Abstract
Jawless vertebrates, which occupy a unique position in chordate phylogeny, employ leucine-rich repeat (LRR)-based variable lymphocyte receptors (VLR) for antigen recognition. During the assembly of the VLR genes (VLRA, VLRB and VLRC), donor LRR-encoding sequences are copied in a step-wise manner into the incomplete germ-line genes. The assembled VLR genes are differentially expressed by discrete lymphocyte lineages: VLRA- and VLRC-producing cells are T-cell like, whereas VLRB-producing cells are B-cell like. VLRA+ and VLRC+ lymphocytes resemble the two principal T-cell lineages of jawed vertebrates that express the αβ or γδ T-cell receptors (TCR). Reminiscent of the interspersed nature of the TCRα/TCRδ locus in jawed vertebrates, the close proximity of the VLRA and VLRC loci facilitates sharing of donor LRR sequences during VLRA and VLRC assembly. Here we discuss the insight these findings provide into vertebrate T- and B-cell evolution, and the alternative types of anticipatory receptors they use for adaptive immunity.
Keywords: Adaptive immunity, αβ and γδ T-cell receptor, Variable lymphocyte receptor, leucine-rich repeat, Jawless vertebrates
Introduction
Jawless vertebrates have been a focus of interest in the search for evolutionary origin of adaptive immunity, because of their pivotal position in chordate phylogeny (reviewed by Pancer and Cooper [1]). In the 1960s and 1970s, several studies of the extant jawless vertebrates, lamprey and hagfish were conducted to investigate their immune responsiveness. Immunization of lampreys with particulate antigens, such as heat-killed bacteria and mammalian erythrocytes, resulted in the production of specific agglutinins against these immunogens [2-6]. Hagfish were also found to be able to produce specific agglutinins to keyhole limpet hemocyanin [7]. Analysis of immune serum suggested an agglutinin molecular weight similar to that of IgM antibodies [2, 6]. In addition to this evidence for humoral immunity in lampreys and hagfish, delayed-type hypersensitivity to tuberculin and accelerated second set allograft rejection were suggestive of cell-mediated immunity [2, 8]. Leukocytes in hagfish were also shown to proliferate in response to allogeneic cells in vitro [9]. Despite this evidence for adaptive immunity in jawless vertebrates, the molecular basis for their immune responses remained unknown until recent years.
The cardinal recognition elements of adaptive immune system, immunoglobulin (Ig), T cell receptors (TCRs) and major histocompatibility complex (MHC) genes, are found in all of the jawed vertebrates, but none of these components could be found in jawless vertebrates [10, 11]. Instead, lampreys and hagfish have been shown to use variable lymphocyte receptors (VLRs) that are composed of somatically-assembled, leucine-rich-repeat (LRR) motifs [12, 13]. Three different types of anticipatory receptors (known as VLRA, VLRB, VLRC) have been identified in jawless vertebrates [12-16]. The VLRB-producing cells are similar to B cells in jawed vertebrates, whereas VLRA- and VLRC- expressing cells resemble the αβ and γδ T cells [17-19]. The definition of three distinct lymphocyte lineages in the two branches of vertebrates suggests that the three major lymphocyte differentiation pathways of the adaptive immune system were already present in a common ancestor of jawed and jawless vertebrates. Here we discuss some of the basic features and evolutionary implications of the existence of two prototypic T-cell lineages and their corresponding anticipatory receptors in jawless vertebrates.
Discovery of variable lymphocyte receptors
As the nearest living phylogenetic relatives of jawed vertebrates, it was anticipated that lampreys and hagfish would have orthologous genes responsible for adaptive immunity. The search for the genetic basis for antigen recognition in jawless vertebrates began with a transcriptome analysis of lamprey and hagfish lymphocyte like cells. This analysis revealed several genes orthologous to those that lymphocytes in jawed vertebrates use for cellular migration, proliferation, differentiation and intracellular signaling [10, 20-24]. An orthologue of CD45, a prototypical transmembrane protein tyrosine phosphatase (PTPase) that plays an essential role in signal transduction through T-cell and B-cell receptors was found in both lampreys and hagfish [20, 25]. A single copy TCR-like gene containing an exon encoding both V and J-like sequences [22, 26] was found in lamprey. Furthermore a VpreB-like gene was shown to be expressed by lamprey lymphocyte-like cells [27] and a family of paired-Ig-like receptor genes encoding transmembrane proteins with activating and inhibitory potential, named agnathan paired receptor resembling Ag receptors (APAR), was identified in hagfish [28]. However, the cardinal elements of adaptive immunity, namely Ig, TCR, RAG1 and 2, and MHC class I and II, were conspicuously missing.
In a renewed search for antigen receptor genes in jawless vertebrates, lampreys were stimulated by an antigen and mitogen mixture to survey the transcriptome of their activated lymphocytes. This strategy was based on the assumption that expression of immune-related genes would be increased during an immune response. Large lymphoblastoid cells were indeed induced by the stimulating mixture, and these activated cells were used to construct a cDNA library, followed by subtracting the myeloid and erythrocyte cDNAs [12]. Although TCR, BCR, and MHC genes still could not be found, the lymphoblastoid cDNA-enriched library contained a large number of transcripts that encoded for highly diverse LRR proteins (Fig.1). These were named variable lymphocyte receptors (VLRs) because of their expression on lymphocytes and remarkable sequence diversity [12]. Each VLR transcript encoded a conserved signal peptide (SP) followed by highly variable LRR modules: a 27–38 residue N-terminal LRR (LRRNT), the first 18-residue LRR (LRR1), up to eight 24-residue variable LRRs (LRRV), one 24-residue end LRRV (LRRVe), one 13-residue connecting peptide (CP), and a 48–65 residue C-terminal LRR (LRRCT). The invariant threonine/proline-rich stalk region contained a predicted glycosyl-phosphatidyl-inositol (GPI) cleavage site, and the phospholipase cleavage of a recombinant VLR from the surface of transduced mouse cell line verified its GPI membrane anchorage.
Figure 1.

Evolution of alternative adaptive immune systems in jawless and jawed vertebrates (modified from [89]). The LRR-based VLR genes are found only in the jawless vertebrates, whereas Ig-based BCR, TCR and MHCI/II genes are limited to the extant jawed vertebrates. Neither of these types of antigen recognition receptors has been found in amphioxus and tunicates, suggesting that the LRR-based VLRs and the Ig-based BCRs and TCRs emerged through convergent evolution. The presence of one and the other of these lymphocyte-based adaptive immune systems attests to their survival advantage.
