Summary
Bacterial motilities participate in biofilm development. However, it is unknown how/if bacterial motility affects formation of the biofilm matrix. Psl polysaccharide is a key biofilm matrix component of Pseudomonas aeruginosa. Here we report that type IV pili (T4P)-mediated bacterial migration leads to the formation of a fibre-like Psl matrix. Deletion of T4P in wild type and flagella-deficient strains results in loss of the Psl-fibres and reduction of biofilm biomass in flow cell biofilms as well as pellicles at air-liquid interface. Bacteria lacking T4P-driven twitching motility including those that still express surface T4P are unable to form the Psl-fibres. Formation of a Psl-fibre matrix is critical for efficient biofilm formation, yet does not require flagella and polysaccharide Pel or alginate. The Psl-fibres are likely formed by Psl released from bacteria during T4P-mediated migration, a strategy similar to spider web formation. Starvation can couple Psl release and T4P-driven twitching motility. Furthermore, a radial-pattern Psl-fibre matrix is present in the middle of biofilms, a nutrient-deprived region. These imply a plausible model for how bacteria respond to nutrient-limited local environment to build a polysaccharide-fibre matrix by T4P-dependent, bacterial migration strategy. This strategy may have general significance for bacterial survival in natural and clinical settings.
Introduction
Biofilms (surface-associated bacterial communities) are found in virtually every habitat. In natural and clinical settings, biofilms enhance survival, enabling organisms to adapt to changing conditions collectively instead of as single cells (Costerton et al., 1995). Biofilm growth is also correlated with starvation-induced tolerance to antibiotics in vivo (Costerton et al., 1999; Lewis, 2007; Nguyen et al., 2011). Bacterial communities mainly rely on extracellular matrix to enmesh bacteria and maintain biofilm structure and integrity (Sutherland, 2001; Whitchurch et al., 2002a; Matsukawa and Greenberg, 2004; Friedman and Kolter, 2004a; Ma et al., 2006; 2009; Kolodkin-Gal et al., 2012). Bacterial motility was reported to contribute to biofilm architecture and development (O’Toole and Kolter, 1998; Klausen et al., 2003a; Shrout et al., 2006; 2011; Conrad et al., 2011). However, it is not clear whether bacterial motility is involved in formation of the biofilm matrix and if there is any link between bacterial migration and biofilm matrix formation.
Exopolysaccharides are a key biofilm matrix component of many Gram-positive and -negative bacteria, as they contribute to the overall biofilm architecture and resistance (Mah et al., 2003; Matsukawa and Greenberg, 2004; Friedman and Kolter, 2004a; Ma et al., 2006; 2009; Flemming and Wingender, 2010; Colvin et al., 2011; Yang et al., 2011; Kolodkin-Gal et al., 2012). Pseudomonas aeruginosa, a Gram-negative environmental bacterium, is a model organism for studying biofilms. It is also an important opportunistic pathogen that can cause lethal acute and chronic persistent infections in cystic fibrosis (CF) patients and individuals with a compromised immune system. P. aeruginosa can form biofilms on a variety of abiotic and biotic surfaces such as the mucus plugs of the CF lung, contaminated catheters and contact lenses (Davies et al., 1998; Singh et al., 2000; Willcox, 2007). It can also form pellicles at the air-liquid interface of standing cultures as does the Gram-positive bacterium Bacillus subtilis (Friedman and Kolter, 2004b; Kolodkin-Gal et al., 2010). Three exopolysaccharides, alginate, Psl and Pel, are involved in P. aeruginosa biofilm formation (Hentzer et al., 2001; Friedman and Kolter, 2004b). P. aeruginosa cells often become mucoid upon prolonged colonization of the CF lung. The mucoid phenotype is due to the overproduction of alginate that provides an advantage for P. aeruginosa in the airway of CF patients (Govan and Deretic, 1996). Pel is a glucose-rich exopolysaccharide, which is required to form air-liquid interface biofilms (pellicles) (Friedman and Kolter, 2004b). Psl is a repeating pentasaccharide containing d-mannose, d-glucose and l-rhamnose (Byrd et al., 2009). This exopolysaccharide is an essential matrix component for non-mucoid and mucoid P. aeruginosa to initiate and maintain biofilms (Matsukawa and Greenberg, 2004; Ma et al., 2006; 2009; 2012; Yang et al., 2012). Data from Yang and colleagues suggest that Psl is more important than Pel in P. aeruginosa PAO1 biofilm microcolony formation and antibiotic resistance (Yang et al., 2011). Our previous studies showed that Psl is arranged in a helical pattern on the bacterial surface and forms fibre-like strands that connect each other to enmesh bacteria in a biofilm (Ma et al., 2009). Overproduction of Psl enhances bacteria-bacteria interaction and results in more mushroom-shaped biofilm structure (MSBS) in a flow cell chamber (Ma et al., 2006). However, it is not clear how the fibre-like Psl strands form, and whether the Psl-fibre strands are important to build the matrix and biofilm structure. It is also unknown whether the formation of the Psl-fibre matrix depends on other exopolysaccharides of P. aeruginosa.
It is reported that bacterial motility affects biofilm formation and architecture (O’Toole and Kolter, 1998; Chiang and Burrows, 2003; Klausen et al., 2003a; Shrout et al., 2006; 2011). Bacteria can move on surfaces via distinct appendage-specific motility modes. Flagella mediate bacterial swimming and surface-bound spinning (Conrad et al., 2011). Flagella and type IV pili (T4P) mediate swarming, a motility mode used for colony expansion along a semisolid surface (Kohler et al., 2000). T4P also mediate twitching that is commonly observed in dense aggregates with cell-to-cell contact (Whitchurch, 2006). The T4P on the bacterial cell works by extension and retraction of the pili to pull the cell forward (Skerker and Berg, 2001; Bertrand et al., 2010). In P. aeruginosa, the polar flagella and T4P are both located at the same cell pole, but drive bacteria in opposite directions (Conrad et al., 2011). Flagella and T4P-mediated motility are both important to build a biofilm structure in vitro (Klausen et al., 2003a,b). T4P are important for the formation of the MSBS cap (Barken et al., 2008). Barken and colleagues suggested that T4P might function as niche-specific adhesins/matrix components in P. aeruginosa biofilms (Barken et al., 2008). O’Toole and colleagues have also reported that T4P play an important role in microcolony formation while flagella have a role in the initial cell-to-surface interaction (O’Toole and Kolter, 1998). In Myxobacteria, extracellular polysaccharides are an anchor for T4P, triggering pili retraction and enabling social motility (Mauriello et al., 2010). In P. aeruginosa, it is unclear whether there is a link between bacteria motility and exopolysaccharide.
In this study, we use non-destructive in situ detection technique to investigate whether T4P/flagella and their directed motility affect the exopolysaccharide matrix in P. aeruginosa biofilms, how a fibre-like Psl matrix is formed, and the contribution of the Psl-fibres to biofilm formation. Our data show that T4P-mediated bacterial migration is critical for the formation of a Psl-fibre matrix in biofilms and reveal that the Psl-fibre matrix is formed through a strategy similar to the spider web formation. We also provide a fundamental and significant understanding about how bacteria respond to nutrient-poor environments to maintain its community structure.
Results
The fibre-like Psl polysaccharide strands in the biofilms of P. aeruginosa PAO1-derived strains
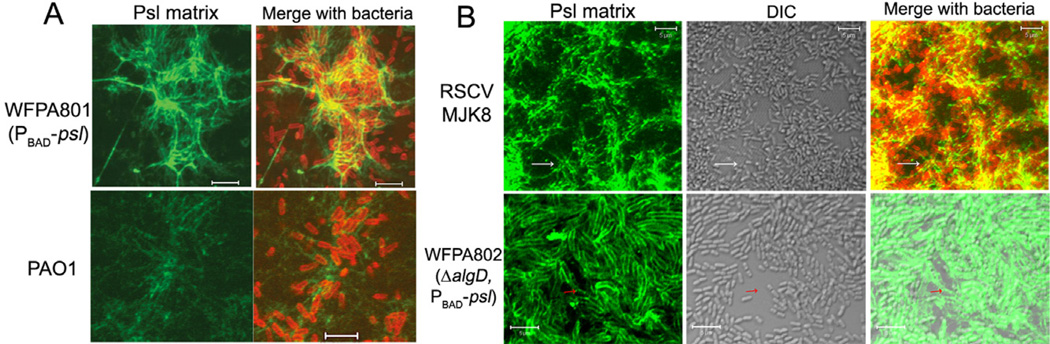
Our previous study showed that P. aeruginosa can form fibre-like Psl strands enmeshing bacteria within a biofilm (Ma et al., 2009). The Psl-fibre strands were present in the biofilm of both P. aeruginosa wild type strain PAO1 and Psl-inducible strain WFPA801 and most clearly visualized at the early stages of biofilm development (Figs 1A and S1). The Psl-fibres with a strong fluorescent signal were observed in the biofilm of WFPA801, surrounding the multiple-cell aggregates, connecting microcolonies, and forming a spider web-like matrix (Fig. S1). Strikingly, many Psl-fibres were present at areas where there were few bacteria (indicated by arrows in Fig. 1A and Fig. S1). Thus, these Psl-fibre strands did not appear to be formed by bacterial cell–cell interaction as we assumed previously (Ma et al., 2009). To investigate whether the similar phenomenon is present in other PAO1-derived strains, and whether the other polysaccharides produced by P. aeruginosa affect the formation of the Psl-fibre matrix, we examined a rugose small-colony variant (RSCV) derived from a laboratory-grown biofilm of PAO1, MJK8 and its isogenic pel and psl mutants (Table 1) (Kirisits et al., 2005). Growth of P. aeruginosa in biofilms and chronic CF airway infections produces RSCVs (Starkey et al., 2009). Both clinical and in vitro derived biofilm RSCVs display increased production of the Pel and Psl polysaccharides and an elevated capacity to form biofilms (Starkey et al., 2009). Psl staining showed that the fibrelike Psl was also found in the biofilm of MJK8 and was clearly visualized at the area with few bacterial cells (Fig. 1B, white arrow). No staining was present in the MJK8 psl mutant (Fig. S2). The Psl matrix in the biofilms of MJK8 and MJK8Δpel was indistinguishable (Fig. S2), indicating that formation of the Psl-fibre matrix is independent of Pel.
