Abstract
Gender-preferential gene expression is a widespread phenomenon in humans. It is important to study how gender differences influence the pathogenesis of various diseases and response to specific drugs. The aim of this study is to determine if the mouse albumin enhancer/promoter may serve as the promoter to introduce gender-preferential gene expression in transgenic animals. We created four independent transgenic rat lines in which the human C-reactive protein (CRP) transgene was under the control of mouse albumin enhancer/promoter. Quantitative real time RT-PCR analysis showed that transgene expression in the liver of male rats was significantly higher than transgene expression in the female rats (p<0.05). There was a 5.3-fold (male/female) difference in line-519, and a 12.2-fold (male/female) difference in line-488. ELISA showed that the serum of male transgenic rats had a 13 to 679-fold difference at the protein level on transgene production compared with female transgenic rats. The male-to-female difference in gene expression was 10 to 17-fold in the liver of transgenic rats. Orchiectomy dramatically reduced protein production from the transgene in the liver. Testosterone administration into female rats did not increase the transgene expression, but estrogen administration into the male rats reduced transgene expression. This study provides a valuable tool for investigating the pathological roles of genes that are expressed in a gender-preferential manner in human disease.
Keywords: Gender-preferential, transgenic, animal model, gene expression
Introduction
Gender-preferential differences in gene expression area widespread phenomenon in both human and animal genomes (Heidecker et al. 2010; Kimoto et al. 2005; Vawter et al. 2004; Zhang et al. 2007). It has been shown by microarray analysis that 27 genes had sex-specific expression in the glomeruli of healthy men and women and 57 genes were found to be differentially expressed in the tubulointerstitial compartment of those same healthy men and women (Si et al. 2009). Gender-preferential gene expression is not only common; it has been shown to be closely related to certain human disease processes. A study performed on the dopamine neurons of postmortem brains from sporadic Parkinson’s disease patients found that males suffering from the disease had an enrichment of upregulated genes related to many aspects of cellular homeostasis including mitochondrial activity and Ca2+homeostasis. This data suggested a bias towards men developing Parkinson’s disease at the molecular level and supported some of the clinical observations showing disease progression and presentation are gender-preferential (Simunovic et al. 2010).
In order to examine the functional role of gender-preferential gene expression in various human diseases, a tool is needed to induce gender-preferential expression using an animal model. Here, we demonstrate that the mouse albumin enhancer/promoter may serve as a promoter to introduce gender-preferential gene expression in transgenic animals. We anticipate that our strategy will provide a valuable tool to investigate the molecular and pathophysiological roles of genes expressed in a gender-preferential manner and how differential expression affects drug metabolism.
Materials and Methods
Transgene construct
A fragment containing 21 nucleotides (nt) upstream of the transcription initiation site, exons and introns, and a 1.2 kb segment of the 3′-flanking region of the human CRP gene was amplified from human genomic DNA and cloned into a TA cloning vector (Life Technologies). This fragment was excised by with restriction enzymes then inserted into the vector downstream of the mouse albumin enhancer/promoter element. The construct was confirmed by restriction enzyme mapping and DNA sequencing. The plasmid mALB-hCRP contained a 2.7-kb mouse albumin enhancer element (−12.171 to −9.469 kb) and a 334-bp promoter element (−305 to +29) as well as the human CRP gene (Fig. 1). The 4.9-kb fragment was excised from the ALB-hCRP construct, purified by agarose gel electrophoresis and QIA quick gel extraction (Qiagen).
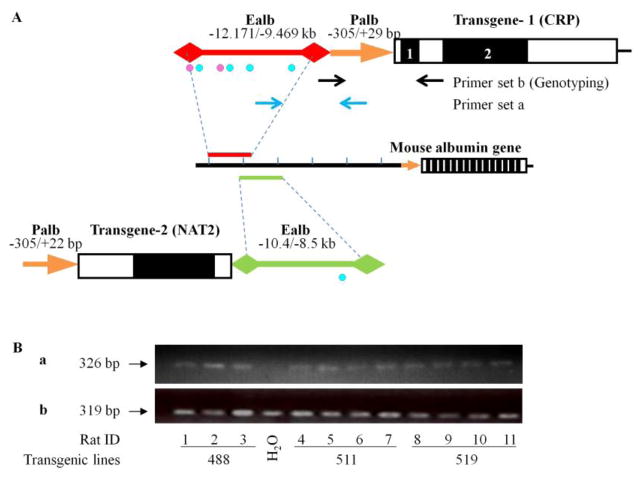
Figure 1. The transgene.
