Abstract
Handedness is a feature of human motor control that is still not fully understood. Recent work has demonstrated that the dominant and nondominant arm each excel at different behaviors, and has proposed that this behavioral asymmetry arises from lateralization in the cerebral cortex: the dominant side specializes in predictive trajectory control, while the nondominant side is specialized for impedance control. Long-latency stretch reflexes are an automatic mechanism for regulating posture, and have been shown to contribute to limb impedance. To determine whether long-latency reflexes also contribute to asymmetric motor behavior in the upper limbs, we investigated the effect of arm dominance on stretch reflexes during a postural task that required varying degrees of impedance control. Our results demonstrated slightly but significantly larger reflex responses in the biarticular muscles of the nondominant arm, as would be consistent with increased impedance control. These differences were attributed solely to higher levels of voluntary background activity in the nondominant biarticular muscles, indicating that feedforward strategies for postural stability may differ between arms. Reflex sensitivity, which was defined as the magnitude of the reflex response for matched levels of background activity, was not significantly different between arms for a broad subject population ranging from 23–51 years of age. These results indicate that inter-arm differences in feedforward strategies are more influential during posture than differences in feedback sensitivity, in a broad subject population. Interestingly, restricting our analysis to subjects under 40 years of age revealed a small increase in long-latency reflex sensitivity in the nondominant arm relative to the dominant arm. Though our subject numbers were small for this secondary analysis, it suggests that further studies may be required to assess the influence of reflex lateralization throughout development.
Keywords: long-latency, reflex, arm dominance, posture, stability, asymmetry, lateralization
I. INTRODUCTION
In most individuals, there is an observed preference for using one arm over the other for a variety of skilled motor tasks. The side that is preferred for most tasks is known as the dominant arm/hand, and the dominance of a particular side is often termed “handedness”. A simple view of handedness is that the dominant arm is more skilled in general, due to a combination of genetic predisposition and accumulated practice. Indeed, the dominant arm tends to demonstrate superior speed, strength, and dexterity in many tasks (Goble & Brown 2008a). However, the assumed inferiority of the nondominant arm is challenged by numerous studies that suggest each arm excels at different types of tasks (Haaland & Harrington 1989; Elliott et al. 1993; Winstein & Pohl 1995; Sainburg 2002). For instance, in right-handers it has been shown that the nondominant arm excels at impedance-based positional control, with superior performance in stabilizing tasks and corrective movements (Bagesteiro & Sainburg 2003). Meanwhile, the dominant arm excels at complex trajectories and tasks that require coordination of limb dynamics (Bagesteiro & Sainburg 2002, Sainburg 2002). This theory of arm specialization agrees with a pattern observed in bimanual tasks, in which the nondominant arm tends to stabilize while the dominant arm performs more complex movements (e.g. writing on paper, opening a jar).
The observed asymmetry in right-handers’ arm performance likely stems from a lateralization of control systems in the cortical hemispheres. Lateralization is a common feature in the arrangement of neural structures, with one well-known example being that of language processing in humans (Broca 1861; Wernicke 1974). In this case, the left hemisphere is often specialized for basic components of language such as phonology and semantics (Vigneau et al. 2006), with the right hemisphere being crucial for more complex linguistic interpretation (Bottini et al. 1994). Neural centers for different types of motor control may also be lateralized to a particular hemisphere, although perhaps not to the same degree as language processing. The theory of motor lateralization is supported by studies of unilateral brain damage. For example, when comparing the corrective movements of subjects with unilateral stroke, reaching deficits are displayed in both the paretic and non-paretic arm (Schaefer et al. 2009). Furthermore, these deficits correspond with the proposed specialization of the lesioned hemisphere (Schaefer et al. 2012). These studies and others suggest that in right-handed individuals, the dominant (left) hemisphere is specialized for predictive, feedforward control and movement planning. Meanwhile, the nondominant (right) hemisphere specializes in feedback-mediated error correction mechanisms. It should be noted that these theories of lateralization have been developed specifically for the case of right-handedness, as left-handed subjects display less lateralization (Knecht et al. 2000, Przybyla et al. 2011a) and are often excluded from participation.
Recent work has quantified the behavioral asymmetry of the upper limbs (Bagesteiro & Sainburg 2002, Bagesteiro & Sainburg 2003, Sainburg & Kalakanis 2000), and has illuminated task features that highlight asymmetry (Yadav & Sainburg 2014a), as well as potential sites of lateralized controllers (Mutha et al. 2011, Schaefer et al. 2012). However, the neural mechanisms contributing to these behavioral asymmetries remain in question. The nondominant side has been shown to rely more heavily on proprioceptive feedback (Goble & Brown 2008b) and exhibits advantages in closed-loop feedback control (Winstein & Pohl 1995). The stretch reflex is one of the most rapid feedback control mechanisms that could contribute to behavioral asymmetry between limbs. Short-latency stretch reflexes are constrained to spinal pathways (Liddell & Sherrington 1924), and would therefore not be expected to exhibit asymmetries arising from lateralization in the cortex. However, long-latency stretch reflexes can involve supraspinal structures, specifically the cerebral cortex (Matthews 1991; Shemmell et al. 2009; Pruszynski et al. 2011). Thus, transcortical stretch reflexes are one mechanism that we propose may underlie the behavioral differences between the dominant and nondominant limb.
During postural tasks, perturbations of arm position will elicit stretch reflexes that tend to restore the limb to the desired posture (Gielen et al. 1988; Kurtzer et al. 2008). When the stability of the arm is challenged by the inherent instability of a task, the long-latency portion of the stretch reflex shows increased sensitivity (Akazawa et al. 1983; Krutky et al. 2010). For example, inherent instability in a task is demonstrated by holding the arm in a posture straight above the head, where gravity will destabilize the arm in all directions. Compare this to hanging the arm straight down, a posture that is stabilized by gravity. In less stable conditions, increased sensitivity of the long-latency reflex should enhance limb stability.
The component of the long-latency reflex that is sensitive to task stability is what we here call the stabilizing reflex. Modulation of stabilizing reflexes is shown to rely on involvement of the motor cortex, indicating a transcortical pathway (Kimura et al. 2006; Shemmell et al. 2009) that is specialized for stabilizing tasks. Based on the theory of hemispheric specialization, we might expect the nondominant left arm to display superior activation of stabilizing reflexes when counteracting a disturbance, yet previous studies have almost exclusively tested the right arm of right-handed subjects (Gielen et a. 1988; Shemmell et al. 2009; Krutky et al. 2010). Therefore, this study aimed to compare the stabilizing reflexes of the dominant and nondominant arm of right-handers during posture, in order to assess the contribution of long-latency reflexes as a potential mechanism underlying asymmetrical motor performance between the dominant and nondominant limb.
