Abstract
Purpose
Along with other childhood cancer survivors (CCS), hematopoietic cell transplantation (HCT) survivors are at high risk of treatment-related late effects, including cardiovascular disease and diabetes. Cardiometabolic risk factor abnormalities may be exacerbated by inadequate physical activity (PA). Relationships between PA and cardiometabolic risk factors have not been well described in CCS with HCT.
Methods
PA (self-report), mobility (Timed Up and Go test), endurance (six-minute walk test), handgrip strength, and cardiometabolic risk factors were measured in 119 HCT survivors and 66 sibling controls aged ≥18 years. Adjusted comparisons between HCT survivors and controls and between categories of low and high PA, mobility, endurance, and strength were performed with linear regression.
Results
Among HCT survivors, the high PA group had lower waist circumference (WC) (81.9±2.5 v 88.6±3.1 cm±standard error (SE), P=.009) than the low PA group, while the high endurance group had lower WC (77.8±2.6 v 87.8±2.5 cm±SE, P=.0001) and percent fat mass (33.6±1.8 v 39.4±1.7 %±SE, P=.0008) and greater insulin sensitivity (IS) (10.9±1.0 v 7.42±1.14 mg/kg/min±SE via euglycemic insulin clamp, P=.001) than the low endurance group. Differences were greater in HCT survivors than in controls for WC between low and high PA groups, triglycerides between low and high mobility groups, and WC, systolic blood pressure, and IS between low and high endurance groups (all Pinteraction <.05).
Conclusions
Higher endurance was associated with a more favorable cardiometabolic profile in HCT survivors, suggesting that interventions directed to increase endurance in survivors may reduce the risk of future cardiovascular disease.
BACKGROUND
Nearly 45 years since the first successful allogeneic bone marrow transplant was conducted,1 hematopoietic cell transplantation (HCT) has become a standard treatment for a number of malignant and non-malignant conditions in children. As the number of transplants being performed has increased, survival after HCT has been increasing, resulting in a growing population of long-term survivors.2 Unfortunately, along with other childhood cancer survivors (CCS), HCT survivors are at high risk for numerous adverse late effects, including cardiovascular disease, abdominal obesity, insulin resistance, and diabetes mellitus.3-5 Although the etiology of these late effects is multifactorial and not well understood, exposure to total body irradiation and other forms of prolonged immunosuppressive treatment during the HCT process and post-transplant endocrine dysfunction and/or leptin resistance have been suggested to play a role.3,5,6
In recent years, more attention has been placed on the prevention of adverse late effects among CCS by lifestyle behaviors such as diet, physical activity (PA), and smoking cessation. For example, physical inactivity is thought to exacerbate several late effects such as cardiovascular disease, abdominal visceral obesity, hypertension, dyslipidemia, and insulin resistance.7,8,9 Most studies have reported low levels of PA among CCS, especially those who are now adults.10 In fact, fewer than half of CCS engage in regular PA or meet guidelines for regular PA, and they are less likely to be physically active compared to non-cancer controls.10 Perhaps more importantly, reduced cardiorespiratory endurance (maximal oxygen uptake (VO2 max)), mobility, and muscle strength have also been reported among adult survivors of childhood cancer.11,12 Such deficits might result from and/or contribute to low PA levels.
Among the general population, all-cause mortality, cardiovascular disease, dyslipidemia, insulin resistance, and type 2 diabetes have all been shown to be inversely associated with PA.13,14 In addition, greater cardiorespiratory endurance and muscle strength have been inversely associated with several cardiometabolic risk factors.15-23 Relationships between PA/fitness and cardiometabolic risk factors among HCT survivors are not well understood; only two prior studies of HCT survivors have examined possible associations between PA and cardiometabolic risk factors and/or outcomes.24,25 Therefore, we examined cross-sectional associations of PA and fitness (i.e., mobility, endurance, and muscle strength) measures with cardiometabolic risk factors among CCS who underwent HCT and their sibling controls.
METHODS
Study Design
The study was approved by the Institutional Review Board Human Subjects Committees at the University of Minnesota and the Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance (FHCRC/SCCA). All participants provided written informed consent. HCT survivors were selected from transplant databases at each institution and were eligible to participate if they were diagnosed with a primary hematologic malignancy at age ≤21 years, received HCT, were treated at either Fairview-University Medical Center or the FHCRC/SCCA, were ≥9 years of age at the time of study participation, survived a minimum of two years post-transplant, and were currently in remission. Sibling controls were eligible to participate if they were ≥9 years of age at the time of examination and had never had cancer. Controls were frequency matched to HCT survivors by age and sex. Pregnant women were excluded until three or more months after the end of their pregnancy. Of the 339 potentially eligible survivors identified, 60 refused participation, and we were unable to establish contact (passive refusal) with an additional 125 subjects. The remaining 154 (45% of those potentially eligible, 72% of those contacted) provided informed written consent to participate along with 92 of their siblings. Three HCT survivors were found to be ineligible at the time of study due to previously undiagnosed diabetes (n=1), severe hypertension (n=1), and multiple medical issues (n=1) that all required immediate medical attention. This left the final study population of 151 subjects. For the purposes of this analysis we excluded the 32 HCT survivors and 26 controls who were less than 18 years of age, leaving a total of 119 HCT survivors and 66 controls.
