Abstract
Rho GTPase regulated contractile signaling in the trabecular meshwork (TM) has been shown to modulate aqueous humor (AH) outflow and intraocular pressure (IOP). To explore whether elevated IOP, a major risk factor for primary open angle glaucoma (POAG) influences Rho GTPase signaling in the TM, we recorded AH outflow in enucleated contralateral porcine eyes perfused for 4–5 hours at either 15 mm or 50 mm Hg pressure. After perfusion, TM tissue extracted from perfused eyes was evaluated for the activation status of Rho GTPase, myosin light chain (MLC), myosin phosphatase target substrate 1 (MYPT1), myristoylated alanine-rich C-kinase substrate (MARCKS) and paxillin. Eyes perfused at 50 mm Hg exhibited a significant decrease in AH outflow facility compared with those perfused at 15 mm Hg. Additionally, TM tissue from eyes perfused at 50 mm Hg revealed significantly increased levels of activated RhoA and phosphorylated MLC, MYPT1, MARCKS and paxillin compared to TM tissue derived from eyes perfused at 15 mm Hg. Taken together, these observations indicate that elevated IOP-induced activation of Rho GTPase-dependent contractile signaling in the TM is associated with increased resistance to AH outflow through the trabecular pathway, and demonstrate the sensitivity of Rho GTPase signaling to mechanical force in the AH outflow pathway.
Keywords: Trabecular meshwork, Rho GTPase, Mechanotransduction, Intraocular Pressure, Outflow resistance
Glaucoma is the second leading cause of blindness globally, and if untreated, can lead to irreversible blindness due to optic nerve degeneration and loss of retinal ganglion cells (Kwon et al., 2009). Primary open-angle glaucoma (POAG), the most prevalent form of glaucoma in the United States, is associated with elevated intraocular pressure (IOP), which is considered a definitive risk factor for POAG (Kwon et al., 2009; Quigley, 1993; Weinreb and Khaw, 2004). Importantly, lowering IOP has been shown to delay vision loss in glaucoma patients, and has remained only treatment available for all types of glaucoma (Kwon et al., 2009; Lee and Goldberg, 2011; Weinreb and Khaw, 2004). However, the pathobiology of elevated IOP and glaucoma has remained elusive. Therefore, a thorough understanding of molecular basis of both physiological and pathological IOP is expected to offer novel insights into the development of efficacious new IOP lowering medication.
It is commonly believed that elevated IOP derives primarily from the increased resistance to AH outflow through the conventional or trabecular pathway consisting of the trabecular meshwork (TM), Schlemm's canal (SC), and juxtacanalicular connective tissue (JCT) (Gabelt and Kaufman, 2005; Lee and Goldberg, 2011). Although the molecular basis for increased resistance to AH outflow is not completely clear, it is believed that accumulation of extracellular matrix, tissue stiffness, compromised phagocytosis and cell death are some of the factors associated with increased resistance to AH outflow and elevated IOP (Stamer and Acott, 2012; Stamer et al., 2015). Various growth factors (e.g. TGF-β, connective tissue growth factor, Wnt, Nitric oxide, lysophosphatidic acid, sphingosine-1-phosphate and endothelin-1), cytokines, extracellular matrix (ECM) proteins and ECM degrading enzymes have also been shown to modulate AH outflow through the TM by influencing actomyosin organization, cell adhesive interactions and contractile properties of the TM (Stamer and Acott, 2012). Importantly, Rho GTPase and its downstream effector, Rho kinase, have been demonstrated to play a critical role in the signaling mechanisms triggered by physiological factors and to thereby regulate TM contractile properties, ECM synthesis and AH outflow through the TM (Inoue and Tanihara, 2013; Pattabiraman et al., 2013; Pattabiraman and Rao, 2010; Pattabiraman et al., 2014; Rao et al., 2001; Zhang et al., 2008). Additionally, mechanical stretch, matrix rigidity, fluid flow force and tissue fibrosis have also been shown to regulate Rho GTPase signaling activity in vascular and other smooth muscle tissues and in endothelial cells suggesting involvement of Rho GTPase in mechanotransduction (Boopathi et al., 2014; Chiquet et al., 2009; Higashida et al., 2013; Huang et al., 2012; Mammoto et al., 2008; Mattias et al., 2014; Provenzano and Keely, 2011; Tan et al., 2013; Zhao et al., 2007; Zhou et al., 2013). Conversely, elevated IOP has been reported to influence the expression profile of various genes in TM as a homeostatic response to control fluctuations in AH outflow and IOP (Borras, 2003). Since dysregulated Rho GTPase signaling in the trabecular AH outflow pathway has been demonstrated to increase resistance to AH outflow under experimental conditions (Junglas et al., 2012; Pattabiraman et al., 2014; Zhang et al., 2008), and Rho kinase inhibitors are in advanced clinical trials as agents for lowering IOP in human patients (Inoue and Tanihara, 2013; Williams et al., 2011), in this study, we sought to address whether elevated IOP influences the activity of Rho GTPase and its downstream effector proteins involved in contractile and cell adhesive responses in TM in the context of AH outflow resistance.
