Abstract
Memory involves the storage of information at synapses by an LTP-like process. This information storage is synapse specific and can endure for years despite the turnover of all synaptic proteins. There must, therefore, be special principles that underlie the stability of LTP. Recent experimental results suggest that LTP is maintained by the complex of CaMKII with the NMDAR. Here we consider the specifics of the CaMKII/NMDAR molecular switch, with the goal of understanding the biochemical principles that underlie stable information storage by synapses. Consideration of a variety of experimental results suggests that multiple principles are involved. One switch requirement is to prevent spontaneous transitions from the off to the on state. The highly cooperative nature of CaMKII autophosphorylation by Ca2+ (Hill coefficient of 8) and the fact that formation of the CaMKII/NMDAR complex requires release of CaMKII from actin are mechanisms that stabilize the off state. The stability of the on state depends critically on intersubunit autophosphorylation, a process that restores any loss of pT286 due to phosphatase activity. Intersubunit autophosphorylation is also important in explaining why on state stability is not compromised by protein turnover. Recent evidence suggests that turnover occurs by subunit exchange. Thus, stability could be achieved if a newly inserted unphosphorylated subunit was autophosphorylated by a neighboring subunit. Based on other recent work, we posit a novel mechanism that enhances the stability of the on state by protection of pT286 from phosphatases. We posit that the binding of the NMNDAR to CaMKII forces pT286 into the catalytic site of a neighboring subunit, thereby protecting pT286 from phosphatases. A final principle concerns the role of structural changes. The binding of CaMKII to the NMDAR may act as a tag to organize the binding of further proteins that produce the synapse enlargement that underlies late LTP. We argue that these structural changes not only enhance transmission, but also enhance the stability of the CaMKII/NMDAR complex. Together, these principles provide a mechanistic framework for understanding how individual synapses produce stable information storage.
Keywords: Memory, LTP, Kinase, Phosphatase
Introduction
The storage of memory in the brain involves activity-dependent increases in the strength of synapses, a process termed long-term potentiation (LTP). The longest recordings show that LTP can persist for at least a year (Abraham, 2003). Several lines of evidence indicate that LTP contributes to learning. Notably, learning can trigger LTP (Gruart et al., 2006; Whitlock et al., 2006); moreover, artificial induction of LTP saturates synapses and thereby interferes with memory (Moser et al., 1998). This and other recent work (Nabavi et al., 2013; Nabavi et al., 2014) leaves little doubt that LTP is at least one of the mechanisms that underlie memory.
Achieving the required stability required for such long-term information storage is nontrivial. Covalent modifications are generally reversed within minutes or hours. Furthermore, experiments show that all synaptic proteins undergo protein turnover within less than a week (Cohen et al., 2013). There must thus be special mechanisms that allow a biochemical system to store information in a stable way.
In discussing the maintenance processes that make LTP persistent, it is important to differentiate the maintenance process from the induction and expression processes that are also aspects of LTP. Induction has to do with the biochemical events directly triggered by the strong synaptic activity responsible for initiating LTP. For example, at the CA1 hippocampal synapses that have served as the primary model system for understanding LTP, induction involves the opening of NMDA receptors (NMDAR), the influx of Ca2+, the binding of Ca2+ to calmodulin, and the rapid activation (and autophosphorylation) of the abundant synaptic protein, calcium calmodulin-dependent protein kinase II (CaMKII) (reviewed in (Bliss and Collingridge, 1993; Lisman et al., 2012)). These processes trigger the long-lasting maintenance processes that persist for the duration of LTP. Expression processes couple the maintenance process to the presynaptic and postsynaptic channels that enhance synaptic transmission.
A critical test of any putative maintenance mechanism is the “erasure” test, a test that distinguishes maintenance from induction and expression. In this test, saturated LTP is first induced. Then, the putative maintenance mechanism is transiently inhibited, with the goal of resetting the molecular switch that underlies maintenance. If such a transient inhibition produces a persistent reversal of LTP, this indicates that the molecule being inhibited is critical for the maintenance process. With this procedure, the reversal of LTP cannot be due to interference with induction processes, because the inhibition isn’t performed until after induction is completed. Furthermore, the reversal of LTP cannot be due to an effect on expression processes because the inhibition was transient; if the effect were on an expression process, LTP would recover after removal of the inhibiting agent. A final aspect of the erasure test is to reinduce LTP after erasure. If LTP can be reinduced, this proves that reversal is not due to cell damage but, rather, to resetting the LTP switch. As described below, the CaMKII/NMDAR complex has passed this erasure test.
The hypothesis that LTP maintenance is due to the CaMKII/NMDAR complex is based on several findings. Biochemical experiments showed that strong synaptic activity can lead to an increase in the amount of complex (Leonard et al., 1999; Strack and Colbran, 1998). (Barria and Malinow, 2005) showed that a mutation in NR2B that interferes with binding to CaMKII prevents the induction of LTP. This work, however, did not resolve the question of whether the complex is important only in induction or also in maintenance. To resolve this issue, (Sanhueza et al., 2007) performed the erasure test described above. To perform this test, they used a peptide derived from an endogenous CaMKII inhibitor, CaMKIIN, that is highly specific for CaMKII (Chang et al., 1998). This protein (and its peptide derivatives, CN21 and CN19) binds to what is termed the “T site” of CaMKII (Vest et al., 2007). Importantly, the NR2B tail also binds to this site (Bayer et al., 2001). Thus, the CN peptides interfere with the binding of CaMKII to NR2B (Vest et al., 2007). (Sanhueza et al., 2011) used a form of CN peptides that was made cell permeable by addition of a tat sequence. Thus, transient application of the peptide could be achieved by applying the peptide to the bath and then removing it.
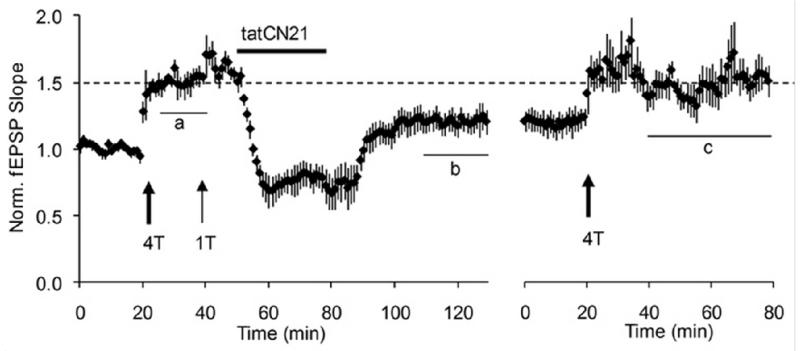
The results of the erasure test are shown in Fig.1. Transient application of the peptide CN19 erased LTP. It was then possible to reinduce LTP, indicating that the CN19 had produced true erasure. Control biochemical experiments showed that, under the conditions of the experiment, the CaMKII/NMDAR complex, as measured by co-immunoprecipitation, was actually reduced. Furthermore, the CaMKII content of spines, which is due in part to the binding of CaMKII to NMDAR, was persistently reduced by CN21. Subsequent controls showed that the persistent depression produced by CN21 did not depend on glutamatergic function and thus is not a form of LTD and that it did not occur in very young animals (P7-P10) at an age before CaMKII is substantially present at synapses (Gouet et al., 2012).
