Abstract
Assessment of zinc status remains a challenge largely because serum/plasma zinc may not accurately reflect an individual’s zinc status. The comet assay, a sensitive method capable of detecting intracellular DNA strand breaks, may serve as a functional biomarker of zinc status. We hypothesized that effects of zinc supplementation on intracellular DNA damage could be assessed from samples collected in field studies in Ethiopia using the comet assay. Forty women, from villages where reported consumption of meat was less than once per month and phytate levels were high, received 20 mg zinc as zinc sulfate or placebo daily for 17 days in a randomized placebo-controlled trial. Plasma zinc concentrations were determined by inductively coupled plasma mass spectrometry (ICPMS). Cells from whole blood at the baseline and endpoint of the study were embedded in agarose, electrophoresed, and stained before being scored by an investigator blinded to the treatments. Although zinc supplementation did not significantly affect plasma zinc, mean (± SEM) comet tail moment measurement of supplemented women decreased from 39.7 ± 2.7 to 30.0 ± 1.8 (p<0.005) indicating a decrease in DNA strand breaks in zinc-supplemented individuals. These findings demonstrated that the comet assay could be used as a functional assay to assess the effects of zinc supplementation on DNA integrity in samples collected in a field setting where food sources of bioavailable zinc are limited. Furthermore, the comet assay was sufficiently sensitive to detect changes in zinc status as a result of supplementation despite no significant changes in plasma zinc.
Keywords: zinc deficiency, comet assay, women, Ethiopia, randomized controlled trial, biomarkers
1. Introduction
Zinc is a required cofactor for over two hundred enzymes involved in most major metabolic pathways and supports a wide range of biochemical and immunological functions [1] including maintenance of DNA integrity and antioxidant defense in cells [2]. Lack of adequate dietary zinc and effects of the subsequent zinc deficiency remain worldwide health problems disproportionately affecting populations in developing counties whose diets are heavily reliant upon plant-based foods [3, 4]. Inadequate dietary zinc continues to contribute to increased risk of infectious disease in the developing world [5].
While dietary intakes of zinc may correlate with plasma zinc concentrations, there are numerous dietary and non-dietary factors that negatively affect zinc bioavailability, such as inhibitory ligands like phytic acid, as well as gastrointestinal diseases and malabsorptive syndromes [4, 6–11]. In a meta-analysis of three observational studies involving 1184 participants, Lowe and colleagues found a non-significant relationship between dietary Zn intake and plasma zinc concentration [12]. Furthermore, based on a meta-analysis of ten zinc supplementation RCTs which included 1285 participants, Lowe and colleagues concluded that doubling of zinc intake would increase plasma/serum zinc concentrations by only 6% [12]. Hess and coworkers and the iZiNCG suggested that plasma/serum zinc concentrations are useful to assess a population’s risk of zinc deficiency or response to zinc supplementation but may not be reliable indicators of zinc status [13]. Thus no single biochemical assay has been validated that accurately reflects tissue or cellular zinc concentrations of individuals [4].
A particularly important function of zinc is protection of the cell against DNA damage through its role in proteins involved in DNA repair pathways. Zinc is required for optimal function of the repair proteins poly (ADP-ribose) polymerase 1 (PARP1), p53, and apurinic endonuclease (APE) [14–16]. In a rat glioma C6 brain cell model, increased oxidative DNA damage and impaired nucleic acid repair mechanisms appeared to result, at least in part, from low zinc availability [17]. Indeed, zinc deficiency in both in vitro and in vivo models is associated with increased oxidative stress and increased DNA damage [2, 18].
As a result of this relationship between cellular zinc levels and DNA damage, the comet assay, a method that measures DNA strand breaks in cells, may represent a sensitive functional tool to assess response to zinc supplementation. Due to the fact that zinc is found predominantly as a cofactor or structural component in intracellular proteins, including transcription factors and proteins involved in DNA damage repair, changes in intake may affect intracellular processes such as DNA structure and repair more than plasma zinc concentration [17, 19]. In a zinc depletion-repletion study conducted in healthy men, the comet assay reflected zinc status as a function of alterations in DNA integrity [20]. Importantly, increases in DNA damage preceded any change in fasting plasma zinc, suggesting that DNA damage is a more sensitive marker of zinc status.
The comet assay is based on a micro-electrophoretic technique [21–23] and has been used in cancer research, as well as in toxicological studies, to assess damage to nucleic acids [24, 25]. The assay allows quantification of DNA damage within single agarose-embedded cells [26]. Under the appropriate assay conditions, electrophoresis of lysed cells may result in the generation of comet-like structures. Intact DNA is retained in the “head”, while fragmented DNA forms the tail, resulting in the formation of so-called “comets”. The comets are stained and visualized using fluorescence microscopy [27, 28]. Because of the essential role of zinc in DNA repair mechanisms, changes in zinc status can contribute to changes in the comet size and morphology.
