Abstract
Excess dosing of anticoagulant agents has been linked to increased risk of bleeding after PCI for women as compared with men, but these studies have largely included older patients. We sought to determine the prevalence and sex-based differences of excess dosing of anticoagulants including glycoprotein inhibitors (GPIs), bivalirudin and unfractionated heparin (UFH), in young patients with acute myocardial infarction (AMI) undergoing percutaneous coronary intervention (PCI) and to examine its association with bleeding. Among 2076 patients enrolled in the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study who underwent PCI, we abstracted doses of UFH, bivalirudin and GPIs administered during PCI from the medical records. At least 47.2% received at least one excess dose of an anticoagulant, which did not differ by sex. We used logistic regression to determine the predictors of excess dosing and the association of excess dosing with bleeding. In multivariable analysis, only lower body weight and younger age were significant predictors of excess dosing. Bleeding was higher in young women who received excess dosing versus those who did not (9.3% vs. 6.0%, P=0.03); but was comparable among men (5.2% vs. 5.9%, P=0.69) in univariate analysis. In multivariable analysis, there was a trend to an association between excess dosing and bleeding [OR, 1.33; 95% CI 0.92–1.91]; although not statistically significant. In conclusion, approximately half of the patients received excess dosing of anticoagulant drugs during PCI, which did not vary based upon sex. There was a trend towards an association between excess dosing and increased bleeding, although not statistically significant.
Keywords: anticoagulants, excess dosing, percutaneous coronary intervention, sex differences
Introduction
Anticoagulants and antiplatelet agents are commonly utilized in the management of patients undergoing percutaneous coronary intervention (PCI), but are prone to dosing errors.1–3 Despite weight-based and renal dosing guidelines, anticoagulants continue to be administered in excess of the recommended dose in almost half of the patients with both non ST-elevation4 and ST-elevation acute coronary syndromes,5 including a high proportion of patients undergoing PCI. Dosing errors have been linked to increased risk of bleeding complications after PCI for women as compared with men, but these studies largely focused upon older patients.6–9 To investigate potential dosing errors in younger patients, we analyzed data from the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study that recruited patients with AMI who were ≤ 55 years of age.10 Specifically, we identified VIRGO participants who underwent PCI during their initial hospitalization with AMI. In this population, we determined the prevalence and sex-based differences in excess dosing of anticoagulant drugs including, glycoprotein IIb/IIIa inhibitors (GPIs) (eptifibatide and abciximab), bivalirudin and unfractionated heparin (UFH). We also examined the association between excess dosing of these drugs and in-hospital bleeding events.
Methods
Between August 8th, 2008 and January 5th, 2012, a total of 3,572 patients with AMI from 103 US hospitals, 24 Spanish hospitals, and 3 Australian hospitals were enrolled in the VIRGO study. The study used a 2:1 female to male enrollment design to enrich the study’s inclusion of previously understudied young women. We limited our study to the 2985 patients enrolled from the US only. The methods of VIRGO have been described previously.10 In brief, participants were 18–55 years old and AMI was confirmed by increased cardiac biomarkers within 24 hours of admission and with either ischemic symptoms or electrocardiographic evidence of AMI. Participants must have presented directly to the enrolling site, or been transferred within 24 hours of presentation, thus ensuring that primary clinical decision-making occurred at the enrolling site. Exclusion criteria included (a) non-English/non-Spanish speaking patients (b) inability to provide informed consent, (c) incarceration and (d) those patients who developed elevated cardiac markers as a result of elective coronary revascularization. Institutional Review Board approval was obtained at each participating institution, and patients provided informed consent for their study participation including baseline and follow-up interviews.
Out of 2985 patients enrolled from the US, 2172 patients underwent PCI during their initial hospitalization in the study cohort and were included in our study. Out of these, we excluded patients with missing information regarding creatinine and weight which was necessary to calculate renal- or weight-based drug dosing, leading to a final population of N=2076. For patients who underwent PCI more than once, we only considered information from the initial PCI. We abstracted data for the administration of GPIs, bivalirudin and UFH administered prior to and during PCI from the patients’ medical records. For each medication, recommended dosing was defined in accordance with AHA guidelines, package inserts, and clinical trial publications. We characterized excess dosing into minor and major categories (Table 1). Minor excess dosing was defined as administration of the drug in up to 10% excess of the recommended dose. Major excess dosing was defined as administration of the drug in more than 10% excess of the recommended dose.4,5
Table 1.
