Abstract
Tetramethylenedisulfotetramine (TETS) is a potent convulsant GABAA receptor blocker. Mice receiving a lethal dose of TETS (0.15 mg/kg i.p.) are rescued from death by a high dose of diazepam (5 mg/kg i.p.) administered shortly after the second clonic seizure (∼20 min post-TETS). However, this high dose of diazepam significantly impairs blood pressure and mobility, and does not prevent TETS-induced neuroinflammation in the brain. We previously demonstrated that TETS alters synchronous Ca2+ oscillations in primary mouse hippocampal neuronal cell cultures and that pretreatment with the combination of diazepam and allopregnanolone at concentrations having negligible effects individually prevents TETS effects on intracellular Ca2+ dynamics. Here, we show that treatment with diazepam and allopregnanolone (0.1 μM) 20 min after TETS challenge normalizes synchronous Ca2+ oscillations when added in combination but not when added singly. Similarly, doses (0.03–0.1 mg/kg i.p.) of diazepam and allopregnanolone that provide minimal protection when administered singly to TETS intoxicated mice increase survival from 10% to 90% when given in combination either 10 min prior to TETS or following the second clonic seizure. This therapeutic combination has negligible effects on blood pressure or mobility. Combined treatment with diazepam and allopregnanolone also decreases TETS-induced microglial activation. Diazepam and allopregnanolone have distinct actions as positive allosteric modulators of GABAA receptors that in combination enhance survival and mitigate neuropathology following TETS intoxication without the adverse side effects associated with high dose benzodiazepines. Combination therapy with a benzodiazepine and neurosteroid represents a novel neurotherapeutic strategy with potentially broad application.
Keywords: allopregnanolone, combination therapy, diazepam, neurosteroid, seizures, tetramethylenedisulfotetramine
1. Introduction
Tetramethylenedisulfotetramine (TETS) is a highly lethal convulsant toxicant that is believed to induce seizures by blocking GABAA receptors in the central nervous system (Banks et al., 2014). Tens of thousands of deaths due to TETS poisoning have been documented, primarily in China (Li et al., 2014), and there is significant concern regarding its potential use as a chemical threat agent outside of China (Whitlow et al., 2005; Jett and Yeung, 2010). There is no established antidote for TETS intoxication in humans. GABAA receptor positive modulators, including benzodiazepines (Olsen, 1981) and neurosteroids (Belelli et al., 1989; Kokate et al., 1994; Wieland et al., 1995; Czlonkowska et al., 2000; Singh et al., 2010), protect against seizures induced by other GABAA receptor antagonists, such as picrotoxin, bicuculline and pentylenetetrazol (Meldrum and Rogawski, 2007). Experimental evidence suggests that benzodiazepines provide some protection against TETS-induced seizures. For example, in mice, treatment with a high (5 mg/kg) dose of diazepam following exposure to a lethal dose of TETS reduced seizure manifestations and prevented lethality (Shakarjian et al., 2012; Vito et al., 2014), but did not eliminate ictal activity in the electroencephalogram (Shakarjian et al., 2012) or mitigate neuroinflammatory responses (Vito et al., 2014). Benzodiazepines are the standard-of-care treatment for acute seizures and high doses of diazepam or another benzodiazepine are often administered to humans experiencing TETS-induced seizures (Whitlow et al., 2005). However, the few available anecdotal reports indicate that benzodiazepines alone do not effectively terminate established TETS-induced seizures in humans or prevent subsequent neurologic deficits (Barrueto et al., 2003; Chau et al., 2005; Whitlow et al., 2005; Deng et al., 2012; Li et al., 2012).
As part of a screen to identify improved treatment approaches for TETS intoxication, we have studied the action of GABAA receptor positive modulators on TETS-evoked physiological responses in an in vitro tissue culture system. Primary cultures of mouse hippocampal neurons exhibit synchronous Ca2+oscillations (Cao et al., 2012). These synchronous Ca2+ oscillations (SCOs) are dependent upon action potentials, as they are eliminated by tetrodotoxin (Ogura et al., 1987), and require glutamate-mediated synaptic transmission, as they are suppressed by AMPA and to a lesser extent by NMDA receptor antagonists (Tanaka et al., 1996; Dravid and Murray, 2004; Cao et al., 2012). We previously reported that addition of TETS to such cultures triggers an immediate rise in intracellular Ca2+, which is followed by decrease in the frequency and an increase in the amplitude of Ca2+ oscillations (Cao et al., 2012). Pretreatment with diazepam (0.03–1 μM) partially counteracted the effect of TETS, but a major reversal only occurred at the highest concentration tested (1 μM). Similarly, allopregnanolone (0.03–1 μM), a neurosteroid that acts as a positive allosteric modulator of GABAA receptors (Reddy and Rogawski, 2012), also partially mitigated the action of TETS, but a pronounced effect only occurred at 1 μM. In contrast, pretreatment with the combination of diazepam and allopregnanolone each at concentrations of 0.1 μM completely blocked the action of TETS. These results raised the possibility that diazepam and allopregnanolone act synergistically as a treatment for TETS poisoning. In the present study we further investigated this combination treatment, and report that diazepam and allopregnanolone administered after TETS exposure protects against the action of TETS on cultured hippocampal neurons and that the synergy observed in the in vitro model extends to in vivo therapeutic advantages in mice. We further demonstrate that the combination does not exhibit the excessive sedation and hypotension that limit treatment with high dose diazepam (Kitajima et al., 2004).
2. Materials and methods
2.1. Chemicals
TETS was synthesized as previously described (Zolkowska et al., 2012). A final recrystallization step was performed to ensure no water remained in the crystals. The final product was estimated to be > 98% pure by gas chromatography-mass spectrometry with total ion monitoring. TETS was dissolved in 100% DMSO and stored as stock solutions at 10 mg/ml. USP grade diazepam in 40% propylene glycol, 10% alcohol, 5% sodium benzoate and 1.5% benzyl alcohol manufactured by Hospira was purchased from Western Medical Supply (Arcata, CA). Allopregnanolone was synthesized by SAFC Pharma (Madison, WI) and characterized as >99% pure by HPLC. 2-Hydroxypropyl-β-cyclodextrin was from Sigma-Aldrich (St Louis, MO). Paraformaldehyde, sulfamide, hydrochloric acid, acetone, and hexane of the highest purity available were obtained from Thermo Fisher Scientific (Waltham, MA). The Ca2+ fluorescence dye Fluo-4 was purchased from Life Technology (Grand Island, NY). GFAP antibody was from Dako Chemical (Carpentaria, CA); Iba-1 antibody, from Wako Chemical (Richmond, VA); and Fluoro-Jade B, from Millipore (Billerica, MA).
2.2. Animals
Animals were maintained in facilities fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all experiments involving animals were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023) as adopted and promulgated by the U.S. National Institutes of Health and as approved by the Institutional Animal Care and Use Committee of the University of California, Davis. Young adult (6-8 wk old) male NIH Swiss mice (22–25 g) were obtained from the Animal Resource Program, Center for Cancer Research, National Cancer Institute (Bethesda, MD) and were housed four per cage. All animals were kept in a vivarium under controlled environmental conditions (22–26 °C; 40– 50% humidity) with a norm al 12 h light/dark cycle and free access to food and water. Animals were allowed to acclimate to the vivarium for at least 5 d prior to experimentation. Experiments were performed during the light phase of the light/dark cycle after at least 30 min of acclimation to the laboratory setting.
2.3. Dosing paradigm
On the day of the experiment, stock solutions of TETS were sequentially diluted in 10% DMSO in saline at 40-60 °C to a final concentration of 0.015 mg/ml in 10% DMSO. TETS solutions were kept at 35-36 °C and administered in traperitoneally (i.p.) in a volume of 10 ml/kg. Stock concentrations of diazepam (5 mg/ml) were diluted in 10% DMSO in saline to 0.01 or 0.003 mg/ml. Allopregnanolone was dissolved to a stock concentration of 10 mg/ml in 40% 2-hydroxypropyl-β-cyclodextrin in saline and further diluted in 10% DMSO in saline to 0.01 and 0.003 mg/ml. In studies assessing the efficacy of pretreatment with diazepam and/or allopregnanolone, mice were injected with diazepam (0.1 mg/kg i.p.), allopregnanolone (0.1 mg/kg i.p.) or a combination of the two drugs (total volume of 5 ml/kg) 10 min prior to receiving TETS (0.15 mg/kg i.p.). In studies assessing the efficacy of diazepam and/or allopregnanolone when administered after mice had been exposed to TETS, diazepam (0.03 mg/kg i.p.) and/or allopregnanolone (0.03 mg/kg i.p.) were administered within 2 min after the second clonic seizure. Immediately after the TETS injection, animals were observed for 1 h for seizure behavior, and the latency to seizure onset and duration of each seizure were recorded for each animal as previously described (Zolkowska et al., 2012).
