Abstract
Introduction
Children with systemic lupus erythematosus (SLE) have an increased prevalence of kidney disease compared to their adult counterparts. Our goal was to identify potential clinical and laboratory predictors of renal disease.
Methods
We performed a cohort study of incident and prevalent patients with SLE aged ≤ 19 years. Retrospective data from initial presentation until study enrollment was also collected. Laboratory and clinic data were recorded from each clinic visit including disease activity indices, autoantibodies, urinalyses, blood counts, and metabolic profile. Kidney disease was defined as the presence of abnormal renal biopsy or by American College of Rheumatology case definition for lupus nephritis. Logistic regression analyses were used to determine the association between clinical and laboratory data with kidney disease in those who had renal involvement within 30 days of SLE diagnosis. We also performed a time to event analysis to identify antecedents of renal disease.
Results
47 children and adolescents with SLE were followed in the cohort, 91% female and 68% Black. All of the males in the cohort developed renal disease, and all within one month of the diagnosis of SLE. In logistic regression, low serum albumin (Odds Ratio: 4.8, 95% CI: 1.9–12.5) and positive dsDNA antibodies (OR: 3.2, 95% CI: 1.7–5.9) were associated with kidney disease. In longitudinal analyses, isolated sterile pyuria (Hazard Ratio (HR): 3, 95% CI: 1.1–6.4) and low serum albumin (HR: 3.4, 95% CI: 1.7–6.9) were predictors of future kidney disease. The presence of antibodies against Ro were protective against renal disease (HR: 0.2, 95% CI: 0.05–0.5).
Conclusion
We identified variables associated with kidney disease, both at initial diagnosis of SLE and in longitudinal follow-up in a cohort of children with SLE. The recognition of these abnormal laboratory values may help clinicians identify patients at risk for kidney disease before its onset thus preventing long-term complications.
Introduction
Systemic lupus erythematosus (SLE) is a multi-system autoimmune disease that accounts for up to 10% of all pediatric rheumatic disease in the United States. Between 50–80% of children and adolescents with SLE will have kidney involvement at some point during their lifetime 1–6. Recognition and treatment of kidney disease is important because survival has been shown to be decreased in pediatric patients with SLE and kidney disease compared to those without kidney disease 6.
Kidney biopsy is the gold standard method for detection of lupus nephritis. However, it is an invasive procedure, often requiring sedation in younger patients. The American College of Rheumatology (ACR) has recently proposed guidelines for case definition of lupus nephritis 7. These guidelines were developed using systematic literature review and the opinions of qualified experts including nephrologists, pathologists, and rheumatologists as well as independent review panels. Our goal was to define predictors of kidney involvement in a cohort of pediatric patients with SLE using both the ACR case definition of lupus nephritis and biopsy-confirmed renal involvement.
Methods
SLE Cohort
Incident and prevalent pediatric patients, defined as age ≤19 years at SLE onset, were recruited into this cohort study from Johns Hopkins Children’s Center and Children’s Hospital of Philadelphia. Institutional review board approval was obtained at both institutions and all subjects/families enrolled in the cohort study consented for participation. Subjects enrolled in the cohort have study follow up in conjunction with clinic visits every 3 months or more frequently if medically necessary. Patients presented with SLE from January 2002 through December 2012. All patients met the American College of Rheumatology (ACR) classification criteria for SLE 8–10.
Demographic data including age at visit, age at lupus diagnosis, gender, race, and ethnicity were collected for enrolled subjects (both incident and prevalent SLE diagnosis) at cohort entry. At prospective q 3 month visits, medication and laboratory data including complete blood count, erythrocyte sedimentation rate, basic metabolic profile, urinalysis, spot urine protein to creatinine ratio, complement levels (C3, C4), and autoantibodies including ANA, dsDNA, Ro, La, Smith (Sm), and RNP antibodies were collected. Disease activity indices were also calculated at each visit. The SLE Disease Activity Index (SLEDAI) and the Systemic Lupus Activity Measure have been validated in children. The SLEDAI is a weighted, cumulative index that is grouped into 9 organ systems. The organ systems include central nervous system, vascular, renal, musculoskeletal, serosal, dermal, immunologic, constitutional, and hematologic. The SLAM is used to assess degree of disease activity within the preceding month and includes clinical manifestations and 8 laboratory parameters, each weighted.