Data Collection
All participants underwent a two-day examination at the University of Minnesota Clinical Research Center/Clinical and Translational Science Institute or the Clinical Research Center at FHCRC/SCCA. Height, weight, waist circumference (WC), and blood pressure were assessed according to a standard protocol, as previously described.26 Fat mass and lean body mass were measured using dual-energy X-ray absorptiometry (DXA, Lunar Prodigy scanner, software version 9.3; General Electric Medical Systems, Madison, WI).
After a 10-12-hour overnight fast, the hyperinsulinemic euglycemic clamp method was used to assess insulin sensitivity (IS), as described previously.26,27 Insulin infusion was started at time 0 at a rate of 1 mU/kg/min for 3 hours. An infusion of 20% glucose was given and adjusted to maintain euglycemia (serum glucose level of 100 mg/dL [5.6 mmol/L]) with plasma glucose determined every 10 minutes. IS was determined by the amount of glucose required to maintain euglycemia in the final 40 minutes of the clamp study and expressed as mg/kg/min of glucose with adjustment for lean body mass. Lower IS values are indicative of greater insulin resistance.
Fasting blood samples obtained at the start of the insulin clamp were analyzed for serum lipid levels (low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides), serum insulin, and plasma glucose using a Vitros 5600 (Ortho-Clinical Diagnostics, Inc., Rochester, NY), a chemoluminescence immunoassay (Immulite Insulin DPC, Los Angeles, CA), and a Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA), respectively. LDL-C was calculated by the Friedewald equation. Homeostasis model assessment insulin resistance (HOMA-IR) was calculated with fasting insulin and glucose values using the equation HOMA-IR = [(fasting glucose units of mmol/L * insulin units in μU/mL)/22.5].28
To assess PA, participants completed the Modifiable Activity Questionnaire (MAQ). Participants reported activities in which they had participated at least ten times during the past year in their leisure time, along with the number of months over the year, the average number of times per month, and the average minutes per time that each activity was performed. In addition, participants reported average minutes per day spent walking or bicycling to/from work along with the number of days per week and months per year they attended their job(s). The MAQ-A has been shown to provide valid and reproducible estimates of past year leisure-time PA in adult populations.29,30
A subset of participants (82 HCT survivors, 33 controls) completed physical functioning assessments to objectively measure mobility, endurance, and strength. Functional mobility was evaluated by the validated “Timed Up and Go” measure,31 which is the time in seconds taken by an individual to stand up from a 46 cm height arm chair, walk three meters, turn, walk back to the chair and sit down again. Endurance, a surrogate for was measured by a six-minute walking test (a validated modified Cooper test32,33) in which the total distance (in meters) traveled in six minutes is recorded by a pedometer. Handgrip strength was measured in both hands using a mechanical hand-held dynamometer, a valid tool for measuring overall muscle strength.34,35
Statistical Analysis
All analyses were conducted with SAS version 9.2 (SAS Institute, Inc., Cary, NC). Hours per week spent walking/biking to work and engaging in leisure-time PA were summed to obtain total hours per week of PA. Participants who met the U.S. federal recommendation of at least 2.5 hours per week of moderate-intensity PA in adults36 were categorized as high PA while those reporting less than 2.5 hours per week were categorized as low PA. Participants were categorized into low and high groups for mobility, endurance, and handgrip strength based upon location either below or at/above the median for HCT survivors and controls combined (median mobility = 4.66 seconds, median endurance = 588.9 meters, median handgrip strength = 27.5 kg).
Descriptive statistics are expressed as frequencies and percents or mean ± standard error (SE), as appropriate. HCT survivors were grouped into three treatment groups based on their HCT preparative regimen: total body irradiation and central nervous system irradiation (TBI+CNS), TBI but no CNS irradiation (TBI not CNS), and no TBI nor CNS irradiation (chemotherapy only). Analysis of covariance models adjusted for multiple pairwise comparisons by the post hoc Tukey-Kramer test were used to detect differences in PA, fitness, and cardiometabolic risk factors between the three treatment groups. All analyses including data from sibling controls were implemented in SAS's GENMOD procedure using generalized estimating equations (GEE) to account for intra-family correlation, with the default working correlation (independence) and robust variance estimates. All adjusted comparisons used multivariable linear regression models with adjustments for age, sex, and race/ethnicity. As indicated, models were further adjusted for percent fat mass, height, weight, and/or treatment when appropriate. Primary diagnosis, time since HCT, presence of growth hormone deficiency, and presence of thyroid hormone deficiency were also considered as potential covariates but were not included in final models because their inclusion did not alter the results in any substantive way. Adjusted means were evaluated at the mean levels of covariates included in the models. A two-sided P-value <0.05 was considered to be statistically significant, although because of the high number of statistical tests carried out, those between 0.01 and 0.05 should be viewed with caution.
