Abstract
The prevalence of maternal obesity is rising rapidly worldwide and constitutes a major obstetric problem, increasing mortality and morbidity in both mother and offspring. Obese women are predisposed to pregnancy complications such as gestational diabetes mellitus (GDM), and children of obese mothers are more likely to develop cardiovascular and metabolic disease in later life. Maternal obesity and GDM may be associated with a state of chronic, low-grade inflammation termed “metainflammation”, as opposed to an acute inflammatory response. This inflammatory environment may be one mechanism by which offspring of obese women are programmed to develop adult disorders. Herein we review the evidence that maternal obesity and GDM are associated with changes in the maternal, fetal and placental inflammatory profile. Maternal inflammation in obesity and GDM may not always be associated with fetal inflammation. We propose that the placenta ‘senses’ and adapts to the maternal inflammatory environment, and plays a central role as both a target and producer of inflammatory mediators. In this manner, maternal obesity and GDM may indirectly program the fetus for later disease by influencing placental function.
Keywords: Metainflammation, obesity, gestational diabetes mellitus, placenta, cytokines
1. Introduction
Obesity in pregnancy is rapidly becoming more common worldwide and constitutes a major medical problem. In the United States, 35% of women of reproductive age are obese (body mass index, BMI>30kgm/m2) [1]. Maternal obesity has a significant economic impact, estimated to cost $106.8 million annually in the US alone [2]. Obesity during pregnancy is associated with increased mortality and morbidity for both mother and offspring. Obese women are at greater risk for developing pregnancy complications such as preeclampsia, thromboembolism, and gestational diabetes mellitus (GDM), as well as cardiovascular and metabolic disorders in later life [3, 4]. The risk of developing GDM is increased 1.3–3.8 times in obese women compared to women of normal BMI [5], and ~70% of women with GDM may go on to develop type 2 diabetes up to 28 years post-partum [6].
Infants of obese mothers have a higher incidence of congenital abnormalities and are more likely to be large for gestational age (LGA) at birth [4, 7, 8]. Children of obese mothers, in particular if they are born LGA, are prone to develop metabolic disease [9–12]. The hypothesis that the fetal environment may influence the development of disease in adulthood, now termed the ‘developmental origins of adult disease’, was proposed by Barker and based on the observation that low birth weight correlated positively with the development of cardiovascular disease in later life [13]. Considerable epidemiological evidence has accumulated demonstrating that maternal obesity is predictive for the development of obesity [14], cardiovascular disease [15], and type 2 diabetes in offspring [10]. Children of obese women with GDM are more likely to have increased adiposity and be insulin resistant, propagating the vicious cycle of metabolic disorders into the next generation [16]. There are currently limited options for early prevention of the metabolic syndrome in children born to obese women. Improving the metabolic environment of the obese pregnant mother to break this vicious cycle may be an attractive approach to decrease the future economical, societal, and personal burden of obesity. However, to make progress in this area, better understanding of the mechanisms linking obesity in pregnancy to adverse outcomes in the infant is necessary.
In recent years, there has been increased interest in the role of inflammation as a mediator of programming of metabolic disorders following exposure to the adverse intrauterine environment in maternal obesity. In the non-pregnant state, obesity is associated with a chronic, low-grade inflammatory state, termed ‘metainflammation’, or metabolically induced inflammation [17]. Metainflammation is distinct from an acute pro-inflammatory response and is triggered primarily by metabolites and nutrients, leading to systemic insulin resistance [17].
Placental inflammation has been observed in pregnancies complicated by obesity [18] and GDM [19], and may play a central role in determining the fetal environment in these pregnancies. Normal pregnancy is associated with a highly regulated inflammatory response that is vital to the process of placentation, from implantation through to labor at term [20]. Maternal obesity and GDM have been associated with changes in placental nutrient transporter expression and activity [21, 22]. These changes in placental function may be caused by altered inflammatory profiles in the mother, placenta and fetus in obesity and GDM, leading to the co-morbidities observed in these pregnancies [23, 24]. This article summarizes the literature reporting inflammatory profiles in the maternal, placental and fetal compartments in association to maternal obesity and GDM. Unless otherwise stated, we have focused specifically on studies documenting inflammation in women. While vascular and endothelial changes, oxidative stress, and tissue damage do occur in response to inflammation, we have focused on primary changes in inflammatory mediators and immune cells and pathways in obese pregnancies and GDM. A better understanding of how maternal obesity and metainflammation relates to the fetal intrauterine environment may lead to the development of better therapeutic interventions to prevent the development of metabolic disorders in later life.
2. Metainflammation, Obesity and Insulin Resistance in the Non-Pregnant State
The acute inflammatory response induced by infection or trauma is typically a rapid response, characterized by vasodilation, infiltration of tissue by neutrophils, accumulation of macrophages and lymphocytes, and resolution. Acute inflammation is also characterized by an increased basal metabolic rate due to the focused and rapid response to the insult. Once the insult is neutralized, inflammation subsides. Inflammation induced by obesity in non-pregnant individuals is distinct from the classical inflammatory response in that (1) it is metabolically induced by excessive consumption of nutrients; (2) it is a modest and low-grade response; (3) it alters the profile of immune cells favoring a pro-inflammatory environment in tissues such as adipose, liver and pancreas; (4) it is chronically maintained by metabolic cells such as adipocytes without resolution, and (5) it is associated with a reduced metabolic rate [17]. For these reasons, the term ‘metaflammation’ or ‘metainflammation’ was coined to describe this particular profile associated with obesity [17].
The link between adiposity, inflammation and insulin resistance was first identified when it was observed that levels of the proinflammatory cytokine TNF-α were increased in adipose tissue of obese individuals, and that TNF-α antagonism led to increased insulin sensitivity [25]. Weight-loss is associated with a reduction of TNF-α and reversal of insulin resistance [26]. It is now evident that, in the obese state, several adipokines, chemokines, and cytokines released from adipose tissue and immune cells may interact in an autocrine and paracrine network, causing impaired insulin sensitivity in the metabolic syndrome. Adipose tissue thus behaves as one of the largest endocrine organs in the body [27].