For individuals entering the cohort with a prior diagnosis of SLE, retrospective chart reviews were performed to record clinical and laboratory data from the time of initial SLE diagnosis to the time of cohort entry.
Definition of Renal Disease
The ACR criteria for kidney involvement include case definition of lupus nephritis and recommendations for kidney biopsy. The indications of lupus nephritis are the following: 1) persistent proteinuria of >0.5 gram (gm) per day or greater than 3+ by dipstick, and/or 2) urinary cellular casts including red blood cells [RBCs], hemoglobin, granular, tubular, or mixed. An “active urinary sediment” defined as >5 RBC/high power field (hpf), > 5 white blood cells (WBC)/hpf in the absence of infection, or cellular casts limited to RBC or WBC casts can be substituted for cellular casts. The authors noted that a spot urine protein/creatinine ratio of >0.5 can be substituted for the 24 hour urinalysis. The ACR recommendations for kidney biopsy include: increasing serum creatinine from unknown cause, or proteinuria ≥1 gm per 24 hours (either in 24 hour urine specimen or spot protein/creatinine ratio) or a combination of the following: proteinuria ≥0.5 gm per 24 hours plus hematuria defined as ≥ 5 red blood cells per high power field or proteinuria ≥ 0.5 gm per 24 hours plus cellular casts 7. Renal disease was also defined by biopsy proven lupus nephritis as classified by current International Society of Nephrology/Renal Pathologic Society (ISNRPS) classification criteria 11. The pathology in these children has a broad range of activity, from minimal mesangial involvement (ISNRPS class I) to diffuse proliferative glomerulonephritis (ISNRPS class IV) and advanced sclerosing lupus nephritis (ISNRPS class VI). We included all types of renal pathology in these analyses as there were too few numbers in each individual category for independent analyses. Biopsies were performed if deemed clinically necessary by the treating physician.
Statistical Analysis
Using cross sectional analysis, we compared clinical and demographic characteristics for individuals with and without kidney involvement at first presentation to rheumatology or nephrology clinic. The group without kidney disease at initial clinic presentation was further divided to include children who eventually developed kidney disease vs. those who never developed renal disease during follow-up.
Univariate logistic regression analyses were used to determine the association between clinical and laboratory variables with kidney disease in patients who presented to the cohort with renal involvement (renal disease diagnosed within 30 days of initial SLE diagnosis). Power calculations indicated that with this sample size of patients, population effect size of change in values by three points, and significance level of 0.05, there was 80% power to detect significant associations between variable and kidney disease. In order to avoid Type I error, we calculated the power of the analyses at a lower significance level. For significance level of 0.01 with the same sample size and effect size, we had 72% power to detect variables associated with kidney disease. Multivariate analyses controlling for race as a potential confounder were also calculated.
Time to event analyses were used to determine the association between clinical and laboratory variables and SLEDAI AND SLAM to predict and the onset of renal disease. Time at risk was calculated using date of SLE diagnosis as the start date and last visit before the patient either met the ACR case definition for LN and/or had diagnostic kidney biopsy as the end date. Failure, or the event of interest, was defined as the development of kidney disease. Pathologic evidence of kidney disease was used for those patients who underwent biopsy. For those who did not undergo biopsy, kidney disease was defined using the ACR criteria as noted above.
Cox proportional hazard ratios were used to determine predictors of kidney disease. Both univariate and multivariate analyses were performed, controlling for race in the multivariate analysis. Clustering by identification number was used to account for multiple measures within the same patient. Power analyses show that with this sample size, we had 82% power to detect a change in variables over time and association with kidney disease.
Demographic characteristics included gender, race, and ethnicity. Laboratory data used in these analyses included autoantibodies including dsDNA, Ro, La, Smith, RNP, complement levels, hemoglobin, albumin, and sedimentation rate, and urinalysis. Laboratory variables were defined as abnormal if they were noted to be outside of the recommended ranges. dsDNA titers were noted as either positive or negative; C3 was defined as low if <79 milligrams (mg) /deciliter (dl); C4 was low if < 12 mg/dl ; low hemoglobin was defined as <11 grams (g)/dl, low albumin as <3.5 g/dl, and sedimentation rate was noted to be elevated if > 20 mm/hour. Mean SLEDAI and SLAM disease activity scores were also included in the analyses. There was no missing data from the visit prior to the onset of renal disease; other missing data were imputed. Data were analyzed using STATA, version 12 (Stata Corporation, College Station, TX). P-values less than 0.05 were considered significant.