Previous studies showed that rural women in subsistence farming households in the Sidama region consumed meat very rarely (less than once per month) [29] , and that phytate:zinc molar ratios in women’s diets averaged 17:1 [30]. Staple foods in southern Ethiopia are maize and enset (Enset ventricosum). Therefore we hypothesized that women from the area would be at high risk of chronic zinc deficiency. Due to the limitations of assessing zinc status by measuring plasma zinc, we examined the use of the comet assay to assess the effects of a double-blind placebo-controlled supplementation with zinc sulfate in Ethiopian women drawn from this population. We hypothesized that effects of zinc supplementation on intracellular DNA damage could be assessed from samples collected in field studies in Ethiopia using the comet assay. To test this hypothesis, changes in DNA strand breaks in peripheral blood cells at baseline and endpoint were compared between the placebo and zinc-supplemented groups.
2. Methods and materials
2.1. Study design, participants, and ethical approvals
This double-blind placebo-controlled zinc supplementation trial was conducted in the Finichawa community of the Sidama region in Ethiopia in January, 2010. Women were recruited through community health workers by researchers from Hawassa University. A healthcare assistant excluded volunteers who self-reported pregnancy, malarial infection, or exhibited other clinical signs of impaired health. Details of the study were explained in the local language in an information session, and women between 18 and 50 years of age who chose to volunteer returned on another day to give consent and to be enrolled in the study. For women who could not read or write, the consent form was read in the local language, and oral consent was witnessed by a community volunteer able to sign her name as witness.
Approval for this study was provided by Institutional Review Boards at both Oklahoma State University and Hawassa University. Approval was also granted through the Southern Nations, Nationalities and People’s Regional Health Bureau and the zonal health office of Hawassa. At the national level in Ethiopia, approval was granted by the National Health Research Ethics Review Committee and the Drug Administration and Control Authority.
2.2. Anthropometric measurements and zinc supplementation
Weight and height of participants were measured twice at baseline, and BMI was calculated as wt/ht2 (kg/m2). Demographic information was collected individually from each subject at the time of enrollment using a questionnaire administered in the participant’s primary language.
Participants were assigned to either zinc supplement (n=20) or placebo (n=20) using a random number table. Study personnel were blinded to the treatment. Each morning for the duration of the supplementation period (17 days), participants received either 20 mg of zinc as zinc sulfate (ZinCfant, Nutriset S A S, Malaunay, France) or a placebo (Nutriset S A S) that was taken under the supervision of study personnel at a local healthcare facility. All women completed the supplementation.
2.3. Blood collection
Fasting blood samples were drawn by venipuncture in lithium-heparin-coated polypropylene tubes designed for trace-element research (Sarstedt AG & Co., Newton, NC) between 9:00 and 10:00 am both at baseline (Day 0) and on the day after the last supplement was administered (Day 18). Immediately following collection, whole blood samples were stored on ice. For plasma samples, blood was centrifuged in a clinical centrifuge at 1000×g within one hour of collection. Plasma was separated, stored on ice, and frozen at −20°C at Hawassa University until shipped to Oklahoma State University for analyses. Two women were eliminated from the placebo group because their blood samples were damaged in storage/shipment. Three women were eliminated from the zinc-supplemented group for the following reasons: one sample could not be obtained at endpoint, one sample was badly hemolyzed and one sample was damaged in storage/shipment.
2.4 Zinc measurement in plasma
Plasma was diluted 1:20 in 0.1% high-purity nitric acid (GFS Chemicals, Inc., Powell, OH) using trace element free plasticware (Sarstedt Inc., Newton, NC). Zinc concentrations in plasma were analyzed by inductively-coupled plasma mass spectrometry (ICP-MS) (Elan 9000, Perkin Elmer Life and Analytical Sciences, Norwalk, CT) with gallium as internal standard. Commercial quality control standards (Utak Laboratories Inc., Valencia, CA) were analyzed after every tenth sample. A plasma zinc concentration below 10.7 µmol/L (700 µg/L) was considered deficient as recommended [4, 13] for morning fasting samples collected from non-pregnant women.
2.5. Comet assay
Alkaline single cell gel-electrophoresis was used to assess DNA single-strand breaks [27, 28] as described by Song and colleagues [20] with slight modifications. After centrifugation, an aliquot of the resuspended cell pellet was mixed with 0.5% low melting-point agarose (Thermo Fisher Scientific Inc., Hanover Park IL) dissolved in phosphate-buffered saline (Sigma-Aldrich, Inc., St. Louis, MO). In each well of the 3-well comet assay slides (Trevigen, Gaithersburg, MD), 50 µL of the cell-agarose mixture was pipetted and the slides were stored on ice for 15 min.
The prepared comet slides were then placed in ice-cold lysis buffer (10% DMSO, Sigma-Aldrich, Inc., St. Louis, MO) in Trevigen lysis solution (Trevigen Inc., Gaithersburg, MD) to allow DNA to denature for 1 hr. The slides were placed in alkaline buffer (0.3 M NaOH/1 mM EDTA) in an electrophoresis chamber and stored for another 20 min. The alkalized cells were electrophoresed at 300 mA (constant current) for 30 min using ice cold buffer. Fourteen slides from a mixture of baseline and endpoint slides from both the placebo and zinc-supplemented groups were electrophoresed simultaneously. After electrophoresis the alkali buffer was neutralized with a 0.4 M Tris buffer, pH 7.5 (Trizma Base, Sigma-Aldrich, Inc., St. Louis, MO) applied to the slides three times for 5 min each. The slides were then immersed 5 min in ice-cold methanol, followed by 5 min in ice-cold ethanol, and then air-dried for storage at room temperature. Slides were stained (ethidium bromide 20 µg/mL) and visualized using fluorescence microscopy (Nikon E400, Nikon Instruments Inc. Melville, NY).