Insulin resistance is a primary feature of the metabolic syndrome, caused by reduced insulin sensitivity in adipose tissue, muscle and liver. Pancreatic β-cells increase insulin secretion, but when the demand for insulin exceeds the secretory capacity of the β-cells, hyperglycemia and diabetes may ensue. Insulin exerts its actions on muscle, liver and adipose tissue by binding insulin receptors, which leads to tyrosine kinase phosphorylation of insulin receptor substrates (IRS). IRS binds the regulatory subunit of phosphoinositide 3-kinase (PI3K), activating protein kinase 1 (PDK1) which in turn phosphorylates Akt, resulting in translocation of GLUT4 (glucose transporter 4) to the plasma membrane in adipose tissue and muscle to facilitate glucose uptake. Insulin-stimulated Akt phosphorylation also activates glycogen synthase and glycogen synthesis. Mitogen activated protein kinase (MAPK) and mammalian target of rapamycin complex (mTORC) pathways downstream of insulin signaling play a role in promoting protein synthesis and cell growth and differentiation [28]. In obesity, nutrients or inflammatory signals cause activation of the kinases Janus kinase (JNK) and Iκβ kinase (IKK), leading to serine phosphorylation of IRS-1 and inhibition of the insulin receptor-signaling cascade, thus causing insulin resistance [17]. Knockout mouse models have highlighted the importance of inflammatory signaling in the development of insulin resistance, as deletion of IKK2 and JNK-1 prevents insulin resistance, while deletion of suppressor of cytokine signaling 1 (SOCS1) or PPAR-γ causes insulin resistance [28, 29].
The immune cell profile is altered in obesity. An increase in macrophages, neutrophils, T cells, B cells and mast cells is observed in visceral adipose tissues (VAT) in non-pregnant mice with diet-induced obesity. Conversely, adipose tissue T-helper cells, regulatory-T cells and eosinophils are decreased. In the non-obese state, macrophages comprise 10–15% of the VAT immune cell population, while 40–50% of all immune cells in VAT are macrophages in obese humans and mice. Macrophages may be activated by the classical or the alternative pathway (M1 and M2 respectively) [30]. In non-obese individuals, IL-4 and IL-13 maintain macrophages in the M2 state, resulting in the secretion of the anti-inflammatory cytokines IL-10 and IL-1Ra. In obesity, the M2 phenotype switches to M1, a proinflammatory state, leading to the secretion of TNF-α and IL-6. IFN-γ and endogenous Toll-like receptor (TLR) ligands maintain the M1 state [31]. Obesity in pregnancy and GDM have been linked to the disruption in several inflammatory mediators in the maternal and fetal compartments. These will be discussed below.
3. Maternal and Fetal Inflammation in Obesity in Pregnancy and GDM
Maternal Inflammation
Pregnancy itself is characterized by an altered inflammatory profile compared to the non-pregnant state. A tightly regulated balance between pro- and anti-inflammatory cytokines may be necessary for normal implantation, trophoblast invasion and placentation. A pro-inflammatory response localized to the uterine site of implantation may be necessary for this process [32]. In contrast, the post-implantation period is associated with an ‘immunosuppressive’ bias towards producing Th2 cytokines, which is believed to be necessary to prevent immune rejection of the fetus [33]. Normal pregnancy is also characterized by a state of insulin resistance, with a 50% reduction in insulin-mediated glucose clearance, and a ~250% increase in insulin production to maintain maternal euglycemia [34]. Pregnant women with obesity or GDM are insulin-resistant compared to normal pregnant women. However, the widely accepted dogma that increased adiposity equates to increased maternal inflammation may not be as evident during pregnancy as in the non-pregnant state.
Evidence from Longitudinal Studies of Maternal Obesity
Longitudinal studies have provided information on the temporal changes in maternal cytokine profiles during normal pregnancy, and alterations of this profile in obesity. Recently, Christian et al. comprehensively measured proinflammatory cytokines IL-6, IL-8, CRP, TNF-α and IL-1β in a total of 57 women of normal BMI, overweight and obese women in the first, second and third trimesters as well as 4–6 weeks post-partum [35]. In normal pregnant women, levels of IL-6 and TNF-α increased at each visit and postpartum, while IL-8 and IL-1β decreased from the first to the third trimester, and increased postpartum. CRP decreased throughout pregnancy and post-partum. In obese women IL-6 and CRP were elevated during pregnancy and postpartum compared to controls. While the patterns of change in cytokines were similar in normal, overweight, and obese women, the women with higher BMI did show a trend towards elevation in some (CRP, IL-6), but not all inflammatory markers [35]. Similarly, in a study by Stewart et al., an elevation in CRP was observed in obese women at each trimester, while IL-6 was significantly increased only in the second and third trimester compared to controls. TNF-α did not differ at any time-point measured in obese women compared to controls [36].
Friis et al. determined the levels of CRP, MCP1, IL-6 and IL-1Ra in maternal plasma of 240 overweight and obese women using enzyme-linked immunosorbent assay (ELISA). It was found that while levels of these circulating inflammatory mediators were increased with increasing BMI in early to mid-pregnancy, this elevation was not evident towards the end of pregnancy [37]. The authors speculate that perhaps increased adiposity during pregnancy is not associated with enhanced inflammation, as opposed to the widely held belief.