RESULTS
On average, HCT survivors were slightly older and shorter and lighter than sibling controls, but BMI was similar (Table 1). Controls and HCT survivors as a whole had similar levels of PA, mobility, endurance, and strength, as well as WC, HDL-C, and systolic and diastolic blood pressure (Table 2). HCT survivors had greater percent fat mass, triglycerides, LDL-C, and HOMA-IR and lower lean body mass and IS than controls. These patterns differed somewhat across the three treatment groups. Pairwise analyses revealed that the TBI+CNS group had lower mobility, strength, lean body mass, HDL-C, and higher triglycerides compared to one or both of the other treatment groups, particularly the chemotherapy only group. We also considered possible effects of HCT type (allogeneic versus autologous) and graft versus host disease (3 categories: chronic only or chronic plus acute, acute grades II-III, none or acute grade I). There were no statistically significant differences in PA, fitness, or cardiometabolic risk factors between HCT types or between categories of graft versus host disease severity.
Table 1.
Characteristics of HCT Survivors and Sibling Controls
| Survivors (n=119) | Controls (n=66) | ||||
|---|---|---|---|---|---|
| N (%) | Mean ± SE | N (%) | Mean ± SE | P | |
| Age at Study (years) | 27.4 ± 0.7 | 25.0 ± 1.0 | .02 | ||
| Sex | |||||
| Male | 67 (56.3) | 36 (54.6) | .80 | ||
| Female | 52 (43.7) | 30 (45.5) | |||
| Race/ethnicity a | |||||
| White Non-Hispanic | 109 (91.6) | 61 (92.4) | .83 | ||
| Other | 10 (8.4) | 5 (7.6) | |||
| Diagnosis | |||||
| ALL | 32 (26.9) | ||||
| AML | 39 (32.8) | NA | |||
| CML | 15 (12.6) | ||||
| HOD | 10 (8.4) | ||||
| MDS | 11 (9.2) | ||||
| Others | 12 (10.1) | ||||
| HCT Preparative Regimen | |||||
| TBI+CNS | 24 (20.2) | NA | |||
| TBI not CNS | 62 (52.1) | ||||
| Chemotherapy onlyb | 33 (27.7) | ||||
| HCT Type | |||||
| Allogeneic | 87 (73.1) | NA | |||
| Autologous | 32 (26.9) | ||||
| Age at Most Recent HCT (years) | 12.7 ± 0.6 | NA | |||
| Height (cm) | 166.1 ± 1.0 | 173.7 ± 1.2 | <.0001 | ||
| Weight (kg) | 68.6 ± 1.8 | 73.8 ± 2.0 | .04 | ||
| Body Mass Index (kg/m2) | 24.6 ± 0.5 | 24.3 ± 0.5 | .71 | ||
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; CNS, central nervous system; HCT, hematopoietic cell transplantation; HOD, Hodgkin's lymphoma; MDS, myelodysplastic syndrome; NA, not applicable; NHL, non-Hodgkin lymphoma; SE, standard error; TBI, total body irradiation.
White Hispanic, black, and other categories were collapsed for the comparison between survivors and controls
Some received other radiation prior to or after HCT: Mantle/mediastinal (n=10 for HD), arm, orbit (n=2 for chloromas), temple (n=1 with history of sarcoma and HCT for secondary AML), abdominal (n=1 for NHL).
Table 2.
Physical Activity, Fitness, and Cardiometabolic Risk Factors in HCT Survivors and Sibling Controls
| All Survivors (n=119) | TBI + CNS (n=24) | TBI not CNS (n=62) | Chemotherapy only (n=33) | Controls (n=66) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SE | P a | Mean ± SE | P a | Mean ± SE | P a | Mean ± SE | P a | Mean ± SE | |
| Physical Activity/Fitness | |||||||||
| Physical Activity (hours/week) | 7.25 ± 1.29 | .62 | 6.16 ± 1.73 | .73 | 7.71 ± 0.95 | .61 | 8.04 ± 1.73 | .97 | 6.70 ± 1.47 |
| Mobility (seconds) | 5.38 ± 0.26 | .07 | 5.71 ± 0.24A | .002 | 5.07 ± 0.32B | .29 | 5.28 ± 0.43B | .48 | 4.98 ± 0.29 |
| Endurance (meters) | 583.1 ± 29.8 | .69 | 608.2 ± 36.9 | .11 | 585.7 ± 72.9 | .78 | 627.2 ± 36.6 | .77 | 591.9 ± 39.1 |
| Handgrip Strength (kg) | 30.5 ± 2.1 | .21 | 31.6 ± 3.2A | .17 | 24.9 ± 1.2A | .02 | 34.4 ± 2.8B | .05 | 32.8 ± 2.4 |
| Cardiometabolic Risk Factors | |||||||||
| Waist Circumference (cm) | 83.0 ± 2.5 | .62 | 82.3 ± 3.9 | .82 | 81.9 ± 4.7 | .79 | 86.1 ± 3.6 | .13 | 82.1 ± 3.3 |
| Percent Fat Mass (%) | 36.0 ± 1.2 | <.0001 | 36.7 ± 2.1 | .002 | 34.2 ± 2.1 | .0007 | 36.5 ± 1.7 | .004 | 30.2 ± 1.5 |
