Abstract
Background
Transforming growth factor beta-1 (TGF-β1) is an important regulator of inflammation. Platelets are a major source of TGF-β1, and are reduced in severe malaria. However, the relationships between TGF-β1 concentrations and platelet counts, pro- and anti-inflammatory cytokine and chemokine concentrations, and disease severity in malaria have not been characterized.
Methods
Platelet counts and serum concentrations of TGF-β1 and interleukin-1beta (IL–1β), IL-6, IL-10, interferon (IFN)–γ, tumor necrosis factor (TNF)- α and RANTES were measured at the time of presentation in Ugandan children with cerebral malaria (CM, n=75), uncomplicated malaria (UM, n=67) and healthy community children (CC, n=62).
Results
TGF-β1 concentrations decreased with increasing severity of disease (median concentrations (25th, 75th percentile) in ng/ml in CC, 41.4 (31.6, 57.4), UM, 22.7 (14.1, 36.4), CM, 11.8 (8, 21), P for trend<0.0001). In children with CM or UM, TGF-β1 concentrations correlated positively with platelet count (CM, P<0.0001, UM, P=0.0015). In children with CM, TGF-β1 concentration correlated negatively with IFN-γ, IL-6, and IL-10 and positively with RANTES concentrations (all P<0.01). TGF-β1 concentration was not associated with death or adverse neurologic or cognitive outcomes in children with CM.
Conclusions
TGF-β1 concentrations decrease with increasing P. falciparum disease severity. In cerebral malaria, thrombocytopenia correlates with decreased TGF-β1, and decreased TGF-β1 correlates with cytokine/chemokine changes associated with increased disease severity and death. Thrombocytopenia may mediate disease severity in malaria through reduced TGF-β1-mediated regulation of cytokines associated with severe disease.
Keywords: cerebral malaria, platelets, TGF-beta, tumor growth factor beta
Introduction
In 2010 there were an estimated 1.24 million deaths from malaria world-wide, most of which were due to infection with Plasmodium falciparum [1]. The clinical presentation of malaria ranges from asymptomatic parasitemia and mild illness to cerebral malaria (CM), a condition with a mortality rate of ~15% [2]. Host immune response directly impacts the severity and outcome of disease in murine models of severe malaria [3–7], and appears to be similarly important in severe malaria in humans [8–11].
A balance between pro-inflammatory and anti-inflammatory properties appears to be necessary to combat P. falciparum parasitemia. Cytokines such as interferon- gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α) and interleukin 1-beta (IL-1β), promote inflammation and reduce parasitemia [6, 7, 12, 13]. However, an exaggerated pro-inflammatory response is associated with increased morbidity and mortality in severe malaria [10, 13, 14]. Although interleukin-10 (IL-10) is generally considered an anti-inflammatory cytokine, elevated concentrations of IL-10 have also correlated with increased mortality in several studies (14–16), highlighting the complexity of the human immune response to P. falciparum and its effects on disease severity.
TGF-β1 is a multi-functional protein that is important in regulating the inflammatory response. It is produced by multiple sources, including monocytes, T cells, and B cells, in an inactive precursor form with various activation pathways [15, 16]. Large quantities of TGF-β1 are stored in the alpha granules of platelets [17]. In malaria, TGF-β1 may play an important role in the regulation of the immune response and tolerance of parasitemia [18, 19]. Upon activation, TGF-β1 has concentration and environment-dependent pro- and anti-inflammatory properties. At low concentrations, TGF-β1 pro-inflammatory properties include modulating the concentrations of endothelial cell adhesion molecules, as well as recruiting monocytes, T-cells and neutrophils to sites of early infection [20]. At high concentrations, TGF-β1 has anti-inflammatory properties and assists in transition from Th-1 type to Th-2 type responses through mechanisms that include the suppression of TNF-α production from macrophages, and the inhibition of IFN-γ and TNF-α production from NK cells [21, 22]. TGF-β1 has also been shown to induce FOXp3, which is required for regulatory T cell (Treg) development and function [23]. Tregs act as important mediators of the host immune response. They are associated with increased rates of P. falciparum growth in vivo [24] and are increased in individuals with asymptomatic malaria compared to individuals who are not infected [25].
Multiple studies have demonstrated that TGF-β1 concentrations are low in P. falciparum malaria, with disease severity increasing as TGF-β1 levels decrease [13, 26, 27], but the factors that lead to lower TGF-β1 concentrations in malaria are uncertain. Thrombocytopenia could contribute to the low TGF-β1 concentrations seen in severe malaria, as platelets are a major storage site for TGF-β1, and thrombocytopenia is common in malaria and associated with disease severity and death [28]. We conducted the present study to determine the relationship of TGF-β1 concentrations to disease severity and to platelet count and parasite density in children with cerebral malaria (CM), uncomplicated malaria (UM) and community children (CC).
