Abstract
Background
Deficient levels of 25-hydroxyvitamin D [25(OH)D] have been associated with increased fracture risk. Racial differences in fracture risk may be related to differences in bioavailable vitamin D due to single nucleotide polymorphism (SNP) variations in the Vitamin D Binding Protein (DBP).
Methods
We measured 25(OH)D levels in 12,781 middle-aged white and black participants [mean age 57 years (SD 5.7), 25% black] in the ARIC Study who attended the second examination from 1990–1992. Participants were genotyped for two DBP SNPs (rs4588 and rs7041). Incident hospitalized fractures were measured by abstracting hospital records for ICD-9 codes. We used Cox proportional hazards models to evaluate the association between 25(OH)D levels and risk of fracture with adjustment for possible confounders. Interactions were tested by race and DBP genotype.
Results
There were 1,122 incident fracture-related hospitalizations including 267 hip fractures over a median of 19.6 years of follow-up. Participants with deficient 25(OH)D (<20 ng/ml) had a higher risk of any fracture hospitalization [HR=1.21 (95% CI 1.05–1.39)] and hospitalization for hip fracture [HR=1.35 (1.02–1.79)]. No significant racial interaction was noted (p-interaction=0.20 for any fracture; 0.74 for hip fracture). There was no independent association of rs4588 and rs7041 with fracture. However, there was a marginal interaction for 25(OH)D deficiency with rs7041 among whites (p-interaction=0.065). Whites with both 25(OH)D deficiency and the GG genotype [i.e. with predicted higher levels of DBP and lower bioavailable vitamin D] were at the greatest risk for any fracture [HR=1.48 (1.10–2.00)] compared to whites with the TT genotype and replete 25(OH)D (reference group).
Conclusions
Deficient 25(OH)D levels are associated with higher incidence of hospitalized fractures. Marginal effects were seen in whites for the DBP genotype associated with lower bioavailable vitamin D, but results inconclusive. Further investigation is needed to more directly evaluate the association between bioavailable vitamin D and fracture risk.
Keywords: vitamin D, vitamin D binding protein polymorphisms, fracture, race, epidemiology
1. Introduction
Vitamin D insufficiency is common, affecting approximately 1 billion people worldwide.1,2 According to the Institute of Medicine, the recommended levels of 25-hydroxyvitamin D [25(OH)D] should be equivalent to or greater than 20 ng/mL.3 Persons who have deficient levels of 25(OH)D are at higher risk for fractures.4–7 Prevention of low-trauma fractures and hip fractures are of key importance since each year greater than 1.5 million people in the United States experience fractures with its attendant morbidity and mortality.7 Whether vitamin D supplements independently reduce fractures is unclear as evidence in the literature has shown conflicting results.8–14 Even the IOM and the US Preventive Services Task Force have altering opinions regarding supplementation for fracture reduction.3,15 However, the overall effect of supplementation on incidence of fractures may be multi-factorial and depend on factors such as dosage, concomitant treatment with calcium, and 25(OH)D levels at baseline.16
Observational data allows the ability to explore the risk of fracture across a range of 25(OH)D levels, stratified by race. Many observational studies, including the Women’s Health Initiative (WHI), have recognized a racial paradox in the association of vitamin D with fracture: blacks tend to have higher bone mineral density and lower risk of fractures, despite having lower average 25(OH)D levels than whites.17 One possible explanation is that blacks have lower levels of both 25(OH)D and vitamin D binding protein (DBP) compared to whites, and as a result both racial populations have similar levels of estimated bioavailable vitamin D.18
Most of 25(OH)D (~85–90%) circulates predominantly tightly bound to DBP, which may impair the ability of vitamin D to act on target cells.19 The remainder, known as bioavailable vitamin D, circulates mostly bound to albumin, with <1% in the free form.20 Approximately 80% of the variation in serum DBP levels is due to the single nucleotide polymorphisms (SNP) rs7041 and rs4588.18 These SNP variants alter the protein’s binding affinity for vitamin D, and their allele frequencies vary greatly by race,18,20 resulting in blacks being more likely to have lower levels of DBP. Prior studies that have analyzed the association between the DBP SNPs and risk of fractures have yielded conflicting findings.21–25 It is presently unknown whether DBP genotypes modify the association of low levels of 25(OH)D and risk of fracture. Were this the case, it may provide insight into the paradoxical finding of blacks having low 25(OH)D levels and lower fracture risk than whites.
Our objective was to examine the associations, as well as the potential interactions, between 25(OH)D levels, DBP SNPs, and race with the risk of incident fracture hospitalizations occurring over 20 years of follow-up among white and black participants of the Atherosclerosis Risk in Communities (ARIC) Study. Our main hypothesis was that 25(OH)D deficiency would be independently associated with higher risk of hospitalized fractures, and that this association would be influenced by race and genetic variations of DBP SNPs rs7041 and rs4588. We hypothesized that those with the DBP genotypes associated with higher DBP levels (and thus lower levels of bioavailable vitamin D) would be at greater risk for fractures in the setting of low total 25(OH)D.
2. Methods
2.1 Participants
The ARIC Study is an ongoing community-based prospective cohort of 15,792 middle-aged adults recruited from four locations: Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota, and Washington County, Maryland.26 Participants took part in several clinical visits: 1987–1989 (Visit 1), 1990–1992 (Visit 2), 1993–1995 (Visit 3), 1996–1998 (Visit 4), and 2011–2013 (Visit 5). Participants provided written consent both for their involvement in the study and for use of their genetic data. The Institutional Review Boards (IRBs) from all four locations and the Coordinating Center have approved the ARIC study.
The population for this study was comprised of participants attending visit 2 (baseline for the present analysis, n=14,348). Exclusion criteria included incident fracture-hospitalization before visit 2 (n=353), race other than black or white (n=41), blacks from the Minnesota and Maryland centers (n=49; because of the small numbers these individuals are not thought to represent their communities), and missing values for 25(OH)D (n=1,173). After all exclusions were accounted for, we had a final sample size of 12,781 participants (89% of source population).
2.2 Laboratory Analyses
Serum samples (after an overnight fast) were collected at visit 2 (1990–1992) and stored at −70oC. Levels of 25(OH)D2 and 25(OH)D3 were measured in 2012–2013 using liquid chromatography-tandem high-sensitivity mass spectrometry (Waters Alliance e2795, Milford, Massachusetts). Using samples collected in duplicate tubes and stored, the coefficient of variation (processing plus assay variation) for 25(OH)D2 was 20.8% and for 25(OH)D3 was 6.9%. The Pearson correlations from these blind duplicate samples were 0.98 for 25(OH)D2 and 0.97 for 25(OH)D3. 25(OH)D2 and 25(OH)D3 were added together for total 25(OH)D concentration.
