Abstract
Household air pollution from biomass fuel use affects three billion people worldwide; however, few studies have examined the relationship between biomass fuel use and blood pressure. We sought to determine if daily biomass fuel use was associated with elevated blood pressure in high altitude Peru and if this relationship was affected by lung function. We analyzed baseline information from a population-based cohort study of adults aged ≥35 years in Puno, Peru. Daily biomass fuel use was self-reported. We used multivariable regression models to examine the relationship between daily exposure to biomass fuel smoke and blood pressure outcomes. Interactions with sex and quartiles of forced vital capacity (FVC) were conducted to evaluate for effect modification. Data from 1004 individuals (mean age 55.3 years, 51.7% female) were included. We found an association between biomass fuel use with both prehypertension (adjusted relative risk ratio 5.0, 95% CI 2.6 to 9.9) and hypertension (adjusted relative risk ratio 3.5, 95% CI 1.7 to 7.0). Biomass fuel users had a higher SBP (7.01 mmHg, 95% CI 4.4 to 9.6) and a higher DBP (5.9 mmHg, 95% CI 4.2 to 7.6) when compared to nonusers. We did not find interaction effects between daily biomass fuel use and sex or percent predicted FVC for either SBP or DBP. Biomass fuel use was associated with an increased risk of hypertension and higher blood pressure in Peru. Reducing exposure to household air pollution from biomass fuel use represents an opportunity for cardiovascular prevention.
Keywords: Air Pollution, Indoor, Blood Pressure, Latin America, Global Health, Health Status Disparities, Rural Health
INTRODUCTION
Household air pollution (HAP) has been identified as the third leading risk factor for death worldwide1, and it is estimated to affect three billion people each year. HAP results from the burning of wood, animal dung, and other organic debris for cooking and heating. The negative health effects disproportionately burden households in resource-poor settings, as cleaner, more efficient fuels, such as liquefied petroleum gas (LPG), are more commonly used in households in urban settings and with higher incomes2.
The relationship between exposure to HAP and respiratory conditions including chronic obstructive pulmonary disease, lung cancer, and pneumonia has been well established3. However, given the evidence of the association between ambient air pollution exposure and cardiovascular outcomes, including heart failure, myocardial infarction, arrhythmia and cardiovascular-related mortality, there has been increased interest in examining the cardiovascular effects of HAP exposure2,4–7. Conflicting data exist regarding the association between HAP exposure and adverse cardiovascular outcomes. Observational studies of HAP exposure and cardiovascular disease have shown that biomass fuel use is associated with increased systemic blood pressure and an increased prevalence of hypertension8–10. On a cellular and biochemical level, biomass fuel use is associated with a pro-thrombotic, pro-inflammatory biological state, with increased platelet aggregation, reactive oxygen species and anti-cardiolipin IgG11.
Although it is estimated that HAP from biomass fuel use is a considerable risk factor for cardiovascular disease in resource-poor settings worldwide, additional evidence regarding the specific ways by which HAP increases systemic blood pressure is needed. Moreover, both pulmonary disease and systemic blood pressure are associated with biomass fuel use, yet the interaction between pulmonary function and systemic blood pressure in the setting of biomass fuel use has not been adequately characterized. While several large cohort studies have described an association between reduced lung function with blood pressure and arterial elasticity, it remains unclear whether cardiovascular disease from HAP occurs with concomitant reduced lung function12–15. We sought to determine the association between daily biomass fuel use with systemic blood pressure, prehypertension and hypertension in a population-based cohort of adults in Puno, Peru and if lung function, as measured by spirometry, was an effect modifier of the relationship between daily biomass fuel use and systemic blood pressure.
METHODS
Study setting
We conducted a longitudinal cohort study designed to characterize the prevalence of and risk factors for chronic disease in three geographically distinct settings in Peru16. This study utilized the baseline round of questionnaire and clinical data in Puno, a city in southeastern Peru at 3,825 meters above sea level with 150,000 inhabitants where biomass fuels are used almost exclusively in rural villages. Puno primarily consists of indigenous, Andean people with both Quechua and Aymara speaking populations.
Study design
Individuals aged ≥35 years, full-time residents in the area, that provided informed consent were invited to participate in the study. We identified a sex, age-stratified (35–44, 45–54, 55–64 and ≥65 years) and location-stratified (urban vs. rural) sample, and only one participant per household was enrolled. The study was approved by the Institutional Review Boards at Universidad Peruana Cayetano Heredia and A.B. PRISMA, in Lima, Peru, and at the Bloomberg School of Public Health, Johns Hopkins University, in Baltimore, USA.