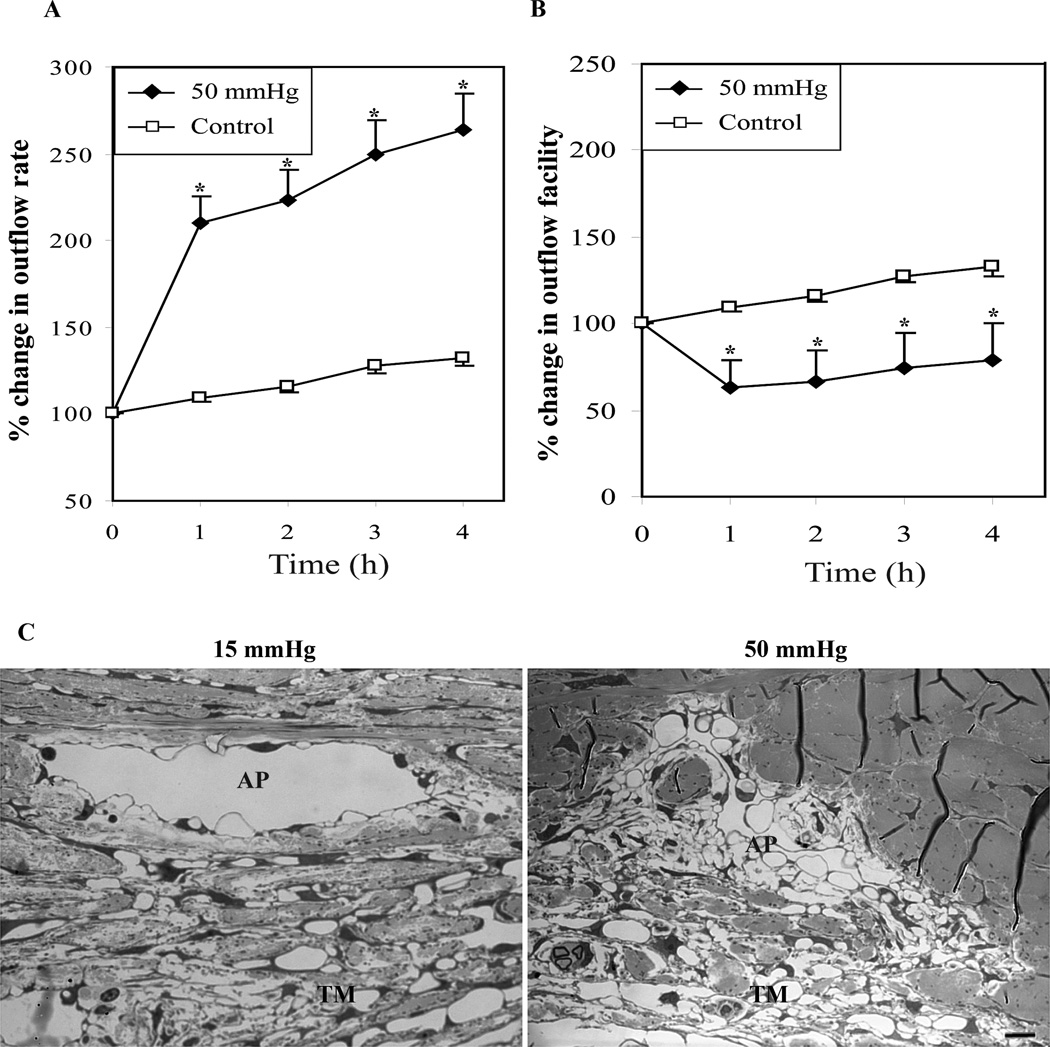
To this end, experiments were conducted on freshly enucleated cadaver porcine eyes (contralateral pairs) perfused with Dulbecco’s Phosphate Buffered Saline, pH7.4 containing 5.5 mM d- glucose at 32 °C, using a Grant perfusion system as we described previously (Epstein et al., 1999; Rao et al., 2001). Eyes were initially perfused at 15 mm Hg for 1 hour to record baseline AH outflow, following which one eye from each pair continued to be perfused at 15 mm Hg, while the contralateral eye was perfused at 50 mm Hg for 4 hours. Aqueous outflow was recorded at hourly intervals and after completion of perfusion, aqueous outflow rate (µl/min) and facility (µl/min/mm Hg) data were analyzed by a paired two-tailed Student’s t-test or ANOVA (analysis of variance) to determine significance of any observed changes. Fig. 1A which illustrates percent change in aqueous outflow rate from baseline, shows a significant increase in aqueous outflow (p<0.05; n=7) rate in eyes perfused at 50 mm Hg compared to control eyes perfused at 15mm Hg. On the other hand, aqueous outflow facility in eyes perfused at 50 mm Hg showed a significant decrease compared to eyes perfused at 15 mm Hg (Fig. 1B). This decrease in aqueous outflow facility in eyes perfused at 50 mm Hg was evident from the first hour and persisted till the 4th hour post baseline perfusion, indicating increased resistance to aqueous outflow in response to elevated perfusion pressure. This observation was found to be consistent with several previous reports in which an inverse association has been confirmed between IOP and AH outflow facility (Battista et al., 2008; Becker and Constant, 1956; Brubaker, 1975; Dai et al., 2009; Hashimoto and Epstein, 1980). These data also indicate that under conditions of acutely increased perfusion pressure, the AH outflow tissues appear to adapt to facilitate increased AH drainage through the conventional pathway within the first hour, based on the observed increase in the aqueous outflow rate in the eyes perfused at 50 mm Hg.