After the discovery of the first lamprey VLR gene (now called VLRB), two hagfish VLR homologues, VLRB and VLRA (recently reclassified as VLRC) were identified in an expressed sequence tag database of hagfish leukocyte transcripts [13]. A lamprey VLRA orthologue was identified in a subsequent search of the draft sequence database of the sea lamprey genome [14], and a third VLR gene known as VLRC was then discovered in lamprey [15]. More recently, a third hagfish VLR gene was identified. Comparative analysis of the three types of jawless vertebrate VLR genes indicated that the third hagfish VLR is the true lamprey VLRA counterpart and the previously identified hagfish “VLRA” is orthologous to the lamprey VLRC [16]. Thus, three orthologous VLR genes (VLRA, VLRB and VLRC) have now been characterized in both lampreys and hagfish, suggesting that this anticipatory receptor system evolved in a common ancestor of the two cyclostome lineages approximately 480 million years ago [16].
Germline VLR genes and assembly mechanism
In addition to the V(D)J recombination events in the recombinatorial assembly of antibody genes, gene conversion of the V region using pseudo V segment sequences contributes to the generation of antibody diversity in some jawed vertebrates, such as birds and rabbits [29-31]. The vast repertoire of the VLRs, which is estimated to be comparable to that of Igs and TCRs [32], is generated by a unique gene assembly mechanism taking place only in lymphocytes. Before their assembly, the incomplete germline VLR genes have non-coding intervening sequences, instead of the variable LRR-encoding regions (Fig.2). A large number of donor LRR-encoding sequences are located near the VLR genes. These are not rearranged, but instead are used as template donors to replace the non-coding intervening sequences in a segmental stepwise manner to complete the VLR gene assembly. This assembly process is guided by short overlapping homologies (10-30 bp) between donor and acceptor LRR sequences and may beginfrom either the 5' or 3' -end of the diversity region (Fig. 3).
Figure 2.
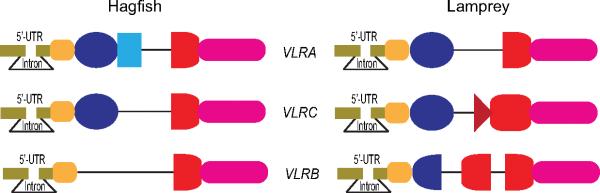
Germline configuration of VLR genes in lampreys and hagfish (modified from [89]). The three pairs of orthologous germline VLRA, VLRC, and VLRB genes are different in lampreys and hagfish. Notably, sea lamprey VLRB has a 5' LRRCT- encoding donor sequence in the large intervening region, whereas the other VLR genes do not and they possess shorter intervening sequences. (not drawn to scale)
Figure 3.

Schematic diagram of an VLR gene and the postulated assembly mechanism. (A) Domain organisation of a functional VLR protein. The diversity region is composed of a 3'-end of the N-terminal LRR (LRRNT), LRR1, multiple LRRVs, connecting peptide (CP) and a 5'-end of the C-terminal LRR (LRRCT). (B) Hypothetical scheme of VLR gene assembly process. Multiple LRR cassettes are located upstream and downstream of a pre-assembled germline VLR gene with its non-coding intervening sequence. At the beginning of the gene assembly process, a cytosine deaminase (CDA) converts cytosine (C) to uracil (U) in the germline VLR gene. The uracil is then removed by uracil-DNA glycosylase (UNG), leaving an apurinic (AP) site, blank residue in DNA. The AP site activates nicking activity of AP endonuclease (APE) which leads to a DNA double strand break. To repair this break, homologous recombination starts from the 5'-end and/or 3'-end of the break, based on the sequence homology of 10-30bp between the donor and acceptor LRR cassettes. This process is repeated along with deletion of the intervening sequence until a functional VLR is expressed on the cell surface.
The assembly of each VLR is lymphocyte lineage specific: VLRB assembly does not occur in the VLRA+ and VLRC+ lymphocytes and, conversely, VLRA and VLRC assembly does not occur in VLRB+ lymphocytes. On the other hand, VLRC-assembly may occur in a minor portion of the VLRA+ cells and VLRA-assembly can sometimes be found in VLRC+ cells, although cells with productive assembly of both VLRA and VLRC genes are rare [19]. Our analysis suggests a hypothetical model in which a common progenitor in the thymoid begins assembly first of one VLRC allele and, if productive assembly fails, then the other allele undergoes assembly. If VLRC assembly on both alleles is unproductive, the thymoid cell can undergo assembly of VLRA alleles in order to achieve a productive VLRA gene assembly [19].
The VLR assembly mechanism is thought to involve enzymatic activity similar to that previously shown to contribute to antigen receptor diversity in jawed vertebrates. In jawed vertebrates, activation induced cytidine deaminase (AID) converts cytosine to uracil leading to the activation of DNA repair pathways that promote somatic hypermutation, class switching and gene conversion in immunoglobulin genes. Two AID/APOBEC orthologues, cytidine deaminase (CDA) 1 and CDA2, have been identified in jawless vertebrates [14]. CDA1 expression at a level that can be detected by in situ hybridization is restricted to cells undergoing VLRA and VLRC assembly in thymoid region. Conversely, CDA2 expression is restricted to cells of the VLRB lineage, predominantly those in the hematopoietic tissues, typhlosole and kidney [33]. Notably, non-functional VLRA and VLRC assemblies are much more frequent in the thymoid region of the gills than elsewhere [19, 33]. These findings support the idea that a functional VLRA or VLRC assembly is essential for survival of lymphocytes developing in the thymoid. Moreover, analysis of the VLRC sequence in the thymoid and peripheral tissues suggests that receptor selection occurs in the thymoid, wherein the VLRC repertoire is selected for more uniform VLRC length and N-terminal diversity, although the mechanism for the selection is presently unknown.