Subjects, Materials, and Methods
Study population and recruitment
The study was conducted at Mulago Hospital, Kampala, Uganda. Children of 4–12 years of age were recruited as part of a study assessing the complications of CM. A total of 88 children with CM, 76 children with UM, and 100 community children (CC) without evidence of acute illness were recruited. Complete details of the study cohorts were previously reported [29]. Briefly, children with CM were enrolled if they were admitted to Mulago Hospital and met the WHO criteria for CM: coma, P. falciparum on blood smear, and no other cause for coma. CM was treated with quinine for 7 days. Children with UM were enrolled from the Acute Care clinic or an outpatient malaria clinic at the hospital sponsored by the University of California, San Francisco (UCSF). Children were considered to have UM if they had signs and symptoms of malaria, P. falciparum infection on blood smear, and no evidence of malaria complications or other acute illness. Children with UM were treated in an Acute Care clinic (chloroquine plus sulfadoxine/pyrimethamine) or with combination therapy at the UCSF outpatient clinic (either amodiaquine plus sulfadoxine/pyrimethamine or amodiaquine plus artesunate). Community children were recruited from the household and extended households of children with CM or UM. Community children and children with UM were recruited to be in the same age range (4–12 years) as children with CM, and a history and physical exam was performed to ascertain that the community children were healthy at the time of enrollment.
Blood samples of 2–5 ml were obtained by venipuncture utilizing a tourniquet with the initial blood draw for routine laboratory testing prior to or as anti-malarial treatment was being given. A second sample was drawn from children with CM at 72 hours after initiation of treatment. The separated serum was pipetted into aliquots and frozen at −70 °C until testing was performed. Serum samples from 75 children with CM and 67 children with UM were available for testing, and serum samples of 62 of the 100 community children were randomly chosen for testing. Serum samples from 65 of the 75 children with CM at 72 hours after admission were available for testing. Seven of the 62 community children had asymptomatic parasitemia. All were treated and were not excluded from the study. Stool was requested from all enrolled subjects and obtained from 133 children. Stool helminth infections were assessed by microscopic examination of a stool wet mount preparation.
Written, informed consent was obtained from the parents or guardians of study participants. Ethics approval for the study was granted by the institutional review boards at Makerere University, Case Western Reserve University and the University of Minnesota.
Cytokine testing
Concentrations of total TGF-β1 (R&D Systems) and interleukin-10 (IL-10) (BD Pharmingen) were assessed by standard cytokine ELISA according to the manufacturers’ instructions. Results were interpolated from standard curves generated with SoftMax software (Molecular Devices). Concentrations of interleukin-1beta (IL–1β) [3], interleukin 6 (IL-6) [8], interferon gamma (IFN–γ) [9], tumour necrosis factor alpha (TNF- α) [30], macrophage inflammatory protein alpha (MIP–1α) [31], and macrophage inhibitory protein beta (MIP-1β) [32], which have been associated with increased risk of severe disease and/or death in malaria, and of RANTES, which have been associated with a decreased risk of severe disease and death in malaria [11], were determined by a commercially available cytometric bead assay (CBA)(R&D Systems) using the Luminex system and have been previously published [11].
Neurologic and cognitive testing
A complete neurologic exam was done on children with CM at discharge, 3 months, and 6 months after hospitalization. Cognitive testing assessed attention, working memory, and tactile-based learning, as described previously [33]. Age-adjusted z-scores calculated using the scores from the healthy community children.
Statistical analysis
Data analyses were performed using STATA 10.1 (Stata Corp). Variables were assessed for normality in STATA, and those that were not normally distributed were compared using the Wilcoxon rank-sum test, and those normally distributed were compared using Student’s t-test. As is typically the case, values of all cytokines/chemokines were not normally distributed. Values across groups (including TGF-β1) were compared by the Wilcoxon rank-sum test, while values comparing cytokines and T TGF-β1 were compared using Spearman’s rank correlation, which does not rely on a linear relationship between the variables. P values for multiple comparisons were adjusted for with the Bonferroni correction.
Results
Demographic and clinical characteristics of study participants
Children with CM were significantly more likely to younger than children with UM or CC (Table 1). Children with CM also had significantly lower hemoglobin concentrations and platelet counts than children with UM or CC, but median parasite density in children with CM and UM did not differ. Children with UM had significantly lower hemoglobin concentrations, platelet counts and higher parasitemia than CC (Table 1). Seven of the 62 CC had asymptomatic parasitemia. These asymptomatic children did not differ from the community controls in any of the variables included in Table1.
Table 1.