Results
47 pediatric patients with SLE were recruited into this cohort study. 90% of patients were enrolled in the cohort at the time of initial diagnosis of SLE. In the remaining 10% of subjects, enrollment in the cohort occurred within six months-one year from the time of SLE diagnosis. For these patients, data from the time of SLE diagnosis to time of cohort enrollment were collected through chart review. The number of visits in this cohort was 12.9 ± 7.6 with over 126 person-years of data. Demographic and clinical characteristics by the presence or absence of kidney disease at initial presentation are shown in Table 1.
Table 1.
Characteristics of Cohort at Initial Clinic Presentation
| No renal disease during follow-up N=19 |
Renal disease at Baseline N=14 |
Renal disease one year or more From Baseline N=14 |
p-value Comparing No renal disease to Renal disease at Baseline |
p-value Comparing Renal disease at Baseline to Renal Disease one year or more from Baseline |
|
|---|---|---|---|---|---|
| Clinical Characteristics | |||||
| Age, years (SD) | 12.9 (3.4) | 11.5 (3.4) | 12.2 (4.4) | 0.2 | 0.6 |
| Gender, n (%) F | 19 (100) | 11 (78) | 14 (100) | 0.04 | 0.04 |
| Hispanic, n (%) | 0(0) | 1(7) | 1 (7) | 0.7 | 0.4 |
| Race, n (%) AA | 14 (74) | 9 (64) | 9 (64) | 0.6 | 0.2 |
| Laboratory Parameters | |||||
| ANA, n (%) positive | 19 (100) | 14 (100) | 14 (100) | 1 | 1 |
| dsDNA, n (%) positive | 13 (68) | 12 (85) | 8 (57) | 0.3 | 0.2 |
| Ro, n (%) positive | 5/12 (40) | 2/7 (29) | 2/7 (29) | 0.6 | 1 |
| La, n (%) positive | 1/7 (14) | 2/7 (29) | 1/7 (14) | 0.7 | 0.6 |
| RNP, n (%) positive | 5/12 (40) | 5/11 (45) | 4/11 (36) | 0.7 | 0.8 |
| Sm, n (%) positive | 1/7 (14) | 7/12 (58) | 4/10 (40) | 0.03 | 0.4 |
| Low C3, n (%) | 47 | 64 | 64 | 0.3 | 0.9 |
| Low C4, n (%) | 56 | 71 | 63 | 0.4 | 0.7 |
| Elevated ESR, n (%) | 74 | 57 | 82 | 0.3 | 0.1 |
| Low Hgb, n (%) | 16 (84) | 6 (42) | 4 (28) | 0.9 | 0.7 |
| Low Albumin, n (%) | 10 (52) | 6 (42) | 3 (21) | 0.9 | 0.4 |
| Active Urinary Sediment, n (%) | 0 (0) | 11 (78) | 0 (14) | 0.01 | 0.01 |
| Isolated Sterile Pyuria, n (%) | 0 (0) | 0 (14) | 0 (14) | 1 | 1 |
| Disease Activity Indices | |||||
| Mean SLEDAI | 13.1 ± 9.6 | 10.7 ±11.7 | 12.8 ± 14.2 | 0.6 | 0.4 |
| Mean SLAM | 6.1 ± 5.5 | 5.4 ± 3.1 | 5.1 ± 3.6 | 0.8 | 0.6 |
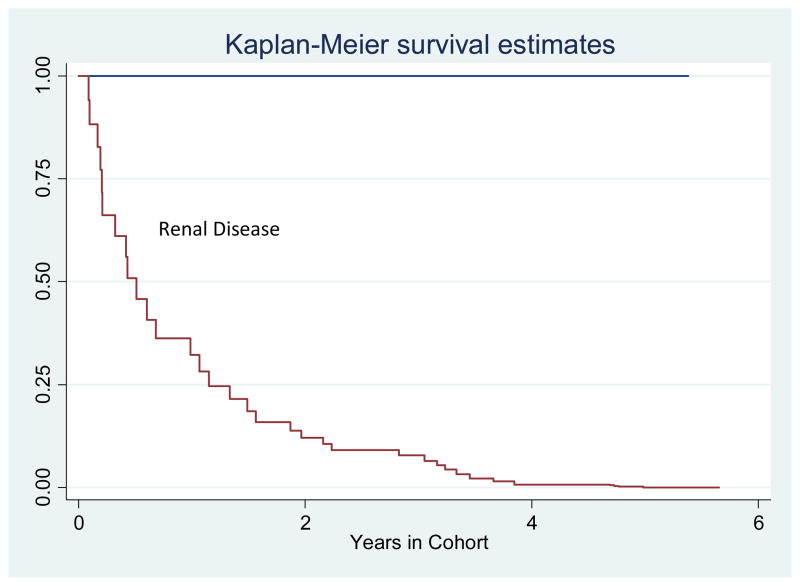
Twenty-eight (60%) of the cohort met the case definition for renal involvement either at presentation or in follow up. These included 24 patients who underwent renal biopsy and 4 patients with lupus nephritis by ACR. These 4 patients were deemed not stable enough to undergo renal biopsy by the physician, and were subsequently treated for presumed renal disease. In 14 of these 28 patients, renal disease developed within one month of the diagnosis of SLE. In the other 14 patients, kidney disease manifested a median of 3.3 ± 2 years from the initial SLE diagnosis (Figure 1).