(A). The mAlb-hCRP transgene construct is composed two sequences fragments from the 5′-flanking region of mouse albumin gene, and the human CRP structure gene without promoter as well as the 3′-end of the human CRP gene. Blue dots indicate the locations of androgen receptor binding sites predicted by MAPPER and PROMO software, the pink dots indicate the locations of predicted estrogen receptor binding sites. (B). The confirmation of transgene structure in the genomes of three transgenic lines by two specific PCRs. The first PCR used an upstream primer in the albumin enhancer and a downstream primer in the albumin promoter (primer set a). The second PCR used an upstream primer in the albumin promoter and a downstream primer in the CRP gene (primer set b). Totally 11 transgenic rats from three transgenic lines were examined for re-arrangement after transgene incorporation into the rat genome.
Generation of transgenic rats
To generate transgenic founders, purified hCRP DNA was microinjected into fertilized eggs obtained by mating CD IGS Sprague-Dawley rats from Charles River Laboratory (Wilmington, MA). Pronuclear microinjection was performed by the University of Michigan Transgenic Animal Model Core facility following a procedure described previously (Filipiak and Saunders 2006). Transgenic founders were identified by genotyping with primers shown in the Figure 1 and mated with non-transgenic rats to produce F1 progeny. Male and female transgenic rats of 6 to 10 weeks old weighing 170 to 250 grams were used. All rats were maintained under a 12-hour light-dark cycle and given food and water. All animal experiments were performed with the approval of the Animal Care Committee of the University of Morehouse School of Medicine, and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
We identified human CRP transgenic rats by genotyping tail snip samples. Briefly, rats were given inhalation anesthesia and tail biopsies were performed by removing 2 mm of each tail for genomic DNA isolation. PCR was performed in 20 μl reaction mixtures containing 5 ng genomic DNA, 5 μM each primer set, 5 mM dNTP mixture, 2 μl of 10X PCR buffer, and 1 unit of Taq polymerase, with the primers (forward: 5′-ACA TAC GCA AGG GAT TTA GTC-3′, reverse: 5′-AAC AGC TTC TCC ATG GTC AC-3′). Amplification was performed for 30 cycles using the following PCR conditions: 12 min at 95°C, 30 sec at 94°C, 30 sec at 55°C, 1 min at 72°C, on a GeneAmp PCR System 9600 (PE Applied Biosystems). PCR products were separated electrophoretically on 1% agarose gels and visualized after ethidium bromide staining.
Ovariectomy and Orchiectomy
All surgical procedures were performed using sterile surgical techniques. Rats were anesthetized with 3% isoflurane. A single mid-dorsal incision in the skin was made for ovariectomy by entering the abdominal cavity in a region of a periovarian fat pad. Both ovaries were exteriorized by grasping the periovarian fat with tissue forceps. The pedicle was excised between the uterine horn and the fallopian tubes. For orchiectomy, a small incision was made at the tip of the scrotum. The tunic was opened and the testis, cauda epididymis, vas deferens, and the spermatic blood vessels were exteriorized. Blood vessels and the vas deferens were then ligated with 4-0 absorbable suture or alternatively cauterized. Testis and the epididymis were removed. The remaining tissue was returned to the sac and the procedure repeated for the other testis. The wound and skin were closed with absorbable and non-absorbable suture, respectively. Sham surgery was performed on the sham group. Buprenorphine (0.1–0.5 mg/kg) was administered subcutaneously twice daily for 24–72 hours for treatment of pain. Blood was drawn one day before surgery and 1 week post- surgery.
Preparation of total RNA and protein samples
Blood was drawn from rat tails and allowed to clot at ambient temperature before centrifugation at 5,000 rpm to collect serum. Serum samples were stored at −20°C prior to further analysis. Rat liver was carefully dissected and immediately homogenized with FastPrep Lysing Matrix Tubes D (MP Biomedicals, Germany). Total RNA was isolated using RNeasy Mini Kits (Qiagen). The concentration of total RNA samples was measured with Nanodrop (ND-1000, NanoDrop, USA).
