Abstract
Metabotropic glutamate receptor 4 (mGlu4) negatively modulates GABA and glutamate release in the ‘indirect pathway’ of the basal ganglia, and has thus been proposed as a potential target to treat motor symptoms in Parkinson's disease. Here, we present an extensive comparison of the behavioural effects produced by the mGlu4 positive allosteric modulator (PAM), VU0364770, and the mGlu4 orthosteric agonist, LSP1-2111, in rats with unilateral 6-OHDA lesions. The compounds' activity was initially assessed in a test of haloperidol-induced catalepsy in intact rats, and effective doses were then evaluated in the hemiparkinsonian animal model. Neither of the two compounds modified the development of dyskinetic behaviours elicited by chronic treatment with full doses of l-DOPA. When given together with l-DOPA to rats with already established dyskinesias, neither VU0364770 nor LSP1-2111 modified the abnormal involuntary movement scores. VU0364770 potentiated, however, the motor stimulant effect of a sub-threshold l-DOPA dose in certain behavioural tests, whereas LSP1-2111 lacked this ability. Taken together, these results indicate that a pharmacological stimulation of mGlu4 lacks intrinsic antidyskinetic activity, but may have DOPA-sparing activity in Parkinson's disease. For the latter indication, mGlu4 PAMs appear to provide a better option than orthosteric agonists.
Keywords: Parkinson's disease, Glutamate, Dopamine, Movement disorders, Plasticity, Dyskinesias, Metabotropic receptor
1. Introduction
Current pharmacotherapies for Parkinson's disease (PD) aim at ameliorating deficits in central dopaminergic tone. Among these therapies, l-DOPA remains the most effective option (Salat and Tolosa, 2013) but its therapeutic window narrows during the progression of PD, leading to motor fluctuations and abnormal involuntary movements (dyskinesias) (recently reviewed in Cenci et al., 2011; Schneider and Obeso, 2014). These motor complications are perceived as debilitating by the affected patients (Palfreman, 2014) and continue to be a major problem to the clinical management of PD. For this reason, there is a great interest in developing non-dopaminergic treatments that can be added to l-DOPA to reduce these untoward effects (Brotchie,1998; Cenci et al., 2011; Finlay and Duty, 2014). Moreover, new approaches are being explored to prevent the maladaptive neuroplasticity underlying the long-term development of dyskinesias (Cenci, 2014; Cerasa et al., 2014).
The progressive nigrostriatal degeneration typical of PD leads to an overactivity of the “indirect pathway”, which originates from dopamine D2 receptor-positive striatal neurons projecting to the external globus pallidus (GPe). The ensuing overactivity of the subthalamic nucleus (STN) plays a critical role in generating parkinsonian motor features (Albin et al., 1989). Accordingly, surgical lesions or functional inactivation of the STN produce marked antiparkinsonian effects (reviewed in Sgambato-Faure and Cenci, 2012).
There is great interest in the possibility of reducing the overactivity of the indirect pathway by targeting specific receptors, such as adenosine A2a receptors (Morelli et al., 2009) or group III metabotropic glutamate receptors (mGlu receptors) (Johnson et al., 2009). Group III mGlus (mGlu4, -7, and -8) can presynaptically inhibit GABA and glutamate release at several nodes of the indirect pathway (reviewed in Conn et al., 2005). Studies based on intracerebroventricular administration of a group III mGlu agonist reported motor stimulant effects in both acute and chronic animal models of PD, and these effects were linked to an inhibitory action on GABAergic transmission at striatopallidal synapses (reviewed in Conn et al., 2005; Finlay and Duty, 2014). A seminal study identified mGlu4 as the crucial mediator of these effects (Valenti et al., 2003). Accordingly, subsequent studies reported that selective agonists of mGlu4 can improve motor deficits in rodent models of PD (Lopez et al., 2007; Niswender et al., 2008; Beurrier et al., 2009).
Until recently, it has been difficult to develop selective agonists for mGlu4, as the orthosteric ligand-binding site of group III mGluR subtypes is highly conserved. On the other hand, mGlus have modulatory sites with less homology between subtypes, the so-called allosteric sites. Highly selective mGlu4 ligands that bind an allosteric modulatory site (positive allosteric modulators, PAMs) have recently been explored as alternative therapeutic options. The earliest mGlu4 PAMs had limited potential for in vivo use due to unsuitable chemical properties (Marino et al., 2003). In 2012, the compound, N-(3-chlorophenyl)picolinamide (VU0364770) was reported as a systemically active mGlu4 PAM. VU0364770 was found to reverse haloperidol-induced catalepsy when given alone, and exhibited anti-akinetic efficacy when given to unilaterally 6-OHDA-lesioned rats either alone or in combination with subthreshold doses of either the A2a antagonist, preladenant or l-DOPA (Jones et al., 2012). Soon thereafter, additional mGlu4 PAMs, including LuAF21934 and ADX88178 (developed at Lundbeck Pharmaceuticals and Addex Pharmaceuticals, respectively) were reported to alleviate haloperidol-induced catalepsy and potentiate the anti-akinetic effect of l-DOPA (Le Poul et al., 2012; Bennouar et al., 2013).
In parallel with these efforts, Acher and colleagues developed a group III-mGlu orthosteric agonist with preferential activity at mGlu4 (Acher et al., 2007). This compound, termed LSP1-2111, was found to reverse haloperidol-induced catalepsy after intrapallidal administration and to ameliorate deficits in a reaction-time task in rats with partial, bilateral nigrostriatal dopamine lesions (Beurrier et al., 2009). LSP1-2111 was recently evaluated in mice with unilateral 6-OHDA lesions treated with l-DOPA, where it was reported to attenuate the development of dyskinesias upon chronic de novo treatment (Lopez et al., 2011).
The purpose of this study was to determine whether a PAM and an orthosteric agonist at mGlu4 have comparable properties in either potentiating the anti-akinetic action of l-DOPA or attenuating its dyskinesiogenic potential. To this end, we selected VU0364770 and LSP1-2111, well-characterized compounds that are centrally active upon systemic administration (Beurrier et al., 2009; Jones et al., 2012). The compounds were administered to rats with unilateral 6-OHDA lesions, either alone or in combination with l-DOPA, according to different treatment schedules. Our findings suggest that selective potentiation of mGlu4 may provide some mild l-DOPA-sparing activity without inducing dyskinesia.
2. Materials and methods
2.1. Animals
Studies of haloperidol-induced catalepsy were performed at Vanderbilt University using male Sprague–Dawley rats (body weight, 275–300 g) (Harlan Laboratories, Inc Indianapolis, IN). The experimental protocols were approved by the Institutional Animal Care and Use Committee of Vanderbilt University and conformed to the guidelines established by the National Research Council Guide for the Care and Use of Laboratory. Studies on hemiparkinsonian animals were performed at Lund University using adult female Sprague–Dawley rats (body weight 225–250 g) (Charles River, Germany), according to our standard procedures (Lindgren et al., 2010; Rylander et al., 2010). The experimental protocols were approved by the Malmö/Lund Ethical Committee on Animal Research. In all studies, rats were housed under 12 h light/dark cycle with free access to food and water. Behavioural tests were performed during the light hours. A total of 240 rats were used in this study (including animals that had to be excluded because of technical failure, and those used in preliminary pharmacokinetic experiments).