Study procedures
Participants responded to a questionnaire on sociodemographics, cardiopulmonary risk factors, and history of cardiopulmonary symptoms. Fieldworkers measured weight, height, waist circumference, blood pressure, and spirometry. Spirometry was conducted using the Easy-On-PC spirometer (ndd, Zurich, Switzerland) before and after 200 mcg of inhaled salbutamol via a spacer following standard guidelines17. Systolic (SBP) and diastolic (DBP) blood pressure were measured in triplicate using a digital sphygmomanometer (OMRON HEM-780, Osaka, Japan) that was previously validated for an adult population16. We used the right arm for all measurements using an appropriately fitted cuff size. Participants were asked to sit comfortably for five minutes before the first measurement was taken, and the set interval of time between measurements was five minutes. The right forearm was supported on a table for all measurements. Final SBP and DBP were calculated as the average of the second and third measurements.
Definitions
The following variables were determined by self-report in a structured questionnaire: Daily biomass fuel use; hypertension diagnosis; use of anti-hypertensive medications; pack-years of tobacco smoking. Daily biomass fuel use was defined as self-reported daily burning of wood or dung for cooking or heating for more than six months at any time during the participant’s lifetime. Prehypertension was defined as SBP of 120–139 mmHg or DBP of 80–89 mmHg in the absence of anti-hypertensive medication use18; and, hypertension as SBP ≥140 mmHg, DBP ≥90 mmHg, or self-reported diagnosis by a physician with concomitant use of anti-hypertensive medications. Forced vital capacity (FVC) was defined as the total volume of air (in liters) expired during forced expiration. Percent predicted values were determined using the predictions derived from a healthy Mexican-American population19.
Physical activity was determined based on leisure time and transport time domains of IPAQ as recommended for Latin American populations20. Alcohol abuse was measured using the Alcohol Use Disorders Identification Test21. Depressive symptoms were defined as a score of ≥23 in the Spanish-validated version of the Center for Epidemiologic Studies Depression Scale22,23. Education was categorized by schooling years (<6 years, 7–11 years and 12 or more years). Socioeconomic status was defined as wealth index based on household income, assets and household facilities as previously described24.
Biostatistical methods
Our primary aim was to compare blood pressure outcomes in participants with and without daily exposure to biomass fuel smoke. We used multivariable linear and multinomial logistic regressions to model continuous blood pressure outcomes and categories of hypertension, respectively. In a minimally-adjusted model, we controlled for differences in age, sex, and BMI. In the fully-adjusted model, we controlled for age, sex, BMI, height, tertiles of wealth index, categories of education years, presence or absence of depressive symptoms, pack-years of smoking, alcohol abuse, and low physical activity. Antihypertensive medication use was included in the model for continuous blood pressure outcomes only. We examined for interaction effects between biomass fuel use and either sex or post-bronchodilator percent-predicted forced vital capacity (FVC) on blood pressure, and used the likelihood ratio test to evaluate for effect modification. Statistical analyses were conducted in STATA 12 (STATA Corp, College Station, USA) and R (www.r-project.org)
RESULTS
Participant Characteristics
A total of 1004 participants were included in this analysis (Table 1). Participants with daily exposure to biomass fuel smoke were more likely to be female and live in a rural setting; had a lower prevalence of obesity; lower values of lung function; lower prevalence of tobacco smoking use; increased physical activity; fewer years of education; and, a lower wealth index than did participants who did not have daily exposure to biomass fuel smoke. Overall prevalence of daily tobacco smoking was low in both groups.
Table 1.
Participants characteristics stratified by daily exposure to biomass fuel use status.