Dosing Recommendations and Categories for Antiplatelet and Antithrombotic agents for patients undergoing percutaneous coronary intervention adapted from the American College of Cardiology/American Heart Association guidelines – 2009 update
| Drug | Recommendations during PCI | Minor Excess Dosing | Major Excess Dosing |
|---|---|---|---|
| GPIs | |||
| Abciximab | Bolus 0.25 mg/kg IV; Infusion 0.125 mcg/kg/min (maximum 10 mcg/min) | 0.25mg/kg> Bolus <0.275 mg/kg; 0.125 mcg/kg/min > Infusion <0.1375 mcg/kg/min | Bolus > 0.275 mg/kg; Infusion >0.1375 mcg/kg/min OR Infusion > 10 mcg/min whichever is less |
| Eptifibatide | Bolus 180 mcg/kg IV; Infusion 2.0 mcg/kg/min, reduce to 1.0 mcg/kg/min if CrCl<50 mL/min | 180 mcg/kg > Bolus < 198 mcg/kg; 2.0 mcg/kg/min > Infusion rate < 2.2 mcg/kg/min | Bolus > 198 mcg/kg; Infusion rate > 2.2 mcg/kg/min OR; Full dose if CrCl< 50 mL/min |
| Bivalirudin | Bolus 0.75 mg/kg; Infusion 1.75 mg/kg/hour | 0.75 mg/kg > Bolus < 0.825 mg/kg; 1.75 mg/kg/hour > Infusion rate < 1.925 mg/kg/hour | Bolus > 0.825 mg/kg; Infusion rate > 1.925 mg/kg/hour |
| Unfractionated Heparin | With GPIs : 50–70 U/kg No GPIs: 70–100 U/kg; Infusion rate: 12–15 U/kg/hour | With GPIs: 70U kg >UFH bolus<77 U/kg ; No GPIs: 100 U/kg> UFH bolus< 110 U/kg; 15 U/kg/hour> Infusion <16.5 U/kg/hour | With GPIs : UFH bolus> 77 U/kg; No GPIs: UFH bolus> 110 U/kg; Infusion>16.5 U/kg/hour |
Abbreviations: CrCl – Creatinine clearance
For GPIs, the strength of the solution administered (eptifibatide – 2 mg/mL or 0.75 mg/mL; abciximab – 2mg/mL or 9 mg/250 mL or other) was recorded to determine the dose of bolus (eptifibatide – mcg/kg; abciximab – mg/kg) and infusion (mcg/kg per minute for both). For bivalirudin, the bolus (mg or mL) and infusion (mg/h or mL/h) doses as recorded in the medical record abstraction tool were converted into the bolus (mg/kg) and infusion (mg/kg/h) doses administered using strength of bivalirudin (5 mg/mL) and total body weight. For UFH, bolus (U) and infusion doses (U/h) were divided by total body weight (kg) to determine the doses in U/kg and U/kg per hour respectively. For UFH boluses, we classified patients as receiving excess dosing if any individual bolus, not cumulative dose, was in more than 10% excess of standard dosing guidelines.11
We estimated glomerular filtration rate (GFR) using the CKD-EPI equation which has been shown to estimate GFR with the highest precision and accuracy, particularly in the younger age groups and in women.12 We defined patients as having renal dysfunction if their eGFR value was ≤60ml/min/1.73 m2.
We defined bleeding as (1) occurring at percutaneous entry site, during or after catheterization laboratory visit until discharge, which may be external or a hematoma > 10 cm for femoral, > 5 cm for brachial, or > 2 cm for radial access; (2) retroperitoneal; (3) gastrointestinal; (4) genitourinary; and (5) other/unknown origin during or after catheterization laboratory visit until discharge. All bleeding events required a transfusion, prolonged hospital stay, and/or a drop in hemoglobin > 3.0 g/dL. This was in accordance with the commonly used definitions (CathPCI registry).13
Patient characteristics, presentation and treatment are shown for overall patients, and are stratified by sex and whether they received excess dosing of anticoagulants or not. Categorical variables are reported as numbers (percentages) and continuous variables are reported as medians [interquartile ranges (IQR)] or mean (SD). We tested the significance of observed differences with chi-square tests for categorical variables and Wilcoxon rank sum tests or t-tests for continuous variables. We explored factors associated with excess dosing using multivariable logistic regression to adjust for patient demographics and co-morbidities previously shown to be associated with increased risk of both excess dosing and bleeding.4
We explored factors associated with bleeding in patients receiving these therapies by using multivariable logistic regression and adjusted for excess dosing and other socio-demographic and clinical covariates previously shown to affect risk of bleeding.14 These included sex, age, weight, eGFR, diabetes mellitus, PCI urgency, systolic blood pressure and prior peripheral arterial disease. We repeated the analysis to examine the effect of ‘major’ excess dosing in place of ‘any’ excess dosing on bleeding, and repeated the bleeding models including a gender-excess dosing interaction. We considered a 2-sided P<0.05 as statistically significant. All analyses were done using SAS 9.3 (SAS Institute Inc., Cary, NC) and VIRGO data version 1.0.
Results
The final cohort consisted of 2076 AMI patients who underwent PCI, 1311 women and 765 men. A total of 1172 (56.5%) patients received GPIs, 692 (33.3%) patients received bivalirudin and 1623 (78.2%) patients received UFH. Of these, 352 patients received a combination of bivalirudin and UFH, 152 patients received a combination of bivalirudin and GPIs, and 982 patients received a combination of UFH and GPIs.