Fig. 1.
Spider-web-like Psl-fibre matrixes in the biofilms of a variety of P. aeruginosa PAO1-derived strains. Shown are biofilms of PAO1 (A), Psl-inducible strain WFPA801 (A), RSCV MJK8 (B) and ΔalgD Psl-inducible strain WFPA802 (B) after 22 h of growth under flow condition and stained with HHA-FITC (green, Psl matrix) and FM4-64 (red, bacteria membrane stain). Grey images are corresponding differential interference contrast (DIC) image of biofilms. Merged images were either merge of Psl matrix (green) with FM4-64 labelled biofilm (red) or with DIC image (lower right image in B). Arrows indicate the Psl-fibres located on the area with few bacteria. Scale bar: 2 µm for biofilm images of PAO1 and WFPA801, 5 µm for MJK8 and WFPA802.
Table 1.
The strains used in this study and their motility phenotype.
|
P. aeruginosa PAO1- derived strains |
Relevant characteristics | Swimminga | Twitchingb | Source/reference |
|---|---|---|---|---|
| PAO1 | Non-mucoid, wild type strain, | ++ | ++ | |
| MJK8 | Rugose small-colony variant, isolated from aged biofilm of PAO1. | − | − | Kirisits et al. (2005) |
| MJK8-Dpel | MJK8 Δpel | − | − | Kirisits et al. (2005) |
| MJK8-Dpsl | MJK8 Δpsl | + | ± | Kirisits et al. (2005) |
| WFPA801 | psl-inducible strain, PBAD-psl | ++ | ++ | Ma et al. (2006) |
| WFPA1 | ΔalgD, Tcr | ++ | ++ | Wozniak et al. (2003) |
| WFPA802 | ΔalgD, PBAD-psl, Tcr | ++ | ++ | This study |
| WFPA850 | ΔfliC, in frame no mark deletion strain | − | ++ | Byrd et al. (2010) |
| ΔfliMΔpilA | Gfp-tagged PAO1, ΔfliMΔpilA, | − | − | Klausen et al. (2003b) |
| ΔrelAΔspoT | relA and spoT double deletion mutant | ND | ND | Nguyen et al. (2011) |
| AWO | ΔpilA | ++ | − | Watson et al. (1996) |
| IMPA31 | In-frame deletion of pilH | ++ | + | This study |
| IMPA33 | In-frame deletion of pilT | + | − | This study |
| IMPA13 | ΔfliC, PBAD-psl | − | ++ | This study |
| IMPA34 | In-frame deletion of fliC and pilT | − | − | This study |
++, motility zone ≥ 20 mm; +, motility zone ≥ 10 mm; –, no motility zone/the zone similar to negative control.
++, motility zone ≥ 15 mm; +, 40–60% of wt motility zone; ±, < 40% of wt motility zone; –, no motility zone/the zone similar to negative control.
ND, not done.
To determine whether the formation of Psl-fibres required alginate, we constructed WFPA802, a Psl-inducible strain in an alginate synthesis deficient background (Table 1). The Psl-fibres were observed in the biofilm of WFPA802 (Fig. 1B) and the Psl matrix of WFPA802 (Fig. S2F) was similar to WFPA801 (Ma et al., 2009). In addition, the Psl matrix of an alginate-synthesis-deficient strain, WFPA1 (Fig. S2E) was also similar to that of the wild type strain PAO1 (Fig. S2D). Taken together, these data showed that RSCV MJK8, Pel-deficient RSCV, and alginate-deficient strains can form the Psl-fibres, indicating the formation of the Psl-fibre matrix does not require Pel polysaccharide or alginate.
The formation of Psl-fibre matrix depends on T4P, but not flagella
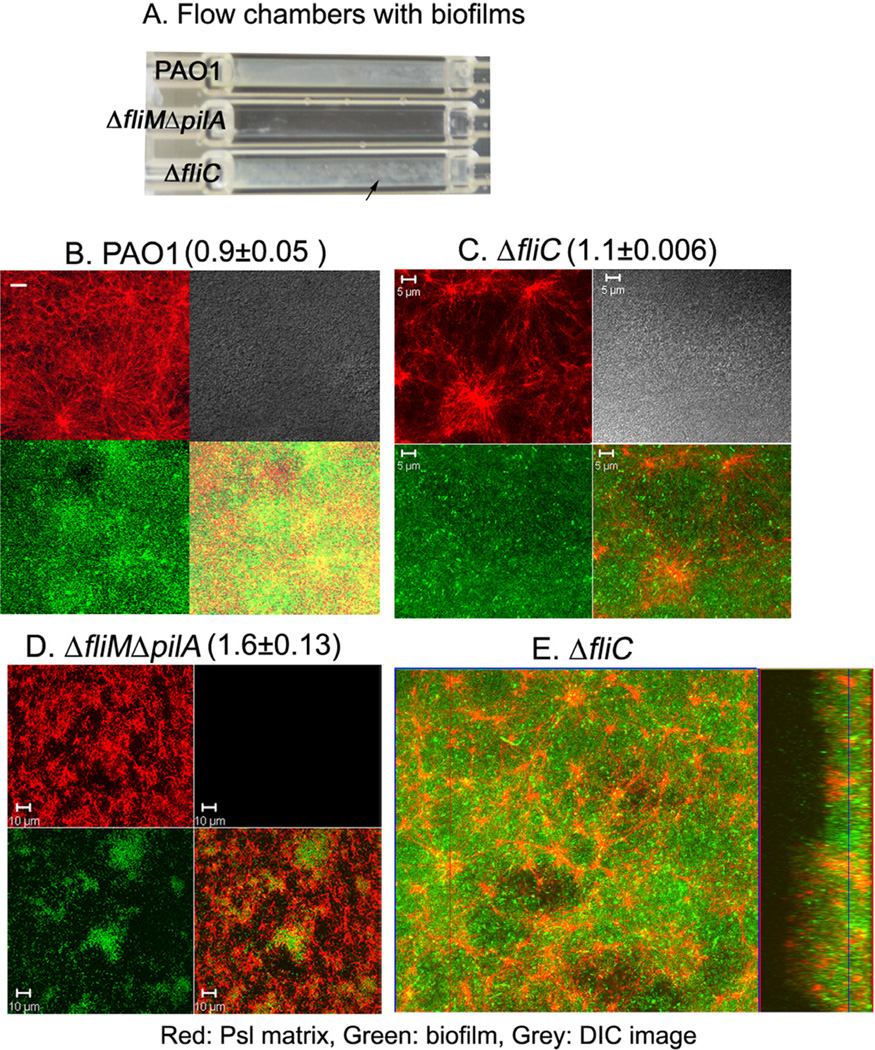
Previous reports showed both flagella and T4P were important for biofilm development (Klausen et al., 2003a). Recently, we found that non-motile mucoid strain FRD1 did not form the fibre-like Psl strands (Ma et al., 2012), tempting us to hypothesize that motility may be involved in the formation of the Psl-fibre. To test this, we first examined the Psl-fibre matrix formation of a PAO1-derived fliC (encodes flagellin, the main subunit of flagellum) deletion mutant, which lacks bacterial surface flagella and flagella-mediated motility (Klausen et al., 2003b). After 4-day growth in a flow chamber, the fliC mutant formed a similar flat biofilm (25 ± 5 µm thickness) as compared with that of wild type PAO1, which covered the entire chamber surface (Fig. 2A). However, biofilms formed by the fliC mutant had few MSBS but some irregular-shaped large macrocolonies were visible as white spots in the flow chamber (Fig. 2A, C and E). Strikingly, the fliC mutant showed extensive fibre-like Psl strands (Fig. 2C, red panel) forming a radial-pattern matrix with Psl-fibres coming out from a centre where there was concentrated Psl material. This radial Psl-fibre matrix was clearly observed in the middle of biofilm, connecting microcolonies, and continued for a few micrometers within a biofilm (Fig. 2E and Video S1). The similar radial matrix pattern could be observed in the biofilms of the wild type (Fig. 2B), but was more pronounced in the fliC mutant. This revealed that the generation of Psl-fibre matrix did not require flagella.
Fig. 2.
Type IV pili are necessary for the formation of the Psl-fibre matrix.