| Participant Characteristics | Non-user of biomass fuel | Daily users of biomass fuels | p- value |
|---|---|---|---|
| Sample size | 495 | 509 | |
| Sociodemographics | |||
| Age in years, mean (SD) | 55.0 (12.3) | 55.6 (12.4) | 0.46 |
| Men, n (%) | 255 (51.5) | 230 (45.2) | 0.05 |
| Rural site, n (%) | 3.4 | 95.1 | <0.001 |
| Lowest wealth tertile, n (%) | 109 (22.0) | 362 (71.7) | <0.001 |
| <6 years of education, n (%) | 61 (12.3) | 325 (63.9) | <0.001 |
| Anthropometrics | |||
| BMI in kg/m2, mean (SD) | 27.9 (4.3) | 25.2 (3.8) | <0.001 |
| BMI ≥ 30 kg/m2, n (%) | 130 (26.3) | 57 (11.2) | <0.001 |
| Waist circumference in cm, mean (SD) | 92.9 (10.6) | 84.6 (11.4) | <0.001 |
| Height in cm, mean (SD) | 157.1 (9.0) | 155.3 (7.9) | <0.001 |
| Hypertension outcomes | |||
| Prehypertension, n (%) | 33 (7.6) | 79 (18.1) | <0.001 |
| Hypertension, n (%) | 56 (12.9) | 51 (11.7) | 0.58 |
| Use of anti-hypertensive medications, n (%) | 39 (7.9) | 8 (1.6) | <0.001 |
| Lung function | |||
| Post-bronchodilator FVC in L, mean (SD) | 3.75 (1.06) | 3.61 (1.02) | 0.04 |
| % predicted FVC, mean (SD) | 111.0 (15.8) | 112.0 (17.3) | 0.36 |
| Factors that may modify blood pressure | |||
| Tobacco smoking in pack-years, mean (SD) | 0.93 (4.4) | 0.30 (2.1) | 0.004 |
| Alcohol abuse, n (%) | 97 (19.6) | 84 (16.5) | 0.21 |
| Diabetes, n (%) | 30 (6.3) | 17 (3.5) | 0.05 |
| Low physical activity, n (%) | 395 (80.1) | 379 (74.6) | 0.04 |
| Depressive symptoms, n (%) | 93 (18.6) | 168 (33.1) | <0.001 |
| Use of lipid-lowering medication, n (%) | 16 (3.2) | 0 | <0.001 |
| Use of diabetes medication, n (%) | 17 (3.4) | 6 (1.2) | 0.02 |
Systolic and Diastolic Blood Pressure by Daily Biomass Fuel Use
In unadjusted analysis, SBP and DBP were higher by 3.0 mmHg and 2.5 mmHg between participants with and without daily biomass fuel use, respectively (Table 2). In the minimally-adjusted model, SBP and DBP were higher by 6.1 mmHg and 4.7 mmHg between participants with and without daily biomass fuel use, respectively. The fully adjusted model further strengthened this relationship: SBP and DBP were higher by 7.0 mmHg and 5.9 mmHg between participants with and without daily biomass fuel use, respectively. After stratification by sex, the association between daily biomass fuel use with SBP and DBP remained statistically significant in both men and women; however, there was no interaction effect between sex and daily biomass fuel use on either SBP (p=0.10) or DBP (p=0.56).
Table 2.
Single variable and multivariable analysis of the association between daily biomass fuel use and blood pressure outcomes. Multivariable models were either minimally-adjusted (for age, sex and body mass index) or fully-adjusted (for age, sex, BMI, height, tertiles of wealth index, categories of education years, presence or absence of depressive symptoms, pack-years of smoking, alcohol abuse and low physical activity).
| Blood Pressure Variable | Single variable |
p- value |
Minimally-Adjusted Model |
p- value |
Fully-Adjusted Model |
p- value |
|---|---|---|---|---|---|---|
| Increase in SBP, mmHg (95% CI) | ||||||
| Overall | 3.0 (1.0 to 5.1) | 0.004 | 6.1 (4.2 to 8.0) | <0.001 | 7.0 (4.4 to 9.6) | <0.001 |
| Men | 5.8 (3.3 to 8.4) | <0.001 | 8.9 (6.3 to 11.4) | <0.001 | 8.7 (5.4 to 12.1) | <0.001 |
| Women | 1.4 (−1.6 to 4.4) | 0.37 | 3.8 (1.0 to 6.6) | 0.007 | 5.4 (1.4 to 9.4) | 0.008 |
| Increase in DBP, mmHg (95% CI) | ||||||
| Overall | 2.5 (1.3 to 3.8) | <0.001 | 4.7 (3.5 to 6.0) | <0.001 | 5.9 (4.2 to 7.6) | <0.001 |
| Men | 3.4 (1.7 to 5.1) | <0.001 | 5.5 (3.7 to 7.3) | <0.001 | 6.0 (3.6 to 8.3) | <0.001 |
| Women | 2.1 (0.4 to 3.9) | 0.02 | 4.1 (2.4 to 5.8) | <0.001 | 5.6 (3.1 to 8.1) | <0.001 |
| Prehypertension, RRR (95% CI) | 2.7 (1.7 to 4.2) | <0.001 | 4.0 (2.4 to 6.5) | <0.001 | 5.0 (2.6 to 9.9) | <0.001 |
| Hypertension, RRR (95% CI) | 1.0 (0.7 to 1.5) | 0.91 | 2.0 (1.2 to 3.3) | 0.005 | 3.5 (1.7 to 7.0) | <0.001 |
Prevalence of Prehypertension and Hypertension by Daily Biomass Fuel Use
In unadjusted analysis, daily biomass fuel use was associated with having prehypertension (Table 2). Both the minimally-adjusted and fully-adjusted model increased the strength of the association between daily biomass fuel use and having prehypertension. In unadjusted analysis, we did not find a relationship between biomass fuel use and having hypertension (Table 2); however, after minimal adjustment, an association between daily biomass fuel use and having hypertension became apparent. This suggests that age, sex, and body mass index were important confounders of the relationship between biomass fuels and having hypertension. The fully-adjusted model further strengthened the relationship between biomass fuel use and having hypertension. Due to the low prevalence of prehypertension and hypertension, we were unable to adequately stratify these analyses by sex.
