Abstract
Familial breast cancer occurs in about 10% of breast cancer cases. Germline mutation in BRCA1 is the most penetrant predisposition for the disease. The mutated BRCA1 leads to the disease by causing genome instability via multiple mechanisms including epigenetic changes. This review summarizes recent progress in studying the correlation between BRCA1 predisposition and epigenetic alterations in BRCA1-type familial breast cancer.
Breast cancer is the most frequent cancer in women (1). In the United States, the chance of a woman being diagnosed with breast cancer is 1 in 8 in her lifetime (2). Of the breast cancer cases, about 10% or so are familial breast cancer, in which multiple members across generations in the family are affected in an autosomal dominant manner. Germline predisposition has been determined as the major factor for familial breast cancer. The best-known predisposition is the germlne mutation in BRCA1 (BRCA1+) (3, 4, 5). A woman who has inherited a pathogenic mutation in BRCA1 (BRCA1+) has up to 80% probability of developing breast cancer by the age of 70 (6).
BRCA1 is a tumor suppressor gene that plays essential roles in maintaining genomic stability through multiple mechanisms including homologous double strand DNA break repair, transcriptional regulation, chromatin remodeling, ubiquitin ligation, cell cycle and apoptosis regulation (7). Germline mutations in BRCA1 cause premature truncation, translational frame-shift, altered splice sites, and nonsense-mediated RNA decay. These changes damage the normal function of BRCA1, cause genome instability, and lead to the development of breast and ovarian cancer (8, 9). It was previously shown that the BRCA1 gene can be regulated by promoter DNA methylation (10). Additionally, more recent studies show that BRCA1 also plays important roles in multiple types of epigenetic modifications and mutated BRCA1 can cause epigenetic abnormalities, which contribute to the development of breast cancer.
The role of BRCA1 in DNA methylation
Of the four types of DNA methyltransferases, DNMT1, DNMT3a DNMT3b and DNMT3L, DNMT1 is essential in methylation of the nascent replicated DNA strand to maintain the original DNA methylation pattern of DNA (11). In contrast, DNMT3a and DNTM3b act as de novo methyltransferases to methylate previously unmethylated DNA (12, 13). DNMT3L is involved in maternal genomic imprints (14 13). Approximately 60% of protein-coding genes harbor CpG islands in their promoters (15). In general, hypomethylated promoters are transcriptionally more active than hypermethylated promoters, which are often silenced (16). Many of those genes silenced by DNA methylation are involved in important functions including cell cycle control, steroid receptors, metastasis, oncogene and tumor suppressor. The relationship between BRCA1 and DNA methylation has been extensively studied in familial breast cancer (17–18).
Followings are examples showing the relationship between BRCA1 and methylation:
-
-
BRCA1 and hypermethylation
Abnormal expression of estrogen receptor plays important role in breast cancer development, and the majority of BRCA1+ familial breast cancer is estrogen receptor negative. The promoter of estrogen receptor alpha (ERα) in BRCA1+ familial breast cancer is highly methylated (19), explaining why ERα is low in BRCA1+ familial breast cancer. Certain tumors exhibit aberrant concurrent hypermethylation of specific genes, a phenomenon known as CpG island methylator phenotype (CIMP) (20). Hypermethylation across the genome of blood cells in BRCA1+ carriers had significant correlation with breast cancer development and can be used accurately for disease prediction (21).
-
-
BRCA1 and hypomethylation
In BRCA1+ familial breast cancer, DNA methylation is less abundant compared to sporadic breast cancers with normal BRCA1 (22). Hypomethylation leads these genes, such as the oncogene Rad9, to have increased expression, and thus promotes breast cancer proliferation and invasion (23). Hypomethylation can also cause unbalanced chromosomal translocation with breakpoints in pericentromeric DNA of specific chromosomes (24). Hypomethylation is also common in noncoding Alu and LINE-1 repetitive sequences in breast cancer (25). Studies in a Brca1-deficient mouse model showed that Brca1 can bind directly to the OCT1 site of DNMT1 promoter to activate DNMT1 expression at transcriptional level to maintain genomic methylation. Mutated BRCA1 lost this function, led to global DNA hypomethylation, loss of genomic imprinting, and over-expression of multiple oncogenes including c-Fos, Ha-Ras, and c-Myc (26).