T-like and B-like cell lineages in lampreys and hagfish
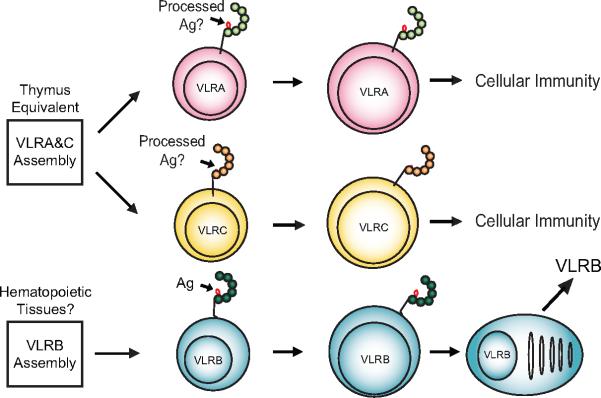
Two T cell-like populations of cells, which express VLRA and VLRC respectively, and a B cell-like lineage of lymphocytes, which express VLRB, have been defined in lampreys (Fig.4) [12, 17-19]. As mentioned above, the VLRA+ and VLRC+ cells are generated in the thymus-equivalent or thymoid region at the tip of each gill fold [33]. Both of these cell types resemble the T lymphocytes in jawed vertebrates. They express their receptors only on the cell surface, respond preferentially to the classical T cell mitogen, phytohemaglutinin, and express similar cytokine, chemokine and transcription factor gene profiles [18, 19]. Conversely, the VLRB+ cells appear to be generated in hematopoietic tissues, express their antigen-binding receptors on the cell surface and they respond to antigenic stimulation by differentiating into plasma cells that secrete multimeric VLRB antibodies [18, 19, 32, 34, 35].
Figure 4.
Model of B- cell and T-cell like lineages in jawless vertebrates (modified from [90]). Cognate antigens (Ag) stimulate VLRA+, VLRC+ and VLRB+ lymphocytes, but whether or not VLRA and VLRC see native or processed antigens is unknown. Antigen-stimulated VLRB cells differentiate into VLRB-secreting plasma cells, whereas activated VLRA and VLRC cells do not secrete their receptors.
Hagfish also have three discrete lymphocyte lineages which express VLRA or VLRB or VLRC. These have not yet been well characterized, but the presently available data suggest that, like their lamprey counterparts, hagfish VLRB+ lymphocytes are B-like and the VLRC+ lymphocytes are T-like cells.
Signature gene profiles for T- and B-like lineages
Discriminating gene expression profiles observed for the three different lymphocyte populations involve molecules for transcription factors, cytokine/chemokines and their receptors, integrins, Toll-like receptors and various signaling proteins. VLRB+ lymphocytes in jawless vertebrates preferentially express genes orthologous to many of those that are expressed by B cells in jawed vertebrates [18, 19]; these include the haematopoietic progenitor homing receptor CXCR4, the B cell transcription factors E2A, paired box protein 5 (Pax5) [36], PR domain zinc finger protein 1 (also called BLIMP1) [37], and B-cell CLL/lymphoma 6 (BCL6) [38], the herpes virus entry mediator/tumor necrosis factor receptor superfamily member 14 (TNFRSF14) that binds to LIGHT on T cells, two components of the BCR-mediated signaling cascades, the spleen tyrosine kinase (Syk) and B cell adaptor protein (BCAP) [39, 40], the interleukin (IL)-8 chemotactic inflammatory cytokine [41], IL-17 receptor, and Toll-like receptors TLR2, TLR7 and TLR10. In contrast, the VLRA+ lymphocytes preferentially express genes orthologous to those typically expressed by T cells in jawed vertebrates: several transcription factors that may be used for T-cell differentiation such as GATA2/3 [42], c-Rel [43], aryl hydrocarbon receptor (AHR) [44] and BCL11b transcriptional factors used for T cell differentiation [45], the CCR9 chemokine receptor involved in thymus homing of lymphocyte progenitors [46], the T-cell fate determining molecule Notch1 [47], the tyrosine phosphatase receptor CD45 that is essential for T cell development [48], the IL-8 receptor CXCR2 [49], and pro-inflammatory cytokines IL-17 [50, 51] and Macrophage migration inhibitory factor (MIF) [52]. The transcriptional profile of VLRC+ cells is similar to that of VLRA+ cells, except that the VLRC+ cells differ [19] in their preferential expression of the SRY-box containing gene 13 (SOX13) encoding a fate-determining transcription factor [53, 54], which is used for γδ T-cell lineage commitment, an integrin αL (ITGAL), one component of the heterodimeric lymphocyte function associated antigen 1 (LFA1), the very late antigen 4 (VLA4) components integrins α4 and β1 (ITGA4 and ITGB1), whose expression correlates with the adherence of human γδ T cells to epithelial cells [55], TLR3 [56] and a modulator of T-cell activation IL-16 [57]. The transcriptional programs for lamprey VLRA and VLRC cells are thus reminiscent of those of mammalian αβ and γδ T cells, respectively.
Structural organization of VLRA and VLRC
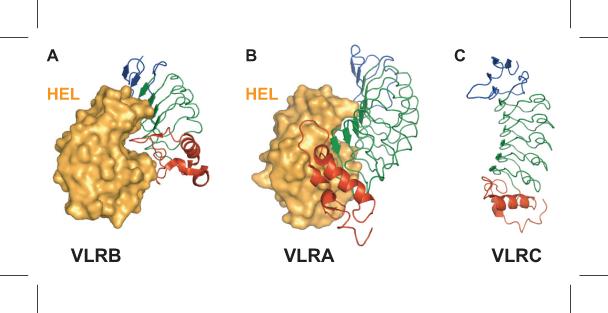
Structures of lamprey and hagfish VLRA, VLRB and VLRC antigen-binding domains have been characterized by X-ray crystallography[58-66]. The antigen-binding domain of all three VLR isotypes is crescent-shaped with β-strands contributed by the LRR modules lining the concave surface; however, there are notable differences in the capping LRR modules of the three VLR types (Fig. 5). The LRRNT module caps the N-terminus that encodes for two anti-parallel β-strands, and it contains two disulfide bonds that stabilize the structure. The LRRNTs of VLRA and VLRC have limited sequence variability compared to the LRRNT of VLRB, and a short protruding loop in the LRRNT was observed in the crystal structure of lamprey VLRC that was not present in the structures of other VLR isotypes[58]. The LRRCT caps the C-terminus and also contains two disulfide bonds for stability like the LRRNT. The LRRCT of VLRA and VLRB isotypes have a variable insert loop that faces the concave surface, whereas the LRRCT of VLRC is almost invariant and does not include an extended insert loop. The VLRA and VLRB LRRCT loops are highly variable in amino acid sequence and in length, which ranges from 9 to 13 amino acids in VLRA and 0 to 13 amino acids in VLRB [16]. In all three VLR isotypes, the LRR1, LRRV, LRRVe, and LRRCP modules each encode a highly variable β-strand to collectively form a continuous β-sheet on the concave face[58, 63, 65, 66]. The average number of β-strands varies for each VLR isotype and this feature is conserved in lampreys and hagfish[16]. VLRB is smaller with an average of 2.5 LRRV-encoded β-strands, while VLRA and VLRC are slightly larger with an average of 4 LRRV-encoded β-strands. Collectively, the isotypic differences in the LRRNT, LRRCT and the average number of β-strands suggest that the different VLR isotypes may bind to antigens in different ways or to different forms of antigen.
