Lentiviral shRNA Constructs
DNA oligonucleotides were annealed and ligated into pENTR/H1/TO using the manufacturer’s kit and instructions (Invitrogen). The sequences are provided in the Supplemental Information. shRNA cassettes were recombined into a destination vector with puromycin resistance. Lentiviruses were produced and melanoma cells were transduced, as above.
Animal Studies
A375TR cells (1 × 106/mouse) were intradermally injected into female athymic mice (NU/J: Jackson) and allowed to grow for 7 days to reach palpable tumor size (40-100 mm3). Mice were then exposed to drinking water containing doxycycline (2 mg/ml) and three days later fed with control chow or PLX4720 chow (90 mg/kg PLX4720). The first measure of tumor size was taken (day 0). Subsequently, measurements were taken twice a week using digital calipers, and tumor volume was determined by the following formula: volume = (length × width2) × 0.52. Mice were euthanized when tumor volume reached 1000 mm3.
Statistical Analysis
For in vitro experiments, statistical significance of differences between the results was evaluated using a student two-tailed t test assuming non-equal variance. For in vivo data, statistical analysis was conducted using a mixed-effects model and Tukey’s corrected pairwise comparisons of mean fold change in volume between treatment groups (SAS statistical software).
Animal Study approval
All animal experiments were approved by the Institutional Animal Care and Use Committee and conducted in an Association for the Assessment and Accreditation of Laboratory Animal Care accredited facility at Thomas Jefferson University.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Drs. Antoni Ribas (UCLA) and Meenhard Herlyn (Wistar Institute, Philadelphia) for the provision of cells and Dr. Gideon Bollag and Plexxikon Inc. (Berkeley, CA, USA) for RAF inhibitor. We thank Dr. Tingting Zhan for help with statistics. This study was supported by NIH grants R01-GM067893 (AE Aplin), an Outrun the Sun fellowship (Y Shao), a grant from the Pennsylvania Department of Health (AE Aplin) and a Fundamental Research Funds for the Central Universities of China (Y Shao). The Department specifically disclaims responsibility for any analysis, interpretation or conclusion. The Sidney Kimmel Cancer Center is supported by National Cancer Institute Support Grant 1P30CA56036.
Abbreviations
- NFκB
nuclear factor κB
- c-FLIP
cellular caspase 8 (FLICE)-like inhibitory protein
- ERK1/2
extracellular signal-regulated kinase 1/2
- RTK
receptor tyrosine kinase
- TAM
tumor-associated macrophages
- siRNA
small interfering RNA
- shRNA
short hairpin RNA
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest
REFERENCES
- Abel EV, Basile KJ, Kugel CH, III, et al. Melanoma adapts to RAF/MEK inhibitors by FOXD3-dependent upregulation of ERBB3 by FOXD3. J Clin Invest. 2013;123:2155–68. doi: 10.1172/JCI65780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri KI, Horton LW, LaFleur BJ, et al. Augmenting chemosensitivity of malignant melanoma tumors via proteasome inhibition: implication for bortezomib (VELCADE, PS-341) as a therapeutic agent for malignant melanoma. Cancer Res. 2004;64:4912–8. doi: 10.1158/0008-5472.CAN-04-0673. [DOI] [PubMed] [Google Scholar]
- Boisvert-Adamo K, Longmate W, Abel EV, et al. Mcl-1 is required for melanoma resistance to anoikis. Mol Cancer Res. 2009;7:549–56. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullani RR, Huard B, Viard-Leveugle I, et al. Selective expression of FLIP in malignant melanocytic skin lesions. J Invest Dermatol. 2001;117:360–4. doi: 10.1046/j.0022-202x.2001.01418.x. [DOI] [PubMed] [Google Scholar]
- Burke JR, Pattoli MA, Gregor KR, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem. 2003;278:1450–6. doi: 10.1074/jbc.M209677200. [DOI] [PubMed] [Google Scholar]
- Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene. 2001;20:7342–51. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- Chang DW, Xing Z, Pan Y, et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. Embo J. 2002;21:3704–14. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Kamata H, Solinas G, et al. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–13. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol Cell Biol. 2000;20:2687–95. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Stinski MF. Role of regulatory elements and the MAPK/ERK or p38 MAPK pathways for activation of human cytomegalovirus gene expression. J Virol. 2002;76:4873–85. doi: 10.1128/JVI.76.10.4873-4885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Eng J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geserick P, Drewniok C, Hupe M, et al. Suppression of cFLIP is sufficient to sensitize human melanoma cells to TRAIL- and CD95L-mediated apoptosis. Oncogene. 2008;27:3211–20. doi: 10.1038/sj.onc.1210985. [DOI] [PubMed] [Google Scholar]