2.4. Measurement of Ca2+ dynamics and SCO patterns in cultured hippocampal neurons
Primary cultures of dissociated hippocampal neurons were derived from C57BL/6J mice of mixed sex at postnatal day 0-1 and maintained in Neurobasal medium (Invitrogen, Life Technologies, Carlsbad, CA) supplemented with 5% fetal bovine serum (Atlanta Biologicals, Atlanta, GA) as previously described (Cao et al., 2012). Hippocampal neurons between 13-17 days in vitro (DIV) were used to investigate TETS-induced Ca2+ responses and the influence of diazepam and allopregnanolone singly or in combination on TETS-induced Ca2+ dysregulation. Intracellular Ca2+ levels were measured simultaneously in all wells of a 96-well plate using FLIPRTETRA as described previously (Cao et al., 2012). After recording the basal Ca2+ oscillations for 2 min, TETS (3 μM) or vehicle (0.01 % DMSO) were added to the cultured hippocampal neurons and the response recorded for 20 min. Diazepam and allopregnanolone singly or in combination were added 20 min after the addition of TETS and the response recorded for an additional 20 min.
2.5. Measurement of blood pressure
Blood pressure was measured in mice using a CODA non-invasive blood measuring system from Kent Scientific (Torrington, CT). This system employs volume pressure recording (VPR) technology to detect changes that correspond to systolic and diastolic blood pressures. As specified by the manufacturer, the minimum and maximum values that can be measured with this system according to the manufacturer are 50-150 mm. Blood pressure below 50 mm Hg does not produce sufficient blood flow through the tail to obtain an accurate reading. Mice were acclimated to the restraining device for 30 min per d for 6 d to ensure a stable blood pressure baseline prior to drug administration. Blood pressure values represent the average of 20 measurements of 30 sec each with a 10 sec delay between each measurement. On the day of the experiment, BP measurements were initiated 5 min after drug administration to allow the animal's BP to stabilize after the injection of the drug and to provide time for the drugs to distribute to the brain. Data are presented as the mean blood pressure in mm Hg.
2.6. Open field activity
Horizontal locomotion was assessed as an indication of sedation using the open field test (Silverman et al., 2011; Yang et al., 2012). Mice were placed in the middle of an acrylic chamber (40 cm long, 40 cm wide and 20 cm high), the floor of which was marked off into 100 cm2 (10 cm × 10 cm) boxes. The number of crossings (both hind paws crossing into a neighboring cell) was manually recorded for a duration of 2 min. The test was performed under ambient lighting conditions prior to treatment (pretreatment performance) and 20 min following administration of vehicle or drug(s). Data are presented as a percent of pretreatment performance.
2.7. Wire hang test
To evaluate motor function, a slight modification of a standard wire hang test (Crawley, 2007) was performed. Untrained mice were administered vehicle, diazepam and/or allopregnanolone i.p.; 20 min later mice were individually placed in the middle of a clean wire cage lid. The lid was gently shaken to stimulate grasping and then rapidly inverted and suspended over the animal's home cage for 1 min. The latency to fall was recorded. Each animal was tested in three consecutive trials. The data are presented as the mean latency time to fall in sec; animals that did not fall were scored as 60 s.
2.8. Histological analysis of neuroinflammation
Mice were perfused transcardially with phosphate buffered saline followed by 4% paraformaldehyde. Brains were removed and cryoprotected with 30% sucrose prior to cutting with a cryostat into 10 μm sagittal sections. To quantify neuronal cell damage, sections were stained with Fluoro-Jade B, and to assess reactive gliosis and microglial cell activation, sections were immunostained for GFAP and Iba-1, respectively, as previously described (Zolkowska et al., 2012). Images of the hippocampus and frontal cortex were obtained on an Olympus IX-81 spinning disk confocal microscope and the area of immunoreactivity quantified using Metamorph Advanced Software (version 7.8.1.0, Molecular Devices Sunnyvale, CA) as previously described (Zolkowska et al., 2012). These values were log-transformed. All endpoints were examined in at least 3 serial sections per animal and data were collected from the same level of the brain across all animals.
2.9. Statistical analyses
Data regarding seizure behavior in mice, as well as data of SCOs in cultured neurons were analyzed using one-way ANOVA (with statistical significance set at P < 0.05) and differences between treatment groups were identified using post hoc Tukey's test (GraphPad, Version 5.01, Graphpad Software Inc., La Jolla, CA). Survival data in mice were analyzed using logistic regression using Stata 13 software (StataCorp, College Station, TX) with the Akaike Information Criterion (AIC), a parsimony-favoring measure of model goodness-of-fit used to choose between a model with only main effects for the two experimental factors (DZP+ versus DZP- and AlloP+ versus AlloP-) versus a model with separate effects for each of the four treatment groups (Burnham and Anderson, 2002). Histological data were also analyzed using Stata 13. Graphics were produced using the R 3.0.1 software suite (Vienna, Austria; package ggplot2 version 0.9.3.1 by Hadley Wickham). Because data regarding area of immunoreactivity were performed on multiple sections from different brain regions of each animal, mixed-effects regression models employing repeated measures taken from the same animal were used to account for within-animal correlations and to improve the efficiency of estimates. Alternative repeated measures analysis of variance (ANOVA) methods were inappropriate due to missing observations and to their inability to deal with count outcomes. Exploratory analysis of the area of GFAP/Iba-1 immunoreactivity indicated a logarithmic transformation of the data was required to stabilize the variance and to allow for linear model specification by normalizing the distribution of the outcome. Linear mixed effects models with animal random effects were applied unless residual scatterplots indicated that the error variance was approximately proportional to the mean. In the latter case, Poisson mixed effects models (log link) were used. In every model, candidate fixed effects were as follows: treatment condition, brain region (cortex or hippocampus), number of days after treatment administration, and their interactions. Variable selection was performed using the AIC and Wald tests for pairwise comparisons of interaction effects; when interaction terms were not significant the model was simplified by removing them. Glial activation data were expressed as counts and Poisson mixed effects models were applied with variable selection proceeding.
3. Results
3.1. Pretreatment with combined diazepam and allopregnanolone protects against TETS-induced lethality at doses that are ineffective singly
Previously, we demonstrated that pretreatment with a combination of diazepam and allopregnanolone was more effective than either drug alone in reducing TETS-induced Ca2+ dysregulation in cultured mouse hippocampal neurons (Cao et al., 2012). To determine whether these in vitro observations are predictive of in vivo results, we assessed the efficacy of pretreatment with diazepam and allopregnanolone singly or in combination in preventing death of mice intoxicated with a lethal dose of TETS. The lethal dose of TETS was 0.15 mg/kg i.p., as determined previously (Zolkowska et al., 2012; Vito et al., 2014), and doses of diazepam and allopregnanolone that were ineffective or partially effective when administered singly were determined in pilot dose-range finding studies (data not shown). Mice were treated (i.p.) with vehicle (10% DMSO in saline), 0.03 - 0.1 mg/kg diazepam, 0.03 - 0.1 mg/kg allopregnanolone, or a combination of the two agents at these same doses 10 min prior to i.p. injection of a lethal dose of TETS (Fig. 1A). Treatment with diazepam or allopregnanolone alone at either 0.03 or 0.1 mg/kg did not significantly alter the number of TETS-induced clonic seizures relative to TETS intoxicated mice treated with vehicle (Fig. 1B and 1D). However, combined treatment with 0.1 mg/kg diazepam and 0.1 mg/kg allopregnanolone significantly decreased the number of clonic seizures (Fig. 1D) relative to TETS-intoxicated animals pretreated with vehicle or either drug singly. Pretreatment with the combination significantly increased the time to seizure onset relative to TETS intoxicated mice treated with vehicle or with diazepam only; however, it did not significantly alter seizure duration (Table 1). The percent survival at 24 h was 0-10% in vehicle-treated animals (Fig 1C and 1E). Diazepam alone at 0.1 mg/kg increased survival to 30% whereas allopregnanolone alone at 0.1 mg/kg increased survival to 20% (Fig. 1E). To determine statistical significance, the AIC selected a main-effects only model over a model with separate effects for each of the four treatment groups. Each treatment significantly incrementally enhanced survival (Adjusted Odds Ratio (AOR) [95% CI] for main effect of DZP (DZP+ versus DZP-) = 14.5 [2.3, 93.1] and for main effect of AlloP = 9.1 [1.5, 56.0]. The combined treatment was more effective than either treatment alone, increasing survival to 90% (Fig. 1E).