| Lean Body Mass (kg)b | 42.7 ± 0.7 | <.0001 | 39.3 ± 1.1A | <.0001 | 42.0 ± 0.9AB | <.0001 | 46.9 ± 1.0B | .04 | 48.8 ± 0.4 |
| Triglycerides (mg/dL)c | 181.3 ± 19.4 | .0001 | 268.1 ± 46.4A | .002 | 179.3 ± 27.0AB | .002 | 136.0 ± 21.7B | .13 | 98.2 ± 14.0 |
| HDL-C (mg/dL)c | 43.1 ± 1.7 | .12 | 37.5 ± 2.2A | .004 | 44.0 ± 2.3B | .29 | 44.7 ± 2.6AB | .74 | 45.9 ± 1.8 |
| LDL-C (mg/dL)c | 106.8 ± 4.4 | .007 | 115.4 ± 6.3 | .001 | 102.2 ± 5.9 | .07 | 109.5 ± 6.8 | .03 | 93.5 ± 3.9 |
| Systolic Blood Pressure (mmHg)c | 114.2 ± 2.0 | .25 | 119.6 ± 3.7 | .32 | 115.7 ± 2.9 | .19 | 110.6 ± 2.2 | .13 | 116.4 ± 2.3 |
| Diastolic Blood Pressure (mmHg)c | 68.7 ± 1.6 | .47 | 69.9 ± 3.5 | .27 | 68.3 ± 1.9 | .63 | 66.1 ± 1.5 | .75 | 67.7 ± 1.6 |
| HOMA-IRc | 2.95 ± 0.31 | .0007 | 4.28 ± 0.61 | .0006 | 3.37 ± 0.52 | .02 | 2.15 ± 0.35 | .16 | 1.57 ± 0.38 |
| Insulin Sensitivity (mg/kg/min)c | 9.98 ± 0.62 | .03 | 7.76 ± 1.03 | .003 | 9.61 ± 0.91 | .08 | 11.3 ± 0.8 | .51 | 11.9 ± 0.7 |
CNS, central nervous system; HCT, hematopoietic cell transplantation; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment insulin resistance; LDL-C, low-density lipoprotein cholesterol; SE, standard error; TBI, total body irradiation.
All models adjusted for age, sex, and race/ethnicity.
For comparisons between TBI+CNS, TBI not CNS, and chemotherapy only groups, means within bolded rows that do not share the same letter are significantly different from each other (Tukey-Kramer test, P < .05). There were no significant differences within non-bolded rows.
P value for comparison between survivors (or subgroup) and controls, on the basis of adjusted linear regression models.
Additional adjustment for height and weight.
Additional adjustment for percent fat mass.
Among HCT survivors, the high PA group had lower WC than the low PA group (Table 3). Among controls, the high PA group had lower diastolic blood pressure than the low PA group. Among HCT survivors, the high endurance group had lower WC and percent fat mass, and greater IS than the low endurance group (Table 4). There were no statistically significant differences between the low and high mobility groups or between the low and high handgrip strength groups within HCT survivors or controls (data not shown).
Table 3.
Cardiometabolic Risk Factors by Physical Activity Level in HCT Survivors and Sibling Controls
| Survivorsa | Controls | Interaction Model | |||||
|---|---|---|---|---|---|---|---|
| Low PA (<2.5hr/wk) (N=33) | High PA (≥2.5hr/wk) (N=72) | Low PA (<2.5hr/wk) (N=13) | High PA (≥2.5hr/wk) (N=31) | Survivor/control × PA group | |||
| Mean ± SE | Mean ± SE | P | Mean ± SE | Mean ± SE | P | P interaction | |
| Waist Circumference (cm) | 88.6 ± 3.1 | 81.9 ± 2.5 | .009 | 72.7 ± 3.3 | 75.7 ± 3.0 | .21 | .008 |
| Percent Fat Mass (%) | 38.8 ± 2.0 | 35.8 ± 1.6 | .07 | 24.3 ± 3.3 | 25.5 ± 2.6 | .63 | .28 |
| Lean Body Mass (kg)b | 42.2 ± 1.2 | 42.6 ± 0.9 | .63 | 49.3 ± 1.2 | 50.1 ± 0.6 | .55 | .55 |
| Triglycerides (mg/dL)c | 221.2 ± 51.3 | 192.2 ± 41.0 | .48 | 108.8 ± 12.5 | 97.5 ± 6.6 | .38 | .67 |
| HDL Cholesterol (mg/dL)c | 41.0 ± 2.8 | 42.4 ± 2.2 | .54 | 40.8 ± 3.4 | 44.8 ± 2.7 | .19 | .71 |
| LDL Cholesterol (mg/dL)c | 116.8 ± 9.3 | 114.1 ± 7.3 | .71 | 94.8 ± 6.5 | 90.6 ± 3.2 | .63 | .68 |
| Systolic Blood Pressure (mmHg)c | 118.0 ± 3.6 | 114.3 ± 2.9 | .21 | 113.3 ± 3.0 | 111.2 ± 2.1 | .44 | .24 |
| Diastolic Blood Pressure (mmHg)c | 71.8 ± 2.7 | 71.1 ± 2.2 | .77 | 66.6 ± 1.3 | 61.6 ± 0.8 | .02 | .30 |
| HOMA-IRc | 2.82 ± 1.69 | 3.19 ± 1.33 | .79 | 2.38 ± 0.61 | 1.51 ± 0.23 | .11 | .48 |
| Insulin Sensitivity (mg/kg/min)c | 8.19 ± 1.37 | 9.46 ± 1.07 | .26 | 10.3 ± 1.4 | 12.2 ± 0.9 | .18 | .70 |
HCT, hematopoietic cell transplantation; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment insulin resistance; LDL-C, low-density lipoprotein cholesterol; PA, physical activity; SE, standard error.