Although both women and men were of similar age, women had a lower mean body weight than men (86.7 vs. 97.8 kg; p<0.01) (Table 2). Overall, 47.2% of the patients received at least one dose of an anticoagulant in excess, and 6.6% of patients received more than one class of medications in excess. Within individual categories of medications, 40% of the patients receiving GPIs, 49% of the patients receiving bivalirudin and 18.9% of the patients receiving UFH received at least one excess dose of these drugs (Table 3).
Table 2.
Baseline Characteristics by Sex in Patients ≤ 55 Years of Age with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention
| Patient characteristics | Overall (n=2076) | Women (n=1311) | Men (n=765) | P value |
|---|---|---|---|---|
| Age (years), median (IQR) | 48.0 (44.0 – 52.0) | 49.0 (44.0 – 52.0) | 48.0 (44.0 – 52.0) | 0.13 |
| Obesity (BMI ≥ 30 kg/m2) | 1133 (54.6%) | 764 (58.3%) | 369 (48.2%) | <0.01 |
| Weight (kg), mean (std) | 90.8 (23.1) | 86.7 (22.6) | 97.8 (22.2) | <0.01 |
| Nonwhite race | 469 (22.6%) | 335 (25.6%) | 134 (17.5%) | <0.01 |
| Diabetes mellitus | 720 (34.7%) | 532 (40.6%) | 188 (24.6%) | <0.01 |
| Hypertension | 1368 (65.9%) | 885 (67.5%) | 483 (63.1%) | 0.04 |
| Current smokers | 1194 (57.5%) | 788 (60.1%) | 406 (53.1%) | <0.01 |
| Prior stroke | 84 (4.0%) | 69 (5.3%) | 15 (2.0%) | <0.01 |
| Prior myocardial infarction | 319 (15.4%) | 190 (14.5%) | 129 (16.9%) | 0.15 |
| Prior revascularization | 362 (17.5%) | 211 (16.1%) | 151 (19.9%) | 0.03 |
| Prior heart failure | 68 (3.3%) | 58 (4.4%) | 10 (1.3%) | <0.01 |
| eGFR (ml/min/1.73m2), mean (std) | 89.1 (23.1) | 88.9 (24.7) | 89.4 (20.2) | 0.64 |
| eGFR<50 ml/min/1.73m2 | 98 (4.7%) | 80 (6.1%) | 18 (2.4%) | <0.01 |
| Renal Dysfunction (eGFR<=60) | 211 (10.2%) | 151 (11.6%) | 60 (7.9%) | 0.01 |
| Serum Creatinine (mg/dL), median (IQR) | 0.9 (0.7 – 1.0) | 0.8 (0.7 – 0.9) | 1.0 (0.9 – 1.1) | <0.01 |
| Creatinine >2mg/dL | 45 (2.2%) | 33 (2.5%) | 12 (1.6%) | 0.15 |
| Presentation variables | ||||
| Signs of heart failure | 69 (3.5%) | 51 (4.1%) | 18 (2.5%) | 0.06 |
| Systolic blood pressure (mm Hg), mean (std) | 144.7 (31.0) | 143.5 (32.4) | 146.7 (28.4) | 0.02 |
| Heart rate (bpm), mean (std) | 82.8 (19.6) | 83.6 (19.2) | 81.4 (20.1) | 0.02 |
| ST depression | 855 (41.5%) | 544 (41.8%) | 311 (40.9%) | 0.66 |
| Treatment | ||||
| Aspirin | 2012 (97.3%) | 1263 (96.9%) | 749 (98.2%) | 0.07 |
| Unfractionated heparin | 1623 (78.2%) | 1009 (77.0%) | 614 (80.3%) | 0.08 |
| Glycoprotein IIb/IIIa inhibitors | 1172 (56.5%) | 717 (54.7%) | 455 (59.5%) | 0.03 |
| Eptifibatide | 899 (43.3%) | 556 (42.4%) | 343 (44.8%) | 0.28 |
| Abciximab | 273 (13.2%) | 161 (12.3%) | 112 (14.6%) | 0.12 |
| Clopidogrel | 1718 (89.2%) | 1080 (89.5%) | 638 (88.6%) | 0.55 |
| Prasugrel | 256 (13.3%) | 156 (12.9%) | 100 (13.9%) | 0.55 |
| Ticlopidine | 3 (0.2%) | 2 (0.2%) | 1 (0.1%) | 1.00 |
| Bivalirudin | 692 (33.3%) | 459 (35.0%) | 233 (30.5%) | 0.03 |
: All tables percentages are calculated by excluding missing values
Abbreviations: BMI – Body mass index; std – standard deviation; bpm – beats per minute; eGFR – estimated glomerular filtration rate; IQR – interquartile range
Table 3.