Results were analyzed using software designed for comet analysis (III software, Perceptive Instruments, Haverhill, UK) and investigators were blinded to the treatments. Images of 150 randomly selected nuclei (~50 nuclei per well in triplicate) were recorded and comet measurements were determined for further analysis. The tail moment is calculated by expressing the percentage of migrated DNA in the tail multiplied by the displacement of the tail center of mass relative to the center of the head. This parameter, which is compiled from several comet measurements, is most commonly used for reporting comet assay results [20, 27].
2.6 Analysis of α-1-acid glycoprotein (AGP)
Plasma AGP was quantified using an ELISA kit (GenWay Biotech, Inc., San Diego, CA) to assess chronic inflammation. The calibrators, quality control material (Kentrol, Kent Laboratories, Inc. Bellingham, WA) and plasma samples were added to the coated wells. The 96-well plate was then read at 450 nm (Synergy HT, BioTek Instruments, Inc., Winooski, VT). The standard curve was calculated with a four parameter logistic curve fit (GraphPad Software, Inc., La Jolla, CA) and the AGP concentrations in controls and plasma were interpolated from the standard curve. The controls lay within the expected range.
2.7. Statistical analyses
Statistical analyses were conducted using SAS, v. 9.3 (SAS Institute, Inc., Cary, NC, USA). Sample characteristics at baseline of the two groups were compared using two-tailed student’s t-tests. Pearson’s correlation coefficients were calculated using the PROC CORR procedure, with p<0.05 considered significant. Treatment effects on plasma zinc and the comet assay tail moment were analyzed as repeated measures (PROC MIXED) with an autoregressive period 1 error structure. The simple effect of treatment as a function of time (SLICE option in an LSMEANS statement) was used to identify significant changes from baseline with supplementation using SAS, v. 9.3 (SAS Institute, Inc., Cary, NC, USA). Results are expressed as means ± standard error of the mean (SEM). Differences with p<0.05 were considered significant.
3. Results
3.1. Subject characteristics
Participant characteristics are summarized in Table 1. Mean (± SEM) age was 32.3 ± 1.2 years and mean BMI was 20.6 ± 0.3 kg/m2. There were no significant differences in measured characteristics of the placebo and zinc-supplemented groups at baseline.
Table 1.
Subject characteristics of non-pregnant women participants from Finchawa, southern Ethiopia1
| Characteristics | Mean ± SEM | Range |
|---|---|---|
| Age (y) | 32.3 ± 1.2 | 20.0 – 45.0 |
| Weight (kg) | 51.3 ± 1.2 | 33.5 – 68.0 |
| Height (m) | 1.58 ± 0.01 | 1.43 – 1.78 |
| BMI (wt(kg)/ht(m)2) | 20.6 ± 0.3 | 16.5 – 24.1 |
| Education (y) | 1.3 ± 0.4 | 0 – 10 |
Values are means ± SEM, n = 35
3.2. Plasma zinc and AGP concentrations
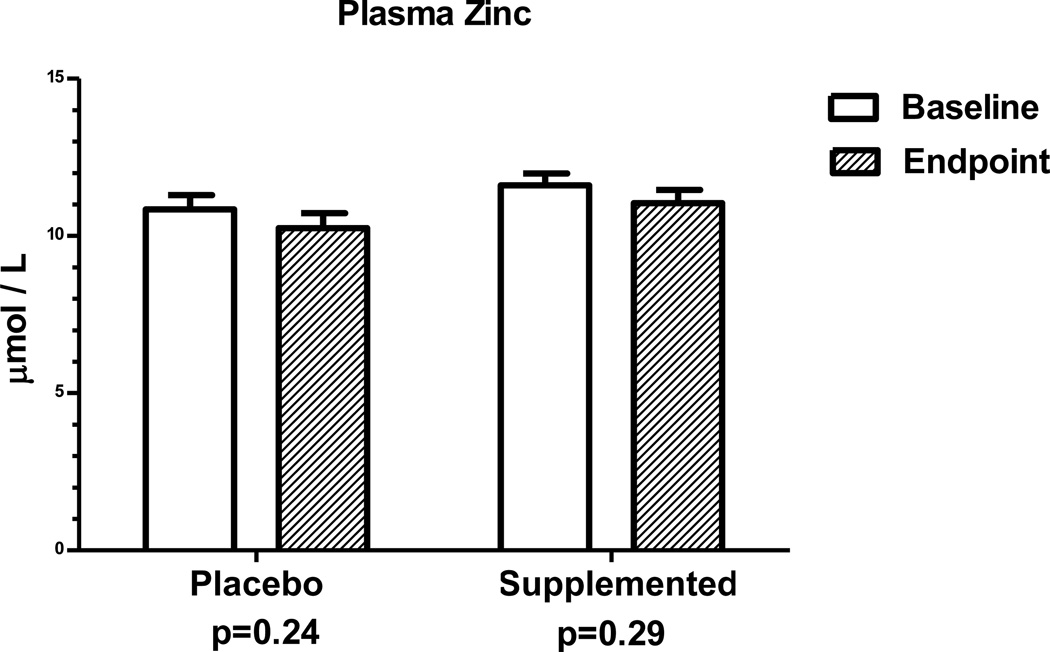
Mean plasma zinc concentration at baseline was 11.2 ± 0.3 µmol/L (734 ±20 µg/dL) and 37% of the women had values below 10.7 µmol/L. Plasma zinc concentrations of baseline and endpoint samples from both groups were not statistically different from each other (Figure 1). Also, no woman had plasma α-1-acid glycoprotein (AGP) concentrations above 1 g/L which would have indicated chronic inflammation (data not shown).