All models adjusted for age, sex, and race/ethnicity.
Additional adjustment for treatment (HCT preparative regimen) in all models of survivors.
Additional adjustment for height and weight.
Additional adjustment for percent fat mass.
Table 4.
Cardiometabolic Risk Factors by Endurance Level in HCT Survivors and Sibling Controls
| Survivorsa | Controls | Interaction Model | |||||
|---|---|---|---|---|---|---|---|
| Low Endurance (N=40) | High Endurance (N=42) | Low Endurance (N=17) | High Endurance (N=16) | Survivor/control × endurance group | |||
| Mean ± SE | Mean ± SE | P | Mean ± SE | Mean ± SE | P | Pinteraction | |
| Waist Circumference (cm) | 87.8 ± 2.5 | 77.8 ± 2.6 | .0001 | 76.7 ± 2.3 | 79.3 ± 1.5 | .30 | .001 |
| Percent Fat Mass (%) | 39.4 ± 1.7 | 33.6 ± 1.8 | .0008 | 30.1 ± 3.0 | 29.3 ± 1.1 | .79 | .17 |
| Lean Body Mass (kg)b | 40.4 ± 1.1 | 41.7 ± 1.0 | .25 | 46.2 ± 1.8 | 49.0 ± 0.9 | .20 | .77 |
| Triglycerides (mg/dL)c | 217.0 ± 45.6 | 160.1 ± 44.4 | .21 | 91.9 ± 9.4 | 107.9 ± 7.0 | .26 | .10 |
| HDL Cholesterol (mg/dL)c | 43.0 ± 2.2 | 45.0 ± 2.1 | .37 | 39.5 ± 3.0 | 41.8 ± 1.3 | .47 | .88 |
| LDL Cholesterol (mg/dL)c | 112.8 ± 9.0 | 118.0 ± 8.2 | .52 | 91.2 ± 6.0 | 90.8 ± 3.3 | .95 | .90 |
| Systolic Blood Pressure (mmHg)c | 120.3 ± 3.2 | 114.6 ± 3.1 | .08 | 109.9 ± 2.1 | 113.7 ± 0.9 | .15 | .02 |
| Diastolic Blood Pressure (mmHg)c | 71.5 ± 2.6 | 71.2 ± 2.5 | .92 | 62.9 ± 1.7 | 62.5 ± 1.0 | .83 | .80 |
| HOMA-IR c | 3.63 ± 1.79 | 1.80 ± 1.60 | .27 | 2.05 ± 0.19 | 1.59 ± 0.10 | .05 | .24 |
| Insulin Sensitivity (mg/kg/min)c | 7.42 ± 1.14 | 10.9 ± 1.0 | .001 | 14.4 ± 1.3 | 13.5 ± 0.6 | .49 | .02 |
HCT, hematopoietic cell transplantation; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment insulin resistance; LDL-C, low-density lipoprotein cholesterol; SE, standard error.
All models adjusted for age, sex, and race/ethnicity.
Additional adjustment for treatment (HCT preparative regimen) in all models of survivors.
Additional adjustment for height and weight.
Additional adjustment for percent fat mass.
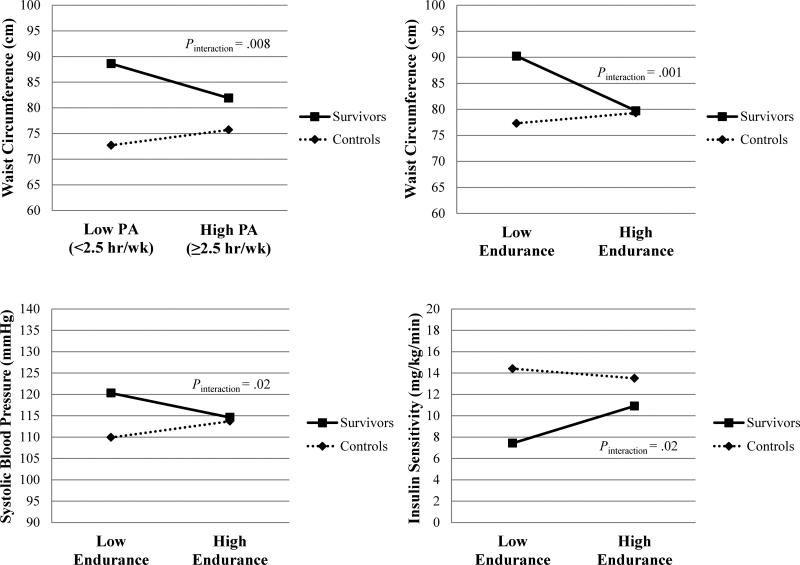
We tested the significance of the interaction between survivor/control status and PA/fitness level for each cardiometabolic risk factor. As depicted by the interaction plots in Figure 1 and displayed in the rightmost column of Tables 3 and 4, differences in WC between low and high PA and differences in WC, systolic blood pressure, and IS between low and high endurance depended on survivor/control status (all Pinteraction < .05). In general, HCT survivors had larger differences in WC, systolic blood pressure, and IS at the higher PA/endurance levels compared to controls. There was no such evidence of effect modification by survivor/control status for the other risk factors examined (all Pinteraction > .05).