In 2012–2013 using stored samples from visit 2, we also measured calcium, phosphorus, and parathyroid hormone (PTH) as follows: (calcium and phosphorus: Roche Modular P-Chemistry Analyzer [Roche Diagnostics, Indianapolis, Indiana], PTH: Elecsys 2010 [Roche Diagnostics, Indianapolis, Indiana]). Cystatin C was also measured in 2012–2013 from stored samples collected at visit 2 using the Gentian cystatin C assay on the Roche Modular P Chemistry analyzer. Serum creatinine was measured at visit 2 using a modified kinetic Jaffé reaction. Estimated glomerular filtration rate (eGFR) was estimated using the 2012 CKD EPI combined equation, which incorporates both cystatin C and creatinine.27 Fasting lipids were measured at the time of ARIC visit 2, and plasma total cholesterol and triglycerides were determined by enzymatic methods. HDL-cholesterol was measured after dextran-magnesium precipitation, and the Friedewald equation was used to calculate LDL-cholesterol in those with triglyceride levels under 400 mg/dL.
2.3 Ascertainment of incident fracture-related hospitalizations
The ARIC study makes annual telephone calls asking study participants about any hospitalizations, and medical records from hospitalizations are then obtained and abstracted. This procedure is supplemented by continuous surveillance of discharge lists from hospitals in the ARIC communities.28 We classified ICD-9 codes from hospital discharge summaries using the Clinical Classification Software (CCS) developed at the Agency for Healthcare Research and Quality (AHRQ). Incident-fracture hospitalization was any hospitalization that occurred after the second visit (1990–1992) and before 12/31/2011 and had an International Classification of Diseases, 9th revision (ICD-9) discharge code of 733.1, 733.19, 733.93–733.98, or 800–829. Hip fracture had an ICD-9 discharge code of 820.00–821.39.
2.4 Genotyping
Participants’ DNA samples were used to genotype the two SNPs of interest (rs7041 and rs4588), which are found in the coding region of the vitamin D-binding protein gene.18 A (50K) SNP genotyping array, ITMAT-Broad-CARE Chip, from the Broad Institute of Massachusetts Institute of Technology and Harvard was used for genotyping. We tested whether rs7041 and rs4588 were in Hardy Weinberg Equilibrium, separately by race. There was no evidence that equilibrium was violated (Chi-square tests all had p-values >0.8).
We analyzed blacks and whites separately and labeled TT (rs7041) and CC (rs4588) as the respective references for each SNP, since these genotypes associate with predicted lower DBP levels and thus higher levels of bioavailable vitamin D. Few blacks had GG (rs7401) (n=93/3154) and AA (rs4588) (n=35/3154) genotypes, as seen in prior studies,18 and among these, only 2 blacks with GG and 5 blacks with AA had incident fracture. Because of the small sample size, results including these individuals were unwieldy, with extremely large confidence intervals. Thus, we categorized genotypes for blacks as either TG or TT for rs7041 and either AC or CC for rs4588 and for whites as either TG or GG or TT for rs7041 and either AC or AA or CC for rs4588.
2.5 Covariates
Most of the variables analyzed for this study were measured at visit 2 (1990–1992), except for education, family income, and physical activity, which were assessed at visit 1 (1987–1989). Medication usage, demographical, and behavioral variables were obtained through standard ARIC questionnaires administered by trained interviewers. Use of —vitamin D supplements at visit 2 was determined through medication codes for products that contain vitamin D including multivitamins.
Our main covariates include: age (continuous; years), race/center (Minneapolis-Whites; Washington County-Whites; Forsyth County-Whites; Forsyth County-Blacks; Jackson County-Blacks), sex (male; female), education (<high school; high school; college, graduate or professional school), family income for the past 12 months (<$16,000; $16,000–34,999; $35,000– 49,999; >$50,000), physical activity (measured on a scale of 1–5 based on a modified Baecke Physical Activity questionnaire29), smoking status (never; former; current), drinking status (never; former; current), BMI (kg/m2), waist-hip ratio, diabetes (yes/no; defined as a self-reported physician diagnosis, current diabetes medication use, fasting serum glucose ≥126 mg/dl or non-fasting glucose ≥200 mg/dl), sitting systolic and diastolic blood pressure (continuous; measured in triplicate with random-zero sphygmomanometer and used mean of the second and third measurements), anti-hypertensive medication (yes/no), HDL-C (continuous, mg/dl), total cholesterol (continuous, mg/dl), use of thiazide diuretics (yes/no), estimated glomerular filtration rate (continuous, ml/min/1.73m2), and hormone replacement therapy (yes/no). These covariates were considered as potential confounders of the association between vitamin D levels and fracture risk. In a supplemental model, we also adjusted for the vitamin D related biomarkers of PTH, calcium, and phosphate.
2.6 Statistical Analysis
We adjusted serum 25(OH)D concentrations for seasonal variation, since prior data has shown that 25(OH)D levels are affected by season.30 To adjust for seasonal variation, we calculated residuals using a linear regression model such that 25(OH)D was the dependent variable and the month of blood drawn was the independent variable. Residuals were calculated separately for blacks and whites. The calculated residuals were then added to the total mean and an estimated annual 25(OH)D value was obtained.
Visit 2 (1990–1992) was the baseline for our analysis. Baseline characteristics of the study participants are reported using means (SDs) and proportions stratified by the clinical cutpoint for deficiency3 (deplete <20, replete ≥20 ng/ml) and also stratified by race.
We used Cox proportional hazards models to estimate the hazards ratio (95% confidence intervals) for the association of 25(OH)D levels with risk of hospitalized fractures (either all fractures or just hip fractures) by vitamin D status. Additionally, to investigate for possible dose-response relationship, we modeled estimated annual 25(OH)D using restricted cubic splines with knots at the 5th, 35th, 65th, and 95th percentiles of their baseline distributions to show the continuous relationship between 25(OH)D levels and risk of hospitalized fractures.31 Study participants contributed follow-up time from the date of the participant’s second visit until the date of their incident hospitalization fracture, death, loss-to-follow-up, or the end of ARIC follow-up for hospitalization (12/31/2011), whichever came first. To adjust for potential confounders, we used models with increasing degrees of adjustments. Model 1 adjusted for demographic factors: age, sex, and race/center. Model 2 further adjusted for potentially confounding variables of education, annual household income, physical activity, smoking status, alcohol drinking status, body mass index, waist-to-hip ratio, diabetes, systolic and diastolic blood pressure, use of hypertension medication, total and HDL cholesterol, estimated glomerular filtration rate, thiazide diuretic usage, and hormone replacement therapy. Model 3 adjusted for all the variables in model 2 plus calcium, phosphate, and PTH levels that may be mediators in the vitamin D and fracture pathway.
