Abstract
Rationale
The role of purinergic signaling in the human ENS is not well understood. We sought to further characterize the neuropharmacology of purinergic receptors in human ENS and test the hypothesis that endogenous purines are critical regulators of neurotransmission.
Experimental Approach
LSCM-Fluo-4-(Ca2+)-imaging of postsynaptic Ca2+ transients (PSCaTs) was used as a reporter of neural activity. Synaptic transmission was evoked by fiber tract electrical stimulation in human SMP surgical preparations. Pharmacological analysis of purinergic signaling was done in 1,556 neurons from 234 separate ganglia 107 patients; immunochemical labeling for P2XRs of neurons in ganglia from 19 patients. Real-time MSORT (Di-8-ANEPPS) imaging was used to test effects of adenosine on fast excitatory synaptic potentials (fEPSPs).
Results
Synaptic transmission is sensitive to pharmacological manipulations that alter accumulation of extracellular purines. Apyrase blocks PSCaTs in a majority of neurons. An ecto-NTPDase-inhibitor 6-N,N-diethyl-D-β,γ-dibromomethyleneATP or adenosine deaminase augments PSCaTs. Blockade of reuptake/deamination of eADO inhibits PSCaTs. Adenosine inhibits fEPSPs and PSCaTs (IC50=25μM), sensitive to MRS1220-antagonism (A3AR). A P2Y agonist ADPβS inhibits PSCaTs (IC50=111nM) in neurons without stimulatory ADPβS responses (EC50=960nM). ATP or a P2X1,2,2/3 (α,β-MeATP) agonist evokes fast, slow, biphasic Ca2+ transients or Ca2+ oscillations (EC50=400μM). PSCaTs are sensitive to P2X1 antagonist NF279. Low (20nM) or high (5μM) concentrations of P2X antagonist TNP-ATP block PSCaTs in different neurons; proportions of neurons with P2XR-ir follow the order P2X2>P2X1≫P2X3; P2X1+ P2X2 and P2X3+P2X2 are co-localized. RT-PCR identified mRNA-transcripts for P2X1-7,P2Y1,2,12-14R. Responsive neurons were also identified by HuC/D-ir.
Conclusions
Purines are critical regulators of neurotransmission in the human enteric nervous system. Purinergic signaling involves P2X1, P2X2, P2X3 channels, P2X1+P2X2 co-localization and inhibitory P2Y or A3 receptors. These are potential novel therapeutic targets for neurogastroenterology.
Keywords: ATP, endogenous adenosine, human enteric nervous system, submucous plexus, purinergic synaptic transmission, P2X channels, inhibitory P2Y receptors, A3 inhibitory receptors
1. Introduction
The concept of purinergic signaling stems from studies that were designed to identify the non-adrenergic, non-cholinergic (NANC) inhibitory neurotransmitter in the gut (Burnstock, 1972). Purine receptors are broadly classified as P1 for nucleosides (adenosine) and P2 for nucleotides (ATP). They can be subdivided into adenosine (A1,A2A,A2B,A3), P2X1-7 and P2Y1,2,4,6,11-14. Most, if not all of these receptors, are expressed in the gastrointestinal tract where they are known to be involved in the physiological regulation of gut reflexes in animal models (Burnstock, 2008; Burnstock, 2012; Christofi, 2008). For example, purine release from enterochromaffin cells (EC) modulates serotonin release that triggers gut neural reflexes (Liñán-Rico et al, 2013). ATP is also released from epithelial cells to influence gut reflexes (Burnstock, 2008; Burnstock, 2009). In animal models, purines (ATP, ADP, AMP, β-NAD+, ADP ribose) are potential transmitter(s) in the enteric nervous system (ENS) and gut reflexes, and are known to act at all levels of gut motor reflexes (Burnstock, 2008; Burnstock, 2012; Christofi et al, 2004; Cooke et al, 2004; Christofi, 2008; Gomes et al, 2009; Gulbransen and Sharkey, 2009; Gulbransen et al, 2012). Emerging evidence supports the concept that purine nucleotides other than ATP such as β-NAD+ or its metabolite ADP ribose are involved in P2Y1 – mediated inhibitory neuromuscular transmission in rodents, primates and humans (Durnin et al, 2013; Gallego et al, 2011; Hwang et al, 2011; Hwang et al, 2012). Adenosine, a metabolite of ATP, acts as an inhibitory neuromodulator in the ENS (via A1 AR or A3AR; Bozarov et al, 2009; Christofi, 2001; Christofi, 2008). In contrast to neuromuscular transmission, our knowledge of purinergic signaling between neurons in the human ENS for adenosine or nucleotides is rather limited.
It is difficult to translate data on purinergic signaling mechanisms and neural receptors identified in animal models to human ENS, since species differences are known to exist in neurochemistry, neurophysiology, and receptor pharmacology of enteric neurons (Breunig et al, 2007; Burnstock 2012; Furness, 1995; Schemann et al, 2002; Schemann and Neunlist, 2004; Timmermans, 2001). Our earlier studies identified species differences in purinergic receptors. The difference was that A3, but not A1, adenosine receptors can be identified in human ENS, whereas both functional receptors are present in guinea pig (Bozarov et al, 2009; Christofi et al, 1993; Wunderlich et al, 2008). Marked species differences in the distribution of P2X2 and P2X3 receptors exist in mouse, guinea-pig, rat and human (Van Nassauw et al,2002; Yangou et al,2000; Xiang & Burnstock,2006; Poole et al, 2002; Ren et al, 2003). In addition, mouse and guinea pig P2X receptors are known to have different pharmacological properties (Burnstock, 2012).
Neuroimaging techniques for monitoring intracellular free Ca2+ levels ([Ca2+]i) or multi-site optical recording (MSORT) of membrane potential (Vm) have made it possible to perform in vitro neuropharmacology studies in human submucous plexus (a component of the ENS) from surgical tissue or biopsy, and identified neuronal receptors for histamine (H1-H4), serotonin (5-HT3), proteases (PAR-2), purine nucleotides (e.g. P2Y1) and noradrenaline in surgical tissues (Cirillo et al, 2013; Michel et al, 2011; Neunlist et al, 1999; Schemann et al, 2010; Wunderlich et al, 2008;). In contrast to animals, little information exists on receptor subtypes or purinergic signaling mechanisms of activation/inactivation in the human ENS (other than a single study by our group; Wunderlich et al, 2008). A better understanding of the neuropharmacology of purinergic receptors and signaling pathways is essential, as they are potential therapeutic targets for Inflammatory Bowel Diseases, Irritable Bowel Syndrome and diarrheal disorders (Antonioli et al, 2008; Bozarov et al, 2009; Burnstock, 2008; Cockayne et al, 2000; Eltzschig et al, 2012; Gulbransen et al, 2012; Guzman et al, 2006; Linan-Rico et al, 2013; Ren et al, 2011).
We further tested the general hypothesis that endogenous purines are key regulators of neurotransmission in the human submucous plexus, acting at ATP-gated P2X ion-channels, metabotropic P2Y or P1 receptors, as they are in animal models. Experiments were done using in vitro micro-dissected submucous plexus (SMP) from surgical cases. Ca2+ neuroimaging was used to study purinergic synaptic transmission by pharmacological manipulation with selective drugs (agonists or antagonists) for P2X-ion channels, metabotropic P2Y and A3 receptors, as well as enzymes or inhibitors that either increase or decrease extracellular levels of endogenous purines. Responsive neurons were identified by their immunoreactivity for HuC/D, response to high K+ depolarization, size/shape and location; protein and mRNA expression was used to identify receptors. Novel findings support the hypothesis that endogenous purines are critical regulators of neurotransmission in human ENS acting at P2X1, P2X2, P2X3 channels, as well as inhibitory P2Y or A3 receptors. Purine nucleotides and adenosine, together with tachykinin(s) and ACh are key regulators of neurotransmission or co-activation in human SMP.
2. Materials and methods
2.1 Chemicals
Drugs were purchased from either Sigma-Chemical Company (ARL67156, ATP, adenosine, EHNA, TNP-ATP, suramin, ADPβS, PPADS, apyrase, hexamethonium, adenosine deaminase, NF279) or Tocris (α,β-Me-ATP, MRS1220, MRS2179, SB218795) and 1–10mM stocks were dissolved in distilled water or DMSO (final concentration ≤0.1%) and diluted into Krebs buffer. Drugs > 0.2mM concentrations were adjusted for pH if needed.
2.2 Institutional IRB approval of tissue procurement and LSCM Fluo-4 AM Ca2+ imaging in human SMP
In vitro Ca2+ imaging was conducted on freshly procured surgical tissue from minimal invasive Roux-en-Y gastric-bypass after patient consent as outlined in IRB protocols 2004H0165 and 2012H0231 approved at The Ohio State University. The surgical specimen was placed directly into 4°C oxygenated Krebs solution and the time elapsed from tissue removal from the patient to the laboratory bench was kept to ~15 min. For SMP dissections in the lab, whole-thick tissue was placed in oxygenated Krebs buffer at room temperature and micro-dissected to remove mucosa, after initial blunt dissection of muscular layers with attached myenteric plexus (to remove longitudinal muscle – myenteric plexus and attached circular muscle). The SMP with intact networks of ganglia was cut into ~1 cm2 pieces for loading with Ca2+ indicator to run several experiments (Wunderlich et al, 2008).
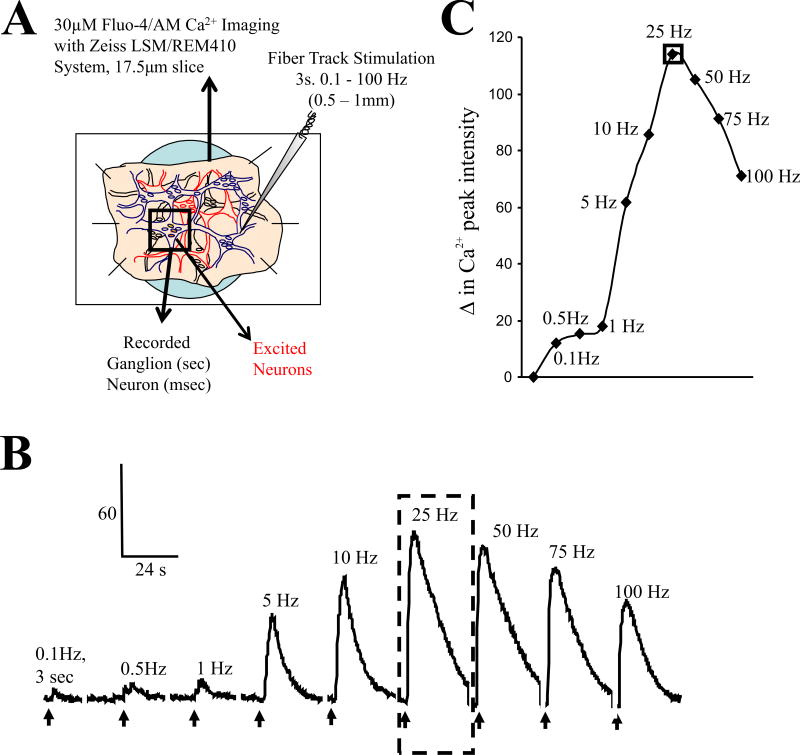
Each piece of SMP was loaded with 30μM Fluo-4/AM (Molecular Probes, Eugene, OR) for 2 hr and then incubated an additional 1 hr in oxygenated Krebs at room temperature to cleave the AM. The time that elapsed from tissue procurement to neuroimaging was ~5 hr. Ca2+ imaging was done using a modified-custom fitted Zeiss LSCM 410/REN laser scanning confocal imaging system. A solution in-line heater was used to maintain perfusion temperature at 36.5 °C ± 0.5 °C at a perfusion-rate of 9 ml/min. Time-series analysis of [Ca2+]i at 1-sec intervals was done in Fluo-4/AM loaded submucous neurons imaged through a 40X oil immersion apofluor objective (numerical aperture 1.3, working distance = 170μm). Ca2+ imaging was carried out using an Ar-Kr laser to excite the cells at 488 nm, and fluorescence emissions were passed through a FT510 dichroic mirror and collected through a photomultiplier tube equipped with a BP 505–550 filter, positioned in front of pinhole and light path. Scanned optical sections had a thickness of 17.5 μm. Figure 3 describes the recording technique for fiber tract stimulation (FTS) to evoke a synaptic response observed as a post-synaptic Ca2+ transient (PSCaT). After an equilibration period, a 3-sec FTS at 0.1–100Hz could be applied by a 25-μm Teflon coated platinum electrode (at 50V, 400 μsec). The effects of superfusion of agonists, antagonists, enzymes or enzyme inhibitors on basal or FTS-evoked PSCaTs were assessed before and 10–30 min after treatment. TTX, high Mg2+/low Ca2+ Krebs or hexamethonium were used to confirm synaptic transmission. At the end of each experiment, the recorded ganglion was exposed to modified Krebs buffer containing 75mM high-potassium (K+). Neurons were included in the Ca2+ analysis if they could respond to FTS before drug treatment and to high-K+ depolarization at the end of experiments (Wunderlich et al, 2008).
Figure 3. Fiber tract electrical stimulation (FTS) elicits a frequency dependent postsynaptic Ca2+ transient (PSCaT) in human submucous neurons.
