Abstract
Background
Lymphatic dysfunction has been linked to inflammation since the 1930’s. Lymphatic function in the gut and mesentery is grossly underexplored in models of IBD despite the use of lymphatic occlusion in early models of IBD. Activation of the innate and adaptive immune system is a hallmark of TNBS-induced inflammation and is linked to disruption of the intrinsic lymph pump. Recent identification of crosstalk between lymphatic vessel resident immune cells and regulation of lymphatic vessel contractility underscore the importance of the timing of lymphatic dysfunction during tissue inflammation in response to TNBS.
Methods
To evaluate lymphatic function in TNBS induced inflammation, lymph was collected and flow measured from mesenteric lymphatics. Cellularity and cytokine profile of the lymph was also measured. Histopathology was performed to determine severity of injury and immunofluorescent staining of the mesentery was done to evaluate changes in the population of immune cells that reside near and on gastro-intestinal collecting lymphatics.
Results
Lymph transport fell 24hrs after TNBS administration and began recovering at 72hrs. Significant reduction of lymph flow preceded significant increase in histopathological score and occurred simultaneously with increased MPO activity. These changes were preceded by increased MHCII+ cells surrounding mesenteric lymphatics leading to an altered lymphatic environment that would favor dysfunction.
Conclusions
Alterations in environmental factors that effect lymphatic function occur before the development of gross GI inflammation. Reduced lymphatic function in TNBS-mediated inflammation is likely an early factor in the development of injury and that recovery of function is associated with resolution of inflammation.
Keywords: inflammation, lymphatic dysfunction, ileitis, colitis, lymph flow
Introduction
Regulation of immune cell signaling and antigen presentation through altered cellular environmental cues is a well-defined mechanism used by the immune system to generate an appropriate response to an antigen (1–3). This phenomenon is employed in laboratories to force differentiation and alteration of immune cell behavior in vitro to either inflammatory or tolerogenic responses and this same scheme holds in true in vivo (2). Alteration of the cellular milieu in vivo is regulated by a complex integration of the responses of cells making up the tissue resident innate and adaptive immune cells (4, 5). This mechanism of regulation is critical for controlling the response to antigens by the adaptive immune system and establishing which antigens will be tolerated (tolerance) and which will elicit an inflammatory response (6–8).
Traditionally, antigens are sampled and presented by dendritic cells to and with various T and B cell subsets in the lymph node (9–18). The node and this process are fed by the lymphatic system, which collects antigens, cytokines and immune cells from the tissue spaces (17, 19, 20). Activation and regulation of the adaptive immune response is commonly thought to be a secondary function of the lymphatics with fluid and macromolecule recovery and return to the blood vasculature being the primary function (13–18). However, mice that lack an adaptive immune response have reduced or absent secondary lymphoid organs and therefore have an incomplete lymphatic system (21). This suggests that the primary function of the lymphatic system is to serve and support the adaptive immune system. Indeed anatomically the body’s network of lymphatic vessels is centered on the lymph nodes, with the afferent lymphatic network delivering immune elements from the interstitium to the node and the efferent lymphatic network transporting immune elements from the node to the blood for eventual distribution throughout the body (15, 22–26). CSF1R and PU.1 knockout mice show that interruptions of monocyte development and differentiation leads to the formation of a dysplastic lymphatic network, supporting this principle since the monocyte lineage is responsible for regulating innate and adaptive immunity (27, 28). Furthermore it has been show that certain macrophage cells express a common lymphatic endothelial marker LYVE-1, and associate with and integrate into lymphatic vessels, which further strengthens the argument that the lymphatic system primarily serves the immune system (24, 29, 30). Our group has reported that a unique population of innate immune cells resides on muscular lymphatic vessels and that the mesenteric collecting lymphatics have a significant investment of these cells that are very dynamic (22, 30). What roles these cells are playing for the lymphatic system during different immune responses are not known. However, many of these innate immune cells (e.g. mast cells, macrophages, etc.) are capable of producing mediators that reduce or stop lymphatic function (31–33). We hypothesize that they provide mechanisms to sample and condition lymph as well as regulate lymphatic function, thereby orchestrating the lymphatic systems response to immune reactions to induce either tolerance or inflammation. To do this, important immune elements must be carried to and from the nodes and these must be carried in the fluid component of lymph. Thus when taken as a whole, there is evidence that if either parts of the innate immune system or adaptive immune system are lost then the lymphatic system does not fully develop and perform one of it’s critical functions. Therefore it stands to reason that the lymphatics function not only to maintain fluid homeostasis, but also as a critical temporal and spatial matrix for the coordination and regulation of the immune system.
In addition to the cells and antigens that lymph transports, it also contains a collection of elements of the cellular milieu such as cytokines, chemokines, adipokines, cells and extracellular matrix signaling molecules. Therefore lymph is also a key indicator of tissue status (34–39). When lymphatic flow from a tissue is interrupted in an otherwise healthy animal, an inflammatory response develops at the tissue that is normally served by that lymphatic and node (14, 40–43). This suggests that in the absence of normal lymph flow local immunological homeostasis is interrupted, which fits with the hypothesis that the lymphatics exist to serve the immune system under both normal and inflamed conditions. When lymphatic dysfunction occurs under otherwise ‘normal’ conditions”, a general cellulitis can occur that then can develop further into a more complex inflammatory disease (44–47). However, if lymph flow is interrupted in the intestine, inflammation is theorized to occur due to contamination of the intestinal wall with gut contents. This creates an inflammatory injury that, while mechanistically may be similar to cellulitis, is much more complex due to environmental factors (43, 48–50). Experimentally, this inflammation appears to bridge the descriptions of Crohn’s disease and sclerosing lymphangitis (42, 43, 51–53). This has lead to debate about how to classify Crohn’s Disease, with some groups suggesting that the disease resembles a form of lymphedema with complications resulting from contamination with intestinal contents (49, 50, 54). If this hypothesis is accurate then lymphatic transport from the gut should decrease or stop prior to the development of the chronic gut inflammation. As we have stated before, others have described lymphatic abnormalities during chronic gut inflammation including the initial description of Crohn’s disease in 1932 (51). Furthermore, studies confirmed that interruption of lymph flow was sufficient to yield an injury similar to that seen in the tissues of IBD patients. This was experimentally accomplished in a number of different ways including surgical obstruction, fine particulate feeding and node fixation. All of these experimental procedures resulted in similar injury across multiple species (42, 43, 48). Recent findings on the alterations of lymph transport in models of intestinal inflammation demonstrate that inflammation of the gut is associated with the reduction of contractile activity of the muscularized lymphatics (55). This work was done in a model of ileitis using TNBS ileal injections, and while it provides evidence that lymphatic transport is interrupted during gross inflammation it does not provide information as to which develops first. Our work presented here is an attempt to address this major issue. We have found that lymphatic transport is reduced prior to the development of gross tissue injury and inflammation in our model. This suggests that lymphatic dysfunction is an initial component of intestinal inflammation and contributes to disease development.
Materials and Methods
All animal protocols were approved by the Scott and White Healthcare and Texas A&M University IACUCs and conform to the NIH guide for the care and use of laboratory animals.
Induction of TNBS inflammation
Non-fasted animals were anesthetized by isoflurane inhalation following which an 18-gauge catheter with a blunted end was inserted 7.5cm into the rectum and TNBS (in 500uL of 30% ethanol in PBS) was instilled at a dose of 30mg/kg of body weight at 0 and 120hrs. The anus was loosely held closed for 5min after instillation to allow for coating and absorbance of the TNBS, after which the animals were allowed to recover from anesthesia.
Collection of immune cells in the lymph
Control and TNBS treated animals were anesthetized as previously described and a midline laparotomy was performed with a loop of mesentery and associated intestine exposed and attached to a dissection board. The downstream portion of a lymphatic vessel originating in the gut wall was cannulated using a heparinized glass pipet pulled to a tip diameter of 80um that was fixed to maintain the height at heart level. Lymph was collected and the time and volume of collection was recorded and normalized to the weight of the animal in grams to determine the rate of lymph flow. The collected lymph was diluted to 900uL using PBS and spun at 2500×g for 5min to pellet cells present in the lymph. Then the supernatant was removed and saved for further analysis. The cells were resuspended in 50uL of PBS and smeared on a glass slide in a 1cm circle and allowed to dry at room temperature for 30min. The smears were fixed in methanol at 4°C for 30 sec and stained with DiffQuik differential stain. The slides were imaged at 40 and 100× magnification and the total number of cells per 40× field of view was counted, Cell counts were adjusted for the original lymph volume collected. N=3 for all time points as determined by power analysis except for 96hrs which displayed an extreme variability in data at that time point had an N=5.