Effect Modification of Blood Pressure by Forced Vital Capacity
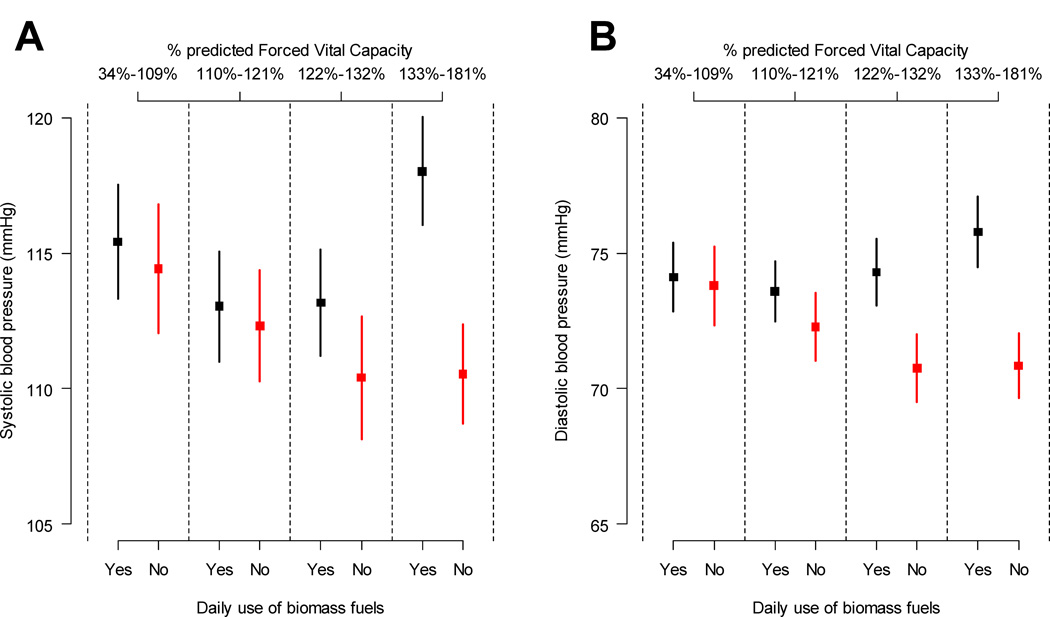
In unadjusted analyses, the difference in SBP and DBP between participants with and without daily exposure to biomass fuel appeared to be greater in participants in the two highest quartiles of post-bronchodilator percent-predicted FVC (Figure 1). Despite this trend, after multivariable analyses, we did not find interaction effects between daily exposure to biomass fuels smoke and percent predicted FVC for either SBP (p=0.47) or DBP (p=0.57).
Figure 1.
Differences in systolic blood pressure and diastolic blood pressure in daily biomass fuel users and non-users stratified by quartiles of percent predicted forced vital capacity. Panels A shows unadjusted differences for systolic blood pressure. Panel B shows unadjusted differences for diastolic blood pressure.
DISCUSSION
In a high altitude region of Peru, participants who reported daily use of biomass fuel had higher blood pressures and an increased risk of having prehypertension or hypertension than did those without daily biomass fuel use. Both men and women appeared to be equally affected. The relationship between biomass fuel use and blood pressure was not modified by lung function.
Our study adds to the growing body of literature on the cardiovascular effects of HAP from biomass fuel use, suggesting that while daily biomass fuel use is associated with increased blood pressure in multiple settings, the magnitude of the association varies8,9. Baumgartner et al. studied PM2.5 personal exposure in rural Chinese women and found a 2.2 mmHg increase in SBP and 0.5 mmHg increase in DBP for each 1-log-µg/m3 increase in 24-hour mean PM2.5 exposure10. Lee et al. examined the cardiovascular effects of biomass fuel use in Chinese adults and found that self-reported biomass fuel use was associated with a 1.7 increased odds of hypertension8, which is substantially less than the adjusted relative risk ratio of 6 from the analysis of our cohort. Additionally, Lee et al. reported that women were more likely to have a doubling in the odds hypertension attributed to biomass fuel use than did men8. In contrast, the results from our study show that daily exposure to biomass fuel smoke was associated with higher SBP and DBP in both men and women. These results run contrary to the generally held assumption that the health effects of biomass fuel use might only be present in women due to the larger role women often have in cooking. There are no prior large studies of hypertension or any other cardiovascular disease in relation to biomass fuel use in Latin America, other than two small improved cookstove interventions in Guatemala and Nicaragua25,26.