Figure 1.
Comparison in Ethiopian women of plasma zinc concentrations at baseline and at endpoint after daily supplementation for 17 days with 20 mg zinc as zinc sulfate or placebo. For zinc supplemented-group, n=17. For placebo, n=18.
3.3. Comet assay measurements
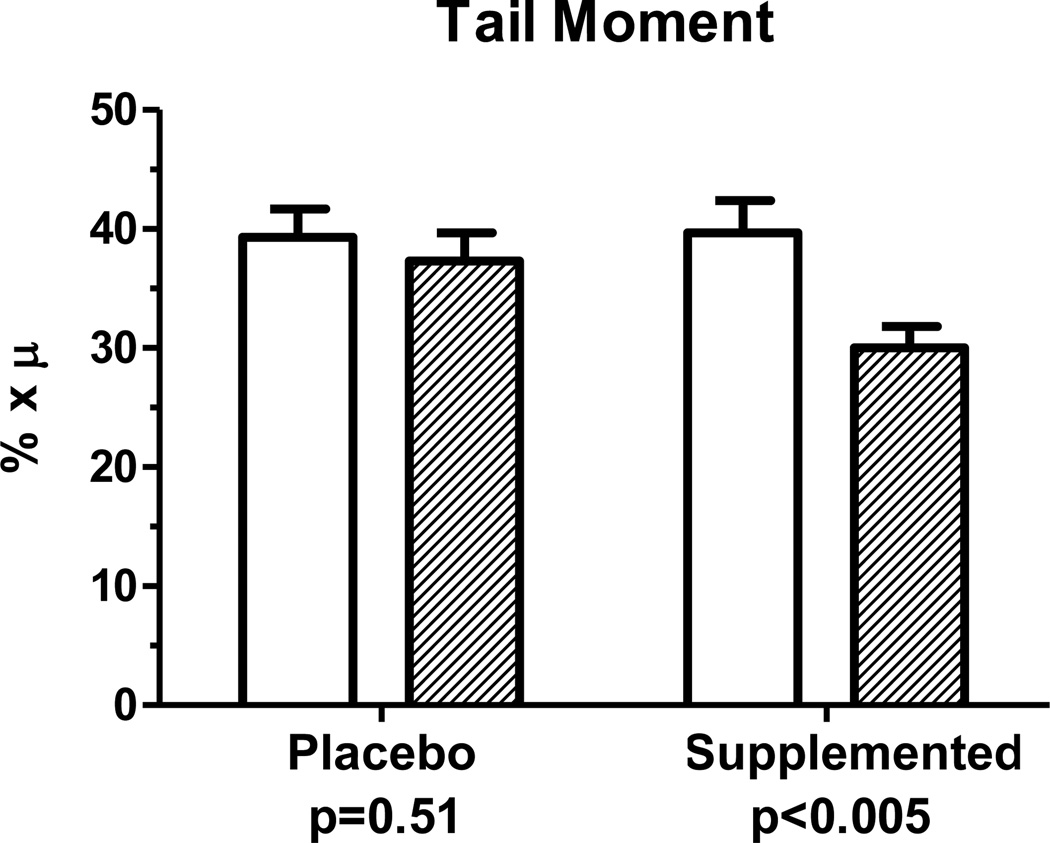
Multiple morphological measures were evaluated in the comet assay samples and used to calculate the tail moment measure. There were no significant differences in the calculated tail moment between baseline and endpoint samples of the placebo group (p = 0.51) while the 17 days of 20 mg zinc supplementation reduced (p<0.01) the comet tail moment (Figure 2).
Figure 2.
Comparison in Ethiopian women of comet tail moment measurements at baseline and at endpoint after daily supplementation for 17 days with 20 mg zinc as zinc sulfate or placebo. For zinc supplemented-group, n=17. For placebo, n=18.