TNF-α, IL-6 and CRP in Obesity and GDM
TNF-α is arguably the most extensively studied cytokine in relation to inflammation and the development of insulin resistance in obesity and GDM. Given the relationship between increased adiposity, TNF-α, and insulin resistance, it would be expected that levels of TNF-α correlate with adiposity in pregnancy, similar to obesity in the non-pregnant state. However, this may not be the case. While increased circulating TNF-α in maternal serum correlate with increasing BMI in some studies [18, 38], several other studies do not report a significant increase in circulating TNF-α in obesity with [39] or without GDM [35, 36, 40–43]. In fact, one study documented a decrease in TNF-α production by T-cells isolated from obese women without GDM [44]. An increase in TNF-α mRNA levels in maternal stromal vascular cells [40] as well as TNF-α mRNA and protein in the placenta of obese women has been observed, but this may not correlate to increased circulating TNF-α levels in maternal blood [40, 41, 45]. Increased circulating TNF-α may be related to the development of GDM, and several groups have therefore postulated that circulating maternal TNF-α levels are an independent predictor for the development of GDM regardless of BMI [46–48].
In contrast, elevated circulating levels of IL-6 in maternal plasma and serum have been consistently observed in maternal obesity as well as in GDM in the presence or absence of obesity [47, 49–52]. An increase in IL-6 mRNA in subcutaneous adipose tissue (SAT) of women with GDM has also been reported [19]. IL-6 induces the acute-phase response, which is characterized by the release of CRP from the liver [35]. Increased CRP levels have frequently been observed in conjunction with increased IL-6 in obesity in pregnancy [36, 40, 41, 53].
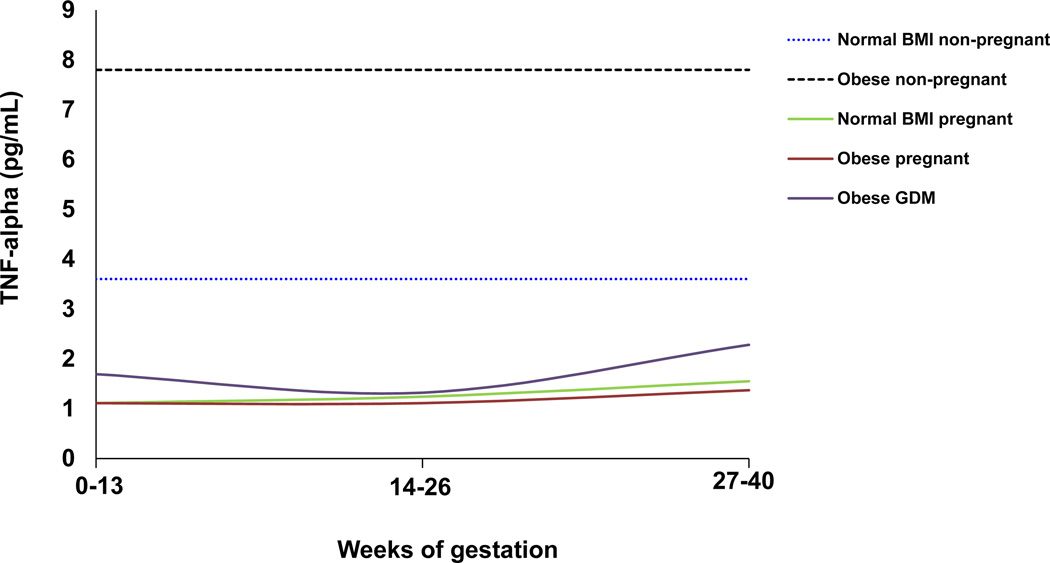
Changes across gestation in maternal serum TNF-α and CRP, two inflammatory markers that are most commonly assessed in relation to obesity and insulin resistance, are summarized in Figures 1A and B. These representative schematics show the trends in these two cytokines in women of normal BMI and obese non-pregnant women, compared to normal and obese pregnant women and obese women with GDM. In Figure 1A, levels of TNF-α in obese and normal non-pregnant individuals are presented as an average across three studies in women [54–56]. TNF-α levels are reduced in the pregnant state compared to non-pregnancy, in both obese and normal women. That TNF-α levels are lower in pregnancy compared to non-pregnant obese women and non-pregnant women of normal BMI, is consistent with the Th1/Th2 paradigm of pregnancy, which postulates that a lower Th1:Th2 cytokine ratio is necessary for the maintenance of pregnancy. Recurrent spontaneous abortion is frequently associated with high levels of Th1 cytokines, such as TNF-α, in early pregnancy [57]. During pregnancy, TNF-α levels are lower in the first and second trimester, and modestly elevated in the third trimester. In Figure 1A, TNF-α values across the first, second and third trimesters in obese and normal women are averaged across three studies [35–37]. TNF-α levels may be elevated in GDM, consistent with the long-standing hypothesis that TNF-α is involved in propagating insulin resistance leading to GDM [46, 58, 59].
Figure 1.
A: Representative schematic of changes in maternal serum TNF-α levels throughout pregnancy in obesity and GDM, compared with normal pregnant, normal non-pregnant, and obese non-pregnant individuals. TNF-α levels are decreased in normal pregnant women compared to non-pregnant individuals, and appear to increase from the first to third trimester. In obese women, TNF-α levels may be lower than in normal pregnant women, and are increased in GDM. TNF-α is highest in non-pregnant obese individuals. Data represents absolute TNF-α levels in pg/mL in (1) normal and obese non-pregnant individuals averaged across three studies in women [54–56]; (2) normal and obese pregnant women averaged across 3 longitudinal studies measured in the first, second and third trimester [35–37]; and (3) women with GDM (BMI≥30kg/m2) in the first [46], second [57] and third [46, 57, 58].