Differences in the magnitude of association and the effect of sex across the various studies are likely influenced by a number of factors including gene by environment interactions, cultural practices, altitude and other environmental variables. Aspects of the cooking environment, including ventilation of the cooking space and the types of biomass fuels used, influence the intensity of the exposure as well as the relative concentration of noxious gases and particulate matter released during combustion. Additionally, our study was conducted in a population living at very high altitude, and thus it is unclear to what extent the relationship between biomass fuel use and systemic blood pressure was affected by this environmental exposure. While individuals who ascend to high altitude from sea level experience an increase in blood pressure, epidemiological studies of the blood pressure effect of chronic exposure to high altitude have had mixed results27,28. The combined effect of altitude and exposure to HAP on blood pressure has not been well described and deserves further study, potentially increasing our understanding of blood pressure regulation.
There are several potential mechanisms by which biomass fuel use might affect blood pressure. Biomass fuel combustion releases gases and fine particulate matter. Fine particulate matter exposure in controlled studies has been shown to increase blood pressure, affecting vasomotor tone through nitric oxide pathways29–35. Acute exposure to air pollution also exerts autonomic effects, resulting in increased heart rate and decreased heart rate variability35,36. Moreover, exposure to fine particulate matter (2.5 µm in size) has been associated with systemic inflammation, increased oxidative stress, and decreased endothelial function, including increased plasma fibrinogen, serum C-reactive protein, and changes in leukocyte adhesion molecule expression37–40. One hypothesis is that the presence of fine particulate matter in the lung interstitium causes systemic inflammation that in turn increases blood pressure41,42. In looking at the role of lung function as measured by FVC as a potential mediator in the relationship of daily biomass fuel use with SBP and DBP, our study found no effect modification by quartiles of lung function. However, the trend towards increased difference in SBP and DBP between daily biomass fuel users and nonusers in the higher two quartiles of FVC suggests that increased lung function might be associated with increased SBP and DBP in setting of HAP exposure from biomass fuel use. The biological mechanism by which HAP from biomass fuel increases blood pressure might involve translocation of ultrafine particulate matter (0.1 µm in size) via the alveolar capillaries, although studies of ultrafine particle translocation via the lungs in healthy human volunteers have had conflicting results43,44. Particulate matter other than PM2.5 may also account for the association between air pollution exposure and increased in blood pressure. One study in rural China found that exposure to black carbon among women was more greatly associated with increased blood pressure than PM2.5 exposure45. Additional studies of the interaction between individual components of air pollution, lung function and systemic blood pressure are needed.
The results of our study support the need for additional research on the effect of HAP from biomass fuel use on cardiovascular disease. Globally, there are initiatives to reduce HAP exposure by replacing traditional cook-stoves with cleaner technology that either replaces biomass fuel use with liquefied-petroleum gas or electricity, or effectively vents the smoke from biomass fuel combustion out of the home. However, as these initiatives are evaluated, evidence regarding both the cardiovascular and pulmonary health benefits of each cook-stove initiative is needed in order to better direct investment. Cultural acceptance and economic sustainability are key components of clean cook-stove technology initiatives, however, the precise understanding of potential health benefits is needed in order to encourage public and private investment and avoid wasting resources on ineffective cook-stove technologies. Given the widespread use of biomass fuels globally, reducing exposure to HAP presents a potential opportunity to reduce morbidity and mortality from cardiovascular disease in resource-poor settings.