(A) Schematic diagram of the submucous plexus preparation used for optical recording of changes in [Ca2+]i of the neurons. SMP is loaded with Fluo-4/AM and imaged on a Zeiss LSM/REM410 system – Fluo-4/Ca2+ imaging is used as a reporter of neural activity by recording postsynaptic Ca2+ transients. An electrical stimulating electrode is positioned on a fiber tract ≥0.5mm away from the recorded ganglion using a piezo Eppendorff 5171 micromanipulator and a train stimulus is applied to evoke a synaptic response. (B) Frequency dependent PSCaT in a single neuron with a peak response at 25Hz. A 3 sec train stimulus is applied to the fiber tract. (C) Peak Ca2+ responses in ‘B’ are plotted at frequencies from 0.1–100 Hz fiber tract stimulation. A train stimulus at higher frequencies (e.g. 25Hz) activates many fibers in the fiber tract from different neurons located in other submucous ganglia in the network leading to transmitter(s) release and activation of the postsynaptic neurons in the recording ganglion. (Wunderlich et al, 2008).
2.3 Immunochemical identification of responsive neurons
In selected experiments, the SMP was immediately fixed at the end of the recording period and processed for immunochemical staining with antibodies for the neuronal marker HuC/HuD, and subsequent relocation to identify responsive neurons. These neurons were routinely identified by their HuC/D immunoreactivity, high K+ Ca2+response, size/location of calcium transient. Antisera characteristics for HuC/D labeling are included in Table 1 (supplemental information).
2.4 Fast neuroimaging with voltage-sensitive dyes
Human surgical samples from colectomy patients at the Medical Clinics in Freising and Rechts der Isar were taken from macroscopically unaffected areas. Procedures were approved by the ethics committee of the Technische Universität München (1746/07). After removal, specimens were placed in cold oxygenated sterile Krebs solution and immediately transferred to the laboratory. Dissection of all tissues was done as described previously for fast imaging recording of the inner submucosal plexus (Michel et al, 2005). Final preparations were approximately 5 × 10mm and pinned onto a sylgard ring (Dow Corning) that was placed in a recording chamber and continuously perfused with 37°C oxygenated Krebs buffer. The tissue chamber was mounted onto an inverted epifluorescence Olympus IX 50 microscope (Olympus, Hamburg, Germany) and individual ganglia were stained with the voltage sensitive dye Di-8-ANEPPS (20 μM; 1-(3-sulfonatopropyl)-4-[beta[2-(di-n-octylamino)-6-naphthyl]vinyl]pyridinium betaine; Invitrogen, Germany). Stained neurons were visualised with x40 or x100 oil immersion objectives (x40: UAPO/340, x100: SPlanApo100, both from Olympus, Hamburg, Germany) by using a filter cube (excitation: 545±15 nm, dichroic mirror: 565 nm, emission: 580 nm, AHF Analysentechnik, Tübingen, Germany). Fluorescent images of the ganglia were acquired at a rate of 1k Hz and processed by a NeuroCCD system (RedShirtImaging, Decatur, GA, USA). The setup measures the fluorescence intensity in arbitrary units and calculates the relative change in fluorescence (ΔF/F) which is linearly related to changes in the membrane potential Vm (Kao et al, 2001; Neunlist et al, 1999). Interganglionic fiber tracts were stimulated electrically with a Teflon coated platinum electrode (25μm diameter, Medwire 10Ir1T, Science Products, Hofheim, Germany) connected to a constant current stimulator (A360, WPI, Berlin, Germany). Electrical pulses had durations of 300μs and amplitudes varying from 10–150μA. Di-8-ANEPPS was solubilized in Krebs solution containing pluronic F-127 (final concentration in the application pipette 0.014%; Molecular Probes) dissolved in DMSO (final concentration 0.125% in the application pipette, Sigma-Aldrich, Steinheim, Germany).
2.5 RT-PCR
Total RNA preparation and RT-PCR analysis from human SMP preparations was done as we previously reported for human mucosal preparations using primers for P2X1-7 and P2Y1,2,4,6,11-14 listed in Supplemental Table 4.
2.6 P2X1, P2X2, P2X3 and HuC/HuD Immunofluorescent Labeling in SMP
Immunofluorescent labeling was used to evaluate the expression and distribution of P2X1, P2X2, and P2X3 receptor immunoreactivity and colabeling for P2X1/P2X2 or P2X2/P2X3 receptors in micro-dissected human SMP. Colabeling with anti-HuC/HuD mouse monoclonal antibody was used to confirm identity of neurons in these experiments.
SMP preparations were fixed overnight at 4°C in Zamboni’s fixative. After fixation, the tissues were rinsed on a shaker for 1 hr with cold PBS, changing the buffer every 10 min. Tissue was blocked for 3 to 4 hours with 10% normal donkey serum, 0.75% Triton X-100 in PBS. Primary antibody incubation was done for 48 hours at 4°C. After incubation with the primary antibodies, the tissue was rinsed for 70 min with cold PBS, changing the buffer every 10 min. Secondary antibody incubation was done for 3 to 4 hours at room temperature, protected from light. Tissue was rinsed for 50 min with cold PBS, changing the buffer every 10 min and mounted to a microscope slide with Fluoromount G (Southern Biotech, Birmingham, Ala, USA). Source and dilutions for primary and secondary antibodies are in Supplemental Table 1. For relocation of calcium-recorded neurons in Fluo-4/AM calcium imaging studies, tissue remained in the recording chamber, and was stained and visualized in the same position (and was not mounted on a microscope slide). With this approach, relocation of the recorded ganglion and cells could be performed with > 95% accuracy. Primary antibodies were omitted for negative control experiments (or control peptides used to block the primary antisera for staining).
2.7 Statistical analysis
Mean values ± SEM are reported. SPSS 17.0, GraphPad Prism 3.02, Stat-View 54.51 and LSCM Zeiss software or MSORT analysis software were used for analysis of Ca2+ transients, PSCaTs or changes in Vm. In some experiments, X-Y Excel data was adjusted to eliminate minor movement artifacts in some of the tissues. In rare cases of major movement resulting in out of focus images, data was discarded and not included in the analysis. Data was normalized as % change in the peak-Ca2+ response. Analysis of variance (ANOVA) was used for multiple comparisons followed by Newman-Keuls Multiple comparison test. For other parameters, simple unpaired/2-tail or one tail t-test or student’s paired t test were used where appropriate. Differences were considered statistically significant at p<0.05.
3. Results
Ca2+ transients were analyzed in 1,556 single neurons from 107 Roux-en-Y surgical cases (jejunum) for 234 separate experiments on recorded ganglia in SMP. An additional 19 surgical cases/patients (N=19) were used in immunochemical analysis of P2X1, P2X2 and P2X3 receptors, and counterstained for HuC/HuD to identify neurons expressing the receptors. Key results are summarized in Figures 1–12, Table 1 and Tables 1–4 in supplemental information. Data is reported for N, number of surgical cases (patients); n, number of neurons; and numbers of ganglia (used in separate imaging experiments).
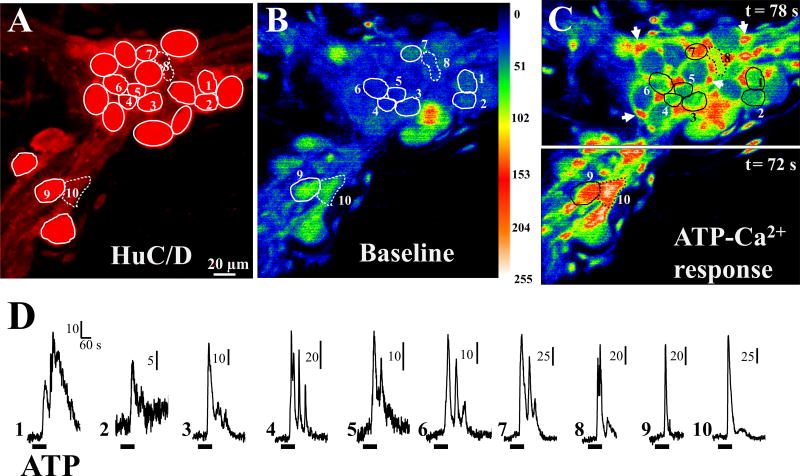
Figure 1. ATP calcium responses in immunochemically identified neurons of the human submucous plexus (SMP).
SMP was loaded with Fluo4/AM to test effects of ATP on calcium responses. Immediately after recording calcium responses, the tissue was fixed in the recording-chamber and processed for immunochemical identification of neurons by their HuC/D immunoreactivity, relocation and identification of neurons responding to ATP. (A) Neurons with HuC/D immunoreactivity in the recorded ganglion; solid circles, HuC/D-positive neurons; neurons 8 and 10, no labeling for HuC/D. (B) Baseline calcium levels in the ganglion for neurons 1 to 10. (C) ATP calcium response in the neurons and (D) individual calcium transients in each neuron numbered 1 to 10; neurons display different types of responses ranging from a simple monophasic response (cells 9, 10) to a biphasic response (cell 1) to cells with several calcium oscillations (cells 4, 6, 7); split image in C is from 2 different time points to more clearly illustrate ATP calcium responses in all the neurons that responded (as peak responses occur at slightly different time points); arrows in C indicate glial cells surrounding the much larger neurons. Images in B and C are pseudo-colored based on a scale ranging 0 to 255.
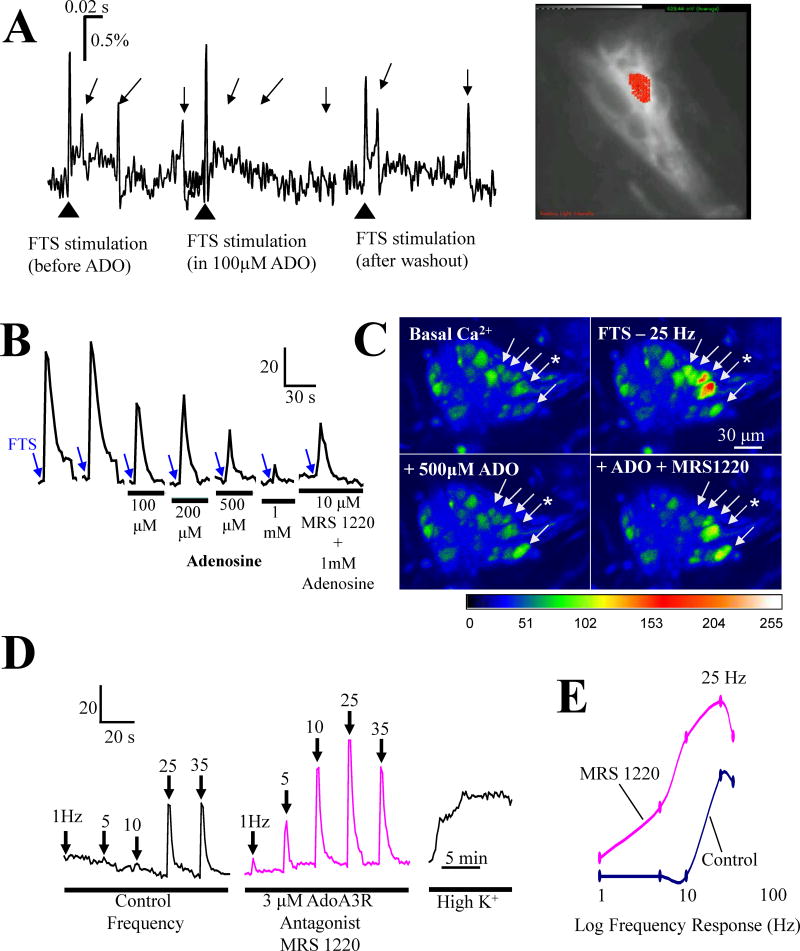
Figure 12. Adenosine inhibits synaptic transmission in human submucous ganglia via low affinity A3 AR.
(A) Multi-site optical recording (MSORT imaging) reveals that adenosine suppresses fast EPSPs in a reversible manner in colonic submucous neurons (two tailed t-test, p=0.006 for control, 20±3 versus 100μM ADO, 5±1, n=3); image of ganglion stained with the voltage sensitive dye Di-8-ANEPPS (bright white cell with recording site depicted in red. (B) LSCM Ca2+ imaging of postsynaptic Ca2+ transients in jejunum submucous neurons indicates that the inhibitory effect of adenosine is sensitive to adenosine A3 receptor blockade with MRS 1220 (10μM); * neuron used in the analysis. (C) Representative images to show effects of adenosine and MRS1220 in submucous neurons; pseudocolor scale is based on intensity values from 0–255. (D) MRS1220 (3μM) alone can augment the frequency dependent synaptic response evoked by fiber tract stimulation. (E) Effect of MRS 1220 (3μM); curves are constructed from peak values in ‘D’.
Table 1.