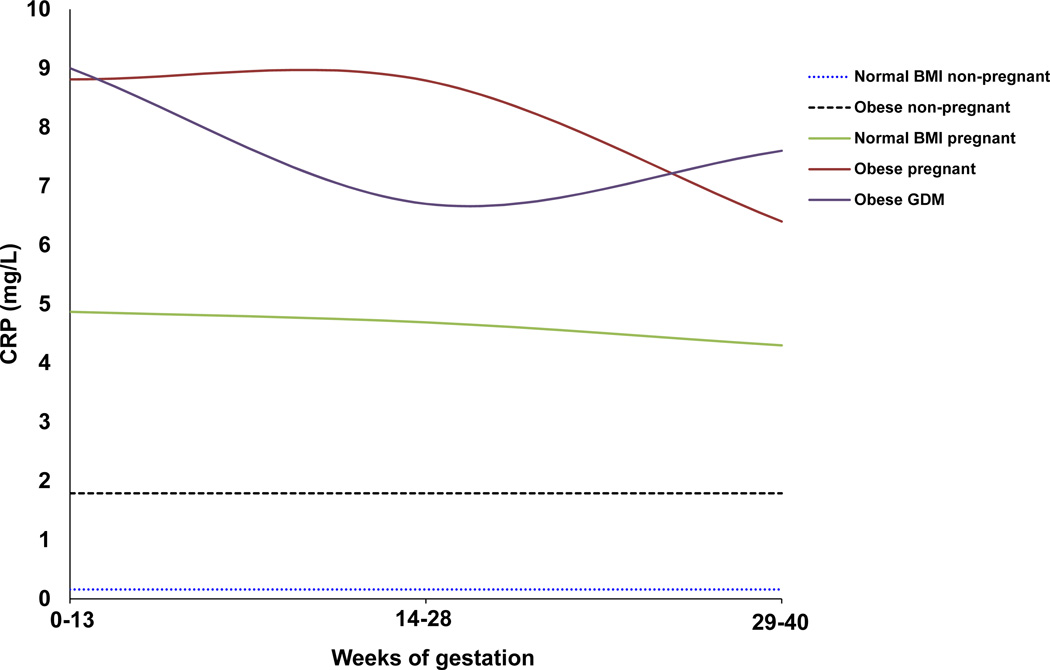
B: Representative schematic of changes in maternal serum CRP levels throughout pregnancy in obesity and GDM, compared with normal pregnant, normal non-pregnant, and obese non-pregnant individuals. CRP levels are increased in normal pregnant women compared to normal non-pregnant and obese individuals, and appear to decrease slightly from the first to third trimester. In obese women, CRP levels are increased compared to normal pregnant women and decrease in late pregnancy. The opposite pattern is observed in GDM, with a decrease in mid-gestation and a rise at term. Data represents absolute CRP levels in mg/mL in (1) normal and obese non-pregnant women averaged across 2–3 studies in women [55, 59, 60]; (2) normal and obese pregnant women averaged across 2 longitudinal studies measured in the first, second and third trimester [35, 36]; and (3) women with GDM (BMI≥30kg/m2) in the first [74], second [75] and third [61, 75] trimester.
In contrast, CRP levels in normal, non-pregnant individuals are low, and are slightly raised in obese non-pregnant individuals. Levels of CRP in normal, non-pregnant women are averaged across 2 studies [55, 60], while levels in non-pregnant obese women are averaged across three studies [55, 60, 61]. CRP increases when women of normal BMI or obese women become pregnant (Figure 1B). Levels of CRP, albeit higher than in normal pregnant women, tend to decrease when an obese woman becomes pregnant and may decrease further at the end of pregnancy [35, 36], while in GDM CRP may increase at the end of pregnancy [62].
Lack of concordance in the profile of inflammatory mediators circulating in maternal serum in obese and/or GDM pregnancies between different studies can be attributed to several factors. (1) Some studies do not exclude other comorbidities related to obesity and the inflammatory mediators observed may be related to other pathologies of pregnancy apart from obesity alone or obesity with GDM. (2) Different study designs (cross-sectional or longitudinal) make it more difficult to directly compare levels of inflammatory mediators between studies. (3) The exact time of sampling, the type of sample (plasma or serum, SAT, VAT, immune cell subsets) as well as assay method may influence the levels of inflammatory mediators measured in each study. (4) Selection of subjects according to race and ethnicity and age may play a role in the responses observed in maternal obesity and GDM. (5) These studies may be confounded by a ‘negative publication bias’, with many studies showing no changes in inflammatory mediators never being published. Further well-designed, appropriately controlled large studies are required to more definitely establish the maternal inflammatory profile in obesity and GDM. Table 1 summarizes the studies investigating inflammation in the maternal, fetal, and placental compartments in obesity with and without GDM. Changes in maternal serum cytokines levels across gestation have not been presented in this table. The representative schematics in Figures 1A and B provide an indication of the differential inflammatory changes that occur in maternal circulating cytokine levels across gestation in obesity and GDM.
Table 1.
Overview of changes in cytokines in the maternal and fetal/placental compartments in obese and GDM pregnancies.
| Reference | Criteria | TNF-α | CRP | IL6 | IL1β | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Basu et al. [40] | Obese | ↔ | ↑ | ↑ | - | Fetal/placental cytokines | |||||
| Challier et al. [41] | Obese | ↔ | ↑ | ↑ | - | ||||||
| Christian et al. [35] | Obese | ↔ | ↑ | ↑ | ↔ | ||||||
| Farah et al. [42] | Obese | ↔ | - | ↑ | ↔ | ||||||
| Ramsay et al. [53] | Obese | - | ↑ | ↑ | - | ||||||
| Stewart et al. [36] | Obese | ↔ | ↑ | ↑ | - | ||||||
| Stone et al. [38] | Obese | ↑ | - | ↔ | - | ||||||
| Korkmazer et al. [76] | GDM | ↑ | ↔ | - | - | ||||||
| Kuzmicki et al. [52] | GDM | - | ↑ | ↑ | - | ||||||
| Friis et al. [37] | Obese and GDM |
- | ↑ | ↑ | - |
Sample studied |
TNF-α | IL6 | IL10 | MCP1 |
Birthweight in obese/GDM (kg) |
| Vega-Sanchez et al. [43] | Obese | ↔ | - | ↔ | - | Cord blood | ↔ | - | ↔ | - | * |
| Aye et al. [18] | Obese | ↑ | - | ↔ | ↔ | Cord blood | ↔ | ↔ | ↔ | ↔ | 3.45±0.05 (↑) |
| Ategbo et al. [47] | Obese and GDM |
↑ | - | ↑ | - | Cord blood | ↓ | ↓ | ↑ | - | 4.35±0.6 (↑) |
| Van der Burg et al. [62] | Obese neonates |
- | - | - | - | Neonatal blood spots |
↔ | ↑ | - | ↔ | * |
| Roberts et al. [77] | Obese | ↔ | - | ↑ | ↔ | Placenta (mRNA) |
↔ | ↔ | - | ↑ | 3.38±.48 (↔) |
| Oliva et al. [45] | Obese | - | - | - | - | Placenta (protein) |
↑ | ↑ | - | - | 3.78±.106 (↑) |
| Kleiblova et al. [19] | GDM | - | - | - | - | Placenta (mRNA) |
↔ | ↔ | - | - | 3.47±0.27 (↔) |
Note: (↑) indicates cytokines that were increased in obese/GDM pregnancies compared to pregnancies with normal BMI; (↓) indicates cytokines that were decreased compared to normal pregnancies; (↔) indicates no significant difference in cytokine levels compared to normal pregnancies; (-) denotes cytokines that were not measured in that study. Birthweights are indicated as mean weight of neonates born to obese and/or GDM mothers in kilograms. (↑) indicates an increase; (↓) indicates a decrease; and (↔) indicates no change in birthweights of neonates compared to neonates born to control mothers.