Demographic and clinical characteristics of study participants.
| CC (n = 62) |
UM (n = 67) |
CM (n = 75) |
|
|---|---|---|---|
| Sex, % male | 46.7 | 38.8 | 57.3 |
| Age, yearsa | 7.8 (2.1) | 7.6 (2.6) | 6.4 (2.4)c,d |
| Hemoglobin mg/dLa | 11.9 (1.5) | 11.2 (1.8)b | 8.4 (2)c,d |
| Platelet counta 10(9)cells/L | 345.9 (103.5) | 144 (85)b | 81 (75.6)c,d |
| Parasite density, median (IQR) | 0 (0) | 33,920 (111,625)b | 29,760 (136,060)d |
Mean, SD,
P<0.05 for UM vs. CC,
P<0.05 for CM vs. UM,
P<0.05 for CM vs. CC
TGF-β1 concentrations according to severity of disease and clinical outcomes
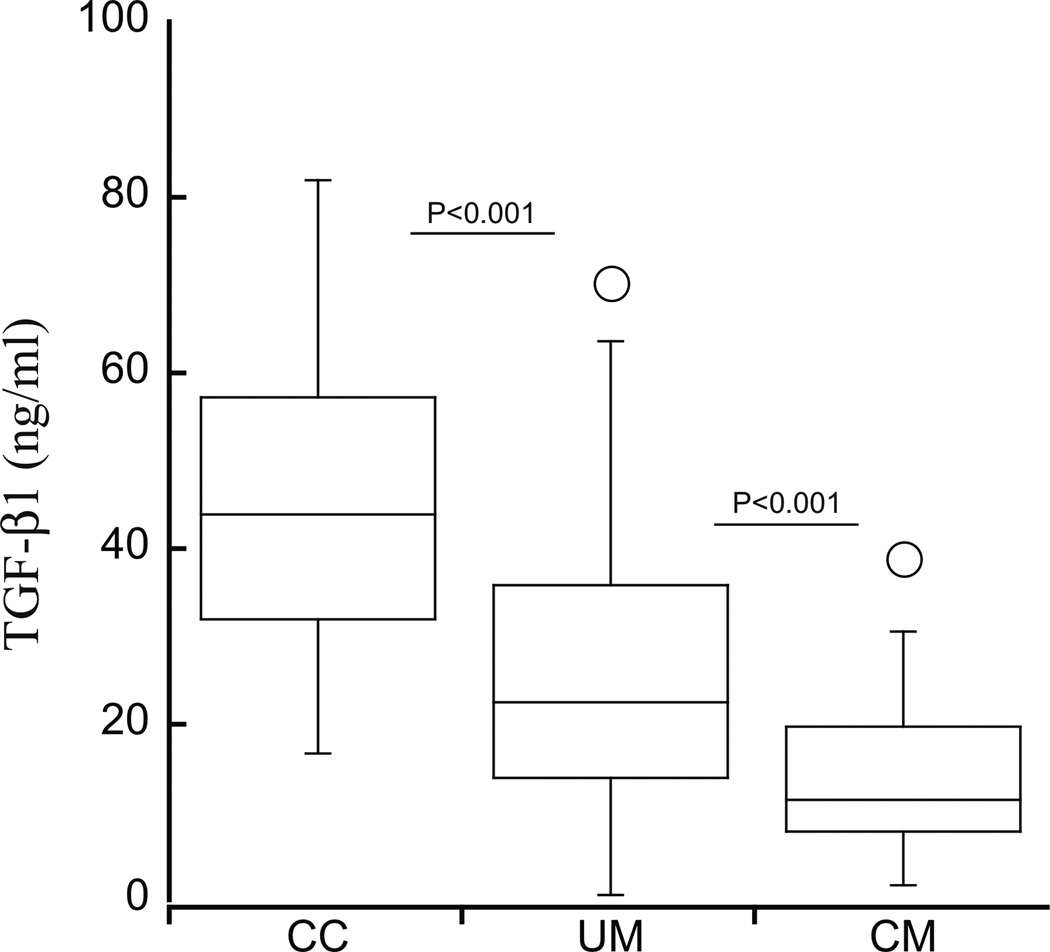
Serum concentrations of TGF-β1 decreased with increasing severity of disease (median concentrations (25th, 75th percentile)), ng/ml: CC, 41.4 (31.6, 57.4), UM, 22.7 (14.1, 36.4), CM, 11.8 (8, 21), P for trend<0.0001, Figure 1). After adjustment for age, hemoglobin level and sex with multivariate linear regression analysis, log-transformed TGF-β1 levels remained higher in children with UM than CM (P=0.016, beta coefficient 0.39, 95% CI, 0.07–0.74) and higher in children with community children than UM (P= 0.001, beta coefficient 0.59, 95% CI, 0.35–0.833). There was no significant difference in concentrations of TGF-β1 in CC with asymptomatic parasitemia 55.7 (32.15–60.5) and CC with no detectable parasitemia (41.4 (31.6–57.4), P=0.5). Seventy-two hours after initiation of treatment, TGF-β1 concentrations in children with CM or UM had normalized to the concentrations seen in community children at enrollment (CM, 37.9 (27–48.1), UM 40.7 (28.6–53)).