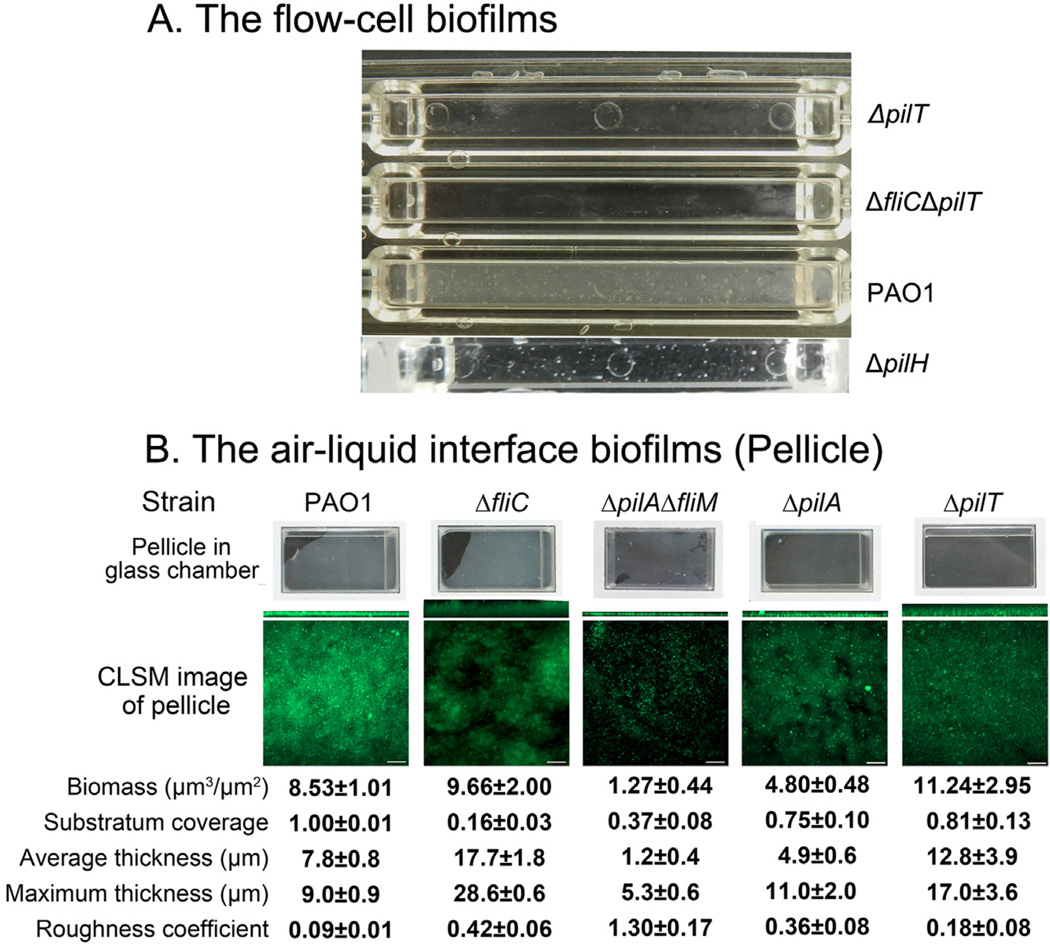
A. Photograph of a flow cell with the 4-day-old biofilms of strain PAO1, ΔfliMΔpilA and ΔfliC. The black arrow indicates the irregular-shaped large macrocolonies.
B–D. Shown are selected CLSM images of the flow cell biofilms (green) and the corresponding Psl matrix (red). Psl matrix was stained by HHA-TRITC (red). The bacteria in the ΔfliC biofilms were stained by SYTO9 (green) and the ΔfliMΔpilA mutant was labelled by GFP. The lower right images of each panel were merged image of green (bacteria in biofilms) and red (matrix).
E. A selected optical section image (large square) showed how the web-like radial pattern Psl-fibre matrix (red) enmeshed bacteria in the biofilm (green) of a ΔfliC strain. The blue line on the rectangle image shows the section located in the middle of biofilm, which is depicted in the large square. Scale bar: 5 µm for PAO1 and ΔfliC, 10 µm for ΔfliMΔpilA. The numbers with deviation above the images B–D indicated the Psl relative to biofilm biomass.
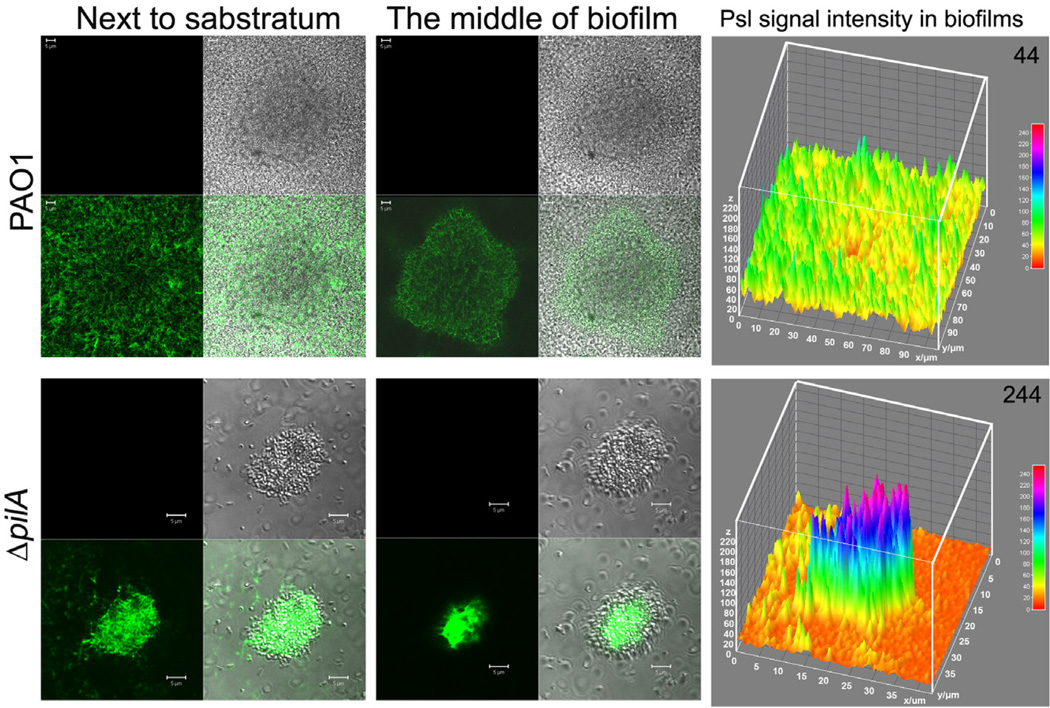
To investigate whether T4P or T4P-mediated bacterial migration is involved in the formation of the radial Psl-fibre matrix within the biofilms, we utilized a fliM and pilA double deletion mutant, which lacked T4P-mediated motility and flagella-mediated motility (Klausen et al., 2003b). After 4-day growth in a flow chamber, the biofilm of the ΔfliMΔpilA strain was mainly composed of thin multicellular aggregates (thickness approximately 10 µm) that were not readily observed in the flow chamber (Fig. 2A). Furthermore, no clear fibre-like Psl structures or typical biofilm matrix patterns were observed in the biofilm of the ΔfliMΔpilA strain (Fig. 2D). Loss of Psl fibres was not due to the reduction of Psl in the biofilm because there was more Psl in the biofilm of ΔfliMΔpilA than that of PAO1 and fliC mutant according to COMSTAT analysis (indicated by the number above each panel in Fig. 2B–D). To determine if mutation of T4P are sufficient to account for the loss of the fibre-like Psl structure, we examined the biofilm of ΔpilA strain since PilA is the major structural subunit of T4P and is essential for T4P-mediated twitching motility (Table 1) (Whitchurch, 2006). Compared with the biofilm of wild type PAO1 strain, ΔpilA bacteria formed a few microcolonies with concentrated Psl, which appeared fibre-less especially at the middle to the top of the microcolonies (Fig. 3). As pilA mutants cannot generate a Psl-fibre matrix, indicating the formation of a Psl-fibre matrix required T4P.
Fig. 3.
The biofilm microcolonies and Psl matrix of P. aeruginosa PAO1 and its isogenic ΔpilA mutant. Shown are selected optical sectioned images of PAO1 and ΔpilA biofilms stained by HHA-FITC after 3 days of growth in a flow cell. The lower right small square images at the left and middle panels are merged images of corresponding green (Psl) and DIC (biofilm) image. Scale bar, 5 µm. The right panel images depict the Psl fluorescence intensity in the corresponding biofilm. The average Psl signal intensity in per µm3 biofilm is shown at the upper right corner of the corresponding image.
In MSBS, the radial Psl-fibre matrix was located at the stalk area above the substratum (Fig. S3). This suggested that formation of the radial Psl-fibre matrix did not rely on the adherence on a solid surface. Consistent with this, we observed a similar radial pattern Psl-fibre matrix in an air-liquid interface biofilm (pellicle) of the fliC mutant grown under static conditions (Fig. 4). This indicated that the radial-pattern Psl-fibre matrix can be formed in the biofilm grown under either flow or static conditions.
Fig. 4.