indicates studies that did not provide mean birthweight values. All changes indicated are significant (P<0.05). Only studies in which ≥2 cytokines were measured have been presented. Changes in maternal serum cytokine levels across gestation are not presented.
Fetal Inflammation
Alterations in maternal inflammatory markers may not be reflected by similar changes in the fetal circulation. In one study, levels of inflammatory proteins were investigated 1, 7 and 14 days after delivery in a total of 939 infants born to non-obese, overweight, and obese mothers showed that the levels of IL-6, IL-8, ICAM3, TNFR1 and VEGFR2 were positively correlated to maternal BMI. However, day-14 concentrations were not elevated in the obese group [63]. Ategbo et al. investigated circulating levels of cytokines and adipokines in 59 women with GDM and their macrosomic infants, compared to 60 age-matched controls [47]. Maternal serum levels of adiponectin and Th1 cytokines (IL-2 and IFN-γ) were decreased, while in their macrosomic neonates, adiponectin was decreased and Th1 cytokines were increased (Table 1). Leptin, IL-6, TNF-α, and IL-10 were increased in GDM mothers, while in their neonates, leptin, TNF-α and IL-6 were decreased (Table 1). Birthweights were significantly increased in neonates born to obese women with GDM [47]. Previous work by our group has also shown that umbilical vein cytokine levels were unaffected by maternal obesity [18]. Birthweight was also increased in the obese group in this study [18]. It is therefore possible that the placenta acts as a mediator and an adaptor in pregnancy, sensing and responding to the maternal inflammatory environment in order to maintain pregnancy. Several studies have assessed inflammation in the placenta in obesity and GDM.
4. The Placenta as an Inflammatory Organ: Not just a Silent Observer
It is well established that placental cytokine production is critical for the maintenance of pregnancy. Cytotrophoblasts, syncytiotrophoblast and Hofbauer cells are known to secrete cytokines necessary at various stages of pregnancy from implantation to delivery [20]. It has been suggested that the placenta plays an active role in mediating inflammation in women with obesity and GDM. Placental structure and function may be altered in an adaptive response to obesity, and the placenta may act as a target and a source of inflammatory cytokines in these pregnancies. Challier at al. have reported a 2–3-fold increase in the number of placental macrophages in obese women, characterized by an increase in IL-1, TNF-α and IL-6 mRNA expression [41]. One study comparing the transcriptome of active monocytes isolated from the placenta, maternal venous, and umbilical cord blood, found that monocytes isolated from maternal blood and the placenta showed 73% homology, suggesting an inflammatory phenotype at the placental interface [64].
Placental mRNA and protein expression of inflammatory mediators in obesity and GDM have been explored in a number of studies. Saben and coworkers sequenced placental RNA and found that levels of IL-12RB2, IL-21R, and CX3CR1 were increased, while IL-1R1, IL-1RAP, CXCR2, CXCR1, CCR3 and ADIPOR1 were decreased in placentas from obese women compared to placentas from women with normal BMI [65]. A number of studies have documented an increase in IL-6 [41] and TNF-α [41, 66] in placentas from obese women, and an increase in IL-8 [67] and leptin [68] in placentas from women with GDM. Other studies found limited indications of inflammation [18].
Some of the changes observed in the placenta in maternal obesity may represent an adaptation, which could contribute to limit exposure of the fetus to inflammation and oxidative stress. For example, Lappas and co-workers reported that exposure of placental tissue from women with and without GDM to oxidative stress resulted in the release of only 3 out of 16 cytokines (IL-1β, TNF-α, M1P1B) and no changes in antioxidant gene expression. This was in contrast to women with normal BMI who exhibited an increase in 13 out of 16 cytokines and alterations in antioxidant genes in placenta exposed to oxidative stress [69]. Collectively, these studies highlight the importance of the placenta as a source of inflammatory mediators, a site of inflammation and an adaptive mediator.
Cytokines produced by the placenta may be responsible for the elevated levels observed in the maternal circulation in GDM, as 94% of TNF-α produced by in vitro perfused placental cotyledons is released to the maternal side and only 6% to the fetal side [46]. In cultured primary human trophoblasts, inflammatory cytokines IL-6 and TNF-α have been shown to upregulate amino acid transporter system A activity [23], while IL-1β down-regulates insulin-stimulated system A transport in primary trophoblasts [70]. This may be one mechanism by which inflammatory mediators influence placental nutrient transport, thereby linking inflammation in maternal obesity to changes in fetal growth.
Previous work by our laboratory has shown that while maternal serum MCP1 and TNF-α is increased, and placental p38-MAPK and STAT3, but not NFκB are activated in maternal obesity, levels of inflammatory markers in umbilical blood are unaltered [18]. It has therefore been suggested that maternal inflammation in obese women or women with GDM may influence fetal development by impacting placental function, rather than directly influencing the fetal inflammatory profile [18].