- Golks A, Brenner D, Fritsch C, et al. c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem. 2005;280:14507–13. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- Gray-Schopfer VC, Karasarides M, Hayward R, et al. Tumor necrosis factor-alpha blocks apoptosis in melanoma cells when BRAF signaling is inhibited. Cancer Res. 2007;67:122–9. doi: 10.1158/0008-5472.CAN-06-1880. [DOI] [PubMed] [Google Scholar]
- Hartsough E, Shao Y, Aplin AE. Resistance to RAF inhibitors revisited. J Invest Dermatol. 2014;134:319–25. doi: 10.1038/jid.2013.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Jensen TO, Schmidt H, Moller HJ, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27:3330–7. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–7. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Kawakami H, Tomita M, Matsuda T, et al. Transcriptional activation of survivin through the NF-kappaB pathway by human T-cell leukemia virus type I tax. International journal of cancer. 2005;115:967–74. doi: 10.1002/ijc.20954. [DOI] [PubMed] [Google Scholar]
- Kreuz S, Siegmund D, Scheurich P, et al. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–73. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger A, Schmitz I, Baumann S, et al. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633–40. doi: 10.1074/jbc.M101780200. [DOI] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston P, Roy N, Tamai K, et al. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996;379:349–53. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- Lito P, Pratilas CA, Joseph EW, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 2012;22:668–82. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–95. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- Magne N, Toillon RA, Bottero V, et al. NF-kappaB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett. 2006;231:158–68. doi: 10.1016/j.canlet.2005.01.022. [DOI] [PubMed] [Google Scholar]
- May MJ, D'Acquisto F, Madge LA, et al. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–4. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- Meng Y, Beckett MA, Liang H, et al. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Research. 2010;70:1534–43. doi: 10.1158/0008-5472.CAN-09-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyskens FL, Jr., Buckmeier JA, McNulty SE, et al. Activation of nuclear factor-kappa B in human metastatic melanomacells and the effect of oxidative stress. Clin Cancer Res. 1999;5:1197–202. [PubMed] [Google Scholar]
- Rasper DM, Vaillancourt JP, Hadano S, et al. Cell death attenuation by 'Usurpin', a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 1998;5:271–88. doi: 10.1038/sj.cdd.4400370. [DOI] [PubMed] [Google Scholar]
- Safa AR, Day TW, Wu CH. Cellular FLICE-like inhibitory protein (C-FLIP): a novel target for cancer therapy. Curr Cancer Drug Targets. 2008;8:37–46. doi: 10.2174/156800908783497087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safa AR, Pollok KE. Targeting the anti-apoptotic protein c-FLIP for cancer therapy. Cancers (Basel) 2011;3:1639–71. doi: 10.3390/cancers3021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Schmitz I, Krammer PH, et al. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274:1541–8. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- Shao Y, Aplin A. Akt3-mediated resistance to apoptosis in B-RAF targeted melanoma cells. Cancer Res. 2010;70:6670–81. doi: 10.1158/0008-5472.CAN-09-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Aplin AE. BH3-only protein silencing contributes to acquired resistance to PLX4720 in human melanoma. Cell Death Differ. 2012;19:2029–39. doi: 10.1038/cdd.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Eng J Med. 2012;366:707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik C, de Martin R, Binder BR, et al. Cytokine induced expression of porcine inhibitor of apoptosis protein (iap) family member is regulated by NF-kappa B. Biochem Biophys Res Commun. 1998a;243:827–32. doi: 10.1006/bbrc.1998.8185. [DOI] [PubMed] [Google Scholar]
- Stehlik C, de Martin R, Kumabashiri I, et al. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998b;188:211–6. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–4. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MP, Sanchez-Laorden B, O'Brien K, et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFalpha. Cancer Dis. 2014;4:1214–9. doi: 10.1158/2159-8290.CD-13-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torisu H, Ono M, Kiryu H, et al. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer. 2000;85:182–8. [PubMed] [Google Scholar]
- Wang Z, Boudjelal M, Kang S, et al. Ultraviolet irradiation of human skin causes functional vitamin A deficiency, preventable by all-trans retinoic acid pre-treatment. Nat Med. 1999;5:418–22. doi: 10.1038/7417. [DOI] [PubMed] [Google Scholar]
- Wilson TR, Fridlyand J, Yan Y, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–9. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Richmond A. Constitutive kappa B kinase activity correlates with nuclear factor-kappa B Activation in human melanoma cells. Cancer Res. 2001;61:4901–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.