Descriptive summary of findings on effects of purinergic compounds on PSCaTs or basal Ca2+responses in human in vitro SMP preparations from surgical specimens
| Treatment (mechanism) | Effect | #Patients | #ganglia | #neurons | |
|---|---|---|---|---|---|
| FTS response | Basal Ca2+levels | ||||
| ADPβS (10nM–50μM, P2Y agonist) | − | + | 18 | 22 | 161 |
| ADO (P1, A3 agonist) | − | ? | 4 | 16 | 152 |
| α,β-MeATP (5,10μM,P2X1,2/3,3 agonist) | ? | + | 9 | 10 | 29 |
| ATP (10μM –2mM, P2 agonist) | + | + | 9 | 19 | 152 |
| TNP-ATP (20nM, P2X3 antagonist) | − | Φ | 5 | 15 | 101 |
| TNP-ATP (5μM, P2X2 antagonist, In different cells) | − − |
Φ |
3 |
10 |
73 |
| Apyrase (1–8U/ml, ecto-ATPase) | − | Φ | 8 | 20 | 152 |
| AdoDase (metabolize eADO→ino) (0.5–8U/ml) | + | + | 9 | 24 | 138 |
| ARL67156 (5μM, ATPase inhibitor) | + | Φ | 3 | 8 | 42 |
| MRS1200 (3,10μM, A3, antagonist) | + | Φ | 8 | 21 | 151 |
| SB218795 (100nM, NK3 antagonist) | − | Φ | 3 | 5 | 37 |
| TTX | − | Φ | 13 | 27 | 138 |
| High Mg2+/low Ca2+Krebs (inhibit transm.) | − | Φ | 2 | 9 | 50 |
| Hexamethonium (antagonist, nAChR) | − | Φ | 4 | 8 | 40 |
| DMPP (agonist, nAChR) | n/a | + | 6 | 18 | 128 |
| NF279 (100nM, P2X1 antagonist) | + | Φ | 3 | 3 | 12 |
| 107 surgical specimens | 235 ganglia | 1556 neurons | |||
(+), stimulation (−) inhibition (Φ), no effect (?), not tested (n/a) not applicable
3.1 Identification of submucous neurons responding to ATP with a Ca2+transient
Ca2+-responsive neurons in the submucous plexus are identified by immunochemical staining for the neuronal marker HuC/HuD. As shown in the example of Figure 1, SMP was loaded with Fluo4/AM to test effects of ATP on Ca2+ responses. Immediately after the recordings, the tissue was fixed in the recording-chamber and stained for HuC/D immunoreactivity, for relocation and identification of neurons responding to ATP. HuC/D immunoreactivity is shown in Figure 1A; ATP calcium responses in neurons with HuC/D immunoreactivity are shown for neurons 1 to 7 and 9 in Figures 1B–C (representative images). Figure 1D shows the Ca2+ transients in the neurons. Neurons display different types of Ca2+ responses ranging in shape and kinetic profiles. In cases where a cell loaded with Fluo-4/AM calcium indicator is not labeled for HuC/D (neurons 8 and 10 of Figure 1A), neurons were identified by size, location of the Ca2+ response and responses to high K+ depolarization (see Figure 2).
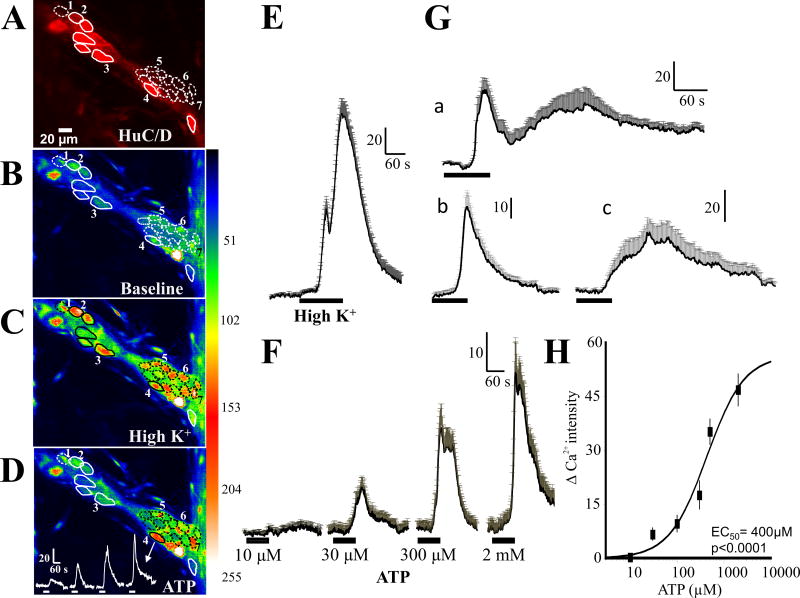
Figure 2. ATP causes a concentration-dependent response in human submucous neurons.
(A) Neurons with HuC/D immunoreactivity in the recorded ganglion; solid circles, HuC/D-positive neurons, dotted circles represent neurons that do not label for HuC/D, (B and C) but respond to high K+ depolarization with a calcium transient (representative image). (D) Representative image to illustrate some of the neurons (1–6) that respond to ATP; only neurons numbered 2, 3, 4 are positive for HuC/D immunoreactivity and neurons numbered 1, 5 and 6 are sensitive to high K+ depolarization but are not positive for HuC/D. A representative concentration – dependent Ca2+ response is shown for neuron number 4. (E) Average high K+ depolarization induced Ca2+ response in 22 neurons (20 of which responded to ATP as well). (F) Pooled data for the concentration – dependent effect of ATP in 20 neurons from 1 ganglion that responded to ATP. (Ga) Biphasic response to ATP (n=12 neurons in 2 ganglia from different patients); (Gb) monophasic response to ATP with fast kinetics (n=15 neurons in 2 ganglia from different patients); (Gc) monophasic response with slower kinetics (n=5 neurons from 2 ganglia in different patients). (H) Concentration-response curve for ATP from several ganglia. Data were fitted with a logistic equation and each symbol represents the average Ca2+ intensity value of 10–57 neurons. Vertical lines are S.E.M. The IC50 = 400μM (57 neurons, n=5 ganglia from 4 surgical cases/patients). ANOVA, p<0.001 for the curve.
3.2 Concentration – dependent calcium effect of ATP in human submucous ganglia
ATP causes a concentration-dependent response in submucous neurons. As shown in Figure 2, neurons that have ATP evoked Ca2+ responses can be identified by both HuC/D immunoreactivity and responses to high K+ depolarization. Neuron number 4 for example, responds to ATP with a non-cumulative-concentration dependent increase in intracellular free Ca2+ levels, responds to high K+ depolarization and expresses HuC/D immunoreactivity. However, in some instances cells loaded with calcium indicator did not stain for HuC/D, but did respond to high K+ depolarization and had the size and location characteristic of neurons (dotted circles represent such cells in Figures 2B,C); such cells were characterized as neurons in the analysis below.
ATP causes a concentration dependent Ca2+ response in submucous neurons. The average ATP concentration-dependent Ca2+ transients are shown for 20 of 22 high K+ responsive neurons (Figure 2E) in a single ganglion (Figure 2F). A concentration effect curve constructed from data from 57 neurons (5 ganglia form 4 patients, Fig 2H) gave an EC50 = 400μM. Three types of Ca2+ transients are observed in submucous neurons, based on their kinetic profiles. Neurons exhibit biphasic responses to ATP (Figure 2Ga, n=12 neurons), monophasic responses with fast kinetics (Figure 2Gb, n=15 neurons) or monophasic responses with slower kinetics (Figure 2Gc, n=5 neurons).
3.3 Effect of Apyrase and ARL 67156 on synaptic transmission
FTS elicits a frequency dependent increase in [Ca2+]i in single neurons of the human submucous plexus (Figure 3). The peak of the PSCaT occurs at 25Hz. This frequency was used routinely to study synaptic transmission as it produced a robust and reproducible Ca2+ transient for analysis.
The PSCaT elicited by a 3s/25 Hz train stimulus (FTS) is abolished by 1μM TTX or high Mg2+/low Ca2+ Krebs (Figure 4A; p<0.01; TTX, 37 neurons, 7 ganglia, N=5 surgical cases; Mg2+/low Ca2+, 29 neurons, 6 ganglia, N=4 patients).
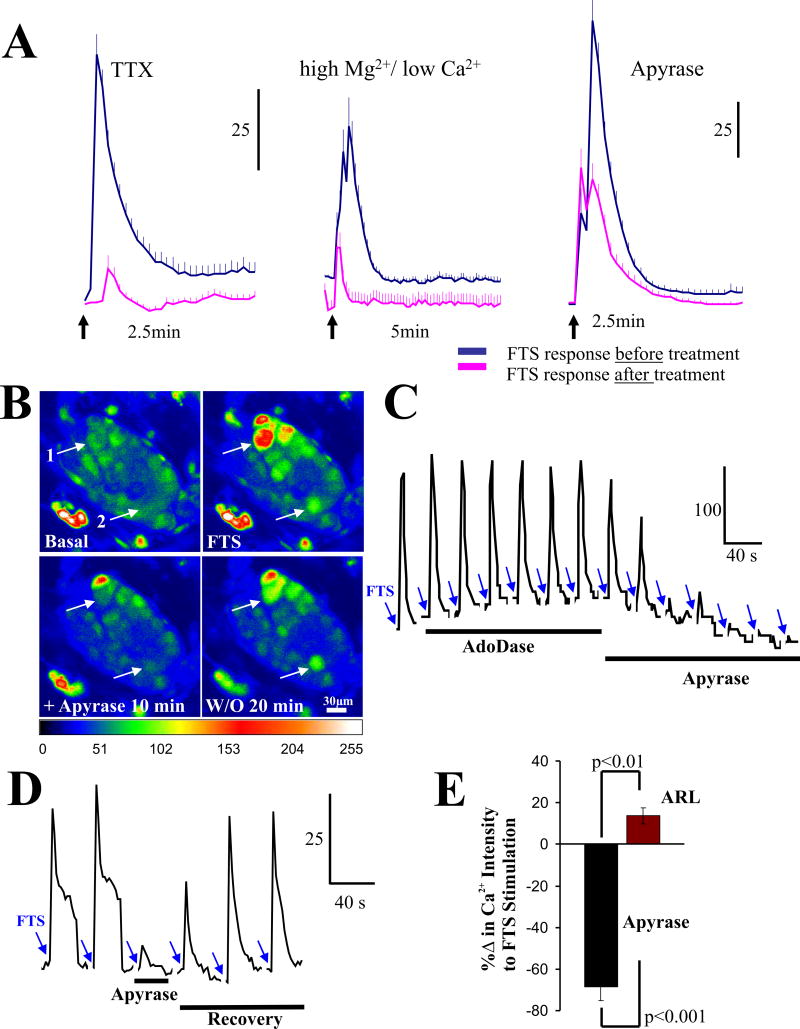
Figure 4. The ectoNTPD1 enzyme apyrase blocks synaptic transmission in human submucous ganglia.
(A) The PSCaT is abolished by 1μM TTX or high Mg2+/low Ca2+ Kreb’s buffer. Apyrase (5U/ml) reduces the peak PSCaT response (see Figure 4E for %Δ in Ca2+ response). (B) Representative pseudocolor images to show inhibitory effect of apyrase on FTS-Ca2+ responses and washout recovery; pseudocolor scale is based on intensity values from 0–255. (C) Inhibitory effect of apyrase at a lower concentration (1.5U/ml) on synaptic transmission in a single neuron. Adenosine deaminase (1U/ml), an enzyme that inactivates adenosine does not have any effect on the cell. FTS responses were repeated at 5 min intervals after full recovery. (D) Apyrase blocks in a reversible manner the PSCaT. (E) Apyrase (5U/ml) or ARL 67156 (100 μM) have opposite effects on PSCaTs; see results for details.
The NTPDase1 enzyme apyrase reduced the peak-Ca2+ response of the PSCaT in some neurons by ~40% to 100%. The effect of 5U/ml apyrase is shown in Figure 4A for the PSCaT (n=23 neurons, 4 ganglia, p<0.001). It was effective in the concentration range of 0.5 – 5 U/ml. In different cells it reduced, abolished or had no effect on PSCaTs (Figures 4B–D). The effect was reversible with washout (Figure 4B, D). Analysis of changes in peak-Ca2+ response with apyrase are shown in Figure 4E. Apyrase was tested in N=8 surgical cases, 20 ganglia and 152 neurons. Apyrase caused inhibition of PSCaTs in ~63% of the neurons analyzed in 20 ganglia (representing 20 separate experiments); ~ 70% of the response in these neurons is sensitive to apyrase. ARL 67156 (5μM), an inhibitor of 5′-ectonucleotidases, enhanced the PSCaT in contrast to apyrase (Figure 4E). It augmented the peak-Ca+2 response by 14±4%, p=0.018. ARL 67156 enhanced PSCaTs in 58% of neurons analyzed (for ARL67156, analysis was done for N=3 surgical cases, 8 ganglia and 42 neurons).