Myeloperoxidase assay of the ileum and colon
Tissue samples were harvested from sites of the small intestine 2cm distal to the junction of the stomach and small bowel, 2cm proximal to the junction of the ileum and caecum and 2cm distal of the junction of the caecum and colon. The tissue was split length wise in cold PBS and washed of any luminal contents. It was then homogenized in PBS using a glass dauncer followed by two freeze thaw cycles. The resulting suspension was centrifuged at 13,000×G for 10min and the resulting enzymatic extraction (supernatant) was removed for use in the assay. A fluorometric MPO assay from AbCAM (485/528nm excitation emission) was used and 1µL of the enzymatic suspension was used for each sample and read after 5min and 30min incubation. The MPO activity was calculated as specified by the assay kit and normalized to total protein content in 1µL of enzyme suspension and expressed as the MPO activity (units)/mg of protein. N=3 for all samples.
Wet to dry weight ratios
Tissues were harvested from the same areas as the histopathology and was split and washed of all contents in cold PBS. The tissue was blotted dry and weighed prior to being placed in a convection drying oven at 45°C for 72hrs. The tissue was then reweighed and the percent water weight calculated. N=3 for all samples
Mesenteric Lymphatic vessel Diameter
Lymphatic vessels from the mesentery of control and 48hrs TNBS-treated rats were isolated from the ileum and cannulated on resistance matched glass pipettes (inner diameter ~80µm, outer diameter ~100µm) in a Living Systems microvessel apparatus. Vessels (n=3) were pressurized to 3cm/H2O and allowed to equilibrate for 30min at 37°C and the maximum outer diameter were recorded. No vessel used in this study displayed signs of damage (stricture sites, etc.) during isolation, manipulation or pressurization.
Histopathology
After induction with TNBS, the animals were sacrificed at 1, 6, 24, 48, 72, 96, 120 and 144hrs for sample collection and analysis. Tissues from the terminal ileum and colon (3cm distal to the caecum) were collected from control and TNBS animals, flushed of luminal contents and fixed in 4% PFA overnight. The fixed tissues were processed and embedded in paraffin and cut into 5µm sections, de-paraffinized and stained with hematoxylin and eosin. Tissues were scored on a scale of 0–4 (0 no damage, 1 least damage/severity, 4 worst damage/severity) for each of the following criteria; epithelial cell loss/damage, crypt loss, tissue cellularity, edema and vascularity. During the process of scoring, we examined both normal and transitional ileal sections (proximal and adjacent to the caecum) to determine overall alterations in the ileum. The individual scores for each criterion were compared and scores for overall histopathology were totaled. Colon sections were examined to confirm that there was injury but were not scored since our experiment focused on the ileal inflammation that occurred proximal to the site of immediate TNBS exposure and injury.
IHC of immune cells/contractile markers in, on and around the lymphatics
After the animals were sacrificed, the mesentery and surrounding intestine was removed, washed in PBS and pinned to SilGuard142© coated dishes. The tissue was fixed in 4% PFA for 2hrs followed by 3 washes in PBS and a final rinse in PBS with protease inhibitor cocktail overnight. The mesentery was trimmed away from the gut the following day and permeabilized in 0.01% triton-X100 in PBS for 1hr followed by a blocking step in 5% normal goat serum in PBS for 2hrs. Tissues were incubated overnight with primary antibodies at 4°C in antibody amplifying dilution buffer (ProHisto) followed by 3 washes in that buffer. Then the tissues were incubated for 2hrs with secondary antibody at room temperature. The dilutions and technical information for the antibodies used are listed in Table 1. Whole mount mesentery tissues from control and 48hr TNBS treated animals were harvested and fixed in 4% PFA as described and stained for smooth muscle actin and SM22α following the same procedure used for immune cell IHC. N=6 for all time points.
Table 1.
Manufacturer, catalog number and dilution factors of antibodies used in this study.
| Target | Manufacturer | Catalog # | Dilution |
|---|---|---|---|
| α Smooth muscle Actin | Abcam | ab54723 | 1 µg/mL |
| SM22α | Abcam | ab14106 | 2 µg/mL |
| MHCII (10-3.6) | Sant Cruz | sc-53721 | 0.5 µg/mL |
PCR of mesenteric lymphatic vessels from TNBS treated animals
Lymphatic vessels were isolated from exteriorized loops of mesentery from control, 1 and 6hr TNBS treated animals (n=3) after fixation in RNAlater. The isolated lymphatic vessels from one mesenteric loop per animal were pooled and RNA was extracted using a Qiagen mini spin column kit. RNA quantity and quality were check by NanoDrop measurements prior to reverse transcription (RT) reaction. An equal amount of RT DNA was reacted with primer sets for iNOS, TNF-α, IL-6 and GAPDH. Fold change in expression was determined by the ΔΔCT method.
Measurement of cytokines in lymph
Lymph samples were taken from the resulting supernatant of the pre-nodal lymph collected for flow measurement and cell analysis. Cytokine expression was measured using a rat specific Proteome Profiler™ kit from R&D Systems (cat. # ARY008). Total sample volumes of cell free lymph were diluted to 250µL total volume and used to determine levels of cytokines. The blot strips were imaged on a Fuji LS4000 imaging system and analyzed using ImageJ software (NIH). Integrated densities were normalized to control spots and then further normalized to sample volume (non-diluted lymph volume). N=5 for all time points.
Statistical Analysis
Statistical analysis was performed using ANOVA with Dunnett’s post hoc testing where applicable (all multiday comparisons compared to control (t=0)). In the cases of two-way comparisons with single variables Student’s two-tailed t-tests were used. All bars represent standard error of the mean (SE)
Ethical Considerations
Animals were monitored daily for signs of disease and weight loss. Due to the nature of the study analgesics and anti-inflammatory agents were not used, as a majority of these compounds are known to interfere with the mechanisms that were studied. No animals meet removal criteria (20% body weight loss, hunched position for more than 1 day and/or frank blood at time of TNBS delivery) for this study and no adverse events were encountered.
Results
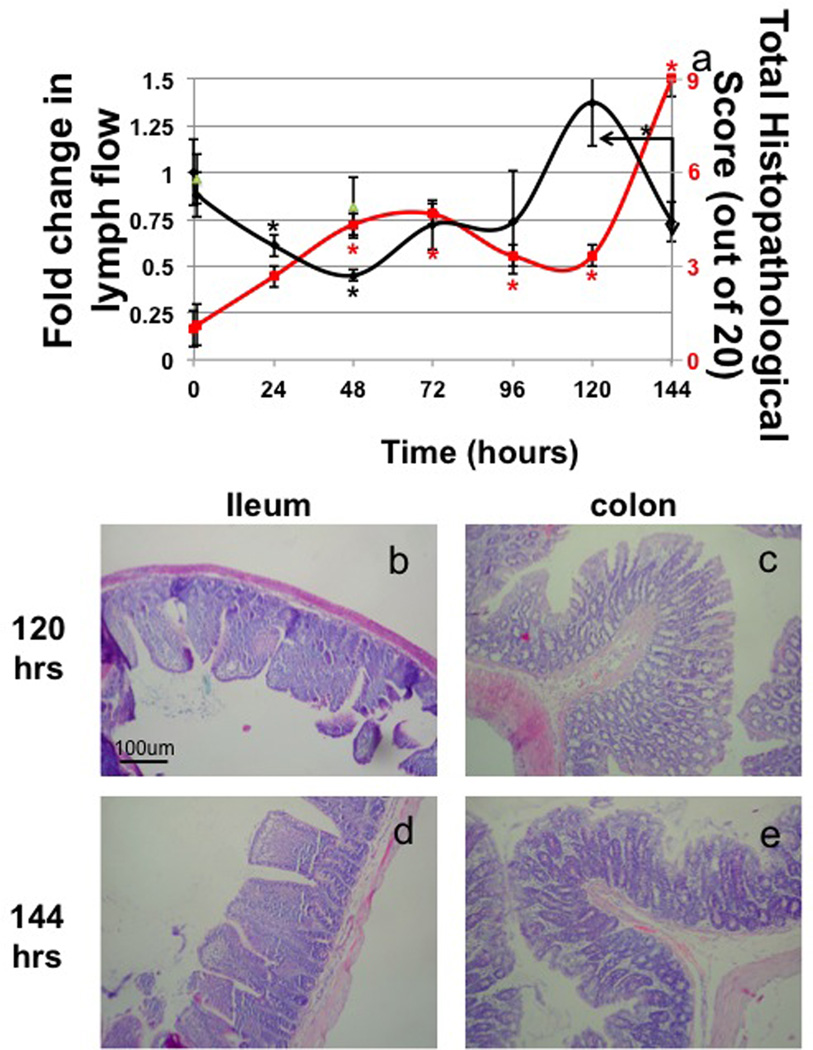
Lymph flow significantly fell 40% at 24hrs. It then declined further to a 50% decrease at 48 hours that recovered partially by 72 and 96hrs and continued to rise at 120hrs but did not reach a significantly elevated level. After the second TNBS treatment, (144hrs) lymph flow again dropped significantly from that measured at 120hrs (Fig 1a black line). This second interruption of lymph flow occurred quicker after the TNBS instillation than the first interruption, occurring within 24hrs after the exposure instead of 48hrs in the case of the initial instillation. Total histopathology scores revealed that injury in the ileum rose significantly at 48hrs and began a trend towards recovery at 96hrs (Fig 1a red line) but remained significantly elevated and never returned to control levels. Injury scores then peaked again at 144hrs after the second TNBS treatment at levels much higher than the previous post insult increase in histological score. Colon injury appeared more severe (Fig 1c, e) than ileal injury (Fig 1b, d) but appeared to follow the same temporal pattern as the ileum with injury being most apparent at 48 and 144hrs. Cellularity of the ileum significantly increased at 48hrs and remained elevated at 72hrs. The peak of ileal damage occurred after significant reduction in lymph flow from the gut and highest cell content of the lymph. Histological edema scores were elevated in all tissues (ileum and colon) at 48hrs and 72hrs but began to recover at 96hrs and were fully recovered at 120hrs although again the increases were milder in the ileum. The edema score then rose significantly again at 144hrs after the second treatment of TNBS. There was little epithelial damage and crypt loss in the ileum until after the second administration of TNBS, however it was mild compared to the epithelial damage in the colon.