Fig.1. Erasure of LTP by tatCN21, a peptide that interferes with the binding of CaMKII to NR2B.
The postsynaptic response of CA1 neurons was measured by the slope of the field EPSP. At 20 min, LTP was induced by four tetani (100 Hz, 1 s) given to the input axons. This LTP is saturated, as evidenced by the lack of effect of an additional tetanus given at 40 min. tatCN21 was applied and then removed. It produced both a reversible effect and a persistent erasure of a large fraction of LTP. At right, a period of further recording showed that LTP could be reinduced in the same slices. In control experiments in which tatCN19 was not applied, LTP was not erased and no further LTP could be induced. From Fig.2 of (Sanhueza et al., 2011). Note that the application of tatCN21, in addition to persistently reversing LTP, has a reversible effect, probably due to the inhibition of presynaptic release (Waxham et al., 1993).
One potential objection to the idea that the CaMKII/NMDAR complex (and specifically the complex of CaMKII with the NR2B subunit) has an important role in the maintenance of memory is that LTP at CA1 synapses produces a switch from NR2B function to NR2A function (Barria and Malinow, 2002; Bellone and Nicoll, 2007). However, these results are based on measurements of synaptic current and so could be due to modulation of channel function rather that channel number. Indeed, direct measurements of synaptic NR2B content (relative to NR1) show no developmental changes in the amount of NR2B (Swulius et al., 2010).
Biochemical principles of a memory switch
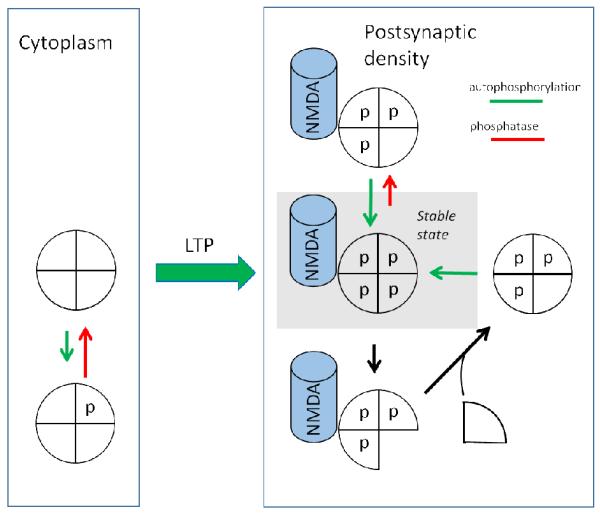
We now turn to a discussion of the biochemical principles that could underlie information storage by the CaMKII/NMDAR complex. For simplicity, let us consider the system a two-state (on/off) switch (the mechanism of gradation of synaptic strength will be discussed later). One must then ask what molecular change underlies the transition from the off to the on state during LTP induction and why these states are otherwise stable. Fig.2 shows the scheme of the CaMKII switch based on earlier models (Lisman and Goldring, 1988; Lisman, 1985) but updated to include the idea that, in the on state, CaMKII is bound to the NMDAR. CaMKII is a holoenzyme consisting of 12 catalytic subunits arranged in two rings of six (simplified in Fig.2). In outline, the switch works in the following way. In the off state, CaMKII is in the cytoplasm, primarily bound to actin. In the cytoplasm, the phosphatase activity is high and thus any CaMKII that becomes spontaneously autophosphorylated will be rapidly dephosphorylated, thereby ensuring the stability of the off state. During LTP induction, Ca2+ elevation is high and CaMKII becomes highly phosphorylated. While in this phosphorylated state, CaMKII may bind to the NMDARs in the postsynaptic density (PSD), a structure directly attached to the postsynaptic membrane. In this phosphorylated state, several processes serve to maintain the phosphorylated on state: 1) When a CaMKII subunit is phosphorylated, it is active even in the absence of Ca2+. Thus an autocatalytic reaction can maintain the on state in the following way: a subunit that gets dephosphorylated can be rephosphorylated by a neighboring phosphorylated subunit; 2) This maintenance of the on state is promoted by a variety of factors that lower the rate of dephosphorylation of CaMKII when it is bound to NMDARs in the PSD. Thus the balance of phosphorylation vs dephosphorylation lies highly in favor of phosphorylation, making it highly unlikely that the switch will be reset to the off state by phosphatase; 3) If a phosphorylated subunit is replaced by an unphosphorylated one in the process of protein turnover, the newly inserted subunit will be phosphorylated by a neighboring subunit. CaMKII can therefore stay phosphorylated and bound to the NMDAR, and the on state of the switch is therefore stable. A detailed discussion of these mechanisms and their experimental support is given in the following sections.
Fig.2. Contribution of autophosphorylation and subunit exchange to maintenance of the on state.
(Left) Simplified model of CaMKII holoenzyme with only four shown subunits, each with a t286 site unphosphorylated. Such holoenzymes are in the cytoplasm, usually bound to actin. (Right) Upon activation during LTP induction, CaMKII translocates to the postsynaptic density, where it binds to the NMDAR. In this on state, each T286 site is phosphorylated. Two types of reactions must be counteracted to make the switch stable. (Right/Upper) Phosphatase may dephosphorylate a subunit; this is counteracted by autophosphorylation of that site by a neighboring active subunit. (Right/Lower) If protein turnover occurs by subunit exchange, a phosphorylated subunit may be replaced by an unphosphorylated subunit. This subunit is then phosphorylated by a neighboring subunit.
Principle 1: Intersubunit autophosphorylation of T286 maintains the phosphorylated on state
Ca2+/Calmodulin activates kinase activity, leading the kinase to phosphorylate itself on T286. This phosphorylation has the important consequence of making the kinase active even after Ca2+ levels fall (this is termed “autonomous” activity), thereby forming a biochemical trace of the Ca2+ elevation (Miller and Kennedy, 1986). Most of the activated CaMKII in the cytoplasm of a dendritic spine becomes dephosphorylated within minutes (Lee et al., 2009). However, a small fraction (Feng et al., 2011) translocates to the synapse and binds to the NMDAR, and it is this fraction that is thought to be important for LTP maintenance. This fraction may be at least partially phosphorylated (Halt et al., 2012), a phosphorylation that affects binding to the NMDAR. This tightness will depend on how many binding interactions occur with the NMDAR (there are multiple binding sites on NR2B and at least one on NR1; two of these require phosphorylation of T286) (Bayer et al., 2001; Leonard et al., 1999). The importance of T286 phosphorylation is demonstrated by the fact that when a mutation is made that prevents this phosphorylation, the CaMKII content of the PSD is low, LTP is greatly reduced, and long-term memory is severely affected (Giese et al., 1998; Gustin et al., 2011).