Wald tests were used to formally test for two-way multiplicative interactions by race, sex, age, and DBP gene polymorphisms (rs7041 and rs4588) in the association of 25(OH)D with the risk for hospitalized fracture, by including cross-product terms in the model. We decided a priori that any interactions with p<0.1 would be further explored. However, based on prior studies,17,32 we a priori planned to present the results overall and stratified by race, regardless of whether a race interaction was present.
Two sensitivity models were also performed. First, we limited our analysis to only those NOT taking vitamin D supplements at ARIC visit 2. Secondly, we conducted a competing risk analysis (taking into account the competing risk of death),33 as vitamin D deficiency is also associated with increased risk of death from cardiovascular disease.34
Two sided P-values less than or equal to 0.05 were considered statistically significant. We performed statistical analyses using Stata version 12 (StataCorp, College Station, TX).
3. Results
In the overall study population (n=12,781), the mean age of participants was 57.1 years (SD 5.7), 55% were females, and 25% were black. The median 25(OH)D levels for whites, blacks, and overall were 25.6, 18.2, and 23.7 ng/mL, respectively. Among blacks, the majority had deplete levels of vitamin D (61.4%), whereas among whites fewer (23.1%) were deplete. Population characteristics stratified by vitamin D status and race are presented in Table 1. Compared to participants with replete levels, participants with deplete levels of vitamin D (<20 ng/ml) were more likely to be younger, current smokers, females, less physically active, and have lower education and higher BMI (p<0.001). Additionally, participants with deplete levels of vitamin D were more prone to having diabetes and hypertension (p<0.001).
Table 1.
Baseline characteristics of study participants by Vitamin D status during visit 2, 1990–1992
| 25(OH)D | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Overall | Whites | Blacks | ||||
|
|
||||||
| (<20.0 ng/ml) (n=416 0) | (≥20 ng/ml) (n=862 1) | (<20.0 ng/ml) (n= 2224) | (≥20 ng/ml) (n=740 3) | (<20.0 ng/ml) (n=193 6) | (≥20 ng/ml) (n=1218) | |
| 25(OH)D (ng/ml) | 15.3 ± 3.4 | 28.6 ± 6.7 | 15.9 ± 3.3 | 29.1 ± 6.8 | 14.6 ± 3.5 | 25.7 ± 4.8 |
| Vitamin D supplement Use | 426 (10.2) | 2,020 (23.4) | 237 (10.7) | 1,755 (23.7) | 189 (9.8) | 265 (21.8) |
| Vitamin D binding protein gene polymorphisms | ||||||
| rs4588 | ||||||
| CC | 2,268 (57.8) | 4,848 (58.7) | 889 (41.4) | 3,900 (54.7) | 1,379 (77.8) | 948 (84.1) |
| AC/AA | 1,654 (42.2) | 3,409 (41.3) | 1,260 (58.6) | 3,230 (45.3) | 394 (22.2) | 179 (15.9) |
| rs7041 | ||||||
| TT | 1,855 (47.2) | 1,964 (23.7) | 559 (26.0) | 1,220 (17.1) | 1,296 (72.9) | 744 (65.7) |
| TG/GG | 2,076 (52.8) | 6,312 (76.3) | 1,595 (74.0) | 5,923 (82.9) | 481 (27.1) | 389 (34.3) |
| Age (years) | 56.3 ± 5.7 | 57.2 ± 5.7 | 56.8 ± 5.7 | 57.2 ± 5.7 | 55.7 ± 5.6 | 57.0 ± 5.9 |
| Male | 1,334 (32.1) | 4,251 (49.3) | 750 (33.7) | 3,702 (50.0) | 584 (30.2) | 549 (45.1) |
| Race/center | ||||||
| Minneapolis, MN Whites | 801 (19.4) | 2,628 (30.5) | 801 (36.0) | 2,628 (35.5) | - | - |
| Washington County, MD Whites | 822 (19.9) | 2,462 (28.6) | 822 (37.0) | 2,462 (33.3) | - | |
| Forsyth County, NC Whites | 601 (14.6) | 2,313 (26.9) | 601 (27.0) | 2,313 (31.2) | - | - |
| Forsyth County, NC Blacks | 222 (5.4) | 112 (1.3) | - | - | 222 (11.7) | 112 (9.3) |
| Jackson, MS Blacks | 1,680 (40.7) | 1,093 (12.7) | - | - | 1,680 (88.3) | 1,093 (90.7) |
| ¶ Education | ||||||
| <High School | 1,035 (24.9) | 1,718 (20.0) | 339 (15.3) | 1,176 (15.9) | 696 (36.0) | 542 (44.7) |
| High School or Vocational School | 1,661 (40.0) | 3,668 (42.6) | 1,087 (49.0) | 3,347 (45.3) | 574 (29.7) | 321 (26.5) |
| College, Graduate, or Professional School | 1,457 (35.1) | 3,221 (37.4) | 794 (35.8) | 2,872 (38.8) | 663 (34.3) | 349 (28.8) |
| Smoking Status | ||||||
| Never | 1,713 (41.3) | 3,414 (39.6) | 823 (37.0) | 2,873 (38.8) | 890 (46.3) | 541 (44.6) |
| Former | 1,327 (32.0) | 3,480 (40.4) | 788 (35.4) | 3,113 (42.1) | 539 (28.0) | 367 (30.3) |
| Current | 1,106 (26.7) | 1,720 (20.0) | 613 (27.6) | 1,415 (19.1) | 493 (25.7) | 305 (25.1) |
| Alcohol consumption | ||||||
| Never | 1,150 (27.7) | 1,755 (20.4) | 449 (20.2) | 1,351 (18.3) | 701 (36.5) | 404 (33.3) |
| Former | 959 (23.1) | 1,693 (19.7) | 395 (17.8) | 1,289 (17.4) | 564 (29.4) | 404 (33.3) |
| Current | 2,036 (49.1) | 5,166 (60.0) | 1,380 (62.1) | 4,760 (64.3) | 656 (34.1) | 406 (33.4) |
| ¶ Physical activity index | 2.2 ± 0.7 | 2.6 ± 0.8 | 2.3 ± 0.7 | 2.6 ± 0.8 | 2.1 ± 0.7 | 2.2 ± 0.7 |
| Body mass index (kg/m2) | 29.4 ± 6.3 | 27.3 ± 4.8 | 28.5 ± 5.9 | 27.0 ± 4.6 | 30.5 ± 6.6 | 29.2 ± 5.6 |
| Diabetes | 842 (20.3) | 1,050 (12.2) | 364 (16.4) | 753 (10.2) | 478 (24.9) | 297 (24.6) |
| Hypertension | 1,821 (44.0) | 2,776 (32.3) | 727 (32.7) | 2,125 (28.8) | 1,094 (56.9) | 651 (53.7) |
| Total Cholesterol (mg/dl) | 209.6 ± 40.3 | 209.3 ± 37.8 | 209.8 ± 39.6 | 209.0 ± 37.1 | 209.5 ± 41.2 | 211.2 ± 41.7 |
| HDL (mg/dl) | 50.4 ± 16.7 | 49.8 ± 16.7 | 48.1 ± 16.0 | 49.2 ± 16.6 | 53.2 ± 17.1 | 53.5 ± 16.6 |
| LDL (mg/dl) | 134.1 ± 38.1 | 133.4 ± 36.0 | 134.2 ± 37.2 | 133.1 ± 35.3 | 134.0 ± 39.1 | 135.6 ± 40.0 |
| Triglycerides (mg/dl) | 125.6 ± 64.0 | 130.6 ± 65.8 | 137.6 ± 67.3 | 133.8 ± 66.4 | 111.6 ± 56.8 | 110.7 ± 58.0 |
| Magnesium (mEq/L) | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 |
| Estimated GFR <60 ml/min/1.73 m2 | 95 (2.3) | 179 (2.1) | 37 (1.7) | 133 (1.8) | 58 (3.0) | 46 (3.8) |
| Thiazide | 366 (8.8) | 569 (6.6) | 147 (6.6) | 385 (5.2) | 219 (11.3) | 184 (15.1) |
| Hormone replacement therapy | 658 (15.8) | 1,522 (17.7) | 381 (17.1) | 1,366 (18.5) | 277 (14.3) | 156 (12.8) |
| ** PTH (pg/ml) | 48.5 ± 31.9 | 39.7 ± 17.0 | 44.5 ± 17.1 | 39.0 ± 13.6 | 53.2 ± 42.8 | 44.4 ± 30.1 |
| ** Calcium (mg/dl) | 9.4 ± 0.5 | 9.3 ± 0.4 | 9.3 ± 0.4 | 9.3 ± 0.4 | 9.4 ± 0.5 | 9.5 ± 0.5 |
| ** Phosphate (mg/dl) | 3.6 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.5 | 3.6 ± 0.5 |
Data are means ± SDs or number (%)
Assessed at ARIC Visit 1 (1987–1989).