BRCA1 can regulate gene expression through both DNA hypomethylation and hypermethylation. In general, BRCA1-regulated hypermethylation or hypomethylation is not a uniformed changes for all genes but gene type-dependent. For example, hypermethylation can suppress the expression of tumor suppressor genes and hypomethylation can promote the expression of oncogenes (26). It remains unclear for how BRCA1 plays such a selective role.
Regulation of BRCA1 expression by promoter DNA methylation
In certain familial breast cancer, BRCA1 gene coding structure remains intact but the CpG islands in BRCA1 promoter can be highly hypermethylated (27). Methylation in multiple CpG islands of the BRCA1 promoter was observed in nearly a third of breast cancer cases. The event was more frequent in breast cancer in younger women, with high-grade pathology, and triple-negative breast cancer (estrogen receptor, progesterone receptor, Her2/Neu) (28). Compared with the BRCA1 promoter without methylation, hypermethylation led to decreased or even completely abrogated expression of BRCA1. This event correlated with poor survival, and is present in both BRCA1+ familial breast cancer and sporadic breast cancer (29, 30). The significance of BRCA1 methylation is that BRCA1 promoter methylation in sporadic breast cancer can lead to functional consequence of loss of BRCA1 function similar to BRCA1 mutation in hereditary breast cancer (31, 32).
Relationship between BRCA1 and histone modification
Histone proteins constitute the nucleosome core around which DNA is tightly packaged. Amino-terminal tails which protrude from the histone core harbor numerous sites for protein post-translational modification, such as acetylation, methylation, phosphorylation, sumoylation, ubiquitylation, or ADP-ribosylation (33). The type of modification and the affected amino acid residue determine the DNA-histone or other protein-histone interactions, leading to either an open chromatin state (active transcription) or compact chromatin (transcriptional repression). BRCA1 ubiquitylates histone H2A in both major and minor satellite regions and the RING finger domain in the N-terminal of BRCA1 is determined to be essential for this function (34). Mutation in the RING finger domain of BRCA1 results in the loss of histone H2A ubiquitylation, leading to increased expression of satellite DNA, deficient homologous recombination and genome instability. BRCA1 also deacetylates H2A and H3 through interaction between its BRCT domain and histone deacetylases HDAC1 and HDAC2 (35). Without BRCA1, HDAC2 cannot properly deacetylate H2A and H3, leaving histones in their active form. BRCA1 also interacts with HDAC1 to deacetylate the genes involved in Ku70-involved non-homologous recombination pathway for DNA damaging repair (36). Lysine-specific histone demethylase 5B (KDM5B) is involved in DNA homologous damage repair through recruiting BRCA1 to the damaged sites (37). BRCA1+ abolishes this interaction. Without functional BRCA1, It is likely that KDM5B will not be able to facilitate repair the damaged DNA.