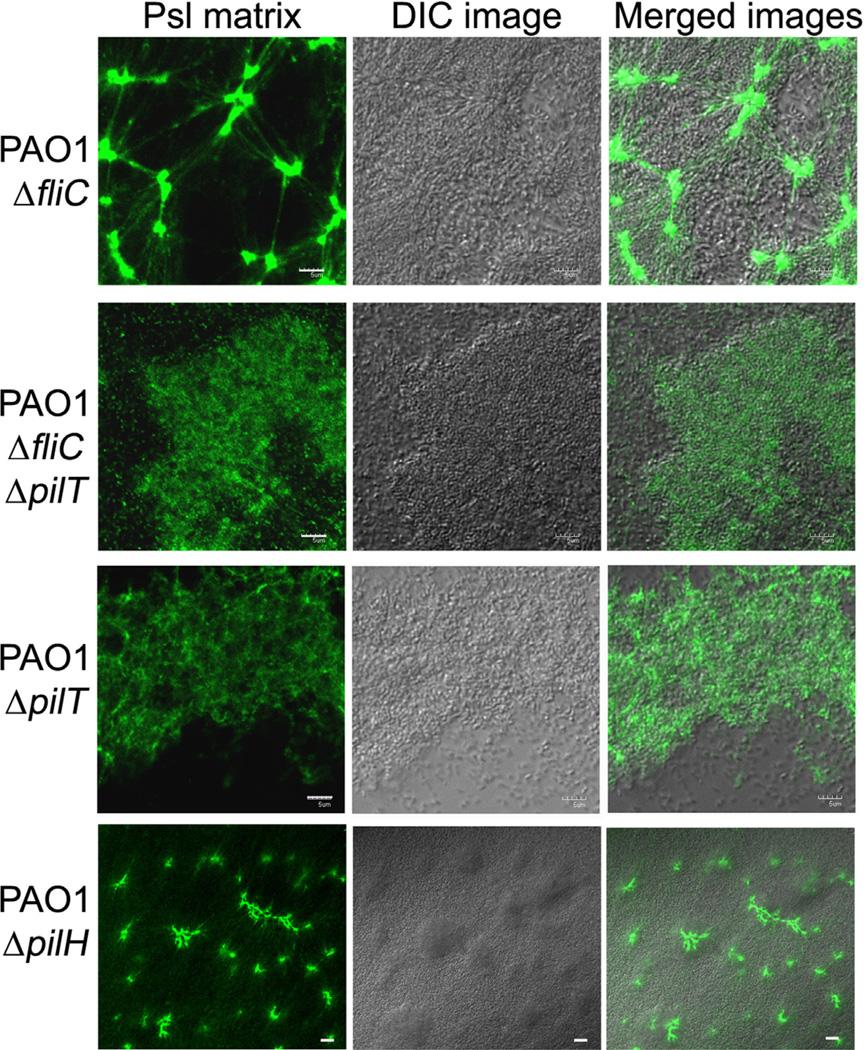
The Psl matrix in the pellicles of ΔfliC, ΔfliCΔpilT, ΔpilT and ΔpilH strain. Shown are selected CLSM images located in the middle of pellicles. The pellicles were stained by FITC-HHA for Psl matrix (green) after 44 h of growth under a static growth condition. Grey image was the corresponding DIC image of biofilm. Scale bar, 5 µm for all images.
Type IV pili-mediated bacterial migration builds a Psl-fibre matrix
To determine whether formation of the Psl fibre matrix required the presence of T4P structure or T4P-mediated motility, we utilized three pilus-retraction-deficient strains, ΔpilT, ΔfliCΔpilT and ΔpilH (Table 1) (Bertrand et al., 2010). As controls, we also examined ΔfliC, ΔpilA and ΔfliMΔpilA strains. Since Psl-fibre matrix can form in pellicles, we grew pellicles to examine the Psl matrix of above mutant strains. All these strains were able to form pellicles at air-media interface with various biofilm bio-masses after 2-day growth (Fig. 4). The Psl staining results did show a correlation between T4P-mediated motility and the Psl-fibre formation in pellicles. The ΔfliC strain retained T4P-mediated twitching motility at a level similar to wild type strain (Table 1) and this strain can form a typical radial Psl-fibre matrix in pellicles and flow-cell biofilms (Figs 4, 2C, and 2E). The pilT and pilH genes are both involved in pilus retraction in P. aeruginosa. PilH is a CheY-like response regulator, which interacts with ATPase PilT to mediate pilus retraction (Bertrand et al., 2010). Deletion of either pilT or pilH results in increased levels of surface T4P relative to wild type, yet ΔpilT strains were completely defective for twitching motility and ΔpilH bacteria retained some twitching motility (Table 1) (Bertrand et al., 2010). Consistent with the phenotype of twitching motility, Psl staining results showed that the DpilH strain can form Psl-fibre structure in pellicle (Fig. 4). However, no typical Psl-fibres were found in the pellicle of ΔpilT and ΔfliCΔpilT strains, which had totally loss of twitching motility (Fig. 4). Similar fibreless Psl matrixes were found in the pellicles of ΔpilA and ΔfliMΔpilA strains that lacked T4P and T4P-mediated twitching motility (Fig. S4). Since the ΔpilT and ΔfliCΔpilT strains retain surface expression of T4P yet lack twitching motility and biogenesis of the Psl-fibres, this result indicates that T4P-mediated motility, but not T4P structure, plays a key role for the formation of a Psl fibre matrix.
The contributions of Psl-fibres in P. aeruginosa biofilm formation
Neither the pilA mutant nor the fliMpilA double mutant can form the Psl-fibres (Fig. S4). The flow cell biofilms derived from these strains also had much less biomass compared with Psl-fibre-proficient strains PAO1 and ΔfliC (Figs 2 and S4). The hyperpiliated strains ΔpilT and ΔfliCΔpilT also cannot form the Psl-fibres, and their biofilm had little biofilm biomass in a flow cell chamber as that of the pilA mutants (Figs 5A and 2A). These suggested that the Psl-fibres had contributions on the maintaining of biofilm biomass. Furthermore, there are more Psl presented in the microcolonies of ΔpilA strain than PAO1 microcolonies according to the fluorescent intensity of Psl (Fig. 3). Psl signal per µm3 biofilm biomass of ΔpilA microcolonies was sixfold higher than PAO1 (the right panels of Fig. 3 and compare the number on the upper right corner in each image). Independently, Yang and colleagues also detected a large amount of matrix material in the flow cell biofilm of pilA mutant (Yang et al., 2011). In addition, the fliMpilA mutant also had more Psl relative to its biofilm biomass, compared with that of PAO1 and fliC mutant (Fig. 2B – D). This suggested that the Psl-fibre-deficient strains, such as pilA mutants, required more Psl to keep biomass in a microcolony than the Psl-fibre-proficient strains (Fig. 3). This also implied that forming Psl fibres may be an efficient way for bacteria to occupy a surface and build the biofilm biomass, which allows bacteria to use less Psl to gain more biomass. These data suggested that the formation of Psl-fibres was important for efficient biofilm formation.
Fig. 5.
The contribution of Psl fibres in the biofilm formation.
A. A photograph of flow cells with the 2-day-old biofilms of strain PAO1 and T4P mutants. The white cloud and spot in the lower two chambers indicate biofilms and microcolonies.
B. The pellicles of PAO1 and PAO1-derived T4P mutants. The pellicles are stained by SYTO9 (green). The upper panel shows the photos of glass chamber with pellicles. The lower panel show representative 3-dimesion-reconstructed CLSM image of each pellicle. Squares are top-down view and rectangles are side view. A COMSTAT analysis of the data from each pellicle is indicated below.
The Psl-fibre matrixes were also located at the stalk area of MSBS (Fig. S3), suggesting their potential contributions to the formation of MSBS, perhaps serving as a focal point for T4P-mediated motility to form a MSBS cap. Consistently, ΔpilA and ΔfliMΔpilA were unable to form typical MSBS with mushroom-shaped caps (Barken et al., 2008; Yang et al., 2011). In contrast, ΔpilH bacteria retained the ability to form Psl-fibres in a pellicle biofilm (Table 1 and Fig. 4), which can also form mushroom-shape caps with minor defects in a flow-cell biofilm as reported previously (Barken et al., 2008). Taken together, these data suggested the formation of Psl-fibres were critical for maintaining the biomass and three-dimensional structure of biofilm.
The Psl-fibre matrixes were also observed in the middle of pellicles formed at the air-media interface during static growth, leading us to investigate the contribution of Psl-fibre in the pellicle formation. We grew pellicles at air-media interface for 20 h, and then compared the pellicles of T4P-deficient and/or flagellar-deficient strains with wild type strain PAO1. Surprisingly, the Psl-fibre-deficient T4P mutants, such as ΔpilA and ΔpilAΔfliM, formed pellicles although most of them had less biomass compared with PAO1 (Fig. 5B). The results showed PAO1 formed a flat and dense pellicle with the highest substratum coverage and lowest roughness coefficient (Fig. 5B), an indicator of biofilm heterogeneity (Heydorn et al., 2000). Consistent with the flow cell biofilm data, the pellicle of the Psl-fibre-proficient ΔfliC strain had similar biomass as PAO1, but more structure variation indicated by the high roughness (5 times of PAO1) and maximum thickness (3 times of PAO1) (Fig. 5B). The pellicle of the Psl-fibre-deficient ΔpilAΔfliM strain had reduced biomass but high roughness, which was similar to the result of flow cell biofilm. In contrast to the flow cell data, the ΔpilA strain formed a pellicle that had half of the biomass of PAO1 level while the pellicle of the ΔpilT strain was thicker and had slightly more biomass than PAO1. This was consistent with previous suggestion about structural support of T4P in biofilms (Chiang and Burrows, 2003; Barken et al., 2008). Despite this, the pellicles of these two mutants were unstable during the pellicle staining process compared to the wild type pellicle, suggesting loosely bacterial connections within the pellicles of mutants. In addition, the structural support of T4P is not sufficient to promote a stable biofilm in a flow cell as mentioned above (Fig. 5A).