Figure 5.
Structures of the three VLR isotypes. (A) VLRB binds to hen egg lysozyme (HEL) using multiple β-strands on its concave surface and insertion of the LRRCT variable loop into the catalytic cleft. (VLRB.2D, PDB ID: 3G3A) (B) VLRA also binds to HEL using its concave surface, but the LRRCT loop packs against the side of HEL, instead of inserting in the catalytic site. (VLRA.R2.1, PDB ID: 3M18) (C) Unlike VLRA and VLRB, VLRC does not have a protruding loop in the LRRCT, and the LRRCT sequence is almost invariant. (PDB ID: 3WO9, LRRCT is indicated in red and LRRNT in blue)
Multiple examples of VLRB binding to native antigens have been described. Immunization with mammalian cells, bacteria and viruses results in the secretion of antigen-specific VLRB antibodies that circulate in the blood. Using HEK-293T cells transfected with VLRB cDNAs [67]and yeast surface display approaches[68], VLRB monoclonal antibodies have been isolated that bind to the BclA spore-coat protein of Bacillus anthracis [61, 67], the BCR idiotope of human B cell chronic lymphocytic leukemia cells[69], human CD5[70], hen egg lysozyme (HEL) [64] and multiple carbohydrates [59, 65, 68, 71]. VLRB-antigen structures have been solved for four of these VLRB monoclonal antibodies: anti-H trisaccharide (RBC36) [65], anti-BclA (VLR4) [61], anti-HEL (VLRB.2D) [64] and anti-TFα disaccharide (aGPA.23) [59]. In all four structures, the antigen makes contacts with multiple residues on the β-strands encoded by LRR1, LRRV(s), LRRVe and LRRCP modules, as well as the LRRCT insert loop, which is essential for the interaction. Although the VLRB LRRNT has high sequence variability, it does not contact the antigen in three of the four structures and only a single amino acid from the LRRNT of VLR4 binds to BclA. A notable feature of the VLRB.2D-HEL structure is that the LRRCT loop is inserted into the catalytic cleft of HEL [64] (Fig. 5A). Other naturally occurring single-chain antibodies, camelid VHH (cAb-Lys3) [72] and shark IgNAR (PBLA8) [73], recognize a similar epitope in the HEL catalytic site, whereas conventional VHVL antibodies typically bind to flatter epitopes instead of the catalytic cleft [60].
Compared to VLRB, little is known about the nature of the antigens recognized by VLRA and VLRC receptors. VLRA and VLRC cells proliferate after immunization, but unlike VLRB cells, they do not secrete their antigen receptors [18, 19]. VLRA cells were unable to bind to B. anthracis, S. typhimurium, E. coli and S. pneumonia before or after immunization, whereas a small percentage of VLRB cells were able to bind to the bacteria and the frequency of binders increased significantly after immunization [18]. One interpretation of the inability of VLRA lymphocytes to bind to bacteria is that VLRA may generally recognize antigens in a processed form similar to jawed vertebrate TCRs, instead of native antigens. However, VLRA clones that bind to native HEL have been isolated by yeast surface display, and the structure of one of the VLRA clones (VLRA.R2.1) in complex with the antigen has been determined [63, 68] (Fig. 5B). In the structure, the concave surface of VLRA.R2.1 binds to a relatively flat epitope on HEL away from the catalytic cleft. Residues from LRRNT, LRR1, LRRV1-4, LRRVe and LRRCP make contacts with HEL and the 10 amino acid residues of the LRRCT loop packs to the side of HEL, instead of inserting into a crevice like VLRB.2D. In the VLRA.R2.1 structure, β-strands on the concave surface account for the majority of the surface area buried by HEL. In contrast, the LRRCT of VLRB.2D contributes the highest proportion of buried surface area[60, 64]. However, mutations in the LRRCT loop of VLRA.R2.1 abolished HEL binding, thereby demonstrating that the LRRCT loop is essential for the HEL interaction for both VLRA and VLRB[63]. The affinity of VLRA.R2.1 for HEL (KD=180 pM) is ~2,400-fold higher than VLRB.2D (KD=0.43μM). VLRA.R2.1 has better shape complementarity for HEL than VLRB.2D, which likely accounts for most of the differences in affinity. VLRA.R2.1 demonstrates that it is possible for VLRA receptors to bind with high affinity to a native protein, but other experimental approaches have been unable to detect VLRA binding to other native antigens, and only VLRA binders to HEL have been isolated by yeast surface display, while VLRB binders to multiple native protein and carbohydrate antigen have been isolated using the same technique [68, 71]. VLRA is similar to a TCR in that it is only found on the lymphocyte surface and never secreted like an antibody. At least in some cases, VLRA receptors can bind unprocessed antigens and are therefore more reminiscent of γ/δ TCRs, some of which also bind native antigens, rather than α/β TCRs that bind to processed and presented antigens.
VLRC is only expressed as a membrane-bound receptor and defines a second population of T cell-like lymphocytes[16, 19, 74]. Unlike VLRA and VLRB, no antigen-specific VLRC receptors have been identified. The LRRCT of VLRC receptors is almost invariant, and it does not have the LRRCT insert loop that is critical for antigen binding in all of the VLRA and VLRB structures characterized so far [13, 16, 58, 66, 74] (Fig. 5C). The lack of the insert loop and sequence diversity in the LRRCT suggests that VLRC binds to antigens in a different way than the other VLR isotypes and possibly to different forms of antigens. The VLRC LRRNT also has limited sequence diversity, hinting at the intriguing possibility that the LRRNT and LRRCT capping modules may be used interact with invariant regions of a cognate MHC-like receptor that presents processed antigen, while highly variable amino acids in LRR1, LRRV, LRRVe and LRRCP contact the antigen.
Similarities of the VLRA/VLRC and TCR α /TCR δ assembly mechanisms and genomic organization
In jawed vertebrates, T cells develop along two primary differentiation pathways that are characterized by expression of either αβ or γδ TCRs. The TCR α and TCR δ loci are interlinked, whereas the TCR β and TCR γ loci are located in two different genomic regions. Since the TCRδ locus is located within the TCRα locus, this allows a common pool of V genes to be shared by TCRα and TCRδ , so that certain V genes may recombine either to a DJδ or directly to a Jα gene segment [75-79]. T cells do not rearrange their Ig genes, and there is no shared usage of Ig and TCR V(D)J components [80], except for rare trans rearrangement between juxtaposed Ig and TCR loci in elasmobranches [81].