In this study, we assessed the sensitivity of stretch reflexes in the upper limbs of unimpaired right-handers. Subjects performed a single-joint postural task within two simulated haptic environments: one that stabilized the arm by pushing it towards the postural target, and one that challenged stability by pushing the arm away from the postural target. Rapid perturbations of the elbow posture were used to elicit reflexes in muscles crossing the elbow joint as subjects interacted with these environments. We hypothesized that the nondominant left arm would display more effective long-latency stabilizing reflexes, as compared to the dominant right arm. We defined “more effective” as higher reflex sensitivity in all conditions, or by a larger increase in reflex sensitivity when the haptic environment challenged postural stability. Thus, dual environments allowed us to test for an interaction effect between environment and arm. Specifically, we supposed that inter-arm reflex differences might be greater in an unstable environment, when there is a strong need for robust impedance control. Conversely, inter-arm differences might be greater in the stable environment, if the nondominant arm is optimized for impedance control in all conditions, while the dominant arm relies heavily upon it only when postural stability is challenged.
II. MATERIALS AND METHODS
A. Subjects & Experimental Setup
Eighteen right-handed, able-bodied individuals (age: 23–51, 9 females) with no known neurological disorders volunteered to participate in the experiment. Right-handedness was confirmed using the Edinburgh Handedness Inventory (Oldfield 1971; handedness score (mean±SD)= 86±13). All protocols were approved by the Northwestern University Institutional Review Board and required informed written consent.
Participants were seated with the trunk secured and the experiment arm posture at approximately 90° shoulder abduction, 0° shoulder flexion, and the elbow at 90° flexion. The upper arm was restrained by an adjustable trough and the wrist was immobilized in a pronated, neutral position using a thermoplastic cast. The cast was attached to a linear motor (Copley ThrustTube TB3806) instrumented with a force sensor and linear encoder to track force and position. The linear motor was oriented orthogonal to the forearm, so that small displacements of the motor caused rotation about the elbow joint. The linear motor was configured as an admittance servo, allowing us to simulate a range of haptic environments. The simulated environments had a stiffness of either 500 N/m (stable) or -500 N/m (unstable). We chose these stiffness magnitudes based on a previous study (Krutky et al. 2010), which demonstrated that similar changes in a haptic environment led to consistent changes in the sensitivity of the long-latency stretch reflex. Each environment was further configured as a second-order mechanical system with a mass of 2 kg and a damping of 10 Nm/s.
Surface electromyographic (EMG) activity was recorded from the brachioradialis, biceps, triceps lateralis, and triceps longus using bipolar Ag/AgCl electrodes (Noraxon). EMGs were amplified and conditioned using a Bortec AMT-8 with a band-pass filter of 10–1,000 Hz. The resulting signals were anti-alias filtered using 5th order Bessel filters with a 500-Hz cut-off frequency and sampled at 2,500 Hz using an analog to digital converter (PCI-DAS1602/16). Visual feedback of the current endpoint position was provided on a computer monitor placed directly in front of the participant.
B. Protocol
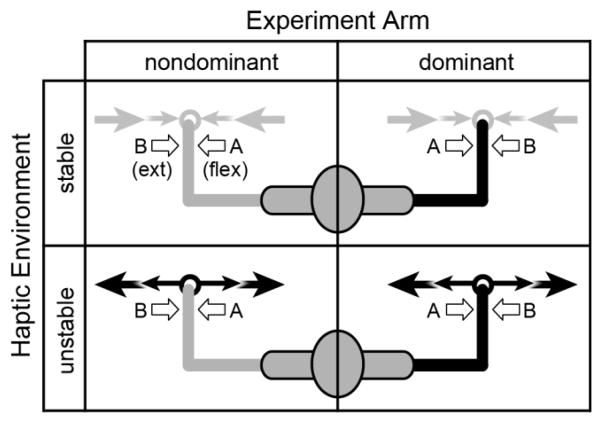
Our experiment was designed to elicit stabilizing long-latency reflexes in the elbow muscles. This was done by perturbing the elbow while subjects were engaged in a postural task. We tested each arm in a separate experiment, with the order of arms randomized for each subject. For each arm, the subject completed eight blocks of twenty trials. Each block presented either flexion or extension perturbations, and either a stable or an unstable haptic environment (Fig. 1). The order of blocks was randomized for each experiment.
Fig. 1.
Experimental block design used in this study. Each subject performed the experiment in the dominant (black) and nondominant (gray) arm on separate days, in a randomized order. For each arm, the subject was required to maintain elbow posture while interacting with the stable (light arrows) or unstable (dark arrows) haptic environment. In each condition, a bias force and perturbations were applied at the wrist to elicit stretch reflexes in the flexor (A) or extensor (B) muscles
At the beginning of each experiment, we collected a series of isometric maximum voluntary contractions (MVCs). These data were used to normalize the EMGs of each muscle in subsequent analyses. We also performed one MVC with the subject’s arm attached to the motor to determine each subject’s maximum elbow torque in the experimental arm posture. For the postural task, subjects were asked to maintain the motor position (i.e. the wrist) within a 4 mm target. In each block of trials, a bias force of 10% MVC was applied in the appropriate direction to preload either flexor or extensor muscles. This was done in an effort to obtain larger and clearer feedback responses by creating a baseline input to the motoneuron pool. The bias force, as well as the forces created by the haptic environment, had to be overcome in order to maintain the target posture. Each block included only perturbations in the appropriate direction to stretch the preloaded muscles. While this made the direction of perturbations predictable, there was a variable delay before the perturbations so that the timing was unpredictable. This method has been shown to produce robust reflexes that exhibit significant modulation, regardless of whether the subject is required to react to the perturbation (Shemmell et al. 2009).
A trial was initiated after the target posture was maintained for two seconds. Then there was a short, variable delay before a ramp-and-hold perturbation was applied to stretch the muscles. At the onset of the perturbation, the position feedback was frozen so that visual feedback of the perturbation did not affect the reflex response. All perturbations had a speed of 400 mm/s with a duration of 100 ms (4 cm amplitude), which is sufficient to activate the long-latency stretch reflex (Lewis et al. 2005). Perturbations in each environment were matched by transiently switching the linear motor to a position servo mode, as we have done previously (Perreault et al. 2008). Subjects were instructed not to react to the perturbation but to continue the ongoing muscle activity until instructed to relax. Instructions to actively resist a perturbation may activate multiple long-latency reflex pathways, and we wanted to limit our investigation to the proposed transcortical pathway, which shows sensitivity to environment stability (Shemmell et al. 2009).