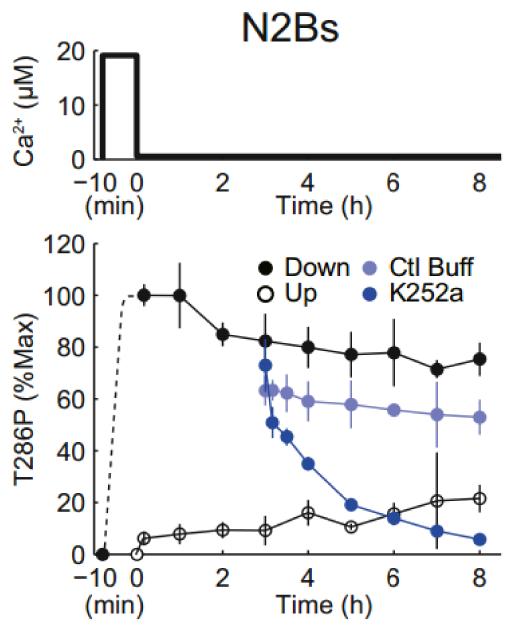
Modelling studies (Lisman, 1985; Miller et al., 2005) have suggested that intersubunit autophosphorylation can maintain the phosphorylated state of the kinase once it is located in the PSD; each subunit is catalytic and, if itself phosphorylated at T286, can phosphorylate T286 on a nearby subunit that might become dephosphorylated (this reaction requires that the substrate subunit has calmodulin bound as a result of basal Ca2+ levels (Hanson et al., 1994)). Recent reconstitution experiments directly demonstrate this mechanism (Urakubo et al., 2014). These experiments utilized purified CaMKII, protein phosphatase 1 (PP1), and a peptide fragment of NR2B. What was measured was the state of switch, as monitored by the fraction of CaMKII phosphorylated at T286. In the experiment shown in Fig.3, the CaMKII switch was initially turned on by a brief pulse of Ca2+/Calmodulin. After Ca2+ was lowered, the switch stayed on for at least 8 hours because any T286 site that became dephosphorylated by PP1 was rephosphorylated by autophosphorylation. The requirement for such autophosphorylation is demonstrated (Fig.3) by the fact that inhibiting the kinase with a catalytic site inhibitor led, over several hours, to switch failure (dephosphorylation of T286).
Fig.3. In vitro reconstitution of memory switch using purified CaMKII-alpha, protein phosphatase (PP1) and peptide corresponding to CaMKII binding site of NR2B (1289-1310).
Switch state is determined by the fraction of CaMKII phosphorylated on T286. Switch starts out off. At −10 min, Ca2+ is added and then lowered at t=0 to basal levels (0.56 micromolar). Without this Ca2+ elevation, the switch stays off (open circles). With Ca2+ elevation, the switch remains on after Ca2+ removal (closed black circles). This persistence requires autophosphorylation because if kinase is inhibited with K252a, the switch does not remain on (closed dark blue circles). Light blue circles show that the persistence is maintained if the solution change does not introduce a kinase inhibitor. From Fig.1 of (Urakubo et al., 2014).
Principle 2: In the on state, dephosphorylation of T286 becomes less efficient
Simulations of the CaMKII switch show that a requirement for a bistable switch is that the dephosphorylation of T286p becomes less efficient when the switch is in the on state as compared to the off state. One possible mechanism for this effect explored in previous models is the reduction in phosphatase efficiency because the phosphatase becomes saturated by the high concentration of phosphorylated T286 (Lisman and Zhabotinsky, 2001). This saturation reduces the “per site” rate of dephosphorylation. As will be described later, there are now indications that phosphatase may also become less efficient by a second process that depends on the NMDAR.
An important finding of the reconstitution experiments of (Urakubo et al., 2014) is that the switch was not stable in the absence of NR2B (see also (Bradshaw et al., 2003)). From a theoretical standpoint, this is not unexpected, given that the experiments were conducted under conductions of CaMKII holoneyzme concentration (2.7 micromolar) much less than the concentration in the PSD (~50 micromolar); thus, the saturation of PP1 that promotes the stability of the on state was below what would be expected in the PSD (Zhabotinsky, 2000). However, if the NR2B binding site for CaMKII was present, the switch was bistable (Fig.3), indicating that there is another mechanism for reducing phosphatase efficiency in the on state of the switch.
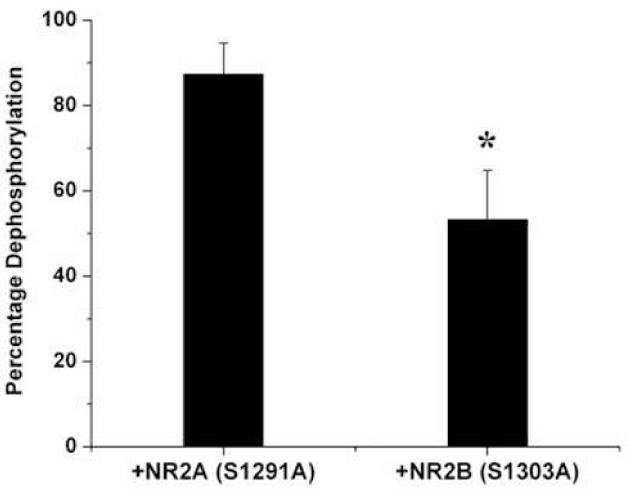
The first hint of an additional phosphatase control mechanism was revealed in efforts to study the switching properties of CaMKII in the PSD. It was found that the phosphatases within the PSD could dephosphorylate some phosphorylation sites on CaMKII (there are many in addition to T286) but that the T286 site could not be significantly dephosphorylated during the one hour of the assay (Mullasseril et al., 2007). Thus, the T286 sites were somehow protected from phosphatase. (Cheriyan et al., 2011) obtained evidence that the binding of the NR2B to CaMKII somehow provides such protection. As shown in Fig.4, in experiments with purified CaMKII and PP1, the addition of NR2B peptide inhibited pT286 dephosphorylation. A model of how NR2B might produce this inhibition will be presented in a later section.
Fig.4. In vitro experiments showing that dephosphorylation of CaMKII by PP1 is reduced by action of NR2B, but not by NR2A.
Substrate is phospho-Thr286 of α-CaMKII. Phosphorylation sites on NR2A and NR2B have been mutated to alanines to prevent their phosphorylation (and associated complexity). Measurements were made with the kinase inhibited with staurosporine. Data normalized to level of CaMKII phosphorylation in absence of PP1. From Fig.4B of (Cheriyan et al., 2011).