5. Inflammation and Developmental Programming
Inflammatory mediators may act in utero to program fetal adipose tissue, liver and skeletal muscle for insulin resistance later in life. Because it is not possible to examine fetal and neonatal tissues in humans, animal models have been utilized to investigate inflammation in specific tissues in offspring of obese dams. Maternal obesity is associated with adipogenesis, increased adiposity and insulin resistance in fetal tissues in several animal models [71]. In one study, activation of the NF-κB pathway was observed in skeletal muscle from fetuses of obese ewes. Increased adipogenesis, intramuscular adipocytes and insulin resistance were also observed, constituting one mechanism by which maternal obesity may predispose to metabolic diseases in offspring [72]. Fetuses of mice fed a high fat diet display higher levels of TNF-α, CD68 and MCP-1 mRNA in adipose tissue, suggesting that maternal obesity may cause inflammation in fetal adipose tissue [73]. Maternal over-nutrition has been reported to be associated with elevated triglyceride levels, increased inflammatory markers and fatty livers in offspring [74]. The effects of maternal obesity and inflammation on the fetus in animal models have been reviewed recently [71]. More detailed studies are required to investigate the exact mechanisms by which localized inflammation in fetal tissues is linked to metabolic disease later in life.
Conclusions and Future Directions
In conclusion, metainflammation, or chronic, low-grade metabolically induced inflammation may play a role in maternal obesity and GDM, leading to developmental programming in utero. The maternal inflammatory profile associated with GDM may be more pronounced than in obese women pregnant women without GDM. Studies investigating inflammatory mediators in the maternal, placental and fetal compartments in obesity and GDM are not always concordant. There is insufficient evidence that maternal inflammation equates to inflammation in fetal serum. Circulating cytokines may not be truly reflective of inflammatory status, and more data is required detailing cytokine production from specific maternal and fetal tissues. The placenta may play a role as a mediator in inflammation in obesity and GDM. Further, adequately powered, well-designed studies are required to help us better understand (1) the placental mechanisms by which inflammation may be involved in developmental programming, and (2) the effects of inflammation on fetal tissues in utero. It is necessary to understand these processes in order to develop treatments to reverse the effects of maternal obesity on the developmental programming of metabolic and cardiovascular diseases.
Highlights.
Inflammation occurs in maternal obesity and gestational diabetes mellitus (GDM)
The placenta plays a key role in inflammation in obesity and GDM
Maternal inflammation may not equate to fetal inflammation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of Obesity and Trends in the Distribution of Body Mass Index Among US Adults, 1999–2010. Jama-J Am Med Assoc. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Trasande L, Lee M, Liu Y, Weitzman M, Savitz D. Incremental charges, costs, and length of stay associated with obesity as a secondary diagnosis among pregnant women. Medical care. 2009;47(10):1046–1052. doi: 10.1097/MLR.0b013e31819c94b8. [DOI] [PubMed] [Google Scholar]
- 3.Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, et al. Maternal obesity and pregnancy outcome: a study of 287 213 pregnancies in London. Int J Obesity. 2001;25(8):1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 4.Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001;91(3):436–440. doi: 10.2105/ajph.91.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SY, Sappenfield W, Sharma AJ, Wilson HG, Bish CL, Salihu HM, et al. Racial/ethnic differences in the prevalence of gestational diabetes mellitus and maternal overweight and obesity, by nativity, Florida, 2004–2007. Obesity (Silver Spring) 2013;21(1):E33–E40. doi: 10.1002/oby.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 7.Watkins ML, Botto LD. Maternal prepregnancy weight and congenital heart defects in the offspring. Epidemiology. 2001;12(4):439–446. [PubMed] [Google Scholar]
- 8.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191(3):964–968. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 9.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The Hyperglycemia and Adverse Pregnancy Outcome Study Associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35(4):780–786. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SY, Sharma AJ, Sappenfield W, Wilson HG, Salihu HM. Association of Maternal Body Mass Index, Excessive Weight Gain, and Gestational Diabetes Mellitus With Large-for-Gestational-Age Births. Obstet Gynecol. 2014;123(4):737–744. doi: 10.1097/AOG.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowers K, Laughon SK, Kiely M, Brite J, Chen Z, Zhang C. Gestational diabetes, pre-pregnancy obesity and pregnancy weight gain in relation to excess fetal growth: variations by race/ethnicity. Diabetologia. 2013;56(6):1263–1271. doi: 10.1007/s00125-013-2881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker DJ. The fetal and infant origins of disease. European journal of clinical investigation. 1995;25(7):457–463. doi: 10.1111/j.1365-2362.1995.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 14.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New Engl J Med. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 15.Gaillard R, Steegers EAP, Duijts L, Felix JF, Hofman A, Franco OH, et al. Childhood Cardiometabolic Outcomes of Maternal Obesity During Pregnancy The Generation R Study. Hypertension. 2014;63(4):683–691. doi: 10.1161/HYPERTENSIONAHA.113.02671. [DOI] [PubMed] [Google Scholar]
- 16.Jones CW. Gestational diabetes and its impact on the neonate. Neonatal network :NN. 2001;20(6):17–23. doi: 10.1891/0730-0832.20.6.17. [DOI] [PubMed] [Google Scholar]