Fig. 1.
Elevated IOP in enucleated porcine eyes increases aqueous outflow rate and decreases aqueous outflow facility. A and B shows percent change in aqueous outflow rate and aqueous outflow facility from baseline in eyes perfused at 50 and 15 mm Hg, respectively. The eyes perfused at 50 mm Hg showed a significant increase in aqueous outflow rate (µl/min) from the base line value compared to eyes perfused at 15 mm Hg (A). On the other hand, the aqueous outflow facility (µl/min/mm Hg) was found to be decreased significantly from base line facility in eyes perfused at 50 mm Hg compared with eyes perfused at 15 mm Hg (B). Values represent mean ± standard error. n = 7, *p < 0.05, **p < 0.01. C. Porcine eyes perfused at 50 mm Hg for 5 hours revealed collapsed aqueous plexi (AP) and disorganized/compressed TM compared with eyes perfused at 15 mm Hg, based on histological evaluation by transmission electron microscopy. Bar indicates 10µm image magnification.
Additionally, when perfusion pressure was lowered to 15 mm Hg in eyes initially perfused at high pressure (50 mm Hg for 4 hours), the outflow facility began to increase significantly within three hours, indicating that perfusion pressure exerts a reversible response on outflow facility in the short term perfusion model (data not shown). In Fig. 1A and B, porcine eyes perfused at 15 mm Hg also show a steady increase in aqueous outflow rate and facility with increased perfusion time, a response attributed to the washout effect (Epstein et al., 1999). Although, the molecular mechanism involved in washout effect is not thoroughly understood, enucleated eyes from certain species exhibit increased outflow facility with time during perfusion with normal perfusion medium (Battista et al., 2008; Epstein et al., 1999). Since contralateral paired eyes were used to evaluate the effects of 15 and 50 mm Hg intraocular pressure on outflow facility, the washout response observed in controls eyes was subtracted from the values of outflow facility measured in eyes perfused at 50 mm Hg.