Frequency of Excess Dosing of Anticoagulants in Young Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention
| Drugs | Any excess dosing (>0%) | Minor excess dosing (0–10%) | Major excess dosing (>10%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | P value | Female | Male | P value | Female | Male | P value | |
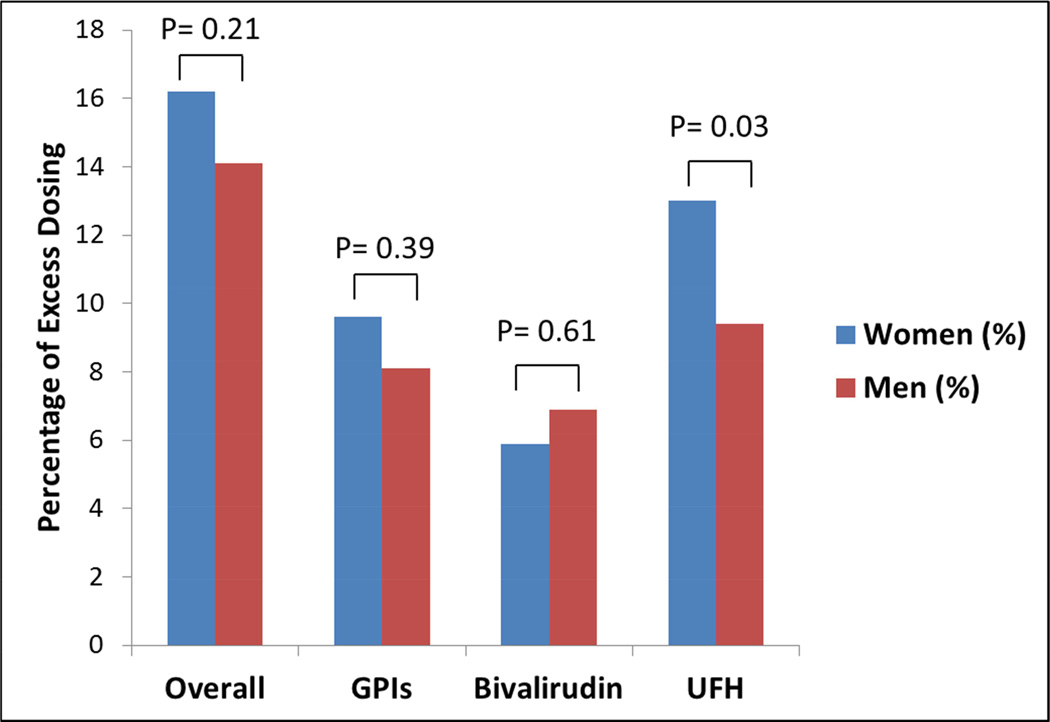
| Overall | 621 (47.4%) | 359 (46.9%) | 0.85 | 409 (31.2%) | 251 (32.8%) | 0.45 | 212 (16.2%) | 108 (14.1%) | 0.21 |
| GPIs | 287 (40.0%) | 187 (41.1%) | 0.72 | 218 (30.4%) | 150 (33.0%) | 0.36 | 69 (9.6%) | 37 (8.1%) | 0.39 |
| Bivalirudin | 220 (47.9%) | 119 (51.1%) | 0.43 | 193 (20.4%) | 103 (19.7%) | 0.75 | 27 (5.9%) | 16 (6.9%) | 0.61 |
| UFH | 206 (20.4%) | 100 (16.3%) | 0.04 | 75 (10.0%) | 42 (8.5%) | 0.37 | 131 (13.0%) | 58 (9.4%) | 0.03 |
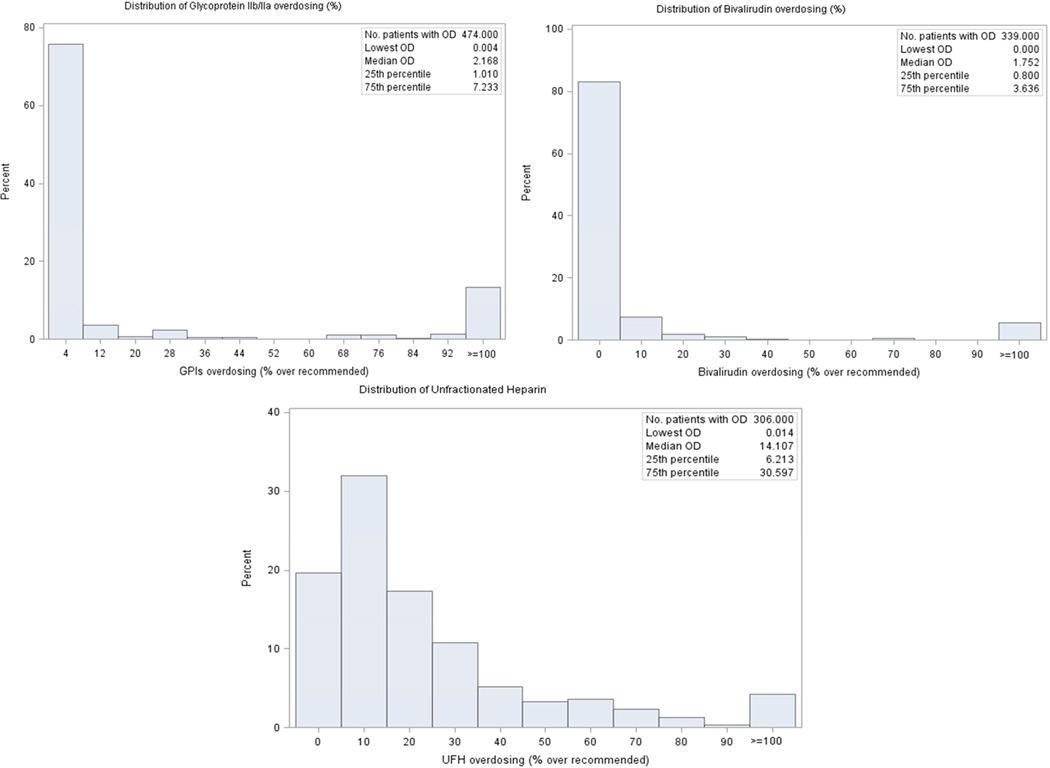
Of note, 25.9% (n=51) of the patients who were administered excess UFH boluses received a dose of exactly 5000 units and 21.9% (n=30) of the patients who were infused UFH in excess received a dose of exactly 1000 U/h when heparin therapy was initiated. For GPIs and bivalirudin, excess dosing was most frequently minor (77.6% and 87.3% respectively). However, 61.8% of excess dosing for UFH was major (Figure 1). Overall, the proportion of women who received at least one excess dose of these anticoagulant agents was not significantly different from men (Figure 2). However, UFH was administered in excess more frequently in women [n=206 (20.4%)] as compared with men [n=100 (16.3%)] (P value = 0.04) (Table 3).
Figure 1.
Distribution of Excess Dosing of GPIs, Bivalirudin and UFH above the Recommended Range in Young Patients with AMI Undergoing PCI
Figure 2.
Sex Differences in ‘Major’ Excess Dosing of Anticoagulant Agents in Young Patients with AMI Undergoing PCI