Principle 3: Protein turnover by subunit exchange allows the on state to be stable despite protein turnover
The turnover of all synaptic proteins occurs within less than a week, yet LTP can last at least a year. How can the CaMKII switch remain on despite this turnover? A conceptually simple solution proposed in an early model (Lisman, 1985) assumed that CaMKII undergoes protein turnover by subunit exchange. In this case, the replacement of a phosphorylated subunit by an unphosphorylated one would be followed by intersubunit autophosphorylation, thereby returning the holoenzyme to a fully phosphorylated on state. The phosphorylated subunits that are lost from an activated holoenzyme are rapidly dephosphorylated (because of high phosphatase activity in the cytoplasm) and are thereby returned to a basal (off) state (Lee et al., 2009). In this way, undesirable spread of activation to nearby synapses is prevented.
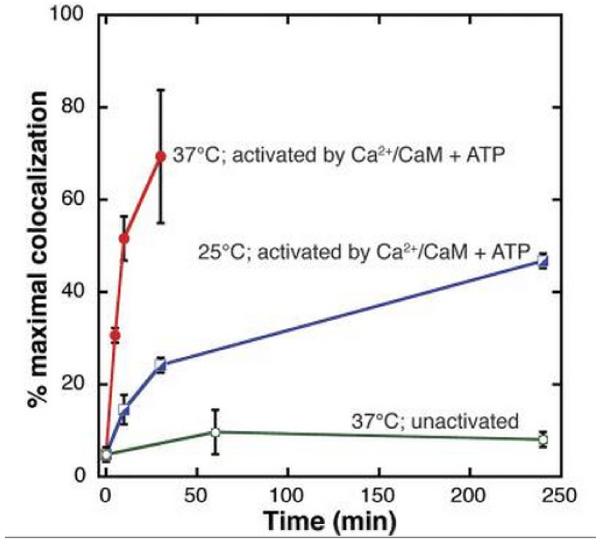
A recent in vitro study (Stratton et al., 2014) provided the first indication that CaMKII might undergo turnover by subunit exchange. Holoenzymes were used in which subunits were labeled uniformly with either a yellow or green fluorescent tag. Holoenzymes of the two colors were mixed, activated with Ca2+, and then diluted for examination of single-holoenzyme fluorescence. As shown in Fig.5, single holoenzymes could display both colors, thus indicating that subunit exchange had occurred. These in vitro results raise the possibility that subunit exchange may be the mechanism of CaMKII turnover in vivo, but this remains to be directly tested.
Fig.5. Single-molecule assay reveals activation-dependent subunit exchange between CaMKII holoenzymes.
TIRF was used to measure colocalization of labels within single holoenzymes that were originally labeled by a single fluorophore. The rate of increase in colocalization was faster at 37°C (red) compared to 25°C (blue) when Ca2+/CaM and ATP were added. At 37°C, the unactivated sample (i.e., with no addition of Ca2+/CaM and ATP) showed only a low level of exchange (green). From Fig.2B of (Stratton et al., 2014).
Additional principles underlying switch stability
The experimental data summarized above provides support for the model of Fig.2 but leaves important questions unanswered. As described above, the presence of the NMDAR somehow inhibits dephosphorylation of T286. In the next section, we suggest a novel mechanism for this effect. A second question regards the issue of protein turnover. If memory storage is mediated by the complex of CaMKII with the NMDAR, then it is necessary to consider not only the turnover of CaMKII, but also the turnover of the NMDAR itself. This may involve additional principles. Finally, a complete model must specify how the CaMKII/NMDAR complex leads to enlargement of the synapse and enhancement of transmission. We will review the evidence that this process is structural and will describe a working hypothesis regarding the proteins involved. This framework raises a third question: whether the structure that is built as a result of the binding of CaMKII to the NMDAR then contributes to the stability of the CaMKII/NMDAR complex. These questions are dealt with below.
Principle 4: NMDAR protects pT286 from dephosphorylation by forcing this amino acid into the catalytic site of the kinase
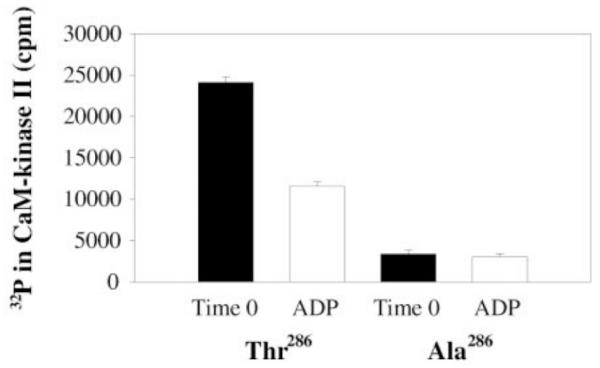
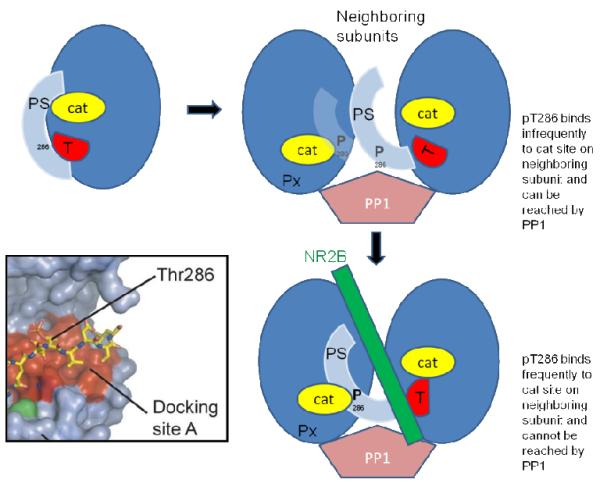
As discussed above, in connection with Figs. 3 and 4, the NMDAR somehow inhibits the ability of phosphatase to dephosphorylate T286p, but not other sites on CaMKII. In principle, NR2B could act by changing the conformation of CaMKII, making pT286 not accessible or recognizable as a substrate. However, on the basis of other experimental results, we suggest that the NMDAR, when bound to CaMKII, promotes the binding of pT286 to the catalytic region on the neighboring subunit, thereby hiding pT286 from phosphatase. This hypothesis is suggested by the results of (Kim et al., 2001) (Fig.6). When ADP was added to CaMKII containing radioactive phosphate at T286, pT286 became dephosphorylated (and radioactive ATP was synthesized; not shown). It was also found that ATP and pT286 can occupy the catalytic site, and this would be the more likely situation in a living cell, where the concentration of ATP greatly exceeds that of ADP. What these results demonstrate is that there is a substantial probability that the product of the kinase reaction, pT286, can rebind to the catalytic site and not be dephosphorylated at that site (because ATP is present) but be protected from phosphatase. Such product rebinding is generally unlikely for enzymes but is more likely for CaMKII because the product is held nearby as a result of the holoenzyme structure. Indeed, a crystal structure has been obtained with pT286 bound to the catalytic site, suggesting that this complex is a low energy state (Chao et al., 2010). Based on these findings, we propose that there is an equilibrium between free pT286 and pT286 bound to the catalytic region. When the NMDAR binds to the T site on CaMKII, it shifts the equilibrium so that the bound form becomes prevalent, thereby protecting pT286 from PP1. This concept is illustrated in Fig. 7.