After each perturbation, the motor returned to the starting position in servo mode and then switched back to the haptic environment. Visual feedback of the arm position was also restored. The subject was then free to initiate the next trial by entering the target. Randomly, before ten percent of trials, subjects were required to explore the workspace by moving the arm back and forth over the entire range of motion (4 cm at the wrist). This was done to ensure a continued familiarity with the haptic environment.
C. Data Analysis
Motor position, endpoint force in the direction of motion, and muscle activity were recorded in each experiment. To quantify the muscle activity, we mean-corrected and rectified the EMG, and then normalized by the maximum mean rectified EMG (0.5 sec moving average) recorded during the MVCs. We aligned all data to the onset of the perturbation. Trials were excluded if the mean pre-perturbation position was not in the target, or if the subject had not achieved a steady posture (standard deviation of pre-perturbation position >2mm); less than 2% of all trials were excluded. One subject’s data were excluded from the analysis of flexion tasks because they could not sufficiently stabilize the left arm during unstable flexion trials. Another subject’s data were excluded from the analysis of extension tasks because they did not complete all extension blocks in the right arm. Thus, for each perturbation direction there were 17 subjects included in the analysis.
For each trial, task performance and muscle activity were quantified. To quantify subject performance of the postural task, we computed the variability of the endpoint position. This was computed as the standard deviation of the measured endpoint position during the one second prior to the perturbation. This metric has previously been used to quantify performance of a postural task in compliant and unstable environments (Finley et al. 2012). Muscle activity was quantified for four different time windows: background activity (BGA: −100–0 ms), short-latency reflex (SLR: 25–50 ms), early long-latency reflex (LLR1: 50–75 ms) and late long-latency reflex (LLR2: 75–100 ms). We chose these time bins to be consistent with our previous work. In each window we calculated the average rectified amplitude. For each muscle, only perturbations that stretched the muscle were considered.
D. Statistics
Our hypothesis was that arm dominance would have a significant effect on the sensitivity of the long-latency reflex response during posture. Specifically, we expected the nondominant left arm to display larger reflexes in both environments, or to show a greater modulation of long-latency reflex sensitivity when postural stability was challenged by an unstable haptic environment.
The amplitude of a reflex response depends upon the size of the input stimulus (i.e. muscle stretch velocity), the sensitivity of the reflex pathway, and the level of pre-existing muscle activity (Matthews 1986). With carefully controlled perturbations, we have constrained the effect of the input stimulus. We expect the amplitude of stabilizing reflexes in this experiment to be modulated via mechanisms affecting feedback reflex sensitivity, as has been previously demonstrated (Krutky et al. 2010). However, increased feedforward muscle drive is an alternative strategy for enhancing postural stability and amplifying the reflex responses. To consider both feedback and feedforward effects on reflexes, we computed the mean reflex amplitudes both before and after a background-matching procedure. To enable a background-matched comparison, we manually selected a subset of trials for each muscle of each subject. Trials with overlapping levels of background activity were iteratively selected until paired t-tests showed no significant difference in the background activity between arms or environments for that subject-muscle. At least 10 trials were required for each arm-environment condition. If there was insufficient overlap of background activity for any condition, that subject-muscle was excluded from the background-matched analysis. All selected trials of background-matched data were then averaged to obtain the mean EMG activity in each condition.
We tested the null hypothesis that EMG amplitude was equivalent in the dominant and nondominant arm. This hypothesis was tested separately for each muscle and each time window analyzed (BGA, SLR, LLR1, LLR2) using a linear mixed-effects model. Arm (left/nondominant, right/dominant) and environment (stable, unstable) were fixed factors and subject was treated as a random factor. Analysis of variance (ANOVA) was used to assess the statistical significance of each factor, with significance evaluated against a p-value of 0.05. Post-hoc comparisons were used to evaluate the difference between levels of all significant factors, using Tukey’s honestly significant difference to correct for multiple comparisons. All statistics were done in MATLAB (2010a, MathWorks).
III. RESULTS
A. Arm strength and task performance are not affected by arm dominance
Our main goal was to compare reflex responses for each arm during a postural task. To compare appropriately across arms, we wanted to ensure that subjects were equally able to perform the postural task with each arm. A paired t-test of subject arm strength (using MVC force) showed that there was not a significant difference between dominant and nondominant arm strength in elbow flexion (p=0.28) or extension (p=0.64), as measured in the experimental arm position (Fig. 2). In addition, performance on the postural task was not significantly different between arms. This was assessed by quantifying the variability of the endpoint position prior to the perturbation, and testing the null hypothesis of equivalent variability in each arm and environment (using a linear mixed effects model as described previously for EMG analysis). There were not significant differences in endpoint variability between arms, during either the flexion (FLEX: F1,49=0.01; p=0.94) or extension task (EXT: F1,49=0.15; p=0.7). However, the endpoint variability was profoundly influenced by the haptic environment (FLEX: F1,49=275.16; p<0.001, EXT: F1,49=263.19; p<0.001), with an approximately threefold increase from the stable to unstable environment. Using two-sample t-tests, it was further confirmed that endpoint variability was significantly higher in the unstable environment for both arms of each subject (FLEX: all p<0.001, EXT: all p<0.01). This provides evidence that the destabilizing haptic environment challenged postural arm stability to a greater degree than the stable environment, for all subjects tested.
Fig. 2.