The eyes perfused for five hours at 15 and 50 mm Hg were fixed for histological analysis of changes as we described earlier (Rao et al., 2001), then examined for the changes in the integrity of aqueous outflow pathway tissues including TM and aqueous plexi (equivalent to SC in humans), using transmission electron microscopy (Fig. 1C). These analyses revealed a much reduced lumen space in aqueous plexi (AP) of eyes perfused at 50 mm Hg compared to those perfused at 15 mm Hg. Similarly, there was an obvious disorganization and compression of TM in eyes perfused at 50 mm Hg relative to eyes perfused at 15 mm Hg (Fig. 1C). Consistent with previously reported observations (Battista et al., 2008), these histological changes observed in the TM and AP of porcine eyes subjected to high pressure perfusion demonstrate the collapsible nature of AP and disorganization of TM under high pressure perfusion and correlated well with increased resistance to aqueous humor outflow.
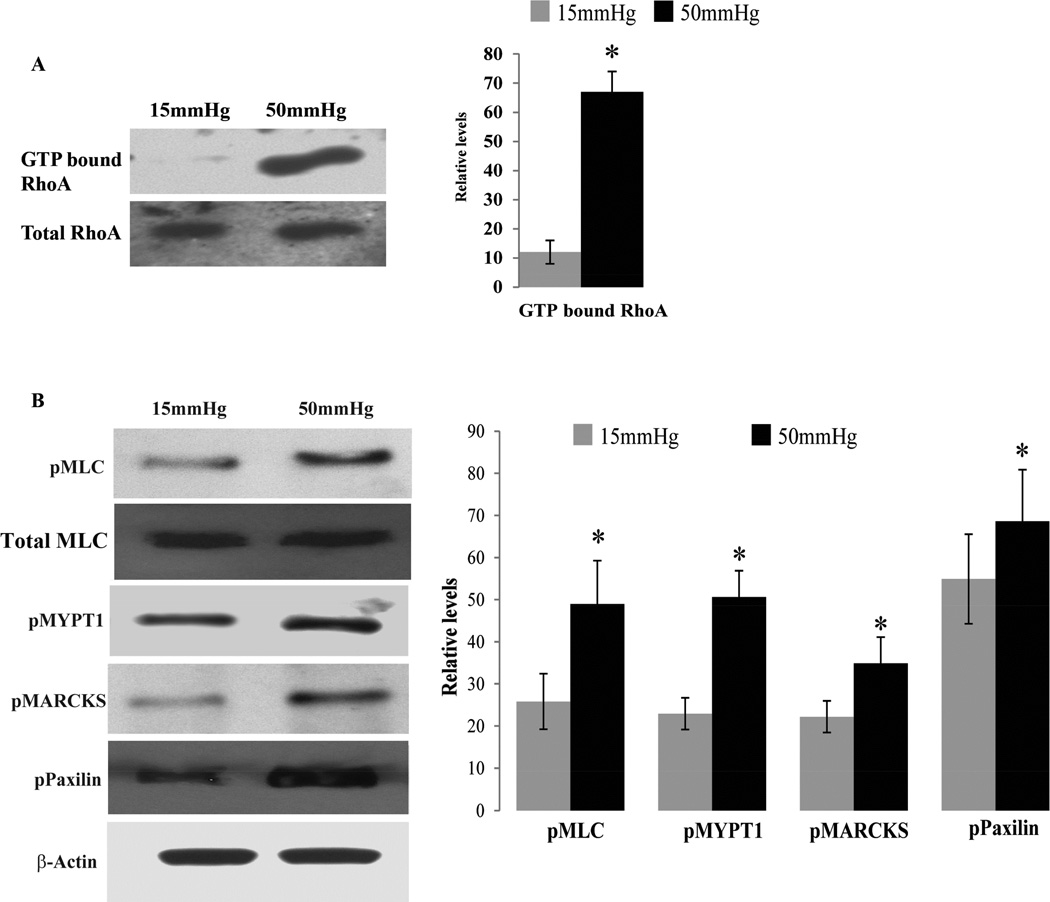
The Rho GTPase/Rho kinase signaling pathway has been shown to modulate AH outflow by regulating the contractile properties of TM and SC cells in both, enucleated eyes and in live animals of different species, including humans (Inoue and Tanihara, 2013; Pattabiraman et al., 2014; Rao et al., 2001). Moreover, Rho GTPase regulates the formation of focal adhesions which are well-recognized mechanosensors for transduction of mechanical forces into biological signals (mechanotransduction) involving integrins, ECM, focal adhesion adaptor proteins, actomyosin cytoskeleton and tyrosine kinases (Goldmann, 2014; Provenzano and Keely, 2011; Schwarz et al., 2006; Shemesh et al., 2005). Therefore, to explore the plausible influence of elevated IOP on Rho GTPase activation, contractile activity and focal adhesion formation in TM tissue, we extracted TM tissue from enucleated porcine eyes perfused either at 15 or 50 mm Hg for 5 hours as described earlier. For these analyses, each sample was comprised of pooled TM tissue from 4 eyes, and 3 pooled samples were analyzed per experimental condition – i.e., the basal (15 mm Hg) and the high (50 mm Hg) pressure conditions. Such pooled samples were collected separately for the Rho GTPase activation assay (Set 1) and phosphorylation status of