Figure 1.
Timeline of Renal Disease Development
In those patients with kidney disease, renal involvement presented within 5 years of the initial diagnosis of SLE.
All of the males in the cohort developed kidney disease, and all within one month of the diagnosis of SLE (Table 1). There were no differences noted in race or Hispanic ethnicity between those who presented with either early or late onset kidney disease. There was an increased predominance of anti-Sm antibodies and active urinary sediment in subjects presenting with renal disease at baseline.
We sought to identify associations between clinical or laboratory data and presence of kidney disease at SLE diagnosis (Table 2). In logistic regression analyses, low serum albumin (Odds Ratio (OR): 3.7, 95% Confidence Interval (CI): 1.4–9.5) and presence of dsDNA antibodies (OR: 3.4, 95% CI: 1.5–7.6) were associated with kidney involvement. Patients with a malar rash were less likely to have concomitant renal disease and this relationship remained significant after adjusting for race (OR: 0.5, 95% CI: 0.2–0.9).
Table 2.
Factors Associated with Renal Disease at Cohort Entry
| Logistic Regression Odds Ratio Univariate (95% Confidence Interval) | Logistic Regression Odds Ratio Controlling for Race (95% Confidence Interval) | |
|---|---|---|
| Age, years | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) |
| Male Gender | 2.5 (1.1–5.9) | 1.3 (0.5–3.5) |
| Black Race | 0.8 (0.3–6.4) | -- |
| dsDNA | 3.4 (1.5–7.6) | 3.2 (1.7–5.9) |
| Ro | 0.5 (0.1–2.2) | 0.8 (0.1–4.1) |
| La | 3.8 (0.3–43.2) | 5.1 (0.5–53.4) |
| RNP | 0.9 (0.4–2.3) | 1.1 (0.4–2.3) |
| Sm | 1.3 (0.3–5.4) | 1.2 (0.3–5.5) |
| Low C3 | 1.7 (0.8–3.8) | 1.7 (0.8–3.8) |
| Low C4 | 1.8 (0.8–4.5) | 2.5 (0.9–6.5) |
| Elevated ESR | 1.1 (0.4–2.7) | 1.5 (0.7–3.3) |
| Anemia | 0.7 (0.4–1.4) | 0.5 (0.4–1.1) |
| Thrombocytopenia | 0.6 (0.1–5.7) | 0.4 (0.1–5.1) |
| Leukopenia | 2.1 (0.4–9.5) | 1.4 (0.3–6.8) |
| Low Albumin | 3.7 (1.4–9.5) | 4.8 (1.9–12.5) |
| Isolated Sterile Pyuria | 1.2 (0.8–1.7) | 1.3 (0.8–1.9) |
| Malar Rash | 0.4 (0.2–0.8) | 0.5 (0.2–0.9) |
| Arthritis | 2.1 (0.8–5.9) | 2.1 (0.7–6.4) |
| SLEDAI score | 2.1 (0.9–2.9) | 2.3 (0.8–3.2) |
| SLAM score | 1.2 (0.9–1.9) | 2.5 (0.8–3.3) |
Out next goal was to determine clinical or laboratory factors that may be predictive of kidney disease in longitudinal analyses, focusing on the subjects with late onset renal disease (Table 3). In univariate analyses, isolated sterile pyuria was predictive of future kidney disease (Hazard Ratio (HR): 3, 95% CI: 1.3–7) and this relationship remained significant after controlling for race (HR: 2.5, 95% CI: 1.1–6.4). The mean time between noting isolated sterile pyuria and development of kidney involvement was 3 months (range 2–6 months). A low serum albumin was also predictive of kidney disease (HR: 3.4, 95% CI: 1.7–6.9). The mean time between findings of low serum albumin and kidney involvement was 2.5 months (range 2–4 months). The presence of Ro antibody was protective against future renal disease in this cohort (HR: 0.2, 95% CI: 0.05–0.5).