Figure 1.
Survivor/control status x PA level (low versus high PA) interaction plot for WC and survivor/control status x endurance level (low versus high endurance) interaction plots for WC, systolic blood pressure, and IS. These plots assess whether the difference in WC between high and low PA was the same for HCT survivors and controls and whether the differences in WC, systolic blood pressure, and IS between high and low endurance were the same for HCT survivors and controls.
Abbreviations: HCT, hematopoietic cell transplantation; IS, insulin sensitivity; PA, physical activity; WC, waist circumference.
DISCUSSION
This study found that endurance, to a greater degree than mobility, handgrip strength, or self-reported PA, was associated with certain cardiometabolic risk factors in HCT survivors. Specifically, high endurance was associated with lower WC and percent fat mass and greater IS among HCT survivors. Prior studies of healthy individuals have indicated that cardiorespiratory endurance is more strongly associated with obesity37-39 and IS38,40 than measures of PA or muscle strength.
Similar comparisons between PA and fitness measures have not been previously made in CCS. However, a recent 16-week home-based exercise intervention involving seventeen 16- to 30-year-old survivors of childhood acute lymphoblastic leukemia (ALL) without HCT resulted in significant improvements in WC, waist-to-hip ratio, percent body fat, fasting plasma insulin, and HOMA-IR, while simultaneously improving VO2 max, maximal work load, and muscle strength.41 Weight, BMI, triglycerides, total cholesterol, LDL-C, HDL-C, and fasting plasma glucose remained unchanged and the effect on blood pressure was variable. Results were also mixed in a larger study of adult survivors of childhood ALL (6 of 117 had underwent HCT); greater PA energy expenditure was associated with lower percent body fat but not with BMI, WC, HOMA-IR, or metabolic syndrome.42
In a study of 2,300 HCT survivors, those with serious cardiovascular disease and those with only hypertension or diabetes were less likely to meet recommended PA levels.24 A smaller study of 26 HCT and 48 non-HCT survivors found a history of HCT was a significant risk factor for having two or cardiometabolic traits, as well as for meeting criteria for metabolic syndrome, but these risks were not associated with or modified by physical activity levels.25 In general agreement with previous findings,12,43-45 HCT survivors in the current study who had CNS irradiation as a part of their HCT preparative regimen had lower mobility, strength, and lean body mass and appeared to be at higher risk of dyslipidemia (low HDL-C and high triglycerides and/or LDL-C) when compared to one or more of the groups who did not receive CNS irradiation.
Results from our interaction tests implied that interventions, especially those that increase endurance, might work more effectively to decrease and/or maintain WC in HCT survivors than in controls. The interaction models also suggested that increases in endurance may lead to more effective improvement or maintenance of systolic blood pressure and IS, while increases in mobility may lead to more effective improvement or maintenance of triglyceride levels in HCT survivors compared to controls. No known prior studies have made similar types of comparisons; however, it was previously suggested that there may be a threshold effect for the PA—insulin resistance relationship (based on systolic blood pressure or body size) or that the relationship is more subtle and not recognized in leaner adolescents.47 These new findings support further investigation into prospective interventions in HCT survivors that focus on improving endurance as a possible way of ameliorating cardiometabolic complications.
It is possible we lacked power to detect clinically significant differences for some of the risk factors examined in this study; and conversely, some of the findings could have been due to chance. Further research in this population is needed to confirm our results. Using objective measures of PA/fitness, such as the fitness tests utilized in this study, is recommended over the use of less reliable self-report measures. Direct measures of abdominal subcutaneous and visceral fat mass (via computed tomography), vascular health (i.e., endothelial-dependent dilation, intima-media thickness, carotid artery cross-sectional compliance and distensibility), and knee extension strength were not available for a majority of participants in this study, but may be important to consider in future investigations. Lastly, we were not able to check for possible systematic differences between participants and non-participants because information on non-participants was not available.
This study serves to fill important gaps in knowledge regarding associations between PA/fitness and cardiometabolic risk factors among CCS with a history of HCT. Although it is not new to find an association between PA/fitness and cardiometbolic risk factors in healthy populations, results suggesting that these relationships are stronger in HCT survivors than in controls are of importance, since they suggest that PA could serve as a tangible target in mitigating the already high cardiometabolic risks of many HCT survivors. Sustainable PA programs focused on improving endurance and tailored to HCT survivors are worth exploring as a means of reducing early morbidity and mortality.