Figure 1.
Measurements of fold change in lymph flow (lymph collected/time in min./animal weight in grams) (TNBS=black diamonds, 30% ethanol vehicle=green triangles) and histopathological scores calculated from TNBS treated rat ileum (red line) (a) and representative images from 120 and 144 hours (distal ileum b, d and proximal colon c, e). n=3 except 96hrs with n=5, bars=SE and * denotes p value ≤0.05
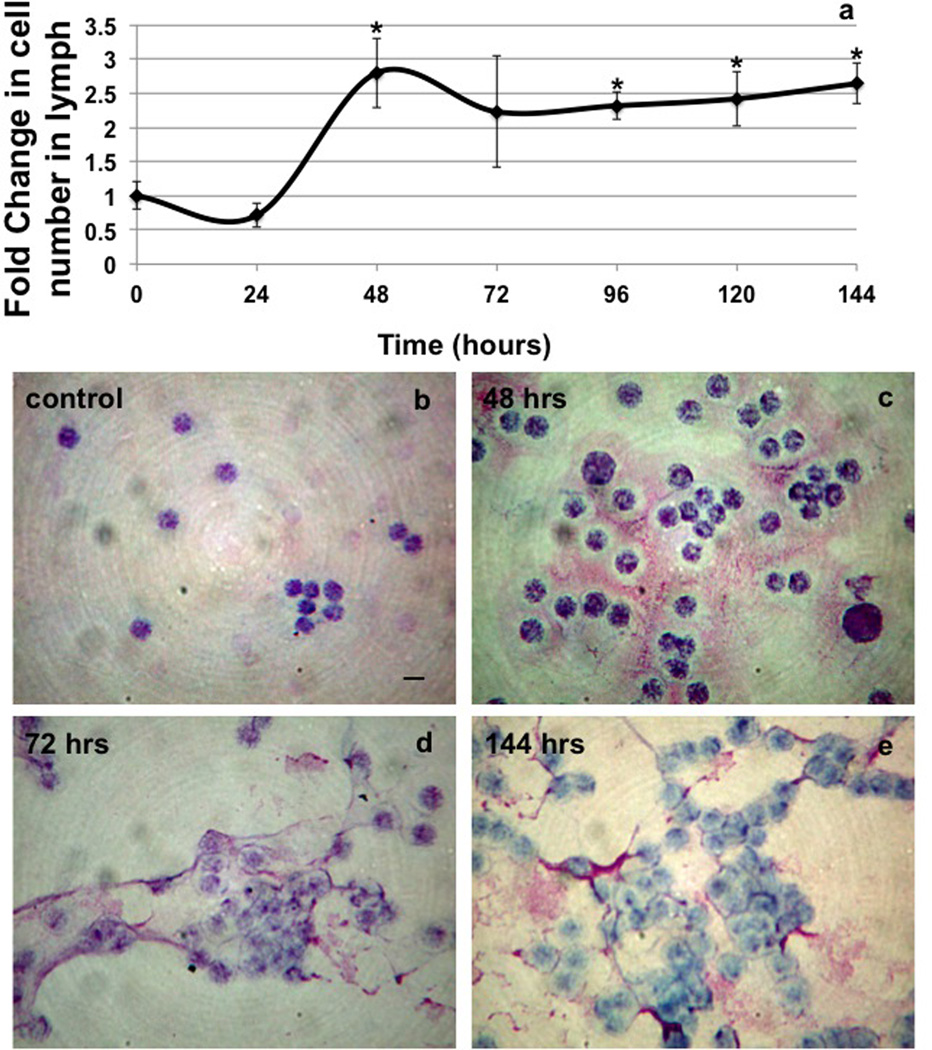
The total number of immune cells carried in the lymph remained at control levels for 24hrs after the first TNBS instillation, but increased 2.75 fold at 48hrs and remained elevated until the latest time point measured 144hrs (Fig 2a). In addition to the increase in the number of cells in the lymph after TNBS administrations there was also evidence of a diffuse fibrous material that was tightly adherent to the cells contained within the lymph (Fig 2c,d,e).
Figure 2.
Measurements of cellularity of the lymph (a) from control and TNBS treated animals from 0–144hrs. Representative images of cells in the lymph stained with DiffQuik differential stain at 0 (b) 48 (c), 72 (d) and 144 hrs (e). Note the accumulation of fibrous material in the 48 and 144hrs stains. n=3 except 96hrs (n=5), bars=SE and * denotes p value ≤0.05
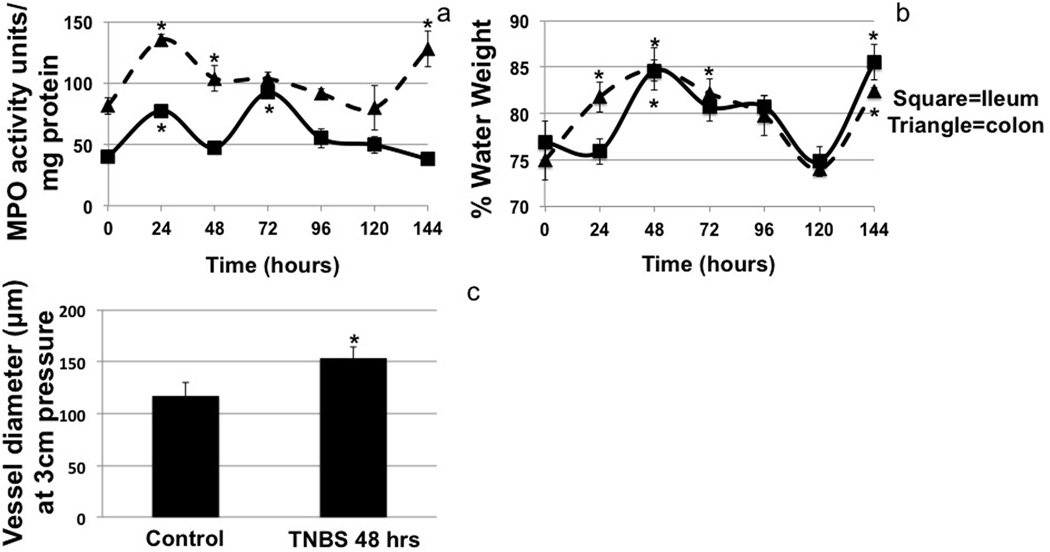
MPO assays confirmed that TNBS treatment increased the number of inflammatory cells in the ileum and colon (Fig 3a). Surprisingly there was also an increase in the MPO score of the jejunum at 48 and 144hrs (data not shown). The MPO scores increased significantly in the colon and ileum at 48hrs and remained elevated but not significantly so until 96hrs. This pattern was repeated at 144hrs in colon samples but not ileal, corresponding to the second administration of TNBS and the associated reduction in lymph flow (Fig 3a).
Figure 3.