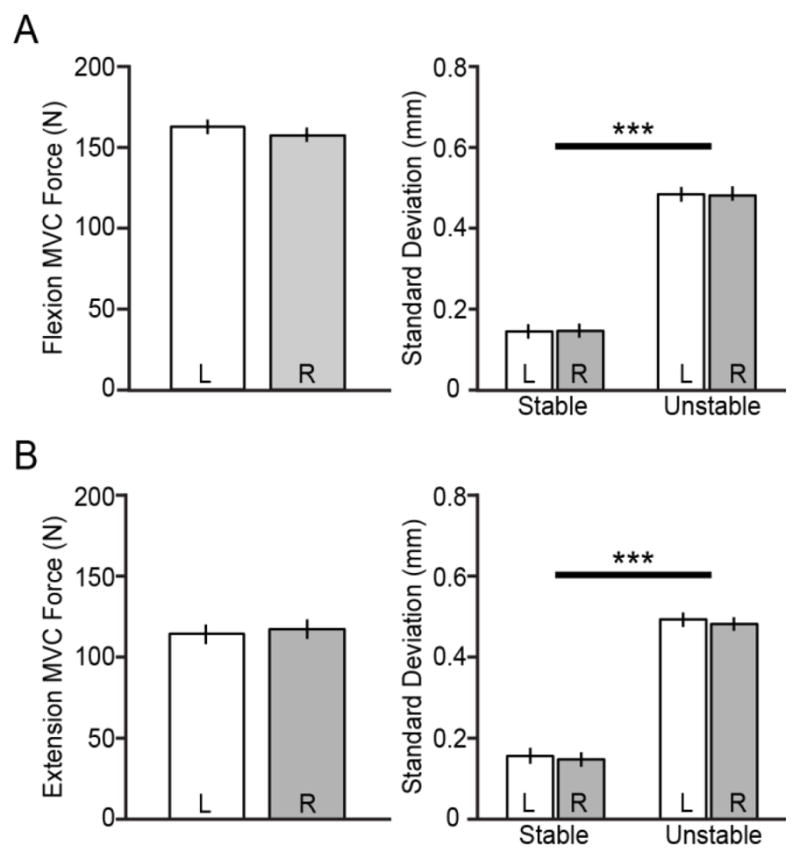
A: In the direction of elbow flexion, arm strength was not significantly different between arms (left), nor was the variability in endpoint position during the target posture (right). However, for both arms the variability of the endpoint position was significantly higher in the unstable environment (*** p < 0.001), indicating a greater challenge to postural arm stability in this environment. B: Likewise for the direction of elbow extension
B. Arm dominance has a small effect on the magnitude of stretch reflexes
Consistent environment-dependent reflexes were elicited in all muscles. Short- and long-latency reflex activity was observed in both arms and both environments (see Fig. 3 for reflex EMG of a representative subject). Across all subjects, there was a small but significant increase in reflex activity for the unstable compared to stable environment. This effect was observed in all reflex time windows, for all muscles tested, except for the short- and early long-latency time windows in brachioradialis (Fig. 4). The significance of this effect was lowest for the short-latency reflex windows (SLR: F1,49=1.70 to 16.39, p=0.2 to <0.001) and highest for the late long latency time windows (LLR2: F1,49=17.43 to 34.28, all p<0.001).
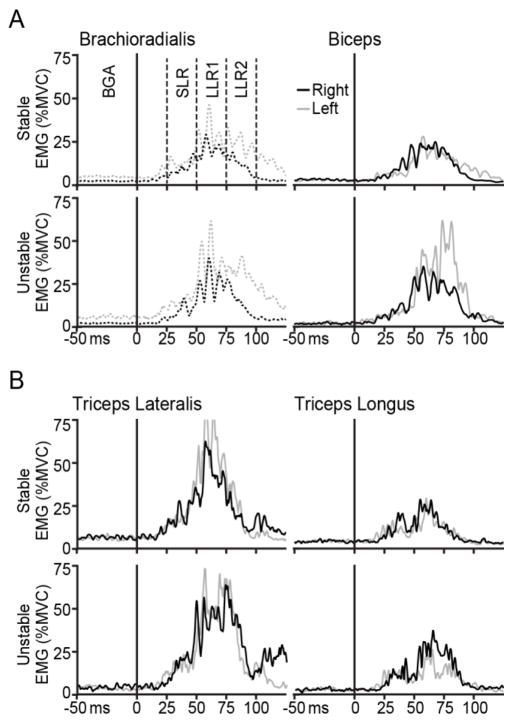
Fig. 3.
A: Average EMG traces for elbow flexion in a representative subject. EMGs from the stable environment are on top, with EMGs from the unstable environment below. The dominant right arm (black) and nondominant left arm (grey) are compared in each case. The onset of the perturbation is at t=0 ms and time windows are shown for background activity (BGA), short-latency reflex (SLR), and early (LLR1) and late (LLR2) long-latency reflex. B: Average EMG traces for elbow extension in this subject. For this subject, all muscles except brachioradialis (dashed) were matched for background activity
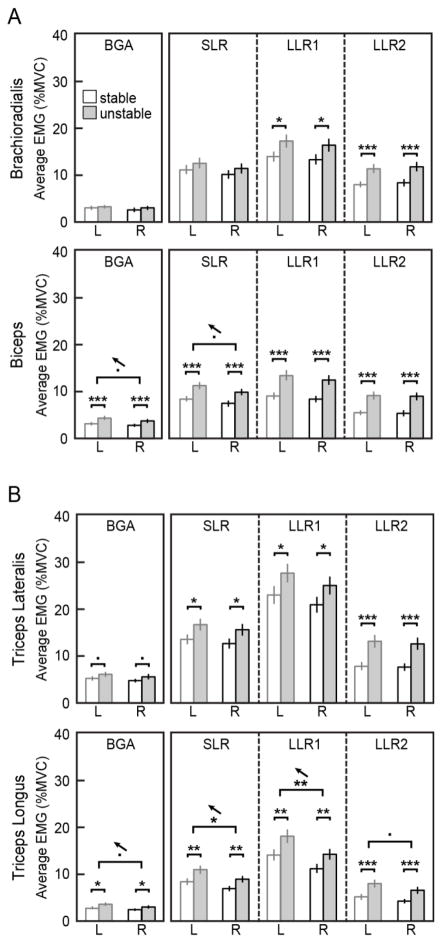
Fig. 4.
A: Across-subject averages for EMG activity in each time window, for flexion tasks. B: Across-subject averages for EMG in extension tasks. Before background matching, reflexes were higher in the nondominant (L) arm for biceps (SLR) and triceps longus (SLR, LLR1, LLR2). However, nondominant background activity was also higher in these muscles. Significant effects between arms and between environments are marked (***p<0.001, **p<0.01, *p<0.05, •p<0.1). Small arrows show the direction of the arm effects
There was a small increase in reflex magnitude in the nondominant arm as compared to the dominant arm, for two of the four tested muscles; specifically in the biarticular muscles. In triceps longus, nondominant EMG activity was significantly higher in the short-latency and early long-latency reflex windows (SLR: F1,49=6.92; p=0.01, LLR1: F1,49=7.49; p=0.009). Nondominant EMG activity was also higher, on average, for the late long-latency time window, but this trend did not reach significance (LLR2: F1,49=3.53; p=0.07). Likewise, there was a trend for higher nondominant EMG activity in the short-latency window in the biceps muscle (SLR: F1,49=3.42; p=0.07). Reflexes in triceps lateralis and brachioradialis did not show any trends or significant effects related to arm dominance. There was no significant interaction between arm and environment in any muscle or time window (F1,48=0.01 to 0.40; p=0.53 to 0.93).