Figure 1.
TGF-β1 concentrations decrease with increasing disease severity. CC, community children; UM, uncomplicated malaria; CM, cerebral malaria. In this box and whisker plot, the box represents the 25th and 75th percentiles, the central line the median, and the whiskers the 5th and 95th percentiles.
Four children with CM died. Serum TGF-β1 concentrations did not differ significantly between the children who died (6.7, (4–11.8)) and those who survived (13.5, (8.3–21.1), P=0.11). TGF-β1 concentrations were not associated with any neurocognitive outcome in children with CM, UM or CC (all P>0.05).
Correlation of TGF-β1 concentrations with platelet count and parasite density
TGF-β1 concentrations correlated strongly with platelet count in children with CM (rho=0.61, P<0.0001) or UM (rho= 0.38, P=0.001). Platelet count correlated negatively with peripheral blood parasite density in children with CM (Spearman’s rho= −0.27, P=0.01) and UM (rho= −0.37, P=0.0003), but TGF-β1 concentrations did not correlate with parasite density in either study group (CM, rho = −0.04, P=0.72, UM, rho= −0.19, P =0.13). Those who died with CM were found to have a lower median platelet count (47.5, (31–65) 10(9) cells/L) than those who survived (84.5, (55–144) 10(9) cells/L), P=0.039.
Correlation of TGF-β1 concentrations with concentrations of other cytokines and chemokines
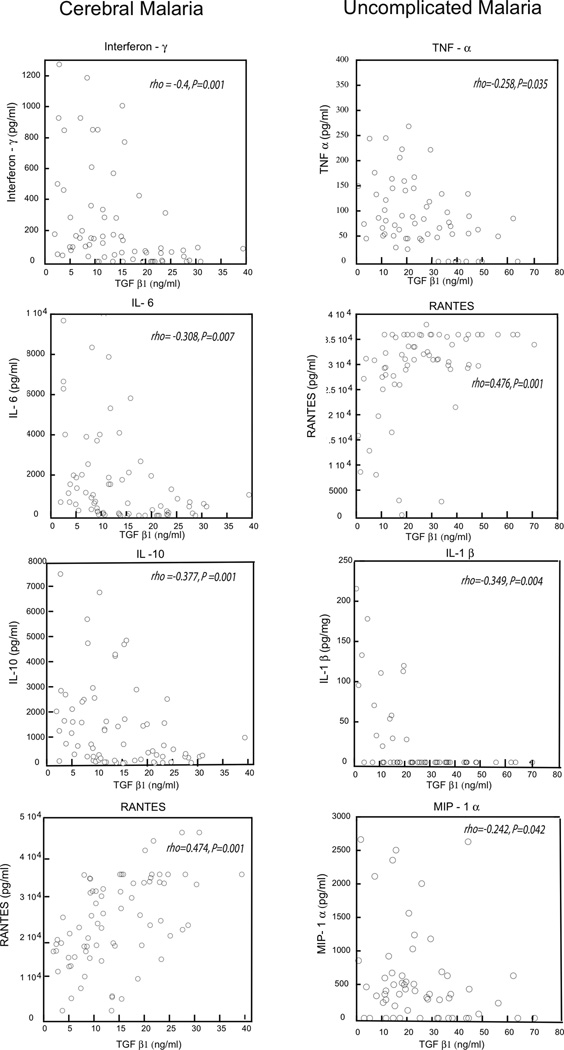
In children with CM, TGF-β1 concentrations correlated negatively with IFN-γ, IL-6, and IL-10 concentrations and positively with RANTES concentrations (Table 2). In children with UM, TGF-β1 concentrations correlated negatively with IL-1β, MIP-1α and TNF-α, and positively with RANTES (Table 2 and Figure 2).
Table 2.