3.4 Relations between plasma zinc concentrations and comet tail moment measurements
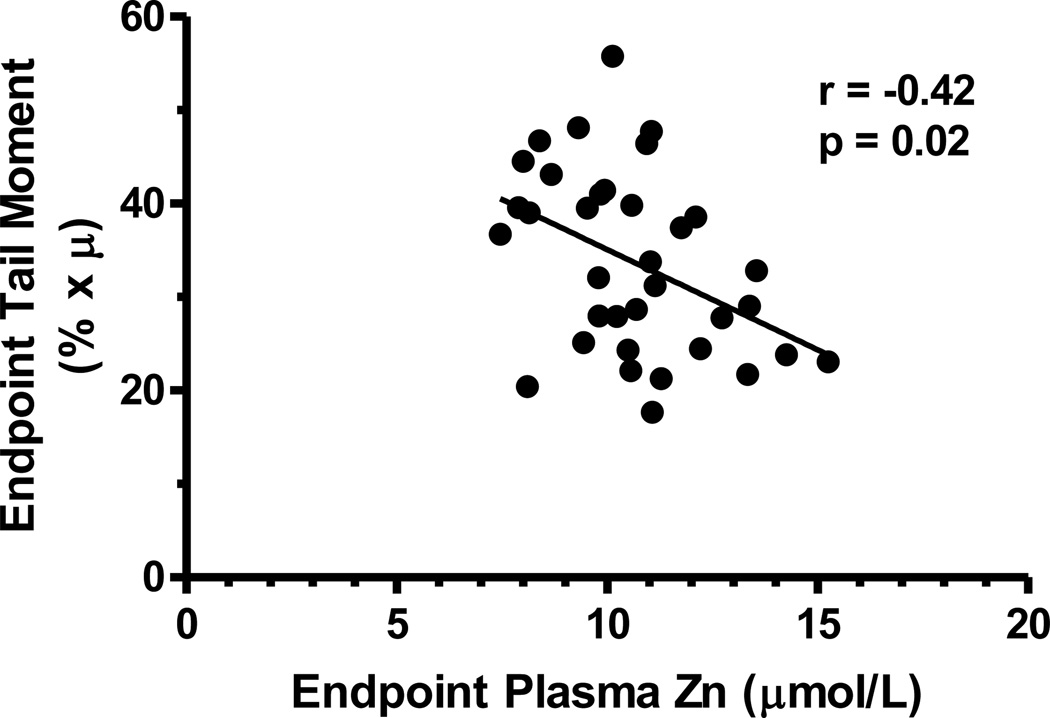
Plasma zinc concentrations at baseline were not correlated with baseline comet tail moments (r=−0.06, p=0.73). However, at endpoint plasma zinc concentration and tail moment were negatively correlated (r= −0.42, p<0.02) (Figure 3).
Figure 3.
Correlation in Ethiopian women of tail moment measurements and plasma zinc concentrations after daily supplementation for 17 days with 20 mg zinc as zinc sulfate or placebo. For zinc supplemented-group, n=17. For placebo, n=18.
4. Discussion
The present study of non-pregnant women of reproductive age in rural Ethiopia found a 37% prevalence of plasma zinc concentrations less than the generally accepted cutoff value of 10.7 µmol/L. In peripheral blood samples from these presumably chronically zinc-depleted women, DNA strand breaks as measured with the comet assay were significantly reduced in the zinc-supplemented group confirming our hypotheses.
The prevalence of zinc deficiency was estimated in 2004 to be 28.2% in Sub-Saharan Africa [4, 31]. Recently published analyses have reduced that estimate of inadequate dietary zinc intakes to 25.6% for Sub-Saharan Africa and have suggested a still lower percentage of deficiency in Ethiopia based on national data [32]. However, Ethiopian national food balance sheets may overestimate the amount of bioavailable zinc consumed by women in subsistence farming households in regions where neither teff nor animal source foods are frequently consumed [29]. A meta-analysis of available studies published in 2010 found that both pregnant and non-pregnant women in Africa are unlikely to meet the recommended nutrient intake levels for zinc, suggesting that fortification or supplementation should target zinc along with iron and folate [33].
Although reports of plasma or serum zinc concentrations throughout Ethiopia have not been uniform [34], two studies of pregnant women in southern Ethiopia have reported high prevalence of zinc deficiency based on the guidelines for pregnant women which allow for the expected hemodilution of pregnancy. A study of 100 women in the third trimester of pregnancy classified more than 70% as zinc deficient (mean plasma zinc of 7.0 µmol/L), while a study of 700 pregnant women conducted in southern Ethiopia in 2011 found 53% to be deficient (mean serum zinc of 8.0 µmol/L) based on the cutoff for their trimester of pregnancy [29, 35, 36].
Our intervention study was conducted with non-pregnant women of reproductive age, but the sample was drawn from rural women with similar dietary patterns as the reports cited above for pregnant Ethiopian women. In the current study 37% of women had plasma zinc concentrations below the lower cut-off for deficiency of 10.7 µmol/L for non-pregnant, non-fasting women. With a mean plasma zinc concentration of only 11.2 µmol/L, this group of women would be presumed to be at substantial risk for zinc deficiency [4].