3.4 P2X antagonists block synaptic transmission in submucous neurons
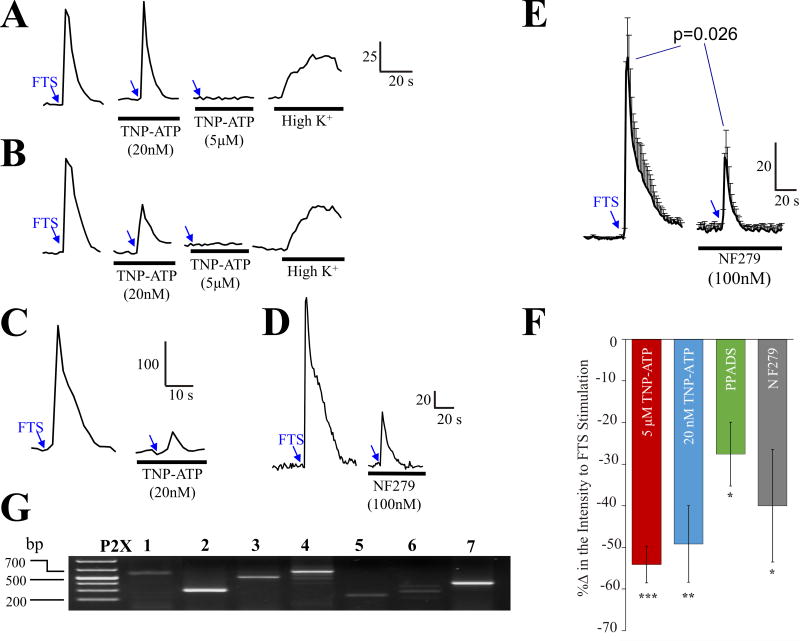
Several P2X antagonists with different selectivities for P2X receptors were used to test whether synaptic transmission involves ATP. Data is summarized in Figure 5 and Table 1. The P2X antagonist 2′3′-O-(2,4,6-trinitrophenyl)-5′adenosine triphosphate (TNP-ATP) blocked synaptic transmission at 5μM TNP-ATP (Figure 5A) or 20nM TNP-ATP (Figure 5C) but in rare cases, consecutive applications of each concentration produce additive effects (Figure 5B); SMP preparations were perfused with 20nM TNP-ATP 15 min to test effect on synaptic transmission, and then followed with 5μM TNP-ATP for another 15 min. In 23% of neurons (15 of 64 neurons) 20nM TNP-ATP blocked the PSCaT (peak Ca2+response) by 49±9% (p=0.005). In a separate group of neurons, 5μM TNP-ATP blocked the PSCaT by 54±4 % in 56% of neurons (31 of 56 neurons, p<0.001). In 9% of neurons (9 of 101 neurons) TNP-ATP caused additive effects and blocked the entire Ca2+ response in several neurons. Data is summarized in Figure 5F for TNP-ATP; data represents N=5 surgical cases, 15 ganglia and 101 neurons. TNP-ATP is a potent antagonist at P2X1, P2X2/3, P2X3 receptors at nanomolar concentrations, and it blocks P2X2 receptors at low micromolar concentrations (2–5μM). The selective P2X1 antagonist NF279 (100nM) depresses synaptic transmission (example in a single neuron, Figure 5D). The average effect of NF279 (100nM) on PSCaTs is shown in Figure 5E for 3 ganglia from 3 patients representing 12 neurons (p=0.026). The non-selective P2X antagonist PPADS (10μM) reduced the PSCaT by 28±8% in a small number of cells tested in 2 patients (3 of 6 neurons, p=0.041). Pooled data for the effects of TNP-ATP, PPADS, NF279 on % blockade of synaptic transmission are shown in Figure 5F.
Figure 5. P2X antagonists block synaptic transmission in human submucous ganglia.
(A–C) The P2X antagonist 2′3′-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP) blocks synaptic transmission in different types of neurons. (A) Example of a neuron in which 20nM TNP-ATP has no effect whereas 5μM TNP-ATP blocks the FTS-induced Ca2+ response; high K+ can still depolarize the neuron to cause an effect. (B) Representative example of a neuron in which TNP-ATP has additive effects at 20nM and 5μM concentrations; the neuron responds to high K+ at end of experiment. (C) Representative example in which a 20nM-concentration TNP-ATP is able to block the PSCaT. Arrows indicate fiber tract stimulation (3 sec, 25Hz stimulus); bars indicate drug treatment group (for details on drug - exposure protocol to TNP-ATP see methods). (D) The selective P2X1 antagonist NF279 (100nM) depresses synaptic transmission. (E) The effect of NF279 (100nM) in 3 ganglia from 3 patients representing 12 neurons. NF279 was pre-incubated for 15 minutes before FTS stimulation. (F) Summary of effects of TNP-ATP, PPADS, NF279 on % blockade of synaptic transmission (p<0.05 for all treatments, see results for details). (G) Agarose gel electrophoresis (1.5%) of RT-PCR P2X1-7 amplification from human SMP using specific sets of primers listed in Table 4 (supplemental information).
3.5 P2X receptor expression and function in submucous neurons
Receptor mRNA transcripts in SMP were identified by RT-PCR (Figure 5G). P2X mRNA transcripts were detected for P2X1-7 although P2X2, P2X3, P2X4 and P2X7 were more prominent.
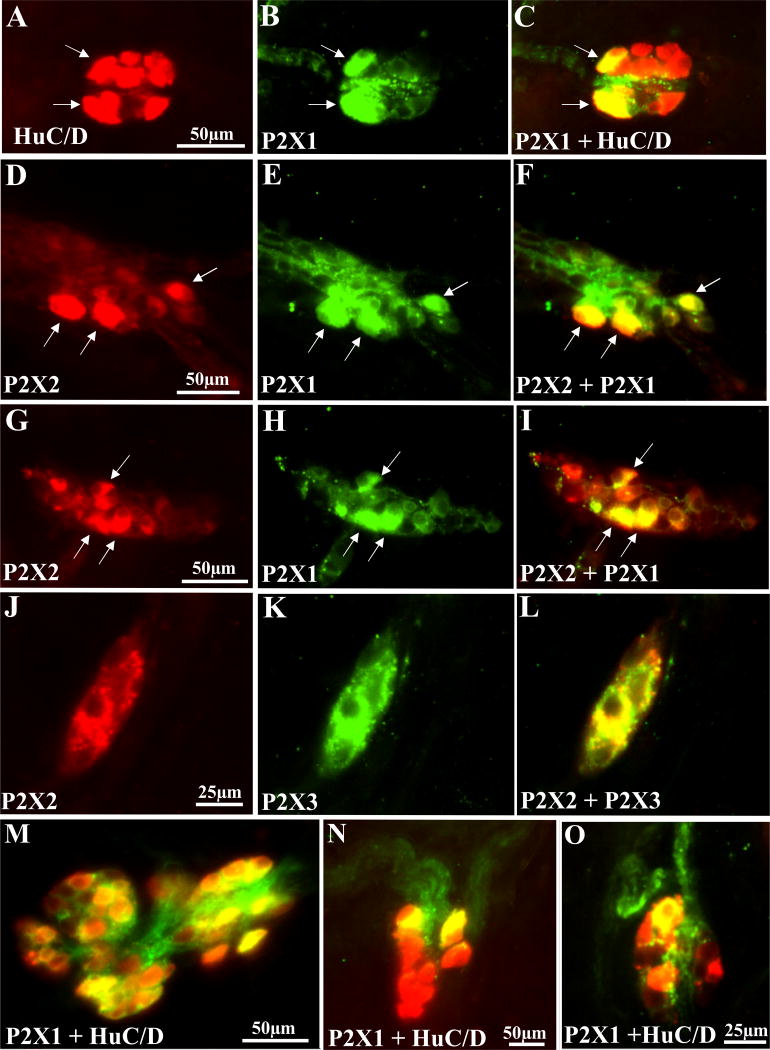
P2X1, P2X2 and P2X3 immunoreactivity was identified in HuC/D immunoreactive SMP neurons (Figure 6). P2X1 receptor immunoreactivity is shown in Figure 6A–C,M–O. P2X2 and P2X1 receptors are colocalized in some neurons (Figure 6D–F; G–I). P2X2 and P2X3 are also colocalized in a subset of SMP neurons (Figure 6J–L).
Figure 6. P2X receptor immunoreactivity in human submucous neurons.
(A–C) P2X1 receptors colocalize with HuC/D in 2 neurons (identified by the arrows). A, HuC/D labeling with secondary antibody conjugated to Alexa Fluor 568 antibody (red); B, P2X1 immunoreactivity with secondary antibody conjugated to Alexa Fluor 488 antibody (green). C, overlay image of A and B with yellow color indicating colocalization. (D–F) P2X2 and P2X1 receptor immunoreactivity in the same neurons. (G–I) Another example of P2X2 and P2X1 receptor colocalization. (J–L) Example of P2X2 and P2X3 receptor colocalization in submucous neurons. (M–O) other examples of P2X1 immunoreactive neurons in the submucous plexus (see results for quantitative analysis).
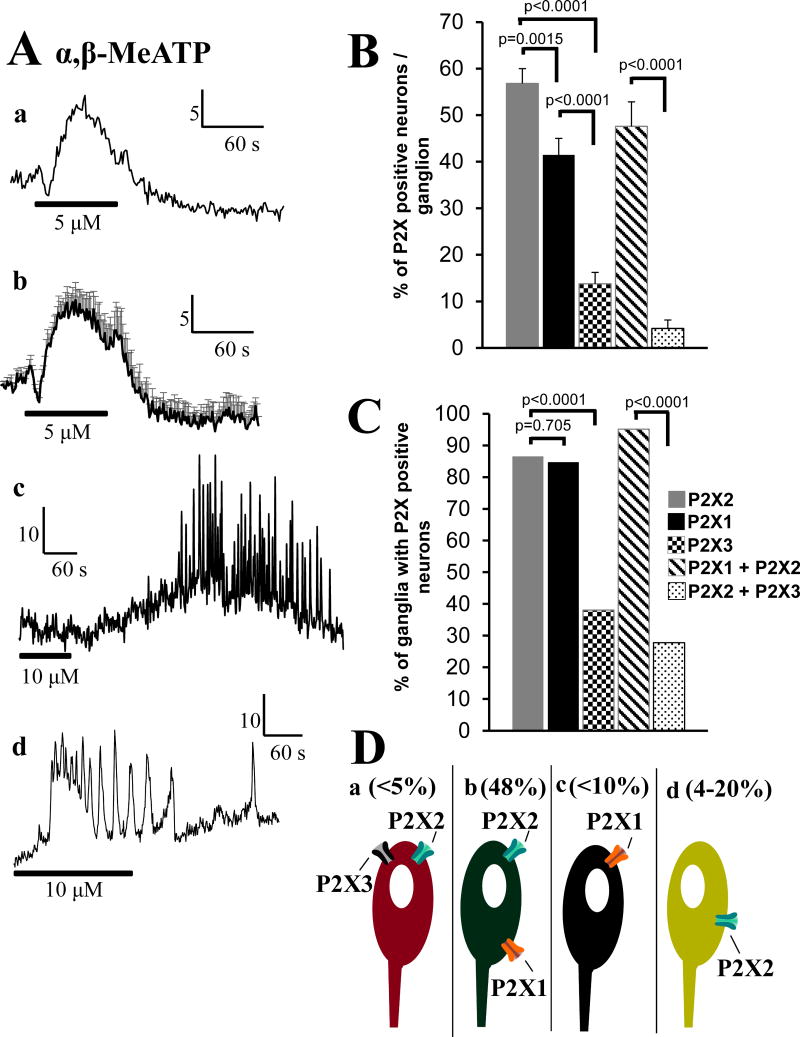
The P2X1,2/3,3 agonist α,β-Me-ATP causes Ca2+ responses in submucous neurons (Figure 7Aa–d). Some of the responses are complex with several oscillations in calcium levels (Figure 7Ac,d) and differ from others suggestive of more than one P2X receptor.
Figure 7. P2X response and P2X receptor quantification in human submucous neurons.
(Aa) The P2X1,2/3,3 agonist α,β-Me-ATP causes a calcium response in a neuron. (Ab) Average calcium response to α,β-Me-ATP (n=4 neurons in a single ganglion. (Ac-d) Other type of responses to α,β-Me-ATP, suggests multiple P2X receptors. (B) Quantitative analysis of P2X+-cells expressed as a % of HuC/D+-neurons that are colabeled for P2X1, P2X2, P2X3; data is also presented for colabeled neurons for P2X1+P2X2 or P2X2+P2X3 receptors. (C) The percentage of ganglia with P2X+-neurons (data and details of antisera used is summarized in Tables 1–3 in supplemental information). (D) Schematic showing 4 types of neurons expressing P2X receptor subtypes: a, P2X2+P2X3; b, P2X2+P2X1; c, P2X1; d, P2X2 receptors.
Quantitative analysis of P2X+ cells expressed as a % of HuC/D+ neurons that are colabeled for P2X1, P2X2, P2X3 is shown in Figure 7B and 7C. Data are also presented for colabeled neurons for P2X1+P2X2 or P2X2+P2X3 receptors. The percentage of ganglia with P2X+ neurons is summarized in Figure 7D (data and details of antisera used are summarized in Tables 1–3 in supplemental information) which is a schematic showing 4 types of neurons expressing P2X receptor subtypes: a, < 5% of neurons express P2X2+P2X3; b, 48% of neurons express P2X2+P2X1; c, <10% of neurons express P2X1 only; d, 4–20% of neurons express P2X2 receptors only; percentages are calculated from data in Figures 7B, 7C and Tables 2 and 3 of the supplemental information.