Correlation with TGF-β1 concentrations with concentrations of other cytokines and chemokines in children with cerebral malaria (CM) or uncomplicated malaria (UM).
| CM Group N, Spearman’s rho, p value |
UM Group N, Spearman’s rho, p value |
|
|---|---|---|
| IL-6 | 75, −0.3082, 0.007 | 67, 0.0214, 0.864 |
| IL-10 | 73, −0.3765, 0.001 | 67, −0.0397, 0.740 |
| IL-1β | 75, 0.1376, 0.239 | 67, −0.3485, 0.004 |
| IFN–γ | 75, −0.4004, 0.004 | 67, −0.1454, 0.240 |
| MIP-1α | 75, −0.1950, 0.094 | 67, −0.2416, 0.042 |
| MIP-1β | 75, −0.1397, 0.232 | 67, −0.2075, 0.092 |
| RANTES | 75, 0.4754, 0.001 | 67, 0.4676, 0.001 |
| TNF-α | 75, 0.1342, 0.251 | 67, −0.2581, 0.035 |
Figure 2.
TGF-β1concentrations and pro- and anti-inflammatory cytokines with in UM, uncomplicated malaria; CM, cerebral malaria. IFN-γ, (Interferon- gamma), TNF-α (tumor necrosis factor-alpha), IL–1β (interleukin-1beta), IL-6 (interleukin 6), MIP–1α (macrophage inflammatory protein alpha), refer to table 2 for spearman correlations.
TGF-β1 concentrations according to stool helminth infection
Eleven of the 133 stool samples obtained across the 3 groups were found to have parasitic infections. Five of the 133 children (4%) had a stool helminth infection (4 Ascaris lumbricoides, 1Trichuris trichiura), including 3 of 40 children with CM, 1 of 46 children with UM and 1 of 47 CC. Six children had Giardia lamblia infection. In children with CM, serum TGF-β1 concentrations did not differ in those with helminth infection (median [IQR]), 13.45 (11.8–13.55) vs. those without helminth infection (11.6 (8–21.1), P=0.94), but the small numbers of children with CM who had helminth infection (n=3) did not allow for meaningful comparison.
Discussion
In the present study, we show that in children with cerebral malaria, thrombocytopenia correlates with decreased TGF-β1, and decreased TGF-β1 correlates with cytokine/chemokine changes associated with increased disease severity and death [8, 9, 11]. We postulate that a reduction in the platelet reservoir of TGF-β1 in severe malaria leads to impaired TGF-β1-mediated regulation of cytokine responses associated with disease severity and death. The study findings suggest a novel mechanism that might explain in part the adverse outcomes associated with thrombocytopenia in severe malaria.
The causes of thrombocytopenia in uncomplicated and severe malaria remain unclear, and the question of whether thrombocytopenia is a reflection of severe disease or contributes to severe disease is also unanswered. A number of factors may be responsible for the thrombocytopenia seen in severe malaria, including host defense mechanisms against parasitemia [34], development of antibodies to platelets [35], sequestration [36], phagocytosis[37] and oxidative stress [38]. It is possible that thrombocytopenia may reflect disease severity but also subsequently contribute to increased disease severity.
In in vitro studies, platelets appear to be important in protection from P. falciparum blood stage infection, through direct anti-parasitic activity and the ability to present antigens to T cells [39, 40]. However, animal models suggest that platelets are part of disease pathogenesis. It has been proposed that timing may be important in determining whether platelets are harmful or helpful in the response to murine malaria. A recent study in a murine model of experimental cerebral malaria showed that platelets were protective early in infection, through limitation of parasite growth, but continued platelet activation contributed to inflammation at later stages of infection [41]. The present study data appear to contradict these findings, since thrombocytopenia was associated with increased disease severity. Several other studies in humans also document an association of thrombocytopenia with disease severity and mortality [28, 42], consistent with a protective and not harmful effect of platelets at later disease stages. The findings of the present study support a novel potential mechanism for platelet-associated protection from severe disease: release of TGF-β1 from platelets, modulating the inflammatory response that may lead to severe disease symptoms.
The finding of lower TGF-β1 concentrations with increasing disease severity in the present study is consistent with most [13, 26, 27] though not all [30, 43] prior studies. TGF-β1 in vitro inhibits development of effector T-cells and macrophage activation and has less effect once those cells have been activated [44, 45]. An initial lack of available TGF-β1 may shift the balance of inflammation towards an exaggerated inflammatory response that continues despite subsequent normalization of TGF-β1 concentrations. In the present study, TGF-β1 correlated negatively with cytokines associated with more severe disease in malaria, and this included not only the pro-inflammatory cytokines IFN-γ and IL-6 [8, 9, 13, 27], but the anti-inflammatory cytokine IL-10[8, 9]. Studies in other disease processes demonstrate TGF-β1 down-regulation of IFN-γ [46, 47]) and IL-6 [48], consistent with our findings, but contrary to our findings, most prior studies show TGF-β1 down-regulation of RANTES [49, 50] and increased levels of IL-10 in individuals with elevated TGF-β1 [51, 52]. The findings could be due to differences in timing and production of cytokines and chemokines, as cytokine concentrations at any single time point collection could reflect concurrent or prior levels of TGF-β1, depending on the cytokine/chemokine. We did not find a correlation between parasitemia and TGF-β1, but other markers such as HRP2, which reflects parasite biomass, might be more appropriate to compare the relation of TGF-β1 concentrations to total parasite burden.