The following variables contained missing data: parathyroid hormone (n=166), calcium (n=170), and phosphate (n=169).
Abbreviations: 25(OH)D, 25-hydroxy-vitamin D; ARIC, Atherosclerosis Risk in Communities; GFR, glomerular filtration rate; HDL, high density lipoprotein; LDL, low density lipoprotein; PTH, parathyroid hormone; SD, standard deviation.
Over a median of 19.6 years of follow-up (maximum 21.9 years), there were 1,122 incident hospitalizations with an ICD-9 code for any fracture, which included 267 hospitalizations with incident hip fractures. Fracture rate was lower among blacks compared to whites. The fracture incidence rate for any fracture was: 5.1 per 1000 person years for overall; 5.9 per 1000 person years for whites; and 2.9 per 1000 person years for blacks. Hip fracture incidence rates were 1.2 per 1000 person years for overall; 1.4 per 1000 person years for whites; and 0.6 per 1000 person years for blacks.
Table 2 shows the risk of hospitalized fracture by vitamin D status. Even after adjustment for demographic, behavioral, and other confounding variables (Model 2), participants with deplete levels of 25(OH)D (<20 ng/mL) were at higher risk of any incident hospitalized fractures [HR=1.21 (95% CI 1.05–1.39)] and hip fractures [HR=1.35 (1.02–1.79)] compared to those with replete levels. Results were similar after additional adjustment for vitamin D related biomarkers (Model 3).
Table 2.
Adjusted hazard ratios for incident fracture hospitalization among study participants from visit 2 during 1990–1992 to Dec 31, 2011
| All fracture | Hip fracture | |||||
|---|---|---|---|---|---|---|
| 25(OH)D Replete (≥20.0 ng/ml) | 25(OH)D Deplete (<20.0 ng/ml) | P- Interaction by race | 25(OH)D Replete (≥20.0 ng/ml) | 25(OH)D Deplete (<20.0 ng/ml) | P- Interaction by race | |
| Overall | ||||||
| Median 25(OH)D (ng/ml) | 27.3 | 15.9 | 27.3 | 15.9 | ||
| n events / n total | 756 / 8621 | 366 / 4160 | 180 / 8621 | 87 / 4160 | ||
| Model 1 | 1.00 (reference) | 1.32 (1.15, 1.50) | 0.08 | 1.00 (reference) | 1.39 (1.06, 1.81) | 0.71 |
| Model 2 | 1.00 (reference) | 1.21 (1.05, 1.39) | 0.20 | 1.00 (reference) | 1.35 (1.02, 1.79) | 0.74 |
| Model 3 | 1.00 (reference) | 1.19 (1.03, 1.37) | 0.20 | 1.00 (reference) | 1.39 (1.04, 1.86) | 0.62 |
| Whites | ||||||
| Median 25(OH)D (ng/ml) | 27.8 | 16.7 | 27.8 | 16.7 | ||
| n events / n total | 696 / 7403 | 274 / 2224 | 168 / 7403 | 66 / 2224 | ||
| Model 1 | 1.00 (reference) | 1.37 (1.19, 1.58) | 1.00 (reference) | 1.40 (1.05, 1.86) | ||
| Model 2 | 1.00 (reference) | 1.22 (1.05, 1.42) | 1.00 (reference) | 1.34 (0.99, 1.82) | ||
| Model 3 | 1.00 (reference) | 1.19 (1.02, 1.39) | 1.00 (reference) | 1.37 (1.01, 1.87) | ||
| Blacks | ||||||
| Median 25(OH)D (ng/ml) | 24.5 | 15.1 | 24.5 | 15.1 | ||
| n events / n total | 60 / 1218 | 92 / 1936 | 12 / 1021 | 21 / 1575 | ||
| Model 1 | 1.00 (reference) | 1.04 (0.75, 1.45) | 1.00 (reference) | 1.28 (0.63, 2.64) | ||
| Model 2 | 1.00 (reference) | 1.06 (0.74, 1.51) | 1.00 (reference) | 1.33 (0.60, 2.92) | ||
| Model 3 | 1.00 (reference) | 1.02 (0.71, 1.48) | 1.00 (reference) | 1.33 (0.59, 3.02) | ||
Model 1: age, sex, race/center or center (race-stratified models).
Model 2: Model 1 + education, income, physical activity, smoking status, alcohol drinking status , body mass index, waist-to-hip ratio, diabetes, systolic and diastolic blood pressure, use of hypertension medication, total cholesterol, HDL cholesterol, estimated glomerular filtration rate, thiazide diuretic usage, hormone replacement therapy
Model 3 (demographic + behavioral + other confounders + possible mediators): Model 2 + calcium (mg/dl, continuous) + phosphate (mg/dl, continuous) + parathyroid hormones (pg/ml, continuous)
The association of vitamin D deficiency with incident fracture was statistically significant in whites but not in blacks. However, no significant racial interaction was seen [p-race interaction=0.20 for any fracture and 0.74 for hip fracture, Model 2]. There was also no significant interaction by sex (p-interaction=0.18 for any fracture and 0.72 for hip fracture). However, there was some evidence for effect modification by age, where older individuals and those with 25(OH)D deficiency were at greater risk of fracture-hospitalization [p-interaction=0.019 for any fracture and 0.053 for hip fracture (Supplemental Table 1)].