2.2. Dopamine denervating lesions
A synopsis of studies using dopamine-denervated rats is provided in Fig. 1. All rats included in these experiments were subjected to a lesion of the right medial forebrain bundle with the neurotoxin 6-hydroxydopamine (6-OHDA-HCl, Sigma Aldrich, Sweden) according to well-standardized procedures (Lindgren et al., 2010; Rylander et al., 2010). Briefly, the toxin was dissolved in 0.02% ascorbate/saline to a concentration of 3.5 μg/μl (free base) and injected at two sites (coordinates given in mm relative to bregma and the dural surface): (1) AP = −4.4, L = −1.2, DV-7.8, with tooth bar at −2.4 (2.5 μl); (2) AP = −4.0, L = −0.8, DV-8.0, tooth bar at +3.4 (2.0 μl). After two weeks, rats were evaluated in the amphetamine-induced rotation test, where the number of rotations was automatically calculated during 90 min after an i.p. injection of dexamphetamine (dexamphetamine-HCl, 2.5 mg/kg, Sigma Aldrich, Sweden, dosed as salt). Rats making more than five full turns/minute in the direction ipsilateral to the lesion were kept for further experiments (Winkler et al., 2002). The amphetamine-induced rotation test resulted in the exclusion of approximately 25% of the 6-OHDA-lesioned animals.
Fig. 1.
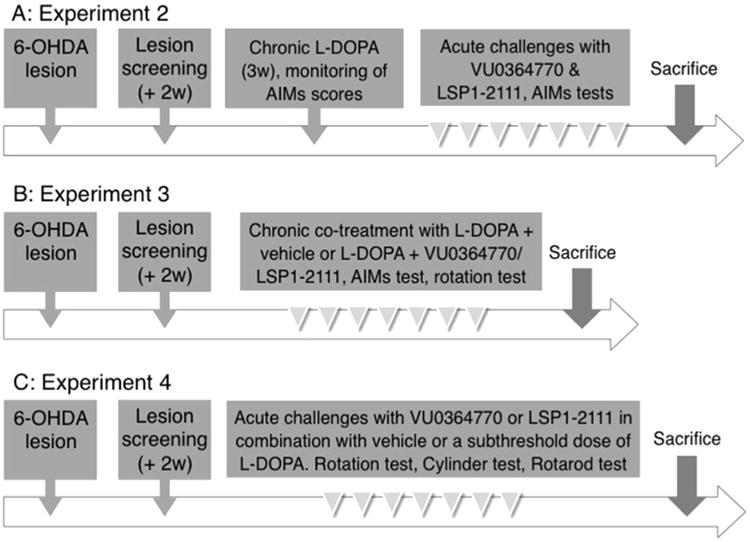
Schematic view of the three experimental designs applied to the 6-OHDA rat model of parkinsonism.
2.3. Drug treatments
All drugs used in these experiments and their administration modalities are reported in Table 1. Drugs were dissolved in the appropriate vehicle (cf. Table 1) immediately prior to use, and administered in a volume of 1 mL/kg; with the exception of VU0364770 which was administered in a volume of 2 mL/kg. l-DOPA was always coadministered with a fixed dose of the peripheral DOPA-decarboxylase inhibitor, benserazide (12 mg/kg, s.c). VU0364770 and LSP1-2111 were custom synthetized for this study at Vanderbilt Center for Neuroscience Drug Discovery. LSP1-2111 was dissolved in sterile water and administered i.p. according to the procedure published in Beurrier et al. (2009). For VU0364770, several vehicles and routes were tested in preliminary experiments, and peroral drug administration in a DMSO/PEG vehicle was chosen for the dyskinesia studies based both on the pilot results and on our previous experience (Rylander et al., 2010). For the last DOPA-sparing study however, the route was changed to s.c. (with the compound dissolved in 10% Tween vehicle), as in Jones et al. (2012). This change of administration route was prompted by the concern that p.o. dosing may provide slightly lower brain exposure.
Table 1.
Drugs, doses and administration procedures used.
| Drug | Mechanism | Suppliera | Route | Dosesb | Vehicle | Timec | Reference |
|---|---|---|---|---|---|---|---|
| Catalepsy studies | |||||||
| Haloperidol | DA antagonist | Sigma–Aldrich | i.p. | 1.5 | 8.5% lactic acid | 90 | Jones et al. (2012) |
| LSP1-2111 | mGluR4 agonist | VCNDDd | i.p. | 0.3–30 | Sterile water | 30 | Beurrier et al. (2009) |
| VU0364770 | mGluR4 PAM | VCNDDd | s.c. | 1–100 | 10% Tween 80 | 30 | Jones et al. (2012) |
| A2A | A2A antagonist | VCNDDd | p.o | 56.6 | 8.5% lactic acid | Jones et al. (2012) | |
| Studies in 6-OHDA rats | |||||||
| l-DOPA methyl ester | DA precursor | Research Organics Inc. | s.c. | 6 | Saline | 0 | Lindgren et al. (2010) |
| Benserazide-HCl | DOPA decarboxylase inhibitor | Sigma–Aldrich | s.c. | 12 | Saline | 0 | Lindgren et al. (2010) |
| VU0364770 | mGluR4 PAM | VCNDDd | p.o. or s.c.e | 100 | 10% Tween 80 or 1:10 DMSO/PEGf | 0 | Jones et al. (2012) |
| LSP1-2111 | mGluR4 agonist | VCNDDd | i.p. | 15 | Sterile water | 0 | Beurrier et al. (2009) |
Source of the compounds used in this study.
Doses in mg/kg.
Time interval relative to testing.
VCNDD: Vanderbilt Center for Neuroscience Drug Discovery.
s.c. route for experiment 1 and 4. p.o. route for experiments 2 and 3.
10% Tween vehicle for experiment 1 and 4, DMSO/PEG 1:10 vehicle for experiments 2 and 3.
2.4. Experimental design
2.4.1. Experiment 1: effect of VU0364770 and LSP1-2111 on haloperidol-induced catalepsy
Rats were administered with haloperidol (1.5 mg/kg, i.p.) 60 min prior to vehicle (10% Tween 80), VU0364770 (1–100 mg/kg, s.c.) or LSP1-2111 (0.3–30 mg/kg i.p.). After an additional 30 min, all rats were assessed for catalepsy. The doses of 100 mg/kg VU0364770 and 15 mg/kg LSP1-2111 produced the largest anti-cataleptic effects, and were thus selected for experiments using 6-OHDA-lesioned rats.
2.4.2. Experiment 2: effect of VU0364770 and LSP1-2111 on already established dyskinesia
The experimental design for experiments 2–4 is outlined in Fig. 1. Two sets of 6-OHDA-lesioned animals received chronic daily treatment with l-DOPA for 3 weeks in order to induce abnormal involuntary movements (AIMs). Dyskinesia rating sessions were carried out 2–3 times per week. Rats that developed moderate/severe and reproducible AIMs were kept for further investigations (11 rats in the study with VU0364770, and 15 rats in the study with LSP1-2111). After the dyskinesia induction phase, rats were kept on a maintenance dosing regimen consisting of 2–4 injections of l-DOPA per week for the rest of the study (Fig. 1A). During this time the rats were acutely challenged with either VU0364770,100 mg/kg, or LSP1-2111,15 mg/kg, or their corresponding vehicles, and the l-DOPA-induced AIM scores were rated after each drug challenge. To assess whether the treatments interfered with the therapeutic benefit of l-DOPA (anti-akinetic effects), rats were evaluated also on other motor tests (cylinder test, in the study with VU0364770, and rotarod test in the study with LSP1-2111; see Supplementary material). Tests and treatments were administered using a randomized latin-square design, with at least 48 h of drug washout between testing/treatment sessions.
2.4.3. Experiment 3: effect of VU0364770 and LSP1-2111 on the development of dyskinesia in previously drug-naive rats
Drug-naïve rats with 6-OHDA lesions were randomized to two groups, which were tested inparallel. One group received daily injections of l-DOPA combined with the drug under investigation (VU0364770, 100 mg/kg, or LSP1-2111, 15 mg/kg), whereas the other group received daily injections of l-DOPA plus vehicle. Ratings of AIM scores were performed three times per week. In addition, rotational behaviour was recorded on one occasion during the last week of the chronic treatment.