Only a small number of children (n=5) in the study had stool helminth infections and it remains unclear if they modulate the TGF-β1 response in malaria. More sensitive testing with PCR to identify stool helminth infections [53] might allow future studies to better explore the relationship between helminth infections and TGF-β1 in malaria. As with most prior human studies, a limitation of the present study is that it can show only association of factors with disease endpoints, and cannot prove causation. However, in light of the contradictory murine model and human findings regarding thrombocytopenia and morbidity in cerebral malaria, it is important to continue with human studies to assess potential mechanisms of thrombocytopenia-associated disease severity, to assess potential pathways for intervention.
In summary, the study data demonstrate that in children with cerebral malaria, thrombocytopenia correlates with decreased TGF-β1 concentrations, which in turn correlates with cytokine/chemokine changes associated with increased disease severity and death. The data suggest that thrombocytopenia may mediate disease severity in malaria in part through reduced TGF-β1-mediated regulation of cytokines associated with severe disease.
ACKNOWLEDGEMENTS
The study was supported in part by grants from the Fogarty International Center and the National Institute of Neurological Disorders and Stroke under Award Numbers R21 TW006794 and R01 NS055349 (PI: John, CC) and from the Eunice Kennedy Shriver National Institute for Child Health and Development, T32 GRANT HD068229 (PI: Schleiss, MR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg. 2001;64:57–67. doi: 10.4269/ajtmh.2001.64.57. [DOI] [PubMed] [Google Scholar]
- 3.Curfs JH, van der Meer JW, Sauerwein RW, Eling WM. Low dosages of interleukin 1 protect mice against lethal cerebral malaria. J Exp Med. 1990;172:1287–1291. doi: 10.1084/jem.172.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Sanni LA, Omer F, Riley E, Langhorne J. Pathology of Plasmodium chabaudi chabaudi infection and mortality in interleukin-10-deficient mice are ameliorated by anti-tumor necrosis factor alpha and exacerbated by anti-transforming growth factor beta antibodies. Infect Immun. 2003;71:4850–4856. doi: 10.1128/IAI.71.9.4850-4856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi F, Ishida H, Matsui T, Tsuji M. Effects of in vivo administration of anti-IL-10 or anti-IFN-gamma monoclonal antibody on the host defense mechanism against Plasmodium yoelii yoelii infection. J Vet Med Sci. 2000;62:583–587. doi: 10.1292/jvms.62.583. [DOI] [PubMed] [Google Scholar]
- 6.Schofield L, Ferreira A, Altszuler R, Nussenzweig V, Nussenzweig RS. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987;139:2020–2025. [PubMed] [Google Scholar]
- 7.Grau GE, Fajardo LF, Piguet PF, Allet B, Lambert PH, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 8.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenisch C, Parschalk B, Narzt E, Looareesuwan S, Graninger W. Elevated serum levels of IL-10 and IFN-gamma in patients with acute Plasmodium falciparum malaria. Clin Immunol Immunopathol. 1995;74:115–117. doi: 10.1006/clin.1995.1017. [DOI] [PubMed] [Google Scholar]
- 10.Day NP, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TT, Mai NT, Phu NH, Sinh DX, White NJ, Ho M. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis. 1999;180:1288–1297. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 11.John CC, Opika-Opoka R, Byarugaba J, Idro R, Boivin MJ. Low levels of RANTES are associated with mortality in children with cerebral malaria. J Infect Dis. 2006;194:837–845. doi: 10.1086/506623. [DOI] [PubMed] [Google Scholar]
- 12.de Kossodo S, Grau GE. Profiles of cytokine production in relation with susceptibility to cerebral malaria. J Immunol. 1993;151:4811–4820. [PubMed] [Google Scholar]
- 13.Perkins DJ, Weinberg JB, Kremsner PG. Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J Infect Dis. 2000;182:988–992. doi: 10.1086/315762. [DOI] [PubMed] [Google Scholar]
- 14.Goncalves RM, Scopel KK, Bastos MS, Ferreira MU. Cytokine balance in human malaria: does Plasmodium vivax elicit more inflammatory responses than Plasmodium falciparum? PLoS One. 2012;7:e44394. doi: 10.1371/journal.pone.0044394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blakytny R, Ludlow A, Martin GE, Ireland G, Lund LR, Ferguson MW, Brunner G. Latent TGF-beta1 activation by platelets. J Cell Physiol. 2004;199:67–76. doi: 10.1002/jcp.10454. [DOI] [PubMed] [Google Scholar]