MLC, MARCKS, MYPT1 and paxillin (Set 2). For the set 1 samples, TM tissue was snap frozen in liquid nitrogen immediately upon extraction from the perfused eye, and stored at −80 °C till further analysis. For assessment of Rho activation status, TM samples were processed for RhoA-GTP pulldown assay using a RhoA activation assay kit (Cytoskeleton Inc, Denver, CO) as we described earlier (Zhang et al., 2008). Assays were done in triplicates with appropriate positive and negative controls as per the manufacturer’s instructions. Tissue lysates containing 500µg of protein were processed in the Rho-GTP pull-down assay to quantify the activated Rho GTPase (Rho-GTP form), using rhotekin-Rho binding domain (RBD) beads, followed by immunoblot analysis using an anti-RhoA monoclonal antibody (Cytoskeleton Inc), as we described earlier (Zhang et al., 2008). Figure 2A shows a representative immunoblot of activated Rho GTPase from both basal (15 mm Hg) and high pressure (50 mm Hg) TM specimens along with total RhoA GTPase which was probed as a loading control using anti-RhoA polyclonal antibody (sc179; Santa Cruz Biotechnology). Quantitative analysis of the levels of activated RhoA in TM tissue from high pressure perfused eyes showed a significant increase (p<0.5, n=3; based on student’s t-test) compared with TM tissue from eyes perfused at basal pressure, demonstrating that elevated IOP triggers activation of RhoA in the TM.
Fig. 2.
Activation of Rho GTPase regulated contractile activity by elevated IOP in the trabecular meshwork of porcine eyes. A. TM derived from eyes perfused under elevated pressure (50 mm Hg) showed significantly increased levels of activated RhoA (RhoA-GTP form) compared to TM derived from eyes perfused at basal pressure (15 mm Hg). Levels of activated RhoA were normalized to total RhoA protein levels. B. TM tissue derived from eyes perfused at 50 mm Hg showed a significant increase in levels of phosphorylated-MLC, MYPT1, MARCKS and Paxillin compared to TM tissue from eyes perfused at 15 mm Hg. pMLC immunoblots were normalized to the total MLC loading control, while other immunoblot data were normalized to the β-actin loading control. Histograms depict values derived from densitometric analysis of immunoblots. Values represent mean± Standard deviation. n = 3, *p < 0.05.