Fig.6. Reversal of phosphorylation reaction by resynthesis of ATP in catalytic site of CaMKII.
CaMKII was initially autophosphorylated, leading to radioactive pT286. This was reduced by subsequent addition of ADP (left). When the same experiment was conducted under conditions in which the 286 site could not be phosphorylated (right), phosphorylation was low and ADP had no effect. From Fig.4A of (Kim et al., 2001).
Fig.7. Diagram showing how the binding of NR2B to the T site of CaMKII pushes pT286 into the catalytic site (cat) of a neighboring subunit, protecting pT286 from dephosphorylation by phosphatase (PP1).
PS is the pseudo-substrate region that is part of the regulatory region and that, in the off state, occupies and inhibits the catalytic site (cat). Inset shows crystal structure of pThr286 bound in the docking site A of the catalytic region of CaMKII (adapted from (Chao et al., 2010)). Px represents an undefined group of other phosphorylated sites that are not protected and which can be dephosphorylated by the PP1 in the PSD (Mullasseril et al., 2007).
The occupancy of the catalytic site by pT286 (and nucleotide) may explain several other findings. First, it has been found that nucleotide (ATP or ADP) strongly enhances the ability of CaMKII to bind to NMDAR in the presence of Ca2+/CaM (Barcomb et al., 2013; O’Leary et al., 2011; Robison et al., 2005). This can be understood as a nucleotide-dependent stabilization of the binding of pT286 to the catalytic site and the consequent availability of the T site (to which T286 normally binds in the off state) for NMDAR binding. Second, the CN class of peptides (derived from an endogenous protein, CaMKIIN) binds to the T site, resulting in a state in which T286 phosphorylation occurs but phosphorylation of exogenous substrates or other sites on CaMKII does not occur (Coultrap et al., 2010). This can be simply understood on the assumption that T286 is forced into the catalytic site; this can result in the phosphorylation of T286 but will prevent phosphorylation of other substrates.
Principle 5: The CaMKII/NMDAR complex leads to structural changes that enhance transmission but also enhance switch stability
As noted earlier, the data demonstrating subunit exchange between CaMKII holoenzymes and the subsequent intersubunit phosphorylation of the newly inserted subunit provides an elegant way to maintain the information stored by CaMKII. However, in considering the stability of the CaMKII/NMDAR complex, one must deal with not only the turnover of CaMKII, but also the turnover of the NMDAR. Measurements show that all synaptic proteins turn over in a matter of days (Cohen et al., 2013). There is thus the concern that the memory switch will become unstable as a result of the protein turnover of the NMDAR.
In addressing this issue, it is necessary to first address another question: how does the formation of the CaMKII/NMDAR complex lead to a stronger synapse? It has long been thought that the later phases of LTP involve protein synthesis and synapse growth. The actual evidence for such growth is now quite strong. The first evidence came from EM analysis of 3-D reconstructions of CA1 synapses. Statistical analysis indicated that synapses from slices that had undergone LTP were larger than those that hadn’t (Bourne and Harris, 2011; Ostroff et al., 2002). Importantly, this difference was only evident several hours after induction, suggesting that synapse growth is slow and, notably, is much slower than the growth of the spine, which occurs within minutes after LTP induction (Tanaka et al., 2008). Recent work adds strength to this conclusion by showing that the accumulation of key PSD proteins in spines indeed occurs, but only after about an hour (Bosch et al., 2014; Meyer et al., 2014). The notion that late LTP involves slow synapse growth is consistent with the finding that late LTP requires protein synthesis (Frey et al., 1988). Thus, the mechanisms that underlie late LTP can be viewed as a structural process in which the synapse growth involves the insertion into the enlarged synapse of newly synthesized proteins. Moreover, given that synapses vary greatly in size, even on the same cell, but that the presynaptic grid and postsynaptic density at any given synapse have the same size (Lisman and Harris, 1993), it can be further concluded that the mechanism of growth must be a trans-synaptic process that involves structural coordination between the presynaptic and postsynaptic cell.
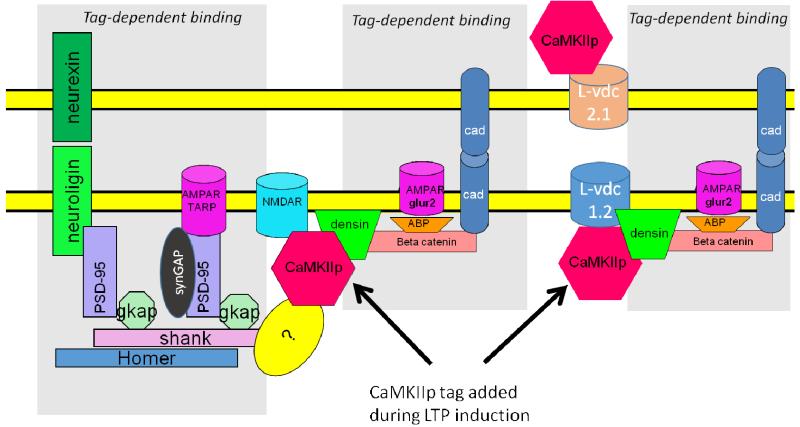
How is this trans-synaptic structural process triggered, what proteins are added, and how do these produce potentiation? According to the Morris and Frey model (Frey and Morris, 1997), a synapse that is activated strongly enough to undergo late LTP is molecularly “tagged” at the time of LTP induction. Proteins, some of which are newly synthesized, are then selectively added to tagged synapses, thereby producing late LTP. The identity of the proteins added and the manner in which they promote synapse growth were not specified in the Morris-Frey model. However, subsequent work suggests that CaMKII is involved in setting the tag and may actually be the tag (Moncada et al., 2011; Redondo et al., 2010). The way in which the tag then organizes other proteins to enhance synapse growth and produce potentiation is suggested by a recent model based on known binding interactions (Fig.8). Readers interested in the evidence for the binding reactions shown in Fig.8 should consult (Sanhueza and Lisman, 2013). According to this model, each CaMKII/NMDAR complex forms a larger structure with other PSD proteins, densin and catenin. Catenin, in turn, binds N-cadherin, an adhesion molecule that organizes a trans-synaptic structure. Among the proteins added postsynaptically to catenin is AMPA binding protein (ABP), which results in an increase in AMPA channels at the synapse. It is assumed that presynaptic N-cadherin organizes additional vesicle release sites, but the details of this process are not specified. As a result of these changes, synaptic strength is increased through presynaptic and postsynaptic changes in a manner consistent with experimental results on quantal transmission (see (Lisman and Raghavachari, 2006)).
Fig.8. Molecular model of trans-synaptic structural unit.