Rat liver tissues, approximately 30 mg in weight, were immediately homogenized using FastPrep Lysing Matrix Tubes D (MP Biomedicals, Germany) with an extraction medium containing 1 volume protease inhibitor cocktail to 29 volume T-Per tissue protein extraction reagent (Thermo Scientific) which was a 10-fold volume of the pellet. After centrifugation for 10 min at 4 C, an aliquot of the supernatant was used to determine the protein concentrations using the BCA assay in Biophotometer (Eppendorf) and a Bio-Rad colorimetric assay kit (Bio-Rad).
Quantitative real time analysis of mRNA expression
Total RNA (0.5 μg) was reverse transcribed with a SuperScriptIII First-Strand Synthesis kit (Invitrogen) according to the manufacturer’s protocol. An aliquot of the cDNA was quantified by real-time PCR on a Light Cycler using a FastStart DNA Master SYBR Green kit (Roche). Two set of primers for the transgene and GAPDH. The sequence of primers were 5′-CATGTCGAGGAAGGCTTTTGTG-3′ and 5′-CACCCACTGTAAAACTGTATCC-3′ for transgene expression, and 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′ for GAPDH gene expression. Real time PCR assays were performed in duplicates using 96-well plates. GAPDH was used as a reference to which all samples were normalized. The relative quantification was calculated using the 2−ΔΔct formula with threshold (Ct) values relative to controls. The results were expressed as fold-change.
Enzyme-Linked Immunosorbent Assay (ELISA) analysis of protein production
Transgenic hCRP protein expression was measured by enzyme-linked immunosorbent assays (ELISA) with a human CRP specific ELISA kit (HELICA Biosystems Inc.). Rat serum and liver samples were assayed according to the manufacturer’s instructions. Briefly, serum samples were diluted 1:6000 and liver samples were diluted as1:250. One hundred microliters of each sample was incubated in a 96-well plate at ambient temperature (20–25°C) for 30 minutes. Plates were gently washed 4–5 times with wash buffer. Stock conjugate (100X) was diluted to 1X with Tris-buffer and 100 μl of conjugate was added into each well. The plate was incubated for 30 minutes at ambient temperature, washed with wash buffer and 100 μl TMB (TMB)/substrate solution was added to the plate. The plate was allowed to hybridize for 5–10 minutes before adding 100μl of Stop solution. A blue color was indicative of a positive reaction. Absorbance (OD) readings were measured using a microplate reader equipped with a 450nm filter. Final CRP levels were calculated by multiplying by 6,000 for serum samples and 250 of liver samples.
Western blot analysis
Western blots was done using serum from transgenic human CRP positive male rats before orchiectomy and 1 week after orchiectomy (n=6). The serum was diluted at 1:1,000, denatured at 70°C for 10 min and 11.7 μl was loaded onto 4–12% non-denaturing polyacrylamide gels before transferring to PVDF membranes. The membranes were blocked with 5% milk for 1 hour and incubated with anti-human CRP antibody overnight. The membranes were washed 3 times with 1×TBST (1% serum albumin in 50 mM Tris-HCl pH 7.4, containing 0.05% Tween-20) at room temperature, and then incubated with a 1:2,000 dilution of goat anti-mouse IgG-HRP antibody (Santa Cruz) at room temperature for 1 hour. Membranes were washed 3 times and incubated with substrate solution (SuperSignal West Pico Chemilluminescent Kit) for 5 minutes before detection on x-ray film. Three independent anti-human CRP antibodies were used in these Western blot analyses, M86284M (Meridian), (M86284M (Meridian), and ab52687 (Abcam).
Injection of estrogen and testosterone
All injection procedures were performed using sterile equipment. Rats were anesthetized with 3% isoflurane. The following hormones were administered daily subcutaneously for 7 days: testosterone cypionate in oil C IIIN (7.5mg/kg/day)(Patterson Veterinary Corporate), estradiol cypionate (10mg/kg/day) (Rood & Riddle Veterinary Pharmacy), and the vehicle(Sesame oil, Sigma). Blood was drawn from rats on the first day before the first injection and 1 day after the last injection.