Highlights.
Childhood cancer survivors with hematopoietic cell transplantation were examined.
We investigated links between physical activity, fitness, and cardiometabolic risk.
High endurance was linked to a more favorable cardiometabolic profile in survivors.
Observed differences were greater in survivors than in controls.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health Grants NCI R01CA112530, T32 CA099936, K05 CA157439, and National Center for Research Resources (NCRR) Grants 1UL1RR033183, 1ULITR000423, and M01-RR00400.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
REFERENCES
- 1.Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–9. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Davies SM, Scott Baker K, Pulsipher MA, Hansen JA. NCI, NHLBI first international consensus conference on late effects after pediatric hematopoietic cell transplantation: etiology and pathogenesis of late effects after HCT performed in childhood--methodologic challenges. Biol Blood Marrow Transplant. 17:1428–35. doi: 10.1016/j.bbmt.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tichelli A, Rovo A, Passweg J, et al. Late complications after hematopoietic stem cell transplantation. Expert Rev Hematol. 2009;2:583–601. doi: 10.1586/ehm.09.48. [DOI] [PubMed] [Google Scholar]
- 4.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356:993–7. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 5.Neville KA, Cohn RJ, Steinbeck KS, Johnston K, Walker JL. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J Clin Endocrinol Metab. 2006;91:4401–7. doi: 10.1210/jc.2006-0128. [DOI] [PubMed] [Google Scholar]
- 6.Arvidson J, Lonnerholm G, Tuvemo T, Carlson K, Lannering B, Lonnerholm T. Prepubertal growth and growth hormone secretion in children after treatment for hematological malignancies, including autologous bone marrow transplantation. Pediatr Hematol Oncol. 2000;17:285–97. doi: 10.1080/088800100276271. [DOI] [PubMed] [Google Scholar]
- 7.Shankar SM, Marina N, Hudson MM, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121:e387–96. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 8.van der Sluis IM, van den Heuvel-Eibrink MM. Osteoporosis in children with cancer. Pediatr Blood Cancer. 2008;50:474–8. doi: 10.1002/pbc.21407. discussion 86. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman KE, Derdak J, Bernstein D, et al. Metabolic syndrome traits in long-term survivors of pediatric sarcoma. Pediatr Blood Cancer. 2008;50:341–6. doi: 10.1002/pbc.21363. [DOI] [PubMed] [Google Scholar]
- 10.Stolley MR, Restrepo J, Sharp LK. Diet and physical activity in childhood cancer survivors: a review of the literature. Ann Behav Med. 2010;39:232–49. doi: 10.1007/s12160-010-9192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonorezos ES, Snell PG, Moskowitz CS, et al. Reduced cardiorespiratory fitness in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:1358–64. doi: 10.1002/pbc.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ness KK, Baker KS, Dengel DR, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:975–81. doi: 10.1002/pbc.21091. [DOI] [PubMed] [Google Scholar]
- 13.Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:76–88. doi: 10.1139/h06-113. [DOI] [PubMed] [Google Scholar]
- 14.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115:1984–94. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artero EG, Ruiz JR, Ortega FB, et al. Muscular and cardiorespiratory fitness are independently associated with metabolic risk in adolescents: the HELENA study. Pediatr Diabetes. 2011;12:704–12. doi: 10.1111/j.1399-5448.2011.00769.x. [DOI] [PubMed] [Google Scholar]
- 16.Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism. 2009;58:1013–22. doi: 10.1016/j.metabol.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Boule NG, Bouchard C, Tremblay A. Physical fitness and the metabolic syndrome in adults from the Quebec Family Study. Can J Appl Physiol. 2005;30:140–56. doi: 10.1139/h05-111. [DOI] [PubMed] [Google Scholar]
- 18.Carnethon MR, Gulati M, Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. Jama. 2005;294:2981–8. doi: 10.1001/jama.294.23.2981. [DOI] [PubMed] [Google Scholar]
- 19.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37:1849–55. doi: 10.1249/01.mss.0000175865.17614.74. [DOI] [PubMed] [Google Scholar]
- 20.Ortega FB, Sanchez-Lopez M, Solera-Martinez M, Fernandez-Sanchez A, Sjostrom M, Martinez-Vizcaino V. Self-reported and measured cardiorespiratory fitness similarly predict cardiovascular disease risk in young adults. Scand J Med Sci Sports. 2012 doi: 10.1111/j.1600-0838.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 21.Sayer AA, Syddall HE, Dennison EM, et al. Grip strength and the metabolic syndrome: findings from the Hertfordshire Cohort Study. Qjm. 2007;100:707–13. doi: 10.1093/qjmed/hcm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stabelini Neto A, Sasaki JE, Mascarenhas LP, et al. Physical activity, cardiorespiratory fitness, and metabolic syndrome in adolescents: a cross-sectional study. BMC Public Health. 2011;11:674. doi: 10.1186/1471-2458-11-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wijndaele K, Duvigneaud N, Matton L, et al. Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med Sci Sports Exerc. 2007;39:233–40. doi: 10.1249/01.mss.0000247003.32589.a6. [DOI] [PubMed] [Google Scholar]