Muscles that exhibited an arm-dependent change in reflex magnitude also had corresponding changes in background muscle activity prior to perturbation onset. Voluntary background EMG showed a trend for higher activity in the nondominant arm for both triceps longus (BGA: F1,49=3.44; p=0.07) and the biceps muscle (BGA: F1,49=3.63; p=0.06). Similarly, the observed environment-dependent changes in each muscle were preceded by environment-dependent differences in background activity, with higher EMG levels in the unstable environment (BGA: F1,49=1.71 to 12.68, p=0.2 to <0.001). We know that the amplitude of stretch reflex responses is substantially affected by the level of background activity in the muscle prior to stretch (Matthews 1986). Therefore, it may be that the observed effect of arm dominance (or environment) is not actually a difference in reflex sensitivity, but simply a consequence of different feedforward muscle activations in each arm, whose effects propagate into the reflex time windows.
C. Arm dominance does not affect reflex sensitivity after background matching in all subjects
Reflex sensitivity was not influenced by arm dominance after responses were matched for the background muscle activity. There was sufficient data to match across both arms and environments in 11 brachioradialis, 9 biceps, 9 triceps lateralis, and 7 triceps longus muscles. After this procedure there was no significant effect of arm dominance on the size of the EMG response in any reflex time window (F1,31/25/25/19=0 to 1.12, p=0.99 to 0.30). There was also no significant interaction between arm and environment in any muscle or time window (F1,31/25/25/19= <0.01 to 1.41; p=0.25 to 0.97). This suggests that the effects of arm dominance observed in the unmatched case are due primarily to differences in feedforward muscle activations, rather than differences in the feedback reflex sensitivities.
In contrast, the influence of environment was not abolished by the matching of background muscle activity. Reflex sensitivity was significantly higher for the less stable environment (Fig. 5), as has been previously shown (Akazawa et al. 1983; Krutky et al. 2010). After background-matching, this effect was only significant in the late long-latency window for most muscles (LLR2: F1,31/25/25/19=6.6 to 14.27, p=0.02 to <0.001), as well as for the short-latency window in triceps longus (SLR: F1,19= 11.06, p=0.004) and the early long-latency in biceps (LLR1: F1,25= 6.08, p=0.02). There were also near-significant trends in the short-latency window for biceps (SLR: F1,25 =3.86; p=0.06) and triceps lateralis (SLR: F1,25=3.11; p=0.09), and in the early long-latency of brachioradialis (LLR1: F1,31=3.55; p=0.07). These results suggest that the effect of environment is not only due to feedforward muscle activation patterns, but is also due to changes in reflex sensitivity, particularly in the late long-latency time period.
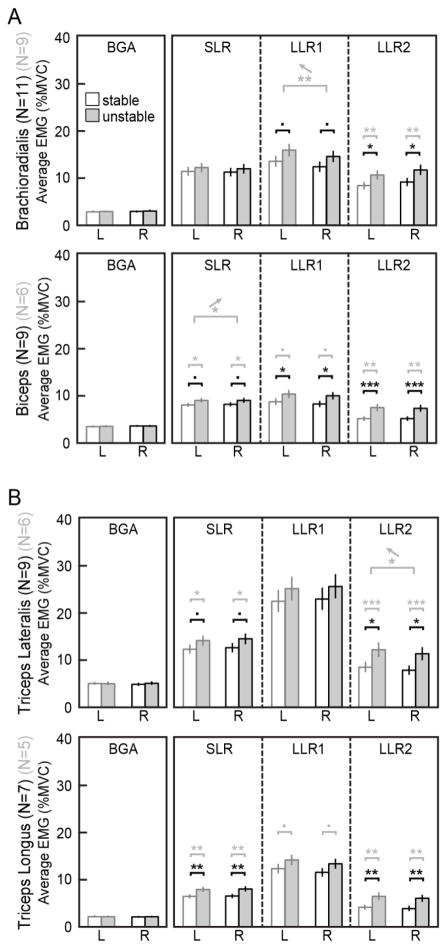
Fig. 5.
A: Across-subject averages for EMG activity in the flexion task, after background matching. N specified for each muscle indicates the number of subjects with matched background in all conditions. Statistical comparisons in black are for all background-matched subjects; those in gray are comparisons excluding older subjects. B: Same, for extension tasks. After background matching, significant effects of environment were still present. However, there were no significant effects of arm until older subjects were excluded (***p<0.001, **p<0.01, *p<0.05, •p<0.1)
D. There is a small increase in reflex lateralization in younger subjects
We included a broad population of subjects in this study, but some research suggests that young individuals display greater effects of lateralization as compared to older individuals (Cabeza 2002, Przybyla et al. 2011b). To investigate this possibility, we repeated our analysis after excluding four subjects who were over 40 years of age. This caused the mean±SD age to drop from 32±9 years old to 28±4 years old. Mean handedness score was not significantly changed.
After excluding older subjects and performing background-matching, long-latency reflex sensitivity was significantly higher in the nondominant arm for two of the four muscles tested (Fig. 5, statistical comparisons in gray). In brachioradialis (N=9 young matched subjects), the early long-latency reflex sensitivity was higher in the nondominant arm by about 22% (LLR1: F1,25=8.9, p=0.006). For comparison, the maximum effect of environment in the same muscle was a 25% increase for the unstable environment. Nondominant reflex sensitivity was also higher by 19% in the late long-latency window for triceps lateralis (N=6; LLR2: F1,16=4.8, p=0.045), compared to a 47% effect of environment in this muscle. These effects are consistent with our original hypothesis of increased long-latency reflex sensitivity in the nondominant arm. An effect in the opposite direction was also observed in the short-latency window of the biceps (N=6; SLR: F1,16=5.2, p=0.037). In this case, reflex sensitivity was higher in the dominant arm by approximately 11%, compared to an effect of environment that was 43%. While this effect is unexpected, it occurs in the short-latency time window, which is spinal rather than cortical and thus not directly in contradiction to our hypothesis. There were no significant interactions between arm and environment, as was found when analyzing the broader subject population.
IV. DISCUSSION
The objective of this study was to determine whether long-latency stabilizing reflexes manifest differently in the dominant and nondominant arm. Because the nondominant hemisphere is specialized for impedance control, we hypothesized that there would be increased sensitivity of stabilizing reflexes in the nondominant limb. We found that stabilizing reflexes were robustly present in both arms, with environment-dependent changes in reflex sensitivities between the stable and unstable environments. Furthermore, reflexes in the biarticular muscles of the nondominant limb were slightly larger than in the dominant limb. However, we found that this effect was not due to a significant difference in reflex sensitivity; rather, it stemmed from subtly different strategies of feedforward muscle activation during the postural task, which influenced reflex amplitude. This study is one of the few to examine the neural mechanisms that may underlie asymmetrical motor performance in the upper limbs, and our results indicate that the mechanism of stretch reflex tuning does not significantly contribute to these motor asymmetries in the broader population, at least for a purely postural task. However, an age-restricted analysis suggests that reflex lateralization may be prevalent at younger ages.