BRCA1 and microRNAs
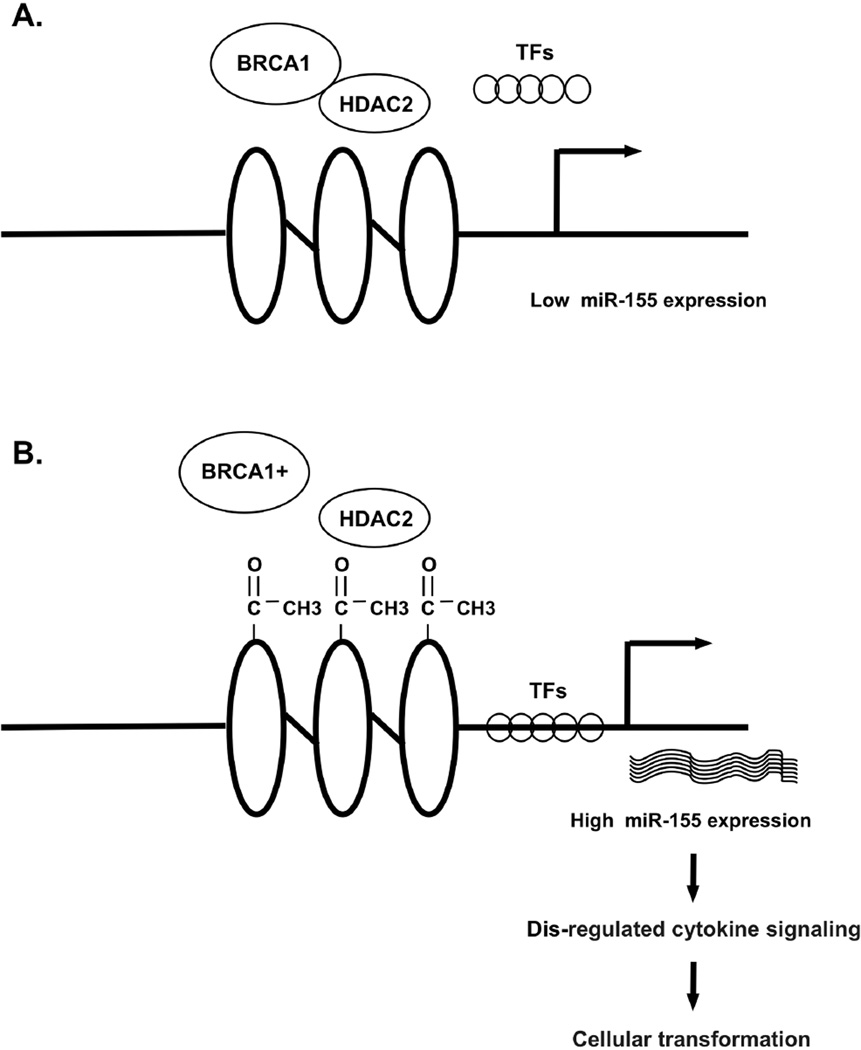
Certain miRNAs have been implicated in BRCA1-related regulation of histone modification. Studies show in breast cancer, at least 16 miRNAs were downregulated (miR-9-1, miR-29a-c, miR-148, let-7, miR-15/16, miR-17-5p, miR-27b, miR-125a/b, miR-126, miR-130a, miR-143, miR-145, miR-155, miR-200c, miR-205, miR-335) and 5 were up regulated (miR-10b, miR-18a, miR-21, miR-27a, miR-206)(38). However, this pattern may not be conclusive. Taking miR-155 as a typical example: miR-155 regulates many genes involved in apoptosis, differentiation, angiogenesis, proliferation, and epithelial-mesenchymal transition (39–40), such as SOCS1, a member of the STAT-induced STAT inhibitor in negative regulators of cytokine signaling (41). BRCA1 represses miR-155 expression by deacetylating histone H2A and H3 on the miR-155 promoter via HDAC2. The mutated BRCA1 leads to acetylation of miR-155 promoter, overexpression of miR-155, dis-regulation of cytokine signaling pathways, and promotes cellular transformation (38–41, Figure 2).
Figure 2.
BRCA1 regulates miR-155 expression. A. BRCA1 maintains intact structure of promoter of miR-155 by suppressing HDAC2 activity, block transcriptional factors binding to promoter elements, resulting in decreased miR-155 transcription; B. Mutated BRCA1 loses its suppression on HDAC2 activity, leading to increased acetylation of miR-155 promoter, increased transcriptional factors binding to promoter elements, and increased miR-155 transcription. The overexpressed miR-155 causes dis-regulation of cytokine signaling pathways, and promotes cellular transformation.