Statistical analysis
All experiments were performed in duplicates and replicated at least three times. Statistical significance was examined by Student’s t test. P values <0.05 were regarded as statistically significant. Data are presented as mean values± standard deviation.
Results
Establishment of transgenic rats
Transgenic rat lines were established, in which the expression of human C-reactive protein (CRP) transgene was under the control of mouse albumin enhancer/promoter (Fig. 1A). Transgenic lines 488, 519 and 511were used in this study. Genotyping was used to identify transgenic animals from their negative littermates (Fig. 1B).
Copy numbers of transgene
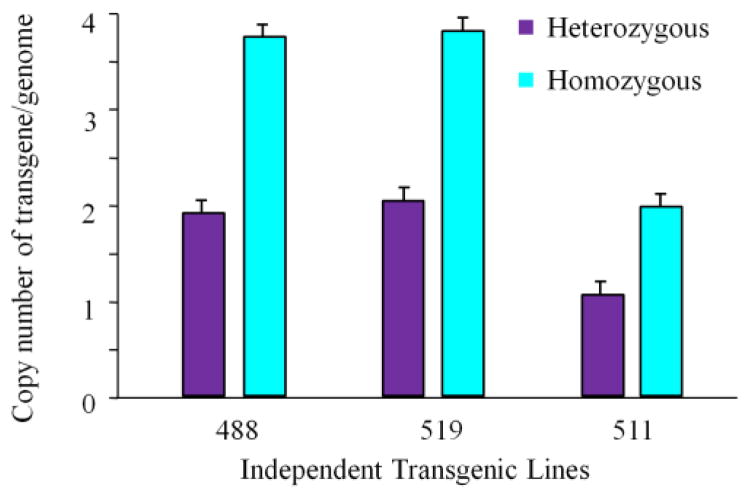
We measured the copy numbers of transgene in three independent transgenic lines by quantitative real-time PCR. As shown in Figure 2, the genomes from transgenic lines 488 [n=6, 1.95 (heterozygote), 3.78(homo)] and 519 [n=6, 2.04(heterozygote), 3.82 (homo)] contain 4 gene copies in their diploid genome; transgenic line-511 [n=6, 1.03(heterozygote), 1.99 (homo)]contains 2 copies of the transgene in the diploid genome.
Figure 2. Copy numbers of transgene in three transgenic lines.
The genomes from transgenic hCRP line 488 (n=6), 519 (n=6), 511 (n=6) were measured with quantitative real time-PCR normalized by the copy number of the CRP gene in the human genome.
Male-preference of transgene expression at the mRNA level
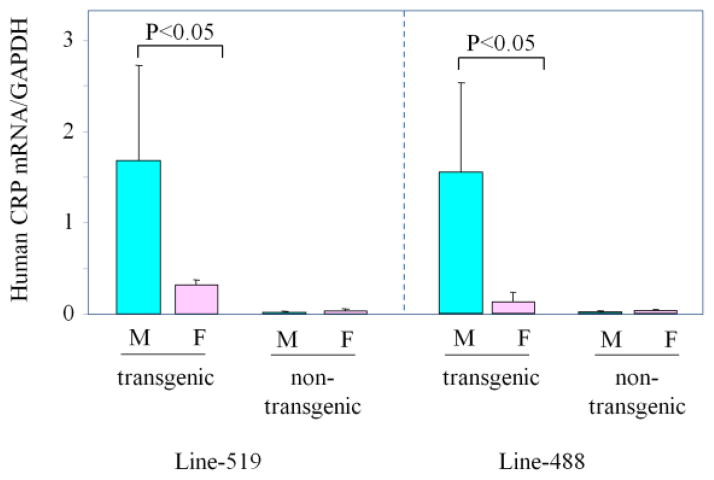
Quantitative real time PCR was carried out to determine the mRNA levels of transgene expression in male rats and female rats. Transgenic rats from line-519 (male=6, female=6) and line-488 (male=6, female=6) were euthanized at the age of 9 weeks and total RNA was extracted from rat livers. As shown in Figure 3, the transgene expression in the male rats was significantly higher than the transgene expression in the female rats (p<0.05). There was a 5.3-fold (male/female) difference in line-519, and a 12.2-fold (male/female) difference in line-488.
Figure 3. The mRNA expression in the liver of transgenic rats.