In univariate analyses, patients receiving excess dosing were less likely to be obese (49.9% vs. 58.8%; p<0.0001) or to have diabetes (32% vs. 37%; p=0.02) than patients who did not receive excess dosing. In multivariate logistic analysis, female sex was not significantly associated with excess dosing (OR 0.89, 95% CI: 0.73–1.08). Both lower body weight (OR 1.07 per 5 kg decrease in weight, 95% CI: 1.05–1.09) and younger age (OR 0.93 per 5 years increase in age, 95% CI: 0.86–0.99) were significantly associated with increased likelihood of excess dosing (Table 4). In secondary analyses, we performed multivariate logistic analysis to identify predictors of major excess dosing, and the findings did not differ significantly from any excess dosing.
Table 4.
Multivariate Logistic Model for the Association between Sex and ‘Any’ Excess Dosing of Anticoagulants in Young Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention
| Any Excess dosing (>0%) | Overall (n=2069) | Glycoprotein IIb/IIIa (n=1169) | Bivalirudin (n=689) | Unfractionated Heparin (n=1618) | ||||
|---|---|---|---|---|---|---|---|---|
| Covariates | Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value |
| Female vs .Male | 0.89 (0.73 – 1.08) | 0.2 | 0.84 (0.65 – 1.09) | 0.2 | 0.91 (0.64 – 1.27) | 0.6 | 0.92 (0.69 – 1.24) | 0.6 |
| White race vs. non-white race | 0.96 (0.77 – 1.19) | 0.7 | 0.87 (0.64 – 1.17) | 0.4 | 0.85 (0.60 – 1.21) | 0.4 | 1.27 (0.91 – 1.78) | 0.2 |
| Diabetes mellitus | 0.93 (0.76 – 1.12) | 0.4 | 1.12 (0.86 – 1.46) | 0.4 | 0.81 (0.58 – 1.14) | 0.2 | 1.01 (0.76 – 1.36) | 0.9 |
| Chronic Heart Failure | 0.99 (0.59 – 1.67) | 1.0 | 0.75 (0.33 – 1.73) | 0.5 | 1.65 (0.74 – 3.66) | 0.2 | 1.36 (0.65 – 2.88) | 0.4 |
| ST- elevation Myocardial Infarctiona | 0.98 (0.81 – 1.17) | 0.8 | 0.85 (0.66 – 1.09) | 0.2 | 0.97 (0.71 – 1.31) | 0.8 | 0.98 (0.75 – 1.29) | 0.9 |
| eGFR (decrease of 10 points) | 1.01 (0.97 – 1.05) | 0.6 | 1.10 (1.04 – 1.16) | <0.01 | 0.94 (0.88 – 1.01) | 0.1 | 1.02 (0.96 – 1.08) | 0.6 |
| Age (increase of 5 years) | 0.93 (0.86 – 0.998) | 0.05 | 0.93 (0.84 – 1.03) | 0.2 | 0.94 (0.83 – 1.07) | 0.3 | 0.98 (0.88 – 1.10) | 0.7 |