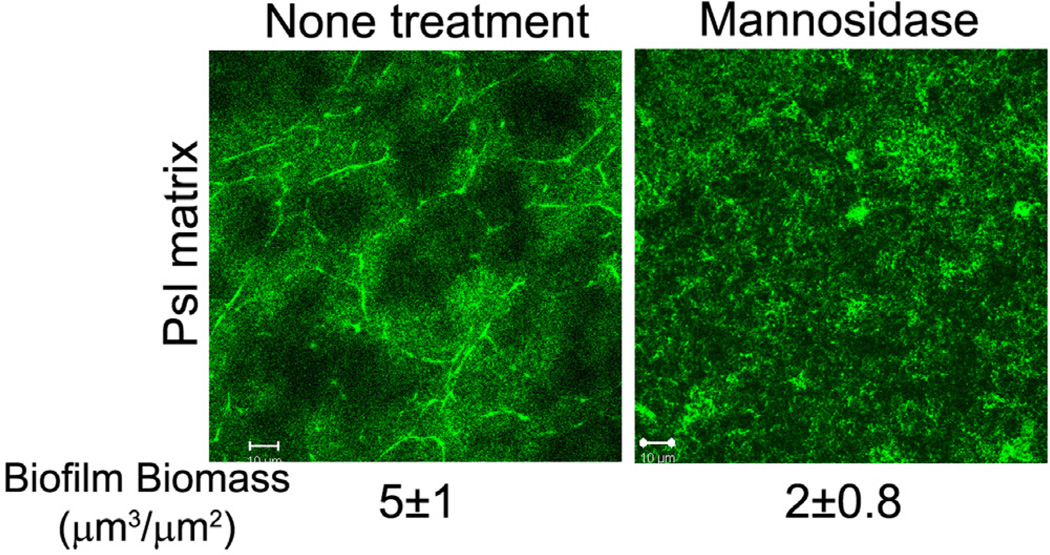
To further investigate the contribution of Psl fibres on maintaining of the pellicle biomass, we performed mannosidase treatment on the PAO1 pellicles as Psl is a mannose-rich polysaccharide (Ma et al., 2007; Byrd et al., 2009). Mannosidase eliminated the most Psl-fibre structure in pellicle and reduce the biomass to 40% of non-treatment control (Fig. 6). This result indicated that the Psl fibre structure was also important for maintaining of pellicle biomass.
Fig. 6.
Mannosidase treatment eliminates the Psl fibres and reduces biofilm biomass. Shown are the HHA-FITC stained Psl matrix of PAO1 pellicles with/without mannosidase treatment. Corresponding biomass are shown under the images. Scale bar, 10 µm.
In summary, our data indicated that the Psl-fibre matrix played a significant role in efficient biofilm formation, especially at the high shear flow conditions. We conclude that the Psl-fibres may help a biofilm by the following: (i) surrounding multiple-cell aggregates and connecting microcolonies to maintain bacterial association (Figs 1–5 and S1); (ii) acting as web-like backbones that strengthen a biofilm; (iii) recruiting bacteria to join a biofilm by functioning as a structure/surface for T4P to adhere and promote a Psl-derived bacterial community (Figs 1 and 5); and (iv) rapidly covering a surface and efficiently gain biofilm biomass (Figs 2 and 3).
Psl-fibres appear to be formed by Psl released from bacterial surface during T4P-mediated migration
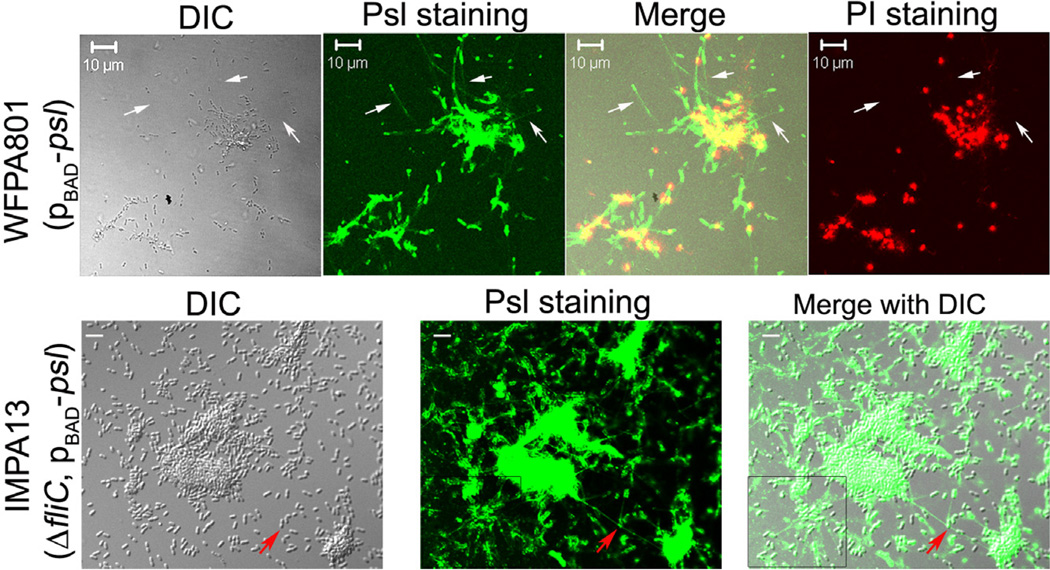
To gain insights into how Psl-fibre strands may form, we stained Psl of bacteria immediately following their attachment to a glass coverslip (green, Fig. 7 upper panels). Psl tracks were observed following bacteria and the length of tracks ranged from 10 to 30 µm (upper panels of Fig. 7, indicated by the white arrow), which is longer than the T4P structure itself (usually ~ 5 µm) (Skerker and Berg, 2001). This suggested that Psl released from bacteria during migration might form the Psl-fibre strands. We also stained the bacteria with pro-pidium iodide (PI), a red fluorescent dye that stains extracellular DNA (eDNA) or DNA in dying/dead bacteria. The result showed that significant cell death and/or eDNA production occurred in multiple-bacteria aggregates. Moreover, few dead bacteria and visible eDNA were overlapping/associated with Psl tracks suggesting that Psl tracks were derived primarily from living bacteria (Fig. 7, upper merged image).
Fig. 7.
Psl tracks were detected after bacterial cells attached on a glass slip. Shown are surface-attached bacterial cells of WFPA801 and IMPA13 stained by HHA-FITC and/or propidium iodide (PI). White arrows pointed out the HHA-FITC stained Psl tracks (green) following bacterial cells. Red fluorescent signals were PI stained DNA of dead/dying bacteria. The red arrow indicates a Psl-fibre strand connecting two microcolonies. The boxed area showed the web-like radial pattern Psl tracks. Grey panel was the corresponding DIC image. Scale bar: 10 mm for WFPA801, 5 µm for IMPA13.
Psl tracks following bacteria were also observed in ΔfliC Psl-overproducing bacteria (Fig. 7, lower panels). Here, Psl-fibre strands appeared to connect two microcolonies and there were a few bacteria joining to the Psl strand (red arrow in Fig. 7 lower panel). A similar phenomenon was also observed in the biofilm of WFPA801 and PAO1 (Figs 1A and S1). Taken together, the above data suggested that Psl released from bacteria during T4P-mediated migration was responsible for forming the Psl-fibre strands.
Starvation triggers Psl release from the bacterial surface and enhances T4P-mediated twitching motility
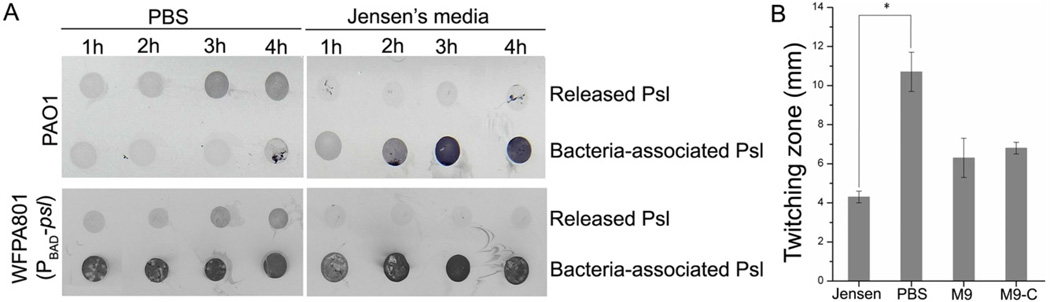
The radial Psl-fibre matrix was present in the middle of biofilms (Fig. 2E), an area with limited nutrients (Stewart and Franklin, 2008). Thus, we hypothesized that nutrient limitation/starvation may trigger Psl release. To test this, we resuspended bacteria from the mid-log phase culture in either PBS buffer or Jensen’s media, and then monitored Psl release during a 5 h period. Our results showed that Psl release from the wild type bacterial surface occurred within 3 h post incubation in PBS (Fig. 8A). In contrast, Psl release was not detected in the samples incubated with Jensen’s media although cell-associated Psl was increased during the incubation (Fig. 8A). Psl release in PBS was not a result of bacterial cell death since the bacteria post 5 h of incubation had the same number of live bacteria as the samples at 0 h of incubation (data not shown). Similar Psl release pattern were observed in Psl-overproducing strain WFPA801 (Fig. 8A), indicating the production of Psl did not affect Psl release. These data showed that starvation triggered the Psl release.
Fig. 8.
Psl release and twitching motility of P. aeruginosa strains under different nutrient conditions.
A. Psl released into PBS buffer and Jensen’s media from mid-log phase bacteria of PAO1 and the Psl-overproducing strain WFPA801 during 4 h of incubation at RT. Released Psl and bacterial surface-associated Psl were detected by immunoblotting with anti-Psl serum.
B. The twitching zone of PAO1 on the Jensen’s media agar plate, PBS agar plate, M9 media plates and M9 media without carbon source (M9-C). *P < 0.001 (one-way ANOVA).
To test whether starvation can also induce the T4P-mediated twitching motility, we compared the bacterial twitching ability in Jensen’s media agar plate and PBS agar plate. Since bacteria cannot grow in PBS agar, to be able to see the twitching zone, we added 15 ml of middle-log phase culture into a hole with 7 mm diameter and examined the twitching zone surrounding the hole. The twitching zone of PAO1 in PBS plate was double than Jensen’s media plate (Figs 8B and S7). This indicated that starvation did enhance T4P-driven twitching motility. We also tested the twitching zone of PAO1 in M9 media and M9 media without carbon source. The results showed that the twitching zones of PAO1 in M9 media were smaller than that of PBS, but larger than that of Jensen’s media (Fig. 8B). Depletion of carbon source in M9 media did not show significant impact on the twitching motility. Since the nutrient in M9 media was less rich than Jensen’s media, this result indicated poor nutrient can also enhance twitching motility of P. aeruginosa. Taken together, our data indicated that starvation can trigger Psl release from bacterial surface as well as T4P-driven bacterial migration. Coupling of these two events may result in the formation of Psl fibres on a surface and in a biofilm.