A. Challenges in comparing muscle activity between arms
We directly compared muscle activity and reflex responses between arms by collecting data from both the dominant and nondominant arm of each subject. While this allowed for pairwise comparison within subjects, there are still potential concerns in making a direct comparison between muscle activity from two distinct limbs. Extrinsic factors that could cause inter-limb differences include electrode placement, arm position or the order of experimental sessions. We controlled for these factors to the best of our ability by standardizing electrode sites and arm position, and by randomizing the order of limb testing for each subject. We also normalized all EMG measures to the value obtained in a maximum voluntary contraction for each muscle, which ameliorates many extrinsic sources of EMG variability (Lehman & McGill 1999).
The two arms could also differ intrinsically in terms of muscle composition or relative strength. For instance, there is evidence for higher percentages of slow twitch fibers for the dominant wrist and hand muscles (Fugl-Meyer et al. 1982; Adam et al. 1998). However, fiber type seems to affect fatigability rather than maximum muscle force, which is frequently shown to be statistically similar between limbs (Adam et al. 1998; Williams et al. 2002). Indeed, we found no significant difference in arm strength for our experimental task, and subjects were instructed to rest between experimental blocks or trials, as needed to minimize muscle fatigue.
Another potential limitation is the use of a single value of environment stiffness for all subjects (±500 N/m). We chose this value based on reported values of endpoint stiffness in a similar arm posture (Krutky et al. 2010), aiming for a negative stiffness that would overcome intrinsic endpoint stiffness in most subjects. However, we found that our weakest subjects found the unstable task quite difficult, while the strongest subjects could easily counteract the unstable field. While this likely introduces variability in our results, we believe that it does not significantly affect the outcome of the study. All subjects were able to complete the postural task after becoming familiar with the field (except left arm flexion for one subject, see Methods), and instability was sufficient to reveal a significant effect of environment on reflex sensitivity (Fig. 5).
B. Environment-dependent reflex sensitivity
In concordance with the existing literature, we found that instabilities in the environment can lead to increased reflex sensitivity. As in previous studies, this effect was found to be most significant in the long-latency time period. However, we also observed an increase in the short-latency reflex sensitivity for some muscles. While this is different than what we and others have reported before (Akazawa et al. 1983, Doemges & Rack 1992, Dietz et al. 1994, Perreault et al. 2008), this effect was small but significant, and was found to be consistent across individuals. Although short-latency reflexes are generally considered less adaptable than long-latency reflexes, short-latency reflex gains in the upper (Nakazawa et al. 1997) and lower limb (Ludvig et al. 2007) have occasionally been shown to modulate according to task or subject intent. We have not observed environment-dependent modulation of short-latency reflexes in our previous work. However, subjects in our previous multi-joint studies exhibited minimal short-latency activity (Perreault et al. 2008, Krutky et al. 2010) and our single-joint studies have previously included only stiff or compliant environments, not unstable (Lewis et al. 2005, Shemmell et al. 2009). It may be that the addition of an instability in this single-joint task has induced slight modulation in the short-latency time period as well. This short-latency modulation could result from a modest increase in gamma drive or from increased heteronymous reflexes in the unstable environment, which are different from the cortical mechanisms that can contribute to the long-latency reflex. Even though the effect here is small, it attests to the multiple mechanisms that can contribute to adaptation of rapid reflex responses, both at short- and long-latency time frames.
C. Feedforward strategies for maintenance of arm posture
We observed small arm-dependent differences in reflex amplitude, which seemed to result from increased feedforward activation of biarticular muscle segments in the nondominant arm. Specifically, we observed an effect of arm dominance for only the biceps and triceps longus, which include segments spanning both the elbow and shoulder joint. This may demonstrate differences in feedforward strategy between limbs. Numerous groups have theorized that muscles spanning multiple joints are essential for regulating impedance and stability in a multijoint limb (Hogan 1985; Franklin et al. 2003), and may be of special importance when interacting with a destabilizing load (McIntyre et al. 1996). We are unaware of any previous work comparing biarticular muscle usage in the dominant and nondominant arms, but our results indicate that the role of biarticular muscles may be more apparent on the nondominant side. However, we observed no significant difference between arms in the performance of the postural task (as quantified by positional variability), so it is unclear if the differences seen here are functionally relevant. Our task was limited, in that motion was restricted to the elbow joint; perhaps a difference in feedforward strategies would become more apparent during postural tasks requiring multi-joint coordination.
D. Lack of reflex asymmetry in the broad population
Our results did not demonstrate asymmetrical reflex sensitivity between the dominant and nondominant arms for a broad subject population. While subject age may be one crucial factor in the lack of asymmetry, we first discuss other potential reasons that reflex sensitivity may not differ between arms for this task. One possibility is that laterality cannot be observed for a purely postural task. Indeed, previous studies that demonstrate lateralized behavior have often focused on reaching or movement paradigms rather than posture (Winstein & Pohl 1995; Bagesteiro & Sainburg 2003; Goble & Brown 2008b). In these studies, stabilizing posture around a final target does seem to be a key feature; thus, we constrained our experiment to posture alone in order to simplify the task. However, it has been shown that posture and movement are controlled distinctly within the cerebral cortex (Kurtzer et al. 2005), so it may be that postural control is not lateralized to the same extent as movement control. Furthermore, the sensory feedback from muscle afferents can be modulated during movement from both peripheral and supraspinal sources (Brooke & Zehr 2006; Staines et al. 2000), which presents a potential mechanism for changing reflex sensitivity. Therefore, perhaps stretch reflexes exhibit arm-dependent modulation during movement control, but not posture alone.
A further theory is that asymmetry will only be present when a task requires a switch between posture and movement modes. A recent simulation study supports the idea of a hybrid control scheme, in which both arms can rely on the predictive controller to initiate movement and the impedance controller to stabilize the end of a movement (Yadav & Sainburg 2014b). Asymmetries in reaching performance may arise from switching between these controllers at different transition times. In a static postural task such as ours, there is no requirement for switching. Thus, each arm might be utilizing the impedance controller equally. If the impedance controller is responsible for modulating the sensitivity of long-latency reflexes, we may observe a different time course of reflex modulation during a task that transitions between movement and posture.