| Weight (decrease of 5kg) | 1.07 (1.05 – 1.09) | <0.01 | 1.06 (1.03 – 1.09) | <0.01 | 1.00 (0.97 – 1.04) | 0.9 | 1.19 (1.15 – 1.24) | <0.01 |
Reference group for this variable is Non ST-elevation Myocardial Infarction
Abbreviations: eGFR – Estimated Glomerular Filtration Rate
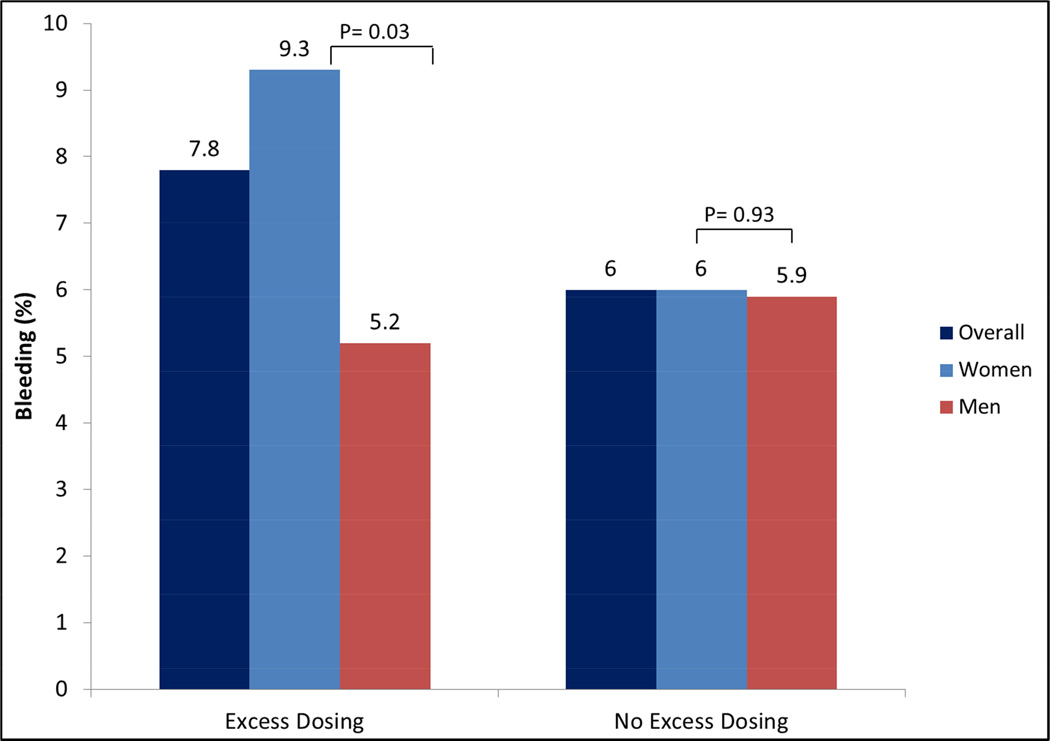
Bleeding occurred in 6.3% (n=131) of the patients who underwent PCI in the VIRGO population. Among patients who were administered GPIs and bivalirudin, bleeding rates were similar in patients receiving excess dosing compared with those who did not (GPIs: 9.8% vs. 7.4%; p=0.2; bivalirudin: 3.6% vs. 3.4%; p=0.9). For UFH, the bleeding rate was slightly higher in patients receiving excess dose though this did not reach statistical significance (10.0% vs. 6.8%; p=0.06). When stratified by sex, frequency of bleeding was higher in women who received excess dosing of UFH compared with women who did not (9.3% vs. 6.0%, P=0.03); however rates in men were similar (5.2% vs. 5.9%, P= 0.69) (Figure 3). In the multivariable model, excess dosing was associated with increased bleeding (OR 1.34, 95% CI: 0.98–1.98), but this was not statistically significant (Table 5). While eGFR per 10 units decrease (OR 1.11, 95% CI: 1.03–1.21), higher systolic blood pressure (OR 2.16, 95% CI: 1.21–3.86) and ST-elevation myocardial infarction (OR 1.92, 95% CI: 1.19–3.10) were associated with an increased likelihood of bleeding, the association with female sex did not attain statistical significance (OR 1.28, 95% CI: 0.91–1.98). In secondary analyses, we replaced ‘any’ excess dosing with ‘major’ excess dosing as a covariate in the multivariable model for bleeding, and the findings did not differ significantly. In addition, we also found that the interaction term for gender-excess dosing was not a significant predictor of bleeding (p=0.17) when included in the multivariable model for bleeding.
Figure 3.
Bleeding Complications in Patients Receiving Excess Dosing of Anticoagulants in Young Patients with AMI Undergoing PCI
Table 5.