Fig. 1.
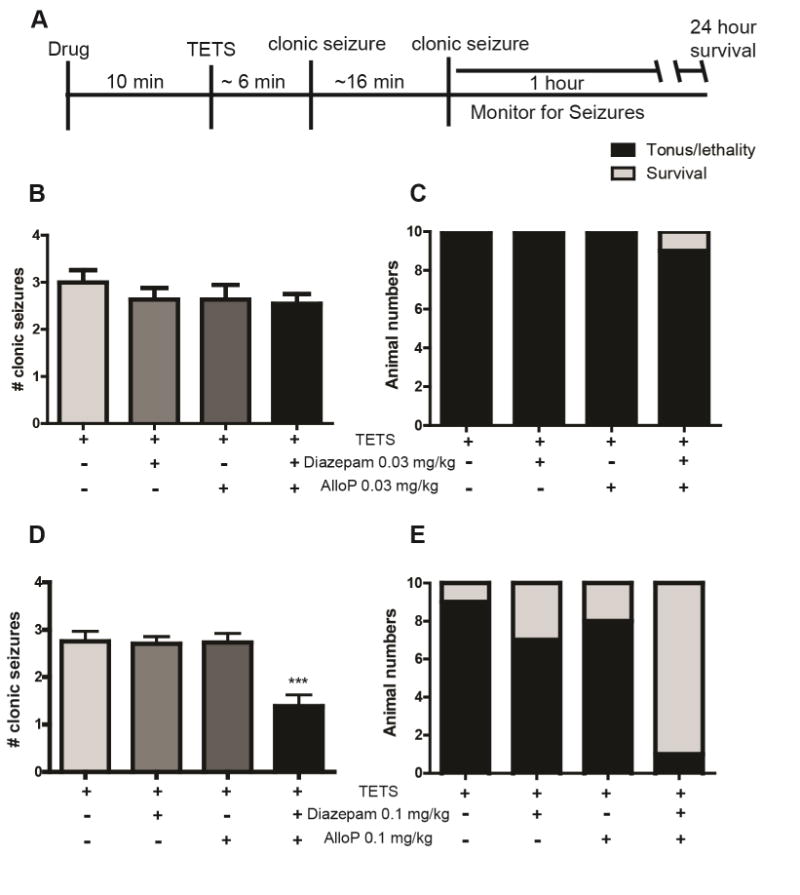
Pretreatment with low dose diazepam and allopregnanolone (AlloP) in combination but not singly protects against TETS-induced lethality. (A) Diazepam and AlloP were administered either singly or in combination 10 min prior to injection of a lethal dose of TETS (0.15 mg/kg i.p.) and the number of clonic seizures was counted during the first hour following TETS injection. Effects of pre-treatment with diazepam and AlloP at 0.03 mg/kg (B, C) or 1.0 mg/kg (D, E) were evaluated for therapeutic efficacy in decreasing the number of clonic seizures (B, D) and in decreasing the number of animals that experienced tonic seizures and died (light gray bars) versus those that survived up to 24 h post-TETS injection (black bars) (C, E). In panels B and D, bars indicate the mean ± SEM of seizure count values for 10 animals; ***significantly different from all other treatment groups at P<0.001 (one-way ANOVA with post hoc Tukey's test). In logistic regression analyses of the data presented in panels C and E, the Akaike Information Criterion selected a main-effects only model as preferable to a model with a 4-level treatment group factor. No significant differences between treatment groups were identified in the pretreatment studies using diazepam and AlloP at 0.03 mg/kg. In contrast, statistically significant differences between treatment groups were identified in the pre-treatment studies using diazepam and AlloP at 0.1 mg/kg. The adjusted odds ratios (AOR) for the incremental main effect of each two-level factor was statistically significant: DZP+ versus DZP- AOR [95% CI] = 14.5 [2.3, 93.1], P=0.005; AlloP+ versus AlloP- AOR [95% CI] = 9.1 [1.5, 56.0], P=0.017. Compared to vehicle alone, the combination of the main effects of DZP and AlloP significantly increases survival: (AOR for “DZP+ & Allo-P+” versus Vehicle =131.8 [5.7, 3057], P=0.002).
Table 1. Effects of pretreatment with diazepam (DZP) and allopregnanolone (AlloP), singly or in combination, on time to onset and duration of TETS-induced seizures.
| Pre-TETS treatment | Time to seizure onset (min) |
Duration of 1st seizure (sec) |
Duration of 2nd seizure (sec) |
Duration of 3rd seizure (sec) |
|---|---|---|---|---|
| Vehicle | 5.9 ±1 | 26 ± 5 sec | 30 ± 4 sec | 30 ± 7 sec |
| 0.1 mg/kg DZP | 6.5 ±4 | 18 ±6 sec | 32 ± 8 sec | 32 ± 6 sec |
| 0.1 mg/kg AlloP | 8.9 ±2 | 29 ± 4 sec | 28 ± 7 sec | 36 ± 4 sec |
| 0.1 mg/kg DZP + 0.1 mg/kg AlloP | 11.7±7**,Δ | 26 ± 3 sec | 33 ± 6 sec | 34 ± 5 sec |
Significantly different from TETS animals pretreated with vehicle at P<0.01;
Δ significantly different from TETS animals treated with 0.1 mg/kg DZP at P<0.05 as determined using one-way ANOVA with post hoc Tukey's test. Data are presented as the mean ± SEM (n=10 animals per group).
3.2. Combined diazepam and allopregnanolone treatment following TETS challenge effectively mitigates TETS dysregulation of Ca2+ dynamics in cultured neurons
The addition of TETS to cultured mouse hippocampal neurons triggers a reproducible, concentration-dependent biphasic change in spontaneous Ca2+ oscillations (Cao et al., 2012). The initial effect (phase 1 response) is an acute increase in intracellular Ca2+ levels followed by a prolonged effect (phase II response) in which the amplitude of Ca2+ oscillations is increased but the frequency of the Ca2+ oscillations is decreased. We previously demonstrated that both phase I and phase II responses to TETS can be prevented by pretreatment with a combination of diazepam and allopregnanolone at low concentrations that have minimal effect when added singly (Cao et al., 2012). Here, we examined whether adding diazepam and allopregnanolone to cultures after TETS exposure would restore normal patterns of synchronous Ca2+ oscillations. Since TETS induces an acute phase I response, we quantified the effects of diazepam and allopregnanolone on the TETS-induced phase II response (Fig. 2A). As illustrated by representative traces of spontaneous Ca2+ oscillations (Fig. 2A), cultures exposed to TETS at a final concentration of 3 μM exhibited an acute phase I response followed by a prolonged phase II response. The addition of either diazepam (0.1 μM) or allopregnanolone (0.1 μM) to the assay buffer ∼20 min after treatment with TETS did not alter the TETS-induced phase II response with respect to the number of Ca2+ oscillations (Fig. 2B). When added singly, diazepam but not allopregnanolone significantly reduced the amplitude of Ca2+ oscillations in TETS-treated cultures but did not fully restore Ca2+ amplitudes to vehicle control levels (Fig. 2C). In contrast, when added in combination, these same concentrations of diazepam and allopregnanolone effectively restored both the frequency (Fig. 2B) and amplitude (Fig. 2C) of spontaneous Ca2+ oscillations to control levels.
Fig. 2.