Finally, it could be that the reflex pathways involved in this postural task do not involve lateralized neural circuitry. We originally hypothesized that long-latency reflex sensitivity could exhibit asymmetry because of cortical involvement in this reflex. Specifically, there is evidence that the stabilizing long-latency reflex involves circuitry of the contralateral primary motor cortex (Kimura et al. 2006; Shemmell et al. 2009), which provides a unique opportunity for cortical lateralization to affect reflex responses. Although the exact pathways of the long-latency reflex remain unclear, there is a prevailing view that this feedback loop involves afferent projection through primary sensorimotor cortex (Phillips 1969; Evarts 1973; Matthews 1991; Pruszynski et al. 2011), to descending corticomotoneuronal projections (Cheney & Fetz 1984). These areas are often thought of as lower levels of brain processing. In contrast, cortical areas that contribute to lateralized motor control may be restricted to higher processing centers. In previous studies showing asymmetrical arm performance, there is often a requirement for more complex cortical processing, such as for trajectory planning (Bagesteiro & Sainburg 2003), multijoint coordination (Sainburg & Kalakanis 2000), or transfer of proprioceptive feedback between hemispheres (Goble & Brown 2008b). Indeed, Mutha et al. (2011) propose that parietal cortex of the left (dominant) hemisphere, and frontal cortex of the right (nondominant) hemisphere, may be key areas for lateralized control. Laterality may not be expressed in long-latency reflexes because the feedback pathway does not depend upon these higher cortical circuits.
E. Influence of age on reflex asymmetry
Subject age is a final feature that may contribute to the lack of asymmetry found in our overall results. Older adults often display reduced hemispheric lateralization when brain activity is monitored in cognitive (Cabeza 2002, Bergerbest et al. 2009) as well as motor (Mattay et al. 2002, Rowe et al. 2006) tasks. Motor performance in the upper limbs also becomes more symmetrical in older adults, such as for reaching trajectories (Przybyla et al. 2011b) and visuomotor adaptation transfer (Wang et al. 2011). Indeed, while subject age is seldom expressly discussed, many studies that demonstrate significant differences between dominant and nondominant arm performance use decidedly young subject populations (Elliott et al. 1993, Bagesteiro & Sainburg 2002, Bagesteiro & Sainburg 2003, Goble & Brown 2008b). Consistent with the above literature, we began to observe small effects of arm dominance only after excluding older subjects from the analysis. However, we are cautious to interpret the strength of these effects or infer a functional significance of the small asymmetries seen here. The exclusion of older subjects left us with fewer background-matched subjects for each of the tested muscles (see Fig. 5). The magnitudes of the arm effects in this younger population were modest, being smaller than the effect of environment in all cases. However, the effects seen in the long-latency time window were consistent with our original hypothesis of heightened reflex sensitivity in the nondominant arm. Further investigations focused on the influence of age may clarify the significance of these reflex asymmetries and how they change through the development process.
Acknowledgments
This work was supported in part by NIH grant NS053813-08 and by NSF grant 0932263. The authors would like to thank Timothy Haswell for his help with the experimental setup and Andrea Beer for her help with data collection.
References
- Adam A, De Luca CJ, Erim Z. Hand dominance and motor unit firing behavior. J Neurophysiol. 1998;80(3):1373–1382. doi: 10.1152/jn.1998.80.3.1373. [DOI] [PubMed] [Google Scholar]
- Akazawa K, Milner TE, Stein RB. Modulation of reflex EMG and stiffness in response to stretch of human finger muscle. J Neurophysiol. 1983;49(1):16–27. doi: 10.1152/jn.1983.49.1.16. [DOI] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol. 2002;88:2408–2421. doi: 10.1152/jn.00901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Nondominant arm advantages in load compensation during rapid elbow joint movements. J Neurophysiol. 2003;90(3):1503–1513. doi: 10.1152/jn.00189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergerbest D, Gabrieli JD, Whitfield-Gabrieli S, Kim H, Stebbins GT, Bennett DA, Fleischman DA. Age-associated reduction of asymmetry in prefrontal function and preservation of conceptual repetition priming. Neuroimage. 2009;45(1):237–246. doi: 10.1016/j.neuroimage.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RSJ, Firth D. The role of the right hemisphere in the interpretation of figurative aspects of language: a positron emission tomography activation study. Brain. 1994;117(6):1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Broca P. Remarks on the seat of the faculty of articulated language, following an observation of aphemia (loss of speech) Bulletin de la Société Anatomique. 1861;6:330–357. [Google Scholar]
- Brooke JD, Zehr EP. Limits to fast-conducting somatosensory feedback in movement control. Exercise Sport Sci R. 2006;34(1):22–28. doi: 10.1097/00003677-200601000-00006. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17(1):85. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol. 1984;349:249–272. doi: 10.1113/jphysiol.1984.sp015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Discher M, Trippel M. Task-dependent modulation of short- and long-latency electromyographic responses in upper limb muscles. Electroen Clin Neuro. 1994;93:49–56. doi: 10.1016/0168-5597(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Doemges F, Rack PM. Task-dependent changes in the response of human wrist joints to mechanical disturbance. J Physiol. 1992;447(1):575–585. doi: 10.1113/jphysiol.1992.sp019019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D, Roy EA, Goodman D, Carson RG. Asymmetries in the preparation and control of manual aiming movements. Can J Exp Psych. 1993;47(3):570–589. [Google Scholar]
- Evarts EV. Motor cortex reflexes associated with learned movement. Science. 1973;179(4072):501–503. doi: 10.1126/science.179.4072.501. [DOI] [PubMed] [Google Scholar]
- Finley JM, Dhaher YY, Perreault EJ. Contributions of feed-forward and feedback strategies at the human ankle during control of unstable loads. Exp Brain Res. 2012;217(1):53–66. doi: 10.1007/s00221-011-2972-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin DW, Osu R, Burdet E, Kawato M, Milner TE. Adaptation to stable and unstable dynamics achieved by combined impedance control and inverse dynamics model. J Neurophysiol. 2003;90(5):3270–3282. doi: 10.1152/jn.01112.2002. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Eriksson A, Sjostrom M, Soderstrom G. Is muscle structure influenced by genetical or functional factors? A study of three forearm muscles. Acta Physiol Scand. 1982;114(2):277–281. doi: 10.1111/j.1748-1716.1982.tb06983.x. [DOI] [PubMed] [Google Scholar]