The mRNA levels of the transgene human CRP was measured by the quantitative real-time PCR. The CRP mRNA levels were then normalized with the mRNA levels of the GAPDH. The data of transgenic lines-519 and 488 were shown. The transgene expression in male is 5.3-fold higher than the transgene expression in the female rats in line 519, the transgene expression in male is 12.2-fold higher than the transgene expression in the female rats in line 488.
Male-preference of transgene expression at the protein level
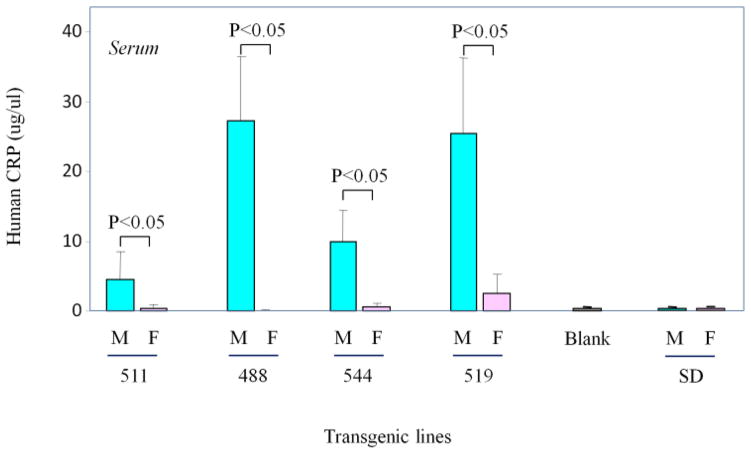
ELISA was carried out to determine the protein levels of transgene production in the serum and liver of male and female rats. Serum was collected from transgenic rats of lines 519 (male=3, female=3), 488 (male=3, female=4), 544 (male=3, female=3) and 511 (male=2, female=3). Livers were collected from transgenic rats of lines 519 (male=7, female=7), and 488 (male=7, female=7) at the age of 9 weeks old. Results indicate that serum from the male rats of lines 511, 488, 544, and 519 showed a 13.6, 679, 19.5 and 9.8 fold increase in transgene protein production respectively than the female rats (Fig. 4). In the livers from lines 519 and 488, protein levels from the human CRP transgene in male rats were 10.5 and 17.1 fold higher than the level in female rats respectively (Fig. 5).
Figure 4. The protein production of transgene in the serum of transgenic rats.
ELISA was performed to measure the protein levels. The human CRP protein levels in the serum of male and female rats of 4 independent transgenic lines are shown. Data are mean±SD. P values are labeled for pairwise comparison with each transgenic line. The male rats showed a 13.6-fold transgene protein production higher than the female rats in line-511, it is 679.1-fold in transgenic line-488, 19.5-fold in transgenic line-544, 9.8-fold in transgenic line-519. Two controls were included in this experiment. In the Blank, PBS was used instead of serum. SD, non-transgenic SD rats.
Figure 5. The protein production of transgene in the liver of transgenic rats.
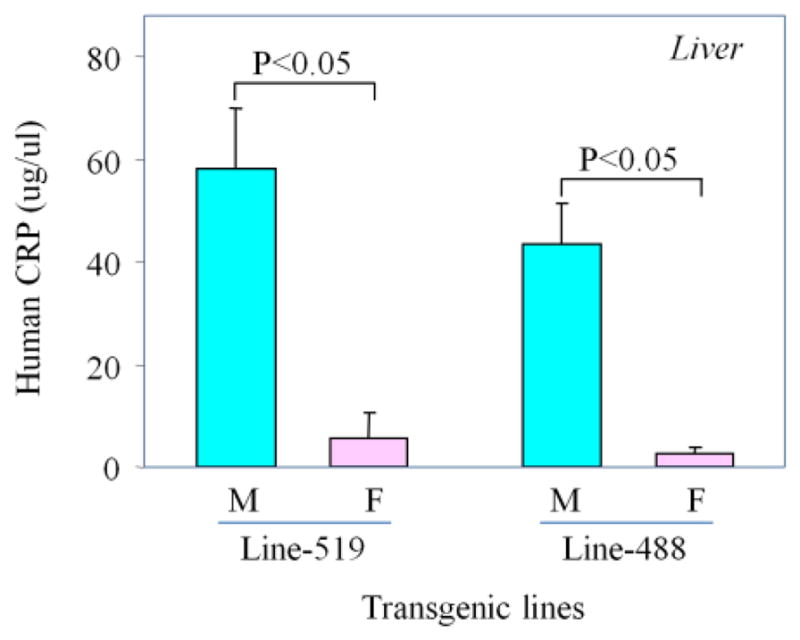
ELISA was performed to measure the protein levels. The human CRP protein levels in the liver of male and female rats of 2 independent transgenic lines are shown. Data are mean±SD. P values are labeled for pairwise comparison with each transgenic line. The male rats showed a 10.5-fold transgene protein production higher than the female rats in line-519, and 17.1-fold in line-488.