Discussion
The spider web strategy
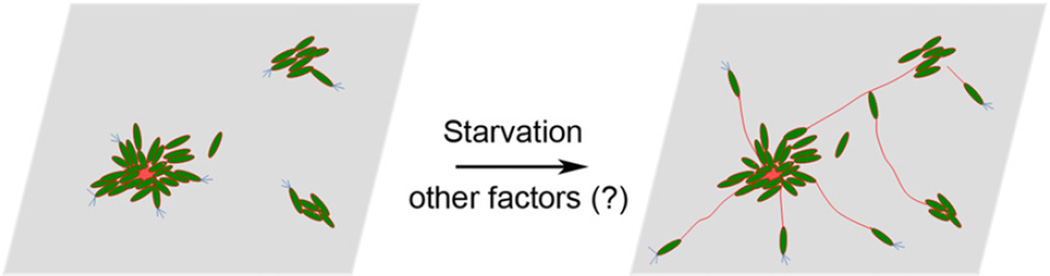
One of the most important features of a biofilm is the extracellular polymeric substance that functions as a matrix, holding bacterial cells together. Understanding how a matrix forms may provide therapeutic targets/ strategies to solve biofilm-related problems, such as persistent infections. Up to date, little is known about how a biofilm matrix forms. Psl is a key scaffolding matrix component for P. aeruginosa to initiate and maintain biofilms (Matsukawa and Greenberg, 2004; Ma et al., 2006; 2009; 2012; Whitchurch, 2006; Yang et al., 2012). This exopoly-saccharide can form fibre-like strands in biofilm. In this study, we have showed that the Psl fibres connecting to each other results in a spider web-shape matrix. The formation of the fibre-like Psl matrix relies on T4P-driven bacterial migration. Bacterial migration within a biofilm is particularly important as nutrients can quickly become limited within the interiors of the dense cell aggregates in biofilms. Bacteria may benefit by being relocated themselves in the biofilm community in response to changing nutritional gradients. Strikingly, Psl-fibres appear to be formed by Psl released from bacterial surface during T4P-driven bacterial crawling. While T4P drives bacteria forward, Psl tracks appear following bacteria, a strategy similar to the formation of spider web lines. Mutants lacking T4P-mediated twitching motility cannot form the Psl-fibre matrix even if retained T4P on bacterial surface and had a regular Psl release pattern (Figs 4 and S5). This suggests that bacterial migration coupled with Psl polysaccharide release is the key to form a Psl fibre. We have also revealed that starvation can trigger both the T4P-driven bacterial migration and Psl release from bacterial surface, which may couple these two evens. Moreover, we have observed a radial web-like Psl-fibre matrix was present in the middle of both flat flow-cell biofilms and pellicles (Figs 2 and 4), which implies that there is directional bacterial migration occurring in the middle of a biofilm. Importantly, this region of the biofilm is nutrient-deprived (Stewart and Franklin, 2008). In addition, the centre of the radial Psl-fibre matrix is also the centre of a microcolony/dense cell aggregate that have concentrated EPS material including Psl polysaccharide and eDNA/ dead bacteria, suggesting starvation occurred in the centre (Fig. S6). Consistent with this, we observed bacteria moving away from a multiple-cell aggregates that had bacterial cell death occurring (upper panels in Fig. 7, red stain indicates the dead bacteria). These provide a plausible model for how the spider web-like Psl fibres matrix may form. In this model, starvation signals in the centre of bacterial cell aggregates may trigger directional bacterial migration across a substratum/biofilm along with Psl release from bacterial surface, leading to the formation of Psl-fibre strands and the radial pattern Psl-fibre matrix (Fig. 9). The radial Psl-fibre matrix was located in the middle of a flow cell biofilm as well as an air-liquid interface biofilm (pellicle) (Figs 2E and 4; Video S1), suggesting T4P may use matrix material as a surface to mediate bacterial migration within biofilm, which may lead to the connection between Psl fibres and microcolonies (Fig. 9). Since the way that the T4P constructs Psl fibres is similar to form a spider web line and the Psl fibres matrix has spider web look, we therefore named this T4P-dependent Psl fibres matrix formation strategy as spider web strategy.
Fig. 9.
A model for how a radial pattern Psl-fibre matrix may form. Starvation within a multiple-cell aggregate triggers Psl polysaccharide release from the bacteria surface and T4P-mediated bacterial migration, resulting in a radial web-like Psl-fibre matrix. Psl and Psl-fibres may function as a structure/surface for T4P to recruit bacteria to join a matrix or biofilm. Green, bacteria; red, Psl; blue, T4P.
Pseudomonas aeruginosa depends on two types of appendages (T4P and flagella) for bacterial migration in biofilm, yet they drive bacteria to opposite directions. This may be one of reasons why a fliC mutant has more typical radial pattern Psl-fibre matrix in biofilm. Because this mutant totally depends on T4P for bacterial migration, the key player for the Psl-fibre matrix formation as proposed above. As a matter of fact, the wild type strain PAO1 does have a more complex Psl matrix pattern than that of the fliC mutant (Fig. 2), which implies that other type of bacterial motility in biofilm may also have some contribution on the formation of Psl matrix.
Rapidly colonizing a surface and biofilm formation is central to bacterial survival among competitors in environmental and clinical settings (Costerton et al., 1995). T4P can function as adhesions for bacteria to rapidly colonize a surface. It can also help bacteria to escape from surfaces when necessary, which is also important for bacterial survival. Psl also promotes surface adherence (Ma et al., 2006), thus Psl release may allow bacteria to rapidly move to a new niches and meanwhile form Psl fibres that help bacterial communities efficiently cover a surface and gain biofilm biomass. The formation of Psl-fibres requires neither the flagella nor other P. aeruginosa biofilm matrix polysaccharides such as Pel or alginate. Flagella-deficient strains were often isolated from CF patients (Wolfgang et al., 2004; Lee et al., 2005) and in vitro biofilms (the oral presentation of Dr Harrison at 6th ASM Conference on Biofilms, September of 2012). This suggests that flagella-deficient bacteria may benefit biofilm formation. As shown in this study that a flagella deficient strain can utilize T4P-dependent bacterial migration strategy to efficiently form a web-like radial pattern Psl-fibre matrix and a flower-shaped multiple-layer biofilm. This might be the reason for how flagella-deficient bacteria may benefit for biofilm formation. Finally, we showed that Psl-fibres were present in the biofilm of RSCVs, which are also often isolated from CF patients and in vitro biofilm (Starkey et al., 2009). Therefore, our data suggest that the T4P-mediated biofilm matrix formation strategy may have general significance for bacterial survival in natural and clinical settings.
Signal molecules involved in Psl release and twitching motility
Starvation-induced polysaccharide release has been reported in marine Pseudomonas species (Wrangstadh et al., 1986). Thus, the starvation-triggered exopolysac-charide release may be a general phenomenon. In this report, we have showed that starvation triggers Psl release from the bacterial surface of P. aeruginosa. A recent report showed that carbon starvation induced biofilm disperse and cAMP was the signal molecule involved (Huynh et al., 2012). Thus, cAMP might be a possible signal that induces Psl release during carbon starvation. However, carbon starvation does not enhance twitching motility. Consistently, the report of Fulcher and colleagues (2010) also suggested that the control of T4P function was in a cAMP-independent manner. The stringent response (SR) is a regulatory system that allows bacteria to sense and adapt to nutrient-poor environments (Cashel et al., 1996). The RelA and SpoT enzymes-synthesized (p)ppGpp is the central mediator of the SR (Battesti and Bouveret, 2009). Vogt and colleagues reported that the relAspoT double mutant of P. aeruginosa was defective in swarming and twitching, but not in swimming motility (Vogt et al., 2011). This suggested that (p)ppGpp may be one of signals to regulate starvation-triggered twitching motility, yet this alarmone was not likely involved in the starvation-induced Psl release as the relAspoT mutant exhibited a similar Psl release pattern as PAO1 (Fig. S5). Therefore, cAMP and (p)ppGpp are not the signal molecules coupled Psl release and twitching motility. Future works are required to elucidate which specific signal coupled Psl release and twitching motility, and how the signals regulate these two phenomena.
Summary
Pseudomonas aeruginosa appears to utilize several strategies to build a biofilm matrix. We have previously shown that Psl is arranged in a helical pattern on the bacterial surface and that the interaction between Psl helices on bacteria may help to promote cell–cell interactions and the matrix formation (Ma et al., 2009). In this study, we provide another Psl matrix formation strategy, a T4P-dependent bacterial migration strategy to build a fibre-like Psl matrix through a mechanism similar to that observed for spider web formation. Pilin expression is reported to be repressed in mucoid P. aeruginosa (Whitchurch et al., 2002b). Thus, alginate-derived bio-films are unlikely to use T4P-mediated migration to form a fibre-like matrix of alginate. A specific staining reagent for Pel has not been developed, so we were unable to determine whether Pel-rich biofilms rely on T4P-mediated bacterial migration.
T4P participate in a number of fundamental bacterial processes, including motility, fruiting body formation, adherence to surfaces/host cells, and biofilm formation (Strom and Lory, 1993). In this report, we gain insight into how T4P participate in the formation of a Psl fibre matrix during biofilm development. In Myxococcus xanthus, extracellular polysaccharides mediate pilus retraction and T4P-mediated social-motility promotes fruiting body formation (Mauriello et al., 2010). In P. aeruginosa, Psl is not necessary for T4P-mediated twitching motility (Table 1), but T4P-mediated motility can help to build a fibre-like Psl polysaccharide matrix within biofilms. Most strikingly, starvation signals can couple Psl release and T4P-mediated twitching motility, leading to the formation of Psl-fibre matrix. Recently, Nguyen and colleagues reported that starvation responses mediated antibiotic tolerance in biofilms and nutrient-limited bacteria (Nguyen et al., 2011). Thus the starving bacteria appear to outperform in biofilm formation and persistence during infections and survival in the environment.