In conclusion, epigenetic abnormality in BRCA1+ breast cancer provides new avenues to understand the mechanism of BRCA1+ caused familial breast cancer, and could provide potentially new targets, for example, targeting the overexpressed miRNAs and DNA methyltransferases, for treatment of familial breast cancer.
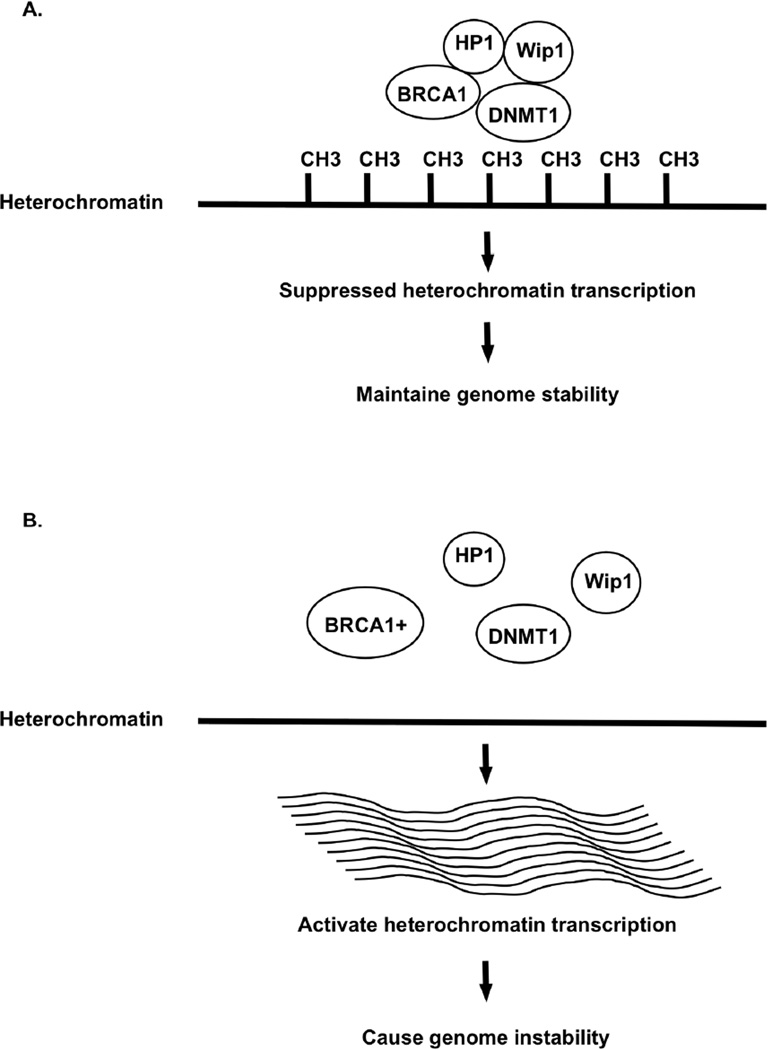
Figure 1.
BRCA1 and heterochromatin methylation. BRCA1 silences heterochromatin transcription by methylating heterochromatin through interaction with Wip1 phosphatase, HP1, and DNA methyltransferase. BRCA1+ loses heterochromatin demethylation, releases the transcriptional silencing, leads overexpression of heterochromatin transcripts, and causes genome instability. CH3: methyl group.