Multivariate Logistic Model for the Association between Excess Dosing of Anticoagulant Agents and Bleeding in Young Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention
| Any Excess Dosing (>0%) | Overall Drug (n=1681) | |
|---|---|---|
| Covariates | Odds Ratio (95% CI) | P value |
| Excess dosing | 1.34 (0.91 – 1.98) | 0.14 |
| Female vs. Male | 1.28 (0.83 – 2.00) | 0.27 |
| Diabetes mellitus | 1.41 (0.93 – 2.13) | 0.11 |
| Percutaneous coronary intervention urgency | 1.07 (0.50 – 2.33) | 0.86 |
| Systolic blood pressure | 2.16 (1.21 – 3.86) | 0.01 |
| Prior peripheral arterial disease | 2.17 (0.83 – 5.63) | 0.11 |
| ST-elevation Myocardial Infarctiona | 1.92 (1.19 – 3.10) | 0.01 |
| Aspirin + prasugrelb | 0.42 (0.19 – 0.92) | 0.03 |
| eGFR (decrease of 10 points) | 1.11 (1.03 – 1.21) | 0.01 |
| Age (increase of 5 years) | 1.03 (0.87 – 1.23) | 0.70 |
| Weight (decrease of 5 kg) | 1.00 (0.96 – 1.05) | 0.98 |
Reference group for this variable is Non ST-elevation Myocardial Infarction
Reference group for this variable is aspirin + clopidogrel/ticlopidine
Abbreviation: eGFR – Estimated Glomerular Filtration Rate
Discussion
Results from this prospective cohort study provide detailed characterizations of dosing of antithrombotic and antiplatelet agents in patients ≤ 55 years of age with AMI undergoing PCI. Of note, approximately half received at least one dose of an anticoagulant agent in excess of the recommended range, and 1 in 5 received more than 10% excess of at least one medication. Low body weight and younger age, but not sex, were noted to be significant predictors of excess dosing. However, excess dosing of these agents among young patients was not significantly associated with a higher likelihood of bleeding.
More than a decade after the Institute of Medicine reported medication errors to be a nationwide epidemic;15 we found dosing errors in more than half of the young patients with AMI undergoing PCI. Appropriate dosing of antiplatelet and anticoagulant agents, in particular, is of critical importance in patients undergoing PCI, as dosing outside of the ‘therapeutic window’ of these drugs may increase the risk of bleeding complications.4,5,16. In a previous study by Alexander et al., 42% of older patients with non ST-elevation myocardial infarction received at least one dose of anticoagulant in excess of the recommended range and older age was strongly associated with higher likelihood of excess dosing.4 Our study suggests that dosing errors are also highly prevalent among younger patients. In our population of patients <=55 years of age, every 5 year decrease in age was associated with increased risk of excess dosing. The reasons why youngest patients are at increased risk of excess dosing are unclear but may warrant further study.
In contrast with previous investigators, we did not identify sex-based differences in the likelihood of receiving excess dose.4,16 Temporal changes in care, possible age-gender interactions and differences in definition of overdosing and inclusion criteria may account for this discordance. While sex differences were prominently noted for excess dosing of GPIs in a relatively elderly population with non ST-elevation myocardial infarction in the CRUSADE study, our cohort is comprised of young patients with AMI undergoing PCI. In addition, our definition of excess dosing was based not only on the administration of a ‘full dose’ in patients with decreased creatinine clearance in their study, but the precise doses of all the drugs administered in the catheterization laboratory.
In unadjusted analyses, UFH was more frequently administered in excess among young women as compared with men, consistent with prior studies.4,5 Of note, the majority of the overdosing for UFH met our definition of major excess dosing. One of the potential reasons for this difference can be attributed to their smaller body size. Low body weight was independently related with excess dosing in the adjusted model which may reflect challenges in accurately estimating the body weight of thinner patients.
Our analysis also suggests that weight-based dosing guidelines for anticoagulants may not have been universally adopted. A high proportion of both men and women received the exact bolus dose of 5000 units and/or infusion dose of 1000 units/hr of UFH. This may be due to chance, but raises the possibility that a ‘one size fits all’ approach may still be employed by many clinicians. Furthermore, high frequency of excess dosing of all three classes of anticoagulants in our study suggests that overdosing is a relatively common occurrence in the catheterization laboratory. Possible solutions to this problem include allocating the responsibility of accurate weight measurement to dedicated staff for all patients going to the catheterization laboratory, use of standardized order sets, closer collaboration with pharmacy staff, and computerized dose calculations for these medications.
When stratified by sex, we found a higher unadjusted rate of bleeding in women who received excess dosing compared with those who did not, while bleeding rates were comparable in men who did and did not receive excess dosing. This is consistent with previous studies that demonstrated the relationship of excess dosing with bleeding to be stronger in women than men.16 However, after adjustment, gender was no longer a significant predictor of bleeding. The young patients enrolled in our study had relatively few bleeding events, and our study was likely underpowered to identify a clinically important association between excess dosing and bleeding outcomes. Based on estimates from previous studies, we anticipated that our study would have power to detect at least 3% difference in bleeding.4,5,16 Nevertheless, the fact that such a large proportion of patients receive excess doses of commonly used medications is of concern and identifies the need to develop strategies that would prevent deviance from accepted dosing guidelines.