2.4.4. Experiment 4: DOPA-sparing potential of VU0364770 and LSP1-2111 in parkinsonian drug-naive rats
Two sets of drug-naive rats with 6-OHDA lesions were used for this study, one set for evaluating VU0364770 and the other one for evaluating LSP1-2111. Drug-naive rats with MFB lesions were subjected to acute challenges with the following treatments: (i) a subthreshold dose of l-DOPA (1.5 mg/kg) and the mGlu4-ligand vehicle; (ii) VU0364770,100 mg/kg (rat set 1) or LSP1-2111,15 mg/kg (rat set 2) and saline; (iii) a combination of l-DOPA and the mGluR4 ligand; (iv) saline and the mGlu4-ligand vehicle. Treatments were administered according to a randomized latin square design. The sequence of treatments was repeated three times in order to evaluate the animals on three behavioural tests (cylinder test, rotarod test, and automated rotation test), with at least 48 h drug washout between tests.
2.5. Behavioural tests
2.5.1. Haloperidol-induced catalepsy test
Rats were administered with haloperidol (1.5 mg/kg, i.p.) 60 min prior to the drug under investigation. After an additional 30 min, rats were assessed for catalepsy. Catalepsy was measured by gently placing the rat's forepaws onto a horizontal bar (6 cm above the testing surface) with the body positioned at an angle of ∼45° to the testing surface. The latency in seconds required for the rat to remove one or both forepaws from the bar was measured using a testing cut-off of 30 s.
2.5.2. Ratings of abnormal involuntary movements
After an injection of l-DOPA, rats were put in individual cages and observed for one minute every 20th over a 180 min testing session. In case of protracted AIMs duration (cf. effect of VU0364770 in study A), the AIMs rating session was prolonged to 220 min. Three subtypes of dyskinesia were simultaneously scored on a severity scale from 0 to 4 based on the proportion of observation time during which dyskinetic movements were present (Cenci et al., 1998). The three subtypes are (1) limb dyskinesia, circular or jerky movements of the forelimb; (2) axial dyskinesia, twisting movements or dystonic posturing of the head, neck or trunk towards the side contralateral to the lesion; (3) orolingual dyskinesia, vacuous chewing movements, jaw opening and tongue protrusion, accompanied by twitching of the facial musculature. In study A, rats exhibiting a severity score ≥2 on at least two AIM subtypes on at least two monitoring periods per session were classified as dyskinetic (Westin et al., 2007), and used to evaluate the antidyskinetic effects of mGluR4 ligands.
To detect possible antidyskinetic effects by these ligands, the basic scale of Cenci et al. (1998) was combined with a scale that rates the amplitude of axial, limb and orolingual AIMs (grades 0–4), as described in Rylander et al. (2010). The basic severity score and the amplitude score of each AIM subtype on each monitoring period were multiplied, and these products were then summed for an entire testing session. This procedure provides greatest sensitivity in detecting antidyskinetic effects of drug treatments (Breger et al., 2013).
2.5.3. Rotation test
Rats were put in hemispherical bowls within an automated rotometry system (AccuScan Instruments, Columbus, OH, USA), and left and right turns were recorded during 180 min. A measure of total motor activation was obtained by summing all left and right turns in a session.
2.5.4. Cylinder test
Thirty and sixty minutes after challenge with each given drug, the rats were placed in a glass cylinder with a diameter of 21 cm and a height of 34 cm to record forelimb use during vertical exploration. All weight-bearing wall contacts of the right and the left forepaws were counted for three minutes. Results were expressed as a percentage use of the forelimb contralateral to the lesion (left) as in Lundblad et al. (2002).
2.5.5. Rotarod test
The rotarod test was performed as previously described (Lundblad et al., 2003; Rylander et al., 2009), with some minor modifications. Briefly, rats were pre-trained on the Rotarod (Panlab, Harvard apparatus), with the rod rotating at an accelerating speed from 4 to 40 rpm over two minutes, until they reached a stable baseline performance (3–4 consecutive days of training). On the days of testing rats were evaluated on the rod at 20, 40 and 120 min after the drug challenge. The mean of the three trials was used for statistical analyses. Data following drug treatment were expressed as a percentage of the baseline performance in each animal.
2.6. Histological verification of the dopamine-denervating lesions
After the behavioural studies, the nigrostriatal lesions were verified using tyrosine hydroxylase (TH) immunohistochemistry. To this end, rats were given an overdose of pentobarbital (240 mg/kg i.p., Apoteksbolaget AB, Sweden) and decapitated. Brains were rapidly extracted, immediately frozen on crushed dry ice, and stored at −80 °C. Coronal sections of 16 μm thickness were cut through the striatum (levels +1.00 mm to −0.20 mm relative to bregma (Paxinos, 2004)) using a cryostat (Microm HM 560, Thermo Fisher Scientific Waltham, MA). Sections were thaw-mounted onto adhesive glass slides (SuperFrost Plus; Menzel Glazer, Braunschweig, Germany) and stored at −20 °C until further analysis. Immunohistochemistry was performed on paraformaldehyde-fixed sections using a peroxidase-based detection method (the procedure is described in Iderberg et al., 2013). A primary antibody against tyrosine hydroxylase (TH) was used to detect DA cells and fibres (rabbit polyclonal anti-TH antibody from Pel-Freeze; 1:1000). Sections were visually inspected on a light table with constant illumination (Kaiser slimlite). Animals displaying a visibly complete loss of TH throughout the striatum ipsilateral to the lesion were included in the study without further quantitative analysis. If some residual DA innervation was visible in any section, optical density measurements were performed according to well-established methods (Francardo et al., 2011), and animals with an average of ≥10% residual TH immunostaining in the caudate-putamen ipsilateral to the lesion were excluded from the study (a total of 2 animals were excluded by these criteria).
2.7. Statistical analysis
Results are presented as group means + S.E.M. Data from the catalepsy tests were analysed by one-way ANOVA; post hoc comparisons with the vehicle control group were performed using Dunnett's test. The calculations were performed using JMP9 statistical software (SAS Institute, Cary, NC) and graphed using SigmaPlot12 (Sangus, MA). Behavioural data from hemiparkinsonian rats were analysed using either one-way ANOVA (and post hoc Student Newman–Keuls test) or repeated measures (RM) ANOVA (and post hoc Bonferroni test) where appropriate. The main effects were verified with non-parametric statistics. The calculations were performed using Graph Pad Prism software. Statistical significance was set at α = 0.05. In the Results, we shall report actual p-values from the ANOVAs, while post-hoc comparisons will be reported as being either statistically significant or non-significant.
3. Results
3.1. Haloperidol-induced catalepsy
Tests of haloperidol-induced catalepsy were performed in order to verify the activity of the batches of VU0364770 and LSP1-2111 that had been synthesized for this study. Moreover, these experiments informed the selection of compound doses for the studies in hemiparkinsonian rats. The dose ranges of VU0364770 (1.0–100 mg/kg, s.c.) and LSP1-2111 (0.3–30 mg/kg, i.p.) were chosen based on published literature (Beurrier et al., 2009; Jones et al., 2012).