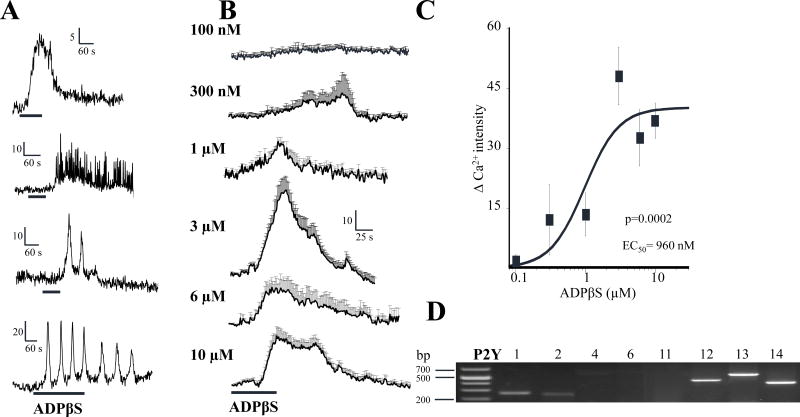
3.6 Dual effect of ADPβS on neuronal responses
The P2Y agonist ADPβS directly stimulates submucous neurons and increases intracellular free Ca2+ levels (Figure 8). Types of calcium transients in response to ADPβS in different neurons are shown in Figure 8A, ranging from a simple response to complex calcium transients associated with multiple oscillations in calcium levels. ADPβS causes a concentration dependent increase in the Ca2+ response from 100nM – 10μM (in cells responding with a simple Ca2+ response as shown in panel A; first calcium transient). The concentration dependent Ca2+ responses are shown in Figure 8B, and the concentration response curve is shown in Figure 8C. The EC50=960nM for the Ca2+ response (ANOVA, p=0.0002). ADPβS stimulation is consistent with P2Y1 receptor activation we reported previously (Wunderlich et al, 2008). However, P2Y mRNA transcripts were detected by RT-PCR for P2Y1, P2Y2, P2Y12, P2Y13 and P2Y14; prominent expression occurred for P2Y12-14, and these represent additional candidates for the response (Figure 8D).
Figure 8. The P2Y agonist ADPβ S stimulates a subset of human submucous neurons.
(A) Types of calcium transients in response to ADPβS (10, 6, 10 and 3μM respectively) in different neurons, ranging from a simple calcium response to complex calcium transients associated with multiple oscillations in calcium. (B) Concentration-effect responses for ADPβS from 100nM – 10μM in cells responding with a simple calcium response shown in panel A (first calcium transient). (C) Concentration dependent Ca2+ response curve to ADPβS in submucous neurons. ANOVA, p=0.0002 and the EC50=960nM. (D) RT-PCR identified mRNA transcripts for P2Y1, P2Y2, P2Y12, P2Y13 and P2Y14 receptors in SMP. ANOVA, p=0.0002 for the curve.
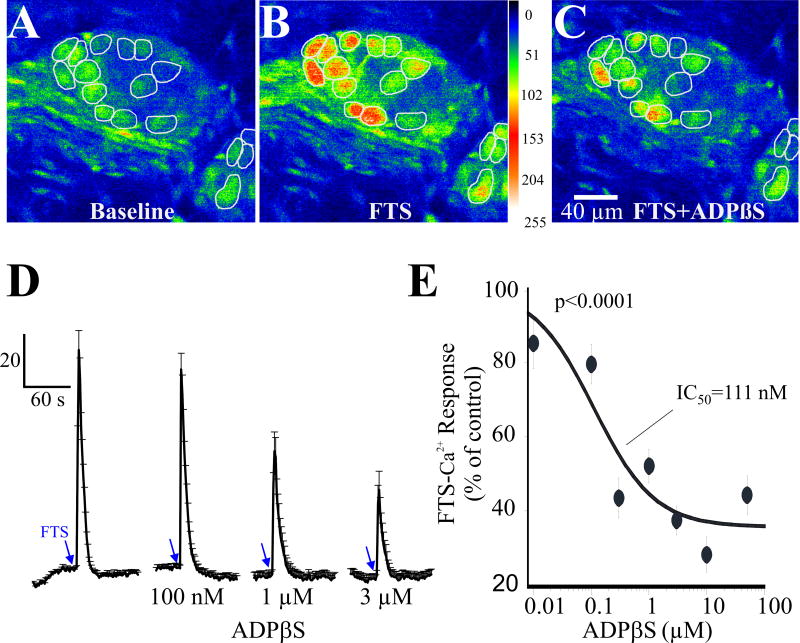
In addition, the P2Y agonist ADPβS inhibits synaptic transmission in a concentration dependent manner in neurons that do not display agonist responses described above. Figure 9s A–C illustrate the inhibitory effect of 3 μM ADPβS on FTS Ca2+ responses like those in a single ganglion. The averaged response for the concentration dependent inhibitory effect of ADPβS in 17 responsive neurons of a single submucous ganglion is shown in Figure 9D. The non-cumulative concentration response curve for ADPβS to inhibit synaptic transmission is shown in Figure 9E from ganglia obtained from 5 surgical cases. The IC50=111nM for inhibition by ADPβS (ANOVA, p<0.0001 for the curve).
Figure 9. The P2Y agonist ADPβS inhibits synaptic transmission in a concentration dependent manner in human submucous ganglia.
(A–C) Representative pseudocolor images of a ganglion showing the inhibitory effect of 3 μM ADPβS on FTS Ca2+ responses; pseudocolor scale is based on intensity values from 0–255. (D) FTS Ca2+ response in 17 responsive neurons represented in A–C. ADPβS was tested on FTS at 15 min intervals; ADPβS was perfused for 5 min prior to each FTS-stimulation and 10 min washout recovery cycle was used between stimulations. (E) Non-cumulative concentration-response curve for ADPβS to inhibit the FTS Ca2+ response. Data were fitted with a logistic equation; each symbol represents the average value of 10 to 18 neurons (from 7 different ganglia and surgical cases/patients) and vertical lines are S.E.M. ANOVA, p<0.0001 for the curve. The IC50=111nM.
3.7 Adenosinergic inhibition of synaptic transmission
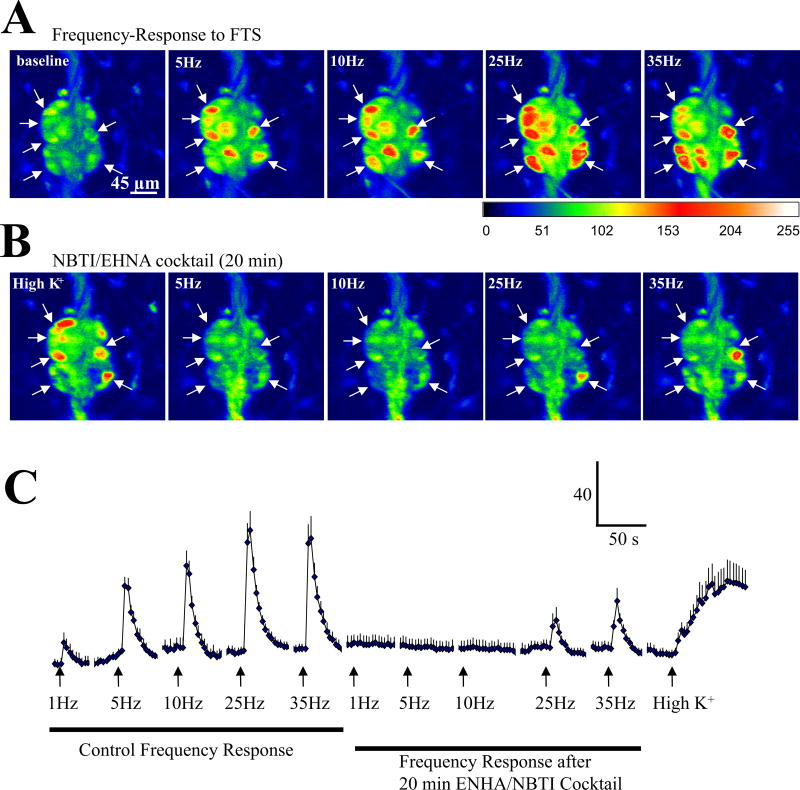
Manipulations of endogenous adenosine (eADO) levels to either increase the accumulation of adenosine or decrease it in the extracellular space were used to test if eADO is involved in the modulation of synaptic transmission. Application of a cocktail of NBTI/EHNA to block re-uptake/deamination of eADO suppressed PSCaTs. The PSCaT was abolished by the cocktail of NBTI/EHNA at 1–10 Hz, and reduced for 25 – 35Hz stimulation. Neurons responded to high K+ depolarization at the end of the experiment indicating that they were still viable (Figure 10, n=7 neurons from 1 ganglion; similar results obtained in 2 other ganglia from different surgical cases).
Figure 10. Accumulation of endogenous adenosine depresses synaptic transmission in human submucous ganglia.
(A) Representative images showing that FTS elicits a PSCaT in specific neurons of a submucous ganglion; images at 5Hz, 10Hz, 25Hz and 35Hz frequencies of stimulation (pseudocolor scale is based on intensity values from 0–255). (B) Representative images of FTS responses in the presence of the cocktail NBTI/EHNA. (C) Representative experiment in 7 neurons from 1 ganglion to illustrate that a cocktail of EHNA (adenosine deaminase uptake inhibitor) and NBTI (a nucleoside uptake inhibitor) can suppress the PSCaT elicited at frequencies ranging from 1 – 35Hz. Neurons are still viable at the end of the study, since they respond to high K+ depolarization. In 2 other ganglia from different surgical cases, it was confirmed that lower frequencies were more sensitive to inhibition than higher frequencies.
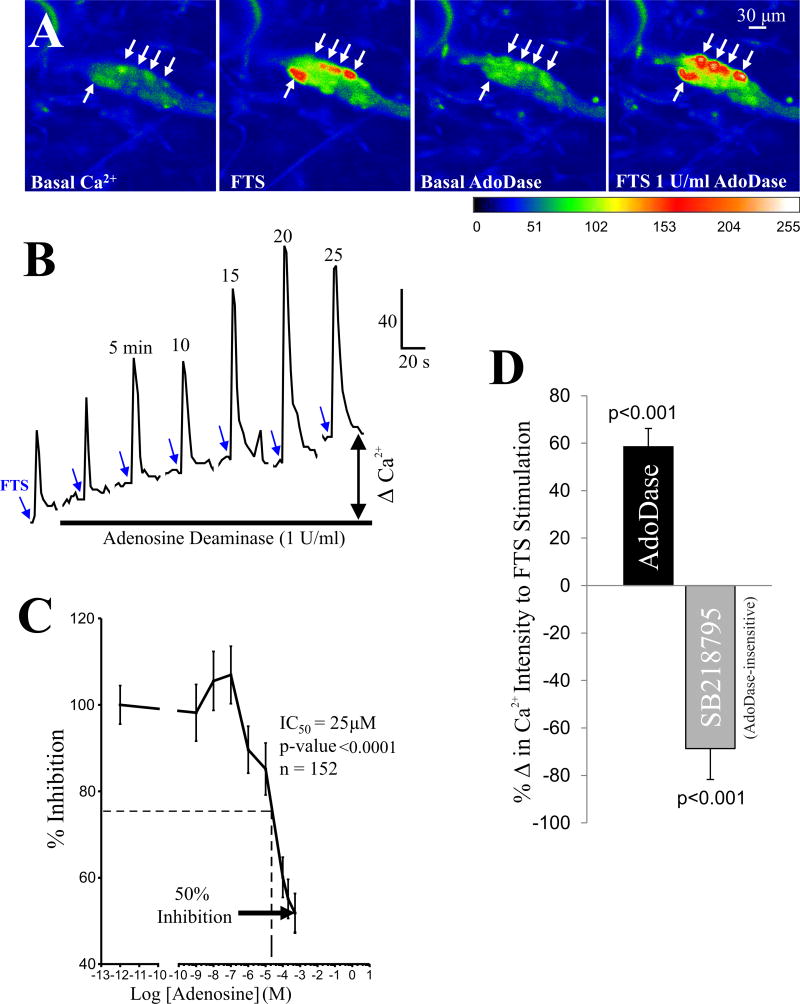
The enzyme adenosine deaminase (AdoDase, that breaks down adenosine to inosine) caused a rise in baseline [Ca2+]i in neurons, and in addition it could augment the peak FTS - Ca2+ response (Figure 11A,B). In other cells, AdoDase only augmented the PSCaT (not shown). AdoDase (0.5–2 U/ml) caused a 58±8% increase in the peak Ca2+ response to FTS in 27 of 78 neurons (35%, p<0.001, 2 tail t-test). In a smaller subset of neurons (14%, 11 of 78), AdoDase suppressed rather than augmented the PSCaT by 45±3% (p<0.05, 2 tail t-test).
Figure 11. Adenosine and adenosine deaminase have opposing effects on synaptic transmission in human submucous ganglia.