The primary limitation of this study is that it is observational in design at a single site and thus cannot determine causation. Although we have adjusted for many confounding variables in our analysis, there may be potentially unmeasured confounders. Specifically, biomass fuel use was closely associated with rural residence, which may be associated with unmeasured environmental variables that might affect blood pressure. Moreover, we did not measure individual exposure to HAP nor the gaseous or particulate matter composition of the biomass fuel smoke, but rather used self-reported daily biomass fuel use as proxy for exposure. However, in prior studies in a subgroup of the same cohort we have determined that 24-hour mean PM2.5 exposure in individuals living in rural Puno, where 95% of the population used biomass fuels daily, was substantially greater than in individuals living in urban Puno, where biomass fuel use was low or non-existent46. Additionally, unpublished data from our group of ambient PM2.5 monitoring in rural Puno suggest that ambient PM2.5 levels are very low and do not significantly contribute to the air pollution exposure of rural Puno residents. Another limitation of our study is that we measured blood pressure in a single examination rather than in multiple visits. We are also unable to account for recent acute environmental exposures that might have transiently affected blood pressure measurement during the day of examination. Given the numerous potentially blood pressure raising exposures found in the urban environment in which most biomass fuel nonusers reside, and the heterogeneity of the biomass fuel smoke exposure captured in our liberal definition of biomass fuel use, our study likely underestimates the true association of biomass fuel smoke exposure and blood pressure in this population. Rural biomass fuel users generally have less access to medical care than urban participants and thus might be less likely to receive a diagnosis of hypertension. The underdiagnosis of hypertension in the rural population may have resulted in further underestimation of the association of biomass fuel use with hypertension.
Perspectives
This study expands on the growing body of literature that examines the cardiovascular risk associated with household air pollution from biomass fuel use. We have demonstrated an association between biomass fuel use with blood pressure and hypertension prevalence, but additional research is needed to understand the degree to which exposure to household air pollution affects hard cardiovascular outcomes, including heart failure, myocardial infarction, and cardiovascular-related mortality. Additionally, further studies are needed to determine whether improved biomass fuel cookstove technology improves cardiovascular outcomes, or if it is necessary to replace biomass fuels with cleaner fuels, such as liquefied petroleum gas, to effectively reduce cardiovascular risk. This area of future research has broad implications for not only public health policy but also energy policy on a national and regional level. Uncovering the totality of cardiovascular and pulmonary disease associated with household air pollution from biomass fuel use is necessary to influence policymakers to enact effective public policy to improve the health of the rural poor.
CONCLUSION
In a high-altitude region of Peru, biomass fuel use was associated with elevated blood pressure and a higher prevalence of hypertension. The association between daily biomass fuel use with SBP and DBP was present and consistent for both men and women. Lung function as measured by forced vital capacity was not an effect modifier in the relationship between biomass fuel use with SBP and DBP. Additional studies of the effect of HAP and cookstove interventions on cardiovascular risk and outcomes are needed.
NOVELTY AND SIGNIFICANCE.
What is New?
This study is one of the few studies that investigate the systemic blood pressure outcomes associated with household air pollution exposure from biomass fuel use. Moreover, there are no other published studies that consider lung function as a potential effect modifier in the relationship between biomass fuel use and systemic blood pressure.
What is Relevant?
While it is estimated that exposure to household air pollution is one of the largest contributors to poor health worldwide, there are few studies that closely examine how household air pollution exposure from biomass fuel use might negatively impact cardiovascular disease outcomes. The results of this study have implications for cardiovascular disease prevention, particularly in resource-poor settings.
Summary
In a high-altitude region of Peru, biomass fuel use was associated with elevated blood pressure and a higher prevalence of hypertension.
Acknowledgments
We are indebted to study participants. Special thanks to all field teams for their commitment and hard work, especially to Lilia Cabrera and David Danz for their leadership in each of the study sites, and Marco Varela for data coordination.
Funding
This project has been funded in whole with Federal funds from the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268200900033C. William Checkley was further supported by a Pathway to Independence Award (R00HL096955) from the United States National Heart, Lung and Blood Institute, National Institutes of Health. Melissa Burroughs Peña was supported by NIH Research Training Grant R25TW009337 funded by the Fogarty International Center and the National Institute of Mental Health in addition to the Vanderbilt-Emory-Cornell-Duke Consortium, the Fogarty International Center and the National Heart, Lung and Blood Institute, National Institutes of Health.
Footnotes
Contributorship
JJM, RHG and WC conceived, designed and supervised the overall study. WC coordinated and supervised fieldwork activities in Puno. MB and WC developed the idea for this manuscript and wrote the first draft. MB, KMR and WC led the statistical analysis. All authors participated in writing of manuscript, provided important intellectual content and gave their final approval of the version submitted for publication.
Disclosure
The authors disclose no conflicts of interest.