As shown in Fig. 2, both VU0364770 (Fig. 2A) and LSP1-2111 (Fig. 2B) produced a dose-dependent reversal of the catalepsy induced byhaloperidol (1.5 mg/kg) (Fig. 2A: F(7,79) = 7.99, p < 0.001; Fig. 2B: F(6,65) = 6.297; p < 0.001). The effect of VU0364770 was significant at the dose range of 30–100 mg/kg. The effect of LSP1-2111 was significant at doses of 1–30 mg/kg, reaching the largest magnitude at a dose range of 10–15 mg/kg (Fig. 2B). As shown in Fig. 2, the anti-cataleptic effect of VU0364770 and LSP1-2111 at the doses of 100 mg/kg and 15 mg/kg, respectively, approximated that produced by preladenant, a potent A2a receptor antagonist (Hodgson et al., 2009; Jones et al., 2012) (cf empty bar Fig. 2B).
Fig. 2.
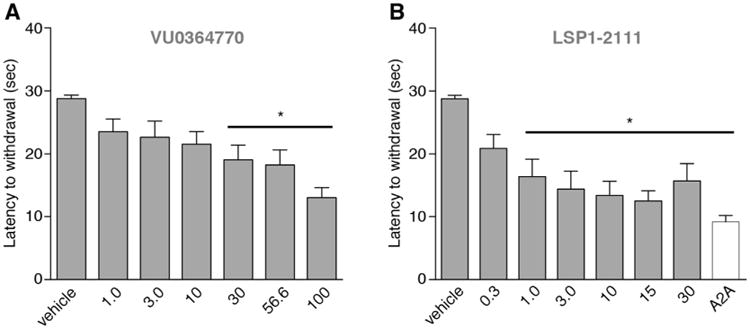
Effect of VU0364770 and LSP1-2111 on haloperidol-induced catalepsy. Both VU0364770 (A, s.c. administration) and LSP1-2111 (B, i.p. administration) dose-dependently reduced catalepsy induced by haloperidol (1.5 mg/kg, i.p.). The maximum effect achieved by both drugs, was comparable to that of the A2A antagonist (56.6 mg/kg; white bar), that was used as a positive comparator drug in this experiment. (n = 10 for VU0364770; n = 10 for LSP1-2111 treated group) p < 0.05, * vs. vehicle, Dunnett's test.
3.2. l-DOPA-induced dyskinesia
In a first study (cf. study design A in Fig.1), 6-OHDA lesioned rats were rendered dyskinetic with daily l-DOPA treatment for 3 weeks (l-DOPA 6 mg/kg + benserazide 12 mg/kg, s.c.). Thereafter, rats with moderate-severe dyskinesias were acutely challenged with VU0364770, LSP1-2111, or their vehicles, together with the full dose of l-DOPA, and AIMs were rated.
VU0364770 did not reduce the severity of established dyskinesias, but prolonged the time–action curve of the AIMs (Fig. 3A, Two-way RM ANOVA, treatment: F(1,20) = 1.80, p = 0.19; time: F(10,200) = 34.9, p < 0.001; interaction: F(10,200) = 4.57, p < 0.001. p < 0.05 for L-DOPA + VU0364770 versus L-DOPA + vehicle at 160–200 min). Despite this prolongation, the sum of l-DOPA-induced AIM scores per session did not differ significantly between VU0364770 and vehicle cotreatment (p = 0.06 and p = 0.10 with paired t-test and Wilcoxon signed ranks test, respectively. Data not shown). LSP1-2111 had no effect on either the severity or the duration of the AIMs (Fig. 3B, Two-way RM ANOVA, treatment: F(2,42) = 0.13, p = 0.88; time: F(10,420) = 124.8, p < 0.001; interaction: F(20,420) = 0.86, p = 0.64).
Fig. 3.
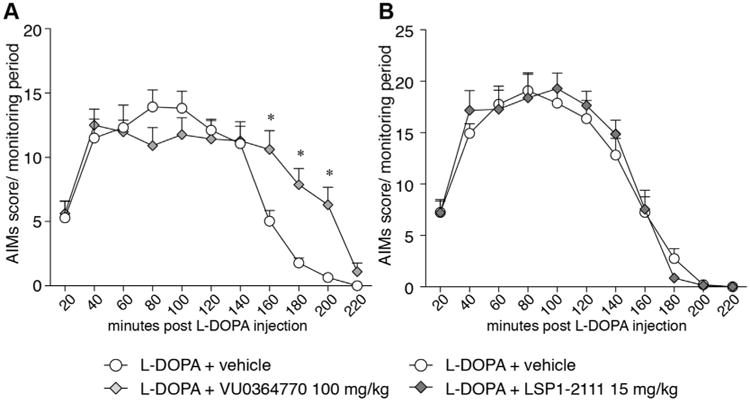
Effect of VU0364770 (100 mg/kg, po) and LSP1-2111 (15 mg/kg, i.p.) on established l-DOPA-induced dyskinesia. Two different sets of l-DOPA-treated dyskinetic rats were acutely challenged with VU0364770 (A, n = 11) or LSP1-2111(B, n = 15) and their AIM scores were rated. None of the compounds had any effect on the peak severity of established dyskinesias but VU0364770 prolonged the duration of the AIMs (grey diamonds in A). p < 0.05, * vs. l-DOPA + vehicle, two-way RM ANOVA and post hoc Bonferroni test.
In a second study (cf. study design B in Fig. 1), drug-naïve animals were randomized to receive daily treatment with either l-DOPA + vehicle or l-DOPA + the mGlu4 ligand for 15–18 days. There was no treatment effect of either VU0364770 or LSP1-2111 on the evolution of the AIM scores over the course of the treatment (Fig. 4A, VU0364770: Two-way RM ANOVA, treatment: F(1,11) = 0.04, p = 0.84; time: F(6,66) = 34.9, p < 0.001; interaction: F(6,66) = 0.94, p = 0.47; and 4D, LSP1-2111: Two-way RM ANOVA, treatment: F(1,11) = 0.17, p = 0.68; time: F(5,105) = 48.28, p < 0.001; interaction: F(5,105) = 1. 66, p = 0.15). An analysis of AIM scores per monitoring period did not reveal any within-session effect of the mGlu4 ligands on the severity or duration of the AIM scores (Fig. 4B VU0364770: Two-way RM ANOVA, treatment: F(1,9) = 0.36, p = 0.55; time: F(9,81) = 15.18, p < 0.001; interaction: F(9,81) = 0.47, p = 0.89; and 4E, LSP1-2111: Two-way RM ANOVA, treatment: F(1,19) = 0.64, p = 0.43; time: F(8,152) = 42.11, p < 0.001; interaction: F(8,152) = 1.58, p = 0.13). Because these results were at variance with the AIMs prolongation by VU0364770 in study design A, the pharmacodynamic profiles of the treatments under investigation were further analysed using automated rotation tests (Fig. 4C, F). This test showed that neither compound had a significant impact on either the total number of turns (a measure of global motor activation) or the duration of turning behaviour induced by l-DOPA (Fig. 4C, VU0364770: Two-way RM ANOVA, treatment: F(1,16) = 0.12, p = 0.73; time: F(24,384) = 10.1, p < 0.0001; interaction: F(24,384) = 0.91, p = 0.91; and 4F, LSP1-2111: Two-way RM ANOVA, treatment: F(1,21) = 1.11, p = 0.30; time: F(17,357) = 18.24, p < 0.001; interaction: F(17,357) = 1.11, p = 0.34). Similar results were obtained upon analyses of contralateral or ipsilateral rotational activity (data not shown).
Fig. 4.