MPO activity (a) and percent water weight (b) of the ileum (square, solid line) and colon (triangle, dashed line) after TNBS administration. MPO activity was measured as MPO activity units per mg of protein and showed a delayed biphasic response in the ileum. Water weight was measured as the dry weight of tissue divided by the wet weight of the tissue. Isolated vessel diameter was measured in lymphatic vessels from the ileum at 3cm/H2O pressure (c). n=3 for all measures, bar=se and * denotes p value ≤0.05
The percent water weight measured from the wet to dry weight ratios of the tissue supports the histological edema scoring very closely (Fig 3b). Increased water weight was seen in the ileum at 48hrs. It began resolving at 72hrs but remained elevated and then returned to normal at 96hrs. Water weight of the ileum again spiked at 144hrs but much more rapidly than the previous increase, again corresponding in time to the reduction in lymph flow associated with the administration of TNBS. We also measured a slight increase in percent water weight in tissues as far upstream as the jejunum at 48hrs (data not shown), suggesting that TNBS is inducing a general inflammation of the gut beginning at 48hrs which corresponds to the largest reduction of lymph flow. The colon percent water weight increased 24hrs after the first TNBS instillation suggesting edema and this pattern repeated after the second TNBS treatment, at 144hrs (Fig 3b).
Average mesenteric lymphatic vessel diameter at 3cm of water pressure was increase after TNBS treatment. Control vessels averaged ~117µm while 48hrs after TNBS treatment the average diameter significantly increased to ~153µm (Fig 3c).
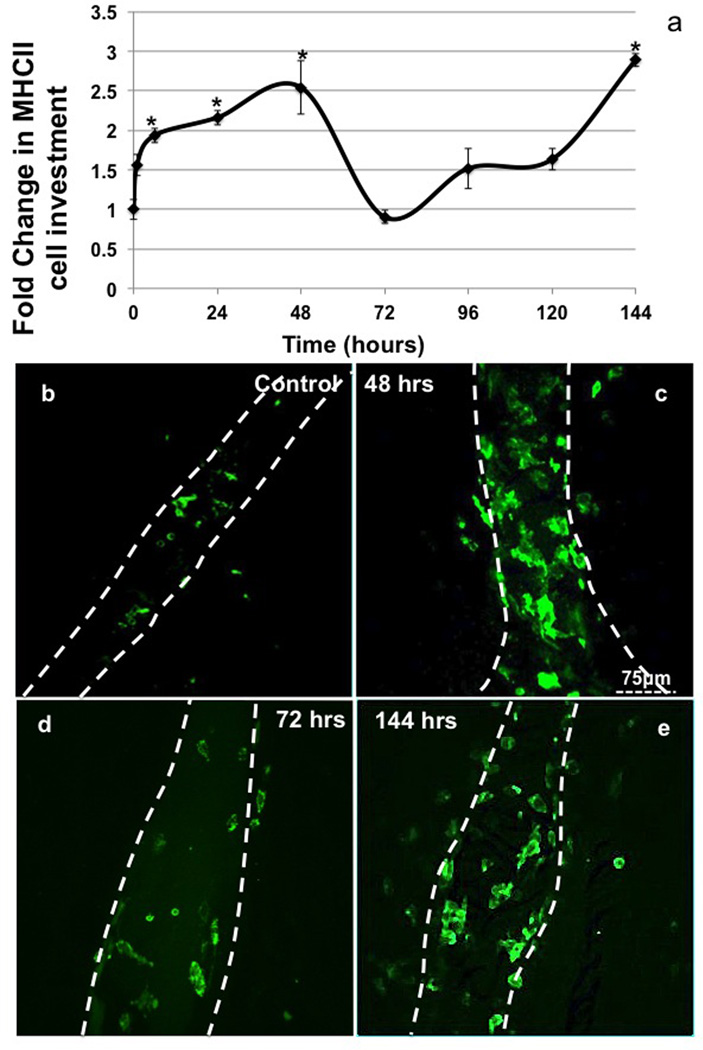
The number of MHCII+ immune cells that surrounded the mesenteric lymphatics increased significantly (>2 fold) in as little as 6hrs post TNBS instillation (Fig 4a). They remained elevated out to 48hrs after treatment compared to control (Fig 4b, c). At 72hrs, the number of MHCII+ cells associated with the vessel dropped to values not different from control numbers (Fig 4d). The number of cells along the vessel remained no different from control values from 72hrs to 120hrs but it then rapidly spiked at 144hrs (24hrs after the second TNBS administration) (Fig 4e).
Figure 4.
The number of MHCII+ cell counted along lymphatic vessels in mesenteric whole mounts and normalized to vessel area from 0 to 144 hrs (including 1 and 6hrs) (a). Representative mesenteric lymphatic vessel staining of MHCII (green, white dashed line showing outline of vessel) 0 (b) and 48hrs (c) n=6 (each n is an average of 3 separate 40× field containing the lymphatic vessel), bars=SE and * denotes p value ≤0.05
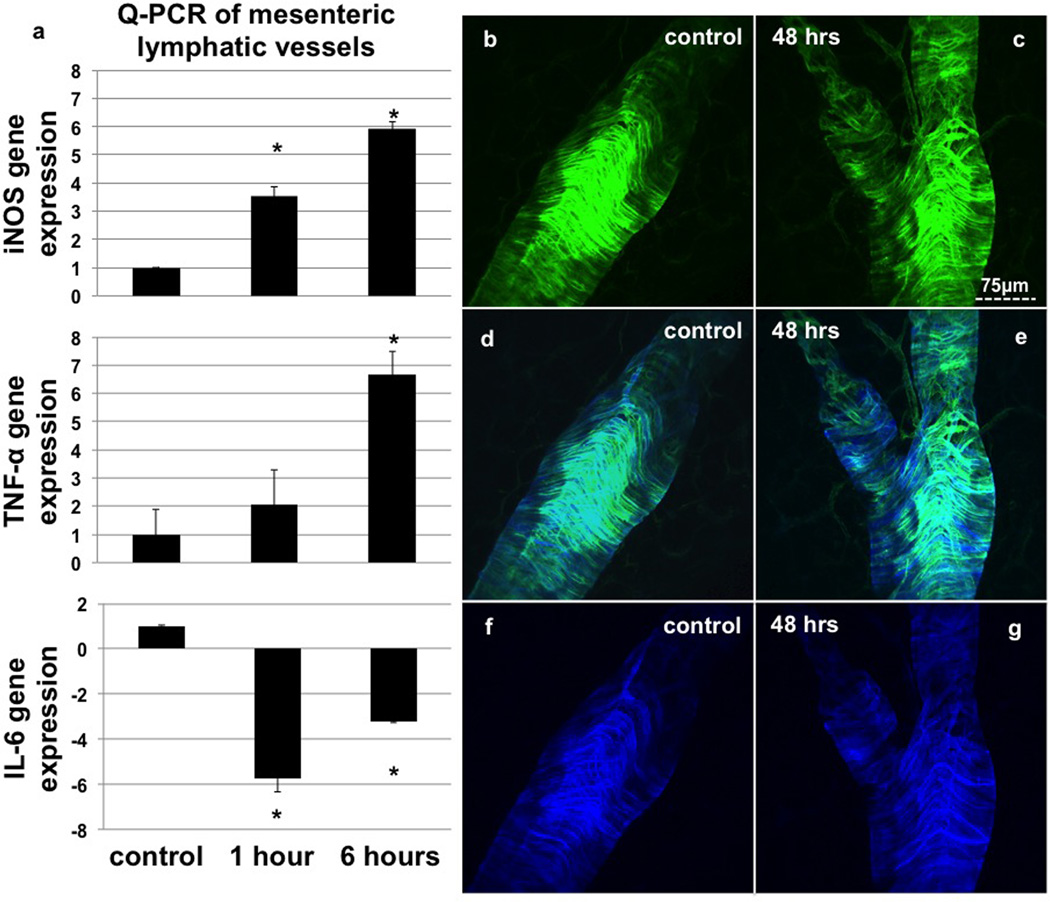
Expression of iNOS and TNF-α mRNA in the lymphatic vessel increased significantly as detected by PCR as early as 1hr after TNBS treatment, which mirrored the increase of MHCII+ cells along the vessel (Fig 5a). Interestingly the levels of IL-6 dropped significantly 1hr after TNBS administration and began recovering at 6 hours (Fig 5a).
Figure 5.
RTPCR data from mesenteric lymphatic vessels in control, 1 and 6hr TNBS animals (a) showing that there is a very rapid change in the expression of inflammatory cytokines and mediators such as iNOS. Representative images of SMA(green) and SM22α (blue) in control (b, d, f) and TNBS treated animals (c, e, g) after 48hrs showing no loss of muscle cells or contractile regulators. n=3 (a), bars=SE and * denotes p value ≤0.05
There was no apparent loss of smooth muscle investment of the vessels at any time point tested, nor was there a loss of the contractile regulatory protein SM22a (Fig 5b–g). Expression patterns also remained similar between TNBS treated and control groups.