Reminiscent of the TCRα and TCRδ genes in jawed vertebrates, some of the donor LRR sequences in both lamprey and hagfish may be shared during the assembly of the VLRA and VLRC genes [82]. In contrast, donor LRR sequence sharing is not evident between assembled VLRA and VLRB genes or between VLRC and VLRB genes. The incomplete germline VLRA and VLRC genes have coding sequences only for the leader peptide, incomplete amino- and carboxy-terminal LRR subunits (LRRNT and LRRCT) and for the stalk region (Fig. 2). Notably, none of these germline VLRA and VLRC gene components is shared during VLR assembly, nor do they serve as donor sequences. For example, assembled sequences that contain the N-terminal portion of VLRA and the C-terminal portion of VLRC are not found and vice versa, even though the requirement of only short stretches of sequence similarity in the step-wise assembly process might suggest that such chimeric sequences could exist. This phenomenon is indicative of a well-tuned genetic control for the utilization of acceptor and donor sequences in VLRA or VLRC locus.
Donor sequence sharing appears to be more frequent in hagfish than in lampreys. Among the different types of genomic cassettes, the 3'LRRV-5' LRRV donor sequences are most frequently shared by VLRAs and VLRCs in both lampreys and hagfish, in part because there are numerically more opportunities for sharing in LRRV-encoding region given that 2-6 LRRV modules are included in the assembled VLRAs and VLRCs. However, functional VLRA and VLRC assemblies do not share LRRCT- or LRRNT-cassettes in common, because the cysteine configurations for the LRRNT and LRRCT portions differ for VLRA and VLRC. The conserved cysteine (Cys) residues in Ig and TCR are important for maintenance of the globulin structure [83]. Similarly, the canonical four cysteines in the LRRNT and LRRCT regions are important for the solenoid structures formed by the LRR modules [65]. Hence, the rule is VLRA-specific and VLRC-specific repertoires for LRRNT and LRRCT regions.
Using the shared and non-shared genomic donor cassettes, Das et al [82] have constructed a partial physical map of the VLRA/VLRC locus in lamprey. The VLRA and VLRC loci are closely linked in the lamprey genome (Fig. 6). Considering the sharing of genomic donor cassettes and the close proximity of VLRA and VLRC genes, the genomic organization of VLRA/C locus somewhat resembles the organization of TCRα/TCRδ locus. Similarly, some of the donor LRR cassettes serve dual function in VLRA and VLRC assembly in jawless vertebrates. Despite the existence of dual-function gene segments, the TCRα and TCRδ in jawed vertebrates and VLRA and VLRC in jawless vertebrates maintain their differential expression in two distinct lymphocyte populations. The TCRα rearrangement occurs after TCRδ rearrangement during T cell development [84, 85] and, similarly VLRA assembly is preceded by VLRC assembly during intrathymoid differentiation of the T-like lymphocytes in jawless vertebrates [19].
Figure 6.

Schematic diagram of the gemomic organization of sea lamprey VLRA/VLRC locus and its resemblance with TCRα/δ loci of jawed vertebrates (modified from [82]). In lamprey a total of 262 genomic donor cassettes have been identified in sea lampreys [82]. Genomic donor cassettes observed only in VLRA or VLRC assemblies are indicated by purple and green dots, respectively, and donor cassettes shared by both VLRA and VLRC are indicated by orange dots.
Conclusion
The origin of the unique structure of TCRα/TCRδ locus remains obscure, but we can deduce the possible mode of origin of the VLRA/VLRC locus. In the sea lamprey genome, multiple duplications comprising different types of donor cassettes are evident in the VLRA/VLRC locus, and some of these duplications appear to be very recent events. Duplication of either the VLRC or VLRA locus, followed by neofunctionalization of the duplicate copies likely gave rise to the complex VLRA/VLRC locus. Multiple duplication events and sequence divergence in the donor LRR cassettes together with the intermingling of different LRR cassette types within the VLRA/VLRC locus are all indicative of a birth-and-death model of evolution, similar to that proposed for the translocon Ig and TCR loci in jawed vertebrates [86-88]. The genomic organization of the VLRA/VLRC locus allows sharing of germline donor LRR cassettes to generate large repertoires of VLRAs and VLRCs in the two T-cell-like populations. The dual-function segments in the TCRα and TCRδ genes in jawed vertebrates and in the VLRA and VLRC genes in jawless vertebrates and their differential expression in two major T cell lineages are indicative of surprising functional convergence. The selective pressures responsible for the conservation of these parallels for >500 million years remain to be explored.
Highlights.
Two T-cell lineages and B-cells evolved before the divergence of jawed and jawless vertebrates.
Lamprey VLRA- and VLRC-producing cells resemble mammalian αβ and γδ T-cells.