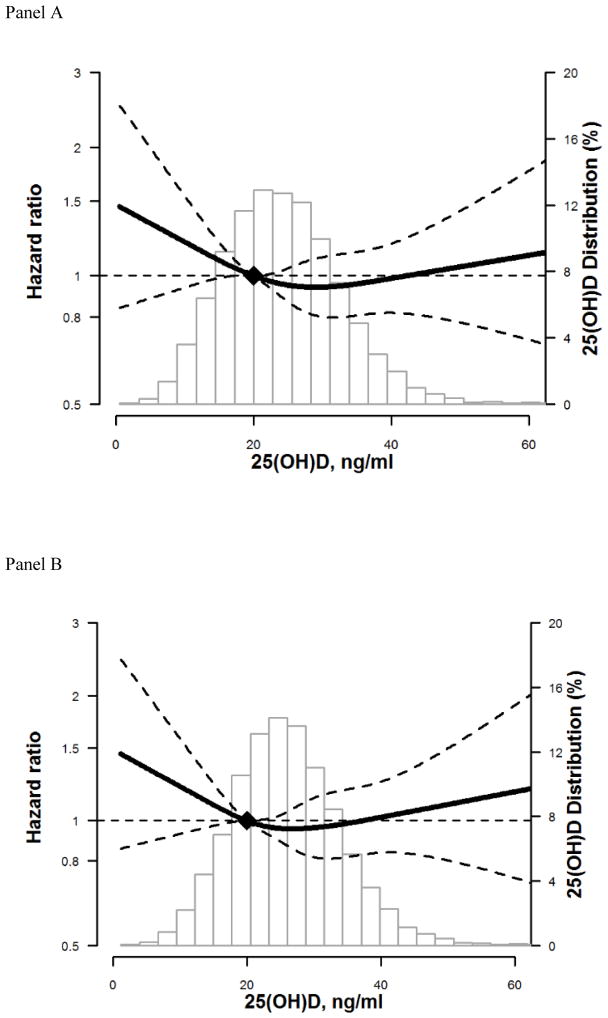
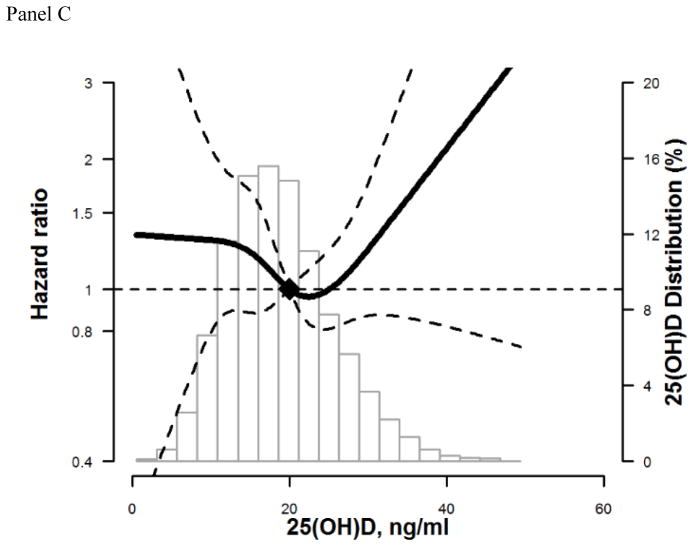
Assessment of the restricted cubic spline model with adjustment for demographic, behavioral, and other modifying variables, suggested a possible non-linear association between 25(OH)D levels and incidence of hospitalized fractures for whites and blacks. The risk appears to increase for levels <20 ng/ml, but confidence intervals overlap the null. (Figure 1: Panel A overall, Panel B whites, Panel C blacks).
Figure 1. Adjusted* restricted cubic spline model showing the hazard ratios (95% confidence intervals) for the association of 25(OH)D with incident hospitalized fracture for overall (Panel A), whites (Panel B), and blacks (Panel C).
The solid line represents the hazard ratios and the dashed lines represents the 95% confidence intervals. Knots at 5th, 35th, 65th, and 95th percentiles. Spline centered at 20 ng/ml. Histogram shows the distribution of concentrations of 25(OH)D. Restricted cubic spline truncated at 1st and 99th percentile of 25(OH)D.
*Model is adjusted for age, sex, race/center or center (race-stratified models), education, income, physical activity, smoking status, alcohol drinking status , body mass index, waist-to-hip ratio, diabetes, systolic and diastolic blood pressure, use of hypertension medication, total cholesterol, HDL cholesterol, estimated glomerular filtration rate, thiazide diuretic usage, hormone replacement therapy
Vitamin D supplements contribute to 25(OH)D levels and thus were not adjusted for in the main models. However, in a sensitivity analysis, we restricted to those not taking vitamin D supplements, and results were essentially unchanged (Supplemental Table 2). We also conducted a competing risk analysis (taking in account the competing risk of death), as older people are more likely to have fracture, and vitamin D deficiency is also associated with increased risk of death from cardiovascular disease.34 The competing risk model gave similar results (Supplemental Table 3).