The T4P are present in several biofilm forming bacteria, such as Neisseria gonorrhoeae, N. meningitidis, Vibrio cholerae, Legionella pneumophila, Salmonella enterica and enteropathogenic E. coli (Strom and Lory, 1993). Future work may provide evidence that T4P-dependent biofilm matrix formation strategy is a general mechanism conserved in the T4P-producing bacteria.
Experimental procedures
Strains and growth conditions
Pseudomonas aeruginosa strains used in this study and their motility phenotype are listed in Table 1. PBAD-psl strains, WFPA802 and IMPA13 were constructed by the exactly same unmarked in-frame deletion strategy used for WFPA801 as described previously (Hoang et al., 1998; Ma et al., 2006). The IMPA34(ΔfliCΔpilT), IMPA33(ΔpilT) and IMPA31(ΔpilH) were constructed by the unmarked in-frame deletion strategy (Hoang et al., 1998), whereby DNA encoding amino acid 5–486 of FliC and/or 4–342 of PilT, and the entire encoded region of pilH were deleted respectively. Unless otherwise indicated, P. aeruginosa was grown at 37°C in Luria–Bertani medium lacking sodium chloride (LBNS) or Jensen’s, a chemically defined medium (Jensen et al., 1980). Biofilms of P. aeruginosa were cultured in Jensen’s medium at room temperature (RT) (Ma et al., 2006). To induce the transcription of the psl operon, 2% arabinose was added to Jensen’s medium.
Immunoblotting of Psl polysaccharide extracts
Bacteria were collected from 450 µl of culture (OD600~0.5). Pellets were resuspended with 450 µl PBS buffer or Jensen’s media and incubated at the RT for 1–5 h. At each hour, Psl was extracted from bacteria and culture supernatant. Bacterial surface-associated Psl was extracted as previously described (Byrd et al., 2009). Released Psl in the supernatant was precipitated by 3 volumes of 100% ethanol, resuspended in 50 µl 0.5 M EDTA and treated with proteinase K for 1 h. Psl extracts were detected by immunoblotting with anti-Psl serum as described previously (Byrd et al., 2009).
Biofilm and Psl matrix staining
The air-liquid interface biofilms were grown in glass chambers (Chambered #1.5 German Coverglass System, Nunc) with glass coverslip at the bottom. Mannosidase (Sigma, 10 u per chamber) was added into glass chambers 1 h post inoculation. For CLSM observation, buffers were gently sucked out from glass chambers to allow pellicles to drop down on the coverslips. The flow cell biofilms were grown at RT in three-channel flow cell with individual channel dimensions of 1 × 4 × 40 mm (Stovall Life Science) as previously described (Ma et al., 2006). The mid-log phase culture was used for inoculation. The biofilms were stained by membrane stain FM4-64 (1 µm final concentration, Molecular Probes, Invitrogen) or DNA stain SYTO9 (Molecular Probes, Invitrogen). Psl matrix was stained by fluorescence-labelled lectin HHA at 100 µg ml−1 (EY lab) as we described elsewhere (Ma et al., 2009).
Psl and PI staining for surface-attached bacteria
Psl expression of WFPA801 and IMPA13 was induced by addition of 2% arabinose for 3 h at 37°C with shaking, 0.5 ml of these cultures was utilized to inoculate glass chambers respectively (Chambered #1.5 German Coverglass System, Nunc). After 1 h of incubation at RT, the bacteria attached on glass coverslip were stained with lectin HHA-FITC for 2 h at RT and stained by PI (Invitrogen) for 10 min in dark.
Microscopy and image acquisition
All fluorescent images were acquired by a Zeiss 510 CLSM (Carl Zeiss, Jena, Germany). Images were obtained using 63×/1.3 objective. A LSM image browser generated the 3D images and optical Z-sections. CLSM-captured images were subjected to quantitative image analysis using COMSTAT software as previously described (Heydorn et al., 2000). The Psl fluorescence intensity of PAO1 and ΔpilA strains were analysed by using Interactive 3D Surface Plot plugin of ImageJ software (version 1.43u, NIH).
Motility assay
Twitching motility was assayed by stab inoculating strains through a thin LBNS/Jensens’s media agar (1% w/v) plate with 24–48 h of incubation at 30°C under humidified condition. Twitching zones were visualized at the agar plate interface (Whitchurch et al., 2002b). Flagellum-mediated swimming motility was assayed by stab inoculating strains onto LBNS agar plates (0.3% agar). After 24 h of incubation at 37°C, motility was assessed by measuring the diameters of the circular zones that the colonies spread from their points of inoculation (Arora et al., 1998). For starvation-induced twitching assay, a 7 mm-diameter hole was obtained on a 1.0% agar plate in Jensen’s media or PBS containing 0.1% tetrazolium red respectively. The 15 ml of mid-log phase culture was inoculated into the holes and the twitch zone was measured after 48 h of incubation at 30°C. In order to assay the influence of carbon source depletion on twitching, M9 media with or without carbon was used under the same condition. All data were obtained from three independent experiments.
Supplementary Material
Acknowledgements
We thank Dr Tolker-Nielsen at University of Copenhagen for providing the ΔfliMΔpilA strain, Dr Engel at University of California for pilH, and pilT mutant strains, Dr Nguyen at McGrill University for relAspoT mutant, and Dr Di Wang, Dr Qing Wei and Dr Palashpriya Das at Institute of Microbiology, Chinese Academy of Sciences for help in English writing. This work was supported by Chinese Academy of Science grant KSCXZ-YW-BR-5 (L.M.), National Natural Science Foundation of China grant 31270177 and 31140041 (L.M.), and Public Health Service grants AI061396 and HL058334 (D.J.W.), USA.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Fig. S1. The fibre-like Psl strands connecting multiple-cell aggregates/microcolonies at biofilm initiation. Shown are bio-films of PAO1 and WFPA801 after 22 h of growth under flow condition and stained with HHA-FITC (green, Psl matrix) and FM4-64 (red, bacteria membrane stain). Black arrows indicate the Psl fibres present in the area with a few bacteria. Scale bar, 5 µm.
Fig. S2. The formation of the Psl-fibre matrix is not required for the production of Pel and alginate. Shown are biofilms of MJK8 (A), MJK8/Dpel (B), MJK8/Dpsl (C), PAO1 (D), WFPA1 (E) and WFPA802 (F) after 22 h of growth under flow condition and stained with HHA-FITC (green, Psl matrix) and FM4-64 (red, bacteria membrane stain). The lower right image in each biofilm panel is the merge of corresponding red and green images. Grey images are DIC image of biofilms. Bar, 2 µm for MJK8 and MJK8/Δpel, 10 µm for MJK8/Δpsl, 2 µm for WFPA1 and 5 µm for PAO1.
Fig. S3. The radial pattern Psl-fibre matrix was located at the stalk area of MSBS. Shown was a selected optical CLSM section of a ΔfliC biofilm. The blue line on the rectangle image shows the section located at the stalk of MSBS, which is depicted in the large square. Arrows indicate the MSBS (square) and the locationofthe radial Psl-fibre matrix within the MSBS (rectangle image). Red: Psl matrix; Green: Bacteria.
Fig. S4. The Psl matrix in the pellicles of PAO1, ΔpilA, and ΔfliMΔpilA strains. Shown are selected CLSM images located in the middle of pellicles. The pellicles were stained by FITC-HHA for Psl matrix (green) after 44 h of growth under a static growth condition. Grey image was the corresponding DIC image of biofilm. Scale bar, 5 µm.
Fig. S5. Comparison of Psl released into PBS buffer and Jensen’s media from mid-log phase bacteria of T4P mutants and relA spoT deletion mutant during 4 h of incubation at RT. Released Psl and bacterial surface-bound Psl were detected by immunoblotting with anti-Psl serum.
Fig. S6. Concentrated Psl polysaccharide (green) and eDNA (red) were presented in the centre of a radial pattern Psl fibre matrix. Shown are selected CLSM images located in the middle of pellicles. The pellicle of ΔfliC strain was stained by FITC-HHA for Psl matrix (green) and PI for eDNA/dead cells after 44 h of growth under a static growth condition. Grey image was the corresponding DIC image of biofilm. Arrows indicate the colocalized Psl and eDNA in the centre of the radial pattern Psl fibre matrix. Scale bar, 5 µm.
Fig. S7. The twitching zone of PAO1 and pilA mutant on the Jensen’s media and PBS agar plate. The hole in the middle is the inoculation hole. The red colour is the result of tetrazolium red stain. The diameter of twitching zone was measured by addition of distance a and b as depicted in the middle image. Scale bar, 7 mm. * indicates statistically significant difference between measurements (P < 0.007, t-test). TZ, Twitching Zone.
Video S1. How the radial pattern Psl-fibre matrix maintain the biofilm structure of a ΔfliC strain. Shown was a serial optical CLSM section of a ΔfliC biofilm from substratum to the top of biofilm. Red: Psl matrix; green: bacteria in the biofilm.