- 17.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 18.Aye IL, Lager S, Ramirez VI, Gaccioli F, Dudley DJ, Jansson T, et al. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol Reprod. 2014;90(6):129. doi: 10.1095/biolreprod.113.116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleiblova P, Dostalova I, Bartlova M, Lacinova Z, Ticha I, Krejci V, et al. Expression of adipokines and estrogen receptors in adipose tissue and placenta of patients with gestational diabetes mellitus. Mol Cell Endocrinol. 2010;314(1):150–156. doi: 10.1016/j.mce.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27(8):794–798. doi: 10.1016/j.placenta.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, et al. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab. 2013;98(1):105–113. doi: 10.1210/jc.2012-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51(7):2214–2219. doi: 10.2337/diabetes.51.7.2214. [DOI] [PubMed] [Google Scholar]
- 23.Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell Physiol. 2009;297(5):C1228–C1235. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]
- 24.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23(1):271–278. doi: 10.1096/fj.08-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of clinical investigation. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: Fall with weight loss. J Clin Endocr Metab. 1998;83(8):2907–2910. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 27.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends in endocrinology and metabolism: TEM. 2000;11(8):327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 28.Cildir G, Akincilar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends in molecular medicine. 2013;19(8):487–500. doi: 10.1016/j.molmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 30.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chawla A, Nguyen KD, Goh YPS. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11(11):738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann Ny Acad Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional Cytokine Interactions in the Maternal-Fetal Relationship - Is Successful Pregnancy a Th2 Phenomenon. Immunol Today. 1993;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 34.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180(4):903–914. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 35.Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine. 2014 doi: 10.1016/j.cyto.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab. 2007;92(3):969–975. doi: 10.1210/jc.2006-2083. [DOI] [PubMed] [Google Scholar]
- 37.Friis CM, Paasche Roland MC, Godang K, Ueland T, Tanbo T, Bollerslev J, et al. Adiposity-related inflammation: effects of pregnancy. Obesity (Silver Spring) 2013;21(1):E124–E130. doi: 10.1002/oby.20120. [DOI] [PubMed] [Google Scholar]
- 38.Stone RA, Silvis A, Jude D, Chaffin D. Increasing body mass index exacerbates inflammation in obese gravidas. Obstet Gynecol. 2014;123(Suppl 1):81S. [Google Scholar]
- 39.Gauster M, Hiden U, van Poppel M, Frank S, Wadsack C, Hauguel-de Mouzon S, et al. Dysregulation of placental endothelial lipase in obese women with gestational diabetes mellitus. Diabetes. 2011;60(10):2457–2464. doi: 10.2337/db10-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basu S, Haghiac M, Surace P, Challier JC, Guerre-Millo M, Singh K, et al. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring) 2011;19(3):476–482. doi: 10.1038/oby.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29(3):274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farah N, Hogan AE, O'Connor N, Kennelly MM, O'Shea D, Turner MJ. Correlation between maternal inflammatory markers and fetomaternal adiposity. Cytokine. 2012;60(1):96–99. doi: 10.1016/j.cyto.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 43.Vega-Sanchez R, Barajas-Vega HA, Rozada G, Espejel-Nunez A, Beltran-Montoya J, Vadillo-Ortega F. Association between adiposity and inflammatory markers in maternal and fetal blood in a group of Mexican pregnant women. Br J Nutr. 2010;104(12):1735–1739. doi: 10.1017/S0007114510002825. [DOI] [PubMed] [Google Scholar]
- 44.Sen S, Iyer C, Klebenov D, Histed A, Aviles JA, Meydani SN. Obesity impairs cell-mediated immunity during the second trimester of pregnancy. Am J Obstet Gynecol. 2013;208(2):139, e1–e8. doi: 10.1016/j.ajog.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Oliva K, Barker G, Riley C, Bailey MJ, Permezel M, Rice GE, et al. The effect of pre-existing maternal obesity on the placental proteome: two-dimensional difference gel electrophoresis coupled with mass spectrometry. J Mol Endocrinol. 2012;48(2):139–149. doi: 10.1530/JME-11-0123. [DOI] [PubMed] [Google Scholar]
- 46.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51(7):2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 47.Ategbo JM, Grissa O, Yessoufou A, Hichami A, Dramane KL, Moutairou K, et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab. 2006;91(10):4137–4143. doi: 10.1210/jc.2006-0980. [DOI] [PubMed] [Google Scholar]
- 48.Xu J, Zhao YH, Chen YP, Yuan XL, Wang J, Zhu H, et al. Maternal circulating concentrations of tumor necrosis factor-alpha, leptin, and adiponectin in gestational diabetes mellitus: a systematic review and meta-analysis. ScientificWorldJournal. 2014;2014:926932. doi: 10.1155/2014/926932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morisset AS, Dube MC, Cote JA, Robitaille J, Weisnagel SJ, Tchernof A. Circulating interleukin-6 concentrations during and after gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2011;90(5):524–530. doi: 10.1111/j.1600-0412.2011.01094.x. [DOI] [PubMed] [Google Scholar]
- 50.Kuzmicki M, Telejko B, Lipinska D, Pliszka J, Wilk J, Wawrusiewicz-Kurylonek N, et al. The IL-6/IL-6R/sgp130 system and Th17 associated cytokines in patients with gestational diabetes. Endokrynol Pol. 2014;65(3):169–175. doi: 10.5603/EP.2014.0023. [DOI] [PubMed] [Google Scholar]