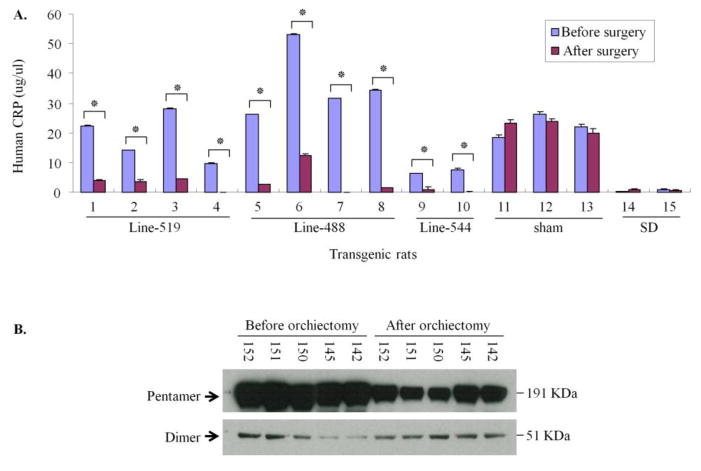
Effect of orchiectomy and ovariectomy on transgene expression
Orchiectomy surgery was carried out in male rats to remove the testes on both sides. As measured by ELISA, the protein production from the transgene was significantly reduced in the liver after orchiectomy (Fig. 6A, B). This phenomenon was observed in all three independent transgenic lines. The average reductions were 6.4-fold in line-519, 16.9-fold in line-488, and 7.0-fold in line-544. Ovariectomy was carried out in female rats to remove the ovaries on both sides. No significant difference in protein levels was observed after ovariectomy (data not shown).
Figure 6. Effect of orchiectomy on transgene expression.
Male transgenic rats of three independent lines were used in this experiment. Testis was removed from male human CRP rats at the age of 7 week. (A). ELISA. The protein levels of the transgene product were significantly reduced after orchiectomy in all rats of three transgenic lines. Mean and SD are shown, * P<0.01. The average reduction was 6.4-fold in line-519, 16.9-fold in line-488, and 7.0-fold in line-544. (B). Western blot analysis. The increase of CRP pentamer in male human CRP transgenic rats after the orchiectomy by Western Blot. CRP dimer did not change after the orchiectomy. The numbers on the top of the gel are the rat ID numbers.
Effects of testosterone and estrogen on transgenic human CRP expression
Following the injection of sex hormones, serum was collected and human CRP levels were measured by ELISA. Estrogen administration (10mg/kg/day) significantly reduced the transgene expression in male rats (P=0.000636, n=6), while testosterone administration (7.5mg/kg/day)did not significantly influence the transgene expression in female rats (n=6) (Fig. 7).
Figure 7. Effects of testosterone and estrogen on the transgene expression in the mALB-hCRP transgenic rats.