VLRA and VLRC genes share donor cassettes during their assembly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Finstad J, Good RA. The Evolution of the Immune Response. 3. Immunologic Responses in the Lamprey. J. Exp. Med. 1964;120:1151–1168. doi: 10.1084/jem.120.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boffa GA, Fine JM, Drilhon A, Amouch P. Immunoglobulins and transferrin in marine lamprey sera. Nature. 1967;214:700–702. doi: 10.1038/214700b0. [DOI] [PubMed] [Google Scholar]
- 4.Marchalonis JJ, Edelman GM. Phylogenetic origins of antibody structure. 3. Antibodies in the primary immune response of the sea lamprey, Petromyzon marinus. J. Exp. Med. 1968;127:891–914. doi: 10.1084/jem.127.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollara B, Litman GW, Finstad J, Howell J, Good RA. The evolution of the immune response. VII. Antibody to human “O” cells and properties of the immunoglobulin in lamprey. J. Immunol. 1970;105:738–745. [PubMed] [Google Scholar]
- 6.Linthicum DS, Hildemann WH. Immunologic responses of Pacific hagfish. 3. Serum antibodies to cellular antigens. J. Immunol. 1970;105:912–918. [PubMed] [Google Scholar]
- 7.Thoenes GH, Hildemann WH. Immunological responses of Pacific hagfish. II. Serum antibody production to soluble antigens. In: Sterzl J, Riha I, editors. Developmental aspects of antibody formation and structure. Academia Publishing House of the Czechoslovak Academy of Science; Prague: 1970. pp. 711–726. [Google Scholar]
- 8.Hildemann WH, Thoenes GH. Immunological responses of Pacific hagfish. I. Skin transplantation immunity. Transplantation. 1969;7:506–521. doi: 10.1097/00007890-196906000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Raison RL, Gilbertson P, Wotherspoon J. Cellular requirements for mixed leucocyte reactivity in the cyclostome, Eptatretus stoutii. Immunol. Cell. Biol. 1987;65(Pt 2):183–188. doi: 10.1038/icb.1987.20. [DOI] [PubMed] [Google Scholar]
- 10.Mayer WE, Uinuk-Ool T, Tichy H, Gartland LA, Klein J, et al. Isolation and characterization of lymphocyte-like cells from a lamprey. Proc. Natl Acad. Sci. USA. 2002;99:14350–14355. doi: 10.1073/pnas.212527499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JJ, Kuraku S, Holt C, Sauka-Spengler T, Jiang N, et al. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat. Genet. 2013;45:415–421. 421e411–412. doi: 10.1038/ng.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, et al. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- 13.Pancer Z, Saha NR, Kasamatsu J, Suzuki T, Amemiya CT, et al. Variable lymphocyte receptors in hagfish. Proc. Natl Acad. Sci. USA. 2005;102:9224–9229. doi: 10.1073/pnas.0503792102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogozin IB, Iyer LM, Liang L, Glazko GV, Liston VG, et al. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat. Immunol. 2007;8:647–656. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]
- 15.Kasamatsu J, Sutoh Y, Fugo K, Otsuka N, Iwabuchi K, et al. Identification of a third variable lymphocyte receptor in the lamprey. Proc. Natl Acad. Sci. USA. 2010;107:14304–14308. doi: 10.1073/pnas.1001910107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Das S, Herrin BR, Hirano M, Cooper MD. Definition of a third VLR gene in hagfish. Proc. Natl Acad. Sci. USA. 2013;110:15013–15018. doi: 10.1073/pnas.1314540110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alder MN, Herrin BR, Sadlonova A, Stockard CR, Grizzle WE, et al. Antibody responses of variable lymphocyte receptors in the lamprey. Nat. Immunol. 2008;9:319–327. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- 18.Guo P, Hirano M, Herrin BR, Li J, Yu C, et al. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirano M, Guo P, McCurley N, Schorpp M, Das S, et al. Evolutionary implications of a third lymphocyte lineage in lampreys. Nature. 2013;501:435–438. doi: 10.1038/nature12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagata T, Suzuki T, Ohta Y, Flajnik MF, Kasahara M. The leukocyte common antigen (CD45) of the Pacific hagfish, Eptatretus stoutii: implications for the primordial function of CD45. Immunogenetics. 2002;54:286–291. doi: 10.1007/s00251-002-0469-1. [DOI] [PubMed] [Google Scholar]
- 21.Uinuk-Ool T, Mayer WE, Sato A, Dongak R, Cooper MD, et al. Lamprey lymphocyte-like cells express homologs of genes involved in immunologically relevant activities of mammalian lymphocytes. Proc. Natl Acad. Sci. USA. 2002;99:14356–14361. doi: 10.1073/pnas.212527699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pancer Z, Mayer WE, Klein J, Cooper MD. Prototypic T cell receptor and CD4-like coreceptor are expressed by lymphocytes in the agnathan sea lamprey. Proc. Natl Acad. Sci. USA. 2004;101:13273–13278. doi: 10.1073/pnas.0405529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothenberg EV, Pant R. Origins of lymphocyte developmental programs: transcription factor evidence. Sem. Immunol. 2004;16:227–238. doi: 10.1016/j.smim.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T, Shin IT, Kohara Y, Kasahara M. Transcriptome analysis of hagfish leukocytes: a framework for understanding the immune system of jawless fishes. Dev. Comp. Immunol. 2004;28:993–1003. doi: 10.1016/j.dci.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Uinuk-Ool T, Nikolaidis N, Sato A, Mayer WE, Klein J. Organization, alternative splicing, polymorphism, and phylogenetic position of lamprey CD45 gene. Immunogenetics. 2005;57:607–617. doi: 10.1007/s00251-005-0019-8. [DOI] [PubMed] [Google Scholar]
- 26.Yu C, Ehrhardt GR, Alder MN, Cooper MD, Xu A. Inhibitory signaling potential of a TCR-like molecule in lamprey. Eur. J. Immunol. 2009;39:571–579. doi: 10.1002/eji.200838846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cannon JP, Haire RN, Pancer Z, Mueller MG, Skapura D, et al. Variable domains and a VpreB-like molecule are present in a jawless vertebrate. Immunogenetics. 2005;56:924–929. doi: 10.1007/s00251-004-0766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki T, Shin IT, Fujiyama A, Kohara Y, Kasahara M. Hagfish leukocytes express a paired receptor family with a variable domain resembling those of antigen receptors. J. Immunol. 2005;174:2885–2891. doi: 10.4049/jimmunol.174.5.2885. [DOI] [PubMed] [Google Scholar]
- 29.McCormack WT, Tjoelker LW, Thompson CB. Avian B-cell development: generation of an immunoglobulin repertoire by gene conversion. Ann. Rev. Immunol. 1991;9:219–241. doi: 10.1146/annurev.iy.09.040191.001251. [DOI] [PubMed] [Google Scholar]