- 51.Kuzmicki M, Telejko B, Szamatowicz J, Zonenberg A, Nikolajuk A, Kretowski A, et al. High resistin and interleukin-6 levels are associated with gestational diabetes mellitus. Gynecol Endocrinol. 2009;25(4):258–263. doi: 10.1080/09513590802653825. [DOI] [PubMed] [Google Scholar]
- 52.Kuzmicki M, Telejko B, Zonenberg A, Szamatowicz J, Kretowski A, Nikolajuk A, et al. Circulating pro- and anti-inflammatory cytokines in Polish women with gestational diabetes. Horm Metab Res. 2008;40(8):556–560. doi: 10.1055/s-2008-1073166. [DOI] [PubMed] [Google Scholar]
- 53.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87(9):4231–4237. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 54.Bullo M, Garcia-Lorda P, Salas-Salvado J. Plasma soluble tumor necrosis factor alpha receptors and leptin levels in normal-weight and obese women: effect of adiposity and diabetes. Eur J Endocrinol. 2002;146(3):325–331. doi: 10.1530/eje.0.1460325. [DOI] [PubMed] [Google Scholar]
- 55.Kondo T, Kobayashi I, Murakami M. Effect of exercise on circulating adipokine levels in obese young women. Endocrine journal. 2006;53(2):189–195. doi: 10.1507/endocrj.53.189. [DOI] [PubMed] [Google Scholar]
- 56.El-Haggar SM, Mostafa TM. Adipokines and biochemical changes in Egyptian obese subjects: possible variation with sex and degree of obesity. Endocrine. 2014 doi: 10.1007/s12020-014-0390-z. [DOI] [PubMed] [Google Scholar]
- 57.Makhseed M, Raghupathy R, Azizieh F, Omu A, Al-Shamali E, Ashkanani L. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Human reproduction. 2001;16(10):2219–2226. doi: 10.1093/humrep/16.10.2219. [DOI] [PubMed] [Google Scholar]
- 58.Noureldeen AF, Qusti SY, Al-Seeni MN, Bagais MH. Maternal leptin, adiponectin, resistin, visfatin and tumor necrosis factor-alpha in normal and gestational diabetes. Indian journal of clinical biochemistry : IJCB. 2014;29(4):462–470. doi: 10.1007/s12291-013-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLachlan KA, O'Neal D, Jenkins A, Alford FP. Do adiponectin, TNFalpha, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes/metabolism research and reviews. 2006;22(2):131–138. doi: 10.1002/dmrr.591. [DOI] [PubMed] [Google Scholar]
- 60.Arikawa AY, Thomas W, Schmitz KH, Kurzer MS. Sixteen weeks of exercise reduces C-reactive protein levels in young women. Medicine and science in sports and exercise. 2011;43(6):1002–1009. doi: 10.1249/MSS.0b013e3182059eda. [DOI] [PubMed] [Google Scholar]
- 61.Browning LM, Krebs JD, Magee EC, Fruhbeck G, Jebb SA. Circulating markers of inflammation and their link to indices of adiposity. Obesity facts. 2008;1(5):259–265. doi: 10.1159/000169832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Retnakaran R, Hanley AJG, Raif N, Connelly PW, Sermer M, Zinman B. C-reactive protein and gestational diabetes: The central role of maternal obesity. J Clin Endocr Metab. 2003;88(8):3507–3512. doi: 10.1210/jc.2003-030186. [DOI] [PubMed] [Google Scholar]
- 63.van der Burg JW, Allred EN, McElrath TF, Fichorova RN, Kuban K, O'Shea TM, et al. Is maternal obesity associated with sustained inflammation in extremely low gestational age newborns? Early Hum Dev. 2013;89(12):949–955. doi: 10.1016/j.earlhumdev.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 64.Basu S, Leahy P, Challier JC, Minium J, Catalano P, Hauguel-de Mouzon S. Molecular phenotype of monocytes at the maternal-fetal interface. Am J Obstet Gynecol. 2011;205(3):265, e1–e8. doi: 10.1016/j.ajog.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saben J, Lindsey F, Zhong Y, Thakali K, Badger TM, Andres A, et al. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35(3):171–177. doi: 10.1016/j.placenta.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varastehpour A, Radaelli T, Minium J, Ortega H, Herrera E, Catalano P, et al. Activation of phospholipase A2 is associated with generation of placental lipid signals and fetal obesity. J Clin Endocrinol Metab. 2006;91(1):248–255. doi: 10.1210/jc.2005-0873. [DOI] [PubMed] [Google Scholar]
- 67.Kuzmicki M, Telejko B, Wawrusiewicz-Kurylonek N, Citko A, Lipinska D, Pliszka J, et al. The expression of suppressor of cytokine signaling 1 and 3 in fat and placental tissue from women with gestational diabetes. Gynecol Endocrinol. 2012;28(11):841–844. doi: 10.3109/09513590.2012.683055. [DOI] [PubMed] [Google Scholar]
- 68.Lepercq J, Cauzac M, Lahlou N, Timsit J, Girard J, Auwerx J, et al. Overexpression of placental leptin in diabetic pregnancy: a critical role for insulin. Diabetes. 1998;47(5):847–850. doi: 10.2337/diabetes.47.5.847. [DOI] [PubMed] [Google Scholar]
- 69.Lappas M, Mitton A, Permezel M. In response to oxidative stress, the expression of inflammatory cytokines and antioxidant enzymes are impaired in placenta, but not adipose tissue, of women with gestational diabetes. J Endocrinol. 2010;204(1):75–84. doi: 10.1677/JOE-09-0321. [DOI] [PubMed] [Google Scholar]
- 70.Aye ILMH, Jansson T, Powell TL. Interleukin-1 beta inhibits insulin signaling and prevents insulin-stimulated system A amino acid transport in primary human trophoblasts. Mol Cell Endocrinol. 2013;381(1–2):46–55. doi: 10.1016/j.mce.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Segovia SA, Vickers MH, Gray C, Reynolds CM. Maternal Obesity, Inflammation, and Developmental Programming. Biomed Res Int. 2014 doi: 10.1155/2014/418975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan X, Zhu MJ, Xu W, Tong JF, Ford SP, Nathanielsz PW, et al. Up-Regulation of Toll-Like Receptor 4/Nuclear Factor-kappa B Signaling Is Associated with Enhanced Adipogenesis and Insulin Resistance in Fetal Skeletal Muscle of Obese Sheep at Late Gestation. Endocrinology. 2010;151(1):380–387. doi: 10.1210/en.2009-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murabayashi N, Sugiyama T, Zhang L, Kamimoto Y, Umekawa T, Ma N, et al. Maternal high-fat diets cause insulin resistance through inflammatory changes in fetal adipose tissue. Eur J Obstet Gyn R B. 2013;169(1):39–44. doi: 10.1016/j.ejogrb.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, Mckee C, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52(6):913–920. doi: 10.1016/j.jhep.2009.12.042. [DOI] [PubMed] [Google Scholar]