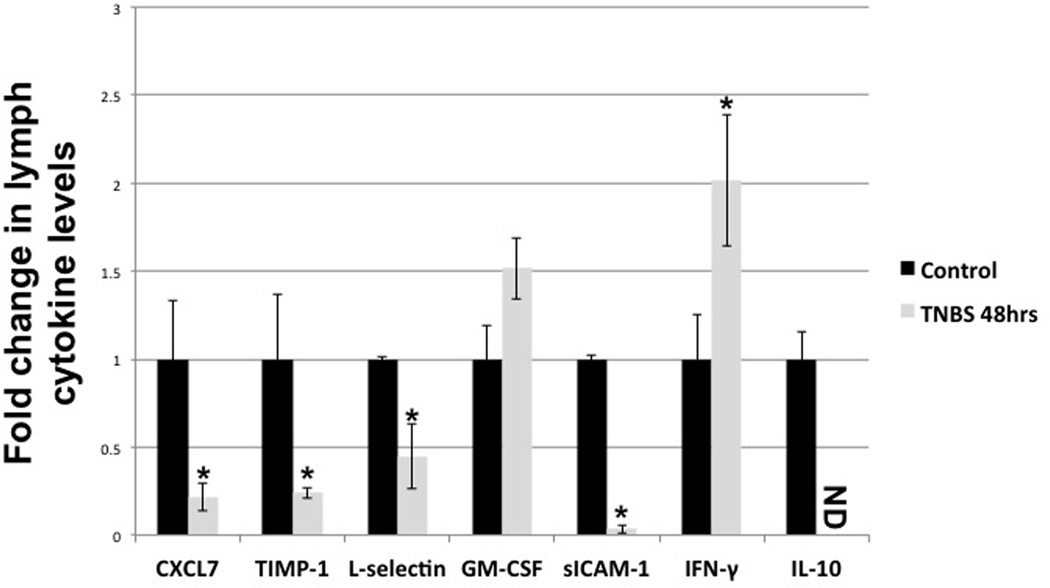
Of the 19 inflammatory cytokines and factors we detected in the lymph, only 5 were significantly changed by TNBS treatment. For example both s-ICAM and s-L-selectin were reduced in the lymph of TNBS treated animals (0.04 and 0.44 fold of control respectively) and TIMP-1 was reduced to 0.23 fold of control (Fig 6). Interestingly IL-10 was reduced in the lymph of TNBS treated animals to levels below detection, while IFN-γ was increased ~2.0 fold and GM-CSF was ~1.5 fold although levels of GM-CSF did not reach significance.
Figure 6.
Cytokine profile of lymph from control and 48hrs TNBS treated animals as detected by DotBlot assay. Lymph samples were collected from flow measurement experiments and centrifuged to remove cells. Statistical analysis was performed using ANOVA. n=5, ND=not detectable, bars=SE and * denotes p value ≤0.05
Discussion
The idea that the status of the lymphatic system is closely tied to inflammation is not new and it was the opinion of B.B. Crohn that lymphatic malfunction in the intestine could lead to inflammation (42, 43, 56). This idea supported by the work of Chess in the 1950’s that found ingestion of silica particles obstructed the intestinal lymphatics and produced tissue damage and inflammation similar to human Crohn’s disease (42). This idea remained prominent through the 1970’s as other methods that may have altered lymphatic function, such as fixation of regional lymph nodes, caused adverse inflammatory events in the GI tissue that node served (43). All of these studies indicated that the experimental impairment of lymph function could lead to inflammation and tissue damage. Clinically, lymphatic dysfunction can be linked to inflammation in the case of lymphedema and cellulitis (44, 47, 57, 58). Cellulitis is a demonstration of cause and effect whereby reduced lymph flow inhibits the ability of the immune system to properly respond to insult resulting in a continuous inflammatory reaction. Interestingly, some rare or underreported forms of IBD often resemble many aspects of cellulitis microscopically and, are associated with bouts of localized edema, particularly in the pelvic and genital areas (52, 59). Recent work in an ileitis model of intestinal inflammation has shown that the intrinsic pumping mechanisms of lymphatics are disrupted by inflammation. However other factors such as the accompanying tissue edema that occurs in that model also play a major role in lymph flow thus complicating interpretation of what happens to total lymph flow (60, 61). To our knowledge this is the first actual quantitative measurement of lymph flow in a model of intestinal inflammation. This study is also unique in examining the very early time points associated with intestinal inflammation and how lymph flow is affected. We have correlated the changes in lymph flow and other parameters of lymphatic function to the development of GI inflammation/injury in an attempt to provide an answer to the question of whether reduced lymph flow is a potential causative force in the inflammation or a secondary result of GI injury.
The model we chose to test this hypothesis in is the rat model of TNBS-induced intestinal inflammation. This is a well established, and the smallest animal in which true pre-nodal lymph can be reliably collected. We are measuring and collecting lymph flow in vessels from the terminal ileum to avoid the secondary effects of direct chemical damage of the lymph vessels by the TNBS administration in the colon. Tissue injury in the ileum was significantly elevated and was not a non-specific result of surgery/damage to the area. TNBS inflammation is dependent on the activation of the immune system by haptenized antigens resulting in a Th1 polarized inflammation. Since we also hypothesized that the immune system might regulate the flow of lymph this was an additional benefit of this model.
The key finding of this study was that TNBS administration significantly reduced lymph flow by 40% 24hrs after the first instillation. Lymph flow continued to fall to a value 45% of control (55% reduction) at 48hrs and began a gradual recovery by 72hrs. Lymph flow then continued to increase to levels above control until the second TNBS treatment at 120hrs. The increase in flow during recovery may be due to the enhancement of lymph production as a result of the preceding ileal edema, a potential increase in lymphangiogenesis and/or recovering or increased ileal motility after the insult (62–66). The second TNBS treatment reduced lymph flow by 64% within 24hrs after administration. There are several possible reasons why the second TNBS treatment resulted in a greater and more rapid inhibition of lymph flow than the first administration; the first possibility is that the inflammation induced by the first TNBS instillation had not completely cleared before the second treatment was given, which is not well supported by other measures of disease such as MPO and wet to dry weight ratio. The second possible explanation is that the immune system in the GI tissues, mesentery and node were primed by the first TNBS treatment and therefore responded much more robustly to the second treatment. It is also possible that TNBS might exert a negative effect on lymphatic function in and of it’s self independent of ethanol vehicle effects which have minimal effects on lymphatic function and potentially a separate mechanism of action (Fig 1a) (67, 68). We feel that the second hypothesis is more plausible since most markers of injury to the gut tissue had dropped to near control levels by the time of the second administration. This leads us to conclude that the immune systems response at the second treatment is more rapid and profound because it is essentially primed to respond. This support the contention that the immune system is not only served by the lymphatic system but that it also partially regulates lymph flow in these vessels through mechanisms that we believe are the result of immune cell interactions with the vessels. We observed that reduced lymph flow in the ileum was matched by an increase in the histopathological score over the first 48hrs. However, the tissue did not show signs of edema until 48hrs suggesting that reduced lymph flow results in accumulation of immune cells prior to complex injury in the tissue. The tissue histopathology remained significantly elevated until after lymph flow recovered. This suggests that reduced lymph flow contributes to sustained tissue injury.
It is interesting to note that at each point lymph flow was decreased, the relative density of cells in the lymph increased. These cells could directly impair lymph flow because of physical changes in the viscosity due to increased cell counts and signs of clotted material and/or alteration of biochemical regulators of lymphatic function. But it appears that these influences are unlikely to be the predominant factor decreasing lymph flow at this time since the number of cells in the lymph remained elevated even after lymph flow recovered. Another factor we suspect may change is the hydrodynamics and resistance of the mesenteric lymph node, which may increase the work that is necessary to move lymph through it. However we did not carefully evaluate this in these studies.
Inflammation in the ileum, in a rectally administered TNBS model has not been well described, even though it is documented that there are many distant effects of TNBS induced colonic inflammation (69, 70). One of the most compelling pieces of evidence that we found of pathological inflammation in the ileum is the alteration in the size of the lymphatic vessels serving this tissue. This phenomenon has been reported previously in a model of TNBS-induced ileitis in which the gut was exteriorized and TNBS injected into the ileum as well as in cases of intestinal lymphangiectasia (55,71).