Activated (phosphorylated) CaMKII binds to the NMDAR, forming the tag. Then other proteins, some newly synthesized, gradually (over the time course of an hour) bind to the synapse in a tag-dependent fashion. Three potential scenarios (not mutually exclusive) are shown in the three grey areas. The addition of adhesion molecules such as N-cadherin may serve to ensure that as the synapse grows, it has the same size presynaptically and postsynaptically. Although beta catenin is shown in the figure, both delta and beta forms bind to densin (Heikkila et al., 2007) and may thus be part of the structural unit. The figure shows a speculative binding interaction (?) that might explain how CaMKII binding to the NMDAR could serve as a seed for the observed addition of Shank and PSD-95.
Fig.8 illustrates several further possible ways that the binding of CaMKII to the synapse might serve as a tag and thereby organize the addition of other synaptic proteins. Recent findings (Bosch et al., 2014; Meyer et al., 2014) show that among the proteins slowly added to the synapse after LTP induction are Shank and PSD-95. However, there is no known linkage of these proteins to either CaMKII, densin, or the proteins that may bind in a densin-dependent way. This raises the possibility that some unknown protein can bind to CaMKII and then organize the addition of Shank and PSD-95 to the synapse. Fig.8 illustrates the further possibility that CaMKII binding to L-type channels, which forms a complex that involves densin, could serve as tag for protein addition (Hell et al., 1996; Hudmon et al., 2005; Jenkins et al., 2010).
In this model, each CaMKII/NMDAR can be considered a binary element; CaMKII is either bound to the NMDAR or not. How then is gradedness of synaptic strength (Enoki et al., 2009) and synapse size (Lisman and Harris, 1993) to be explained? One possibility is that gradation occurs because of variation in the number of CaMKII/NMDAR complexes, each of which organizes a module containing many AMPAR-binding proteins and AMPARs. We previously proposed that the synapse contains such modules (Lisman and Raghavachari, 2006) to account for several puzzling physiological results. Consistent with the concept of structural modules, recent experimental work directly demonstrates that the structure of individual synapses is inhomogeneous; there are clusters of AMPARs within subregions of the synapse (Chen et al., 2011; Fukazawa and Shigemoto, 2012; MacGillavry et al., 2013).
With this background in mind, we can now turn to the question of how information could be stably stored despite turnover of NMDARs. One possibility is that CaMKII binds to two NR2B subunits within the same NMDAR. It might be supposed that NMDARs, like CaMKII, turn over by subunit exchange; in this case, CaMKII could stay bound to one subunit within the NMDAR while the other is replaced. For this to occur, NMDARs would have to contain two NR2B subunits; there are some indications that NMDARs rarely do (Tovar et al., 2013). An alternate possibility is that a single CaMKII holoenzyme could bind to two different NMDARs. In this case, one CaMKII/NMDAR complex could be retained even when the link to the other complex was lost in the process of receptor turnover. Examination of Fig.8 suggests still another possibility. Once such a trans-synaptic structure is built, CaMKII might be held so stably by this structure that CaMKII might be retained at the synapse even after the bonds with all NMDARs are broken. A newly synthesized NMDAR could then bind to CaMKII, restoring the complete structure. It is noteworthy that, in this model, synapse size is initially determined by the number of CaMKII/NMDAR complexes. However, after the trans-synaptic structure is built, one can consider the information to be redundantly stored by the size of presynaptic grid and the postsynaptic density. These paired redundant structures are matched by virtue of the adhesion molecules that cross through the synaptic cleft. This paired structure, which has elements in common with DNA structure, could potentially mean that large elements of either the presynaptic or postsynaptic complexes could dissociate yet be repaired by a pairing operation (Sanhueza and Lisman, 2013).
Principle 6: Inhibition of spontaneous formation of the CaMKII/NMDAR complex
Modest elevation of Ca2+ occurs during action potentials or during mEPSPs. This elevation is much smaller than occurs during LTP induction. If CaMKII activation was linear with Ca2+, these events might cause a very small increase in the CaMKII/NMDAR complex and in synaptic strength. Such increases, when accumulated over years, could lead to synapse saturation that would destroy memory, a process that depends on the heterogeneity of synaptic strengths for information storage. It is thus important that complex formation be selectively stimulated by the strong Ca2+ elevation that occurs during LTP induction, but not during the modest Ca2+ elevations that occur during spontaneous events. Two recently discovered properties of CaMKII produce nonlinear effects that could contribute to this selectivity.
The first property is the highly nonlinear dependence of CaMKII autophosphorylation on Ca2+ levels. Such nonlinearity is described by the Hill coefficient, which for CaMKII is 8 (Bradshaw et al., 2003), an unusually high value for a biochemical process. The Hill coefficient is related to the number of independent reactions that must occur to produce a specific consequence. The mechanisms that contribute to the high Hill coefficient of CaMKII include the need for multiple Ca2+ bindings to activate calmodulin, a form of structural cooperativity that regulates the equilibrium between the condensed and extended structures of CaMKII (Chao et al., 2011), and the requirement for multiple calmodulin bindings to neighboring subunits to produce autophosphorylation of CaMKII (Hanson et al., 1994).
A second property of CaMKII that limits complex formation has to do with the multiple steps required to generate activated CaMKII that is free. Holoenzymes having at least some beta subunits are bound to actin within the spine head at resting Ca2+ levels (Okamoto et al., 2007; Sanabria et al., 2009; Shen et al., 1998). Upon binding of Ca2+/CaM, CaMKII is released from actin and can only then diffuse to the NMDAR. Given that CaMKII cross-links actin, it follows that more than one CaMKII subunit must be activated by Ca2+/CaM before CaMKII becomes diffusible. Because of these factors, the fraction of activated CaMKII that is free will be a nonlinear function of Ca2+ such that even the few CaMKII holoenzymes that are activated by moderate Ca2+ elevation are not free to bind to the NMDAR.
One further possibility is worthy of note. Suppose that a dendritic Ca2+ spike activated CaMKII and liberated it from actin in a spine, but there was no synaptic input to that spine. In this case, the Hebbian condition is not met and LTP is not induced. Thus, one must suppose that CaMKII does not bind to the NMDAR. What could prevent such binding? One mechanism would be if binding to the NMDAR required a conformation of the NMDAR that is dependent on glutamate activation of the receptor (i.e., a metabotropic action of the NMDAR). Recent work suggests that NMDARs have a metabotropic function (Nabavi et al., 2013; Tamburri et al., 2013) (but see (Babiec et al., 2014)). However, it is not yet known whether such metabotropic action facilitates CaMKII binding. An alternative mechanism for which there is suggestive evidence is as follows: activation of NMDAR allows Ca2+ influx that triggers the calmodulin-dependent removal of alpha actinin from the NR1 subunit of the NMDAR (Merrill et al., 2007). Such removal is a prerequisite for CaMKII binding to NR1, a binding that probably contributes to the stability of the CaMKII/NMDAR complex. Thus, in this way, stable formation of the CaMKII/NMDAR complex might only occur at synapses having synaptic input.