Assessment of zinc status remains a challenge and methods that reflect zinc levels in tissues are needed. While plasma zinc of healthy individuals typically increases with supplementation [12, 20, 37], there is little information available on the impact of zinc supplementation in chronically zinc-depleted adults. Plasma zinc comprises a very small percentage of the total body zinc, and plasma zinc may not be a priority pool for repletion in chronically deficient adults. The plasma zinc values in the current study provide an example of the problem with using zinc concentration in the plasma as an indicator of zinc status. The mean plasma zinc concentration of the supplemented group did not change significantly despite supervised intake of the zinc supplement.
Due to zinc’s essential role in maintaining DNA integrity, the comet assay may be a useful tool to assess cellular impacts of alterations in zinc intake and possibly zinc status. Indeed, in an animal model of zinc depletion and repletion, zinc influenced DNA integrity in blood cells [38]; and, oxidative stress produced by zinc deficiency in a human lung fibroblast cell model was associated with increased DNA damage as measured by the comet assay [16]. Zinc deficiency not only affects DNA damage and repair, but also contributes to enhanced cellular oxidative stress. Research has demonstrated that changes in the comet tail moment were significantly correlated to changes in zinc intake in humans as demonstrated in a depletion and repletion study in young men [20] and in dietary restriction in animal models [38]. In our sample the tail moment of the supplemented group decreased significantly by the end of the 17 day experimental period suggesting decreased DNA breaks and presumably improvement in adequacy of intracellular zinc. In the placebo group 50% of the women had lower tail moment values at endpoint while tail moment at endpoint was lower in 76% of the zinc-supplemented women. Furthermore, the tail moment at endpoint was negatively correlated with endpoint plasma zinc concentrations which means that higher plasma zinc concentrations were associated with less DNA strand breakage.
In terms of methodological considerations for use of the comet assay in a field setting, precautions should be taken to provide the most stable post-sampling conditions possible in order to limit the potential to induce artificial DNA breaks [39]. Despite the fact that peripheral blood samples collected in the field were hauled over rough roads, shipped internationally, and not analyzed immediately after collection, we demonstrated that it is possible to obtain results from a field study consistent with improved cellular zinc levels due to zinc supplementation. Regardless of the storage and transport conditions in our study, all samples were handled similarly; thus, the decrease in DNA breaks in the supplemented group, without significant change in the placebo group, demonstrated a positive effect of zinc supplementation.
Strengths of the current study are that the comet assay may represent a functional biomarker of zinc status and that the study was conducted in an area with widespread chronic zinc depletion. Limitations of this study are the relatively small sample size and short supplementation period. Based on this study, a larger sample and improved transport and storage conditions should be arranged. In addition, a general limitation of the comet assay is that at least three other nutrient deficiencies are likely to affect the comet assay: folate, niacin and choline. Our previous analyses have indicated that most Ethiopian women have adequate dietary and circulating folate, but dietary niacin is likely to be low with the maize-based diets which could affect the assay. Dietary choline intakes have not been analyzed. Also, other environmental exposures such as indoor cooking over charcoal might lead to increased DNA strand breaks. Additional research, including screening for limiting nutrients and environmental contaminants, is warranted to further assess the usefulness of the comet assay as a functional biomarker for zinc.
In conclusion, this study demonstrates that the comet assay is a useful tool to identify changes in zinc status. The zinc supplementation trial also revealed that plasma zinc concentrations did not change significantly after a 17 day supplementation with 20 mg of zinc despite the relatively low dietary intakes of bioavailable zinc in the study area. The comet assay appeared to be sensitive enough to detect changes in zinc status as a result of supplementation even when there were no significant changes in plasma zinc.
Supplementary Material
Description of the components of a comet.
Acknowledgment
We thank the women who participated in the study and acknowledge the invaluable technical support of Meron Girma and other research personnel at Hawassa University. This research was supported by the Swiss National Science Foundation (PBSKP3-124358), NIH Grant R01HD053053 (NICHD & Fogarty International Center), Hawassa University, Oregon Agricultural Experiment Station, and the Oklahoma Agricultural Experiment Station. Sponsoring organizations had no involvement in any aspects of the study.
Abbreviations
- ADP
adenosine diphosphate
- AGP
α-1-acid glycoprotein
- APE
apurinic endonuclease
- BMI
body mass index
- DNA
deoxyribonucleic acid
- DMSO
dimethylsulfoxide
- EDTA
ethylenediaminetetraacetic acid
- ICPMS
inductively-coupled plasma mass spectrometry
- IZiNCG
International Zinc Nutrition Consultative Group
- NaOH
sodium hydroxide
- p53
protein 53 or tumor protein 53
- PARP1
poly (ADP-ribose) polymerase 1
- RCT
randomized control trial
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Maya L. Joray, Email: mjoray@gmail.com.
Tian-Wei Yu, Email: david.yu@oregonstate.edu.
Emily Ho, Email: Emily.Ho@oregonstate.edu.
Stephen L. Clarke, Email: Stephen.clarke@okstate.edu.
Zeno Stanga, Email: zeno.stanga@insel.ch.
Tafere Gebreegziabher, Email: taferege@yahoo.com.
K. Michael Hambidge, Email: Michael.hambidge@ucdenver.com.
Barbara J. Stoecker, Email: Barbara.stoecker@okstate.edu.