As stated before there was an increase in the histopathological score of the ileum of TNBS treated rats, but the increase in overall score was due to increases in cellular infiltration 48hrs after insult. There was little to no epithelial erosion and no crypt loss during the acute phase of the injury. This evidence may suggest that the tissue injury during the acute phase is due to trapping of inflammatory cells and edema in the tissue because of the reduced lymph flow, but we cannot exclude the possibility of direct cytotoxic effects of the TNBS treatment. However, it seems less likely that this is due to direct action of TNBS on the epithelium as there was minimal epithelial damage. These histological observations were confirmed by wet to dry weight ratio and MPO assay, which showed the accumulation of fluid and inflammatory immune cells in the ileum. MPO activity in the ileum had a biphasic response after the first TNBS administration with a peak at 24hrs recovering at 48hrs and a second peak at 72hrs corresponding to the initial recovery of lymph flow. While the first peak is likely due to influx of neutrophils into the tissue after the initial TNBS insult, we hypothesize the secondary peak is due to a reaction of the immune system to an efflux of tissue debris, cells and gut related antigens. This delayed lymphatic delivery might cause a secondary inflammatory flair that would account for the prolonged increase in histopathology.
The MHCII+ immune cells we have shown surrounding the vessels are not the only cells that reside along these vessels. In fact we know they share this space with mast cells and CD11c+/MHCII− cells (24, 27, 30) as well as other potential members of the innate immune cell population. However to our knowledge, these MHCII+ cells are the most responsive in terms of rapid population changes immediately after insult. As early as 1hr after TNBS instillation, the number of MHCII+ cells began to increase in the area on and immediately adjacent to the mesenteric lymphatics of the ileum. With the increase in MHCII+ immune cells around the lymphatics we believe that they might be adversely affecting lymphatic function. It is known that intestinal inflammation causes a reduction in the contractile machinery of visceral smooth muscle (62). While this is a different muscle type than that surrounding the lymphatic vessels we hypothesized that there may be a loss of either contractile machinery or total loss of muscle cells (17, 19, 68, 71, 72). However, we found that there was no gross loss of muscle cells or the contractile protein SM22α in the lymphatic muscle cells at 48hrs, suggesting that the loss of contractile machinery was not the reason for the reduction of flow at these time points. However, the contractile function of these cells is extremely complex involving both phasic contractions driving lymph pumping and tonic contractions that regulate outflow resistance. More complete answers to these questions will require further evaluation of the multitude of factors that regulate it, which our collaborators and we are examining now.
We found that the increase in MHCII+ immune cells around the vessels correlated well with increased expression of TNF-α and iNOS mRNA levels. High levels of NO have been implicated as a major inhibitor of lymphatic function in similar models (71, 73–76). This suggests that the immune cells that traffic to the area on and immediately around the lymphatic vessels play an important role in regulating lymphatic function. This supports the notion that NOS is important in modulating lymph flow as found by Gasheva et. al. and Mathias et. al. (71, 74). Immediately after insult, the levels of IL-6 message decreased in the mesenteric lymphatics, which is surprising since IL-6 is a classical pro-inflammatory cytokine. However recent work has shown that IL-6 in conjunction with other cytokines directs the differentiation of macrophages to a tolerogenic/anti-inflammatory phenotype (77–81). We seen that IL-6 message began to recover at 6hrs post treatment, suggesting that IL-6 might be acting as a driver of macrophage phenotype selection along the lymphatics. This data leads us to the question of what other factors might be attracting the immune cells to the lymphatics. It is important to note that these events occurred before the development of lymph flow impairments (≤24hrs) and gross (histological) inflammation (≤48hrs) suggesting that this environmental shift precedes both events.
When we examined the protein content of the lymph and the peritoneal fluid we found that there was evidence of inflammation mediated by TNBS in both the peritoneal and lymphatic compartments. There was an alteration in transport/compartmentalization of critical immunoregulatory molecules induced by TNBS treatment. The soluble forms of cell surface adhesion molecules L-selectin and ICAM were both reduced in lymph. Soluble L-selectin and soluble ICAM can impair normal adhesion and function of immune cells such as macrophages and CD8+ lymphocytes. This suggests a rapid shift to an inflammatory environment in the lymph and possible increased cellular recruitment (82, 83) that could enhance the migration of cells out of the peritoneal space and into the lymphatics. We also found that the levels of IFN-γ, which we previously have shown increases lymphatic endothelial permeability, was elevated in the lymph of TNBS-treated rats at 48hrs (84). This was accompanied by an increase in GM-CSF in the lymph, which suggests that there is a potent chemotactic stimulus for macrophages to migrate to the lymphatic vessel as well as adopt an inflammatory phenotype (85–87). It is likely that these factors are driving recruitment of cells to the lymphatics during inflammation. It is interesting to note that IL-10 was detectable in the lymph of control animals but reduced to levels below detection in the lymph of TNBS-treated animals. These findings suggest that it is likely that there is some mechanism by which the lymph is conditioned prior to reaching the node to assist in directing the immune response. This conditioning may be carried out by either the lymphatic endothelial cells themselves, the immune cell complement that surrounds the lymphatics or by both. This finding could help determine why under normal circumstances antigen presentation via the lymph from the gut induces a tolerogenic state but under states of damage (gut wall injury/breach) or infection (non-normal gut flora) the immune/lymph system can rapidly switch to an inflammatory state (88–91).
In summary we document for the first time that lymph flow from the ileum is rapidly interrupted by insult to the colon and that these decreases in lymph flow occur prior to the development of marked histopathology in the ileal tissues. We hypothesize that failure of the lymphatics to remove immune cells and the offending inflammatory stimuli after the initial insult precipitates further inflammation. This may alter the response of the immune system moving from a ‘normal’ inflammatory response that is necessary to repair epithelial damage and respond to potential pathogens, to one that may become self-perpetuating. Second, we have shown that lymph flow increases prior to tissue recovery. This provides strong support for the principal that the inability of lymph flow to recover after insult promotes the development of a chronic inflammatory state. Thus it appears that the impaired lymphatic function previously observed in other IBD models and patients may not be simply a secondary effect of the inflammation initiated by other events, but may be a major early contributor to disease development. Additionally this data may provide an insight to the development of pathologies such as backwash ileitis and skip lesions as these may result from inhibition of lymphatic function at GI sites distal to the original tissue injury. Currently, we are trying to determine what the triggers early recruitment of immune cells to the tissue surrounding the lymphatic vessels since we believe this is a critical early factor that could modulate lymph flow. However, based on the literature we speculate that it is likely some form of innate immune cells that reside near the vessel releasing chemoattractive substances (22). Additionally we would also like to address if this paradigm is common to other chronic inflammatory states, that is whether or not the reduction of lymph flows from different tissues is a common triggering factor in human inflammatory diseases and if preserving lymphatic flow may attenuate inflammation in those diseases.
Acknowledgments
We would like to thank Dr. Harry Papaconstantinou for his advice in the interpretation of this study. We would also like to thank Dr. Anatoliy Gashev and Dr. Mariappan Muthuchamy for their insight that helped direct the final stages of the design and collection of data.
Sources of support: NIH DK099221, HL096552, R01 HL094269, CA136466, HL096552, The Scott and White Research Foundation, The Texas A&M Health Science Center, The Center for Cell Death and Differentiation.