Conclusions
In this article, we have summarized the growing biochemical evidence relevant to how the CaMKII/NMDAR complex could operate as a long-term memory switch. It has generally been thought that the stability of information storage by synapses is a deeply mysterious process, particularly because information must be stored stably by unstable molecules. Early models of the CaMKII argued that simple biochemical processes, particularly autophosphorylation and subunit exchange, could solve the stability problem. This class of solutions is now strengthened by several lines of experimental results, notably the reconstitution of the CaMKII/NMDAR switch and the increased plausibility of subunit exchange as the mechanism of CaMKII protein turnover. These results suggest that stable information is not deeply mysterious and that it may be possible with further research to provide a biophysically and biochemically constrained explanation of stability.
Although the role of the CaMKII/NMDAR complex in the maintenance of LTP has experimental support, much remains to be learned about the role of this complex in memory storage. Recent work has shown that a putative dominant-negative form of CaMKII can erase a form of memory (addiction based on synaptic changes in the accumbens (Loweth et al., 2013)), suggesting a role of CaMKII in memory maintenance. Other work has more specifically addressed the role of the CaMKII/NMDAR complex. This work, which used a knockin mutation that interferes with the CaMKII/NMDAR complex, showed only a modest reduction in the concentration of complex and only modest reductions in memory (Halt et al., 2012). It would thus appear that other interaction sites between CaMKII and NMDARs (on NR2B and NR1 (Bayer et al., 2001; Leonard et al., 1999)) may allow complex formation in vivo. Other interactions, such as those shown in Fig.8, may produce coimmunoprecipitation of CaMKII with the NMDAR, even when there are is no direct binding. Developing animal models in which the reduction in complex is more marked will be an important step in understanding the role of the complex in behavioral memory.
Some of the principles of switch stability that we have outlined have strong experimental support, but several remain quite speculative. To account for the inhibition of T286p dephosphorylation by the NMDAR, we propose that the NMDAR pushes T286 into the catalytic sites of a neighboring subunit, thereby shielding it from phosphatase. Although it is known that T286p can occupy this site, the dependence of this occupancy on the NMDAR needs to be directly tested. Particularly preliminary are the ideas that we propose about the role of synaptic structure in producing stability. It is often thought that any structural change is stable, but this is not the case. Instability of the structure can arise from the loss of molecules making the structure smaller. Furthermore, because free molecules must be present to deal with protein turnover, there may be spontaneous addition of molecules at the edge of the structure, making it larger. In a seminal paper, (Shouval, 2005) considered abstract rules by which these forms of instability could be minimized, but these rules were not yet formulated in physical terms. Development of analytical tools based on the physical chemistry of protein interactions is needed to understand structural stability.
Highlights.
The CaMKII/NMDAR switch is implicated in the maintenance of LTP.
Autophophosphorylation stabilizes the switch in reconstitution experiments.
Switch stability results from protein turnover by subunit exchange.
A working model for how the CaMKII/NMDAR complex enlarges the synapse.
The catalytic site may protect T286 from phosphatase.
Acknowledgement
We thank Ivan Herreros for comments on the manuscript. This work was supported by NIH/NIDA CRCNS Grant R01DA027807.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John Lisman, Brandeis University, Department of Biology and Volen Center for Complex Systems, 415 South Street – MS008, Waltham, Massachusetts 02454, Lisman@brandeis.edu, Phone: (781) 736-3145, Fax: (781) 736-3107.
Sridhar Raghavachari, Department of Neurobiology, Duke University Medical Center, Durham, NC 27710.
References
- Abraham WC. How long will long-term potentiation last? Philos Trans R Soc Lond B Biol Sci. 2003;358:735–44. doi: 10.1098/rstb.2002.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiec WE, et al. Ionotropic NMDA receptor signaling is required for the induction of long-term depression in the mouse hippocampal CA1 region. J Neurosci. 2014;34:5285–90. doi: 10.1523/JNEUROSCI.5419-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcomb K, Coultrap SJ, Bayer KU. Enzymatic activity of CaMKII is not required for its interaction with the glutamate receptor subunit GluN2B. Mol Pharmacol. 2013;84:834–43. doi: 10.1124/mol.113.089045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–53. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bayer KU, et al. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–5. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–85. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bosch M, et al. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron. 2014;82:444–59. doi: 10.1016/j.neuron.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus. 2011;21:354–73. doi: 10.1002/hipo.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw JM, et al. An ultrasensitive Ca2+/calmodulin-dependent protein kinase II-protein phosphatase 1 switch facilitates specificity in postsynaptic calcium signaling. Proc Natl Acad Sci U S A. 2003;100:10512–7. doi: 10.1073/pnas.1932759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci U S A. 1998;95:10890–5. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LH, et al. Intersubunit capture of regulatory segments is a component of cooperative CaMKII activation. Nat Struct Mol Biol. 2010;17:264–72. doi: 10.1038/nsmb.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LH, et al. A mechanism for tunable autoinhibition in the structure of a human Ca2+/calmodulin-dependent kinase II holoenzyme. Cell. 2011;146:732–45. doi: 10.1016/j.cell.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, et al. PSD-95 is required to sustain the molecular organization of the postsynaptic density. J Neurosci. 2011;31:6329–38. doi: 10.1523/JNEUROSCI.5968-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyan J, et al. Calcium/calmodulin dependent protein kinase II bound to NMDA receptor 2B subunit exhibits increased ATP affinity and attenuated dephosphorylation. PLoS One. 2011;6:e16495. doi: 10.1371/journal.pone.0016495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LD, et al. Metabolic turnover of synaptic proteins: kinetics, interdependencies and implications for synaptic maintenance. PLoS One. 2013;8:e63191. doi: 10.1371/journal.pone.0063191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, et al. CaMKII autonomy is substrate-dependent and further stimulated by Ca2+/calmodulin. J Biol Chem. 2010;285:17930–7. doi: 10.1074/jbc.M109.069351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki R, et al. Expression of long-term plasticity at individual synapses in hippocampus is graded, bidirectional, and mainly presynaptic: optical quantal analysis. Neuron. 2009;62:242–53. doi: 10.1016/j.neuron.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Feng B, Raghavachari S, Lisman J. Quantitative estimates of the cytoplasmic, PSD, and NMDAR-bound pools of CaMKII in dendritic spines. Brain Res. 2011;1419:46–52. doi: 10.1016/j.brainres.2011.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, et al. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–6. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Shigemoto R. Intra-synapse-type and inter-synapse-type relationships between synaptic size and AMPAR expression. Curr Opin Neurobiol. 2012;22:446–52. doi: 10.1016/j.conb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Giese KP, et al. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–3. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Gouet C, et al. On the mechanism of synaptic depression induced by CaMKIIN, an endogenous inhibitor of CaMKII. PLoS One. 2012;7:e49293. doi: 10.1371/journal.pone.0049293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruart A, Munoz MD, Delgado-Garcia JM. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26:1077–87. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin RM, et al. Loss of Thr286 phosphorylation disrupts synaptic CaMKIIalpha targeting, NMDAR activity and behavior in pre-adolescent mice. Mol Cell Neurosci. 2011;47:286–92. doi: 10.1016/j.mcn.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halt AR, et al. CaMKII binding to GluN2B is critical during memory consolidation. EMBO J. 2012;31:1203–16. doi: 10.1038/emboj.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, et al. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron. 1994;12:943–56. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- Heikkila E, et al. Densin and beta-catenin form a complex and co-localize in cultured podocyte cell junctions. Mol Cell Biochem. 2007;305:9–18. doi: 10.1007/s11010-007-9522-6. [DOI] [PubMed] [Google Scholar]