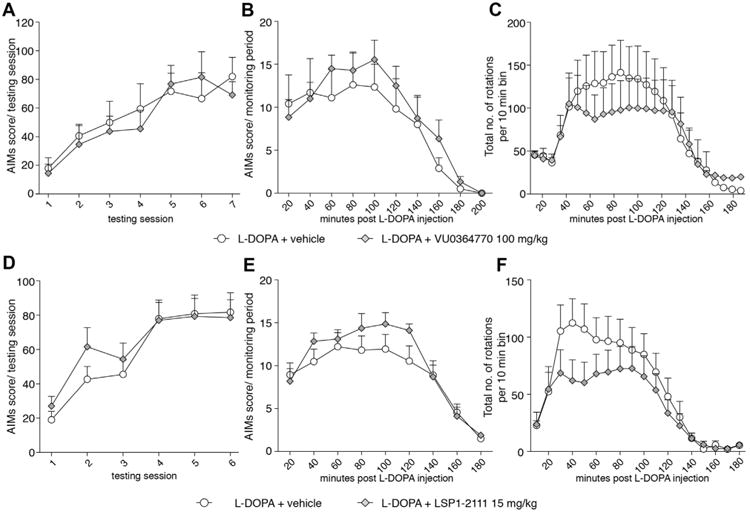
Effect of VU0364770 and LSP1-2111 on the development and severity of dyskinesia and l-DOPA-induced rotations. VU0364770, 100 mg/kg had no effect on the development (AIMs development over chronic treatment period; A) or severity (AIMs during only session 6 of chronic treatment; B) of l-DOPA induced dyskinesia (n = 6/group). VU0364770, 100 mg/kg did not affect the rotational response induced by l-DOPA 6 mg/kg (C). LSP1-2111, 15 mg/kg had no effect on the development (AIMs development over chronic treatment period; D) or severity (AIMs during only session 6 of chronic treatment; E) of l-DOPA induced dyskinesia (n = 11/group). LSP1-2111, 15 mg/kg did not affect the rotational response induced by l-DOPA 6 mg/kg (F).
3.3. l-DOPA-sparing potential
In a third study (cf. design C in Fig. 1), three types of behavioural tests (automated rotation test, cylinder test, and rotarod test) were used to examine the effects of mGlu4 ligands as administered either alone or together with 1.5 mg/kg l-DOPA, a subthreshold dose that does not improve forelimb use asymmetry when given alone (Jones et al., 2012).
This subthreshold dose of l-DOPA had a mild stimulant activity on turning behaviour, although the overall effect did not differ significantly from saline-vehicle treatment (Fig. 5A, B). VU0364770 (100 mg/kg) did not induce any rotational activity on its own, but significantly potentiated the effect of l-DOPA (Fig. 5A, B). Indeed, the total number of rotations per session was, on average, five-fold larger in rats co-treated with VU0364770 and l-DOPA compared to control treatment(Fig. 5B, One-way ANOVA: F(3,28) = 3.96, p = 0.018; p < 0.05 for l-DOPA + VU0364770 versus saline-vehicle).
Fig. 5.
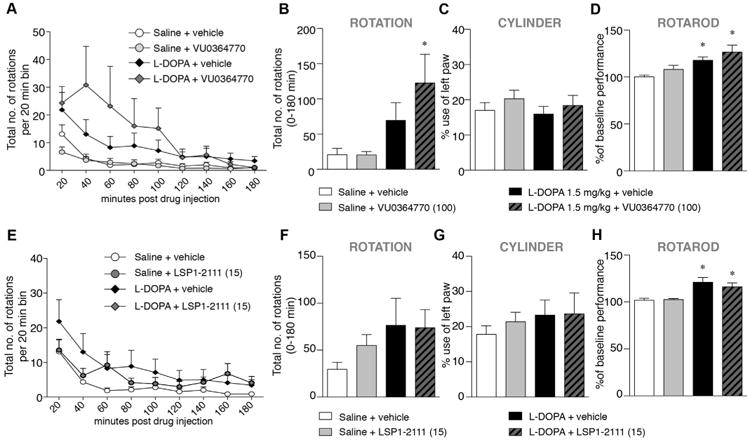
DOPA-sparing effect of VU0364770 and LSP1-2111 on rotation, cylinder and rotarod test when given alone or in combination with l-DOPA, 1.5 mg/kg. l-DOPA alone had no significant effect on rotations but VU0364770 increased rotational behaviour when combined with l-DOPA 1.5 mg/kg (A–B, n = 8). VU0364770 had no effect on forelimb akinesia in the cylinder test (C, n = 18). l-DOPA 1.5 mg/kg slightly improved the rats performance on the rotarod and the combination of l-DOPA 1.5 mg/kg and VU0364770 gave an even stronger and more significant effect (D, n = 18). LSP1-2111 had no effect on rotational behaviour (E–F), or forelimb akinesia in the cylinder test (G, n = 16). While l-DOPA 1.5 mg/kg slightly improved the rats performance on the rotarod, the combination of l-DOPA 1.5 mg/kg and LSP1-2111 had no additional effect (D, n = 16). p < 0.05, * vs. Saline + vehicle, ANOVA and post hoc Student Newman–Keul's test.
In the cylinder test (Fig. 5C), 6-OHDA-lesioned rats used the forelimb contralateral to the lesion only 17% of the times, and l-DOPA did not produce any improvement (15%, Fig. 5C). After treatment with VU0364770, the percentage contralateral forelimb use was raised to 20% (or 19% for the combined treatment with l-DOPA). However, these trends did not reach significance (One-way ANOVA: F(3,67) = 0.59, p = 0.62).
In the rotarod test (Fig. 5D), the time spent on the rod was significantly improved by the drug treatments (one-way ANOVA: F(3,54) = 6.68,; p < 0.001). l-DOPA improved the animals' performance above baseline by 18% (p < 0.01 vs. saline + vehicle). A further improvement was achieved when l-DOPA was combined with VU0364770 (27% above baseline, p < 0.01 vs saline + vehicle), although the difference from l-DOPA-only treatment did not reach significance.
The same study design was then applied to a set of animals treated with LSP1-2111 at the dose of 15 mg/kg (Fig. 5E–H). In the automated rotation test (Fig. 5E, F), LSP1-2111 did not potentiate the number of rotations induced by 1.5 mg/kg l-DOPA (one-way ANOVA: F(5,75) = 1.13, p = 0.35). Similarly to VU0364770, LSP1-2111 had no effect alone nor with l-DOPA on the cylinder test (Fig. 5G, one-way ANOVA: F(3,60) = 0.42, p = 0.74). In the rotarod test, both l-DOPA, 1.5 mg/kg, and the combination of l-DOPA and LSP1-2111 improved the animals' performance above baseline by 21% and 16%, respectively (Fig. 5H, One-way ANOVA: F(5,75) = 13.76, p < 0.001). There was, however, no trend for the combined treatment to achieve a greater effect.