- 16.Clemente A, Caporale R, Sannella AR, Majori G, Severini C, Fadigati G, Cirelli D, Bonini P, Garaci E, Cozzolino F, Torcia MG. Plasmodium falciparum soluble extracts potentiate the suppressive function of polyclonal T regulatory cells through activation of TGFbeta-mediated signals. Cell Microbiol. 2011;13:1328–1338. doi: 10.1111/j.1462-5822.2011.01622.x. [DOI] [PubMed] [Google Scholar]
- 17.Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155–7160. [PubMed] [Google Scholar]
- 18.Omer FM, Kurtzhals JA, Riley EM. Maintaining the immunological balance in parasitic infections: a role for TGF-beta? Parasitol Today. 2000;16:18–23. doi: 10.1016/s0169-4758(99)01562-8. [DOI] [PubMed] [Google Scholar]
- 19.Omer FM, Riley EM. Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J Exp Med. 1998;188:39–48. doi: 10.1084/jem.188.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987;84:5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espevik T, Figari IS, Shalaby MR, Lackides GA, Lewis GD, Shepard HM, Palladino MA., Jr Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987;166:571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellone G, Aste-Amezaga M, Trinchieri G, Rodeck U. Regulation of NK cell functions by TGF-beta 1. J Immunol. 1995;155:1066–1073. [PubMed] [Google Scholar]
- 23.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 24.Walther M, Tongren JE, Andrews L, Korbel D, King E, Fletcher H, Andersen RF, Bejon P, Thompson F, Dunachie SJ, Edele F, de Souza JB, Sinden RE, Gilbert SC, Riley EM, Hill AV. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23:287–296. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Wammes LJ, Wiria AE, Toenhake CG, Hamid F, Liu KY, Suryani H, Kaisar MM, Verweij JJ, Sartono E, Supali T, Smits HH, Luty AJ, Yazdanbakhsh M. Asymptomatic plasmodial infection is associated with increased tumor necrosis factor receptor II-expressing regulatory T cells and suppressed type 2 immune responses. J Infect Dis. 2013;207:1590–1599. doi: 10.1093/infdis/jit058. [DOI] [PubMed] [Google Scholar]
- 26.Chaiyaroj SC, Rutta AS, Muenthaisong K, Watkins P, Na Ubol M, Looareesuwan S. Reduced levels of transforming growth factor-beta1, interleukin-12 and increased migration inhibitory factor are associated with severe malaria. Acta Trop. 2004;89:319–327. doi: 10.1016/j.actatropica.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Wenisch C, Parschalk B, Burgmann H, Looareesuwan S, Graninger W. Decreased serum levels of TGF-beta in patients with acute Plasmodium falciparum malaria. J Clin Immunol. 1995;15:69–73. doi: 10.1007/BF01541734. [DOI] [PubMed] [Google Scholar]
- 28.Gerardin P, Rogier C, Ka AS, Jouvencel P, Brousse V, Imbert P. Prognostic value of thrombocytopenia in African children with falciparum malaria. Am J Trop Med Hyg. 2002;66:686–691. doi: 10.4269/ajtmh.2002.66.686. [DOI] [PubMed] [Google Scholar]
- 29.John CC, Park GS, Sam-Agudu N, Opoka RO, Boivin MJ. Elevated serum levels of IL-1ra in children with Plasmodium falciparum malaria are associated with increased severity of disease. Cytokine. 2008;41:204–208. doi: 10.1016/j.cyto.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esamai F, Ernerudh J, Janols H, Welin S, Ekerfelt C, Mining S, Forsberg P. Cerebral malaria in children: serum and cerebrospinal fluid TNF-alpha and TGF-beta levels and their relationship to clinical outcome. J Trop Pediatr. 2003;49:216–223. doi: 10.1093/tropej/49.4.216. [DOI] [PubMed] [Google Scholar]
- 31.Ochiel DO, Awandare GA, Keller CC, Hittner JB, Kremsner PG, Weinberg JB, Perkins DJ. Differential regulation of beta-chemokines in children with Plasmodium falciparum malaria. Infect Immun. 2005;73:4190–4197. doi: 10.1128/IAI.73.7.4190-4197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armah HB, Wilson NO, Sarfo BY, Powell MD, Bond VC, Anderson W, Adjei AA, Gyasi RK, Tettey Y, Wiredu EK, Tongren JE, Udhayakumar V, Stiles JK. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar J. 2007;6:147. doi: 10.1186/1475-2875-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, Wu B, Boivin MJ. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–e99. doi: 10.1542/peds.2007-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JW, Park SH, Yeom JS, Huh AJ, Cho YK, Ahn JY, Min GS, Song GY, Kim YA, Ahn SY, Woo SY, Lee BE, Ha EH, Han HS, Yoo K, Seoh JY. Serum cytokine profiles in patients with Plasmodium vivax malaria: a comparison between those who presented with and without thrombocytopenia. Ann Trop Med Parasitol. 2003;97:339–344. doi: 10.1179/000349803235002416. [DOI] [PubMed] [Google Scholar]