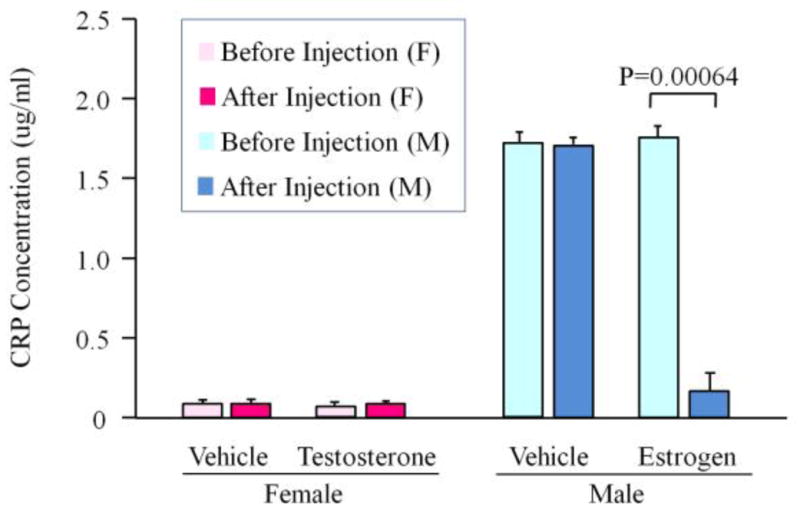
Female transgenic rats were treated with testosterone (7.5 mg/kg/day), male transgenic rats were treated with estrogen (10 mg/kg/day). The hormones were injected subcutaneously every day continuously for 7 days. The serums were collected before the first injection and after the last injection. Human CRP concentrations were measured by ELISA. The results showed that testosterone did not significantly increase the transgene expression in the female rats, but the estrogen significantly reduced the transgene expression in the male rats.
Discussion
In this study we reported that a piece of DNA sequence selected from the 5′-flanking region of mouse albumin gene could drive male-specific gene expression in transgenic rats. This enhancer/promoter provides a valuable tool for investigating the pathological roles of genes that are expressed in a gender-preferential manner in human diseases.
It has been reported previously that the chicken beta-actin promoter could lead a female-specific gene expression in the lung of mice (Pfeifer et al. 2011). For the male-specific gene expression, it was reported that another DNA piece (−8.5 kb to −10.4 kb), also from the mouse albumin gene, generated a 2-fold male/female difference in the liver of transgenic mice (Sugamori et al. 2011). Several key differences exist in our study. First, we used a different DNA sequence (from −12.2 kb to −9.5 kb) although both sequences are located close to the mouse albumin gene (Fig. 1). Second, the enhancer was located at the 5′-end of the transgene in our transgenic animals. In the previous study it was located at the 3′-end of the transgene after integration in the animals (Fig. 1). Although the enhancer and promoter was confirmed to be upstream to the CRP gene by our experimental data in line 511, we could not draw any conclusions on their relative positions in the lines of 488 and 519 due to tandem repeat insertion in each case, so it is unknown if enhancer positioning caused the difference on gender preference between our promoter system and Sugamori system (Sugamori et al. 2011). The gender difference between males and females observed in this study was within the same founder line. The potential position effect from the transgene integration location (Clark et al. 1994; Palmiter and Brinster 1986) has been ruled out in our study by the consistent data from three transgenic lines. The copy numbers of the transgene were measured, as described previously (Clark et al. 1994; Palmiter and Brinster 1986). Interestingly, two transgenic lines (488 and 519) with higher copy numbers of transgene showed higher expression levels of CRP transgene than the transgenic line (511) with a lower copy number of transgene, indicating that the copy number may contribute to the difference of the transgene expression levels.
It is obvious that the native mouse albumin gene will produce enough albumin in female mice. The promoter system we used in this work is not the same as the endogenous mouse albumin gene regulatory elements. Compared with the enhancer/promoter in the transgenic animals, the expression of the endogenous albumin gene is driven additionally by some other upstream or downstream elements. Although it was hypothesized that the albumin enhancer/promoter might be regulated by sex hormones (Sugamori et al. 2011), what we observed in the transgenic rats about the gender preference may not necessarily be the same as what occurred in the endogenous albumin gene regulation. In our transgenic rats, we have observed that ovariectomy on female transgenic animals did not boost the transgene expression, but orchiectomy on male transgenic animals completely abolished the transgene expression (Fig. 6). Furthermore, testosterone administration could not increase the transgene expression in the female rats, but estrogen administration could remarkably reduce the transgene expression in the male rats (Fig. 7). These results indicate that the enhancer-promoter sequence we used to drive the transgene expression is under the tight control of sex hormones. In this control, the presence of estrogen will inhibit the capacity of the enhancer-promoter sequence to drive gene expression, whereas testosterone is essential for the enhancer-promoter sequence to be able to drive transcription of the downstream gene. We have performed a bioinformatic analysis to determine the presence of androgen response elements in the enhancer and promoter we used. The MAPPER software (Multi-genome Analysis of Positions and Patterns of Elements of Regulation) and the PROMO software were used to predict the androgen receptor binding sites and estrogen receptor binding sites. The result showed that multiple androgen receptor binding sites were located in the enhancer/promoter region of our transgene construct. Totally four androgen response elements and two estrogen response elements were discovered in our study (Fig. 1 and 8), while one androgen receptor binding site and no estrogen receptor binding sites were found in the enhancer used by the Sugamori group (Sugamori et al. 2011). In summary, this albumin enhancer/promoter provides a valuable tool for investigating the pathological roles of genes that are expressed in a gender-preferential manner in human diseases.