Acknowledgments
The study was supported by a pilot grant from Fred & Pamela Buffett Cancer Center, University of Nebraska Medical Center and a NIH grant 1R21CA180008 (SMW). The funding bodies play no roles in design, collection, analysis, and interpretation of data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer. J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement. Ann Intern Med. 2005;143(5):355–361. doi: 10.7326/0003-4819-143-5-200509060-00011. [DOI] [PubMed] [Google Scholar]
- 3.Hall JM, Lee MK, Newman B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 4.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 5.Couch FJ, Nathanson KL, Offit K. Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science. 2014;343(6178):1466–1470. doi: 10.1126/science.1251827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoniou A, Pharoah PDP, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. American Journal of Human Genetics. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye Q, Hu YF, Zhong H, et al. BRCA1-induced large-scale chromatin unfolding and allele-specific effects of cancer-predisposing mutations. J Cell Biol. 2001;155:911–921. doi: 10.1083/jcb.200108049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratton MR, Rahman N. The emerging landscape of breast cancer susceptibility. Nat Genet. 2008;40:17–22. doi: 10.1038/ng.2007.53. [DOI] [PubMed] [Google Scholar]
- 9.Welcsh PL, Owens KN, King MC. Insights into the functions of BRCA1 and BRCA2. Trends Genet. 2000;16:69–74. doi: 10.1016/s0168-9525(99)01930-7. [DOI] [PubMed] [Google Scholar]
- 10.Wei M, Grushko TA, Dignam J, et al. BRCA1 promoter methylation in sporadic breast cancer is associated with reduced BRCA1 copy number and chromosome 17 aneusomy. Cancer Res. 2005;65:10692–10699. doi: 10.1158/0008-5472.CAN-05-1277. [DOI] [PubMed] [Google Scholar]
- 11.Yen RW, Vertino PM, Nelkin BD, et al. Isolation and characterization of the cDNA encoding human DNA methyltransferase. Nucleic Acids Res. 1992;20(9):2287–2291. doi: 10.1093/nar/20.9.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998;19(3):219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 13.Xie S, Wang Z, Okano M, et al. Cloning, expression and chromosome locations of the human DNMT3 gene family. Gene. 1999;236(1):87–95. doi: 10.1016/s0378-1119(99)00252-8. [DOI] [PubMed] [Google Scholar]
- 14.Aapola U, Kawasaki K, Scott HS, et al. Isolation and initial characterization of a novel zinc finger gene, DNMT3L, on 21q22.3, related to the cytosine-5-methyltransferase 3 gene family. Genomics. 2000;65(3):293–298. doi: 10.1006/geno.2000.6168. [DOI] [PubMed] [Google Scholar]
- 15.Antequera F. Structure, function and evolution of CpG island promoters. Cell Mol Life Sci. 2003;60:1647–1658. doi: 10.1007/s00018-003-3088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyes J, Bird A. DNA methylation inhibits transcription indirectly via a methyl-CpG binding protein. Cell. 1991;64:1123–1134. doi: 10.1016/0092-8674(91)90267-3. [DOI] [PubMed] [Google Scholar]
- 17.Suijkerbuijk KP, Fackler MJ, Sukumar S, et al. Methylation is less abundant in BRCA1- associated compared with sporadic breast cancer. Ann Oncol. 2008 Nov;9(11):1870–1874. doi: 10.1093/annonc/mdn409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasilatos SN, Broadwater G, Barry WT, et al. CpG island tumor suppressor promoter methylation in non-BRCA-associated early mammary carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2009;18(3):901–914. doi: 10.1158/1055-9965.EPI-08-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Archey WB, McEachern KA, Robson M, et al. Increased CpG methylation of the estrogen receptor gene in BRCA1-linked estrogen receptor-negative breast cancers. Oncogene. 2002;21(46):7034–7041. doi: 10.1038/sj.onc.1205844. [DOI] [PubMed] [Google Scholar]
- 20.Toyota M, Ahnja N, Ohe-Toyota M, et al. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anjum S, Fourkala EO, Zikan M, et al. A BRCA1-mutation associated DNA methylation signature in blood cells predicts sporadic breast cancer incidence and survival. Genome Med. 2014;6(6):47. doi: 10.1186/gm567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernadino J, Roux C, Almeida A, et al. Dutrillaux, B. DNA hypomethylation in breast cancer: An independent parameter of tumor progression? Cancer Genetics and cytogenetics. 1997;97:83–89. doi: 10.1016/s0165-4608(96)00385-8. [DOI] [PubMed] [Google Scholar]