REFERENCES
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajagopalan S, Brook R. Indoor-outdoor air pollution continuum and CVD burden: An opportunity for improving global health. Global Heart. 2012;7:207–213. doi: 10.1016/j.gheart.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortimer K, Gordon SB, Jindal SK, Accinelli RA, Balmes J, Martin WJ., 2nd Household air pollution is a major avoidable risk factor for cardiorespiratory disease. Chest. 2012;142:1308–1315. doi: 10.1378/chest.12-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, Newby DE, Mills NL. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382:1039–1048. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I Expert Panel on P, Prevention Science of the American Heart A. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 6.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 7.Milojevic A, Wilkinson P, Armstrong B, Bhaskaran K, Smeeth L, Hajat S. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart. 2014;100:1093–1098. doi: 10.1136/heartjnl-2013-304963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee MS, Hang JQ, Zhang FY, Dai HL, Su L, Christiani DC. In-home solid fuel use and cardiovascular disease: a cross-sectional analysis of the Shanghai Putuo study. Environ Health. 2012;11:18. doi: 10.1186/1476-069X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutta A, Ray MR. Prevalence of hypertension and pre-hypertension in rural women: a report from the villages of West Bengal, a state in the eastern part of India. Aust J Rural Health. 2012;20:219–225. doi: 10.1111/j.1440-1584.2012.01287.x. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner J, Schauer JJ, Ezzati M, Lu L, Cheng C, Patz JA, Bautista LE. Indoor air pollution and blood pressure in adult women living in rural China. Environ Health Perspect. 2011;119:1390–1395. doi: 10.1289/ehp.1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta A, Ray MR, Banerjee A. Systemic inflammatory changes and increased oxidative stress in rural Indian women cooking with biomass fuels. Toxicol Appl Pharmacol. 2012;261:255–262. doi: 10.1016/j.taap.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs DR, Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S, Smith LJ, Barr RG, Duprez DA. Rate of decline of forced vital capacity predicts future arterial hypertension: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Hypertension. 2012;59:219–225. doi: 10.1161/HYPERTENSIONAHA.111.184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duprez DA, Hearst MO, Lutsey PL, Herrington DM, Ouyang P, Barr RG, Bluemke DA, McAllister D, Carr JJ, Jacobs DR. Associations among Lung Function, Arterial Elasticity and Circulating Endothelial and Inflammation Markers: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2013;61:542–548. doi: 10.1161/HYPERTENSIONAHA.111.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margretardottir OB, Thorleifsson SJ, Gudmundsson G, Olafsson I, Benediktsdottir B, Janson C, Buist AS, Gislason T. Hypertension, Systemic Inflammation and Body Weight in Relation to Lung Function Impairment--An Epidemiological Study. COPD. 2009;6:250–255. doi: 10.1080/15412550903049157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnabel E, Karrasch S, Schulz H, Glaser S, Meisinger C, Heier M, Peters A, Wichmann HE, Behr J, Huber RM, Heinrich J. High blood pressure, antihypertensive medication and lung function in a general adult population. Respir Res. 2011;12:50. doi: 10.1186/1465-9921-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miranda JJ, Bernabe-Ortiz A, Smeeth L, Gilman RH, Checkley W Group CCS. Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open. 2012;2:e000610. doi: 10.1136/bmjopen-2011-000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.Jones DW, Hall JE. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and evidence from new hypertension trials. Hypertension. 2004;43:1–3. doi: 10.1161/01.HYP.0000110061.06674.ca. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 20.Hallal PC, Gomez LF, Parra DC, Lobelo F, Mosquera J, Florindo AA, Reis RS, Pratt M, Sarmiento OL. Lessons learned after 10 years of IPAQ use in Brazil and Colombia. J Phys Act Health. 2010;7:S259–S264. doi: 10.1123/jpah.7.s2.s259. [DOI] [PubMed] [Google Scholar]
- 21.Gomez Arnaiz A, Conde Martela A, Alberto Aguiar Bautista J, Manuel Santana Montesdeoca J, Jorrin Moreno A, Betancor Leon P. Diagnostic usefulness of Alcohol Use Disorders Identification Test (AUDIT) for detecting hazardous alcohol consumption in primary care settings. Med Clin (Barc) 2001;116:121–124. doi: 10.1016/s0025-7753(01)71745-9. [DOI] [PubMed] [Google Scholar]
- 22.LS R. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psyc Meas. 1977;1:385–401. [Google Scholar]