References
- 1.Agrawal S, Agrawal A, Doughty B, et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 2.la Sala A, Ferrari D, Di Virgilio F, et al. Alerting and tuning the immune response by extracellular nucleotides. J Leukoc Biol. 2003;73:339–343. doi: 10.1189/jlb.0802418. [DOI] [PubMed] [Google Scholar]
- 3.Vecchiarelli A. Cytokines and costimulatory molecules: positive and negative regulation of the immune response to Cryptococcus neoformans. Arch Immunol Ther Exp (Warsz) 2000;48:465–472. [PubMed] [Google Scholar]
- 4.Fiocchi C. Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am J Physiol. 1997;273:G769–G775. doi: 10.1152/ajpgi.1997.273.4.G769. [DOI] [PubMed] [Google Scholar]
- 5.Joseph NE, Fiocchi C, Levine AD. Crohn's disease and ulcerative colitis mucosal T cells are stimulated by intestinal epithelial cells: implications for immunosuppressive therapy. Surgery. 1997;122:809–814. doi: 10.1016/s0039-6060(97)90091-x. discussion 814-806. [DOI] [PubMed] [Google Scholar]
- 6.Chen CZ, Schaffert S, Fragoso R, et al. Regulation of immune responses and tolerance: the microRNA perspective. Immunol Rev. 2013;253:112–128. doi: 10.1111/imr.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Getts DR, McCarthy DP, Miller SD. Exploiting apoptosis for therapeutic tolerance induction. J Immunol. 2013;191:5341–5346. doi: 10.4049/jimmunol.1302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki J, Ricordi C, Chen Z. Immune tolerance induction by integrating innate and adaptive immune regulators. Cell Transplant. 2010;19:253–268. doi: 10.3727/096368909X480314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy ST, van der Vlies AJ, Simeoni E, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 10.Robbiani DF, Finch RA, Jager D, et al. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 11.Tacke F, Ginhoux F, Jakubzick C, et al. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Ghoshal S, Ward M, et al. Chylomicrons promote intestinal absorption and systemic dissemination of dietary antigen (ovalbumin) in mice. PLoS One. 2009;4:e8442. doi: 10.1371/journal.pone.0008442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockson SG. The physiology of lymphatic pump therapeutics. Lymphat Res Biol. 2010;8:141. doi: 10.1089/lrb.2010.8301. [DOI] [PubMed] [Google Scholar]
- 14.Rockson SG. Update on the biology and treatment of lymphedema. Curr Treat Options Cardiovasc Med. 2012;14:184–192. doi: 10.1007/s11936-012-0170-0. [DOI] [PubMed] [Google Scholar]
- 15.Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. J Investig Dermatol Symp Proc. 2000;5:14–19. doi: 10.1046/j.1087-0024.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 16.Tso P, Pitts V, Granger DN. Role of lymph flow in intestinal chylomicron transport. Am J Physiol. 1985;249:G21–G28. doi: 10.1152/ajpgi.1985.249.1.G21. [DOI] [PubMed] [Google Scholar]
- 17.Zawieja DC. Contractile physiology of lymphatics. Lymphat Res Biol. 2009;7:87–96. doi: 10.1089/lrb.2009.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zawieja SD, Wang W, Wu X, et al. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H643–H653. doi: 10.1152/ajpheart.00606.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von der Weid PY, Muthuchamy M. Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiology. 2009 doi: 10.1016/j.pathophys.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Xie Y, Bagby TR, Cohen MS, et al. Drug delivery to the lymphatic system: importance in future cancer diagnosis and therapies. Expert Opin Drug Deliv. 2009;6:785–792. doi: 10.1517/17425240903085128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seymour R, Sundberg JP, Hogenesch H. Abnormal lymphoid organ development in immunodeficient mutant mice. Vet Pathol. 2006;43:401–423. doi: 10.1354/vp.43-4-401. [DOI] [PubMed] [Google Scholar]
- 22.Bridenbaugh EA, Wang W, Srimushnam M, et al. An immunological fingerprint differentiates muscular lymphatics from arteries and veins. Lymphat Res Biol. 2013;11:155–171. doi: 10.1089/lrb.2013.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander JS, Ganta VC, Jordan PA, et al. Gastrointestinal lymphatics in health and disease. Pathophysiology. 2010;17:315–335. doi: 10.1016/j.pathophys.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest. 2005;115:2316–2319. doi: 10.1172/JCI26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leak LV, Burke JF. Electron microscopic study of lymphatic capillaries in the removal of connective tissue fluids and particulate substances. Lymphology. 1968;1:39–52. [PubMed] [Google Scholar]
- 26.Casley-Smith JR. How the lymphatic system works. Lymphology. 1968;1:77–80. [PubMed] [Google Scholar]
- 27.Gordon EJ, Rao S, Pollard JW, et al. Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development. 2010;137:3899–3910. doi: 10.1242/dev.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey NL, Gordon EJ. Deciphering the roles of macrophages in developmental and inflammation stimulated lymphangiogenesis. Vasc Cell. 2012;4:15. doi: 10.1186/2045-824X-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruyama K, Ii M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee V, Gashev AA. Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol. 2012;303:H693–H702. doi: 10.1152/ajpheart.00378.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis MJ, Lane MM, Davis AM, et al. Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am J Physiol Heart Circ Physiol. 2008;295:H587–H597. doi: 10.1152/ajpheart.01029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benoit JN, Zawieja DC. Effects of f-Met-Leu-Phe-induced inflammation on intestinal lymph flow and lymphatic pump behavior. Am J Physiol. 1992;262:G199–G202. doi: 10.1152/ajpgi.1992.262.2.G199. [DOI] [PubMed] [Google Scholar]
- 33.Aldrich MB, Sevick-Muraca EM. Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine. 2013;64:362–369. doi: 10.1016/j.cyto.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olszewski WL, Pazdur J, Kubasiewicz E, et al. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum. 2001;44:541–549. doi: 10.1002/1529-0131(200103)44:3<541::AID-ANR102>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Hunziker T, Brand CU, Kapp A, et al. Increased levels of inflammatory cytokines in human skin lymph derived from sodium lauryl sulphate-induced contact dermatitis. Br J Dermatol. 1992;127:254–257. doi: 10.1111/j.1365-2133.1992.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 36.Fraser JR, Kimpton WG, Laurent TC, et al. Uptake and degradation of hyaluronan in lymphatic tissue. Biochem J. 1988;256:153–158. doi: 10.1042/bj2560153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Termeer CC, Hennies J, Voith U, et al. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol. 2000;165:1863–1870. doi: 10.4049/jimmunol.165.4.1863. [DOI] [PubMed] [Google Scholar]
- 38.Miller NE, Michel CC, Nanjee MN, et al. Secretion of adipokines by human adipose tissue in vivo: partitioning between capillary and lymphatic transport. Am J Physiol Endocrinol Metab. 2011;301:E659–E667. doi: 10.1152/ajpendo.00058.2011. [DOI] [PubMed] [Google Scholar]
- 39.Acedo SC, Gotardo EM, Lacerda JM, et al. Perinodal adipose tissue and mesenteric lymph node activation during reactivated TNBS-colitis in rats. Dig Dis Sci. 2011;56:2545–2552. doi: 10.1007/s10620-011-1644-8. [DOI] [PubMed] [Google Scholar]
- 40.Jin da P, An A, Liu J, et al. Therapeutic responses to exogenous VEGF-C administration in experimental lymphedema: immunohistochemical and molecular characterization. Lymphat Res Biol. 2009;7:47–57. doi: 10.1089/lrb.2009.0002. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura K, Radhakrishnan K, Wong YM, et al. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS One. 2009;4:e8380. doi: 10.1371/journal.pone.0008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chess S, Chess D, et al. Production of chronic enteritis and other systemic lesions by ingestion of finely divided foreign materials. Surgery. 1950;27:220–234. [PubMed] [Google Scholar]
- 43.Kalima TV, Saloniemi H, Rahko T. Experimental regional enteritis in pigs. Scand J Gastroenterol. 1976;11:353–362. [PubMed] [Google Scholar]
- 44.Blasiak RC, Morrell DS, Burkhart CN. Pasteurella multocida cellulitis in a 15-year-old male with chronic lymphedema. J Am Acad Dermatol. 2013;68:e183–e184. doi: 10.1016/j.jaad.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Kasai-Sakamoto A, Yokoyama Y, Mizunuma H. A case of cellulitis that complicated lymphedema of the lower limb and produced systemic inflammatory response syndrome (SIRS) Eur J Gynaecol Oncol. 2006;27:419–421. [PubMed] [Google Scholar]
- 46.Shin SU, Lee W, Park EA, et al. Comparison of characteristic CT findings of lymphedema, cellulitis, and generalized edema in lower leg swelling. Int J Cardiovasc Imaging. 2013;29(Suppl 2):135–143. doi: 10.1007/s10554-013-0332-5. [DOI] [PubMed] [Google Scholar]
- 47.Woodruff A, Olivero JJ. Recurrent cellulitis complicating chronic lymphedema. Hosp Pract (Off Ed) 1995;30:87–91. doi: 10.1080/21548331.1995.11443169. [DOI] [PubMed] [Google Scholar]