Rho GTPase induced cellular contraction and focal adhesion formation are regulated by the activation status of MYPT1, MLC, paxillin and MARCKS via phosphorylation-dependent changes in the activity of these proteins. MLC is a regulatory subunit of myosin II, a key component of contractile machinery. The phosphorylation status of MLC regulates myosin II interaction with actin to form actin stress fibers during contraction (Somlyo and Somlyo, 2003). Rho kinase, the key downstream effector of Rho GTPase regulates MLC phosphorylation in a calcium independent manner by inactivating myosin phosphatase (MYPT1 substrate) through phosphorylation (Fukata et al., 2001; Somlyo and Somlyo, 2003; Uehata et al., 1997). MARCKS is one of the actin binding proteins and involved in actin dynamics in a phosphorylation dependent manner regulated by protein kinase C and Rho kinase (Nagumo et al., 2001). It plays an important role in maintaining cell shape, migration and phagocytosis (Aderem, 1995; Arbuzova et al., 2002). Likewise, paxillin is one of the key components of focal adhesions and binds to multiple proteins including integrin, actin cytoskeleton and regulators of Rho GTPase and tyrosine kinases (Turner, 2000). It plays a crucial role in spatial control of activities of Rho GTPases and linking the actin cytoskeleton to ECM through integrins at the focal adhesions in a phosphorylation dependent manner (Deakin and Turner, 2008). To determine the activation status of these different Rho GTPase target proteins in the TM tissue under high pressure perfusion, set 2 TM samples were extracted from perfused eyes after the eye globes were initially washed with 10% trichloroacetic acid (TCA) in PBS for 5 minutes. The purpose of this acid fixation was to stop the metabolic activity and phosphatases activity of tissue as quickly as possible after the eyes were perfused at basal or high pressure. The TCA treated AH outflow pathway tissue (TM and SC) was extracted and processed with urea buffer, pH 7.6 containing (8 M urea, 20 mM Tris, 23 mM glycine, 10 mM dithiothreitol and saturated sucrose.) A Bio-Rad protein dye (Cat# 500–0006) method was used to quantify protein in tissue lysates, as we described earlier (Rao et al., 2001). Urea-glycerol gel electrophoresis and immunblot analysis were performed to detect phospho-MLC, total MLC using anti-phospho MLC and anti-MLC rabbit polyclonal antibodies (Ct# 3671; Cat #3672; Cell Signaling Technology) respectively, as we described earlier (Rao et al., 2001). For detecting changes in phosphorylation status of MYPT1, MARCKS and paxillin, the urea buffer –based tissue lysates described above were subjected to immunoblot analysis using rabbit polyclonal anti-phospho-MYPT1 (Cat# ABS45; Millipore), MARCKS (Cat# 2741; Cell Signaling Technology) and paxillin (Cat# 2541; Cell signaling Technology) antibodies. Immunoblot films were subjected to densitometry analyses using Image J, NIH Image software. β-actin was immunoblotted as a loading control, using anti-monoclonal β-actin (Cat# A5441; Sigma-Aldrich) and all data were normalized against the specified loading control. As shown in Fig. 2B, the phosphorylation of MLC, MYPT1, MARCKS and paxillin was increased significantly (p<0.5, n=3; based on student’s t-test) in TM tissue derived from eyes perfused at 50 mm Hg, compared with TM tissue from eyes perfused at 15 mm, demonstrating an increase in activation of these different proteins under high pressure in TM tissue. In contrast, the levels of total MLC were not found to be different between the two groups of samples described above (Fig. 2B).
The main goal of this study was to explore whether mechanical stretch due to elevated IOP influences the Rho GTPase regulated contractile activity of TM tissue. Our results of significantly increased levels of activated Rho GTPase, phospho-paxillin, phospho-MLC, phospho-MYPT1 and phospho-MARCKs in the TM tissue derived from the eyes perfused under high pressure conditions (50 mm Hg) relative to TM tissue derived from eyes perfused at basal pressure (15 mm Hg), demonstrate that Rho GTPase and paxillin are targets of mechanotransduction within the AH outflow pathway, and suggest that these molecules can in turn influence contractile properties of TM tissue by regulating myosin II activity, actin cytoskeletal organization and focal adhesion formation. These cellular changes have been shown to correlate well with increased resistance to AH outflow in experimental perfusion models (Zhang et al., 2008). Therefore, it is reasonable to speculate that the increased resistance to aqueous outflow observed in this study in eyes perfused at 50 mm Hg could be partly due to increased contractile activity of TM tissue by Rho GTPase signaling and paxillin-associated focal adhesions. In this study we have not addressed whether the observed changes in Rho GTPase activation and phosphorylation of paxillin, MLC, MYPT1 and MARCKS are specific to acute rise in IOP or whether these changes persist over a prolonged period under conditions of elevated IOP. Importantly, it has been demonstrated that the TM has the capacity to support homeostatic response under a prolonged period of elevated IOP (Acott et al., 2014; Borras, 2003). This property of the TM is certainly more relevant and significant as a homeostatic mechanism in the normal eye. However, in glaucomatous eyes with high IOP, in which such a homeostatic mechanism might be expected to have failed, it is likely that elevated IOP drives an increase in Rho GTPase activation and contractile activity in TM, and thus potentially increases resistance to AH outflow. In support of this hypothesis, studies from our laboratory have shown that conditions leading to sustained activation of Rho GTPase activity in the AH outflow pathway increase resistance to AH outflow through increased contractile activity of the TM and SC (Zhang et al., 2008). Therefore, this study provides significant insight into the importance of mechanotransduction in regulating the contractile activity of TM during both physiological and pathological conditions of IOP and AH outflow resistance. This is the first study to demonstrate an elevated IOP-mediated activation of Rho GTPase regulated contractile activity in the TM, which is in turn, associated with increased resistance to AH outflow. Therefore, inhibition of Rho/Rho kinase signaling is expected to suppress the dysregulated mechanotransduction and increase in resistance to AH outflow associated with elevated IOP in glaucoma patients.