4. Discussion
mGlu4 is viewed as a particularly interesting target for the symptomatic treatment of PD because of its strategic localisation in the indirect pathway (Bradley et al., 1999). Indeed, mGlu4 is abundantly expressed presynaptically in the striatum-GPe projection, and it is also expressed at glutamatergic synapses both in the striatum and in the SNr (Wigmore and Lacey, 1998; Wittmann et al., 2001; Bogenpohl et al., 2013). At these locations, mGlu4 can inhibit overactive GABAergic and glutamatergic pathways that play a key role in generating PD motor symptoms (Albin et al., 1989). Furthermore, mGlu4 has been proposed as a possible target for the prevention of l-DOPA-induced dyskinesia (LID) in PD (Beurrier et al., 2009; Lopez et al., 2011). This suggestion was supported bystudies in 6-OHDA-lesioned rodents, inwhich chronic co-administration of l-DOPA and a mGlu4 agonist resulted in a lower incidence (Bennouar et al., 2013) or end-severity of LID (Lopez et al., 2011). However, the severity of already established LID could not be modified by the pharmacological stimulation of mGlu4 (Lopez et al., 2011; Bennouar et al., 2013). How a stimulation of mGlu4 would reduce the development of LID remained unclear. Indeed, a vast literature has emphasized that the direct pathway is pivotal to the genesis of LID, whereas the indirect pathway would play, at best, a modulatory role (Darmopil et al., 2009; Bateup et al., 2010; Heiman et al., 2014). The hypothesis that LID is linked to the stimulation of supersensitive D1 receptors (Cenci, 2007) has now been verified by a large number of independent studies (for review see Cenci and Konradi, 2010; Iderberg et al., 2012). D1 receptors are preferentially expressed in striatal neurons of the ‘direct pathway’, which exhibit genome-wide transcriptional changes in rodent models of LID (Heiman et al., 2014). On the other hand, selective D2 agonists have been reported to induce dyskinesia both in PD patients and in l-DOPA-primed animals (Delfino et al., 2004; Bagetta et al., 2012), indicating that stimulation of either pathway may contribute to dyskinesia at certain disease stages. Moreover, striatopallidal neurons may contribute to generating altered patterns of neural activity in deep basal ganglia nuclei, which have in turn been linked to both akinesia and dyskinesia in animal models of PD and human studies (reviewed in Sgambato-Faure and Cenci, 2012).
In the present study we have evaluated the potential anti-akinetic and anti-dyskinetic effects of two compounds targeting the mGlu4 receptor in the rat model of PD-LID. In particular, we have used the mGlu4 PAM VU0364770 and the orthosteric agonist LSP1-2111 and compared their effects using three different experimental designs. Neither compound modified the development of dyskinetic or antiparkinsonian responses during chronic treatment with full doses of l-DOPA. When given together with l-DOPA to rats with already established dyskinesias, neither VU0364770 nor LSP1-2111 modified the AIMs. A previous study used a mouse model of LID to examine the antidyskinetic properties of LSP1-2111 (Lopez et al., 2011). The compound had no effect on already established dyskinesias. However, chronic de novo treatment withLSP1-2111 as an ‘add-on’ to l-DOPA resulted in somewhat lower AIM scores on the last day of a 9-day treatment course (data from the intermediate days of treatment were not shown). Despite this reduction of AIM scores on the 9th day, LSP1-2111 did not attenuate the hyperphosphorylation of ERK1/2 in the striatum, which provide a robust molecular correlate of LID in all the current animal models (reviewed in Iderberg et al., 2012).
VU0364770 did potentiate the motor stimulant effects of a subthreshold l-DOPA dose in the rotation test, whereas LSP1-2111 lacked this ability. A potentiation of subthreshold l-DOPA doses by mGlu4 PAMs has been reported also by studies assessing forelimb function in rat PD models (Le Poul et al., 2012; Bennouar et al., 2013; Jones et al., 2012). Together with these previous results, our data suggest that the greatest utility of targeting mGluR4 in PD relies on the DOPA-sparing action of its PAMs. The incidence of dyskinesia and motor fluctuations in PD is heavily conditioned by the l-DOPA dosage (Warren Olanow et al., 2013). Thus, reducing the dose requirement for l-DOPA through the use of mGluR4 PAM would result in a reduced incidence of motor complications. In contrast, the orthosteric agonist LSP1-2111 had neither DOPA-sparing effect nor any attenuating effect on LID. Importantly, neither compound worsened the severity of dyskinesias induced by therapeutic doses of l-DOPA.
The differences in DOPA-sparing efficacy of VU0364770 relative to LSP1-2111 remain unclear. One possible explanation may however be found in the lack of complete selectivity of LPS1-2111 relative to the other group III mGlu receptor subtypes (Acher et al., 1997; Beurrier et al., 2009). Systemic administration of LPS1-2111 may also result in the activation of mGlu7 in the SNr, which may be detrimental to an antiparkinsonian effect. This off-target activity was actually hypothesized to underlie the bell-shaped response on haloperidol-induced catalepsy observed in the original report of this compound (Beurrier et al., 2009). For the studies in the 6-OHDA model, we chose a dose of LSP1-2111 that should be selective (Beurrier et al., 2009) while producing brain exposure levels sufficiently high and prolonged to activate the target during the time frame of our behavioural assessments (Cajina et al., 2014). It is however possible that the dose–response of LSP1-2111 is quite steep as the compound crosses over to other receptor subtypes.
That stimulation of group III mGluRs other than mGlu4 may be detrimental to parkinsonism has been suggested by studies using the mGlu4 agonist, ACPT-1. In one study, infusion of this compound in the GP had significant antiakinetic effects both in the haloperidol model and in a bilateral 6-OHDA lesion model (Lopez et al., 2007). However, infusion of the same compound in the substantia nigra resulted in an exacerbation of akinetic features (Lopez et al., 2007). This paradoxical effect was attributed to off-target activity of ACPT-1 at mGlu8, based on the suggestion that different mGlus in the BG circuitry can play sometimes opposite roles in modulating basal ganglia output (Lopez et al., 2007).
As to VU0364770, this compound has been shown not to exert effects on other mGlus nor on a series of GPCRs, transporters, and ion channels, with the exception of potentiation of mGlu6 and antagonism of mGlu5 at micromolar concentrations (potency of 6.8 ± 1.7 mM and 17.9 ± 5.5 μM, respectively). By comparison, the potency of VU0364770 as an mGlu4 PAM was 290 ± 80 nM (Jones et al., 2012). mGlu5 NAMs have been extensively studied for the treatment of l-DOPA-induced dyskinesia (for review see Rascol et al., 2014), however based on previous pharmacokinetic studies (partly published in Jones et al., 2012) we predict that doses of 100 mg/kg VU036770 do not produce brain levels of unbound drug sufficient to affect the activity of mGlu6 and mGlu5. Potential off-target effects of VU0364770 include the inhibition of MAO-A (9.2 μM) and MAO-B (3.0 μM) (Jones et al., 2012). To ensure that the effects of VU0364770 observed in previous studies were not due to MAO inhibition, control experiments were performed using known MAO-A and MAO-B inhibitors. These showed that a 100 mg/kg dose of VU364770 did not affect dopamine levels nor dopamine turnover (Jones et al., 2012). Thus, at the dose used in the present study, VU364770 most likely exerted its effects via mGluR4 only. Indeed, these effects were quite in agreement with those reported for other mGluR4 PAMs (Bennouar et al., 2013; Le Poul et al., 2012).
The animal model used in this study is a unilateral representation of late-stage PD, which is characterized by massive striatal DA denervation, as well as denervation of other monoaminergic systems. Dyskinesia most often occurs in late-stage PD, and our rat model is well validated to study drugs with antidyskinetic potential (reviewed in Iderberg et al., 2012). It is, however, important to compare our results with those obtained in previous studies, which suggest mGluR4 PAMs may be more efficient in animal models with milder parkinsonian features. Thus, the effects of mGlu4 PAMs and orthosteric agonists have been particularly prominent in bilateral pharmacological PD models like haloperidol-induced catalepsy (Jones et al., 2012; Le Poul et al., 2012, and this study) or reserpine-induced akinesia (Austin et al., 2010). In addition, these ligands have produced some potential anti-parkinsonian effects in 6-OHDA models harbouring partial bilateral lesions (Le Poul et al., 2012). In a previous study in rats with unilateral 6-OHDA lesions, VU0364770 slightly improved forelimb use asymmetry in the cylinder test, and potentiated the improvement produced by l-DOPA, 1.5 mg/kg (Jones et al., 2012). In our study, we did not see significant improvements in the cylinder test, however, we found that VU0364770 potentiated the motor stimulant effect of l-DOPA in the rotation test, and tended to further ameliorate the l-DOPA-induced improvement in the rotarod test. The discrepancy between studies relative to the cylinder test data may be due to differences in the lesion model used (cf. injection coordinates and toxin doses) and on the fact that, in the previous study, successful lesions were selected based on a test of apomorphine-induced rotations (Jones et al., 2012). This aspect is important to consider, as apomorphine increases contralateral forelimb use (Olsson et al.,1995) and has long-lasting priming effects.