Table 3.
Risk of Renal Disease in Longitudinal Analyses
| Cox Proportional Hazard Ratio Univariate (95% Confidence Interval) | Cox Proportional Hazard Ratio Controlling for Race (95% Confidence Interval) | |
|---|---|---|
| Age, years | 1.2 (0.9–1.4) | 1.2 (0.9–1.4) |
| Male Gender | 2.2 (0.9–5.1) | 1.4 (0.7–4.5) |
| Black Race | 0.5 (0.1–1.9) | -- |
| dsDNA | 1.3 (0.5–3.4) | 1.3 (0.5–3.1) |
| Ro | 0.1 (0.04–0.5) | 0.2 (0.05–0.5) |
| La | 0.8 (0.1–3.7) | 0.7 (0.2–2.4) |
| RNP | 1 (0.5–2.2) | 1.2 (0.5–2.4) |
| Sm | 0.7 (0.3–1.6) | 0.4 (0.2–1.3) |
| Low C3 | 0.9 (0.4–1.9) | 0.8 (0.3–1.9) |
| Low C4 | 0.6 (0.2–1.6) | 0.6 (0.2–1.5) |
| Elevated ESR | 0.9 (0.4–2.3) | 0.9 (0.4–2.2) |
| Anemia | 0.8 (0.4–1.5) | 0.7 (0.4–1.4) |
| Thrombocytopenia | 1.7 (0.8–3.8) | 1.4 (0.5–3.5) |
| Leukopenia | 0.9 (0.5–1.8) | 0.8 (0.4–1.6) |
| Low Albumin | 3.5 (1.8–7.1) | 3.4 (1.7–6.9) |
| Isolated Sterile Pyuria | 3 (1.3–7) | 2.5 (1.1–6.4) |
| Malar Rash | 1.5 (0.5–5.1) | 1.6 (0.5–5.9) |
| Arthritis | 1.7 (0.4–6.9) | 0.9 (0.2–4.7) |
| SLEDAI score | 1.1 (0.9–1.4) | 0.9 (0.8–1.2) |
| SLAM score | 1.2 (0.9–1.6) | 1.1 (0.8–1.3) |
Discussion
Kidney disease in pediatric patients with SLE can lead to increased morbidity and mortality. Developing biomarkers is highly desirable, as they could provide the basis for developing and testing the efficacy of strategies of treating renal disease before it becomes overt and, thus, theoretically reducing the development or severity of renal damage.
In patients with later onset renal disease, we found that isolated sterile pyuria was predictive of renal disease in longitudinal analyses. Isolated sterile pyuria has been noted in up to 13 percent of 198 adult patients with SLE in a cross-sectional study 12. In a study by Rahman et al, 215 of 946 (23%) had at least one episode of sterile pyuria over the observation period 13. The majority (59%) of these had a past history of at least one other renal manifestation. However, of the 88 patients who had no past history of renal disease, two-thirds subsequently developed kidney involvement over a mean of 1.6 years. Sterile pyuria can be associated with multiple etiologies besides SLE, including infections and medications, particularly non-steroidal anti-inflammatories (NSAIDs). In our cohort, there was no evidence of infection in the patients with sterile pyuria. Additionally, these patients did not report chronic use of NSAIDs; however, intermittent use of NSAIDs is possible.
The presence of malar rash was protective against concomitant renal disease in our analyses. In a longitudinal study of 241 pediatric SLE patients, patients without skin disease, including malar rash, showed higher renal and hematologic involvement both at the time of diagnosis and during follow-up14. This potential inverse relationship between skin and renal disease would be important to confirm in a large cohort.