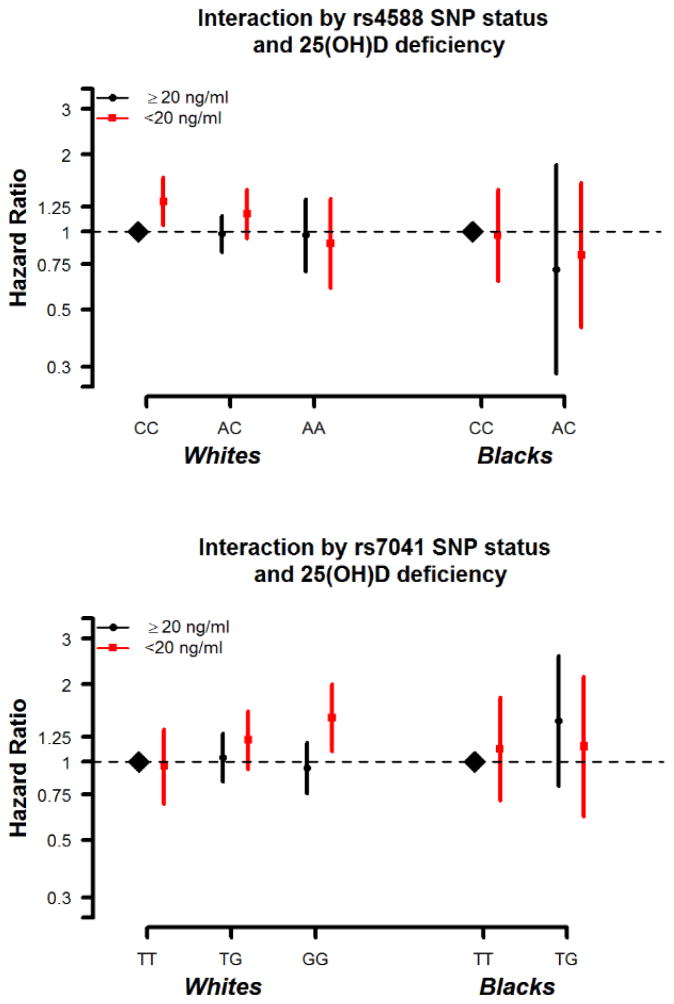
There was no independent association of DBP SNPs rs4588 or rs7041 with risk of fracture (Supplemental Table 4). However, the association of deplete serum 25(OH)D and fracture risk differed slightly by DBP genotype within subgroups of race (Figure 2). There was a marginal interaction between 25(OH)D deficiency and rs7041 among whites (p-interaction=0.065). Whites with both 25(OH)D deficiency and the GG genotype [i.e. with predicted higher levels of DBP and thus lower bioavailable vitamin D] were at the greatest risk for any fracture [HR=1.48 (1.10–2.00)], compared to the reference groups of whites with the TT genotype and replete 25(OH)D (Table 3). No significant interaction between rs7041 and 25(OH)D category was seen for blacks (p-interaction=0.41). However, few blacks had the GG genotype and were excluded from analysis. No significant interaction was seen for rs4588 and deplete 25(OH)D for either whites or blacks (whites: p-interaction=0.43, blacks: p-interaction=0.78 for any fracture).
Figure 2. Interaction between SNP status and 25(OH)D status.
*Model is adjusted for age, sex, race/center or center (race-stratified models), education, income, physical activity, smoking status, alcohol drinking status , body mass index, waist-to-hip ratio, diabetes, systolic and diastolic blood pressure, use of hypertension medication, total cholesterol, HDL cholesterol, estimated glomerular filtration rate, thiazide diuretic usage, hormone replacement therapy
Panel A: rs4588 SNP
Interaction by 25(OH)D status: Blacks: p=0.78; Whites: p=0.43
Panel B: rs7041 SNP
Interaction by 25(OH)D status: Blacks: p=0.41; Whites: p=0.07
Table 3.
Adjusted* Hazard Ratios for incident fracture by 25(OH)D status and rs7041 SNP status (TT versus TG/GG), follow-up: 1990–1992 through Dec 31, 2011.
| All fracture | Hip fracture | ||||
|---|---|---|---|---|---|
|
| |||||
| 25(OH)D Replete (≥20.0 ng/ml) | 25(OH)D Deplete (<20.0 ng/ml) | 25(OH)D Replete (≥20.0 ng/ml) | 25(OH)D Deplete (<20.0 ng/ml) | ||
| n events / n total | |||||
|
| |||||
| Whites | TT | 118 / 1102 | 55 / 504 | 25 / 1102 | 14 / 504 |
| TG | 337 / 3169 | 132 / 931 | 80 / 3169 | 37 / 931 | |
| GG | 219 / 2198 | 79 / 453 | 59 / 2198 | 13 / 453 | |
|
| |||||
| Blacks# | TT | 35 / 709 | 61 / 1235 | 5 / 709 | 18 / 1235 |
| TG | 20 / 336 | 19 / 413 | 6 / 336 | 2 / 413 | |
|
| |||||
| Overall | Model 1 | ||||
| TT | 1.00 (reference) | 1.07 (0.83, 1.37) | 1.00 (reference) | 1.65 (0.99, 2.76) | |
| TG | 1.04 (0.85, 1.26) | 1.31 (1.05, 1.64) | 1.28 (0.84, 1.96) | 1.78 (1.10, 2.87) | |
|
| |||||
| Model 2 | |||||
| TT | 1.00 (reference) | 1.01 (0.77, 1.31) | 1.00 (reference) | 1.68 (0.99, 2.85) | |
| TG | 1.07 (0.88, 1.31) | 1.22 (0.96, 1.55) | 1.32 (0.86, 2.03) | 1.74 (1.06, 2.84) | |
|
| |||||
| Whites | Model 1 | ||||
| TT | 1.00 (reference) | 1.05 (0.76, 1.44) | 1.00 (reference) | 1.31 (0.68, 2.53) | |
| TG | 1.04 (0.85, 1.29) | 1.39 (1.08, 1.78) | 1.20 (0.76, 1.87) | 1.89 (1.14, 3.15) | |
| GG | 0.96 (0.76, 1.20) | 1.73 (1.30, 2.31) | 1.24 (0.78, 1.98) | 1.39 (0.71, 2.72) | |
|
| |||||
| Model 2 | |||||
| TT | 1.00 (reference) | 0.96 (0.69, 1.34) | 1.00 (reference) | 1.32 (0.68, 2.55) | |
| TG | 1.04 (0.84, 1.29) | 1.22 (0.94, 1.58) | 1.18 (0.75, 1.85) | 1.73 (1.03, 2.91) | |
| GG | 0.95 (0.75, 1.20) | 1.48 (1.10, 2.00) | 1.23 (0.77, 1.97) | 1.38 (0.70, 2.72) | |
|
| |||||
| Blacks | Model 1 | ||||
| TT | 1.00 (reference) | 1.04 (0.69, 1.59) | 1.00 (reference) | 2.37 (0.88, 6.42) | |
| TG | 1.15 (0.66, 1.99) | 0.96 (0.55, 1.69) | 2.40 (0.73, 7.87) | 0.73 (0.14, 3.78) | |
|
| |||||
| Model 2 | |||||
| TT | 1.00 (reference) | 1.12 (0.71, 1.77) | 1.00 (reference) | 3.19 (1.01, 10.01) | |
| TG | 1.44 (0.81, 2.58) | 1.15 (0.62, 2.14) | 3.19 (0.86, 11.79) | 0.86 (0.15, 4.97) | |
Model 1: age, sex, race/center or center (race-stratified models).
Model 2: Model 1 + education, income, physical activity, smoking status, alcohol drinking status , body mass index, waist-to-hip ratio, diabetes, systolic and diastolic blood pressure, use of hypertension medication, total cholesterol, HDL cholesterol, estimated glomerular filtration rate, thiazide diuretic usage, hormone replacement therapy
Among the 3154 blacks, there are only 93 blacks with GG and 35 blacks with AA genotypes, and among these, only 2 blacks with GG and 5 blacks with AA had incident fracture. Because of the small sample size, results including these individuals were unwieldy, with extremely large confidence intervals.