References
- Arora SK, Ritchings BW, Almira EC, Lory S, Ramphal R. The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect Immun. 1998;66:1000–1007. doi: 10.1128/iai.66.3.1000-1007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barken KB, Pamp SJ, Yang L, Gjermansen M, Bertrand JJ, Klausen M, et al. Roles of type IV pili, flagellum-mediated motility and extracellular DNA in the formation of mature multicellular structures in Pseudomonas aeruginosa biofilms. Environ Microbiol. 2008;10:2331–2343. doi: 10.1111/j.1462-2920.2008.01658.x. [DOI] [PubMed] [Google Scholar]
- Battesti A, Bouveret E. Bacteria possessing two RelA/SpoT-like proteins have evolved a specific stringent response involving the acyl carrier protein-SpoT interaction. J Bacteriol. 2009;191:616–624. doi: 10.1128/JB.01195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand JJ, West JT, Engel JN. Genetic analysis of the regulation of type IV pilus function by the Chp chemosensory system of Pseudomonas aeruginosa. J Bacteriol. 2010;192:994–1000. doi: 10.1128/JB.01390-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd MS, Sadovskaya I, Vinogradov E, Lu H, Sprinkle AB, Richardson SH, et al. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopoly-saccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol Microbiol. 2009;73:622–638. doi: 10.1111/j.1365-2958.2009.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd MS, Pang B, Mishra M, Swords WE, Wozniak DJ. The Pseudomonas aeruginosa exopolysaccharide Psl facilitates surface adherence and NF-κB activation in A549 Cells. mBio. 2010;1:e00140–e00110. doi: 10.1128/mBio.00140-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M, Gentry D, Hernandez VJ, Vinella D. The stringent response. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, et al., editors. Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Washington, DC, USA: ASM Press; 1996. pp. 1458–1496. [Google Scholar]
- Chiang P, Burrows LL. Biofilm formation by hyperpiliated mutants of Pseudomonas aeruginosa. J Bacteriol. 2003;185:2374–2378. doi: 10.1128/JB.185.7.2374-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GCL, et al. The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011;7:e1001264. doi: 10.1371/journal.ppat.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad JC, Gibiansky ML, Jin F, Gordon VD, Motto DA, Mathewson MA, et al. Flagella and pili-mediated near-surface single-cell motility mechanisms in P. aeruginosa. Biophys J. 2011;100:1608–1616. doi: 10.1016/j.bpj.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial Biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Friedman L, Kolter R. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol. 2004a;51:675–690. doi: 10.1046/j.1365-2958.2003.03877.x. [DOI] [PubMed] [Google Scholar]
- Friedman L, Kolter R. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J Bacteriol. 2004b;186:4457–4465. doi: 10.1128/JB.186.14.4457-4465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher NB, Holliday PM, Klem E, Cann MJ, Wolfgang MC. The Pseudomonas aeruginosa Chp chemosensory system regulates intracellular cAMP levels by modulating adenylate cyclase activity. Mol Microbiol. 2010;76:889–904. doi: 10.1111/j.1365-2958.2010.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan JRW, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, et al. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol. 2001;183:5395–5401. doi: 10.1128/JB.183.18.5395-5401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: applications for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- Huynh TT, McDougald D, Klebensberger J, Al Qarni B, Barraud N, Rice SA, et al. Glucose starvation-induced dispersal of Pseudomonas aeruginosa biofilms is cAMP and energy dependent. PLoS ONE. 2012;7:e42874. doi: 10.1371/journal.pone.0042874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SE, Fecycz IT, Campbell JN. Nutritional factors controlling exocellular protease production by Pseudomonas aeruginosa. J Bacteriol. 1980;144:844–847. doi: 10.1128/jb.144.2.844-847.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisits MJ, Prost L, Starkey M, Parsek MR. Characterization of colony morphology variants isolated from Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2005;71:4809–4821. doi: 10.1128/AEM.71.8.4809-4821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen M, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol Microbiol. 2003a;50:61–68. doi: 10.1046/j.1365-2958.2003.03677.x. [DOI] [PubMed] [Google Scholar]
- Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jorgensen A, Molin S, et al. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol. 2003b;48:1511–1524. doi: 10.1046/j.1365-2958.2003.03525.x. [DOI] [PubMed] [Google Scholar]
- Kohler T, Curty LK, Barja F, van Delden C, Pechere JC. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol. 2000;182:5990–5996. doi: 10.1128/jb.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010;328:627–629. doi: 10.1126/science.1188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin-Gal I, Cao S, Chai L, Böttcher T, Kolter R, Clardy J, et al. A self-produced trigger for biofilm disassembly that targets exopolysaccharide. Cell. 2012;149:684–692. doi: 10.1016/j.cell.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lee B, Haagensen JAJ, Ciofu O, Andersen JB, Hoiby N, Molin S. Heterogeneity of biofilms formed by nonmucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. J Clin Microbiol. 2005;43:5247–5255. doi: 10.1128/JCM.43.10.5247-5255.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- Ma L, Jackson K, Landry RM, Parsek MR, Wozniak DJ. Analysis of Pseudomonas aeruginosa conditional Psl variants reveals roles for the Psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J Bacteriol. 2006;188:8213–8221. doi: 10.1128/JB.01202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Lu H, Sprinkle A, Parsek MR, Wozniak DJ. Pseudomonas aeruginosa Psl is a galactose- and mannose-rich exopolysaccharide. J Bacteriol. 2007;189:8353–8356. doi: 10.1128/JB.00620-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Conover M, Lu H, Parsek MR, Bayles K, Wozniak DJ. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009;5:e1000354. doi: 10.1371/journal.ppat.1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Wang S, Wang D, Parsek MR, Wozniak DJ. The roles of biofilm matrix polysaccharide Psl in mucoid Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol. 2012;65:377–380. doi: 10.1111/j.1574-695X.2012.00934.x. [DOI] [PubMed] [Google Scholar]
- Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 2003;426:306–310. doi: 10.1038/nature02122. [DOI] [PubMed] [Google Scholar]
- Matsukawa M, Greenberg EP. Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J Bacteriol. 2004;186:4449–4456. doi: 10.1128/JB.186.14.4449-4456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriello EMF, Mignot T, Yang Z, Zusman DR. Gliding motility revisited: how do the Myxobacteria move without flagella? Microbiol Mol Biol Rev. 2010;74:229–249. doi: 10.1128/MMBR.00043-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science. 2011;334:982–987. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- Shrout JD, Chopp DL, Just CL, Hentzer M, Givskov M, Parsek MR. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol Microbiol. 2006;62:1264–1277. doi: 10.1111/j.1365-2958.2006.05421.x. [DOI] [PubMed] [Google Scholar]
- Shrout JD, Tolker-Nielsen T, Givskov M, Parsek MR. The contribution of cell-cell signalling and motility to bacterial biofilm formation. MRS Bull. 2011;36:367–373. doi: 10.1557/mrs.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- Skerker JM, Berg HC. Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci USA. 2001;98:6901–6904. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey M, Hickman JH, Ma L, Zhang N, De Long S, Hinz A, et al. Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. J Bacteriol. 2009;191:3492–3503. doi: 10.1128/JB.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 2008;6:199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- Strom MS, Lory S. Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- Sutherland IW. The biofilm matrix - an immobilized but dynamic microbial environment. Trends Microbiol. 2001;9:222–227. doi: 10.1016/s0966-842x(01)02012-1. [DOI] [PubMed] [Google Scholar]
- Vogt SL, Green C, Stevens KM, Day B, Erickson DL, Woods DE, et al. The stringent response is essential for Pseudomonas aeruginosa virulence in the rat lung agar bead and Drosophila melanogaster feeding models of infection. Infect Immun. 2011;79:4094–4104. doi: 10.1128/IAI.00193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AA, Mattick JS, Alm RA. Functional expression of heterologous type 4 fimbriae in Pseudomonas aeruginosa. Gene. 1996;175:143–150. doi: 10.1016/0378-1119(96)00140-0. [DOI] [PubMed] [Google Scholar]
- Whitchurch CB. Biogenesis and function of type IV pillin in Pseudomonas species. In: Ramos JL, Levesque RC, editors. Pseudomonas. Vol. 4. USA: Springer US; 2006. pp. 139–188. [Google Scholar]
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002a;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- Whitchurch CB, Erova TE, Emery JA, Sargent JL, Harris JM, Semmler ABT, et al. Phosphorylation of the Pseudomonas aeruginosa response regulator AlgR is essential for type IV fimbria-mediated twitching motility. J Bacteriol. 2002b;184:4544–4554. doi: 10.1128/JB.184.16.4544-4554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84:273–278. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- Wolfgang MC, Jyot J, Goodman AL, Ramphal R, Lory S. Pseudomonas aeruginosa regulates flagellin expression as part of a global response to airway fluid from cystic fibrosis patients. Proc Natl Acad Sci USA. 2004;101:6664–6668. doi: 10.1073/pnas.0307553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DJ, Sprinkle AB, Baynham PJ. Control of Pseudomonas aeruginosa algZ expression by the alternative sigma factor AlgT. J Bacteriol. 2003;185:7297–7300. doi: 10.1128/JB.185.24.7297-7300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangstadh M, Conway PL, Kjelleberg S. The production and release of an extracellular polysaccharide during starvation of a marine Pseudomonas sp. and the effect thereof on adhesion. Arch Microbiol. 1986;145:220–227. doi: 10.1007/BF00443649. [DOI] [PubMed] [Google Scholar]
- Yang L, Hu Y, Liu Y, Zhang J, Ulstrup J, Molin S. Distinct roles of extracellular polymeric substances in Pseudomonas aeruginosa biofilm development. Environ Microbiol. 2011;3:1705–1717. doi: 10.1111/j.1462-2920.2011.02503.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang H, Wu H, Damkiær S, Jochumsen N, Song Z, et al. Polysaccharides serve as scaffold of biofilms formed by mucoid Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 2012;65:366–376. doi: 10.1111/j.1574-695X.2012.00936.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.