References
- 1.Prasad AS. Discovery and importance of zinc in human nutrition. Fed Proc. 1984;43:2829–2834. [PubMed] [Google Scholar]
- 2.Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15:572–578. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Gibson RS. Zinc: the missing link in combating micronutrient malnutrition in developing countries. Proc Nutr Soc. 2006;65:51–60. doi: 10.1079/pns2005474. [DOI] [PubMed] [Google Scholar]
- 4.International Zinc Nutrition Consultative Group (IZiNCG) Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lönnerdal B, Ruel MT, Sandström B, Wasantwisut E, Hotz C. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004 Mar;25(1 Suppl 2):S99–203. [PubMed] [Google Scholar]
- 5.Black RE. Zinc deficiency, infectious disease and mortality in the developing world. J Nutr. 2003;133:1485S–1489S. doi: 10.1093/jn/133.5.1485S. [DOI] [PubMed] [Google Scholar]
- 6.Oberleas D, Harland BF. Phytate content of foods: effect on dietary zinc bioavailability. J Am Diet Assoc. 1981;79:433–436. PMID 7288050. [PubMed] [Google Scholar]
- 7.Hotz C, Gibson RS. Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J Nutr. 2007;137:1097–1100. doi: 10.1093/jn/137.4.1097. [DOI] [PubMed] [Google Scholar]
- 8.Sreenivasulu K, Raghu P, Ravinder P, Nair KM. Effect of dietary ligands and food matrices on zinc uptake in Caco-2 cells: implications in assessing zinc bioavailability. J Agric Food Chem. 2008;56:10967–10972. doi: 10.1021/jf802060q. [DOI] [PubMed] [Google Scholar]
- 9.Abebe Y, Bogale A, Hambidge KM, Stoecker BJ, Bailey K, Gibson RS. Phytate, zinc, iron and calcium content of selected raw and prepared foods consumed in rural Sidama, Southern Ethiopia, and implications for bioavailability (electronic resource) J Food Comp Anal. 2007;20:161–168. http://dx.doi.org/10.1016/j.jfca.2006.09.003. [Google Scholar]
- 10.Miller LV, Krebs NF, Hambidge KM. Mathematical model of zinc absorption: effects of dietary calcium, protein and iron on zinc absorption. Br J Nutr. 2013;109:695–700. doi: 10.1017/S000711451200195X. PMID 22617116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson RS, Ferguson EL. Assessment of dietary zinc in a population. Am J Clin Nutr. 1998;68:430S–434S. doi: 10.1093/ajcn/68.2.430S. [DOI] [PubMed] [Google Scholar]
- 12.Lowe NM, Medina MW, Stammers A-L, Patel S, Souverein OW, Dullemeijer C, Serra-Majem L, Nissensohn M, Hall Moran V. The relationship between zinc intake and serum/plasma zinc concentration in adults: a systematic review and dose-response meta-analysis by the EURRECA Network. Br J Nutr. 2012;108:1962–1971. doi: 10.1017/S0007114512004382. PMID: 23244547. [DOI] [PubMed] [Google Scholar]
- 13.Hess SY, Peerson JM, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull. 2007;28:S403–S429. doi: 10.1177/15648265070283S303. [DOI] [PubMed] [Google Scholar]
- 14.Ali AAE, Timinszky G, Arribas-Bosacoma R, Kozlowski M, Hassa PO, Hassler M, Ladurner AG, Pearl LH, Oliver AW. The zinc-finger domains of PARP1 cooperate to recognize DNA strand breaks. Nat Struct Mol Biol. 2012;19:685–692. doi: 10.1038/nsmb.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharif R, Thomas P, Zalewski P, Fenech M. Zinc deficiency or excess within the physiological range increases genome instability and cytotoxicity, respectively, in human oral keratinocyte cells. Genes Nutr. 2012;7:139–154. doi: 10.1007/s12263-011-0248-4. PMID: 21935692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho E, Courtemanche C, Ames BN. Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J Nutr. 2003;133:2543–2548. doi: 10.1093/jn/133.8.2543. [DOI] [PubMed] [Google Scholar]
- 17.Ho E, Ames BN. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc Natl Acad Sci USA. 2002;99:16770–16775. doi: 10.1073/pnas.222679399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oteiza PI. Zinc and the modulation of redox homeostasis. Free Radic Biol Med. 2012;53:1748–1759. doi: 10.1016/j.freeradbiomed.2012.08.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan M, Song Y, Wong CP, Hardin K, Ho E. Zinc deficiency alters DNA damage response genes in normal human prostate epithelial cells. J Nutr. 2008;138:667–673. doi: 10.1093/jn/138.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y, Chung CS, Bruno RS, Traber MG, Brown KH, King JC, Ho E. Dietary zinc restriction and repletion affects DNA integrity in healthy men. Am J Clin Nutr. 2009;90:321–328. doi: 10.3945/ajcn.2008.27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 22.Singh NP, Tice RR, Stephens RE, Schneider EL. A microgel electrophoresis technique for the direct quantitation of DNA damage and repair in individual fibroblasts cultured on microscope slides. Mutat Res. 1991;252:289–296. doi: 10.1016/0165-1161(91)90008-v. [DOI] [PubMed] [Google Scholar]