(A) Adenosine deaminase facilitates synaptic transmission in response to FTS. Representative images are shown before and after drug application (1U/ml); pseudocolor scale is based on intensity values from 0–255. Arrows indicate responsive cells after FTS or adenosine deaminase. (B) Adenosine deaminase increases baseline Ca2+ levels (e.g. ΔCa2+) and the peak and duration of the PSCaT in response to fiber tract stimulation (at blue arrows). (C) Concentration-inhibition curve to exogenous adenosine on FTS-induced Ca2+ responses. The maximum inhibition of the peak-Ca2+ response is about 50% of at 1mM adenosine. The IC50=25μM. (D) Adenosine deaminase increased the peak calcium response by 58±8% (n=27 of 78 neurons, p <0.001). Neurons that did not respond to adenosine deaminase were sensitive to blockade of synaptic transmission by the NK3 antagonist SB218795 (100nM, (R)-[[2-Phenyl-4-quinolinyl)carbonyl]amino]-methyl ester benzeneacetic acid). SB218795 reduced the synaptic response by 69±13% (in 7 of 25 neurons).
Exogenous adenosine inhibits the PSCaT in a concentration dependent manner, causing 50% maximum inhibition of the peak Ca2+ response (Figure 11C). The apparent IC50 is 25μM (ANOVA, p<0.0001; n=152 neurons). The NK3 antagonist SB218795 (100nM; (R)-[[2-Phenyl-4-quinolinyl)carbonyl]amino]-methylester benzeneacetic acid) was tested on responses in 25 neurons and depressed the synaptic responses in 7 neurons by 69±13% (p<0.01), all of which were insensitive to adenosine deaminase (Figure 11D). SB218795 was ineffective on synaptic responses in the other 18 adenosine deaminase responsive neurons (p>0.05).
MSORT imaging was used to test the effect of exogenous adenosine on fast excitatory postsynaptic potentials (fast EPSPs) in human SMP. The study confirmed that adenosine inhibits fast EPSPs in a reversible manner (Figure 12A).
Inhibition of the PSCaT by exogenous adenosine is sensitive to blockade by the A3 receptor antagonist MRS 1220 (10μM, Figure 12B,C). A concentration of 10 μM MRS1220 can fully reverse the inhibitory effect of 1mM exogenous adenosine on the PSCaT (N=3 patients, 6 ganglia and 49 neurons). MRS1220 (3μM) alone enhanced the peak-Ca2+ responses to FTS at 1–35Hz, while it sometimes revealed a Ca2+ transient at 1Hz or 5Hz (Figures 12D,E). In other words, it enhances the sensitivity of the cell in response to lower frequencies of stimulation, and also causing a leftward shift in the frequency-response curve.
4. Discussion
This study addresses an important gap in our knowledge of purinergic transmission in the human submucous plexus. Neuroimaging demonstrates that endogenous purines are key regulators of neurotransmission in the human submucous plexus. Support comes from pharmacological studies with agonists or antagonists for these receptors, or interventions that alter extra neuronal levels of ATP or adenosine. We found that neurotransmission in human submucous plexus is tightly regulated by ATP-gated P2X ion channels (P2X1, P2X2 and P2X3), inhibitory P2YRs or A3 AR. Data support the concept that purine nucleotides are major neurotransmitters in the human ENS, whereas the ATP metabolite adenosine provides ongoing inhibitory modulation of synaptic transmission.
4.1 Purinergic transmission revealed by manipulating ecto-NTPDase activity
‘Purinergic transmission’ occurs at most synapses in the human ENS. By manipulating endogenous levels of ATP or related nucleotides with apyrase or ARL 67156 we could show that endogenous nucleotides contribute to PSCaTs. Apyrase converts ATP to ADP and AMP (Lavoie et al, 2011). Apyrase blocked a major component of the PSCaT or abolished the response in most responsive neurons. The ecto-ATPase inhibitor ARL 67156 has the opposite effect to apyrase, and it enhances/prolongs the response by inhibiting the hydrolysis of ATP (Levesque et al, 2007). ARL 67156 is a weak inhibitor of human ATPases (NTPDase1, NTPDase3 and NPP1). At concentrations used in our study (50–100μM) it would be expected to only partially inhibit the human form of these enzymes. Indeed, ARL67156 causes a modest but significant enhancement of the PSCaT. These enzymes are therefore likely to be involved in the physiological inactivation of ATP release (or of related nucleotide) in human ENS. The distribution of NTPDases has been characterized only in mouse ENS (Lavoie et al, 2011).
4.2 P2X1, P2X2, P2X3 receptors and their colocalization
Ionotropic P2X purinoceptors exist in the rodent ENS in motor, sensory and secretomotor neurons and glial cells (Burnstock, 2012). Marked species differences exist in the distribution of neural P2X2 and P2X3 receptors in mouse, rat and guinea pig. This is the first study examining the expression, immunochemical distribution and function of P2X receptors in the human SMP. Multiple P2X receptor mRNA transcripts are expressed in the human submucosal plexus preparations – all but P2X6 were found to be expressed.
The selective P2X antagonists TNP-ATP and NF279 and a more general antagonist, PPADS block synaptic transmission in submucous neurons. Data supports the involvement of multiple P2X receptors in the human ENS. Trinitrophenyl-substituted nucleotides such as TNP-ATP are potent antagonists selective for P2X1, P2X3 and heteromeric P2X2/3 receptors at low nanomolar concentrations used in this study. At higher concentrations (5μM), TNP-ATP blocks P2X2 receptors (Virginio et al, 1998; Thomas et al, 1998). Therefore, data with TNP-ATP is consistent with multiple P2X receptor subtypes in the human SMP. Low (20nM) or high (5μM) TNP-ATP concentrations blocked PSCaTs mainly in different subsets of neurons, consistent with 2 different P2X channels in different classes of neurons. The data suggest that P2X2 and P2X1, P2X2/3 or P2X3 are involved in synaptic transmission. The P2X1,2/3,3 agonist α,β-Me-ATP causes Ca2+ responses in submucous neurons. This is also consistent with a P2X1, P2X2/3 or P2X3 receptor activation. Synaptic transmission was blocked by NF279 at 100nM concentration indicating that P2X1 receptors are involved in purinergic transmission. NF279 is a potent and selective P2X1 antagonist with an IC50 of 20nM, requiring 1–300μM concentrations for P2X2, P2X3 and P2X4 receptors (Rettinger et al., 2000).
Findings with P2X antagonists are supported by immunochemical data on the expression, localization and distribution of P2X1, P2X2 and P2X3 receptors. Quantitative analysis provides evidence for expression of each of these receptors in human submucous plexus, with 56% of neurons expressing P2X2, 41% of neurons expressing P2X1 and 14% of neurons expressing P2X3 receptors in ganglia with positive cells for each receptor. Eighty-five percent of ganglia are positive for P2X1 and P2X2 whereas only 38% of ganglia are positive for P2X3 receptors. There are marked species differences that exist in the distribution of P2X2 and P2X3 receptors in mouse, guinea-pig and rat enteric nervous system (Burnstock, 2012). For instance, in guinea-pig ileum submucous plexus, cholinergic neurons express P2X3 receptors in 12% of the submucous neurons (Van Nassauw et al, 2002). In rat submucous plexus P2X3 is expressed 62% of submucous neurons in the ileum (and 40% in colon). Findings in human indicate that P2X3 receptor expression is similar to guinea-pig and very different from rat submucous plexus. P2X3 receptor immunoreactivity was reported for neurons of the myenteric plexus but not submucous plexus of the human (Yiangou et al, 2000). Our study is the first to provide proof for P2X3 in human submucous plexus. Antisera for P2X3 vary considerably in their specificity and affinity, and 4 different antibodies for P2X3 were tested to identify a suitable antibody for P2X3 in our study. In human, the expression of P2X2 in submucous plexus is the same as in the rat with 56% of neurons expressing the receptor. In guinea-pig, P2X2 were demonstrated in non-cholinergic secretomotor neurons (Burnstock, 2012), suggesting a much lower expression than in the other species. Our finding that P2X1 receptors are highly expressed in human submucous plexus is novel in that P2X1 has not been previously reported in submucous neurons in other species. Another distinguishing feature of the human ENS is that P2X1 is colocalized with P2X2 in a majority of submucous neurons. Therefore, it remains unknown whether it represents a species difference or it also occurs in other species.
The P2X2 and P2X3 subunits were identified in rat submucous plexus (Xiang and Burnstock, 2006). In guinea-pig ENS the localization of the P2X3 subunit was largely distinct from that of the P2X2 subunit. Unlike P2X2, the P2X3 subunit is absent from intrinsic sensory neurons and is expressed in secretomotor neurons (Poole et al, 2002). In our study, a small subset (9%) of human submucous neurons may express functional P2X2 and P2X3 subunits in the same neuron, because antagonism with TNP-ATP was additive after consecutive applications of the drug at 20nM and then at 5μM concentrations. In animals, co-existence of P2X2 and P2X3 subunits occurs in nodose ganglia (Thomas et al, 1998), and in some NOS neurons in enteric ganglia (Poole et al, 2002). On average ~ 50% of the PSCaT is sensitive to TNP-ATP indicating that a nucleotide is a co-transmitter with other excitatory transmitter(s) at these synapses.
Our study provides evidence for colocalization of P2X1/P2X2 receptors and P2X2/P2X3 receptors. There are a number of potentially important implications of these findings. First of all, it complicates the pharmacology of P2X receptors in the human ENS. Secondly, P2X subtypes combine as trimers (Nicke et al, 1998) to form functional homo-and heterotrimeric channels (Burnstock, 2007). P2X1/2, P2X1/4, P2X1/5, P2X2/6 heteromultimers have been identified in different species (Saul et al, 2013). Heteromultimeric P2X1/2 receptors show sensitivity to extracellular pH that is unique to this channel (Brown et al, 2001). However, to date, there is no information on the pharmacology of P2X1/2 channels.
ATP can activate P2X receptors between many types of neurons in the ENS. From electrophysiological and lesion studies in animals, it has been shown that some of the P2X receptors are in descending pathways (Bian et al, 2000; Johnson et al, 1999; LePard and Galligan, 1999). More recent studies in submucous plexus of the guinea-pig have identified P2X-mediated fast EPSPs (Monro et al, 2004). Thus far, only P2X2 receptors have been shown to participate in synaptic transmission in mice, although P2X2, P2X3, and P2X5 receptors are expressed in many submucous neurons (Ren et al, 2003).
4.3 Relationship of Ca2+ events recorded during a PSCaT to neuronal activation
A change in intracellular free Ca2+ levels in the postsynaptic neuron is used as a reporter of neural activity. Electrode recordings of Vm in combination with Ca2+ imaging reveals a close correlation between slow/long lasting changes in [Ca2+]i levels and neuronal excitation; an increase in excitation and numbers of action potentials correlates well with the degree of slow increases in [Ca2+]i responses. Fast Ca2+ imaging (≥ 200Hz sampling rate) can reveal peaks that are associated with single spike discharge recorded by consecutive voltage-sensitive dye imaging. Ca2+ peaks required the opening of voltage-gated Na+ and Ca2+ channels as well as Ca2+ release from stores (Michel et al, 2011). In the current study, our sampling rate for LCSM Ca2+ imaging (1–2Hz/sec) was not fast enough to resolve single spike discharge. The PSCaT is a compound/multimodal Ca2+ response evoked by activation of multi-synaptic pathways, associated with neuronal excitability, voltage-operated Ca2+ channels and spike discharge, P2X-channels, nAChR and/or stimulatory P2Y1-metabotropic receptors. At least in guinea-pig submucous neurons, intracellular recordings proved that activation of the P2Y1 causes a slow EPSP (Hu et al, 2003). Such recordings have yet to be done in human submucous plexus. It is also not possible to know where in the polysynaptic pathways drugs applied by superfusion of the SMP act to modulate the neurotransmission associated with the PSCaT.
4.4 Inhibitory P2Y receptors revealed by ADPβS
Earlier studies show that purinergic synaptic transmission involves activation of P2Y1 receptors in guinea pig (Fang et al, 2006; Hu et al, 2003) and human (Wunderlich et al, 2008). In the human ENS, the P2Y1 antagonist MRS2679 was shown to block or reduce synaptic transmission associated with PSCaTs in a subset of neurons. In the current study, we probed further the role of P2Y receptors in the human submucous plexus using ADPβS which can activate P2Y1, P2Y11, P2Y12 and P2Y13. This was a useful tool to evaluate P2Y receptors in the submucous neurons, since P2Y mRNA transcripts were detected in the submucosa for P2Y1, P2Y2, P2Y12, P2Y13 and P2Y14. A novel finding is that use of ADPβS revealed 2 distinct functional types of P2Y receptors on different groups of human submucous neurons. The P2Y receptor subtypes are distinguished by inhibitory or stimulatory responses and a 10-fold difference in potency. Therefore, ADPβS stimulates intracellular free calcium levels in some neurons, whereas it inhibits synaptic transmission in another group of neurons that lack stimulatory post-synaptic calcium responses. The stimulatory response is consistent with a P2Y1 receptor, suggested to be involved in purinergic synaptic transmission in submucous neurons, although other receptors activated by ADPβS cannot be ruled out. The EC50’s reported for ADPβS (0.1μM, 1μM) and concentrations of ADPβS used in this study (0.1–10μM) can activate P2Y11, P2Y12 and P2Y13 receptors as well (Fang et al, 2002).