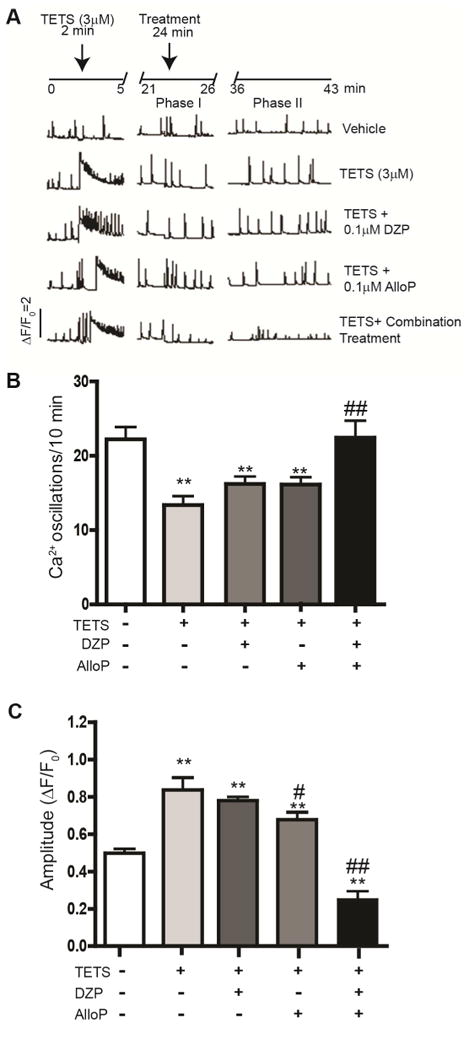
TETS-induced Ca2+ dysregulation in primary cultures of mouse hippocampal neurons is ameliorated by low concentrations of diazepam (DZP) and allopregnanolone (AlloP) added in combination but not singly. (A) Representative traces of spontaneous synchronous Ca2+ oscillations (SCOs) in cultured hippocampal neurons acutely exposed to TETS (3 μM) followed ∼ 20 min later by addition of DZP (0.1 μM) or AlloP (0.1 μM) added singly or in combination. The combination of DZP and AlloP was more effective than either drug alone in ameliorating the effects of TETS on (B) the frequency of SCOs and (C) transient Ca2+ amplitudes. Data presented as the mean ± SEM. Each data point represents at least 8 replicates (wells) and each experiment was repeated in two independent cultures. **Significantly different from vehicle control (white bars) at P<0.01. #Significantly different from TETS alone (light gray bars) at P<0.05; ##P<0.01 (one-way ANOVA with post hoc Tukey's test).
3.3. Post-TETS administration of combined diazepam and allopregnanolone prevents death of TETS-intoxicated mice at doses that are not protective when drugs are given singly
Next, we investigated the in vivo efficacy of combined versus single administration of diazepam and allopregnanolone when administered after TETS exposure. Seizure behavior was scored for up to 1 h post drug administration and survival was assessed 24 h post-TETS exposure in mice injected with a lethal dose of TETS and then administered the drugs singly or in combination within 2 min following the second clonic seizure (∼20 min post-TETS, Fig. 3A).
Fig. 3.
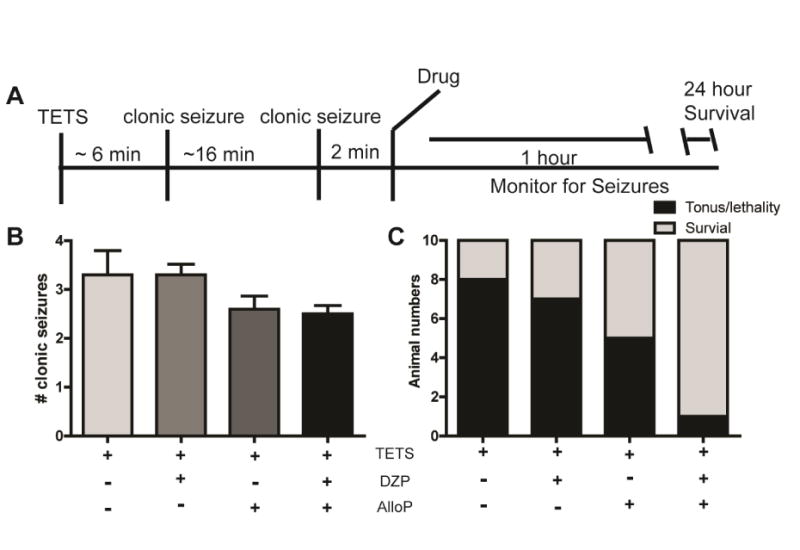
Post-exposure treatment with low dose diazepam (DZP) and allopregnanolone (AlloP) in combination but not singly protects against TETS-induced lethality. (A) Mice injected with a lethal dose of TETS (0.15 mg/kg i.p.) were administered DZP (0.03 mg/kg i.p.) and AlloP (0.03 mg/kg i.p.) singly or in combination ∼2 min after the second clonic seizure. (B) Seizure behavior was monitored for up to 1 h post-drug administration and the number of clonic seizures per animal was scored. Data presented as the mean ± SEM (n=10); one-way ANOVA identified no statistically significant differences between treatment groups with respect to the number of clonic seizures. (C) The number of animals that experienced tonic seizures and died (light gray bars) versus those that survived up to 24 h post-TETS injection (black bars). In logistic regression analyses, the Akaike Information Criterion selected a main-effects only model as preferable to a model with a 4-level treatment group factor: AOR [95% CI] for the incremental main effect of DZP (DZP+ versus DZP-) = 3.8 [0.9, 17.4], P=0.09; AOR [95% CI] for the incremental main effect of AlloP (AlloP+ versus AlloP-) = 8.6 [1.9, 39.3]; P=0.006. Compared to vehicle alone, the combination of the main effects of DZP and AlloP significantly increases survival (AOR for “DZP+ & AlloP+” vs. vehicle = 32.5 [2.8, 381.4], P=0.006).
We previously found this to be the longest therapeutic window for preventing death in TETS-intoxicated mice using diazepam at 5 mg/kg i.p. (Vito et al., 2014). When administered singly or in combination, diazepam or allopregnanolone at 0.03 mg/kg i.p. had no significant effect on the number of TETS-induced clonic seizures (Fig. 3B) or on the time to seizure onset and seizure duration (Table 2). TETS-intoxicated mice administered only diazepam at 0.03 mg/kg i.p. following the second clonic seizure had a survival rate of 30% (3 of 10), which was not significantly different from that of TETS-intoxicated mice treated with vehicle (Fig. 3C). The percent survival at 24 h was 20% in vehicle-treated animals (Fig 3C). Diazepam alone increased survival to 30% whereas allopregnanolone alone increased survival to 50%. For the logistic regression analysis of survival at 24 h, the AIC selected a main-effects only model instead of a model with separate effects for each of the four treatment groups. Administration of diazepam at 0.03 mg/kg i.p. was associated with slight and statistically insignificant incremental enhancement of survival (AOR [95% CI] for main effect of DZP (DZP+ versus DZP-) = 3.8 [0.9, 17.4]; P=0.09), whereas the allopregnanolone incremental enhancement of survival was statistically significant (AOR for main effect of AlloP (AlloP+ versus AlloP-) = 8.6 [1.9, 39.3]; P=0.006]. The combined treatment was more protective than either drug administered singly, increasing survival to 90% (Fig. 3C).
Table 2. Effects of post-treatment with diazepam (DZP) and allopregnanolone (AlloP), singly or in combination, on time to onset and duration of TETS-induced seizures.
| Post-TETS treatment | Time to seizure onset (min) |
Duration of 1st seizure (sec) |
Duration of 2nd seizure (sec) |
Duration of 3rd seizure (sec) |
|---|---|---|---|---|
| Vehicle | 4.6 ±1 | 21 ±5 | 28 ±7 | 25 ±8 |
| 0.03 mg/kg DZP | 5.7 ±2 | 24 ±8 | 35 ±9 | 31 ±9 |
| 0.03 mg/kg AlloP | 4.4 ±1 | 23 ±2 | 39 ±9 | 31 ±7 |
| 0.03 mg/kg DZP + 0.03 mg/kg AlloP | 4.4 ±1 | 19±4 | 29 ±6 | 28 ±7 |
No statistically significant treatment effects were identified using one-way ANOVA (P<0.05).
Data are presented as the mean ± SEM (n=10 animals per group.
3.4. Combined low dose diazepam and allopregnanolone therapy does not cause adverse side effects associated with high dose diazepam therapy
In our initial studies using high dose diazepam to rescue TETS-intoxicated mice (Vito et al., 2014), we observed a pronounced sedative/anesthetic response following diazepam treatment. Treatment with a lower dose of diazepam in combination with a low dose of allopregnanolone did not seem to elicit this response. To quantify these observations, we first used a non-invasive tail cuff method to measure changes in blood pressure. Administration of diazepam at 5 mg/kg i.p. caused mean blood pressure to drop below the limits of detection of the tail cuff method (Fig. 4A). In contrast, treatment with low doses (0.03 mg/kg i.p.) of diazepam or allopregnanolone singly or in combination did not change mean blood pressure relative to control values (Fig. 4A).