Figure 8. Sequence of promoter and enhancer in ALB-hCRP transgene.

(A) Sequence of mouse albumin promoter. (B) Sequence of mouse albumin enhancer. Blue dots indicate the locations of androgen receptor binding sites predicted by MAPPER and PROMO software, the pink dots indicate the locations of predicted estrogen receptor binding sites.
Acknowledgments
Funding: This work was supported by NIH grants (HG006173, MD005964, HL095098, RR003034), American Heart Association grant (09GRNT2300003). We acknowledge Wanda Filipiak for preparation of transgenic rats and the Transgenic Animal Model Core of the University of Michigan’s Biomedical Research Core Facilities.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- Clark AJ, Bissinger P, Bullock DW, Damak S, Wallace R, Whitelaw CB, Yull F. Chromosomal position effects and the modulation of transgene expression. Reprod Fertil Dev. 1994;6 (5):589–598. doi: 10.1071/rd9940589. [DOI] [PubMed] [Google Scholar]
- Filipiak WE, Saunders TL. Advances in transgenic rat production. Transgenic Res. 2006;15 (6):673–686. doi: 10.1007/s11248-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Heidecker B, Lamirault G, Kasper EK, Wittstein IS, Champion HC, Breton E, Russell SD, Hall J, Kittleson MM, Baughman KL, Hare JM. The gene expression profile of patients with new-onset heart failure reveals important gender-preferential differences. Eur Heart J. 2010;31 (10):1188–1196. doi: 10.1093/eurheartj/ehp549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437 (7060):898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Brinster RL. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer C, Aneja MK, Hasenpusch G, Rudolph C. Adeno-associated virus serotype 9-mediated pulmonary transgene expression: effect of mouse strain, animal gender and lung inflammation. Gene Ther. 2011;18 (11):1034–1042. doi: 10.1038/gt.2011.42. [DOI] [PubMed] [Google Scholar]
- Si H, Banga RS, Kapitsinou P, Ramaiah M, Lawrence J, Kambhampati G, Gruenwald A, Bottinger E, Glicklich D, Tellis V, Greenstein S, Thomas DB, Pullman J, Fazzari M, Susztak K. Human and murine kidneys show gender- and species-specific gene expression differences in response to injury. PLoS One. 2009;4 (3):e4802. doi: 10.1371/journal.pone.0004802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simunovic F, Yi M, Wang Y, Stephens R, Sonntag KC. Evidence for gender-preferential transcriptional profiles of nigral dopamine neurons in Parkinson disease. PLoS One. 2010;5 (1):e8856. doi: 10.1371/journal.pone.0008856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamori KS, Brenneman D, Grant DM. Liver-selective expression of human arylamine N-acetyltransferase NAT2 in transgenic mice. Drug Metab Dispos. 2011;39 (5):882–890. doi: 10.1124/dmd.111.038216. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Evans S, Choudary P, Tomita H, Meador-Woodruff J, Molnar M, Li J, Lopez JF, Myers R, Cox D, Watson SJ, Akil H, Jones EG, Bunney WE. Gender-preferential gene expression in postmortem human brain: localization to sex chromosomes. Neuropsychopharmacology. 2004;29 (2):373–384. doi: 10.1038/sj.npp.1300337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Bleibel WK, Roe CA, Cox NJ, Eileen Dolan M. Gender-preferential differences in expression in human lymphoblastoid cell lines. Pharmacogenet Genomics. 2007;17 (6):447–450. doi: 10.1097/FPC.0b013e3280121ffe. [DOI] [PMC free article] [PubMed] [Google Scholar]