- 24.Chow EJ, Baker KS, Lee SJ, et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32:191–8. doi: 10.1200/JCO.2013.52.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow EJ, Simmons JH, Roth CL, et al. Increased cardiometabolic traits in pediatric survivors of acute lymphoblastic leukemia treated with total body irradiation. Biol Blood Marrow Transplant. 2010;16:1674–81. doi: 10.1016/j.bbmt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinberger J, Sinaiko AR, Kelly AS, et al. Cardiovascular risk and insulin resistance in childhood cancer survivors. J Pediatr. 2012;160:494–9. doi: 10.1016/j.jpeds.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moran A, Jacobs DR, Jr., Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–44. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 29.Schulz LO, Harper IT, Smith CJ, Kriska AM, Ravussin E. Energy intake and physical activity in Pima Indians: comparison with energy expenditure measured by doubly-labeled water. Obes Res. 1994;2:541–8. doi: 10.1002/j.1550-8528.1994.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 30.Momenan AA, Delshad M, Sarbazi N, Rezaei Ghaleh N, Ghanbarian A, Azizi F. Reliability and validity of the Modifiable Activity Questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med. 2012;15:279–82. [PubMed] [Google Scholar]
- 31.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 32.Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed) 1982;284:1607–8. doi: 10.1136/bmj.284.6329.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt K, Vogt L, Thiel C, Jager E, Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34:631–6. doi: 10.1055/s-0032-1323746. [DOI] [PubMed] [Google Scholar]
- 34.Febrer A, Rodriguez N, Alias L, Tizzano E. Measurement of muscle strength with a handheld dynamometer in patients with chronic spinal muscular atrophy. J Rehabil Med. 2010;42:228–31. doi: 10.2340/16501977-0507. [DOI] [PubMed] [Google Scholar]
- 35.Leal VO, Mafra D, Fouque D, Anjos LA. Use of handgrip strength in the assessment of the muscle function of chronic kidney disease patients on dialysis: a systematic review. Nephrol Dial Transplant. 2011;26:1354–60. doi: 10.1093/ndt/gfq487. [DOI] [PubMed] [Google Scholar]
- 36.U.S. Department of Health and Human Services . Physical Activity Guidelines for Americans. Washington DC.: 2008. [Google Scholar]
- 37.Moliner-Urdiales D, Ruiz JR, Vicente-Rodriguez G, et al. Associations of muscular and cardiorespiratory fitness with total and central body fat in adolescents: the HELENA study. Br J Sports Med. 2011;45:101–8. doi: 10.1136/bjsm.2009.062430. [DOI] [PubMed] [Google Scholar]
- 38.Chen CN, Chuang LM, Wu YT. Clinical measures of physical fitness predict insulin resistance in people at risk for diabetes. Phys Ther. 2008;88:1355–64. doi: 10.2522/ptj.20080064. [DOI] [PubMed] [Google Scholar]
- 39.Ekblom-Bak E, Hellenius ML, Ekblom O, Engstrom LM, Ekblom B. Independent associations of physical activity and cardiovascular fitness with cardiovascular risk in adults. Eur J Cardiovasc Prev Rehabil. 2010;17:175–80. doi: 10.1097/HJR.0b013e32833254f2. [DOI] [PubMed] [Google Scholar]
- 40.Lin CY, Chen PC, Kuo HK, Lin LY, Lin JW, Hwang JJ. Effects of obesity, physical activity, and cardiorespiratory fitness on blood pressure, inflammation, and insulin resistance in the National Health and Nutrition Survey 1999-2002. Nutr Metab Cardiovasc Dis. 2010;20:713–9. doi: 10.1016/j.numecd.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Jarvela LS, Kemppainen J, Niinikoski H, et al. Effects of a home-based exercise program on metabolic risk factors and fitness in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59:155–60. doi: 10.1002/pbc.24049. [DOI] [PubMed] [Google Scholar]
- 42.Tonorezos ES, Robien K, Eshelman-Kent D, et al. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Causes Control. 2013;24:313–21. doi: 10.1007/s10552-012-0116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker KS, Chow E, Steinberger J. Metabolic syndrome and cardiovascular risk in survivors after hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47:619–25. doi: 10.1038/bmt.2011.118. [DOI] [PubMed] [Google Scholar]
- 44.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–12. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 45.Brennan BM, Rahim A, Blum WF, Adams JA, Eden OB, Shalet SM. Hyperleptinaemia in young adults following cranial irradiation in childhood: growth hormone deficiency or leptin insensitivity? Clin Endocrinol (Oxf. 1999;50:163–9. doi: 10.1046/j.1365-2265.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- 46.Darzy KH, Shalet SM. Radiation-induced growth hormone deficiency. Horm Res. 2003;59(Suppl 1):1–11. doi: 10.1159/000067834. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz KH, Jacobs DR, Jr, Hong C-P, Steinberger J, Moran A, Sinaiko AR. Association of physical activity with insulin sensitivity in children. Int J Obes. 2002;26:1310–16. doi: 10.1038/sj.ijo.0802137. [DOI] [PubMed] [Google Scholar]