P2Y1 receptors have been reported to co-localize with VIP (Fang et al, 2006; Wunderlich et al, 2008) (putative secretomotor neurons) in the human submucous plexus, but other types of neurons expressing P2Y1 receptor immunoreactivity remain unknown. Immunoreactivity for P2Y1 was not detected in presynaptic varicose fibers of the human submucous plexus (Wunderlich et al, 2008). However, inhibitory P2Y receptors have been identified at presynaptic sites in the CNS (Köles et al, 2011), and in the ENS, our study in rat submucous plexus identified P2Y1 immunoreactivity only in the cell somas of submucous neurons, whereas P2Y2 and P2Y4 immunoreactivity was expressed in 5–20% of presynaptic varicose fibers labeled for SP, VIP, NPY and CGRP (Christofi et al, 2004). In contrast, P2Y1, P2Y2 and P2Y4 immunoreactivity was absent from varicose fibers in the guinea-pig submucous plexus, and indicating that species differences exist in presynaptic localization of P2Y receptors. Therefore, it remains unknown whether inhibitory P2Y receptors are distributed at pre-or post-synaptic sites in the human submucous plexus. Overall, a detailed pharmacological analysis will be necessary with selective agonists and/or antagonists available for P2Y1, P2Y2, P2Y12, P2Y13 receptors to resolve the identity of the stimulatory or inhibitory receptors in chemically identified neurons. Immunochemical identification can provide important clues as to the pre-or post-synaptic localization of the 2 distinct P2Y receptor subtypes existing in human submucous neurons.
4.5 Inhibitory modulation by endogenous adenosine
Our study further revealed that eADO is a critical inhibitory modulator of neurotransmission in the human ENS. This is evident from studies with enzyme(s) or nucleoside uptake inhibitors that serve to elevate or decrease extracellular levels of adenosine. AdoDase, which converts eADO to its inactive metabolite inosine, can cause a remarkable increase in [Ca2+]i in neurons and/or augment the PSCaT. Blockade of endogenous AdoDase with EHNA combined with inhibition of nucleoside uptake could block synaptic transmission in the lower frequency range 0.1 – 10 Hz. Such mechanisms tightly regulate the levels of extracellular free eADO near the synapses. Our findings also suggest that eADO release at rest or during transmitter release exerts an inhibitory tone (modulation) on human submucosal neurons. In guinea-pigs, eADO exerts an ongoing inhibitory tone on neurons to modulate their excitability, fast and slow excitatory postsynaptic potentials (EPSPs; Christofi and Wood, 1993). Our MSORT study confirmed that adenosine suppresses the fast EPSP in human submucous ganglia as it does in the guinea-pig (Christofi & Wood, 1993). Alterations in purinergic fast EPSPs may be important in disease states (Ren and Bertrand, 2008).
Exogenous adenosine also inhibits the PSCaT in a concentration dependent manner, but it could only block 50% of the peak response indicating that adenosine is an inhibitory modulator at some, but not all, excitatory synapses in human submucous plexus. This is likely the case since the FTS stimulation used to elicit the PSCaT (e.g. a 3 sec, 25Hz frequency train stimulus) activates polysynaptic pathways in submucous ganglia. In guinea-pig small intestine, adenosine inhibits the excitatory actions of certain transmitters and mediators on neurons (e.g. VIP, GRP, histamine, CCK) but not those of others including tachykinins (e.g. SP) (Palmer et al, 1987; Christofi, 2001). Our findings with the NK3 antagonist show that endogenous adenosine (revealed by adenosine deaminase) operates at synapses where tachykinergic transmission is not involved. Therefore, we were able to show that if adenosine deaminase had an effect on synaptic transmission, the NK3 antagonist could not block synaptic transmission suggesting that tachykinergic transmission is not involved. The converse was true, so that at synapses where adenosine deaminase did not enhance PSCaTs (indicating that endogenous adenosine is not involved) the NK3 antagonist could block synaptic transmission.
The apparent EC50 for adenosine is 25μM consistent with a low affinity A3 receptor. We also show that a selective antagonist at A3 receptors (MRS1220) can reverse or block the effect of adenosine, extending our earlier observations with an A3 agonist Cl-IBMECA (Wunderlich et al, 2008). In rodents, adenosine is released during mucosal reflexes to inhibit the 5-HT and prostaglandin limbs of the secretomotor reflex (Cooke et al, 1999). In an in vitro guinea-pig model of ‘neurogenic diarrhea’, endogenous adenosine provides ongoing inhibitory tone via A3 receptors of the secretomotor responses triggered by histamine at H2 receptors on submucosal neurons (Bozarov et al, 2009).
4.6 Purinergic mediators involved in neurotransmission
Our study did not monitor release of purine nucleotides, and therefore we cannot draw definitive conclusions about which nucleotides or metabolites are involved in purinergic transmission in human submucosal ganglia. However, our results provide some insight. ATP or a related nucleotide is probably the transmitter at some synapses, because PSCaTs are sensitive to NTPDase1 (apyrase) or NTPDase1 inhibitor ARL67156, which have opposing effect on ATP metabolism to ADP or AMP. Furthermore, adenosine is probably the ATP metabolite involved in A3 inhibitory modulation of synaptic transmission, in keeping with the effects of adenosine deaminase, EHNA (adenosine deaminase inhibitor), NBTI (nucleoside uptake inhibitor), and the selective adenosine A3 antagonist MRS 1220; exogenous adenosine mimics actions of endogenous adenosine release. ADP is reportedly the cognate ligand for P2Y1, P2Y11 and P2Y12 receptors, and activation of P2Y1 receptors is involved in PSCaTs at some synapses (Wunderlich et al, 2008). It is unlikely that UDP is involved in neurotransmission since it is the cognate ligand for P2Y6 for which RT-PCR failed to detect mRNA expression for this receptor in SMP. P2X receptors are ATP-gated ion channels that have high affinity for the cognate ligand ATP, and therefore ATP is a primary candidate for activating these channels. Our study provided proof for P2X1, P2X2 and P2X3 receptors.
However, our study has not investigated other purine nucleotides such as ADP-ribose (ADPR) or its precursor β-nicotinamide adenine dinucleotide (β-NAD+(Durnin et al, 2013; Hwang et al, 2011; Hwang et al, 2012; Gallego et al, 2011). The direct actions of these mediators (β-NAD+ or ADPR) or their involvement in ‘purinergic transmission’ in the human enteric nervous system have yet to be determined.
5. Conclusions and inferences
Findings from neuroimaging studies support the hypothesis that ATP-gated P2X1, P2X2 and P2X3 channels contribute to excitatory purinergic signaling or neurotransmission in human submucous plexus. Adenosine A3 and P2Y receptors provide inhibitory modulation in human ENS. Inactivation mechanisms exist for regulating actions of endogenous purines. Together, findings from pharmacological and immunochemical studies support the hypothesis that several types of neurons exist for functional P2X receptor subtypes with the possibility that heteromeric trimers can form. Numbers of neurons with P2XR immunoreactivity follows the order P2X2>P2X1≫P2X3. Four types of neurons were identified based on their expression of P2X receptor subtypes: those with P2X1 alone, P2X2 alone, P2X1+ P2X2 and P2X3+P2X2. Data suggest P2X2/3 heteromeric receptors as well. The level of expression of P2X1 and co-localization of P2X1+P2X2 are novel findings not previously reported for other species. Stimulatory and inhibitory P2Y receptors exist on submucous neurons. A systematic analysis will be required to further study P2X and P2Y receptor mechanisms and the neurochemistry of neurons expressing them. NK3 and A3 mechanisms operate in different types of neurons. Overall, our study shows that endogenous nucleotides, adenosine, tachykinin(s) and ACh are key regulators of neurotransmission in human SMP. Such diversity in the neurochemistry of the synapses suggests a very complex neural circuit behavior in human submucous plexus.
The neurophysiological role of purinergic receptors in the human submucous plexus remains to be studied. In keeping with animal studies, it is likely that these receptors are involved in sensory afferent pathways in gut reflexes, neurosecretion and coordination of motility and secretion reflexes (Antonioli et al, 2008; Burnstock, 2008; Christofi, 2008). Purines have been described to act as ‘danger signals’ in diseases (Elitzschig et al, 2012), purine gene expression profiles are very sensitive to inflammation and signaling pathways may serve as potential ‘biomarkers of disease’ (Rybaczyk et al, 2009). One or more neural receptors identified in our study (P2X1, P2X2, P2X3 channels, A3, inhibitory P2Y, stimulatory P2Y1/other receptors) are potential novel targets of investigation in gastrointestinal diseases, given the therapeutic potential of purinergic targets in GI diseases or disorders (Antonioli et al, 2008; Bozarov et al, 2009; Burnstock, 2008; Cockayne et al, 2000; Elitzschig et al, 2012; Guzman et al, 2006; Linan-Rico et al, 2013; Ren et al, 2011).
Supplementary Material
Table 1 (Supplemental) Antibodies used for immunochemical staining of human submucous neurons
Table 2 (Supplemental) Distribution of P2X1, P2X2, and P2X3 receptors in human submucous neurons
Table 3 (Supplemental) % of submucous ganglia with positive “P2X” cells
Table 4 (Supplemental) Primers for P2X and P2Y receptor transcripts
Purinergic receptors are potential therapeutic targets for IBD and IBS
Optical recording techniques made it possible to study human ENS neuropharmacology
A comprehensive analysis was done in 1,520 submucous neurons from 104 human surgical specimens
Novel neuronal targets identified are P2X1, P2X1 and P2X2 co-localization, P2X3, P2X2/3, P2Y and A3
Purines are critical regulators of neurotransmission at several synapses in human enteric nervous system
Acknowledgments
Grant support from the National Institutes of Health on NIH DK093499-01, DK044179:11-15 and NCRR S10RR11434 to F.L.C; 5K08 DK078035 to K.W., strategic initiative funds from the Department of Anesthesiology & College of Medicine at OSU to F.L.C.
Abbreviations
- ARL67156
6-N,N-diethyl-D-β,γ-dibromomethyleneATP
- ATP
adenosine 5′-triphosphate
- Ca2+
calcium
- [Ca2+]i
intracellular free calcium concentration
- cDNA
cyclic deoxyribonucleic acid
- eADO
endogenous adenosine
- EHNA
erythro-9-Amino-β-hexyl-α-methyl-9H-purine-9-ethanol hydrochloride
- ENS
enteric nervous system
- fEPSPs
fast excitatory synaptic potentials
- FITC
Fluoresceinthiocyanate
- FTS
fiber tract stimulation
- hr
hour
- [Ca2+]i
intracellular free Ca2+ levels
- IBD
inflammatory bowel diseases
- IBS
irritable bowel syndrome
- IP3
inositol-1,4,5-triphosphate
- LSCM
laser scanning confocal microscope
- mRNA
messenger ribonucleic acid
- MSORT
multi-site optical recording technique
- nAChR
nicotinic ACh receptor
- NBTI
N6-nitrobenzyl-thioinosine
- PSCaT
postsynaptic calcium transient
- PBS
phosphate buffer solution
- R(s)
receptor(s)
- RT-PCR
reverse transcription-polymerase chain reaction
- SB218795
(R)-[[2-Phenyl-4-quinolinyl)carbonyl]amino]-methyl ester benzeneacetic acid
- sec
second
- SEM
standard error of the mean
- TNP-ATP
2′3′-o-(2,4,6-trinitrophenyl)-ATP
- TK
tachykinin
Footnotes
Contribution:
Andrómeda Liñán-Rico, PhD Design and perform purinergic Ca2+imaging experiments and RT-PCR on P2X and P2Y mRNA transcripts, contributed to analysis and interpretation of data; contributed to the writing of the article for submission.
Jacqueline Wunderlich, MD, PhD Design and perform FTS - Ca2+experiments, and analyze data on human SMP
Joshua Enneking and Daniel R Tso, Research Assistants Application of co-labeling techniques for P2X1, P2X2, and P2X3 receptors in whole mount tissues; data analysis and interpretation.
Iveta Grants, B.Sc. Collection, analysis and interpretation of data in calcium experiments, created all figures for the manuscript, manuscript writing/editing and submission.
Kent Williams, MD Contributed to the concepts and writing of the manuscript
Andrew Otey, Clinical research coordinator Patient consent, coordinated with surgery and clinical pathology and tissue procurement. Develop a standard approach for procuring viable tissues to be used for calcium imaging studies.
Klaus Michel, PhD design and performance of studies with voltage sensitive dyes, writing on MSORT study.
Michael Schemann, PhD Contributed to the intellectual design and performance of studies on synaptic transmission
Alan Harzman, MD and Bradley Needleman, MD Insure the procurement of viable human tissue from surgical cases to use in calcium neuroimaging studies and RT-PCR analysis. Contribute to the conception of experiments.