In conclusion, this is the first study to systematically compare the effects of a mGlu4 PAM and an orthosteric agonist of the same receptor in the unilateral 6-OHDA rat model of PD. In agreement with other studies using mGlu4 PAMs (Jones et al., 2012; Le Poul et al., 2012; Bennouar et al., 2013), our data suggest that pharmacological potentiation of mGluR4 may be combined with l-DOPA to reduce the latter's dose requirement. This approach may reduce the incidence of dyskinesias if the treatment is initiated early (Warren Olanow et al., 2013). For this purpose, PAMs may be more advantageous than orthosteric agonists. Neither PAM nor agonists of mGlu4 have however any intrinsic antidyskinetic activity.
Supplementary Material
Acknowledgments
The authors are thankful for excellent technical assistance from Michael Sparrenius and Ann-Christin Lindh. The study was supported by grant from the M J Fox Foundation (to MAC and CKJ), NS078262 (to CMN), The Kocks Foundation and the Åhlen Foundation (MAC).
Footnotes
Appendix A. Supplementary data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.neuropharm.2015.02.023.
Contributor Information
Hanna Iderberg, Email: Hanna.Iderberg@med.lu.se.
M. Angela Cenci, Email: Angela.Cenci_Nilsson@med.lu.se.
References
- Acher F, Selvam C, Triballeau N, Pin J, Bertrand H. Hypophosphorous acid derivatives and their therapeutical applications. US Patent Application. 2007;WO2007052169 [Google Scholar]
- Acher FC, Tellier FJ, Azerad R, Brabet IN, Fagni L, Pin JP. Synthesis and pharmacological characterization of aminocyclopentanetricarboxylic acids: new tools to discriminate between metabotropic glutamate receptor subtypes. J Med Chem. 1997;40:3119–3129. doi: 10.1021/jm970207b. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Austin PJ, Betts MJ, Broadstock M, O'Neill MJ, Mitchell SN, Duty S. Symptomatic and neuroprotective effects following activation of nigral group III metabotropic glutamate receptors in rodent models of Parkinson's disease. Br J Pharmacol. 2010;160:1741–1753. doi: 10.1111/j.1476-5381.2010.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagetta V, Sgobio C, Pendolino V, Del Papa G, Tozzi A, Ghiglieri V, Giampa C, Zianni E, Gardoni F, Calabresi P, Picconi B. Rebalance of striatal NMDA/AMPA receptor ratio underlies the reduced emergence of dyskinesia during D2-like dopamine agonist treatment in experimental Parkinson's disease. J Neurosci. 2012;32:17921–17931. doi: 10.1523/JNEUROSCI.2664-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, Fisone G, Nestler EJ, Greengard P. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci U S A. 2010;107:14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennouar KE, Uberti MA, Melon C, Bacolod MD, Jimenez HN, Cajina M, Kerkerian-Le Goff L, Doller D, Gubellini P. Synergy between L-DOPA and a novel positive allosteric modulator of metabotropic glutamate receptor 4: implications for Parkinson's disease treatment and dyskinesia. Neuropharmacology. 2013;66:158–169. doi: 10.1016/j.neuropharm.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Lopez S, Revy D, Selvam C, Goudet C, Lherondel M, Gubellini P, Kerkerian-LeGoff L, Acher F, Pin JP, Amalric M. Electrophysiological and behavioral evidence that modulation of metabotropic glutamate receptor 4 with a new agonist reverses experimental parkinsonism. FASEB J. 2009;23:3619–3628. doi: 10.1096/fj.09-131789. [DOI] [PubMed] [Google Scholar]
- Bogenpohl J, Galvan A, Hu X, Wichmann T, Smith Y. Metabotropic glutamate receptor 4 in the basal ganglia of parkinsonian monkeys: ultra-structural localization and electrophysiological effects of activation in the striatopallidal complex. Neuropharmacology. 2013;66:242–252. doi: 10.1016/j.neuropharm.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Standaert DG, Rhodes KJ, Rees HD, Testa CM, Levey AI, Conn PJ. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J Comp Neurol. 1999;407:33–46. [PubMed] [Google Scholar]
- Breger LS, Dunnett SB, Lane EL. Comparison of rating scales used to evaluate L-DOPA-induced dyskinesia in the 6-OHDA lesioned rat. Neurobiol Dis. 2013;50:142–150. doi: 10.1016/j.nbd.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Brotchie JM. Adjuncts to dopamine replacement: a pragmatic approach to reducing the problem of dyskinesia in Parkinson's disease. Mov Disord. 1998;13:871–876. doi: 10.1002/mds.870130603. [DOI] [PubMed] [Google Scholar]
- Cajina M, Nattini M, Song D, Smagin G, Jorgensen EB, Chandrasena G, Bundgaard C, Toft DB, Huang X, Acher F, Doller D. Qualification of LSP1-2111 as a brain penetrant group III metabotropic glutamate receptor orthosteric agonist. ACS Med Chem Lett. 2014;5:119–123. doi: 10.1021/ml400338f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 2007;30:236–243. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Cenci MA. Presynaptic mechanisms of L-DOPA-induced dyskinesia: the findings, the debate, and the therapeutic implications. Front Neurol. 2014;5 doi: 10.3389/fneur.2014.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, Konradi C. Maladaptive striatal plasticity in L-DOPA-induced dyskinesia. Prog Brain Res. 2010;183:209–233. doi: 10.1016/S0079-6123(10)83011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- Cenci MA, Ohlin KE, Odin P. Current options and future possibilities for the treatment of dyskinesia and motor fluctuations in Parkinson's disease. CNS Neurol Disord Drug Targets. 2011;10:670–684. doi: 10.2174/187152711797247885. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Fasano A, Morgante F, Koch G, Quattrone A. Maladaptive plasticity in levodopa-induced dyskinesias and tardive dyskinesias: old and new insights on the effects of dopamine receptor pharmacology. Front Neurol. 2014;5:49. doi: 10.3389/fneur.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 2005;6:787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- Darmopil S, Martin AB, De Diego IR, Ares S, Moratalla R. Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol Psychiatry. 2009;66:603–613. doi: 10.1016/j.biopsych.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Delfino MA, Stefano AV, Ferrario JE, Taravini IR, Murer MG, Gershanik OS. Behavioral sensitization to different dopamine agonists in a parkinsonian rodent model of drug-induced dyskinesias. Behav Brain Res. 2004;152:297–306. doi: 10.1016/j.bbr.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Finlay C, Duty S. Therapeutic potential of targeting glutamate receptors in Parkinson's disease. J Neural Transm. 2014;121:861–880. doi: 10.1007/s00702-014-1176-4. [DOI] [PubMed] [Google Scholar]
- Francardo V, Recchia A, Popovic N, Andersson D, Nissbrandt H, Cenci MA. Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson's disease. Neurobiol Dis. 2011;42:327–340. doi: 10.1016/j.nbd.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Heiman M, Heilbut A, Francardo V, Kulicke R, Fenster RJ, Kolaczyk ED, Mesirov JP, Surmeier DJ, Cenci MA, Greengard P. Molecular adaptations of striatal spiny projection neurons during levodopa-induced dyskinesia. Proc Natl Acad Sci U S A. 2014;111:4578–4583. doi: 10.1073/pnas.1401819111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson RA, Bertorelli R, Varty GB, Lachowicz JE, Forlani A, Fredduzzi S, Cohen-Williams ME, Higgins GA, Impagnatiello F, Nicolussi E, Parra LE, Foster C, Zhai Y, Neustadt BR, Stamford AW, Parker EM, Reggiani A, Hunter JC. Characterization of the potent and highly selective A2A receptor antagonists preladenant and SCH 412348 [7-[2-[4-2,4-difluorophenyl]-1-piperazinyl]ethyl]-2-(2-furanyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine] in rodent models of movement disorders and depression. J Pharmacol Exp Ther. 2009;330:294–303. doi: 10.1124/jpet.108.149617. [DOI] [PubMed] [Google Scholar]