Highlights.
Elevated intraocular pressure induces RhoA activation in the trabecular meshwork
Elevated intraocular pressure induces the trabecular meshwork contractile activity
RhoA activation associates with increased resistance to aqueous humor outflow
RhoA is sensitive to mechanotransduction in the trabecular meshwork
Acknowledgements
This research is supported by R01 grant (EY018590) from the NIH, and Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
Contributions: PP and TI performed the experiments. PP, TI and PVR designed the experiments and written the manuscript.
References
- Acott TS, Kelley MJ, Keller KE, Vranka JA, Abu-Hassan DW, Li X, Aga M, Bradley JM. Intraocular pressure homeostasis: maintaining balance in a high-pressure environment. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2014;30:94–101. doi: 10.1089/jop.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A. The MARCKS family of protein kinase-C substrates. Biochemical Society transactions. 1995;23:587–591. doi: 10.1042/bst0230587. [DOI] [PubMed] [Google Scholar]
- Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MARCKS proteins. The Biochemical journal. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Investigative ophthalmology & visual science. 2008;49:5346–5352. doi: 10.1167/iovs.08-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Constant MA. The facility of aqueous outflow; a comparison of tonography and perfusion measurements in vivo and in vitro. AMA Arch Ophthalmol. 1956;55:305–312. [PubMed] [Google Scholar]
- Boopathi E, Gomes C, Zderic SA, Malkowicz B, Chakrabarti R, Patel DP, Wein AJ, Chacko S. Mechanical stretch upregulates proteins involved in Ca2+ sensitization in urinary bladder smooth muscle hypertrophy. Am J Physiol Cell Physiol. 2014;307:C542–C553. doi: 10.1152/ajpcell.00033.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borras T. Gene expression in the trabecular meshwork and the influence of intraocular pressure. Prog Retin Eye Res. 2003;22:435–463. doi: 10.1016/s1350-9462(03)00018-1. [DOI] [PubMed] [Google Scholar]
- Brubaker RF. The effect of intraocular pressure on conventional outflow resistance in the enucleated human eye. Invest Ophthalmol. 1975;14:286–292. [PubMed] [Google Scholar]
- Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochimica et biophysica acta. 2009;1793:911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Dai Y, Lindsey JD, Duong-Polk X, Nguyen D, Hofer A, Weinreb RN. Outflow facility in mice with a targeted type I collagen mutation. Invest Ophthalmol Vis Sci. 2009;50:5749–5753. doi: 10.1167/iovs.08-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin NO, Turner CE. Paxillin comes of age. Journal of cell science. 2008;121:2435–2444. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Investigative ophthalmology & visual science. 1999;40:74–81. [PubMed] [Google Scholar]
- Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24:612–637. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Goldmann WH. Mechanosensation: a basic cellular process. Progress in molecular biology and translational science. 2014;126:75–102. doi: 10.1016/B978-0-12-394624-9.00004-X. [DOI] [PubMed] [Google Scholar]
- Hashimoto JM, Epstein DL. Influence of intraocular pressure on aqueous outflow facility in enucleated eyes of different mammals. Investigative ophthalmology & visual science. 1980;19:1483–1489. [PubMed] [Google Scholar]
- Higashida C, Kiuchi T, Akiba Y, Mizuno H, Maruoka M, Narumiya S, Mizuno K, Watanabe N. F- and G-actin homeostasis regulates mechanosensitive actin nucleation by formins. Nature cell biology. 2013;15:395–405. doi: 10.1038/ncb2693. [DOI] [PubMed] [Google Scholar]
- Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. American journal of respiratory cell and molecular biology. 2012;47:340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Tanihara H. Rho-associated kinase inhibitors: a novel glaucoma therapy. Prog Retin Eye Res. 2013;37:1–12. doi: 10.1016/j.preteyeres.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Junglas B, Kuespert S, Seleem AA, Struller T, Ullmann S, Bosl M, Bosserhoff A, Kostler J, Wagner R, Tamm ER, Fuchshofer R. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. The American journal of pathology. 2012;180:2386–2403. doi: 10.1016/j.ajpath.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AJ, Goldberg I. Emerging drugs for ocular hypertension. Expert Opin Emerg Drugs. 2011;16:137–161. doi: 10.1517/14728214.2011.521631. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Mammoto T, Ingber DE. Rho signaling and mechanical control of vascular development. Current opinion in hematology. 2008;15:228–234. doi: 10.1097/MOH.0b013e3282fa7445. [DOI] [PubMed] [Google Scholar]
- Mattias L, Haque A, Adnan N, Akaike T. The effects of artificial E-cadherin matrix-induced embryonic stem cell scattering on paxillin and RhoA activation via alpha-catenin. Biomaterials. 2014;35:1797–1806. doi: 10.1016/j.biomaterials.2013.11.042. [DOI] [PubMed] [Google Scholar]
- Nagumo H, Ikenoya M, Sakurada K, Furuya K, Ikuhara T, Hiraoka H, Sasaki Y. Rhoassociated kinase phosphorylates MARCKS in human neuronal cells. Biochemical and biophysical research communications. 2001;280:605–609. doi: 10.1006/bbrc.2000.4179. [DOI] [PubMed] [Google Scholar]
- Pattabiraman PP, Maddala R, Rao PV. Regulation of Plasticity and Fibrogenic Activity of Trabecular Meshwork Cells by Rho GTPase Signaling. Journal of cellular physiology. 2013 doi: 10.1002/jcp.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010;298:C749–C763. doi: 10.1152/ajpcell.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman PP, Rinkoski T, Poeschla E, Proia A, Challa P, Rao PV. RhoA GTPase-Induced Ocular Hypertension in a Rodent Model Is Associated with Increased Fibrogenic Activity in the Trabecular Meshwork. The American journal of pathology. 2014 doi: 10.1016/j.ajpath.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. Journal of cell science. 2011;124:1195–1205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA. Open-angle glaucoma. N Engl J Med. 1993;328:1097–1106. doi: 10.1056/NEJM199304153281507. [DOI] [PubMed] [Google Scholar]
- Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Investigative ophthalmology & visual science. 2001;42:1029–1037. [PubMed] [Google Scholar]
- Schwarz US, Erdmann T, Bischofs IB. Focal adhesions as mechanosensors: the two-spring model. Bio Systems. 2006;83:225–232. doi: 10.1016/j.biosystems.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Shemesh T, Geiger B, Bershadsky AD, Kozlov MM. Focal adhesions as mechanosensors: a physical mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12383–12388. doi: 10.1073/pnas.0500254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Current opinion in ophthalmology. 2012;23:135–143. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Braakman ST, Zhou EH, Ethier CR, Fredberg JJ, Overby DR, Johnson M. Biomechanics of Schlemm's canal endothelium and intraocular pressure reduction. Prog Retin Eye Res. 2015;44C:86–98. doi: 10.1016/j.preteyeres.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Biechler S, Junor L, Yost MJ, Dean D, Li J, Potts JD, Goodwin RL. Fluid flow forces and rhoA regulate fibrous development of the atrioventricular valves. Developmental biology. 2013;374:345–356. doi: 10.1016/j.ydbio.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Turner CE. Paxillin and focal adhesion signalling. Nature cell biology. 2000;2:E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- Williams RD, Novack GD, van Haarlem T, Kopczynski C, Group ARPAS. Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. American journal of ophthalmology. 2011;152:834–841. e831. doi: 10.1016/j.ajo.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Zhang M, Maddala R, Rao PV. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am J Physiol Cell Physiol. 2008;295:C1057–C1070. doi: 10.1152/ajpcell.00481.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XH, Laschinger C, Arora P, Szaszi K, Kapus A, McCulloch CA. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J Cell Sci. 2007;120:1801–1809. doi: 10.1242/jcs.001586. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. The Journal of clinical investigation. 2013;123:1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]