- 30.Winstead CR, Zhai SK, Sethupathi P, Knight KL. Antigen-induced somatic diversification of rabbit IgH genes: gene conversion and point mutation. J. Immunol. 1999;162:6602–6612. [PubMed] [Google Scholar]
- 31.Das S, Mohamedy U, Hirano M, Nei M, Nikolaidis N. Analysis of the immunoglobulin light chain genes in zebra finch: evolutionary implications. Mol. Biol. Evol. 2010;27:113–120. doi: 10.1093/molbev/msp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, et al. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science. 2005;310:1970–1973. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- 33.Bajoghli B, Guo P, Aghaallaei N, Hirano M, Strohmeier C, et al. A thymus candidate in lampreys. Nature. 2011;470:90–94. doi: 10.1038/nature09655. [DOI] [PubMed] [Google Scholar]
- 34.Alder MN, Herrin BR, Sadlonova A, Stockard CR, Grizzle WE, et al. Antibody responses of variable lymphocyte receptors in the lamprey. Nat. Immunol. 2008;9:319–327. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- 35.Herrin BR, Alder MN, Roux KH, Sina C, Ehrhardt GR, et al. Structure and specificity of lamprey monoclonal antibodies. Proc. Natl Acad. Sci. USA. 2008;105:2040–2045. doi: 10.1073/pnas.0711619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams B, Dorfler P, Aguzzi A, Kozmik Z, Urbanek P, et al. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev. 1992;6:1589–1607. doi: 10.1101/gad.6.9.1589. [DOI] [PubMed] [Google Scholar]
- 37.Turner CA, Jr., Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda T, Yoshida T, Okada S, Hatano M, Miki T, et al. Disruption of the Bcl6 gene results in an impaired germinal center formation. J. Exp. Med. 1997;186:439–448. doi: 10.1084/jem.186.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada T, Maeda A, Iwamatsu A, Gotoh K, Kurosaki T. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity. 2000;13:817–827. doi: 10.1016/s1074-7613(00)00079-0. [DOI] [PubMed] [Google Scholar]
- 40.Jumaa H, Hendriks RW, Reth M. B cell signaling and tumorigenesis. Annu. Rev. Immunol. 2005;23:415–445. doi: 10.1146/annurev.immunol.23.021704.115606. [DOI] [PubMed] [Google Scholar]
- 41.Sims-Mourtada JC, Guzman-Rojas L, Rangel R, Nghiem DX, Ullrich SE, et al. In vivo expression of interleukin-8, and regulated on activation, normal, T-cell expressed, and secreted, by human germinal centre B lymphocytes. Immunology. 2003;110:296–303. doi: 10.1046/j.1365-2567.2003.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat. Rev. Immunol. 2009;9:125–135. doi: 10.1038/nri2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banerjee D, Liou HC, Sen R. c-Rel-dependent priming of naive T cells by inflammatory cytokines. Immunity. 2005;23:445–458. doi: 10.1016/j.immuni.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 45.Wakabayashi Y, Watanabe H, Inoue J, Takeda N, Sakata J, et al. Bcl11b is required for differentiation and survival of alphabeta T lymphocytes. Nat. Immunol. 2003;4:533–539. doi: 10.1038/ni927. [DOI] [PubMed] [Google Scholar]
- 46.Liu C, Saito F, Liu Z, Lei Y, Uehara S, et al. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood. 2006;108:2531–2539. doi: 10.1182/blood-2006-05-024190. [DOI] [PubMed] [Google Scholar]
- 47.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu. Rev. Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 48.Kishihara K, Penninger J, Wallace VA, Kundig TM, Kawai K, et al. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 49.Lippert U, Zachmann K, Henz BM, Neumann C. Human T lymphocytes and mast cells differentially express and regulate extra- and intracellular CXCR1 and CXCR2. Exp. Dermatol. 2004;13:520–525. doi: 10.1111/j.0906-6705.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 50.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 51.Tsutsui S, Nakamura O, Watanabe T. Lamprey (Lethenteron japonicum) IL-17 upregulated by LPS-stimulation in the skin cells. Immunogenetics. 2007;59:873–882. doi: 10.1007/s00251-007-0254-2. [DOI] [PubMed] [Google Scholar]
- 52.Weiser WY, Temple PA, Witek-Giannotti JS, Remold HG, Clark SC, et al. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc. Natl Acad. Sci. USA. 1989;86:7522–7526. doi: 10.1073/pnas.86.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, et al. Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science. 2007;315:230–233. doi: 10.1126/science.1135344. [DOI] [PubMed] [Google Scholar]
- 54.Rothenberg EV, Moore JE, Yui MA. Launching the T-cell-lineage developmental programme. Nat. Rev. Immunol. 2008;8:9–21. doi: 10.1038/nri2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakajima S, Roswit WT, Look DC, Holtzman MJ. A hierarchy for integrin expression and adhesiveness among T cell subsets that is linked to TCR gene usage and emphasizes V delta 1+ gamma delta T cell adherence and tissue retention. J. Immunol. 1995;155:1117–1131. [PubMed] [Google Scholar]
- 56.Wesch D, Beetz S, Oberg HH, Marget M, Krengel K, et al. Direct costimulatory effect of TLR3 ligand poly(I:C) on human gamma delta T lymphocytes. J. Immunol. 2006;176:1348–1354. doi: 10.4049/jimmunol.176.3.1348. [DOI] [PubMed] [Google Scholar]
- 57.Wilson KC, Center DM, Cruikshank WW. The effect of interleukin-16 and its precursor on T lymphocyte activation and growth. Growth Factors. 2004;22:97–104. doi: 10.1080/08977190410001704679. [DOI] [PubMed] [Google Scholar]
- 58.Kanda R, Sutoh Y, Kasamatsu J, Maenaka K, Kasahara M, et al. Crystal structure of the lamprey variable lymphocyte receptor C reveals an unusual feature in its N-terminal capping module. PLoS One. 2014;9:e85875. doi: 10.1371/journal.pone.0085875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo M, Velikovsky CA, Yang X, Siddiqui MA, Hong X, et al. Recognition of the Thomsen-Friedenreich pancarcinoma carbohydrate antigen by a lamprey variable lymphocyte receptor. J. Biol. Chem. 2013;288:23597–23606. doi: 10.1074/jbc.M113.480467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deng L, Luo M, Velikovsky A, Mariuzza RA. Structural insights into the evolution of the adaptive immune system. Annu. Rev. Biophys. 2013;42:191–215. doi: 10.1146/annurev-biophys-083012-130422. [DOI] [PubMed] [Google Scholar]
- 61.Kirchdoerfer RN, Herrin BR, Han BW, Turnbough CL, Jr., Cooper MD, et al. Variable lymphocyte receptor recognition of the immunodominant glycoprotein of Bacillus anthracis spores. Structure. 2012;20:479–486. doi: 10.1016/j.str.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mariuzza RA, Velikovsky CA, Deng L, Xu G, Pancer Z. Structural insights into the evolution of the adaptive immune system: the variable lymphocyte receptors of jawless vertebrates. Biol. Chem. 2010;391:753–760. doi: 10.1515/BC.2010.091. [DOI] [PubMed] [Google Scholar]