4. Discussion
4.1 Summary of key findings
In this large community-based cohort of blacks and whites, participants with deplete levels of 25(OH)D (<20 ng/mL) had a greater risk of fracture, even after adjustment for demographic and behavioral variables, potential confounders, and related medication usages such as thiazide diuretics and hormone therapy. The rs7041 genotype might modify the association between low 25(OH)D levels and fractures, at least in whites, although results were not conclusive. No significant interaction was found for rs4588 and deplete 25(OH)D status.
4.2 25(OH)D levels and fracture risk by race
Risk of fracture due to deficient vitamin D status has been reported to vary by race/ethnicity.17 We found an increased risk of fracture associated with low 25(OH)D among whites but not a statistically significant association among blacks, although we may be underpowered for stratified results with fewer cases among blacks. We did not find any statistically significant interaction by race. This is in contrast to the results from WHI which paradoxically showed that black women with replete 25(OH)D ≥20 ng/ml actually had higher fracture risk compared to black women with levels <20 ng/ml.17 Our study population was both male and female, but we did not find an interaction by sex in our analysis
4.3 DBP genotype and fracture risk
We did not find a main effect of the rs7041 or rs4588 SNP on fracture risk. Individuals with an A allele at rs4588 or a G allele at rs7041 are more likely to have higher levels of DBP.18 Whites are more likely to have this genotype.18,20 Prior studies found that Caucasian women with the Gc2-2 phenotype of DBP (TT at rs7041 and AA at rs4588) had lower levels of 25(OH)D, yet, were three times less likely to have fractures compared to similar women with Gc1-1 phenotype.21,22 Another study found that while the GG genotype of the DBP polymorphism (rs2282679) was associated with lower 25(OH)D levels, it was not associated with past or prospective fractures among Caucasian European adults.25 The contrast among these results suggests that DBP genetics and its association with fracture risk may be dependent on other genetic and environmental factors, such as low 25(OH)D levels, low calcium levels, and the VDR gene.35
4.4 DBP SNPs may modify the risk of low 25(OH)D with fracture
We found some marginal evidence suggesting that the rs7041 DBP genetic polymorphism might modify the association between low 25(OH)D levels and incident fracture. We had hypothesized that those with a rs7041 GG genotype and a rs4588 AA genotype (with predicted lower DBP levels)18 would be at the highest risk for fracture in the setting of low 25(OH)D, due to lower bioavailable 25(OH)D. In accordance with our hypothesis, we did find a marginal statistical interaction, whereby white participants with deplete 25(OH)D levels and the rs7041 GG genotype were at higher risk for hospitalized fractures compared to reference whites (TT genotype with replete vitamin D). Few blacks had the GG genotype, so were unable to examine this relationship in blacks. There was no evidence of interaction between 25(OH)D category and rs4588 polymorphisms on fracture risk.
4.5 Clinical implications of findings
Our results showed a possible non-linear association between 25(OH)D levels and incidence of hospitalized fractures. Thus, it stands to reason that only patients with 25(OH)D deficiency may benefit from vitamin D treatment to prevent fractures.37 Although serum 25(OH)D was once considered the best measurement for vitamin D status, recent discussion of racial differences in serum DBP and bioavailable vitamin D may change this classification scheme. Prior studies have recognized that genetic variations of DBP influence an individual’s response to vitamin D supplementation. For example, a treatment study showed that healthy adults with varying DBP genotypes had different responses in 25(OH)D levels to the same supplemental dose of vitamin D3.36 Clinicians tend to prescribe vitamin D supplements to patients if their serum 25(OH)D levels are below 20 ng/mL; however, the definition for vitamin D deficiency may change based on recent findings about DBP. The level of bioavailable 25(OH)D could potentially be a better indicator of vitamin D status in different racial populations, particularly if it is better correlated with clinical outcomes such as fractures compared to total 25(OH)D.
It is currently inconclusive whether vitamin D treatment can prevent fractures. A review of 19 randomized clinical trials of vitamin D found a decreased fracture incidence in 7 studies, neutral results in 10 studies, and 2 trials with an increased fracture incidence (but the latter tested very high doses of vitamin D).16 Future studies/trials should test whether assessing DBP genotype status or DBP serum levels in combination with 25(OH)D levels influences whether supplements and/or supplement dosage can reduce risk of fracture and other health outcomes.
4.6 Study limitations
Our study has several limitations which must be taken into account in interpretation of our results. We were only able to analyze fractures that resulted in hospitalization, therefore it is likely that milder fractures treated in the out-patient setting were missed; however hip fracture represents a significant clinical event that typically requires hospitalization. Thus, our outcome is highly specific but not very sensitive. We also had only had a single measurement of 25(OH)D, but we did account for seasonal variation. We did not have measurements of serum DBP, so we could not directly calculate bioavailable vitamin D; DBP SNP genotypes were used as a surrogate for predicted DBP levels. Although we analyzed participants’ genotypes for DBP, these results must be interpreted cautiously since the analyses had limited power. Lastly, as with all observational data, inferences about causality must be made with caution.
4.7 Study Strengths
Despite these limitations, our study had several strengths. To the best of our knowledge, no other study has analyzed the interaction between vitamin D levels and incidence of fractures, while simultaneously stratifying by race and genotype (specifically the DBP SNPs of rs4588 and rs7041). We used a large, well-characterized, community-based sample of blacks and whites who were followed for 20 year, and we were able to adjust our results for numerous potential confounders and demographical/behavioral variables.
4.8 Summary and Future Implications
In conclusion, in the observational ARIC cohort, deplete levels of 25(OH)D (<20 ng/ml) was associated with an increased incidence of hospitalized fracture over 20 years of follow-up. We also found evidence, at least in whites, to suggest that an individual’s risk of fracture in the setting of deplete vitamin D may be affected by their genotype, specifically the rs7041 SNP of the DBP gene, but results were not conclusive. Fracture prevention clinical trials should be conducted to evaluate whether vitamin D supplementation is only beneficial for individuals who are vitamin D deficient, and also whether treatment strategies for deplete 25(OH)D should vary by predicted bioavailable vitamin D status.
Supplementary Material
Highlights.
Deficient levels of 25(OH)D (<20 ng/ml) was associated with an increased incidence of hospitalized fracture over 20 years of follow-up.
There was no independent association of key vitamin D binding protein SNPs (rs4588 and rs7041) with fracture risk.
Genetic variation in the rs7041 SNP, at least in whites, may modify risk of fracture in the setting of deficient vitamin D, but results not conclusive.