Antibodies against dsDNA are a classic marker for the diagnosis of SLE. In a longitudinal study of 53 adult SLE patients observed monthly for one year, increases in anti-dsDNA levels were noted before overall disease flares occurred 15. However, this trend did not translate to prediction of renal flares. In fact, concurrent decreases in anti-dsDNA levels were associated with renal involvement. Another study comparing 14 patients with anti-dsDNA antibodies and nephritis to 14 patients with anti-dsDNA antibodies and no nephritis demonstrated that the antibodies were indistinguishable, indicating that the development of renal disease is multi-factorial and not solely predicted by antibody status 16. In our sample, we found that dsDNA antibodies were present in patients with renal disease at SLE diagnosis but were not predictive of future involvement.
Changes in serum complement levels have also been associated with kidney disease. In a study by Ho and Petri, decreases in serum complement levels, C3 and C4, were associated with a concurrent almost 2-fold increase in renal disease activity17. Other studies have shown that persistently low levels of C1q are associated with continued activity in diffuse proliferative glomerulonephritis 18. Yet, these markers do not necessarily correlate with disease flares. In one study, 12 percent of patients with hypocomplementemia and elevated anti-dsDNA antibodies had no evidence of active clinical disease 19.
Anti-Ro antibody, which can also be found in patients with Sjogren’s syndrome, rheumatoid arthritis, and primary biliary cirrhosis, is noted in 30–50% of patients with SLE 20. Anti-Ro antibodies in patients with SLE have been associated with neonatal lupus heart block, photosensitivity, and valvular heart disease 21–23. In a study by Moon et al., investigators noted that adult patients with lupus nephritis and anti-Ro antibody had a decreased chance of renal relapse 24. Another report by Chien et al. showed that kidney disease in adults with SLE was associated with absence of anti-Ro antibody, suggesting a protective effect 25. However, Korbet et al. found that the presence of anti-Ro antibody was associated with progression to end stage renal disease in adult SLE patients with severe lupus nephritis 26. In our study, the presence of Ro antibody was protective against development of future renal disease. As far as we know, this is the first observation of this relationship in pediatric patients with SLE.
As noted, traditional laboratory tests, such as complement and antibody levels, may vary with disease activity. Other investigative laboratory studies have also been reported to correlate with disease activity. An increased expression of interferon-activated genes in peripheral blood mononuclear cells has been associated with increased disease activity 27,28. Increased levels of erythrocyte-bound C4d correlated with increased disease activity in one study 29, but not another 30. Urinary biomarkers for kidney disease including neutrophil gelatinase-associated lipocalin, monocyte chemoattractant protein-1, and hepcidin have also been studied as predictors of renal inflammation 31–38. At the moment, these tests are only available as investigational tools, but there is potential for widespread use in the future.
Our study has some limitations. The sample size was relatively small and included 14 patients with renal disease at the time of SLE diagnosis and 14 patients with renal disease during longitudinal follow-up. We performed power calculations showing that we had 80% power to detect associations between variables and kidney disease and a significance level of 0.05; and 72% power at a lower significance level of 0.01. These power estimates demonstrate that the risk of type I or type II error is relatively low in our cohort. Our findings may not be generalizable to other populations. Our cohort is predominately African American and our findings may not be the same in Caucasians or Hispanics with SLE. There is a need to develop larger cohorts to define reliable biomarkers. Patient data were reported from clinic visits ≤ 3 months before onset of renal disease. It is possible that these same laboratory results would be different if collected closer in time to the onset of kidney disease.
Medication use varied throughout the cohort. There was no significant predictor of any immunosuppressive agent with the future onset of renal disease. However, this may be due to differences in prescribing practice and treatment of SLE between the two centers. Other disease manifestations may prompt earlier immune suppression treatment and alter the expression of kidney disease. Non-steroidal anti-inflammatory drugs (NSAIDs) were used in 20% of patients during the study; however, no NSAID use was noted in patients with isolated sterile pyuria.
In these analyses, we have identified clinical and laboratory indices associated with kidney disease in a cohort of pediatric patients with SLE. The presence of isolated sterile pyuria and low serum albumin were predictive of renal disease; whereas, anti-Ro antibodies were protective. The recognition of these potential indicators may help clinicians identify patients at risk for kidney disease before its onset thus potentially preventing long-term complications.