- 35.Kelton JG, Keystone J, Moore J, Denomme G, Tozman E, Glynn M, Neame PB, Gauldie J, Jensen J. Immune-mediated thrombocytopenia of malaria. J Clin Invest. 1983;71:832–836. doi: 10.1172/JCI110836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skudowitz RB, Katz J, Lurie A, Levin J, Metz J. Mechanisms of thrombocytopenia in malignant tertian malaria. Br Med J. 1973;2:515–518. doi: 10.1136/bmj.2.5865.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coelho HC, Lopes SC, Pimentel JP, Nogueira PA, Costa FT, Siqueira AM, Melo GC, Monteiro WM, Malheiro A, Lacerda MV. Thrombocytopenia in Plasmodium vivax malaria is related to platelets phagocytosis. PLoS One. 2013;8:e63410. doi: 10.1371/journal.pone.0063410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erel O, Kocyigit A, Bulut V, Avci S, Aktepe N. Role of lipids, lipoproteins and lipid peroxidation in thrombocytopenia in patients with vivax malaria. Haematologia (Budap) 1998;29:207–212. [PubMed] [Google Scholar]
- 39.McMorran BJ, Marshall VM, de Graaf C, Drysdale KE, Shabbar M, Smyth GK, Corbin JE, Alexander WS, Foote SJ. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323:797–800. doi: 10.1126/science.1166296. [DOI] [PubMed] [Google Scholar]
- 40.Chapman LM, Aggrey AA, Field DJ, Srivastava K, Ture S, Yui K, Topham DJ, Baldwin WM, 3rd, Morrell CN. Platelets present antigen in the context of MHC class I. J Immunol. 2012;189:916–923. doi: 10.4049/jimmunol.1200580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aggrey AA, Srivastava K, Ture S, Field DJ, Morrell CN. Platelet induction of the acute-phase response is protective in murine experimental cerebral malaria. J Immunol. 2013;190:4685–4691. doi: 10.4049/jimmunol.1202672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogier C, Gerardin P, Imbert P. Thrombocytopenia is predictive of lethality in severe childhood falciparum malaria. Arch Dis Child. 2004;89:795–796. doi: 10.1136/adc.2003.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prakash D, Fesel C, Jain R, Cazenave PA, Mishra GC, Pied S. Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of Central India. J Infect Dis. 2006;194:198–207. doi: 10.1086/504720. [DOI] [PubMed] [Google Scholar]
- 44.Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-beta1-mediated fibrosis. J Exp Med. 2003;198:1179–1188. doi: 10.1084/jem.20030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–64. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 47.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 48.Musso T, Espinoza-Delgado I, Pulkki K, Gusella GL, Longo DL, Varesio L. Transforming growth factor beta downregulates interleukin-1 (IL-1)-induced IL-6 production by human monocytes. Blood. 1990;76:2466–2469. [PubMed] [Google Scholar]
- 49.Cho ML, Min SY, Chang SH, Kim KW, Heo SB, Lee SH, Park SH, Cho CS, Kim HY. Transforming growth factor beta 1(TGF-beta1) down-regulates TNFalpha-induced RANTES production in rheumatoid synovial fibroblasts through NF-kappaB-mediated transcriptional repression. Immunol Lett. 2006;105:159–166. doi: 10.1016/j.imlet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Dai C, Wen X, He W, Liu Y. Inhibition of proinflammatory RANTES expression by TGF-beta1 is mediated by glycogen synthase kinase-3beta-dependent beta-catenin signaling. J Biol Chem. 2011;286:7052–7059. doi: 10.1074/jbc.M110.174821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levings MK, Roncarolo MG. T-regulatory 1 cells: a novel subset of CD4 T cells with immunoregulatory properties. J Allergy Clin Immunol. 2000;106:S109–S112. doi: 10.1067/mai.2000.106635. [DOI] [PubMed] [Google Scholar]
- 52.Doetze A, Satoguina J, Burchard G, Rau T, Loliger C, Fleischer B, Hoerauf A. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol. 2000;12:623–630. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 53.Basuni M, Muhi J, Othman N, Verweij JJ, Ahmad M, Miswan N, Rahumatullah A, Aziz FA, Zainudin NS, Noordin R. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am J Trop Med Hyg. 2011;84:338–343. doi: 10.4269/ajtmh.2011.10-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]