- Gielen CCAM, Ramaekers L, van Zuylen EJ. Long-latency stretch reflexes as co-ordinated functional responses in man. J Physiology. 1988;407:275–292. doi: 10.1113/jphysiol.1988.sp017415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. The biological and behavior basis of upper limb asymmetries in sensorimotor performance. Neurosci Biobehav Rev. 2008a;32:598–610. doi: 10.1016/j.neubiorev.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. Upper limb asymmetries in the matching of proprioceptive versus visual targets. J Neurophysiol. 2008b;99:3063–3074. doi: 10.1152/jn.90259.2008. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington D. The role of the hemispheres in closed loop movements. Brain Cognition. 1989;9(2):158–180. doi: 10.1016/0278-2626(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Hogan N. The mechanics of multi-joint posture and movement control. Biol Cybern. 1985;52:315–331. doi: 10.1007/BF00355754. [DOI] [PubMed] [Google Scholar]
- Kimura T, Haggard P, Gomi H. Transcranial magnetic stimulation over sensorimotor cortex disrupts anticipatory reflex gain modulation for skilled action. J Neurosci. 2006;26:9272–9281. doi: 10.1523/JNEUROSCI.3886-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein E-B, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Krutky MA, Ravichandran VJ, Trumbower RD, Perreault EJ. Interactions between limb and environmental mechanics influence stretch reflex sensitivity in the human arm. J Neurophysiol. 2010;103:429–440. doi: 10.1152/jn.00679.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzer IL, Herter TM, Scott SH. Random change in cortical load representation suggests distinct control of posture and movement. Nature Neurosci. 2005;8(4):498–504. doi: 10.1038/nn1420. [DOI] [PubMed] [Google Scholar]
- Kurtzer IL, Pruszynski JA, Scott SH. Long-latency reflexes of the human arm reflect an internal model of limb dynamics. Curr Biol. 2008;18(6):449–453. doi: 10.1016/j.cub.2008.02.053. [DOI] [PubMed] [Google Scholar]
- Lehman GJ, McGill SM. The importance of normalization in the interpretation of surface electromyography: a proof of principle. J Manip Physiol Ther. 1999;22(7):444–446. doi: 10.1016/s0161-4754(99)70032-1. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Perreault EJ, MacKinnon CD. The influence of perturbation duration and velocity on the long-latency response to stretch in the biceps muscle. Exp Brain Res. 2005;163:361–369. doi: 10.1007/s00221-004-2182-9. [DOI] [PubMed] [Google Scholar]
- Liddell EGT, Sherrington CS. Reflexes in response to stretch (myotatic reflexes) Proc R Soc Lond B. 1924;96:212–242. [Google Scholar]
- Ludvig D, Cathers I, Kearney RE. Voluntary modulation of human stretch reflexes. Exp Brain Res. 2007;183:201–213. doi: 10.1007/s00221-007-1030-0. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58(4):630–635. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol. 1986;374:73–90. doi: 10.1113/jphysiol.1986.sp016066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. The human stretch reflex and the motor cortex. Trends Neurosci. 1991;14(3):87–91. doi: 10.1016/0166-2236(91)90064-2. [DOI] [PubMed] [Google Scholar]
- McIntyre J, Mussa-Ivaldi FA, Bizzi E. The control of stable postures in the multijoint arm. Exp Brain Res. 1996;110:248–264. doi: 10.1007/BF00228556. [DOI] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Critical neural substrates for correcting unexpected trajectory errors and learning from them. Brain. 2011;134(12):3644–3658. doi: 10.1093/brain/awr275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Yamamoto SI, Yano H. Short- and long-latency reflex responses during different motor tasks in elbow flexor muscles. Exp Brain Res. 1997;116:20–28. doi: 10.1007/pl00005740. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Perreault EJ, Chen K, Trumbower RD, Lewis G. Interactions with compliant loads alter stretch reflex gains but not intermuscular coordination. J Neurophysiol. 2008;99(5):2101–2113. doi: 10.1152/jn.01094.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CG. Ferrier lecture, 1968 – motor apparatus of baboons hand. Proc R Soc Lond Ser B Biol Sci. 1969;173:141–174. doi: 10.1098/rspb.1969.0044. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature. 2011;478:387–390. doi: 10.1038/nature10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla A, Good DC, Sainburg RL. Dynamic dominance varies with handedness: reduced interlimb asymmetries in left-handers. Exp Brain Res. 2011a;216(3):419–431. doi: 10.1007/s00221-011-2946-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla A, Haaland KY, Bagesteiro LB, Sainburg RL. Motor asymmetry reduction in older adults. Neurosci Lett. 2011b;489(2):99–104. doi: 10.1016/j.neulet.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Siebner H, Filipovic SR, Cordivari C, Gerschlager W, Rothwell J, Frackowiak R. Aging is associated with contrasting changes in local and distant cortical connectivity in the human motor system. Neuroimage. 2006;32(2):747–760. doi: 10.1016/j.neuroimage.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res. 2002;142(2):241–258. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol. 2000;83(5):2661–2675. doi: 10.1152/jn.2000.83.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia. 2009;47(13):2953–2966. doi: 10.1016/j.neuropsychologia.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Mutha PK, Haaland KY, Sainburg RL. Hemispheric specialization for movement control produces dissociable differences in online corrections after stroke. Cereb Cortex. 2012;22(6):1407–1419. doi: 10.1093/cercor/bhr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemmell J, An JH, Perreault EJ. The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics and verbal instruction. J Neurosci. 2009;29(42):13255–13263. doi: 10.1523/JNEUROSCI.0892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines WR, Brooke JD, McIlroy WE. Task-relevant selective modulation of somatosensory afferent paths from the lower limb. Neuroreport. 2000;11(8):1713–1719. doi: 10.1097/00001756-200006050-00024. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wang J, Przybyla A, Wuebbenhorst K, Haaland KY, Sainburg RL. Aging reduces asymmetries in interlimb transfer of visuomotor adaptation. Exp Brain Res. 2011;210(2):283–290. doi: 10.1007/s00221-011-2631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke C. Der aphasische symptomenkomplex. Springer; Berlin Heidelberg: 1974. [Google Scholar]
- Williams DM, Sharma S, Bilodeau M. Neuromuscular fatigue of elbow flexor muscles of dominant and nondominant arms in healthy humans. J Electomyogr Kines. 2002;12:287–294. doi: 10.1016/s1050-6411(02)00024-x. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Pohl PS. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res. 1995;105:163–174. doi: 10.1007/BF00242191. [DOI] [PubMed] [Google Scholar]
- Yadav V, Sainburg RL. Limb dominance results from asymmetries in predictive and impedance control mechanisms. PloS one. 2014a;9(4):e93892. doi: 10.1371/journal.pone.0093892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V, Sainburg RL. Handedness can be explained by a serial hybrid control scheme. Neurosci. 2014b;278:285–396. doi: 10.1016/j.neuroscience.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]