- 23.Ruiz-Grosso P, Loret de Mola C, Vega-Dienstmaier JM, Arevalo JM, Chavez K, Vilela A, Lazo M, Huapaya J. Validation of the Spanish Center for Epidemiological Studies Depression and Zung Self-Rating Depression Scales: a comparative validation study. PloS One. 2012;7:e45413. doi: 10.1371/journal.pone.0045413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howe LD, Galobardes B, Matijasevich A, Gordon D, Johnston D, Onwujekwe O, Patel R, Webb EA, Lawlor DA, Hargreaves JR. Measuring socio-economic position for epidemiological studies in low- and middle-income countries: a methods of measurement in epidemiology paper. Int J Epidemiol. 2012;41:871–886. doi: 10.1093/ije/dys037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCracken JP, Smith KR, Diaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect. 2007;115:996–1001. doi: 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark ML, Bachand AM, Heiderscheidt JM, Yoder SA, Luna B, Volckens J, Koehler KA, Conway S, Reynolds SJ, Peel JL. Impact of a cleaner-burning cookstove intervention on blood pressure in Nicaraguan women. Indoor Air. 2013;23:105–114. doi: 10.1111/ina.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parati G, Bilo G, Faini A, et al. Changes in 24 h ambulatory blood pressure and effects of angiotensin II receptor blockade during acute and prolonged high-altitude exposure: a randomized clinical trial. Eur Heart J. 2014;35:3113–3122. doi: 10.1093/eurheartj/ehu275. [DOI] [PubMed] [Google Scholar]
- 28.Hurtado A, Escudero E, Pando J, Sharma S, Johnson RJ. Cardiovascular and renal effects of chronic exposure to high altitude. Nephrol Dial Transplant. 2012;27:iv11–iv16. doi: 10.1093/ndt/gfs427. [DOI] [PubMed] [Google Scholar]
- 29.Zanobetti A, Canner MJ, Stone PH, Schwartz J, Sher D, Eagan-Bengston E, Gates KA, Hartley LH, Suh H, Gold DR. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 30.Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, Brook RD. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environl Health Perspect. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Soderberg S, Newby DE, Sandstrom T, Blomberg A. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- 32.Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC, Rajagopalan S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol. 2008;28:1760–1766. doi: 10.1161/ATVBAHA.108.166967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 34.Auchincloss AH, Diez Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, Goff DC, Kaufman JD, O'Neill MS. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2008;116:486–491. doi: 10.1289/ehp.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unosson J, Blomberg A, Sandstrom T, Muala A, Boman C, Nystrom R, Westerholm R, Mills NL, Newby DE, Langrish JP, Bosson JA. Exposure to wood smoke increases arterial stiffness and decreases heart rate variability in humans. Part Fibre Toxicol. 2013;10:20. doi: 10.1186/1743-8977-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brook RD, Urch B, Dvonch JT, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peters A, Frohlich M, Doring A, Immervoll T, Wichmann HE, Hutchinson WL, Pepys MB, Koenig W. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur Heart J. 2001;22:1198–1204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- 38.Pekkanen J, Brunner EJ, Anderson HR, Tiittanen P, Atkinson RW. Daily concentrations of air pollution and plasma fibrinogen in London. Occup Environ Med. 2000;57:818–822. doi: 10.1136/oem.57.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters A, Doring A, Wichmann HE, Koenig W. Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet. 1997;349:1582–1587. doi: 10.1016/S0140-6736(97)01211-7. [DOI] [PubMed] [Google Scholar]
- 40.Frampton MW, Stewart JC, Oberdorster G, Morrow PE, Chalupa D, Pietropaoli AP, Frasier LM, Speers DM, Cox C, Huang LS, Utell MJ. Inhalation of ultrafine particles alters blood leukocyte expression of adhesion molecules in humans. Environ Health Perspect. 2006;114:51–58. doi: 10.1289/ehp.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, Sandstrom T, Blomberg A, Newby DE. Adverse cardiovascular effects of air pollution. Nat Clin Pract Cardiovasc Med. 2009;6:36–44. doi: 10.1038/ncpcardio1399. [DOI] [PubMed] [Google Scholar]
- 42.Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet. 1995;345:176–178. doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 43.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 44.Mills NL, Amin N, Robinson SD, Anand A, Davies J, Patel D, de la Fuente JM, Cassee FR, Boon NA, Macnee W, Millar AM, Donaldson K, Newby DE. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am J Respir Crit Care Med. 2006;173:426–431. doi: 10.1164/rccm.200506-865OC. [DOI] [PubMed] [Google Scholar]
- 45.Baumgartner J, Zhang Y, Schauer JJ, Huang W, Wang Y, Ezzati M. Highway proximity and black carbon from cookstoves as a risk factor for higher blood pressure in rural China. Proc Natl Acad Sci USA. 2014;111:13229–13234. doi: 10.1073/pnas.1317176111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollard SL, Williams DL, Breysse PN, Baron PA, Grajeda LM, Gilman RH, Miranda JJ, Checkley W CRONICAS Cohort Study Group. A cross-sectional study of determinants of indoor environmental exposures in households with and without chronic exposure to biomass fuel smoke. Environ Health. 2014;13:21. doi: 10.1186/1476-069X-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]