- 23.Ostling O, Johanson KJ. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 1984;123:291–298. doi: 10.1016/0006-291x(84)90411-x. [DOI] [PubMed] [Google Scholar]
- 24.Sliwinski T, Czechowska A, Kolodziejczak M, Jajte J, Wisniewska-Jarosinska M, Blasiak J. Zinc salts differentially modulate DNA damage in normal and cancer cells. Cell Biol Int. 2009;33:542–547. doi: 10.1016/j.cellbi.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Baumeister P, Huebner T, Reiter M, Schwenk-Zieger S, Harréus U. Reduction of oxidative DNA fragmentation by ascorbic acid, zinc and N-acetylcysteine in nasal mucosa tissue cultures. Anticancer Res. 2009;29:4571–4574. [PubMed] [Google Scholar]
- 26.Betancourt M, Ortiz R, González C, Pérez P, Cortés L, Rodríguez L, Villaseñor L. Assessment of DNA damage in leukocytes from infected and malnourished children by single cell gel electrophoresis/comet assay. Mutat Res. 1995;331:65–77. doi: 10.1016/0027-5107(95)00052-k. [DOI] [PubMed] [Google Scholar]
- 27.Singh NP, Stephens RE. Microgel electrophoresis: sensitivity, mechanisms, and DNA electrostretching. Mutat Res. 1997;383:167–175. doi: 10.1016/s0921-8777(96)00056-0. [DOI] [PubMed] [Google Scholar]
- 28.Hartmann A, Agurell E, Beevers C, Brendler-Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V, Tice RR. Recommendations for conducting the in vivo alkaline comet assay. 4th International Comet Assay Workshop. Mutagenesis. 2003;18:45–51. doi: 10.1093/mutage/18.1.45. [DOI] [PubMed] [Google Scholar]
- 29.Abebe Y, Bogale A, Hambidge KM, Stoecker BJ, Arbide I, Teshome A, Krebs NF, Westcott JE, Bailey KB, Gibson RS. Inadequate intakes of dietary zinc among pregnant women from subsistence households in Sidama, Southern Ethiopia. Public Health Nutr. 2008;11:379–386. doi: 10.1017/S1368980007000389. [DOI] [PubMed] [Google Scholar]
- 30.Hambidge KM, Abebe Y, Gibson RS, Westcott JE, Miller LV, Lei S, Stoecker BJ, Arbide I, Teshome A, Bailey KB, Krebs NF. Zinc absorption during late pregnancy in rural southern Ethiopia. Am J Clin Nutr. 2006;84:1102–1106. doi: 10.1093/ajcn/84.5.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wuehler SE, Peerson JM, Brown KH. Use of national food balance data to estimate the adequacy of zinc in national food supplies: methodology and regional estimates. Public Health Nutr. 2005;8:812–819. doi: 10.1079/phn2005724. [DOI] [PubMed] [Google Scholar]
- 32.Wessells KR, Brown KH. Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS One. 2012;7:e50568. doi: 10.1371/journal.pone.0050568. Epub 2012 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torheim LE, Arimond M, Penrose K, Ferguson EL. Women in resource-poor settings are at risk of inadequate intakes of multiple micronutrients. J Nutr. 2010;140:2051S–2058S. doi: 10.3945/jn.110.123463. [DOI] [PubMed] [Google Scholar]
- 34.Haidar J, Umeta M, Kogi-Makau W. Effect of iron supplementation on serum zinc status of lactating women in Addis Ababa, Ethiopia. East Afr Med J. 2005;82:349–352. [PubMed] [Google Scholar]
- 35.Stoecker BJ, Abebe Y, Hubbs-Tait L, Kennedy TS, Gibson RS, Arbide I, Teshome A, Westcott J, Krebs NF, Hambidge KM. Zinc status and cognitive function of pregnant women in Southern Ethiopia. Eur J Clin Nutr. 2009;63:916–918. doi: 10.1038/ejcn.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gebremedhin S, Enquselassie F, Umeta M. Prevalence of prenatal zinc deficiency and its association with socio-demographic, dietary and health care related factors in rural Sidama, Southern Ethiopia: A cross-sectional study. BMC Public Health. 2011;11:898–898. doi: 10.1186/1471-2458-11-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wessells KR, Jorgensen JM, Hess SY, Woodhouse LR, Peerson JM, Brown KH. Plasma zinc concentration responds rapidly to the initiation and discontinuation of short-term zinc supplementation in healthy men. J Nutr. 2010;140:2128–2133. doi: 10.3945/jn.110.122812. [DOI] [PubMed] [Google Scholar]
- 38.Song Y, Ho E, Traber MG, Leonard SW. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J Nutr. 2009;139:1626–1631. doi: 10.3945/jn.109.106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faust F, Kassie F, Knasmüller S, Boedecker RH, Mann M, Mersch-Sundermann V. The use of the alkaline comet assay with lymphocytes in human biomonitoring studies. Mutat Res. 2004;566:209. doi: 10.1016/j.mrrev.2003.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of the components of a comet.