Acknowledgments
This work was supported by a National Institutes of Health mentored career award 5K23AR052736 (Sule) and by generous donations from the Hock and Mangione families.
Bibliography
- 1.Barron KS, Silverman ED, Gonzales J, Reveille JD. Clinical, serologic, and immunogenetic studies in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 1993;36(3):348–354. doi: 10.1002/art.1780360310. [DOI] [PubMed] [Google Scholar]
- 2.Hiraki LT, Benseler SM, Tyrrell PN, Hebert D, Harvey E, Silverman ED. Clinical and laboratory characteristics and long-term outcome of pediatric systemic lupus erythematosus: A longitudinal study. J Pediatr. 2008;152(4):550–556. doi: 10.1016/j.jpeds.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Font J, Cervera R, Espinosa G, et al. Systemic lupus erythematosus (SLE) in childhood: Analysis of clinical and immunological findings in 34 patients and comparison with SLE characteristics in adults. Ann Rheum Dis. 1998;57(8):456–459. doi: 10.1136/ard.57.8.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tucker LB, Menon S, Schaller JG, Isenberg DA. Adult- and childhood-onset systemic lupus erythematosus: A comparison of onset, clinical features, serology, and outcome. Br J Rheumatol. 1995;34(9):866–872. doi: 10.1093/rheumatology/34.9.866. [DOI] [PubMed] [Google Scholar]
- 5.Brunner HI, Gladman DD, Ibanez D, Urowitz MD, Silverman ED. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum. 2008;58(2):556–562. doi: 10.1002/art.23204. [DOI] [PubMed] [Google Scholar]
- 6.Hagelberg S, Lee Y, Bargman J, et al. Longterm followup of childhood lupus nephritis. J Rheumatol. 2002;29(12):2635–2642. [PubMed] [Google Scholar]
- 7.Hahn BH, McMahon MA, Wilkinson A, et al. American college of rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012;64(6):797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochberg MC. Updating the american college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 9.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 10.Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15(2):241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 12.Appenzeller S, Clark A, Pineau C, Vasilevsky M, Bernatsky S. Isolated pyuria in systemic lupus erythematosus. Lupus. 2010;19(7):793–796. doi: 10.1177/0961203309358901. [DOI] [PubMed] [Google Scholar]
- 13.Rahman P, Gladman DD, Ibanez D, Urowitz MB. Significance of isolated hematuria and isolated pyuria in systemic lupus erythematosus. Lupus. 2001;10(6):418–423. doi: 10.1191/096120301678646164. [DOI] [PubMed] [Google Scholar]
- 14.Chiewchengchol D, Murphy R, Morgan T, et al. Mucocutaneous manifestations in a UK national cohort of juvenile-onset systemic lupus erythematosus patients. Rheumatology. 2014;53:1504–1512. doi: 10.1093/rheumatology/keu137. [DOI] [PubMed] [Google Scholar]
- 15.Ho A, Magder LS, Barr SG, Petri M. Decreases in anti-double-stranded DNA levels are associated with concurrent flares in patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2342–2349. doi: 10.1002/1529-0131(200110)44:10<2342::aid-art397>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Budhai L, Oh K, Davidson A. An in vitro assay for detection of glomerular binding IgG autoantibodies in patients with systemic lupus erythematosus. J Clin Invest. 1996;98(7):1585–1593. doi: 10.1172/JCI118952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho A, Barr SG, Magder LS, Petri M. A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2350–2357. doi: 10.1002/1529-0131(200110)44:10<2350::aid-art398>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Gunnarsson I, Sundelin B, Heimburger M, et al. Repeated renal biopsy in proliferative lupus nephritis--predictive role of serum C1q and albuminuria. J Rheumatol. 2002;29(4):693–699. [PubMed] [Google Scholar]
- 19.Walz LeBlanc BA, Gladman DD, Urowitz MB. Serologically active clinically quiescent systemic lupus erythematosus--predictors of clinical flares. J Rheumatol. 1994;21(12):2239–2241. [PubMed] [Google Scholar]