- Iderberg H, Francardo V, Pioli EY. Animal models of L-DOPA-induced dyskinesia: an update on the current options. Neuroscience. 2012;211:13–27. doi: 10.1016/j.neuroscience.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Iderberg H, Rylander D, Bimpisidis Z, Cenci MA. Modulating mGluR5 and 5-HT1A/1B receptors to treat l-DOPA-induced dyskinesia: effects of combined treatment and possible mechanisms of action. Exp Neurol. 2013;250:116–124. doi: 10.1016/j.expneurol.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Conn PJ, Niswender CM. Glutamate receptors as therapeutic targets for Parkinson's disease. CNS Neurol Disord Drug Targets. 2009;8:475–491. doi: 10.2174/187152709789824606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Bubser M, Thompson AD, Dickerson JW, Turle-Lorenzo N, Amalric M, Blobaum AL, Bridges TM, Morrison RD, Jadhav S, Engers DW, Italiano K, Bode J, Daniels JS, Lindsley CW, Hopkins CR, Conn PJ, Niswender CM. The metabotropic glutamate receptor 4-positive allosteric modulator VU0364770 produces efficacy alone and in combination with L-DOPA or an adenosine 2A antagonist in preclinical rodent models of Parkinson's disease. J Pharmacol Exp Ther. 2012;340:404–421. doi: 10.1124/jpet.111.187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poul E, Bolea C, Girard F, Poli S, Charvin D, Campo B, Bortoli J, Bessif A, Luo B, Koser AJ, Hodge LM, Smith KM, DiLella AG, Liverton N, Hess F, Browne SE, Reynolds IJ. A potent and selective metabotropic glutamate receptor 4 positive allosteric modulator improves movement in rodent models of Parkinson's disease. J Pharmacol Exp Ther. 2012;343:167–177. doi: 10.1124/jpet.112.196063. [DOI] [PubMed] [Google Scholar]
- Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA. L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson's disease: temporal and quantitative relationship to the expression of dyskinesia. J Neurochem. 2010;112:1465–1476. doi: 10.1111/j.1471-4159.2009.06556.x. [DOI] [PubMed] [Google Scholar]
- Lopez S, Bonito-Oliva A, Pallottino S, Acher F, Fisone G. Activation of metabotropic glutamate 4 receptors decreases L-DOPA-induced dyskinesia in a mouse model of Parkinson's disease. J Parkinson's Dis. 2011;1:339–346. doi: 10.3233/JPD-2011-11066. [DOI] [PubMed] [Google Scholar]
- Lopez S, Turle-Lorenzo N, Acher F, De Leonibus E, Mele A, Amalric M. Targeting group III metabotropic glutamate receptors produces complex behavioral effects in rodent models of Parkinson's disease. J Neurosci. 2007;27:6701–6711. doi: 10.1523/JNEUROSCI.0299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. Eur J Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Vaudano E, Cenci MA. Cellular and behavioural effects of the adenosine A2a receptor antagonist KW-6002 in a rat model of l-DOPA-induced dyskinesia. J Neurochem. 2003;84:1398–1410. doi: 10.1046/j.1471-4159.2003.01632.x. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Williams DL, Jr, O'Brien JA, Valenti O, McDonald TP, Clements MK, Wang R, DiLella AG, Hess JF, Kinney GG, Conn PJ. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson's disease treatment. Proc Natl Acad Sci U S A. 2003;100:13668–13673. doi: 10.1073/pnas.1835724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli M, Carta AR, Jenner P. Adenosine A2A receptors and Parkinson's disease. Handb Exp Pharmacol. 2009:589–615. doi: 10.1007/978-3-540-89615-9_18. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Johnson KA, Weaver CD, Jones CK, Xiang Z, Luo Q, Rodriguez AL, Marlo JE, de Paulis T, Thompson AD, Days EL, Nalywajko T, Austin CA, Williams MB, Ayala JE, Williams R, Lindsley CW, Conn PJ. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol Pharmacol. 2008;74:1345–1358. doi: 10.1124/mol.108.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Bjorklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15:3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfreman J. Who dropped the ball on L-DOPA? A patient's lament. J Parkinson's Dis. 2014;4:313–316. doi: 10.3233/JPD-149003. [DOI] [PubMed] [Google Scholar]
- Paxinos G. The Rat Nervous System. third. Elsevier Academic Press; 2004. [Google Scholar]
- Rascol O, Fox S, Gasparini F, Kenney C, Di Paolo T, Gomez-Mancilla B. Use of metabotropic glutamate 5-receptor antagonists for treatment of levodopa-induced dyskinesias. Park Relat Disord. 2014;20:947–956. doi: 10.1016/j.parkreldis.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Rylander D, Iderberg H, Li Q, Dekundy A, Zhang J, Li H, Baishen R, Danysz W, Bezard E, Cenci MA. A mGluR5 antagonist under clinical development improves L-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol Dis. 2010;39:352–361. doi: 10.1016/j.nbd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Rylander D, Recchia A, Mela F, Dekundy A, Danysz W, Cenci MA. Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signaling. J Pharmacol Exp Ther. 2009;330:227–235. doi: 10.1124/jpet.108.150425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat D, Tolosa E. Levodopa in the treatment of Parkinson's disease: current status and new developments. J Parkinson's Dis. 2013;3:255–269. doi: 10.3233/JPD-130186. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Obeso JA. Clinical and pathological features of Parkinson's disease. Curr Top Behav Neurosci. 2014 doi: 10.1007/7854_2014_317. [DOI] [PubMed] [Google Scholar]
- Sgambato-Faure V, Cenci MA. Glutamatergic mechanisms in the dyskinesias induced by pharmacological dopamine replacement and deep brain stimulation for the treatment of Parkinson's disease. Prog Neurobiol. 2012;96:69–86. doi: 10.1016/j.pneurobio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, Kinney GG, Conn PJ. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J Neurosci. 2003;23:7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, Nissinen H, Leinonen M, Stocchi F Stalevo Reduction in Dyskinesia Evaluation in Parkinson's Disease I. Factors predictive of the development of levodopa-induced dyskinesia and wearing-off in Parkinson's disease. Mov Disord. 2013;28:1064–1071. doi: 10.1002/mds.25364. [DOI] [PubMed] [Google Scholar]
- Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. Spatio-temporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry. 2007;62:800–810. doi: 10.1016/j.biopsych.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigmore MA, Lacey MG. Metabotropic glutamate receptors depress glutamate-mediated synaptic input to rat midbrain dopamine neurones in vitro. Br J Pharmacol. 1998;123:667–674. doi: 10.1038/sj.bjp.0701662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Bjorklund A, Cenci MA. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson's disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2002;10:165–186. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Marino MJ, Bradley SR, Conn PJ. Activation of group III mGluRs inhibits GABAergic and glutamatergic transmission in the substantia nigra pars reticulata. J Neurophysiol. 2001;85:1960–1968. doi: 10.1152/jn.2001.85.5.1960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