- 48.Hendriks HR. Occlusion of the lymph flow to rat popliteal lymph nodes for protracted periods. Z Versuchstierkd. 1978;20:105–112. [PubMed] [Google Scholar]
- 49.Tonelli P. New development in Crohn's disease: ++unravelling the mystery and its reinstatement as a surgically treatable condition. Part 2. Potential etiopathogenesis of "terminal ileitis" and extension of the disease to mesenteric small intestine and the colon. Chir Ital. 2000;52:243–250. [PubMed] [Google Scholar]
- 50.Tonelli P. New developments in Crohn's disease: solution of doctrinal mysteries and reinstatement as a surgically treatable disease. 1. The process is not a form of enteritis but lymphedema contaminated by intestinal contents. Chir Ital. 2000;52:109–121. [PubMed] [Google Scholar]
- 51.Crohn BBGL, Oppenheimer GD. Regional Ileitis : Pathological and Clinical Entity. Jour Amer Med Assoc. 1932 xcix. [Google Scholar]
- 52.Reinders MG, Kukutsch NA. Genital edema in childhood: harbinger of Crohn's disease? J Am Acad Dermatol. 2011;65:449–450. doi: 10.1016/j.jaad.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 53.Alexander JS, Chaitanya GV, Grisham MB, et al. Emerging roles of lymphatics in inflammatory bowel disease. Ann N Y Acad Sci. 2010;1207(Suppl 1):E75–E85. doi: 10.1111/j.1749-6632.2010.05757.x. [DOI] [PubMed] [Google Scholar]
- 54.Monk BE, Mortimer PS. Lymphoedema and Crohn's disease. J R Soc Med. 1999;92:136–137. doi: 10.1177/014107689909200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu TF, Carati CJ, Macnaughton WK, et al. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G566–G574. doi: 10.1152/ajpgi.00058.2006. [DOI] [PubMed] [Google Scholar]
- 56.HE M. Infective Granulomata. Surg, Gyn, and Obst. 1931 lii. [Google Scholar]
- 57.Krywonis N, Kaye VN, Lynch PJ. Cryptococcal cellulitis in congenital lymphedema. Int J Dermatol. 1990;29:41–44. doi: 10.1111/j.1365-4362.1990.tb03754.x. [DOI] [PubMed] [Google Scholar]
- 58.Vaillant L, Titon JP, Gaisne E, et al. Compression for lymphedema and cellulitis. J Am Acad Dermatol. 1990;23:952–953. doi: 10.1016/s0190-9622(08)80713-x. [DOI] [PubMed] [Google Scholar]
- 59.Macaya A, Marcoval J, Bordas X, et al. Crohn's disease presenting as prepuce and scrotal edema. J Am Acad Dermatol. 2003;49:S182–S183. doi: 10.1067/mjd.2003.295. [DOI] [PubMed] [Google Scholar]
- 60.Wu TF, Carati CJ, Macnaughton WK, et al. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G566–G574. doi: 10.1152/ajpgi.00058.2006. [DOI] [PubMed] [Google Scholar]
- 61.Wang DC. Attach importance to research on lymph circulation system after burns. Zhonghua Shao Shang Za Zhi. 2011;27:3–5. [PubMed] [Google Scholar]
- 62.Ohama T, Hori M, Momotani E, et al. Intestinal inflammation downregulates smooth muscle CPI-17 through induction of TNF-alpha and causes motility disorders. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1429–G1438. doi: 10.1152/ajpgi.00315.2006. [DOI] [PubMed] [Google Scholar]
- 63.Van Crombruggen K, Van Nassauw L, Demetter P, et al. Influence of soluble guanylate cyclase inhibition on inflammation and motility disturbances in DSS-induced colitis. Eur J Pharmacol. 2008;579:337–349. doi: 10.1016/j.ejphar.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 64.Capasso R, Borrelli F, Cascio MG, et al. Inhibitory effect of salvinorin A, from Salvia divinorum, on ileitis-induced hypermotility: cross-talk between kappa-opioid and cannabinoid CB(1) receptors. Br J Pharmacol. 2008;155:681–689. doi: 10.1038/bjp.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capasso R, Borrelli F, Zjawiony J, et al. The hallucinogenic herb Salvia divinorum and its active ingredient salvinorin A reduce inflammation-induced hypermotility in mice. Neurogastroenterol Motil. 2008;20:142–148. doi: 10.1111/j.1365-2982.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 66.Cromer WE, Ganta CV, Patel M, et al. VEGF-A isoform modulation in an preclinical TNBS model of ulcerative colitis: protective effects of a VEGF164b therapy. J Transl Med. 2013;11:207. doi: 10.1186/1479-5876-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Souza-Smith FM, Kurtz KM, Molina PE, et al. Adaptation of mesenteric collecting lymphatic pump function following acute alcohol intoxication. Microcirculation. 2010;17:514–524. doi: 10.1111/j.1549-8719.2010.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Souza-Smith FM, Molina PE, Breslin JW. Reduced RhoA activity mediates acute alcohol intoxication-induced inhibition of lymphatic myogenic constriction despite increased cytosolic [Ca(2+)] Microcirculation. 2013;20:377–384. doi: 10.1111/micc.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hathaway CA, Appleyard CB, Percy WH, et al. Experimental colitis increases blood-brain barrier permeability in rabbits. Am J Physiol. 1999;276:G1174–G1180. doi: 10.1152/ajpgi.1999.276.5.G1174. [DOI] [PubMed] [Google Scholar]
- 70.Sans M, Kawachi S, Soriano A, et al. Brain endothelial adhesion molecule expression in experimental colitis. Microcirculation. 2001;8:105–114. [PubMed] [Google Scholar]
- 71.Mathias R, von der Weid PY. Involvement of the NO-cGMP-K(ATP) channel pathway in the mesenteric lymphatic pump dysfunction observed in the guinea pig model of TNBS-induced ileitis. Am J Physiol Gastrointest Liver Physiol. 2013;304:G623–G634. doi: 10.1152/ajpgi.00392.2012. [DOI] [PubMed] [Google Scholar]
- 72.Souza-Smith FM, Kurtz KM, Breslin JW. Measurement of Cytosolic Ca<sup>2+</sup> in Isolated Contractile Lymphatics. J Vis Exp. 2011 doi: 10.3791/3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol. 2011;301:H1897–H1906. doi: 10.1152/ajpheart.00260.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bohlen HG, Wang W, Gashev A, et al. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol. 2009;297:H1319–H1328. doi: 10.1152/ajpheart.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol. 2006;575:821–832. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liao S, Cheng G, Conner DA, et al. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci U S A. 2011;108:18784–18789. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan Y, Wang YH, Diamond B. IL-6 contributes to an immune tolerance checkpoint in post germinal center B cells. J Autoimmun. 2012;38:1–9. doi: 10.1016/j.jaut.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Geisel J, Kahl F, Muller M, et al. IL-6 and maturation govern TLR2 and TLR4 induced TLR agonist tolerance and cross-tolerance in dendritic cells. J Immunol. 2007;179:5811–5818. doi: 10.4049/jimmunol.179.9.5811. [DOI] [PubMed] [Google Scholar]
- 79.Kilmon MA, Wagner NJ, Garland AL, et al. Macrophages prevent the differentiation of autoreactive B cells by secreting CD40 ligand and interleukin-6. Blood. 2007;110:1595–1602. doi: 10.1182/blood-2006-12-061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tilg H, Trehu E, Atkins MB, et al. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- 81.Yasukawa H, Ohishi M, Mori H, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 82.Mendez MP, Monroy YK, Du M, et al. Overexpression of sICAM-1 in the alveolar epithelial space results in an exaggerated inflammatory response and early death in Gram negative pneumonia. Respir Res. 2011;12:12. doi: 10.1186/1465-9921-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimada Y, Hasegawa M, Takehara K, et al. Elevated serum L-selectin levels and decreased L-selectin expression on CD8(+) lymphocytes in systemic sclerosis. Clin Exp Immunol. 2001;124:474–479. doi: 10.1046/j.1365-2249.2001.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cromer WE, Zawieja SD, Tharakan B, et al. The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis. 2013 doi: 10.1007/s10456-013-9393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bozinovski S, Jones JE, Vlahos R, et al. Granulocyte/macrophage-colony-stimulating factor (GM-CSF) regulates lung innate immunity to lipopolysaccharide through Akt/Erk activation of NFkappa B and AP-1 in vivo. J Biol Chem. 2002;277:42808–42814. doi: 10.1074/jbc.M207840200. [DOI] [PubMed] [Google Scholar]
- 86.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 87.Chitta S, Santambrogio L, Stern LJ. GMCSF in the absence of other cytokines sustains human dendritic cell precursors with T cell regulatory activity and capacity to differentiate into functional dendritic cells. Immunol Lett. 2008;116:41–54. doi: 10.1016/j.imlet.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 88.Santambrogio L, DiLorenzo TP. Autoimmunity. The benefit of self-control. Immunol Cell Biol. 2010;88:513–514. doi: 10.1038/icb.2010.59. [DOI] [PubMed] [Google Scholar]
- 89.Clement CC, Rotzschke O, Santambrogio L. The lymph as a pool of self-antigens. Trends Immunol. 2011;32:6–11. doi: 10.1016/j.it.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clement CC, Santambrogio L. The Lymph Self-Antigen Repertoire. Front Immunol. 2013;4:424. doi: 10.3389/fimmu.2013.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Santambrogio L, Stern LJ. Carrying yourself: self antigen composition of the lymphatic fluid. Lymphat Res Biol. 2013;11:149–154. doi: 10.1089/lrb.2013.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]