Fig. 4.
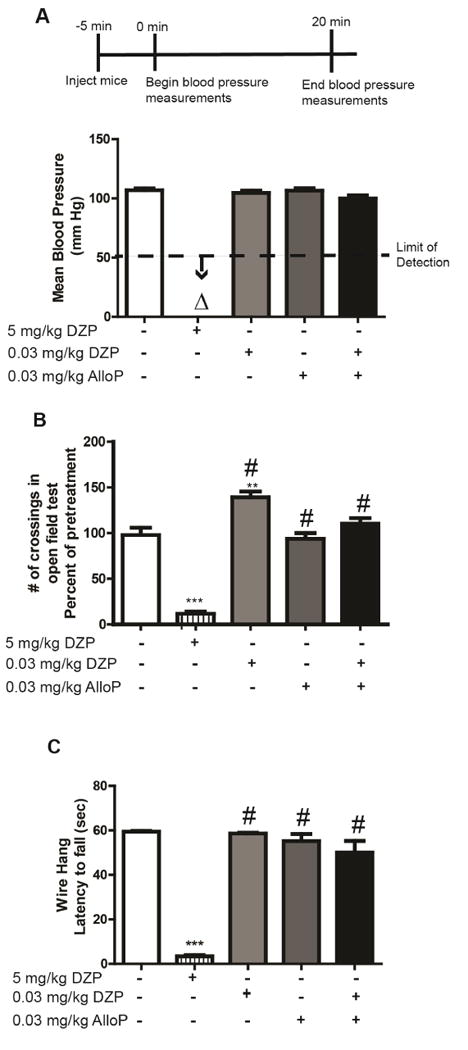
Combined low dose diazepam (DZP) and allopregnanolone (AlloP) does not elicit adverse side effects observed with high dose DZP treatment. Naïve mice (not exposed to TETS) were administered DZP (0.03 mg/kg i.p.) and AlloP (0.03 mg/kg i.p.) singly or in combination. The effects of these low dose treatments were compared to those of high dose of DZP (5 mg/kg i.p.) on: (A) mean blood pressure as measured using a tail cuff system (n=5 per treatment group); (B) locomotor activity as assessed in an open field test (n=10 per treatment group); and (C) motor function as determined using the wire hang test (n=8 per group). Data from each study are presented as the mean ± SEM; **significantly different from vehicle control (white bars) at P<0.01; ***P<0.001; #significantly different from high dose (5 mg/kg) DZP at P<0.05 (one-way ANOVA with post hoc Tukey's test). Δ Below the detection limit of the assay.
In a separate cohort of mice, we assessed potential sedative or neurologically impairing effects of drug treatment by measuring horizontal locomotion in the open field test and motor function as determined using the wire hang test. Mice injected with diazepam at 5 mg/kg i.p. exhibited significantly reduced locomotor activity relative to control mice (Fig. 4B). Locomotor activity was significantly increased in mice administered diazepam at the low dose of 0.03 mg/kg, i.p., relative to control mice, whereas mobility was comparable to that of controls in mice injected with low dose allopregnanolone (0.03 mg/kg, i.p.) singly or in combination with low dose diazepam (Fig. 4B). Consistent with these observations, treatment with high dose diazepam, but not low dose diazepam or allopregnanolone administered singly or in combination significantly impaired performance in the wire hang test (Fig. 4C).
3.5. Combined low dose diazepam and allopregnanolone therapy modulates TETS-induced neuroinflammation
We previously observed that intoxication with sublethal or lethal doses of TETS causes delayed reactive astrogliosis and microglial cell activation in the cortex and hippocampus (Zolkowska et al., 2012), and that administration of high dose diazepam (5 mg/kg i.p.) after the second clonic seizure did not prevent this neuroinflammatory response to a lethal dose of TETS (Vito et al., 2014). Here, we determined whether combinatorial therapy with diazepam and allopregnanolone at low doses (0.03 mg/kg i.p.) administered following the second clonic seizure altered TETS-induced neuroinflammation as determined by GFAP and Iba-1 immunohistochemistry. Brains were collected at 2 or 3 d post-TETS exposure in mice injected with a lethal dose of TETS (0.15 mg/kg i.p.) and rescued following the second clonic seizure with either a single high dose of diazepam (5 mg/kg i.p.) or a combination of low dose diazepam and allopregnanolone (each at 0.03 mg/kg i.p.). Similar to our earlier reports (Zolkowska et al., 2012; Vito et al., 2014), there were no gross pathological lesions or neurodegeneration in the cortex or hippocampus of mice rescued from TETS lethality by high dose diazepam or combined low dose diazepam and allopregnanolone as indicated by H&E staining (Fig. 5) and Fluoro-Jade B staining (data not shown). GFAP immunoreactive cells were observed in the hippocampus and cortex of TETS-intoxicated mice regardless of post-exposure treatment (Fig. 6). Calculation of the geometric mean ratio (GMR) of the area of GFAP immunoreactivity in TETS-intoxicated mice rescued with combined low dose diazepam and allopregnanolone relative to that of TETS-intoxicated mice rescued with high dose diazepam revealed a significant 3-fold and 6-fold increase in reactive astrogliosis in the cortex at 2 and 3 d post-TETS exposure, respectively (Fig. 6). In the hippocampus, the area of GFAP immunoreactivity was not significantly different between treatment groups at 2 d post-TETS exposure, but was increased approximately 1.5 fold in TETS-intoxicated animals receiving the combinatorial therapy relative to the high dose diazepam treatment group at 3 d post-exposure (Fig. 6).
Fig. 5.
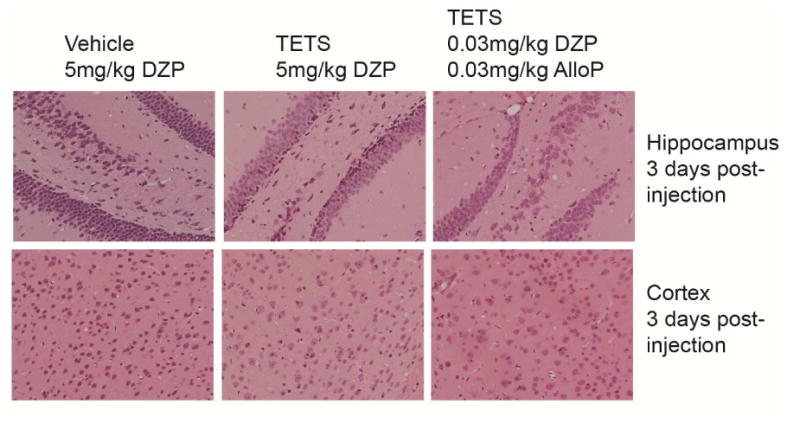
TETS-intoxicated mice rescued with high dose diazepam (DZP) or combined low dose DZP and allopregnanolone (AlloP) do not exhibit gross histological changes in the brain. Representative photomicrographs of hematoxylin and eosin (H&E) staining of the frontal cortex and hippocampus of mice injected with vehicle (saline) or a lethal dose of TETS (0.15 mg/kg i.p.) then rescued from death by high dose DZP (5 mg/kg i.p.) or combined low dose DZP (0.03 mg/kg i.p.) and AlloP (0.03 mg/kg i.p.) administered ∼2 min after the second clonic seizure. No gross pathological abnormalities were noted on the H&E stained sections across all treatment groups.
Fig. 6.