- 63.Deng L, Velikovsky CA, Xu G, Iyer LM, Tasumi S, et al. A structural basis for antigen recognition by the T cell-like lymphocytes of sea lamprey. Proc. Natl Acad. Sci. USA. 2010;107:13408–13413. doi: 10.1073/pnas.1005475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Velikovsky CA, Deng L, Tasumi S, Iyer LM, Kerzic MC, et al. Structure of a lamprey variable lymphocyte receptor in complex with a protein antigen. Nat. Struct. Mol. Biol. 2009;16:725–730. doi: 10.1038/nsmb.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han BW, Herrin BR, Cooper MD, Wilson IA. Antigen recognition by variable lymphocyte receptors. Science. 2008;321:1834–1837. doi: 10.1126/science.1162484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim HM, Oh SC, Lim KJ, Kasamatsu J, Heo JY, et al. Structural diversity of the hagfish variable lymphocyte receptors. J. Biol. Chem. 2007;282:6726–6732. doi: 10.1074/jbc.M608471200. [DOI] [PubMed] [Google Scholar]
- 67.Herrin BR, Alder MN, Roux KH, Sina C, Ehrhardt GR, et al. Structure and specificity of lamprey monoclonal antibodies. Proc. Natl Acad. Sci. USA. 2008;105:2040–2045. doi: 10.1073/pnas.0711619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tasumi S, Velikovsky CA, Xu G, Gai SA, Wittrup KD, et al. High-affinity lamprey VLRA and VLRB monoclonal antibodies. Proc. Natl Acad. Sci. USA. 2009;106:12891–12896. doi: 10.1073/pnas.0904443106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakahara H, Herrin BR, Alder MN, Catera R, Yan XJ, et al. Chronic lymphocytic leukemia monitoring with a lamprey idiotope-specific antibody. Cancer Immunol. Res. 2013;1:223–228. doi: 10.1158/2326-6066.CIR-13-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu C, Ali S, St-Germain J, Liu Y, Yu X, et al. Purification and identification of cell surface antigens using lamprey monoclonal antibodies. J. Immunol. Methods. 2012;386:43–49. doi: 10.1016/j.jim.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong X, Ma MZ, Gildersleeve JC, Chowdhury S, Barchi JJ, Jr., et al. Sugar-binding proteins from fish: selection of high affinity “lambodies” that recognize biomedically relevant glycans. ACS Chem. Biol. 2013;8:152–160. doi: 10.1021/cb300399s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Genst E, Silence K, Decanniere K, Conrath K, Loris R, et al. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc. Natl Acad. Sci. USA. 2006;103:4586–4591. doi: 10.1073/pnas.0505379103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stanfield RL, Dooley H, Flajnik MF, Wilson IA. Crystal structure of a shark single-domain antibody V region in complex with lysozyme. Science. 2004;305:1770–1773. doi: 10.1126/science.1101148. [DOI] [PubMed] [Google Scholar]
- 74.Kasamatsu J, Sutoh Y, Fugo K, Otsuka N, Iwabuchi K, et al. Identification of a third variable lymphocyte receptor in the lamprey. Proc. Natl Acad. Sci. USA. 2010;107:14304–14308. doi: 10.1073/pnas.1001910107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Satyanarayana K, Hata S, Devlin P, Roncarolo MG, De Vries JE, et al. Genomic organization of the human T-cell antigen-receptor alpha/delta locus. Proc. Natl Acad. Sci. USA. 1988;85:8166–8170. doi: 10.1073/pnas.85.21.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chien YH, Iwashima M, Kaplan KB, Elliott JF, Davis MM. A new T-cell receptor gene located within the alpha locus and expressed early in T-cell differentiation. Nature. 1987;327:677–682. doi: 10.1038/327677a0. [DOI] [PubMed] [Google Scholar]
- 77.Kubota T, Wang J, Gobel TW, Hockett RD, Cooper MD, et al. Characterization of an avian (Gallus gallus domesticus) TCR alpha delta gene locus. J. Immunol. 1999;163:3858–3866. [PubMed] [Google Scholar]
- 78.Oltz EM. Regulation of antigen receptor gene assembly in lymphocytes. Immunol. Res. 2001;23:121–133. doi: 10.1385/IR:23:2-3:121. [DOI] [PubMed] [Google Scholar]
- 79.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 80.Krangel MS, McMurry MT, Hernandez-Munain C, Zhong XP, Carabana J. Accessibility control of T cell receptor gene rearrangement in developing thymocytes. The TCR alpha/delta locus. Immunol. Res. 2000;22:127–135. doi: 10.1385/IR:22:2-3:127. [DOI] [PubMed] [Google Scholar]
- 81.Criscitiello MF, Ohta Y, Saltis M, McKinney EC, Flajnik MF. Evolutionarily conserved TCR binding sites, identification of T cells in primary lymphoid tissues, and surprising trans-rearrangements in nurse shark. J. Immunol. 2010;184:6950–6960. doi: 10.4049/jimmunol.0902774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Das S, Li J, Holland SJ, Iyer LM, Hirano M, et al. Genomic donor cassette sharing during VLRA and VLRC assembly in jawless vertebrates. Proc. Natl Acad. Sci. USA. 2014;111:14828–14833. doi: 10.1073/pnas.1415580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu H, May K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. MAbs. 2012;4:17–23. doi: 10.4161/mabs.4.1.18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Livak F, Tourigny M, Schatz DG, Petrie HT. Characterization of TCR gene rearrangements during adult murine T cell development. J. Immunol. 1999;162:2575–2580. [PubMed] [Google Scholar]
- 85.Krangel MS, Carabana J, Abbarategui I, Schlimgen R, Hawwari A. Enforcing order within a complex locus: current perspectives on the control of V(D)J recombination at the murine T-cell receptor alpha/delta locus. Immunol. Rev. 2004;200:224–232. doi: 10.1111/j.0105-2896.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 86.Su C, Jakobsen I, Gu X, Nei M. Diversity and evolution of T-cell receptor variable region genes in mammals and birds. Immunogenetics. 1999;50:301–308. doi: 10.1007/s002510050606. [DOI] [PubMed] [Google Scholar]
- 87.Das S, Nozawa M, Klein J, Nei M. Evolutionary dynamics of the immunoglobulin heavy chain variable region genes in vertebrates. Immunogenetics. 2008;60:47–55. doi: 10.1007/s00251-007-0270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Das S, Nikolaidis N, Klein J, Nei M. Evolutionary redefinition of immunoglobulin light chain isotypes in tetrapods using molecular markers. Proc. Natl Acad. Sci. USA. 2008;105:16647–16652. doi: 10.1073/pnas.0808800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herrin BR, Hirano M, Li J, Das S, Sutoh Y, et al. B cells and antibodies in jawless vertebrates. In: Honjo T, Reth M, Radbruch A, Alt F, editors. Molecular Biology of B Cells. Academic Press; Waltham, Massachusetts: 2015. pp. 121–132. [Google Scholar]
- 90.Hirano M, Das S, Guo P, Cooper MD. The evolution of adaptive immunity in vertebrates. Adv. Immunol. 2011;109:125–157. doi: 10.1016/B978-0-12-387664-5.00004-2. [DOI] [PubMed] [Google Scholar]