Fievos L. Christofi, PhD, AGAF, Principal Investigator on the NIH funded study. Conception and design of experiments, performed some experiments, writing the article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Del Tacca M, et al. Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol Ther. 2008;120:233–253. doi: 10.1016/j.pharmthera.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Bian X, Bertrand PP, Bornstein JC. Descending inhibitory reflexes involve P2X receptor-mediated transmission from interneurons to motor neurons in guinea-pig ileum. J Physiol. 2000;528:551–560. doi: 10.1111/j.1469-7793.2000.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarov A, Wang YZ, Yu JG, Wunderlich J, Hassanain HH, Alhaj M, et al. Activation of adenosine low-affinity A3 receptors inhibits the enteric short interplexus neural circuit triggered by histamine. Am J Gastrointest Liver Physiol. 2009;29:1147–1162. doi: 10.1152/ajpgi.00295.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig E, Michel K, Zeller F, Seidl S, Weyhern CW, Schemann M. Histamine excites neurones in the human submucous plexus through activation of H1, H2, H3 and H4 receptors. J Physiol. 2007;583:731–742. doi: 10.1113/jphysiol.2007.139352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SG, Townsend-Nicholson A, Jacobson KA, Burnstock G, King BF. Heteromultimeric P2X(1/2) receptors show a novel sensitivity to extracellular pH. J Pharmacol Exp Ther. 2002;300:673–680. doi: 10.1124/jpet.300.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic receptors as future targets for treatment of functional GI disorders. Gut. 2008;57:1193–1194. doi: 10.1136/gut.2008.151134. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic mechanosensory transduction and visceral pain. Mol Pain. 2009;305:69. doi: 10.1186/1744-8069-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. P2X receptors in the gut. WIREs Membr Transp Signal. 2012;1:269–279. [Google Scholar]
- Burnstock G, Satchell DG, Smythe A. A comparison of the excitatory and inhibitory effects of non-adrenergic, non-cholinergic nerve stimulation and exogenously applied ATP on a variety of smooth muscle preparations from different vertebrate species. Br J Pharmacol. 1972;46:234–242. doi: 10.1111/j.1476-5381.1972.tb06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofi FL. Unlocking mysteries of gut sensory transmission: is adenosine the key? News Physiol Sci. 2001;6:201–207. doi: 10.1152/physiologyonline.2001.16.5.201. [DOI] [PubMed] [Google Scholar]
- Christofi FL. Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signal. 2008;4:213–236. doi: 10.1007/s11302-008-9104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofi FL, Wood JD. Presynaptic inhibition by adenosine A1 receptors on guinea-pig small intestinal myenteric neurons. Gastroenterology. 1993;104:1420–1429. doi: 10.1016/0016-5085(93)90351-c. [DOI] [PubMed] [Google Scholar]
- Christofi FL, Wunderlich J, Yu JG, Wang YZ, Xue J, Guzman J, et al. Mechanically evoked reflex electrogenic chloride secretion in rat distal colon is triggered by endogenous nucleotides acting at P2Y1, P2Y2, and P2Y4 receptors. J Comp Neurol. 2004;469:16–36. doi: 10.1002/cne.10961. [DOI] [PubMed] [Google Scholar]
- Cirillo C, Tack J, Vanden Berghe P. Nerve activity recordings in routine human intestinal biopsies. Gut. 2013;62:227–235. doi: 10.1136/gutjnl-2011-301777. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Wang YZ, Liu CJ, Zhang H, Christofi FL. Activation of Neuronal Adenosine A1 receptors suppresses secretory reflexes in the guinea-pig colon. Am J Physiol Gastrointest Liver Physiol. 1999;276:G451–G462. doi: 10.1152/ajpgi.1999.276.2.G451. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Xue J, Yu JG, Wunderlich J, Wang YZ, Guzman J, et al. Mechanical stimulation releases nucleotides that activate P2Y1 receptors to trigger neural reflex chloride secretion in guinea pig distal colon. J Comp Neurol. 2004;469:1–15. doi: 10.1002/cne.10960. [DOI] [PubMed] [Google Scholar]
- Durnin L, Hwang SJ, Ward SM, Sanders KM, Mutafova-Yambolieva VN. Adenosine 5-diphosphate-ribose is a neural regulator in primate and murine large intestine along with β-NAD(+) J Physiol. 2012;590:1921–1941. doi: 10.1113/jphysiol.2011.222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Hu HZ, Gao N, Liu S, Wang GD, Wang XY, et al. Neurogenic secretion mediated by the purinergic P2Y1 receptor in guinea-pig small intestine. Eur J Pharmacol. 2006;536:113–122. doi: 10.1016/j.ejphar.2006.02.040. [DOI] [PubMed] [Google Scholar]
- Furness JB, Young HM, Pompolo S, Bornstein JC, Kunze WA, McConalogue K. Plurichemical transmission and chemical coding of neurons in the digestive tract. Gastroenterology. 1995;108:554–563. doi: 10.1016/0016-5085(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Gallego D, Gil V, Aleu J, Martinez-Cutillas M, Clavé P, Jimenez M. Pharmacological characterization of purinergic inhibitory neuromuscular transmission in the human colon. Neurogastroenterol Motil. 2011;23:792–e338. doi: 10.1111/j.1365-2982.2011.01725.x. [DOI] [PubMed] [Google Scholar]
- Gomes P, Chevalier J, Boesmans W, Roosen L, van den Abbeel V, Neunlist M, et al. ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterol Motil. 2009;21:870–862. doi: 10.1111/j.1365-2982.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136:349–358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- Guzman J, Yu JG, Suntres Z, Bozarov A, Cooke H, Javed N, et al. ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm Bowel Dis. 2006;12:766–789. doi: 10.1097/00054725-200608000-00014. [DOI] [PubMed] [Google Scholar]
- Hu HZ, Gao N, Zhu MX, Liu S, Ren J, Gao C, et al. Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol. 2003;15:493–504. doi: 10.1113/jphysiol.2003.041731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, et al. β-nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology. 2011;140:608–617. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol. 2012;590:1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PJ, Shum OR, Thornton PD, Bornstein JC. Evidence that inhibitory motor neurons of the guinea-pig small intestine exhibit fast excitatory synaptic potentials mediated via P2X receptors. Neurosci Lett. 1999;266:169–172. doi: 10.1016/s0304-3940(99)00275-x. [DOI] [PubMed] [Google Scholar]
- Kao WY, Davis CE, Kim YI, Beach JM. Fluorescence emission spectral shift measurements of membrane potential in single cells. Biophys J. 2001;81:1163–1170. doi: 10.1016/S0006-3495(01)75773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köles L, Leichsenring A, Rubini P, Illes P. P2 receptor signaling in neurons and glial cells of the central nervous system. Adv Pharmacol. 2011;61:441–493. doi: 10.1016/B978-0-12-385526-8.00014-X. [DOI] [PubMed] [Google Scholar]
- LePard KJ, Galligan JJ. Analysis of fast synaptic pathways in myenteric plexus of guinea pig ileum. Am J Physiol. 1999;276:G529–G538. doi: 10.1152/ajpgi.1999.276.2.G529. [DOI] [PubMed] [Google Scholar]
- Lavoie EG, Gulbransen BD, Martín-Satué M, Aliagas E, Sharkey KA, Sévigny J. Ectonucleotidases in the digestive system: focus on NTPDase3 localization. Am J Physiol Gastrointest Liver Physiol. 2011;300:G608–G620. doi: 10.1152/ajpgi.00207.2010. [DOI] [PubMed] [Google Scholar]
- Lévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Sévigny J. Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br J Pharmacol. 2007;152:141–150. doi: 10.1038/sj.bjp.0707361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liñán-Rico A, Wunderlich JE, Grants IS, Frankel WL, Xue J, Williams KC, et al. Purinergic autocrine regulation of mechanosensitivity and serotonina release in a Human EC model: ATP-gated P2X3 channels in EC are downregulated in Ulcerative Colitis. Inflamm Bowel Dis. 2013 doi: 10.1097/MIB.0b013e31829ecf4d. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel K, Michaelis M, Mazzuoli G, Mueller K, Vanden Berghe P, Schemann M. Fast calcium and voltage-sensitive dye imaging in enteric neurones reveal calcium peaks associated with single action potential discharge. J Physiol. 2011;589:5941–5947. doi: 10.1113/jphysiol.2011.219550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel K, Zeller F, Langer R, Nekarda H, Kruger D, Dover TJ, et al. Serotonin excites neurons in the human submucous plexus via 5-HT3 receptors. Gastroenterology. 2005;128:1317–1326. doi: 10.1053/j.gastro.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Monro RL, Bertrand PP, Bornstein JC. ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J Physiol. 2004;556:571–584. doi: 10.1113/jphysiol.2004.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Peters S, Schemann M. Multisite optical recording of excitability in the enteric nervous system. Neurogastroenterol Motil. 1999;11:393–402. doi: 10.1046/j.1365-2982.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Nicke A, Bäumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, et al. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JM, Wood JD, Zafirov DH. Purinergic inhibition in the small intestinal myenteric plexus of the guinea-pig. J Physiol. 1987;387:357–369. doi: 10.1113/jphysiol.1987.sp016577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB. The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci. 2002;101:39–47. doi: 10.1016/s1566-0702(02)00179-0. [DOI] [PubMed] [Google Scholar]
- Ren J, Bertrand PP. Purinergic receptors and synaptic transmission in enteric neurons. Purinergic Signal. 2008;4:255–266. doi: 10.1007/s11302-007-9088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Bian X, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, et al. P2X2 subunits contribute to fast synaptic excitation in myenteric neurons of the mouse small intestine. J Physiol. 2003;552:809–821. doi: 10.1113/jphysiol.2003.047944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T, Grants I, Alhaj M, McKiernan M, Jacobson M, Hassanain HH, et al. Impact of disrupting adenosine A3 receptors (A3−/−AR) on colonic motility or progression of colitis in the mouse. Inflamm Bowel Dis. 2011;17:1698–1713. doi: 10.1002/ibd.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettinger J, Schmalzing G, Damer S, Müller G, Nickel P, Lambrecht G. The suramin analogue NF279 is a novel and potent antagonist selective for the P2X(1) receptor. Neuropharmacology. 2000;39:2044–2053. doi: 10.1016/s0028-3908(00)00022-8. [DOI] [PubMed] [Google Scholar]
- Rybaczyk L, Rozmiarek A, Circle K, Grants I, Needleman B, Wunderlich JE, et al. New bioinformatics approach to analyze gene expressions and signaling pathways reveals unique purine gene dysregulation profiles that distinguish between CD and UC. Inflamm Bowel Dis. 2009;15:971–984. doi: 10.1002/ibd.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul A, Hausmann R, Kless A, Nicke A. Heteromeric assembly of P2X subunits. Front Cell Neurosci. 2013;7:250. doi: 10.3389/fncel.2013.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemann M, Hafsi N, Michel K, Kober OI, Wollmann J, Li Q, et al. The beta3-adrenoceptor agonist GW427353 (Solabegron) decreases excitability of human enteric neurons via release of somatostatin. Gastroenterology. 2010;138:266–274. doi: 10.1053/j.gastro.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Schemann M, Michel K, Peters S, Bischoff SC, Neunlist M. Cutting-edge technology. III. Imaging and the gastrointestinal tract: mapping the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2002;282:919–G925. doi: 10.1152/ajpgi.00043.2002. [DOI] [PubMed] [Google Scholar]
- Schemann M, Neunlist M. The human enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 1):55–59. doi: 10.1111/j.1743-3150.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Thomas S, Virginio C, North RA, Surprenant A. The antagonist trinitrophenyl-ATP reveals coexistence of distinct P2X receptor channels in rat nodose neurones. J Physiol. 1998;509:411–417. doi: 10.1111/j.1469-7793.1998.411bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans JP, Hens J, Adriaensen D. Outer submucous plexus: an intrinsic nerve network involved in both secretory and motility processes in the intestine of large mammals and humans. Anat Rec. 2001;262:71–78. doi: 10.1002/1097-0185(20010101)262:1<71::AID-AR1012>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Van Nassauw L1, Brouns I, Adriaensen D, Burnstock G, Timmermans JP. Neurochemical identification of enteric neurons expressing P2X(3) receptors in the guinea-pig ileum. Histochem Cell Biol. 2002;118:193–203. doi: 10.1007/s00418-002-0447-6. [DOI] [PubMed] [Google Scholar]
- Virginio C, Robertson G, Surprenant A, North RA. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3, and heteromeric P2X2/3 receptors. Mol Pharmacol. 1998;53:969–973. [PubMed] [Google Scholar]
- Wunderlich JE, Needleman BJ, Chen Z, Yu JG, Wang Y, Grants I, et al. Dual purinergic synaptic transmission in the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2008;294:G554–G566. doi: 10.1152/ajpgi.00500.2007. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Baecker PA, Ford AP, Knowles CH, Chan CL, et al. ATP-gated ion channel P2X(3) is increased in human inflammatory bowel disease. Neurogastroenterol Motil. 2001;13:365–369. doi: 10.1046/j.1365-2982.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Burnstock G. Distribution of P2Y6 and P2Y12 receptor: their colocalization with calbindin, calretinin and nitric oxide synthase in the guinea pig enteric nervous system. Histochem Cell Biol. 2006;125:327–336. doi: 10.1007/s00418-005-0071-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1 (Supplemental) Antibodies used for immunochemical staining of human submucous neurons
Table 2 (Supplemental) Distribution of P2X1, P2X2, and P2X3 receptors in human submucous neurons
Table 3 (Supplemental) % of submucous ganglia with positive “P2X” cells
Table 4 (Supplemental) Primers for P2X and P2Y receptor transcripts