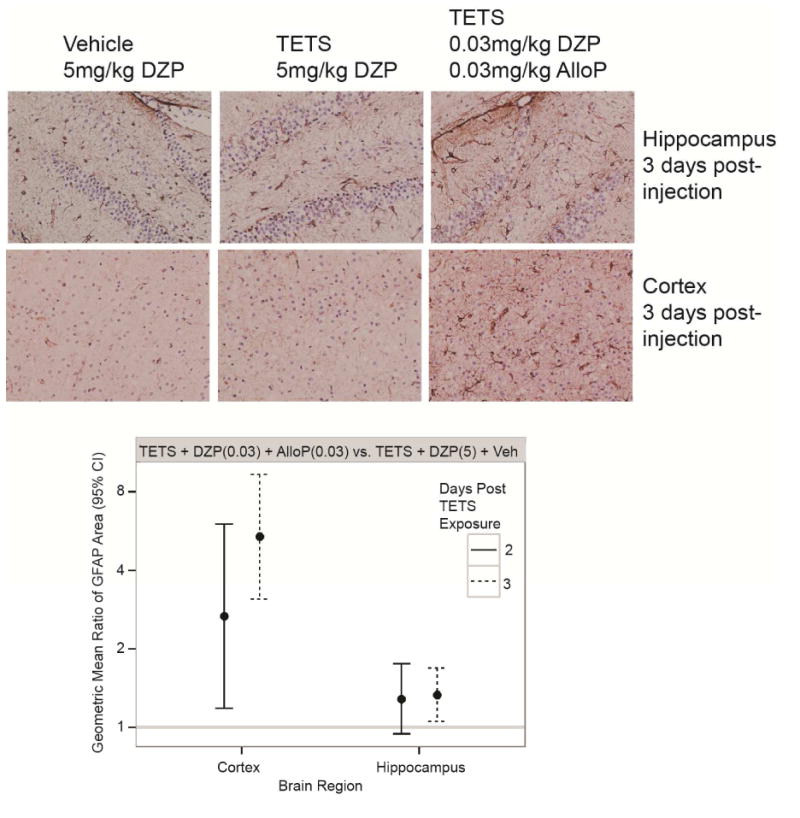
Combined treatment with low dose diazepam (DZP) and allopregnanolone (AlloP) increases reactive astrogliosis in TETS-intoxicated animals. Representative photomicrographs of GFAP immunoreactivity in the hippocampus and prefrontal cortex of vehicle (saline) and TETS-intoxicated mice treated with high dose (5 mg/kg) DZP or a combination of low dose (0.03 mg/kg) DZP and AlloP at 3 d post-injection. The quantitative analyses of these data are represented by dot plots. Dots represent the geometric mean ratio of the area of GFAP immunoreactivity in TETS intoxicated animals treated with combined low dose DZP and AlloP versus TETS intoxicated animals treated with high dose DZP; bars represent 95% confidence intervals (n=3-6 animals per group with 4-7 sections per brain region per animal).
To assess microglial activation, we quantified the area of Iba-1 immunoreactivity in the cortex and hippocampus at 2 and 3 d post-TETS exposure, Iba-1 immunoreactive cells were observed in both brain regions in all treatment groups (Fig. 7). Calculation of the GMR of the area of Iba-1 immunoreactivity in TETS-intoxicated mice rescued with combined low dose diazepam and allopregnanolone versus TETS-intoxicated mice rescued with high dose diazepam indicates that the combinatorial therapy significantly decreases microglial activation relative to the high dose diazepam treatment group in both brain regions at 2 and 3 d post-TETS exposure (Fig. 7).
Fig. 7.
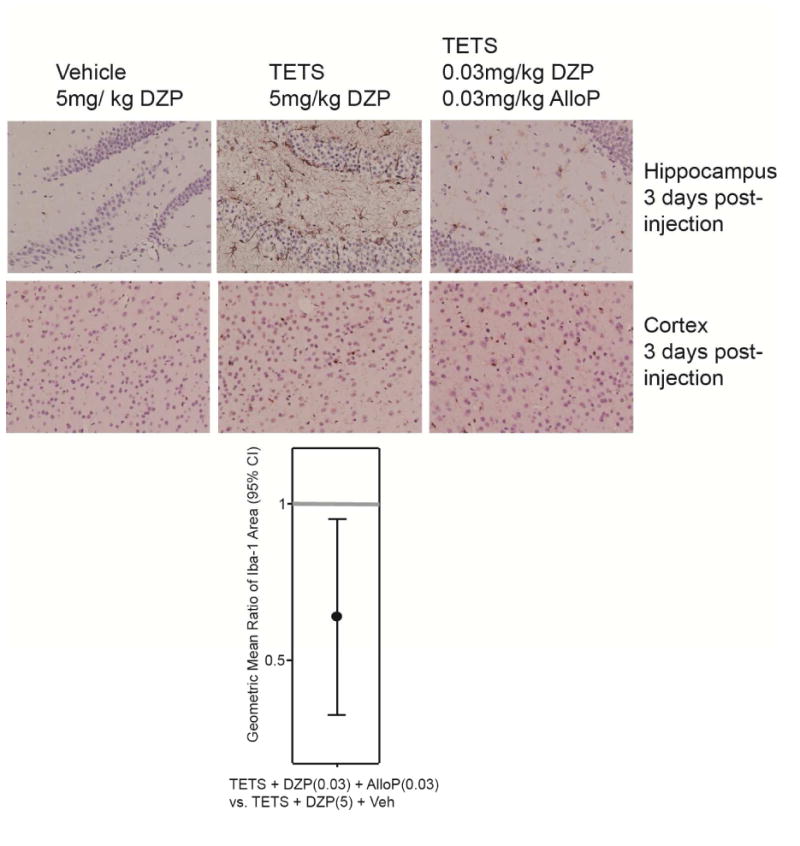
Combined treatment with low dose diazepam (DZP) and allopregnanolone (AlloP) decreases microglial cells in the hippocampus and prefrontal cortex of TETS-intoxicated animals. Representative photomicrographs of Iba-1 immunoreactivity in the hippocampus and prefrontal cortex of vehicle (saline) and TETS-intoxicated mice treated with high dose (5 mg/kg) DZP or a combination of low dose (0.03 mg/kg) DZP and AlloP at 3 d post-injection. The quantitative summary of these data are represented by dot plots. Dots represent the geometric mean ratio of the area of Iba-1 immunoreactivity in TETS intoxicated animals treated with combined low dose DZP and AlloP versus TETS intoxicated animals treated with high dose DZP; bars represent 95% confidence internals (n=3-6 animals per group with 4-7 sections per brain region per animal).
4. Discussion
We previously reported that combined pretreatment with low (0.1 μM) concentrations of diazepam and allopregnanolone markedly reduces TETS-induced Ca2+ dysregulation in mouse hippocampal cultures whereas each agent alone at the same concentration is only minimally effective (Cao et al., 2012). We now confirm a synergistic action of the two agents against TETS-induced seizures in mice. Intraperitoneal injection of mice with 0.15 mg/kg TETS typically causes a sequence of seizure signs consisting of immobility, myoclonic body jerks, clonic seizures, and tonic seizures, followed immediately by death. Neither low dose diazepam nor allopregnanolone alone reduced the frequency of clonic seizures and each had only a modest effect on survival. However, when both agents were administered together clonic seizure frequency was reduced and survival at 24 h post-TETS injection increased significantly from 10% to 90%. A similar synergistic action was observed with post-exposure treatment with respect to both Ca2+ dysregulation in hippocampal cultures and TETS-induced lethality in mice. Interestingly, the doses of diazepam and allopregnanolone required to significantly increase survival of TETS-intoxicated mice when administered in combination were half a log lower when administered post-TETS (0.03 mg/kg each) versus pre-TETS (0.1 mg/kg each). The reason(s) for the different dose-response relationship depending on time of combinatorial drug therapy relative to TETS exposure are not known. One possibility is that acute TETS intoxication increases the permeability of the blood brain barrier, which increases the delivery of the drug(s) to the brain when administered post-TETS exposure relative to pre-TETS exposure.
Hypotension represents a key challenge in the use of benzodiazepines in the treatment of status epilepticus (Treiman et al., 1998; Fritsch et al., 2010). Accordingly, we sought to assess the impact of the combination treatment on blood pressure. Treatment with diazepam at 5 mg/kg, a dose which we (Vito et al., 2014) and others (Shakarjian et al., 2012) previously demonstrated was protective against TETS-induced lethality, markedly reduced blood pressure whereas low doses of either diazepam or allopregnanolone did not cause hypotension. Combination treatment also did not impact blood pressure indicating that protection against TETS-induced lethality can be obtained at doses that allow cardiovascular function to be fully preserved. Combination treatment also did not affect locomotor activity or impair motor performance as assessed by the wire hang test, indicating that protection against TETS-induced lethality can be obtained without disruption of neurologic function.