- Hell JW, et al. N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons. Proc Natl Acad Sci U S A. 1996;93:3362–7. doi: 10.1073/pnas.93.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, et al. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–47. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MA, et al. Ca2+-dependent facilitation of Cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:5125–35. doi: 10.1523/JNEUROSCI.4367-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SA, et al. CaM-kinase II dephosphorylates Thr(286) by a reversal of the autophosphorylation reaction. Biochem Biophys Res Commun. 2001;282:773–80. doi: 10.1006/bbrc.2001.4651. [DOI] [PubMed] [Google Scholar]
- Lee SJ, et al. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, et al. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1999;96:3239–44. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Goldring M. Evaluation of a model of long-term memory based on the properties of the Ca2+/calmodulin-dependent protein kinase. J Physiol (Paris) 1988;83:187–97. [PubMed] [Google Scholar]
- Lisman J, Raghavachari S. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci STKE. 20062006:re11. doi: 10.1126/stke.3562006re11. [DOI] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–82. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proc Natl Acad Sci U S A. 1985;82:3055–7. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Harris KM. Quantal analysis and synaptic anatomy--integrating two views of hippocampal plasticity. Trends Neurosci. 1993;16:141–7. doi: 10.1016/0166-2236(93)90122-3. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Loweth JA, et al. Persistent reversal of enhanced amphetamine intake by transient CaMKII inhibition. J Neurosci. 2013;33:1411–6. doi: 10.1523/JNEUROSCI.4386-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillavry HD, et al. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron. 2013;78:615–22. doi: 10.1016/j.neuron.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill MA, et al. Displacement of alpha-actinin from the NMDA receptor NR1 C0 domain By Ca2+/calmodulin promotes CaMKII binding. Biochemistry. 2007;46:8485–97. doi: 10.1021/bi0623025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Bonhoeffer T, Scheuss V. Balance and stability of synaptic structures during synaptic plasticity. Neuron. 2014;82:430–43. doi: 10.1016/j.neuron.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Miller P, et al. The stability of a stochastic CaMKII switch: dependence on the number of enzyme molecules and protein turnover. PLoS Biol. 2005;3:e107. doi: 10.1371/journal.pbio.0030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44:861–70. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Moncada D, et al. Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation. Proc Natl Acad Sci U S A. 2011;108:12931–6. doi: 10.1073/pnas.1104495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, et al. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–42. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Mullasseril P, et al. A structural mechanism for maintaining the ‘on-state’ of the CaMKII memory switch in the post-synaptic density. J Neurochem. 2007;103:357–64. doi: 10.1111/j.1471-4159.2007.04744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, et al. Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc Natl Acad Sci U S A. 2013;110:4027–32. doi: 10.1073/pnas.1219454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, et al. Engineering a memory with LTD and LTP. Nature. 2014 doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary H, et al. Nucleotides and phosphorylation bi-directionally modulate Ca2+/calmodulin-dependent protein kinase II (CaMKII) binding to the N-methyl-D-aspartate (NMDA) receptor subunit GluN2B. J Biol Chem. 2011;286:31272–81. doi: 10.1074/jbc.M111.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, et al. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci U S A. 2007;104:6418–23. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff LE, et al. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35:535–45. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- Redondo RL, et al. Synaptic tagging and capture: differential role of distinct calcium/calmodulin kinases in protein synthesis-dependent long-term potentiation. J Neurosci. 2010;30:4981–9. doi: 10.1523/JNEUROSCI.3140-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, et al. Differential modulation of Ca2+/calmodulin-dependent protein kinase II activity by regulated interactions with N-methyl-D-aspartate receptor NR2B subunits and alpha-actinin. J Biol Chem. 2005;280:39316–23. doi: 10.1074/jbc.M508189200. [DOI] [PubMed] [Google Scholar]
- Sanabria H, et al. {beta}CaMKII regulates actin assembly and structure. J Biol Chem. 2009;284:9770–80. doi: 10.1074/jbc.M809518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, McIntyre CC, Lisman JE. Reversal of synaptic memory by Ca2+/calmodulin-dependent protein kinase II inhibitor. J Neurosci. 2007;27:5190–9. doi: 10.1523/JNEUROSCI.5049-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, et al. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J Neurosci. 2011;31:9170–8. doi: 10.1523/JNEUROSCI.1250-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, Lisman J. The CaMKII/NMDAR complex as a molecular memory. Mol Brain. 2013;6:10. doi: 10.1186/1756-6606-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, et al. CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- Shouval HZ. Clusters of interacting receptors can stabilize synaptic efficacies. Proc Natl Acad Sci U S A. 2005;102:14440–5. doi: 10.1073/pnas.0506934102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1998;273:20689–92. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- Stratton M, et al. Activation-triggered subunit exchange between CaMKII holoenzymes facilitates the spread of kinase activity. Elife. 2014;3:e01610. doi: 10.7554/eLife.01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swulius MT, et al. Structure and composition of the postsynaptic density during development. J Comp Neurol. 2010;518:4243–60. doi: 10.1002/cne.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburri A, et al. NMDA-receptor activation but not ion flux is required for amyloid-beta induced synaptic depression. PLoS One. 2013;8:e65350. doi: 10.1371/journal.pone.0065350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, et al. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–7. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, McGinley MJ, Westbrook GL. Triheteromeric NMDA receptors at hippocampal synapses. J Neurosci. 2013;33:9150–60. doi: 10.1523/JNEUROSCI.0829-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakubo H, et al. In vitro reconstitution of a CaMKII memory switch by an NMDA receptor-derived peptide. Biophys J. 2014;106:1414–20. doi: 10.1016/j.bpj.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest RS, et al. Dual mechanism of a natural CaMKII inhibitor. Mol Biol Cell. 2007;18:5024–33. doi: 10.1091/mbc.E07-02-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxham MN, et al. Calcium/calmodulin-dependent protein kinase II regulates hippocampal synaptic transmission. Brain Res. 1993;609:1–8. doi: 10.1016/0006-8993(93)90847-g. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, et al. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–7. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Zhabotinsky AM. Bistability in the Ca(2+)/calmodulin-dependent protein kinase-phosphatase system. Biophys J. 2000;79:2211–21. doi: 10.1016/S0006-3495(00)76469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]