- 23.Cheng CK, Chow LWC, Wings WTY, et al. The cell cycle checkpoint gene Rad9 is a novel oncogene activated by 11q13 amplification and DNA methylation in breast cancer. The Journal of Cancer Research. 2005:1916–1930. doi: 10.1158/0008-5472.CAN-04-4243. [DOI] [PubMed] [Google Scholar]
- 24.Qu GZ, Grundy PE, Narayan A. Frequent hypomethylation in Wilms tumors of pericentromeric DNA in chromosomes 1 and 16. Cancer Genetics. 1999;109:34–39. doi: 10.1016/s0165-4608(98)00143-5. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Seo AN, Jung HY, et al. Alu and LINE-1 hypomethylation is associated with HER2 enriched subtype of breast cancer. PLoS One. 2014;9(6):e100429. doi: 10.1371/journal.pone.0100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukla V, Coumoul X, Lahusen T, et al. BRCA1 affects global DNA methylation through regulation of DNMT1. Cell Res. 2010;20(11):1201–1215. doi: 10.1038/cr.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma P, Stecklein SR, Kimler BF, et al. The prognostic value of BRCA1 promoter methylation in early stage triple negative breast cancer. J Cancer Ther Res. 2014;3(2):1–11. doi: 10.7243/2049-7962-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esteller M, Corn PG, Baylin SB, et al. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–3229. [PubMed] [Google Scholar]
- 29.Wu L, Wang F, Xu R, et al. Promoter methylation of BRCA1 in the prognosis of breast cancer: a meta-analysis. Breast Cancer Res Treat. 2013;142:619–627. doi: 10.1007/s10549-013-2774-9. [DOI] [PubMed] [Google Scholar]
- 30.Pang D, Zhao Y, Xue W, et al. Methylation profiles of the BRCA1 promoter in hereditary and sporadic breast cancer among Han Chinese. Med Oncol. 2012;29:1561–1568. doi: 10.1007/s12032-011-0100-0. [DOI] [PubMed] [Google Scholar]
- 31.Turner NC, Reis-Filho JA, Russell AM, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 32.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 33.Ruffner H, Joazeiro CA, Hemmati D, et al. Cancer-predisposing mutations within the RING domain of BRCA1: loss of ubiquitin protein ligase activity and protection from radiation hypersensitivity. Proc Natl Acad Sci U S A. 2001;98(9):5134–5139. doi: 10.1073/pnas.081068398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yarden RI, Brody LC. BRCA1 interacts with components of the histone deacetylase complex. Proc Natl Acad Sci U S A. 1999;96(9):4983–4988. doi: 10.1073/pnas.96.9.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren J, Chu Y, Ma H, et al. Epigenetic interventions increase the radiation sensitivity of cancer cells. Curr Pharm Des. 2014;20(11):1857–1865. doi: 10.2174/13816128113199990529. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Liu L, Yang S, et al. Histone demethylase KDM5B is a key regulator of genome stability. Proc Natl Acad Sci U S A. 2014;111(19):7096–7101. doi: 10.1073/pnas.1324036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veeck J, Esteller M. Breast cancer epigenetics: from DNA methylation to microRNAs. J Mammary Gland Biol Neoplasia. 2010;15:5–17. doi: 10.1007/s10911-010-9165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattiske S, Suetani RJ, Neilsen PM, et al. The oncogenic role of miR-155 in breast cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1236–1243. doi: 10.1158/1055-9965.EPI-12-0173. [DOI] [PubMed] [Google Scholar]
- 39.Chang S, Wang RH, Akagi K, et al. Tumor suppressor BRCA1 epigenetically controls oncogenic microRNA-155. Nat Med. 2011;17(10):1275–1282. doi: 10.1038/nm.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babar IA, Cheng CJ, Booth CJ, et al. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc Natl Acad Sci U S A. 2012;109(26):E1695–E1704. doi: 10.1073/pnas.1201516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshimura A, Ohkubo T, Kiguchi T, et al. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J. 1995;14(12):2816–2826. doi: 10.1002/j.1460-2075.1995.tb07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]