In prior studies, we reported that mice surviving TETS-induced clonic seizures do not exhibit gross histological changes in selected brain regions, such as cell loss or changes to the general brain architecture (Zolkowska et al., 2012; Vito et al., 2014). Similarly, in the present study, no gross structural brain damage was observed in the hippocampus or cortex of mice rescued with high dose diazepam or combination treatment with low doses of diazepam and allopregnanolone. However, GFAP immunoreactivity was increased in these brain regions 2 and 3 days after TETS exposure, and this increase was greater for animals receiving the combination treatment than for those rescued with high dose diazepam. The increase in the area of GFAP immunoreactivity likely reflects astrocyte proliferation. Our earlier work indicated that the TETS-induced increase in GFAP is transitory because levels returned to baseline at 7 days following exposure. The implications of this transient astrogliosis are not known, although it might serve a role in neuroregeneration (Liu et al., 2014). If this is the case, then our data suggesting that combination treatment causes greater astrogliosis than occurs when animals are rescued with high dose diazepam raises the possibility that the combination therapy might allow for improved recovery. This is consistent with the observation of reduced microglial activation with combination therapy as evidenced by decreased Iba-1 immunoreactivity in animals treated with the combination of low dose allopregnanolone and diazepam relative to animals rescued with high dose diazepam. Increased microglial activation in the animals receiving high dose diazepam could reflect the increased stress of seizure activity and reduced cerebral perfusion associated with the hypotensive effect of the treatment. These data suggest that in addition to the lack of negative acute impact on cardiovascular and neurological function, combined treatment with low-dose benzodiazepine and neurosteroid might also reduce any long-term neurologic consequences of TETS-induced seizures in survivors.
Interestingly, we had previously observed in mice with TETS-induced seizures that relative to post-TETS treatment with high dose (5 mg/kg i.p.) diazepam alone, combined treatment with high dose diazepam and a small molecule inhibitor of soluble epoxide hydrolase (sEH) similarly increased GFAP and decreased Iba-1 immunoreactivity compared to high dose diazepam alone (Vito et al., 2014). Pharmacologic inhibition of sEH increases the biologic half-life of epoxyeicosatrienoic acids (EETs) and this action delays the onset of seizures triggered by GABA antagonists (Inceoglu et al., 2013). The effects of sEH inhibition on seizures were enhanced by allopregnanolone and partially blocked by finasteride, which inhibits neurosteroid synthesis, (Inceoglu et al., 2013), suggesting that effects of EETs on GABA activity are mediated in part by neurosteroids. The observation in the present study that combined diazepam and allopregnanolone results in similar neuroinflammatory effects as combined diazepam and sEH inhibition is consistent with the conclusion that neurosteroids play a role in the action of sEH inhibitors.
How does combined treatment with a benzodiazepine and neurosteroid, each at low dose, lead to a synergistic therapeutic action? Benzodiazepines such as diazepam act as positive allosteric modulators of synaptic GABAA receptors that mediate classical phasic inhibition (Mohler et al., 2002; Olsen, 2014); they do not affect most extrasynaptic GABAA receptors that are responsible for tonic inhibition. Nevertheless, benzodiazepines have powerful antiseizure activity although this activity diminishes as seizures continue (Kapur and Macdonald, 1997). There is evidence that extrasynaptic GABAA receptors may also play a role in seizure control (Spigelman et al., 2002; Peng et al., 2004; Brickley and Mody, 2012). Neurosteroids such as allopregnanolone enhance the activity of both synaptic and extrasynaptic GABAA receptors (Herd et al., 2007; Reddy and Rogawski, 2012). Therefore, the combined action on synaptic and extrasynaptic GABAA receptors could contribute to the synergy. Although both benzodiazepines and neurosteroids increase the probability of GABAA receptor channel opening in the presence of GABA, the two types of agents act at different sites on the GABAA receptor-channel complex and they act in different ways (Hosie et al., 2009; Olsen, 2014). Benzodiazepines increase the frequency of channel opening (Bianchi et al., 2009; Bianchi, 2010) whereas neurosteroids increase the mean open channel duration and decrease the mean closed time duration (Akk et al., 2004; Akk et al., 2010). Of particular importance is the reduction in long-lived closed states of the channel. Some investigators have observed benzodiazepines to increase the single channel conductance (Eghbali et al., 1997) whereas neurosteroids do not change the single channel conductance or the number of active channels (Akk et al., 2010). Little is known about the interaction between benzodiazepines and neurosteroids at the single channel level. However, it is well recognized that neurosteroids, including allopregnanolone, enhance benzodiazepine binding to the benzodiazepine recognition site on GABAA receptors as assessed with [3H]flunitrazepam binding (Majewska et al., 1986; Gee et al., 1988; Hawkinson et al., 1994). Therefore, intramolecular interactions within the GABAA receptor that mutually reinforce the actions of GABA, benzodiazepine, and neurosteroid via allosteric mechanisms might account in part for the synergy.
In conclusion, our results demonstrate that combined treatment with diazepam and allopregnanolone, each at low dose, protects mice against lethal seizures induced by the convulsant toxicant TETS, a GABAA receptor antagonist. Although the basis for the synergistic pharmacodynamic interaction between the two allosteric modulators is not yet defined, actions on distinct populations of GABAA receptors and combined modulation of individual GABAA receptors could be relevant. The observation that the dual treatment protects against seizure-induced lethality without systemic side effects indicates that a combination therapy approach might have practical utility. Combined treatment with a GABAA receptor modulating benzodiazepine and neurosteroid could be useful not only for treating victims acutely intoxicated with TETS but may also have broader applicability in seizure therapy.
Highlights.
TETS is a lethal convulsant toxicant for which there is no proven antidote in humans
Combined allopregnanolone and diazepam normalize Ca2+dynamics in cultured neurons
Combined allopregnanolone and diazepam increase survival in TETS-intoxicated mice
Combined treatment does not cause adverse effects associated with high dose diazepam
Combined neurosteroid and benzodiazepine is a novel anticonvulsant strategy
Acknowledgments
We thank Mark McCoy (originally in the laboratory of B.D.H. at University of California, Davis, now at California State University Stanislaus, Turlock, CA) for the synthesis of TETS, and Danielle Harvey (Division of Biostatistics, University of California, Davis) for assistance with statistical analyses and review of early versions of the manuscript. This research was supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Neurological Disorders and Stroke (Grant NS079202). The sponsor was not involved in the study design, the collection, analysis, and interpretation of data, in the writing of the report or in the decision to submit the paper for publication. S.T.V. was supported by a predoctoral fellowship funded by a training grant from the National Institute of General Medical Sciences (Grant GM099608). B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
Abbreviations
- AIC
Akaike Information Criterion
- AlloP
allopregnanolone
- ANOVA
analysis of variance
- CI
confidence interval
- DZP
diazepam
- ESA
electrical spike activity
- GFAP
glial fibrillary acidic protein
- H&E
hematoxylin and eosin
- Iba-1
ionized calcium binding adaptor molecule 1
- i.p.