Further investigation is needed to determine whether treatment strategies for low 25(OH)D should vary by bioavailable vitamin D status.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
FUNDING
Dr. Michos was supported by NIH/NINDS grant R01NS072243. This research was also supported by grants from the NIH/NHLBI (R01HL103706 to Dr. Lutsey), the NIH Office of Dietary Supplements (R01HL103706-S1 to Dr. Lutsey) and the NIH/NIDDK (R01DK089174 to Dr. Selvin). Genotyping was supported through the NHLBI CARe (Candidate Gene Resource) grant N01HC65226. Dr. Schneider was supported by NIH/NHLBI training grant T32HL007024. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF, Chen TC. Vitamin d deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(suppl):1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steingrimsdotti L, Halldorsso TI, Siggeirsdotti K, Cotch MF, Einarsdotti BO, Eiriksdotti G, et al. Hip Fractures and Bone Mineral Density in the Elderly—Importance of Serum 25-Hydroxyvitamin D. PLoS One. 2014;9:1–8. doi: 10.1371/journal.pone.0091122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC, Lee JS, Jackson RD, Robbins JA, Wu C, Stanczyk FZ, LeBoff MS, Wactawski-Wende J, Sarto G, Ockene J, Cummings SR. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008 Aug 19;149(4):242–50. doi: 10.7326/0003-4819-149-4-200808190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai JK, Lucas RM, Clements MS, Roddam AW, Banks E. Hip fracture risk in relation to vitamin D supplementation and serum 25-hydroxyvitamin D levels: a systematic review and meta-analysis of randomised controlled trials and observational studies. BMC Public Health. 2010;10:331. doi: 10.1186/1471-2458-10-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iolascon G, Di Pietro G, Gimigliano F. Vitamin D supplementation in fractured patient: how, when and why. Clin Cases Miner Bone Metab. 2009;6:120–124. [PMC free article] [PubMed] [Google Scholar]
- 8.Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011 Dec 20;155(12):827–38. doi: 10.7326/0003-4819-155-12-201112200-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stähelin HB, Theiler R, Dawson-Hughes B. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012 Jul 5;367(1):40–9. doi: 10.1056/NEJMoa1109617. [DOI] [PubMed] [Google Scholar]
- 10.Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014 Apr;2(4):307–20. doi: 10.1016/S2213-8587(13)70212-2. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin d supplementation: A meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 12.Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database of Systematic Reviews. 2014;(4):1–165. doi: 10.1002/14651858.CD000227.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463. doi: 10.1136/bmj.b5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cauley AJ, Chlebowski RT, Wactawski-Wende J, Robbins JA, Rodabough RJ, Chen Z, Johnson CK, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women's Health Initiative. J Womens Health. 2013;22:915–929. doi: 10.1089/jwh.2013.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moyer VA US Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013;158:691–696. doi: 10.7326/0003-4819-158-9-201305070-00603. [DOI] [PubMed] [Google Scholar]
- 16.Lips P, Gielen E, van Schoor NM. Vitamin d supplements with or without calcium to prevent fractures. BoneKey Rep Int Bone Miner Society. 2014;512:1–6. doi: 10.1038/bonekey.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cauley JA, Danielson ME, Boudreau R, Barbour KE, Horwitz MJ, Bauer DC, et al. Serum 25 hydroxyvitamin (OH)D and clinical fracture risk in a multiethnic cohort of women: the women’s health initiative (WHI) J Bone Miner Res. 2011;26:2378–2388. doi: 10.1002/jbmr.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin d-binding protein and vitamin d status of black americans and white americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 20.Yousefzadeh P, Shapses SA, Wang X. Vitamin d binding protein impact on 25-hydroxyvitamin d levels under different physiologic and pathologic conditions. Int J Endocrinol. 2014;2014:1–6. doi: 10.1155/2014/981581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauridsen AL, Vestergaard P, Hermann AP, Moller HJ, Mosekilde L, Nexo E. Female premenopausal fracture risk is associated with gc phenotype. J Bone Miner Res. 2004;19:875–881. doi: 10.1359/JBMR.040133. [DOI] [PubMed] [Google Scholar]
- 22.Lauridsen AL, Vestergaard P, Hermann AP, Brot C, Heickendorff L, Mosekilde L, Nexo E. Plasma concentrations of 25-hydroxyvitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int. 2005;77:15–22. doi: 10.1007/s00223-004-0227-5. [DOI] [PubMed] [Google Scholar]
- 23.Ryan LM, Chamberlain JM, Singer SA, Wood R, Tosi LL, Freishtat RJ, Gordish-Dressman H, Teach SJ, Devaney JM. Genetic influences on vitamin d status and forearm fracture risk in African American children. J Investig Med. 2012;60:902–906. doi: 10.231/JIM.0b013e3182567e2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida S, Ikari K, Furuya T, Toyama Y, Taniguchi A, Yamanaka H, Momohara S. A GC polymorphism associated with serum25-hydroxyvitamin D level is a risk factor for hip fracture in Japanese patients with rheumatoid arthritis: 10-year follow-up of the Institute of Rheumatology, Rheumatoid Arthritis cohort study. Arthritis Res Ther. 2014;16:1–8. doi: 10.1186/ar4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trummer O, Schwetz V, Walter-Finell D, Lerchbaum E, Renner W, Gugatschka M, Dobnig H, Pieber TR, Obermayer-Pietsch B. Allelic determinants of vitamin d insufficiency, bone mineral density, and bone fractures. J Clin Endocrinol Metab. 2012;97:E1234–1240. doi: 10.1210/jc.2011-3088. [DOI] [PubMed] [Google Scholar]
- 26.The ARIC investigators. The atherosclerosis risk in communities (aric) study: Design and objectives. The aric investigators. Amer J of Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 27.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider AL, Williams EK, Brancati FL, Blecker S, Coresh J, Selvin E. Diabetes and risk of fracture-related hospitalization. Diabetes Care. 2013;36:1153–1158. doi: 10.2337/dc12-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 30.Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, Reid IR. The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am J Clin Nutr. 2007;86:959–964. doi: 10.1093/ajcn/86.4.959. [DOI] [PubMed] [Google Scholar]
- 31.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. Journal of the National Cancer Institute. 1988;80:1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 32.Michos ED, Reis JP, Post WS, Lutsey PL, Gottesman RF, Mosley TH, et al. 25-hydroxyvitamin d deficiency is associated with fatal stroke among whites but not blacks: The nhanes-iii linked mortality files. Nutrition. 2012;28:367–371. doi: 10.1016/j.nut.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 34.Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, et al. Circulating 25-hydroxy-vitamin d and risk of cardiovascular disease: A meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang Y, van Meurs JB, Arp P, et al. Vitamin D binding protein genotype and osteoporosis. Calcif Tissue Int. 2009;85:85–93. doi: 10.1007/s00223-009-9251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu L, Yun F, Oczak M, Wong BYL, Vieth R, Cole DEC. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009:1174–1177. doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet. 2014;383:146–55. doi: 10.1016/S0140-6736(13)61647-5. [DOI] [PubMed] [Google Scholar]
- 38.Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113–1118. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.