- 20.Harley JB, Scofield RH, Reichlin M. Anti-ro in sjogren’s syndrome and systemic lupus erythematosus. Rheum Dis Clin North Am. 1992;18(2):337–358. [PubMed] [Google Scholar]
- 21.Buyon JP, Hiebert R, Copel J, et al. Autoimmune-associated congenital heart block: Demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31(7):1658–1666. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 22.Mond CB, Peterson MG, Rothfield NF. Correlation of anti-ro antibody with photosensitivity rash in systemic lupus erythematosus patients. Arthritis Rheum. 1989;32(2):202–204. doi: 10.1002/anr.1780320213. [DOI] [PubMed] [Google Scholar]
- 23.Shahin AA, Shahin HA, Hamid MA, Amin MA. Cardiac involvement in patients with systemic lupus erythematosus and correlation of valvular lesions with anti-ro/SS-A and anti-la/SS-B antibody levels. Mod Rheumatol. 2004;14(2):117–122. doi: 10.1007/s10165-004-0277-6. [DOI] [PubMed] [Google Scholar]
- 24.Moon SJ, Park HS, Kwok SK, et al. Predictors of renal relapse in korean patients with lupus nephritis who achieved remission six months following induction therapy. Lupus. 2013;22(5):527–537. doi: 10.1177/0961203313476357. [DOI] [PubMed] [Google Scholar]
- 25.Chien JW, Lin CY, Yang LY. Correlation between anti-ro/la titers and clinical findings of patients with systemic lupus erythematosus. Zhonghua Yi Xue Za Zhi (Taipei) 2001;64(5):283–291. [PubMed] [Google Scholar]
- 26.Korbet SM, Lewis EJ, Schwartz MM, Reichlin M, Evans J, Rohde RD. Factors predictive of outcome in severe lupus nephritis. lupus nephritis collaborative study group. Am J Kidney Dis. 2000;35(5):904–914. doi: 10.1016/s0272-6386(00)70262-9. [DOI] [PubMed] [Google Scholar]
- 27.Ronnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54(2):408–420. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- 28.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manzi S, Navratil JS, Ruffing MJ, et al. Measurement of erythrocyte C4d and complement receptor 1 in systemic lupus erythematosus. Arthritis Rheum. 2004;50(11):3596–3604. doi: 10.1002/art.20561. [DOI] [PubMed] [Google Scholar]
- 30.Singh V, Mahoney JA, Petri M. Erythrocyte C4d and complement receptor 1 in systemic lupus erythematosus. J Rheumatol. 2008;35(10):1989–1993. [PubMed] [Google Scholar]
- 31.Chan RW, Lai FM, Li EK, et al. The effect of immunosuppressive therapy on the messenger RNA expression of target genes in the urinary sediment of patients with active lupus nephritis. Nephrol Dial Transplant. 2006;21(6):1534–1540. doi: 10.1093/ndt/gfk102. [DOI] [PubMed] [Google Scholar]
- 32.Kiani AN, Johnson K, Chen C, et al. Urine osteoprotegerin and monocyte chemoattractant protein-1 in lupus nephritis. J Rheumatol. 2009;36(10):2224–2230. doi: 10.3899/jrheum.081112. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki M, Wiers KM, Klein-Gitelman MS, et al. Neutrophil gelatinase-associated lipocalin as a biomarker of disease activity in pediatric lupus nephritis. Pediatr Nephrol. 2008;23(3):403–412. doi: 10.1007/s00467-007-0685-x. [DOI] [PubMed] [Google Scholar]
- 34.Rovin BH, Song H, Birmingham DJ, Hebert LA, Yu CY, Nagaraja HN. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J Am Soc Nephrol. 2005;16(2):467–473. doi: 10.1681/ASN.2004080658. [DOI] [PubMed] [Google Scholar]
- 35.Brunner HI, Mueller M, Rutherford C, et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2577–2584. doi: 10.1002/art.22008. [DOI] [PubMed] [Google Scholar]
- 36.Pitashny M, Schwartz N, Qing X, et al. Urinary lipocalin-2 is associated with renal disease activity in human lupus nephritis. Arthritis Rheum. 2007;56(6):1894–1903. doi: 10.1002/art.22594. [DOI] [PubMed] [Google Scholar]
- 37.Rubinstein T, Pitashny M, Levine B, et al. Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology (Oxford) 2010;49(5):960–971. doi: 10.1093/rheumatology/kep468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinze CH, Suzuki M, Klein-Gitelman M, et al. Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. Arthritis Rheum. 2009;60(9):2772–2781. doi: 10.1002/art.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]