Our study has some limitations. First, participation of the patients in the VIRGO study was voluntary. Patients who agreed to participate may not have been representative of the larger population of young patients with AMI. Second, information about doses of upstream medications, especially for transfer-in patients (41.6% of the total patients) may have been limited by availability of data as this information was derived from the admit notes. However, doses administered in the catheterization laboratory are considered reliable as these were directly abstracted from the catheterization log. Moreover, for patients who underwent more than one PCI or a coronary artery bypass graft surgery after PCI, we included the dosing details of the first PCI only. However, repeat PCI and coronary artery bypass graft surgery occurred only in 4.5% and 1.9% of the patients included in our study and would likely not have influenced our findings substantially. Additionally, risk of bleeding may have been influenced by additional therapies delivered to the patient after the procedure. These may include the need for additional invasive procedures, sheath management, and antithrombotic and antiplatelet therapies. Unfortunately, this information was not collected as part of the VIRGO study and these aspects of care cannot be considered in the analysis. Finally, wide confidence intervals demonstrated in our adjusted results did not allow us to draw definitive conclusions about the relationship of excess dosing with bleeding.
Acknowledgments
Funding: This work was supported by Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO), grant R01 HL081153-06, from the National Heart, Lung, and Blood Institute.
Dr. Curtis receives salary support from the American College of Cardiology Data Registry to provide analytic services and the Centers for Medicare & Medicaid Services to support development of quality measures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
Dr. Curtis holds equity interest in Medtronic.
References
- 1.Kanjanarat P, Winterstein AG, Johns TE, Hatton RC, Gonzalez-Rothi R, Segal R. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm. 2003;60:1750–1759. doi: 10.1093/ajhp/60.17.1750. [DOI] [PubMed] [Google Scholar]
- 2.Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P, Pirmohamed M. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol. 2007;63:136–147. doi: 10.1111/j.1365-2125.2006.02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 4.Alexander KP, Chen AY, Roe MT, Newby LK, Gibson CM, Allen-LaPointe NM, Pollack C, Gibler WB, Ohman EM, Peterson ED. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. JAMA. 2005;294:3108–3116. doi: 10.1001/jama.294.24.3108. [DOI] [PubMed] [Google Scholar]
- 5.Wang TY, Chen AY, Alexander KP, Ohman EM, Gibler WB, Peterson ED, Roe MT. Excess heparin dosing among fibrinolytic-treated patients with ST-segment elevation myocardial infarction. Am J Med. 2008;121:805–810. doi: 10.1016/j.amjmed.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Argulian E, Patel AD, Abramson JL, Kulkarni A, Champney K, Palmer S, Weintraub W, Wenger NK, Vaccarino V. Gender differences in short-term cardiovascular outcomes after percutaneous coronary interventions. Am J Cardiol. 2006;98:48–53. doi: 10.1016/j.amjcard.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 7.Srinivas VS, Garg S, Negassa A, Bang JY, Monrad ES. Persistent sex difference in hospital outcome following percutaneous coronary intervention: results from the New York State reporting system. J Invasive Cardiol. 2007;19:265–268. [PubMed] [Google Scholar]
- 8.Abramson JL, Veledar E, Weintraub WS, Vaccarino V. Association between gender and in-hospital mortality after percutaneous coronary intervention according to age. Am J Cardiol. 2003;91:968–971. A964. doi: 10.1016/s0002-9149(03)00114-0. [DOI] [PubMed] [Google Scholar]
- 9.Radovanovic D, Erne P, Urban P, Bertel O, Rickli H, Gaspoz JM. Gender differences in management and outcomes in patients with acute coronary syndromes: results on 20,290 patients from the AMIS Plus Registry. Heart. 2007;93:1369–1375. doi: 10.1136/hrt.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichtman JH, Lorenze NP, D'Onofrio G, Spertus JA, Lindau ST, Morgan TM, Herrin J, Bueno H, Mattera JA, Ridker PM, Krumholz HM. Variation in recovery: Role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3:684–693. doi: 10.1161/CIRCOUTCOMES.109.928713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5:1003–1009. doi: 10.2215/CJN.06870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 14.Mehta SK, Frutkin AD, Lindsey JB, House JA, Spertus JA, Rao SV, Ou FS, Roe MT, Peterson ED, Marso SP. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. 2009;2:222–229. doi: 10.1161/CIRCINTERVENTIONS.108.846741. [DOI] [PubMed] [Google Scholar]
- 15.Healthcare Cost and Utilization Project. HCUP Methods Series. [Accessed: 12/26/2013]; Available at http://www.hcup-us.ahrq.gov/reports/methods/methods_topic.jsp.
- 16.Alexander KP, Chen AY, Newby LK, Schwartz JB, Redberg RF, Hochman JS, Roe MT, Gibler WB, Ohman EM, Peterson ED. Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) initiative. Circulation. 2006;114:1380–1387. doi: 10.1161/CIRCULATIONAHA.106.620815. [DOI] [PubMed] [Google Scholar]