intraperitoneal
- SCOs
synchronous Ca2+ oscillations
- TETS
tetramethylenedisulfotetramine
Footnotes
Chemical compounds studied in this article: Allopregnanolone (PubChem CID: 262961); diazepam (PubChem CID: 3016); tetramethylenedisulfotetramine (PubChem CID: 64148)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH. Neuroactive steroids have multiple actions to potentiate gabaa receptors. J Physiol. 2004;558:59–74. doi: 10.1113/jphysiol.2004.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Mennerick S, Zorumski CF, Steinbach JH. Kinetic and structural determinants for gaba-a receptor potentiation by neuroactive steroids. Curr Neuropharmacol. 2010;8:18–25. doi: 10.2174/157015910790909458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks CN, Rogawski MA, Yang D, Lein PJ. Tetramethylenedisulfotetramine. In: Wexler P, editor. Encyclopedia of toxicology. Elsevier, Inc., Academic Press; Oxford, U.K.: 2014. pp. 509–511. [Google Scholar]
- Barrueto F, Jr, Furdyna PM, Hoffman RS, Hoffman RJ, Nelson LS. Status epilepticus from an illegally imported Chinese rodenticide: “Tetramine”. J Toxicol Clin Toxicol. 2003;41:991–994. doi: 10.1081/clt-120026523. [DOI] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5 alpha-pregnan-3 alpha-ol-20-one. Eur J Pharmacol. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Bianchi MT. Context dependent benzodiazepine modulation of gaba(a) receptor opening frequency. Curr Neuropharmacol. 2010;8:10–17. doi: 10.2174/157015910790909467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Botzolakis EJ, Lagrange AH, Macdonald RL. Benzodiazepine modulation of gaba(a) receptor opening frequency depends on activation context: A patch clamp and simulation study. Epilepsy Res. 2009;85:212–220. doi: 10.1016/j.eplepsyres.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic gaba(a) receptors: Their function in the ens and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoretic approach. Springer-Verlag; New York: 2002. [Google Scholar]
- Cao Z, Hammock BD, McCoy M, Rogawski MA, Lein PJ, Pessah IN. Tetramethylenedisulfotetramine alters ca(2)(+) dynamics in cultured hippocampal neurons: Mitigation by nmda receptor blockade and gaba(a) receptor-positive modulation. Toxicol Sci. 2012;130:362–372. doi: 10.1093/toxsci/kfs244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau CM, Leung AK, Tan IK. Tetramine poisoning. Hong Kong Med J. 2005;11:511–514. [PubMed] [Google Scholar]
- Crawley JN. What's wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. John Wiley and Sons; New York, NY: 2007. [Google Scholar]
- Czlonkowska AI, Krzascik P, Sienkiewicz-Jarosz H, Siemiatkowski M, Szyndler J, Bidzinski A, Plaznik A. The effects of neurosteroids on picrotoxin-, bicuculline- and nmda- induced seizures, and a hypnotic effect of ethanol. Pharmacol Biochem Behav. 2000;67:345–353. doi: 10.1016/s0091-3057(00)00369-5. [DOI] [PubMed] [Google Scholar]
- Deng X, Li G, Mei R, Sun S. Long term effects of tetramine poisoning: An observational study. Clin Toxicol (Phila) 2012;50:172–175. doi: 10.3109/15563650.2012.657758. [DOI] [PubMed] [Google Scholar]
- Dravid SM, Murray TF. Spontaneous synchronized calcium oscillations in neocortical neurons in the presence of physiological [mg(2+)]: Involvement of ampa/kainate and metabotropic glutamate receptors. Brain Res. 2004;1006:8–17. doi: 10.1016/j.brainres.2004.01.059. [DOI] [PubMed] [Google Scholar]
- Eghbali M, Curmi JP, Birnir B, Gage PW. Hippocampal gaba(a) channel conductance increased by diazepam. Nature. 1997;388:71–75. doi: 10.1038/40404. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Stott JJ, Joelle Donofrio J, Rogawski MA. Treatment of early and late kainic acid-induced status epilepticus with the noncompetitive ampa receptor antagonist gyki 52466. Epilepsia. 2010;51:108–117. doi: 10.1111/j.1528-1167.2009.02205.x. doi:10.1111/j. 1528-1167.2009.02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS. Steroid modulation of the chloride ionophore in rat brain: Structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988;246:803–812. [PubMed] [Google Scholar]
- Hawkinson JE, Kimbrough CL, Belelli D, Lambert JJ, Purdy RH, Lan NC. Correlation of neuroactive steroid modulation of [35s]t-butylbicyclophosphorothionate and [3h]flunitrazepam binding and gamma-aminobutyric acida receptor function. Mol Pharmacol. 1994;46:977–985. [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic gaba(a) receptors. Pharmacol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of gaba a receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Inceoglu B, Zolkowska D, Yoo HJ, Wagner KM, Yang J, Hackett E, Hwang SH, Lee KS, Rogawski MA, Morisseau C, Hammock BD. Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate gaba mediated neurotransmission to delay onset of seizures. PLoS One. 2013;8:e80922. doi: 10.1371/journal.pone.0080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett DA, Yeung DT. The counteract research network: Basic mechanisms and practical applications. Proc Am Thorac Soc. 2010;7:254–256. doi: 10.1513/pats.201001-003SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and zn2+ sensitivity of hippocampal dentate granule cell gabaa receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima T, Kanbayashi T, Saito Y, Takahashi Y, Ogawa Y, Sugiyama T, Kaneko Y, Aizawa R, Shimizu T. Diazepam reduces both arterial blood pressure and muscle sympathetic nerve activity in human. Neurosci Lett. 2004;355:77–80. doi: 10.1016/j.neulet.2003.10.054. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: Correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Li JM, Gan J, Zeng TF, Sander JW, Zhou D. Tetramethylenedisulfotetramine intoxication presenting with de novo status epilepticus: A case series. Neurotoxicology. 2012;33:207–211. doi: 10.1016/j.neuro.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Li Y, Gao Y, Yu X, Peng J, Ma F, Nelson L. Tetramine poisoning in china: Changes over a decade viewed through the media's eye. BMC Public Health. 2014;14:842. doi: 10.1186/1471-2458-14-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li Y, Cui Y, Roberts C, Lu M, Wilhelmsson U, Pekny M, Chopp M. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia. 2014;62:2022–2033. doi: 10.1002/glia.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the gaba receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeutics. 2007;4:18–61. doi: 10.1016/j.nurt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Ogura A, Iijima T, Amano T, Kudo Y. Optical monitoring of excitatory synaptic activity between cultured hippocampal neurons by a multi-site ca2+ fluorometry. Neurosci Lett. 1987;78:69–74. doi: 10.1016/0304-3940(87)90563-5. [DOI] [PubMed] [Google Scholar]
- Olsen RW. The gaba postsynaptic membrane receptor-ionophore complex. Site of action of convulsant and anticonvulsant drugs. Mol Cell Biochem. 1981;39:261–279. doi: 10.1007/BF00232579. [DOI] [PubMed] [Google Scholar]
- Olsen RW. Analysis of gamma-aminobutyric acid (gaba) type a receptor subtypes using isosteric and allosteric ligands. Neurochem Res. 2014;39:1924–1941. doi: 10.1007/s11064-014-1382-3. doi:10.1007/s 11064-014-1382-3. [DOI] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the delta subunit of the gabaa receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroids - endogenous regulators of seizure susceptibility and role in the treatment of epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's basic mechanisms of the epilepsies. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- Shakarjian MP, Veliskova J, Stanton PK, Velisek L. Differential antagonism of tetramethylenedisulfotetramine-induced seizures by agents acting at nmda and gaba(a) receptors. Toxicol Appl Pharmacol. 2012;265:113–121. doi: 10.1016/j.taap.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley JN. Sociability and motor functions in shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Hota D, Prakash A, Khanduja KL, Arora SK, Chakrabarti A. Allopregnanolone, the active metabolite of progesterone protects against neuronal damage in picrotoxin-induced seizure model in mice. Pharmacol Biochem Behav. 2010;94:416–422. doi: 10.1016/j.pbb.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behavior and physiology of mice lacking the gabaa-receptor delta subunit. Epilepsia. 2002;43(Suppl 5):3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N. Intracellular calcium oscillation in cultured rat hippocampal neurons: A model for glutamatergic neurotransmission. Jpn J Pharmacol. 1996;70:89–93. doi: 10.1254/jjp.70.89. [DOI] [PubMed] [Google Scholar]
- Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, Handforth A, Faught E, Calabrese VP, Uthman BM, Ramsay RE, Mamdani MB. A comparison of four treatments for generalized convulsive status epilepticus. Veterans affairs status epilepticus cooperative study group. N Engl J Med. 1998;339:792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- Vito ST, Austin AT, Banks CN, Inceoglu B, Bruun DA, Zolkowska D, Tancredi DJ, Rogawski MA, Hammock BD, Lein PJ. Post-exposure administration of diazepam combined with soluble epoxide hydrolase inhibition stops seizures and modulates neuroinflammation in a murine model of acute tets intoxication. Toxicol Appl Pharmacol. 2014;281:185–194. doi: 10.1016/j.taap.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow KS, Belson M, Barrueto F, Nelson L, Henderson AK. Tetramethylenedisulfotetramine: Old agent and new terror. Ann Emerg Med. 2005;45:609–613. doi: 10.1016/j.annemergmed.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Wieland S, Belluzzi JD, Stein L, Lan NC. Comparative behavioral characterization of the neuroactive steroids 3 alpha-oh,5 alpha-pregnan-20-one and 3 alpha-oh,5 beta-pregnan-20-one in rodents. Psychopharmacology (Berl) 1995;118:65–71. doi: 10.1007/BF02245251. [DOI] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wohr M, Roullet Fl, Katz AM, Abrams DN, Kalikhman D, Simon H, Woldeyohannes L, Zhang JY, Harris MJ, Saxena R, Silverman JL, Buxbaum JD, Crawley JN. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent shank3 null mutant mice. J Neurosci. 2012;32:6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolkowska D, Banks CN, Dhir A, Inceoglu B, Sanborn JR, McCoy MR, Bruun DA, Hammock BD, Lein PJ, Rogawski MA. Characterization of seizures induced by acute and repeated exposure to tetramethylenedisulfotetramine. J Pharmacol Exp Ther. 2012;341:435–446. doi: 10.1124/jpet.111.190579. doi:10.1124/j pet. 111.190579. [DOI] [PMC free article] [PubMed] [Google Scholar]


